Variation Patterns and Climate-Influencing Factors Affecting Maximum Light Use Efficiency in Terrestrial Ecosystem Vegetation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Data
2.2.1. Flux Data
| Site_ID | Latitude | Longitude | Köppen–Geiger Classification | Biome Type | Data Period | Reference |
|---|---|---|---|---|---|---|
| NL-Loo | 52.1666 | 5.7436 | temperate | ENF | 2007–2009 | [44] |
| US-Me2 | 44.4526 | −121.5589 | temperate | ENF | 2013–2014 | [45] |
| IT-Lav | 45.9562 | 11.2813 | continental | ENF | 2007–2009 | [46] |
| CZ-BK1 | 49.5021 | 18.5369 | continental | ENF | 2012–2014 | [47] |
| CH-Dav | 46.8153 | 9.8559 | polar and alpine | ENF | 2007–2009 2012–2014 | [48] |
| PA-SPn | 9.3181 | −79.6346 | tropical | DBF | 2007–2008 | [49] |
| FR-Fon | 48.4764 | 2.7801 | temperate | DBF | 2007–2009 | [50] |
| DK-Sor | 55.4859 | 11.6446 | temperate | DBF | 2012–2014 | [51] |
| US-UMd | 45.5625 | −84.6975 | continental | DBF | 2007–2009 | [52] |
| US-UMB | 45.5598 | −84.7138 | continental | DBF | 2012–2014 | [53] |
| PA-SPs | 9.3138 | −79.6314 | tropical | GRA | 2007–2009 | [49] |
| US-SRG | 31.7894 | −110.8277 | dry | GRA | 2012–2014 | [54] |
| DE-RuR | 50.6219 | 6.3041 | temperate | GRA | 2012–2014 | [55] |
| US-IB2 | 41.8406 | −88.241 | continental | GRA | 2007–2009 | [56] |
| DE-Gri | 50.95 | 13.5126 | continental | GRA | 2012–2014 | [57] |
| CH-Fru | 47.1158 | 8.5378 | polar and alpine | GRA | 2007–2009 | [58] |
| RU-Sam | 72.3738 | 126.4958 | polar and alpine | GRA | 2012–2014 | [59] |
| FR-Gri | 48.8442 | 1.9519 | temperate | CORN | 2012–2014 | [60] |
| DE-Kli | 50.8931 | 13.5224 | continental | CORN | 2012–2014 | [57] |
| US-Myb | 38.0499 | −121.765 | temperate | WET | 2012–2014 | [61] |
| US-LA2 | 29.8587 | −90.2869 | temperate | WET | 2012–2013 | [62] |
| DE-Akm | 53.8662 | 13.6834 | continental | WET | 2012–2014 | [63] |
| GL-NuF | 64.1308 | −51.3861 | polar and alpine | WET | 2012–2014 | [64] |
2.2.2. MODIS Data
2.2.3. Environmental Factors Data
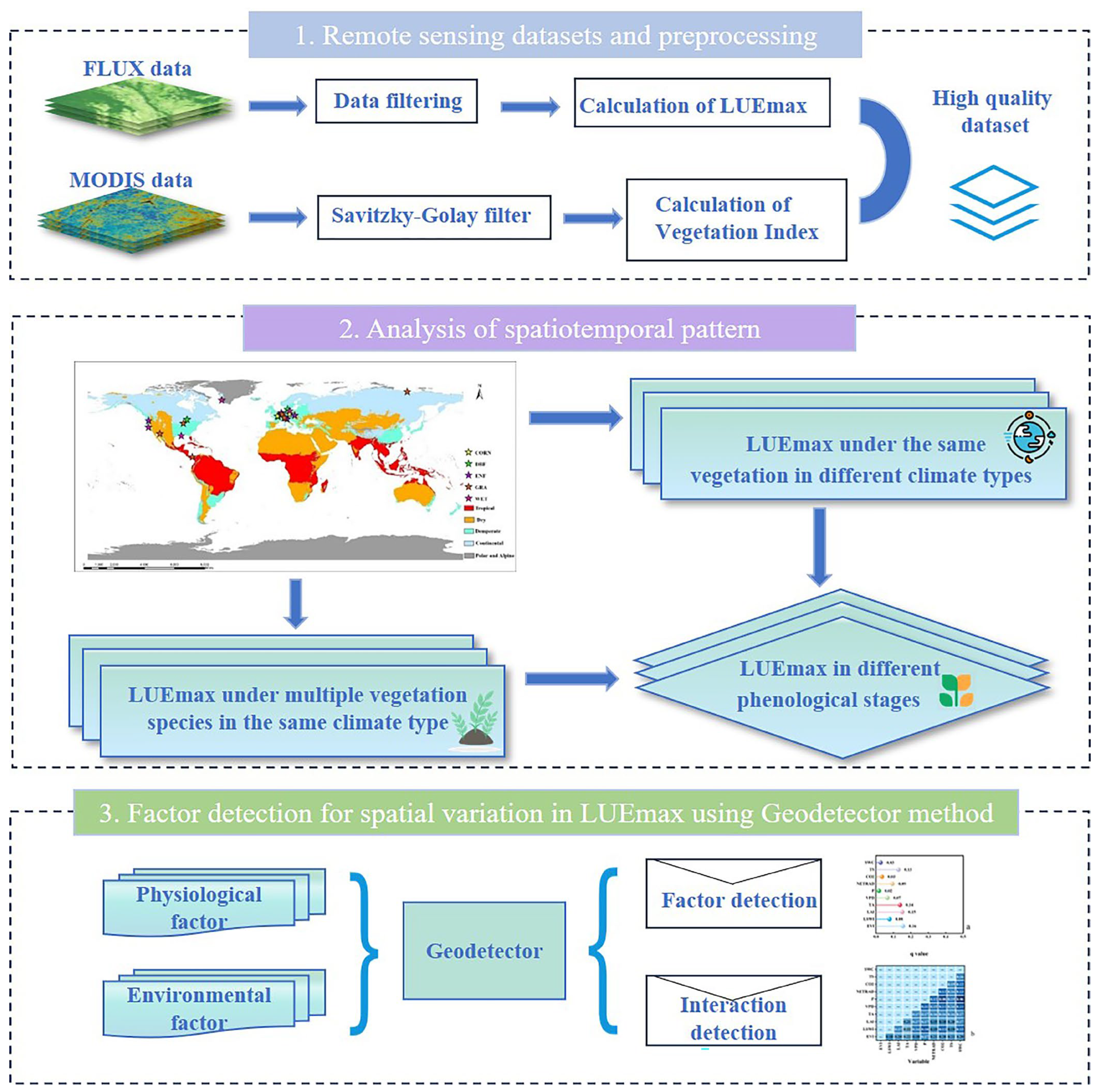
2.3. Methods
2.3.1. Calculation of Maximum Light Use Efficiency
2.3.2. Physical Extraction Methods
2.3.3. Trend Analysis
2.3.4. Geodetector
3. Results
3.1. Spatiotemporal Dynamic Variation Pattern in LUEmax
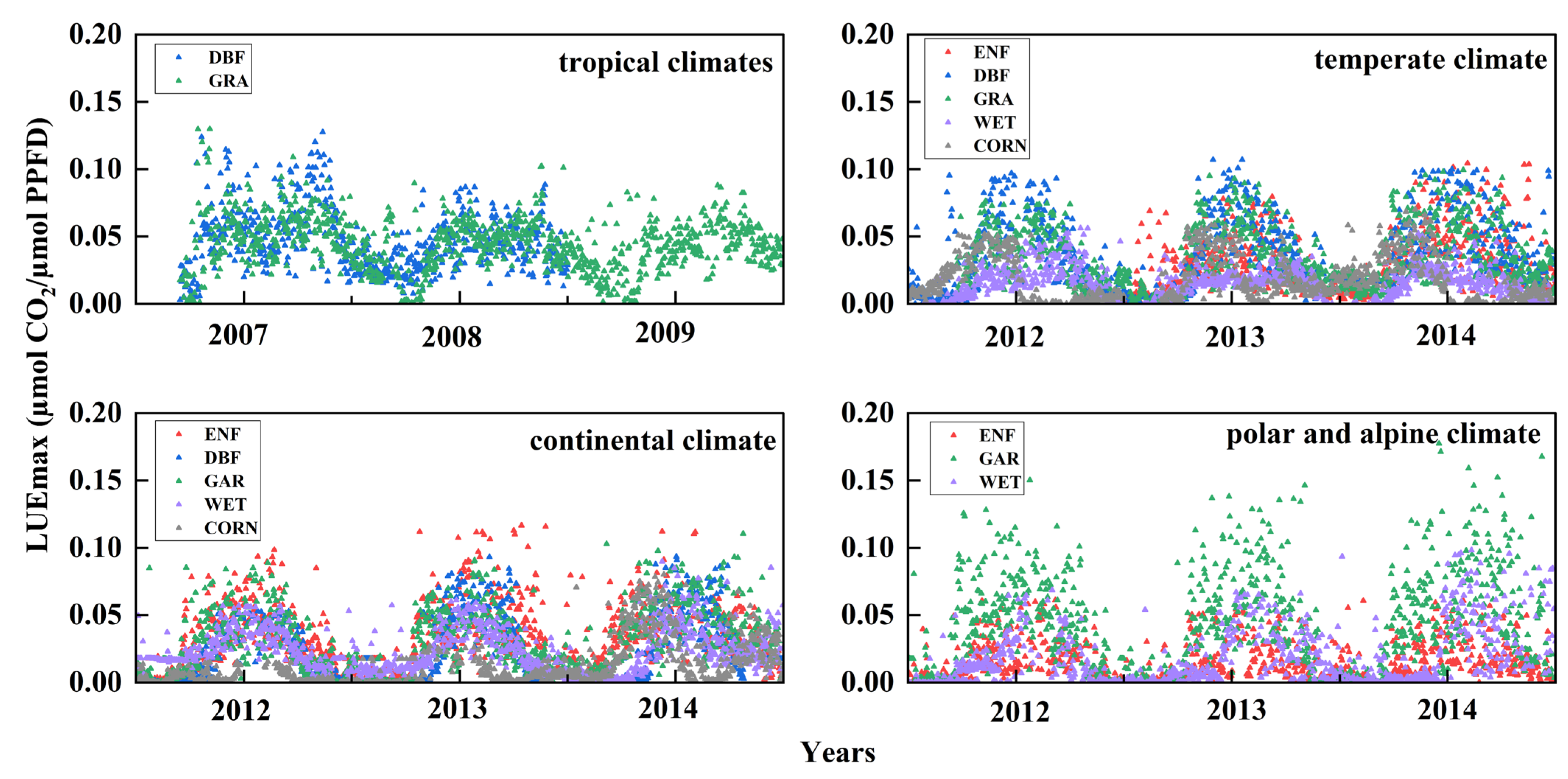
3.1.1. Temporal Dynamics of LUEmax for the Same Vegetation in Different Climate Types
3.1.2. Temporal Dynamics of LUEmax Across Multiple Vegetation Species in the Same Climate Type
3.1.3. Spatial Dynamics of the LUEmax
3.2. Impact of Factors on the LUEmax
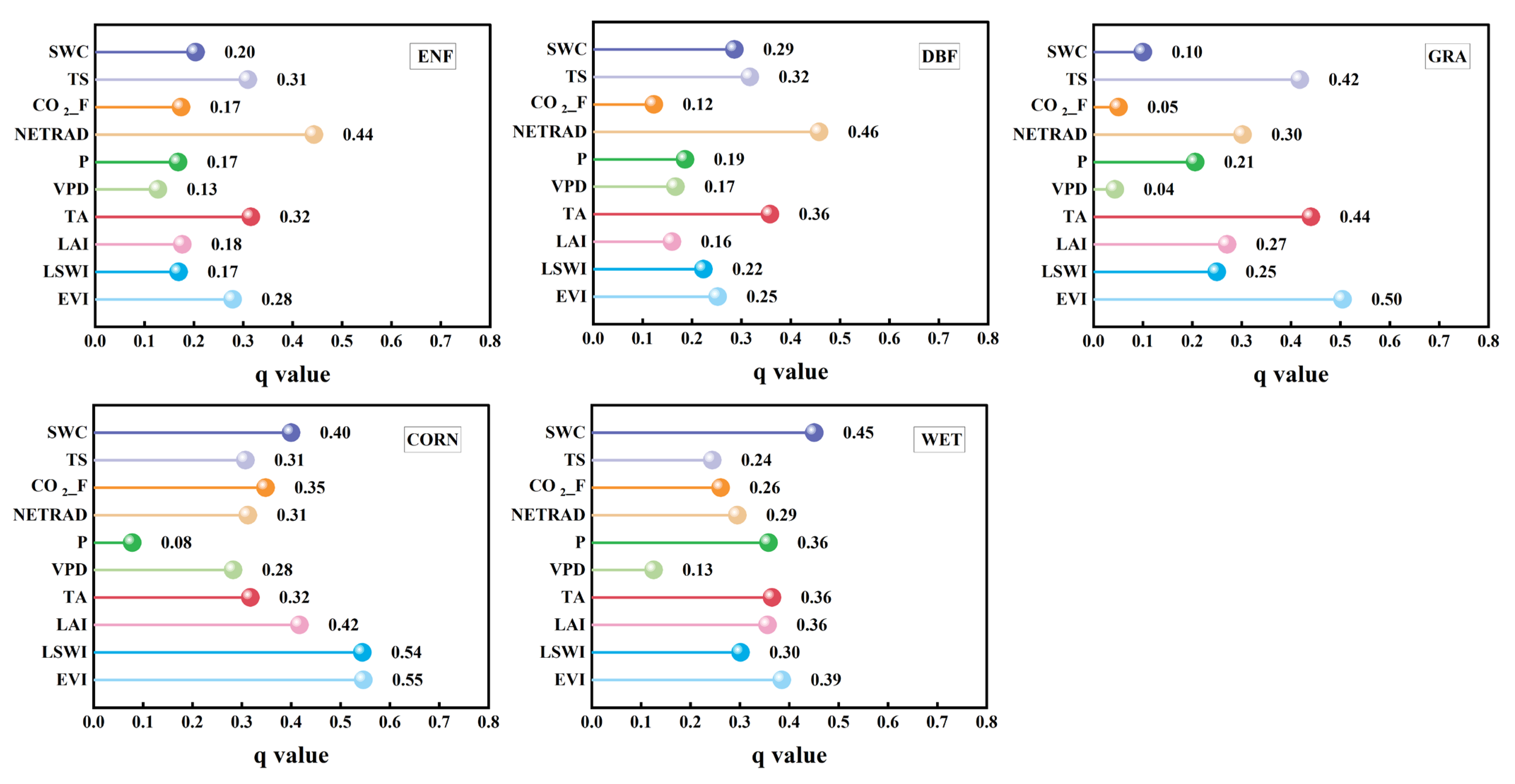
3.2.1. Impact of Factors on the LUEmax Across Different Vegetation Types
3.2.2. Impact of Factors on the LUEmax Across Different Climate Types
3.2.3. Impact of Factors on the LUEmax
4. Discussion
4.1. Dynamics of the LUEmax
4.2. Factors Affecting the Dynamics of LUEmax
4.3. Limitations and Perspectives of the Study
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GPP | gross primary productivity |
| LUEmax | maximum light use efficiency |
| SOS | start of growing season |
| EOS | end of growing season |
| DLF | double logistic function |
| EVI | enhanced vegetation index |
| LSWI | land surface water index |
| LAI | leaf area index |
| SG | Savitzky–Golay |
| NEE | net ecosystem exchange |
| Re | ecosystem respiration |
| PAR | photosynthetically active radiation |
| TS | temperature of the soil |
| TA | temperature of air |
| VPD | vapor pressure deficit |
| SWC | soil water content |
| MODIS | moderate resolution imaging spectroradiometer |
| CO2_F | carbon dioxide molar fraction |
