Enhancement of Biological Durability and Fire Safety in Wood Modified with Maleic Anhydride and Sodium Hypophosphite
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Specimens
| Test | Specimen Dimension (T × R × L, mm) | Replicates per Treatment Group |
|---|---|---|
| Mini block test [24] | 10 × 5 × 30 | 30 (10 per fungal species, 10 control) |
| Terrestrial microcosm test (EN 807) | 10 × 5 × 100 | 40 (10 per harvest) |
| Laboratory mold test | 50 × 4 × 50 | 5 |
| Outdoor mold test | 50 × 4 × 50 | 20 (10 per direction) |
| Bending test | 10 × 10 × 200 | 10 |
2.2. Mini-Block Test—White- and Brown Rot
2.3. Terrestrial Microcosms (ENV 807)—Soft Rot
2.4. Mold Growth in the Laboratory
2.5. Outdoor Weathering Test
2.6. Bending Test
2.7. Thermogravimetric Analysis
2.8. Microscale Combustion Calorimeter (MCC)
2.9. Statistical Analysis
3. Results and Discussion
3.1. Resistance Against Wood-Decaying Fungi
3.2. Resistance Against Surface Fungi
3.3. Outdoor Performance
3.4. Mechanical Properties in Bending Test
3.5. Thermal Behavior
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HRC | Heat release capacity |
| HRR | Heat release rate |
| MA | Maleic anhydride |
| MCC | Microscale combustion calorimeter |
| MOE | Modulus of elasticity |
| MOR | Modulus of rupture |
| pHRR | Peak heat release rate |
| SHP | Sodium hypophosphite |
| TGA | Thermogravimetric analysis |
| THR | Total heat release |
| WPG | Weight percent gain |
References
- Hill, C.A.S. Wood Modification: Chemical, Thermal and Other Processes, 1st ed.; Wiley: Hoboken, NJ, USA, 2006; ISBN 978-0-470-02172-9. [Google Scholar]
- Rowell, R.M. Chemical Modification of Wood: A Short Review. Wood Mater. Sci. Eng. 2006, 1, 29–33. [Google Scholar] [CrossRef]
- Rowell, R.M. Distribution of Reacted Chemicals in Southern Pine Modified with Acetic Anhydride. Wood Sci. 1982, 15, 172–182. [Google Scholar]
- Rowell, R.M. Chemical Modification of Wood. Commonw. For. Bur. 1983, 6, 363–382. [Google Scholar]
- Rowell, R.M. Acetylation of Wood. For. Prod. J. 2006, 56, 4–12. [Google Scholar]
- Lande, S.; Westin, M.; Schneider, M. Development of Modified Wood Products Based on Furan Chemistry. Mol. Cryst. Liq. Cryst. 2008, 484, 367–378. [Google Scholar] [CrossRef]
- Krause, A. Holzmodifiziering Mit N-Methylolvernetzern (Wood Modification with Cross-Linking N-Methylol Compounds). Ph.D. Thesis, University of Göttingen, Göttingen, Germany, 2006. [Google Scholar]
- Behr, G.; Gellerich, A.; Bollmus, S.; Brinker, S.; Militz, H. The Influence of Curing Conditions on Properties of Melamine Modified Wood. Eur. J. Wood Prod. 2018, 76, 1263–1272. [Google Scholar] [CrossRef]
- Larnøy, E.; Karaca, A.; Gobakken, L.R.; Hill, C.A.S. Polyesterification of Wood Using Sorbitol and Citric Acid under Aqueous Conditions. Int. Wood Prod. J. 2018, 9, 66–73. [Google Scholar] [CrossRef]
- Ellis, W.D.; Rowell, R.M. Reaction of Isocyanates with Southern Pine Wood to Improve Dimensional Stability and Decay Resistance. Wood Fiber Sci. 1984, 16, 349–356. [Google Scholar]
- Cruz-Lopes, L.; Sell, M.; Lopes, R.; Esteves, B. Enhancing Pinus Pinaster Wood Durability Through Citric Acid Impregnation. Sustainability 2025, 17, 1979. [Google Scholar] [CrossRef]
- Peydecastaing, J. Chemical Modification of Wood by Mixed Anhydrides. Ph.D. Thesis, University of Toulose, Toulose, France, 2008. [Google Scholar]