References
- As-syakur, A.R.; Osawa, T.; Adnyana, I.W.S. Medium Spatial Resolution Satellite Imagery to Estimate Gross Primary Production in an Urban Area. Remote Sens. 2010, 2, 1496–1507. [Google Scholar] [CrossRef]
- Li, A.; Bian, J.; Lei, G.; Huang, C. Estimating the Maximal Light Use Efficiency for Different Vegetation through the CASA Model Combined with Time-Series Remote Sensing Data and Ground Measurements. Remote Sens. 2012, 4, 3857–3876. [Google Scholar] [CrossRef]
- Hilker, T.; Coops, N.C.; Wulder, M.A.; Black, T.A.; Guy, R.D. The use of remote sensing in light use efficiency based models of gross primary production: A review of current status and future requirements. Sci. Total Environ. 2008, 404, 411–423. [Google Scholar] [CrossRef]
- Monteith, J.L. Solar radiation and productivity in tropical ecosystems. J. Appl. Ecol. 1972, 9, 747–766. [Google Scholar] [CrossRef]
- Madani, N.; Kimball, J.S.; Affleck, D.L.; Kattge, J.; Graham, J.; Van Bodegom, P.M.; Reich, P.B.; Running, S.W. Improving ecosystem productivity modeling through spatially explicit estimation of optimal light use efficiency. J. Geophys. Res. Biogeosciences 2014, 119, 1755–1769. [Google Scholar] [CrossRef]
- Dong, T.; Liu, J.; Qian, B.; Jing, Q.; Croft, H.; Chen, J.; Wang, J.; Huffman, T.; Shang, J.; Chen, P. Deriving Maximum Light Use Efficiency From Crop Growth Model and Satellite Data to Improve Crop Biomass Estimation. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2017, 10, 104–117. [Google Scholar] [CrossRef]
- Bartlett, D.S.; Whiting, G.J.; Hartman, J.M. Use of vegetation indices to estimate indices to estimate intercepted solar radiation and net carbon dioxide exchange of a grass canopy. Remote Sens. Environ. 1989, 30, 115–128. [Google Scholar] [CrossRef]
- Zhao, C.; Zhu, W.; Xie, Z. Comparative evaluation of simulation methods for the maximum light-use efficiency of vegetation. J. Remote Sens. 2024, 28, 649–660. [Google Scholar] [CrossRef]
- Pei, Y.; Dong, J.; Zhang, Y.; Yuan, W.; Doughty, R.; Yang, J.; Zhou, D.; Zhang, L.; Xiao, X. Evolution of light use efficiency models: Improvement, uncertainties, and implications. Agric. For. Meteorol. 2022, 317, 108905. [Google Scholar] [CrossRef]
- Gan, R.; Zhang, L.; Yang, Y.; Wang, E.; Woodgate, W.; Zhang, Y.; Haverd, V.; Kong, D.; Fischer, T.; Chiew, F.; et al. Estimating ecosystem maximum light use efficiency based on the water use efficiency principle. Environ. Res. Lett. 2021, 16, 104032. [Google Scholar] [CrossRef]
- Yuan, W.; Cai, W.; Xia, J.; Chen, J.; Liu, S.; Dong, W.; Merbold, L.; Law, B.; Arain, A.; Beringer, J.; et al. Global comparison of light use efficiency models for simulating terrestrial vegetation gross primary production based on the LaThuile database. Agric. For. Meteorol. 2014, 192–193, 108–120. [Google Scholar] [CrossRef]
- Potter, C.S.; Randerson, J.T.; Field, C.B.; Matson, P.A.; Vitousek, P.M.; Mooney, H.A.; Klooster, S.A. Terrestrial ecosystem production: A process model based on global satellite and surface data. Glob. Biogeochem. Cycles 1993, 7, 811–841. [Google Scholar] [CrossRef]
- Myneni, R.B.; Los, S.O.; Asrar, G. Potential gross primary productivity of terrestrial vegetation from 1982–1990. Geophys. Res. Lett. 1995, 22, 2617–2620. [Google Scholar] [CrossRef]
- Lobell, D.B.; Hicke, J.A.; Asner, G.P.; Field, C.B.; Tucker, C.J.; Los, S. Satellite estimates of productivity and light use efficiencyin United States agriculture. Glob. Change Biol. 2002, 8, 722–735. [Google Scholar] [CrossRef]
- Wang, H.; Jia, G.; Fu, C.; Feng, J.; Zhao, T.; Ma, Z. Deriving maximal light use efficiency from coordinated flux measurements and satellite data for regional gross primary production modeling. Remote Sens. Environ. 2010, 114, 2248–2258. [Google Scholar] [CrossRef]
- Wang, M.; Sun, R.; Zhu, A.; Xiao, Z. Evaluation and Comparison of Light Use Efficiency and Gross Primary Productivity Using Three Different Approaches. Remote Sens. 2020, 12, 1003. [Google Scholar] [CrossRef]
- Zhao, M.; Heinsch, F.A.; Nemani, R.R.; Running, S.W. Improvements of the MODIS terrestrial gross and net primary production global data set. Remote Sens. Environ. 2005, 95, 164–176. [Google Scholar] [CrossRef]
- Prince, S.D.; Goward, S.N. Global primary production: A remote sensing approach. J. Biogeogr. 1995, 22, 815–835. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, X.; Wu, X.; Zhou, S.; Zhang, G.; Qin, Y.; Dong, J. A global moderate resolution dataset of gross primary production of vegetation for 2000–2016. Sci. Data 2017, 4, 170165. [Google Scholar] [CrossRef]
- Huang, X.; Zheng, Y.; Zhang, H.; Lin, S.; Liang, S.; Li, X.; Ma, M.; Yuan, W. High spatial resolution vegetation gross primary production product: Algorithm and validation. Sci. Remote Sens. 2022, 5, 100049. [Google Scholar] [CrossRef]
- Xie, Z.; Zhao, C.; Zhu, W.; Zhang, H.; Fu, Y.H. A radiation-regulated dynamic maximum light use efficiency for improving gross primary productivity estimation. Remote Sens. 2023, 15, 1176. [Google Scholar] [CrossRef]
- Wang, J.; Sun, R.; Zhang, H.; Xiao, Z.; Zhu, A.; Wang, M.; Yu, T.; Xiang, K. New global MuSyQ GPP/NPP remote sensing products from 1981 to 2018. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2021, 14, 5596–5612. [Google Scholar] [CrossRef]
- Groenendijk, M.; Dolman, A.J.; van der Molen, M.K.; Leuning, R.; Arneth, A.; Delpierre, N.; Gash, J.H.C.; Lindroth, A.; Richardson, A.D.; Verbeeck, H.; et al. Assessing parameter variability in a photosynthesis model within and between plant functional types using global Fluxnet eddy covariance data. Agric. For. Meteorol. 2011, 151, 22–38. [Google Scholar] [CrossRef]
- Zhu, X.; Pei, Y.; Zheng, Z.; Dong, J.; Zhang, Y.; Wang, J.; Chen, L.; Doughty, R.B.; Zhang, G.; Xiao, X. Underestimates of grassland gross primary production in MODIS standard products. Remote Sens. 2018, 10, 1771. [Google Scholar] [CrossRef]
- Thanyapraneedkul, J.; Muramatsu, K.; Daigo, M.; Furumi, S.; Soyama, N.; Nasahara, K.N.; Muraoka, H.; Noda, H.M.; Nagai, S.; Maeda, T. A vegetation index to estimate terrestrial gross primary production capacity for the Global Change Observation Mission-Climate (GCOM-C)/Second-Generation Global Imager (SGLI) Satellite Sensor. Remote Sens. 2012, 4, 3689–3720. [Google Scholar] [CrossRef]
- LIMOUSIN, J.M.; Misson, L.; LAVOIR, A.V.; Martin, N.K.; Rambal, S. Do photosynthetic limitations of evergreen Quercus ilex leaves change with long-term increased drought severity? Plant Cell Environ. 2010, 33, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Chen, B.; Chen, J.; Zhang, H.; Sun, S.; Xu, G.; Guo, L.; Ge, M.; Qu, J.; Li, L. Seasonal fluctuations of photosynthetic parameters for light use efficiency models and the impacts on gross primary production estimation. Agric. For. Meteorol. 2017, 236, 22–35. [Google Scholar] [CrossRef]
- Kong, D.; Yuan, D.; Li, H.; Zhang, J.; Yang, S.; Li, Y.; Bai, Y.; Zhang, S. Improving the Estimation of Gross Primary Productivity across Global Biomes by Modeling Light Use Efficiency through Machine Learning. Remote Sens. 2023, 15, 2086. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, Z.; Yu, G.; Yang, M.; Hao, T.; Zhu, X.; Zhang, W.; Han, L.; Liu, Z.; Ma, L.; et al. Spatial patterns of light response parameters and their regulation on gross primary productivity in China. Agric. For. Meteorol. 2024, 345, 109833. [Google Scholar] [CrossRef]