- Abou-Okeil, A.; El-Sawy, S.M.; Abdel-Mohdy, F.A. Flame Retardant Cotton Fabrics Treated with Organophosphorus Polymer. Carbohydr. Polym. 2013, 92, 2293–2298. [Google Scholar] [CrossRef]
- Howell, B.A. Thermal Degradation of Organophosphorus Flame Retardants: Impact of Oxygenation-Level at Phosphorus on Mode of Degradation. In Chemical and Materials Sciences—Developments and Innovations Volume 3; Turkmen, A., Ed.; B P International: Hong Kong, China, 2024; pp. 87–100. ISBN 978-81-974582-7-9. [Google Scholar]
- Aseeva, R.; Serkov, B.; Sivenkov, A. Fire Protection of Timber Building Structures and Constructions. In Fire Behavior and Fire Protection in Timber Buildings; Springer Series in Wood Science; Springer: Dordrecht, The Netherlands, 2014; pp. 199–226. ISBN 978-94-007-7459-9. [Google Scholar]
- Teacă, C.-A.; Spiridon, I. Maleic Anhydride Treatment of Softwood—Effect on Wood Structure and Properties. Cellul. Chem. Technol. 2014, 48, 863–868. [Google Scholar]
- Gao, H.; Xie, Y.; Ou, R.; Wang, Q. Grafting Effects of Polypropylene/Polyethylene Blends with Maleic Anhydride on the Properties of the Resulting Wood–Plastic Composites. Compos. Part A Appl. Sci. Manuf. 2012, 43, 150–157. [Google Scholar] [CrossRef]
- Hameed, S.; Hussain, M.A.; Masood, R.; Haseeb, M.T. Cross-Linking of Cotton Fabric Using Maleic Anhydride and Sodium Hypophosphite. Cellul. Chem. Technol. 2016, 50, 321–328. [Google Scholar]
- Kim, I.; Karlsson, O.; Jones, D.; Mantanis, G.; Sandberg, D. Dimensional Stabilisation of Scots Pine (Pinus sylvestris L.) Sapwood by Reaction with Maleic Anhydride and Sodium Hypophosphite. Eur. J. Wood Prod. 2021, 79, 589–596. [Google Scholar] [CrossRef]
- Kim, I.; Thybring, E.E.; Karlsson, O.; Jones, D.; Mantanis, G.I.; Sandberg, D. Characterisation of Moisture in Scots Pine (Pinus sylvestris L.) Sapwood Modified with Maleic Anhydride and Sodium Hypophosphite. Forests 2021, 12, 1333. [Google Scholar] [CrossRef]
- Kim, I.; Antzutkin, O.N.; Shah, F.U.; Karlsson, O.; Jones, D.; Sandberg, D. Chemical Bonds Formed in Solid Wood by Reaction with Maleic Anhydride and Sodium Hypophosphite. Materials 2024, 17, 4856. [Google Scholar] [CrossRef]
- Thybring, E.E.; Fredriksson, M. Wood Modification as a Tool to Understand Moisture in Wood. Forests 2021, 12, 372. [Google Scholar] [CrossRef]
- EN 84; Durability of Wood and Wood-Based Products—Accelerated Ageing of Treated Wood Prior to Biological Testing—Leaching Procedure. European Committee for Standardization: Brussels, Belgium, 2020.
- Bravery, A.F.; Dcikinson, D.J. Screening Techniques for Potential Wood Preservatives. In Proceedings of the IRG Annual Meeting, Washington, DC, USA, 12–17 February 1978; p. IRG/WP 78-2113. [Google Scholar]
- EN 113-1; Durability of Wood and Wood-Based Products—Test Method Against Wood Destroying Basidiomycetes—Part 1: Assessment of Biocidal Efficacy of Wood Preservatives. European Committee for Standardization: Brussels, Belgium, 2020.
- EN 113-2; Durability of Wood and Wood-Based Products—Test Method Against Wood Destroying Basidiomycetes—Part 2: Assessment of Inherent or Enhanced Durability. European Committee for Standardization: Brussels, Belgium, 2020.
- ENv 807; Wood Preservatives—Determination of the Effectiveness Against Soft Rotting Micro-Fungi and Other Soil Inhabiting Micro-Organisms. European Committee for Standardization: Brussels, Belgium, 2009.