- Lv, Y.; Chi, H.; Shi, P.; Huang, D.; Gan, J.; Li, Y.; Gao, X.; Han, Y.; Chang, C.; Wan, J.; et al. Phenology-Based Maximum Light Use Efficiency for Modeling Gross Primary Production across Typical Terrestrial Ecosystems. Remote Sens. 2023, 15, 4002. [Google Scholar] [CrossRef]
- Turner, D.P.; Gower, S.T.; Cohen, W.B.; Gregory, M.; Maiersperger, T.K. Effects of spatial variability in light use efficiency on satellite-based NPP monitoring. Remote Sens. Environ. 2002, 80, 397–405. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gamon, J.A. The need for a common basis for defining light-use efficiency: Implications for productivity estimation. Remote Sens. Environ. 2015, 156, 196–201. [Google Scholar] [CrossRef]
- Xu, C.; Mao, F.; Du, H.; Li, X.; Sun, J.; Ye, F.; Zheng, Z.; Teng, X.; Yang, N. Full phenology cycle carbon flux dynamics and driving mechanism of Moso bamboo forest. Front. Plant Sci. 2024, 15, 1359265. [Google Scholar] [CrossRef]
- Bao, X.; Li, Z.; Xie, F. Environmental influences on light response parameters of net carbon exchange in two rotation croplands on the North China Plain. Sci. Rep. 2019, 9, 18702. [Google Scholar] [CrossRef]
- Li, J.T.; Zhang, Y.; Chen, H.; Sun, H.; Tian, W.; Li, J.; Liu, X.; Zhou, S.; Fang, C.; Li, B.; et al. Low soil moisture suppresses the thermal compensatory response of microbial respiration. Glob. Change Biol. 2022, 29, 874–889. [Google Scholar] [CrossRef] [PubMed]
- Gilmanov, T.G.; Aires, L.; Barcza, Z.; Baron, V.S.; Belelli, L.; Beringer, J.; Billesbach, D.; Bonal, D.; Bradford, J.; Ceschia, E.; et al. Productivity, Respiration, and Light-Response Parameters of World Grassland and Agroecosystems Derived From Flux-Tower Measurements. Rangel. Ecol. Manag. 2010, 63, 16–39. [Google Scholar] [CrossRef]
- Groffman, P.M.; Baron, J.S.; Blett, T.; Gold, A.J.; Goodman, I.; Gunderson, L.H.; Levinson, B.M.; Palmer, M.A.; Paerl, H.W.; Peterson, G.D.; et al. Ecological Thresholds: The Key to Successful Environmental Management or an Important Concept with No Practical Application? Ecosystems 2006, 9, 1–13. [Google Scholar] [CrossRef]
- Zhang, L.-M.; Yu, G.-R.; Sun, X.-M.; Wen, X.-F.; Ren, C.-Y.; Fu, Y.-L.; Li, Q.-K.; Li, Z.-Q.; Liu, Y.-F.; Guan, D.-X. Seasonal variations of ecosystem apparent quantum yield (α) and maximum photosynthesis rate (Pmax) of different forest ecosystems in China. Agric. For. Meteorol. 2006, 137, 176–187. [Google Scholar] [CrossRef]
- Wei, S.; Yi, C.; Fang, W.; Hendrey, G. A global study of GPP focusing on light-use efficiency in a random forest regression model. Ecosphere 2017, 8, e01724. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Baldocchi, D.; Falge, E.; Gu, L.; Olson, R.; Hollinger, D.; Running, S.; Anthoni, P.; Bernhofer, C.; Davis, K.; Evans, R. FLUXNET: A new tool to study the temporal and spatial variability of ecosystem-scale carbon dioxide, water vapor, and energy flux densities. Bull. Am. Meteorol. Soc. 2001, 82, 2415–2434. [Google Scholar] [CrossRef]
- Pastorello, G.; Trotta, C.; Canfora, E.; Chu, H.; Christianson, D.; Cheah, Y.-W.; Poindexter, C.; Chen, J.; Elbashandy, A.; Humphrey, M. The FLUXNET2015 dataset and the ONEFlux processing pipeline for eddy covariance data. Sci. Data 2020, 7, 225. [Google Scholar] [CrossRef] [PubMed]
- Moors, E.J. Water Use of Forests in the Netherlands; Vrije Universiteit: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Law, B. AmeriFlux AmeriFlux US-Me2 Metolius-Intermediate Aged Ponderosa Pine; Lawrence Berkeley National Laboratory (LBNL): Berkeley, CA, USA, 2016. [Google Scholar]
- Marcolla, B.; Pitacco, A.; Cescatti, A. Canopy architecture and turbulence structure in a coniferous forest. Bound. -Layer Meteorol. 2003, 108, 39–59. [Google Scholar] [CrossRef]
- Acosta, M.; Pavelka, M.; Montagnani, L.; Kutsch, W.; Lindroth, A.; Juszczak, R.; Janouš, D. Soil surface CO2 efflux measurements in Norway spruce forests: Comparison between four different sites across Europe—From boreal to alpine forest. Geoderma 2013, 192, 295–303. [Google Scholar] [CrossRef]
- Zielis, S.; Etzold, S.; Zweifel, R.; Eugster, W.; Haeni, M.; Buchmann, N. NEP of a Swiss subalpine forest is significantly driven not only by current but also by previous year’s weather. Biogeosciences 2014, 11, 1627–1635. [Google Scholar] [CrossRef]
- Wolf, S.; Eugster, W.; Potvin, C.; Turner, B.L.; Buchmann, N. Carbon sequestration potential of tropical pasture compared with afforestation in Panama. Glob. Change Biol. 2011, 17, 2763–2780. [Google Scholar] [CrossRef]
- Delpierre, N.; Berveiller, D.; Granda, E.; Dufrêne, E. Wood phenology, not carbon input, controls the interannual variability of wood growth in a temperate oak forest. New Phytol. 2016, 210, 459–470. [Google Scholar] [CrossRef]
- Pilegaard, K.; Ibrom, A.; Courtney, M.S.; Hummelshøj, P.; Jensen, N.O. Increasing net CO2 uptake by a Danish beech forest during the period from 1996 to 2009. Agric. For. Meteorol. 2011, 151, 934–946. [Google Scholar] [CrossRef]
- Atkins, J.W.; Bohrer, G.; Fahey, R.T.; Hardiman, B.S.; Morin, T.H.; Stovall, A.E.; Zimmerman, N.; Gough, C.M. Quantifying vegetation and canopy structural complexity from terrestrial Li DAR data using the forestr r package. Methods Ecol. Evol. 2018, 9, 2057–2066. [Google Scholar] [CrossRef]
- Aron, P.G.; Poulsen, C.J.; Fiorella, R.P.; Matheny, A.M. Stable water isotopes reveal effects of intermediate disturbance and canopy structure on forest water cycling. J. Geophys. Res. Biogeosciences 2019, 124, 2958–2975. [Google Scholar] [CrossRef]
- Baldocchi, D.; Penuelas, J. The physics and ecology of mining carbon dioxide from the atmosphere by ecosystems. Glob. Change Biol. 2019, 25, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Post, H.; Hendricks Franssen, H.-J.; Graf, A.; Schmidt, M.; Vereecken, H. Uncertainty analysis of eddy covariance CO2 flux measurements for different EC tower distances using an extended two-tower approach. Biogeosciences 2015, 12, 1205–1221. [Google Scholar] [CrossRef]
- Allison, V.J.; Miller, R.M.; Jastrow, J.D.; Matamala, R.; Zak, D.R. Changes in soil microbial community structure in a tallgrass prairie chronosequence. Soil Sci. Soc. Am. J. 2005, 69, 1412–1421. [Google Scholar] [CrossRef]
- Prescher, A.-K.; Grünwald, T.; Bernhofer, C. Land use regulates carbon budgets in eastern Germany: From NEE to NBP. Agric. For. Meteorol. 2010, 150, 1016–1025. [Google Scholar] [CrossRef]
- Imer, D.; Merbold, L.; Eugster, W.; Buchmann, N. Temporal and spatial variations of soil CO2, CH4 and N2O fluxes at three differently managed grasslands. Biogeosciences 2013, 10, 5931–5945. [Google Scholar] [CrossRef]
- Boike, J.; Kattenstroth, B.; Abramova, K.; Bornemann, N.; Chetverova, A.; Fedorova, I.; Fröb, K.; Grigoriev, M.; Grüber, M.; Kutzbach, L. Baseline characteristics of climate, permafrost and land cover from a new permafrost observatory in the Lena River Delta, Siberia (1998–2011). Biogeosciences 2013, 10, 2105–2128. [Google Scholar] [CrossRef]