- EN 15457; Paints and Varnishes—Laboratory Method for Testing the Efficacy of Film Preservatives in a Coating Against Fungi. European Committee for Standardization: Brussels, Belgium, 2022.
- EN 408; Timber Structures—Structural Timber and Glued Laminated Timber—Determination of Some Physical and Mechanical Properties. European Committee for Standardization: Brussels, Belgium, 2012.
- ASTM D 7309; Standard Test Method for Determining Flammability Characteristics of Plastics and Other Solid Materials Using Microscale Combustion Calorimetry. ASTM: Conshohocken, PA, USA, 2021.
- Brischke, C.; Alfredsen, G. On the Use of Miniaturized Wood Specimens in Fungal Decay Experiments—Mini-Blocks versus EN 113 Test Specimens. In Proceedings of the IRG Annual Meeting IRG/WP 24, Knoxville, TN, USA, 19 May 2024. [Google Scholar]
- Iwamoto, Y.; Itoh, T. Vapor Phase Reaction of Wood with Maleic Anhydride (I): Dimensional Stability and Durability of Treated Wood. J. Wood Sci. 2005, 51, 595–600. [Google Scholar] [CrossRef]
- Sailer, M.; van Etten, B. Potential Wood Protection Strategies Using Physiological Requirements of Wood Degrading Fungi. Heron 2004, 49, 327–337. [Google Scholar]
- Kirk, T.K. The Chemistry and Biochemistry of Decay. In Wood Deterioration and Its Prevention by Preservative Treatments; Degradation and Protection of Wood; Syracuse University Press: Syracuse, NY, USA, 1973; Volume 1. [Google Scholar]