- Loubet, B.; Laville, P.; Lehuger, S.; Larmanou, E.; Fléchard, C.; Mascher, N.; Genermont, S.; Roche, R.; Ferrara, R.M.; Stella, P. Carbon, nitrogen and Greenhouse gases budgets over a four years crop rotation in northern France. Plant Soil 2011, 343, 109–137. [Google Scholar] [CrossRef]
- Arias-Ortiz, A.; Oikawa, P.Y.; Carlin, J.; Masqué, P.; Shahan, J.; Kanneg, S.; Paytan, A.; Baldocchi, D.D. Tidal and nontidal marsh restoration: A trade-off between carbon sequestration, methane emissions, and soil accretion. J. Geophys. Res. Biogeosciences 2021, 126, e2021JG006573. [Google Scholar] [CrossRef]
- Krauss, K. AmeriFlux AmeriFlux US-LA2 Salvador WMA Freshwater Marsh; Lawrence Berkeley National Laboratory (LBNL): Berkeley, CA, USA, 2019. [Google Scholar]
- Bernhofer, C.; Grünwald, T.; Moderow, U.; Hehn, M.; Eichelmann, U.; Prasse, H.; Postel, U. FLUXNET2015 DE-Akm Anklam; FluxNet; TU Dresden: Dresden, Germany, 2016. [Google Scholar]
- Westergaard-Nielsen, A.; Lund, M.; Hansen, B.U.; Tamstorf, M.P. Camera derived vegetation greenness index as proxy for gross primary production in a low Arctic wetland area. ISPRS J. Photogramm. Remote Sens. 2013, 86, 89–99. [Google Scholar] [CrossRef]
- Jin, C.; Xiao, X.; Merbold, L.; Arneth, A.; Veenendaal, E.; Kutsch, W.L. Phenology and gross primary production of two dominant savanna woodland ecosystems in Southern Africa. Remote Sens. Environ. 2013, 135, 189–201. [Google Scholar] [CrossRef]
- Xiao, X.; Boles, S.; Liu, J.; Zhuang, D.; Liu, M. Characterization of forest types in Northeastern China, using multi-temporal SPOT-4 VEGETATION sensor data. Remote Sens. Environ. 2002, 82, 335–348. [Google Scholar] [CrossRef]
- Huete, A.; Liu, H.; Batchily, K.; Van Leeuwen, W. A comparison of vegetation indices over a global set of TM images for EOS-MODIS. Remote Sens. Environ. 1997, 59, 440–451. [Google Scholar] [CrossRef]
- Savitzky, A.; Golay, M.J. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Reichstein, M.; Falge, E.; Baldocchi, D.; Papale, D.; Aubinet, M.; Berbigier, P.; Bernhofer, C.; Buchmann, N.; Gilmanov, T.; Granier, A. On the separation of net ecosystem exchange into assimilation and ecosystem respiration: Review and improved algorithm. Glob. Change Biol. 2005, 11, 1424–1439. [Google Scholar] [CrossRef]
- Wang, J.; Wu, C.; Zhang, C.; Ju, W.; Wang, X.; Chen, Z.; Fang, B. Improved modeling of gross primary productivity (GPP) by better representation of plant phenological indicators from remote sensing using a process model. Ecol. Indic. 2018, 88, 332–340. [Google Scholar] [CrossRef]
- Bo, Y.; Li, X.; Liu, K.; Wang, S.; Zhang, H.; Gao, X.; Zhang, X. Three decades of gross primary production (GPP) in China: Variations, trends, attributions, and prediction inferred from multiple datasets and time series modeling. Remote Sens. 2022, 14, 2564. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, E.; Zhang, C.; Han, Y. Impact of seasonal global land surface temperature (LST) change on gross primary production (GPP) in the early 21st century. Sustain. Cities Soc. 2024, 110, 105572. [Google Scholar]
- Wang, J.F.; Li, X.H.; Christakos, G.; Liao, Y.L.; Zhang, T.; Gu, X.; Zheng, X.Y. Geographical Detectors-Based Health Risk Assessment and its Application in the Neural Tube Defects Study of the Heshun Region, China. Int. J. Geogr. Inf. Sci. 2010, 24, 107–127. [Google Scholar] [CrossRef]
- Song, Y.; Wang, J.; Ge, Y.; Xu, C. An optimal parameters-based geographical detector model enhances geographic characteristics of explanatory variables for spatial heterogeneity analysis: Cases with different types of spatial data. GIScience Remote Sens. 2020, 57, 593–610. [Google Scholar] [CrossRef]