- Suttie, E.; Hill, C.; Jones, D.; Orsler, R.J. Chemically Modified Solid Wood. I. Resistance to Fungal Attack. Mater. Und Org. 1999, 32, 159–182. [Google Scholar]
- Nilsson, T. 10. Biological Wood Degradation. In Wood Chemistry and Wood Biotechnology; Ek, M., Gellerstedt, G., Henriksson, G., Eds.; Walter de Gruyter: Berlin, Germany, 2009; pp. 219–244. ISBN 978-3-11-021339-3. [Google Scholar]
- Gunduz, G.; Aydemir, D.; Karakas, G. The Effects of Thermal Treatment on the Mechanical Properties of Wild Pear (Pyrus elaeagnifolia Pall.) Wood and Changes in Physical Properties. Mater. Des. 2009, 30, 4391–4395. [Google Scholar] [CrossRef]
- Korkut, S.; Hiziroglu, S. Effect of Heat Treatment on Mechanical Properties of Hazelnut Wood (Corylus colurna L.). Mater. Des. 2009, 30, 1853–1858. [Google Scholar] [CrossRef]
- Tomak, E.D.; Ustaomer, D.; Yildiz, S.; Pesman, E. Changes in Surface and Mechanical Properties of Heat Treated Wood during Natural Weathering. Measurement 2014, 53, 30–39. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. (Eds.) 12. Influence of Temperature. In Wood; De Gruyter: Berlin, Germany, 1983; pp. 319–344. ISBN 978-3-11-008481-8. [Google Scholar]