- Bradford, J.; Hicke, J.; Lauenroth, W. The relative importance of light-use efficiency modifications from environmental conditions and cultivation for estimation of large-scale net primary productivity. Remote Sens. Environ. 2005, 96, 246–255. [Google Scholar] [CrossRef]
- Zhao, Y.; Niu, S.; Wang, J.; Li, H.; Li, G. Light use efficiency of vegetation: A review. Chin. J. Ecol 2007, 26, 1471–1477. [Google Scholar]
- de Conto, T.; Armston, J.; Dubayah, R. Characterizing the structural complexity of the Earth’s forests with spaceborne lidar. Nat. Commun. 2024, 15, 8116. [Google Scholar] [CrossRef]
- Wellington, M.J.; Kuhnert, P.; Renzullo, L.J.; Lawes, R. Modelling within-season variation in light use efficiency enhances productivity estimates for cropland. Remote Sens. 2022, 14, 1495. [Google Scholar] [CrossRef]
- Lele, N.; Kripa, M.; Panda, M.; Das, S.; Nivas, A.H.; Divakaran, N.; Naik-Gaonkar, S.; Sawant, A.; Pattnaik, A.; Samal, R. Seasonal variation in photosynthetic rates and satellite-based GPP estimation over mangrove forest. Environ. Monit. Assess. 2021, 193, 61. [Google Scholar] [CrossRef] [PubMed]
- Fei, X.H.; Song, Q.H.; Zhang, Y.P.; Yu, G.R.; Zhang, L.M.; Sha, L.Q.; Liu, Y.T.; Xu, K.; Chen, H.; Wu, C.S. Patterns and controls of light use efficiency in four contrasting forest ecosystems in Yunnan, Southwest China. J. Geophys. Res. Biogeosciences 2019, 124, 293–311. [Google Scholar] [CrossRef]
- Houborg, R.; Anderson, M.C.; Norman, J.M.; Wilson, T.; Meyers, T. Intercomparison of a ‘bottom-up’and ‘top-down’modeling paradigm for estimating carbon and energy fluxes over a variety of vegetative regimes across the US. Agric. For. Meteorol. 2009, 149, 2162–2182. [Google Scholar] [CrossRef]
- Teh, C.; Simmonds, L.; Wheeler, T. An equation for irregular distributions of leaf azimuth density. Agric. For. Meteorol. 2000, 102, 223–234. [Google Scholar] [CrossRef]
- Medlyn, B.; Loustau, D.; Delzon, S. Temperature response of parameters of a biochemically based model of photosynthesis. I. Seasonal changes in mature maritime pine (Pinus pinaster Ait.). Plant Cell Environ. 2002, 25, 1155–1165. [Google Scholar] [CrossRef]
- Zhu, G.-F.; Li, X.; Su, Y.-H.; Lu, L.; Huang, C.-L. Seasonal fluctuations and temperature dependence in photosynthetic parameters and stomatal conductance at the leaf scale of Populus euphratica Oliv. Tree Physiol. 2011, 31, 178–195. [Google Scholar] [CrossRef]
- Saito, M.; Miyata, A.; Nagai, H.; Yamada, T. Seasonal variation of carbon dioxide exchange in rice paddy field in Japan. Agric. For. Meteorol. 2005, 135, 93–109. [Google Scholar] [CrossRef]
- Turner, D.; Ritts, W.; Styles, J.; Yang, Z.; Cohen, W.; Law, B.; Thornton, P. A diagnostic carbon flux model to monitor the effects of disturbance and interannual variation in climate on regional NEP. Tellus B Chem. Phys. Meteorol. 2006, 58, 476–490. [Google Scholar] [CrossRef]
- Fisher, J.B.; Huntzinger, D.N.; Schwalm, C.R.; Sitch, S. Modeling the terrestrial biosphere. Annu. Rev. Environ. Resour. 2014, 39, 91–123. [Google Scholar] [CrossRef]
- Baldocchi, D.D. Assessing the eddy covariance technique for evaluating carbon dioxide exchange rates of ecosystems: Past, present and future. Glob. Change Biol. 2003, 9, 479–492. [Google Scholar] [CrossRef]
- Loescher, H.; Law, B.; Mahrt, L.; Hollinger, D.; Campbell, J.; Wofsy, S. Uncertainties in, and interpretation of, carbon flux estimates using the eddy covariance technique. J. Geophys. Res. Atmos. 2006, 111. [Google Scholar] [CrossRef]
- Zhang, L.-M.; Cao, P.-Y.; Zhu, Y.-P.; Li, Q.-K.; Zhang, J.-H.; Wang, X.-L.; Dai, G.-H.; Li, J.-G. Dynamics and regulations of ecosystem light use efficiency in a broad-leaved Korean pine mixed forest, Changbai Mountain. Chin. J. Plant Ecol. 2015, 39, 1156–1165. [Google Scholar]
- Ide, R.; Nakaji, T.; Oguma, H. Assessment of canopy photosynthetic capacity and estimation of GPP by using spectral vegetation indices and the light–response function in a larch forest. Agric. For. Meteorol. 2010, 150, 389–398. [Google Scholar] [CrossRef]
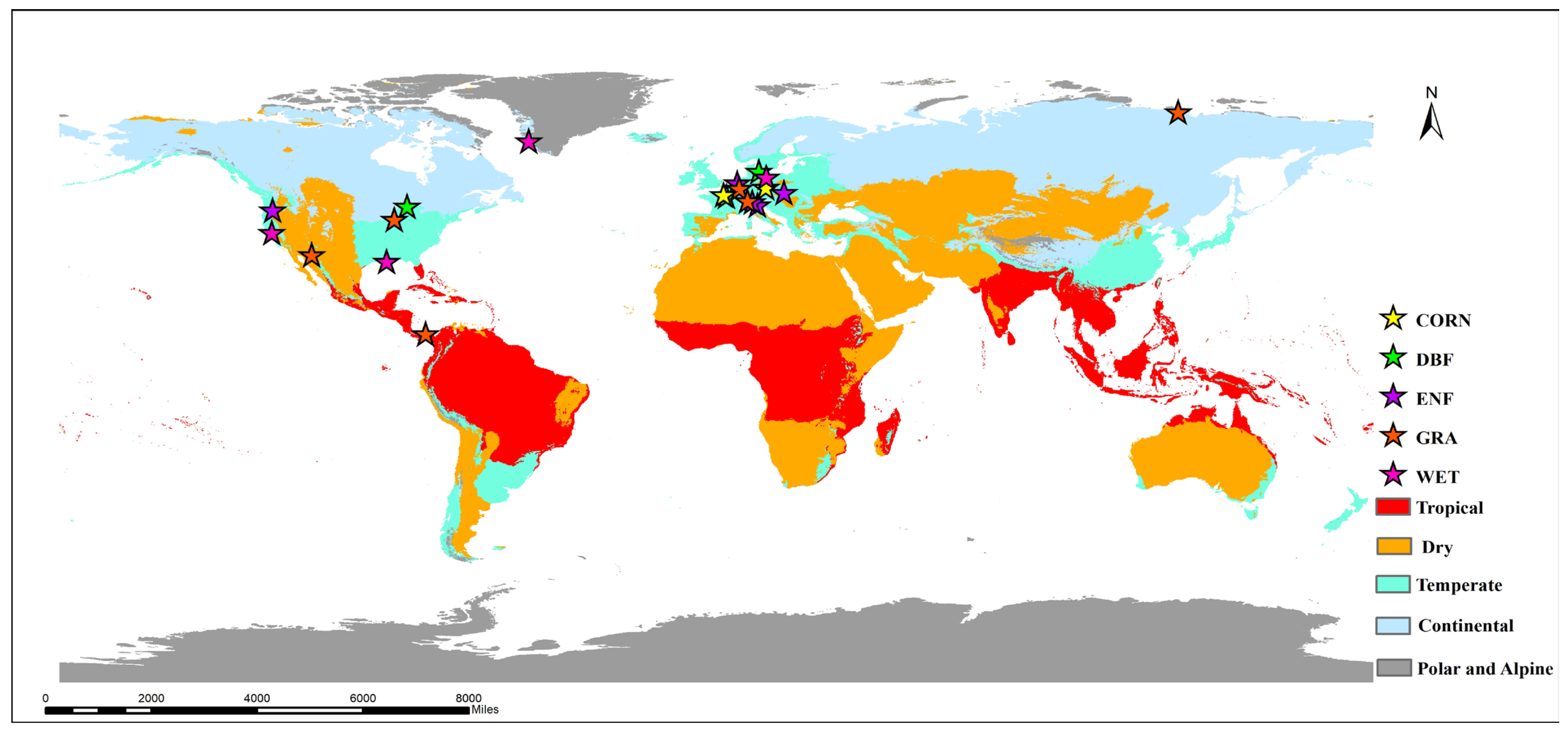


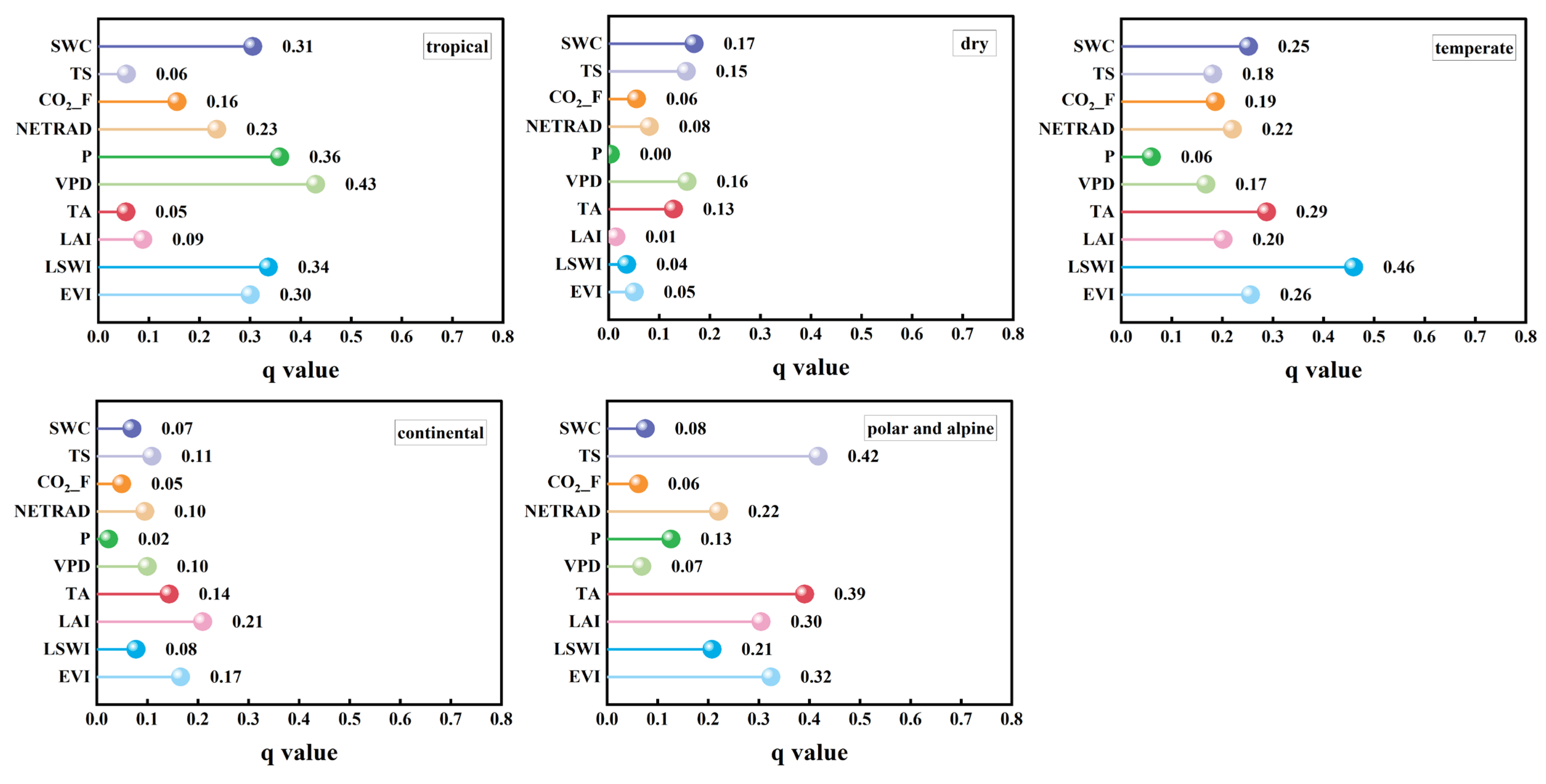
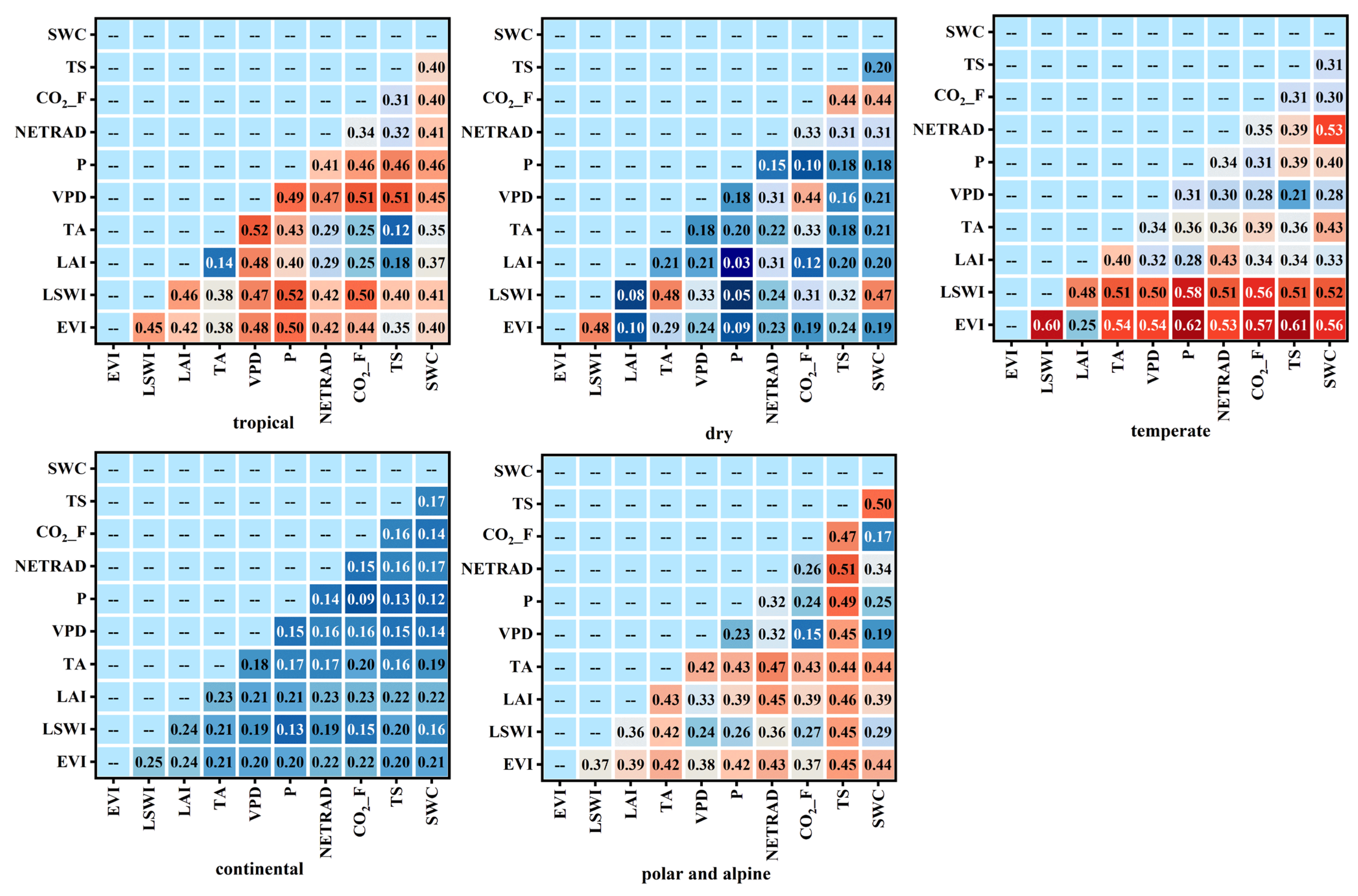
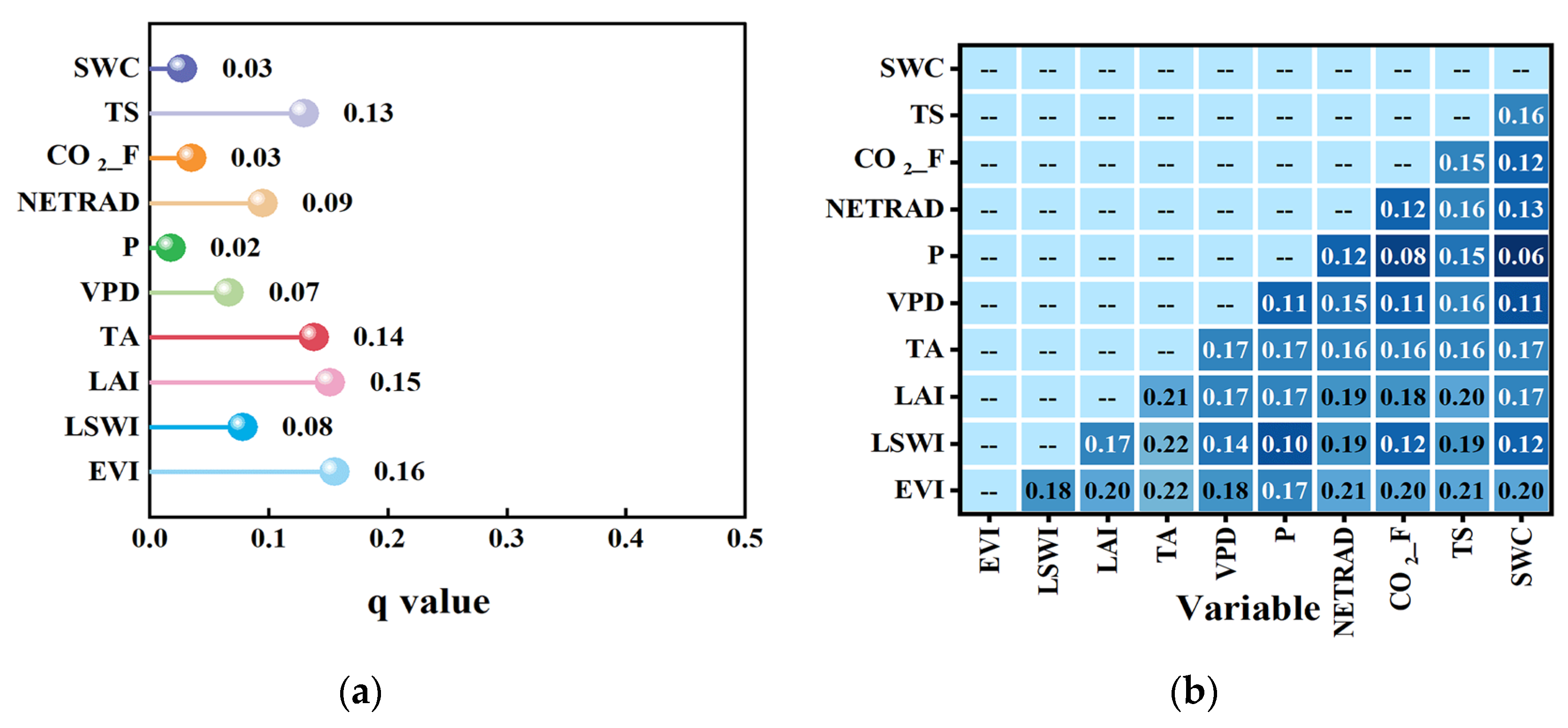
| Variable Type | Influencing Factor Variable |
|---|---|
| Physiological mechanism factor | Vegetation type |
| Enhanced Vegetation Index (EVI) | |
| Leaf Area Index (LAI) | |
| Land Surface Water Index (LSWI) | |
| Environmental mechanisms factor | Temperature of the Air (TA) |
| Temperature of the Soil (TS) | |
| Vapor Pressure Deficit (VPD) | |
| Soil Water Content (SWC) | |
| Net Radiation (NETRAD) | |
| Carbon Dioxide Molar Fraction (CO2_F) | |
| Precipitation (P) |
| Site_ID | Climate Type | Vegetation Type | Before SOS | SOS-Peak | Peak-EOS | After EOS |
|---|---|---|---|---|---|---|
| NL-Loo | temperate | ENF | NSI | VSI | VSD | SSD |
| US-Me2 | temperate | ENF | VSI | VSI | NSD | VSD |
| IT-Lav | continental | ENF | VSI | VSD | SD | VSD |
| CZ-BK1 | continental | ENF | VSI | SSI | SD | VSD |
| CH-Dav | polar and alpine | ENF | NSI | VSI | NSD | NSD |
| PA-SPn | tropical | DBF | NSI | NSI | NSD | SSD |
| FR-Fon | temperate | DBF | VSI | SI | SSD | VSD |
| DK-Sor | temperate | DBF | SI | VSI | VSD | VSD |
| US-UMd | continental | DBF | NSI | VSI | VSD | VSD |
| US-UMB | continental | DBF | VSI | NSI | NSD | NSD |
| PA-SPs | tropical | GRA | NSI | VSI | VSI | SSD |
| US-SRG | dry | GRA | VSI | SSI | SD | VSD |
| DE-RuR | temperate | GRA | SD | VSI | VSD | VSI |
| US-IB2 | continental | GRA | VSI | NSI | SI | NSD |
| DE-Gri | continental | GRA | SSI | NSI | NSI | NSD |
| CH-Fru | polar and alpine | GRA | NSI | VSI | NSD | VSD |
| RU-Sam | polar and alpine | GRA | SSI | VSI | VSD | NSD |
| FR-Gri | temperate | CORN | VSI | SSI | SD | VSI |
| DE-Kli | continental | CORN | SI | NSI | VSD | SSI |
| US-Myb | temperate | WET | NSD | NSI | NSI | VSD |
| US-LA2 | temperate | WET | NSI | NSI | NSD | VSD |
| DE-Akm | continental | WET | VSI | NSI | VSD | SSI |
| GL-NuF | polar and alpine | WET | NSI | VSI | NSD | SSI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, D.; He, Y.; Zou, S.; Song, Y.; Chi, H. Variation Patterns and Climate-Influencing Factors Affecting Maximum Light Use Efficiency in Terrestrial Ecosystem Vegetation. Forests 2025, 16, 528. https://doi.org/10.3390/f16030528
Huang D, He Y, Zou S, Song Y, Chi H. Variation Patterns and Climate-Influencing Factors Affecting Maximum Light Use Efficiency in Terrestrial Ecosystem Vegetation. Forests. 2025; 16(3):528. https://doi.org/10.3390/f16030528
Chicago/Turabian StyleHuang, Duan, Yue He, Shilin Zou, Yuejun Song, and Hong Chi. 2025. "Variation Patterns and Climate-Influencing Factors Affecting Maximum Light Use Efficiency in Terrestrial Ecosystem Vegetation" Forests 16, no. 3: 528. https://doi.org/10.3390/f16030528
APA StyleHuang, D., He, Y., Zou, S., Song, Y., & Chi, H. (2025). Variation Patterns and Climate-Influencing Factors Affecting Maximum Light Use Efficiency in Terrestrial Ecosystem Vegetation. Forests, 16(3), 528. https://doi.org/10.3390/f16030528