- Rowell, R.M.; Dietenberger, M.A. Thermal Properties, Combustion, and Fire Retardancy of Wood. In Handbook of Wood Chemistry and Wood Composites; Roger, M., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 127–149. ISBN 978-0-203-49243-7. [Google Scholar]
- Lin, C.; Karlsson, O.; Das, O.; Mensah, R.A.; Mantanis, G.I.; Jones, D.; Antzutkin, O.N.; Försth, M.; Sandberg, D. High Leach-Resistant Fire-Retardant Modified Pine Wood (Pinus sylvestris L.) by In Situ Phosphorylation and Carbamylation. ACS Omega 2023, 8, 11381–11396. [Google Scholar] [CrossRef]
- Price, D.; Cunliffe, L.K.; Bullett, K.J.; Hull, T.R.; Milnes, G.J.; Ebdon, J.R.; Hunt, B.J.; Joseph, P. Thermal Behaviour of Covalently Bonded Phosphate and Phosphonate Flame Retardant Polystyrene Systems. Polym. Degrad. Stab. 2007, 92, 1101–1114. [Google Scholar] [CrossRef]
- Howell, B.A. Thermal Degradation of Organophosphorus Flame Retardants. Polymers 2022, 14, 4929. [Google Scholar] [CrossRef]
- Sonnier, R. Microscale Forced Combustion: Pyrolysis-Combustion Flow Calorimetry (PCFC). In Analysis of Flame Retardancy in Polymer Science; Elsevier: Amsterdam, The Netherlands, 2022; pp. 91–116. ISBN 978-0-12-824045-8. [Google Scholar]
- Li, Y.; Zheng, H.; Xu, M.; Li, B.; Lai, T. Synthesis of a Novel Phosphonate Flame Retardant and Its Application in Epoxy Resins. J. Appl. Polym. Sci. 2015, 132, 42765. [Google Scholar] [CrossRef]

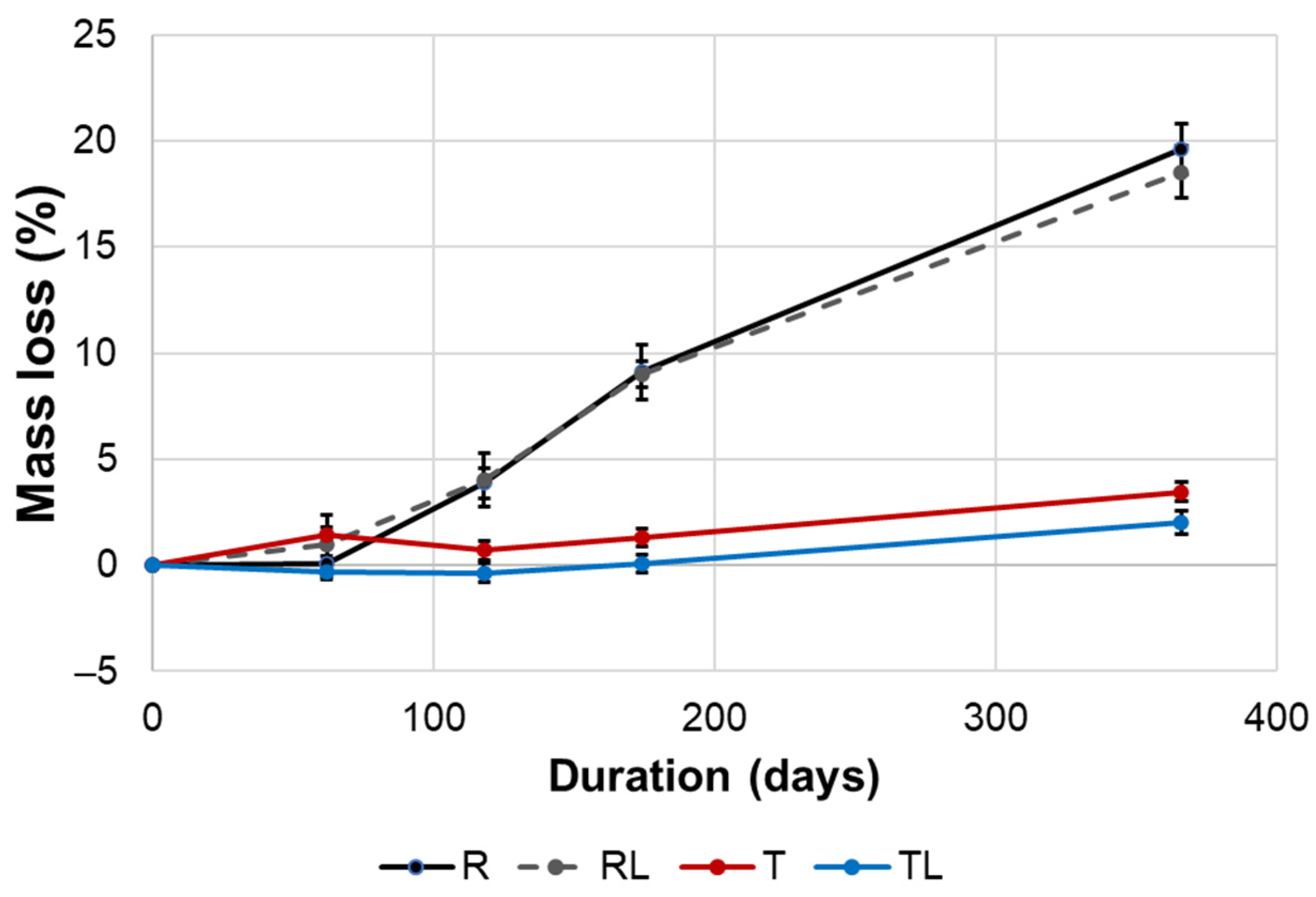

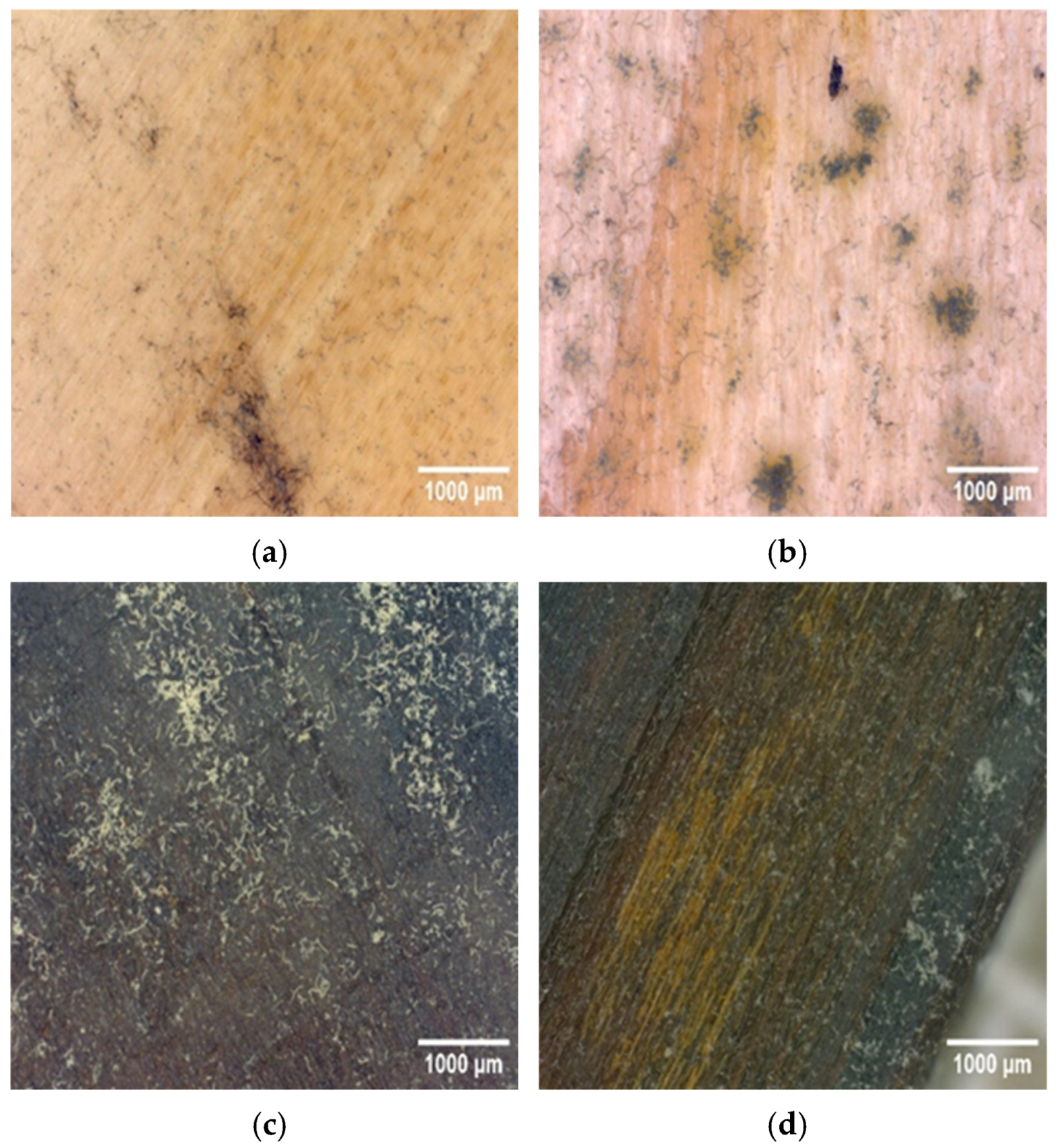


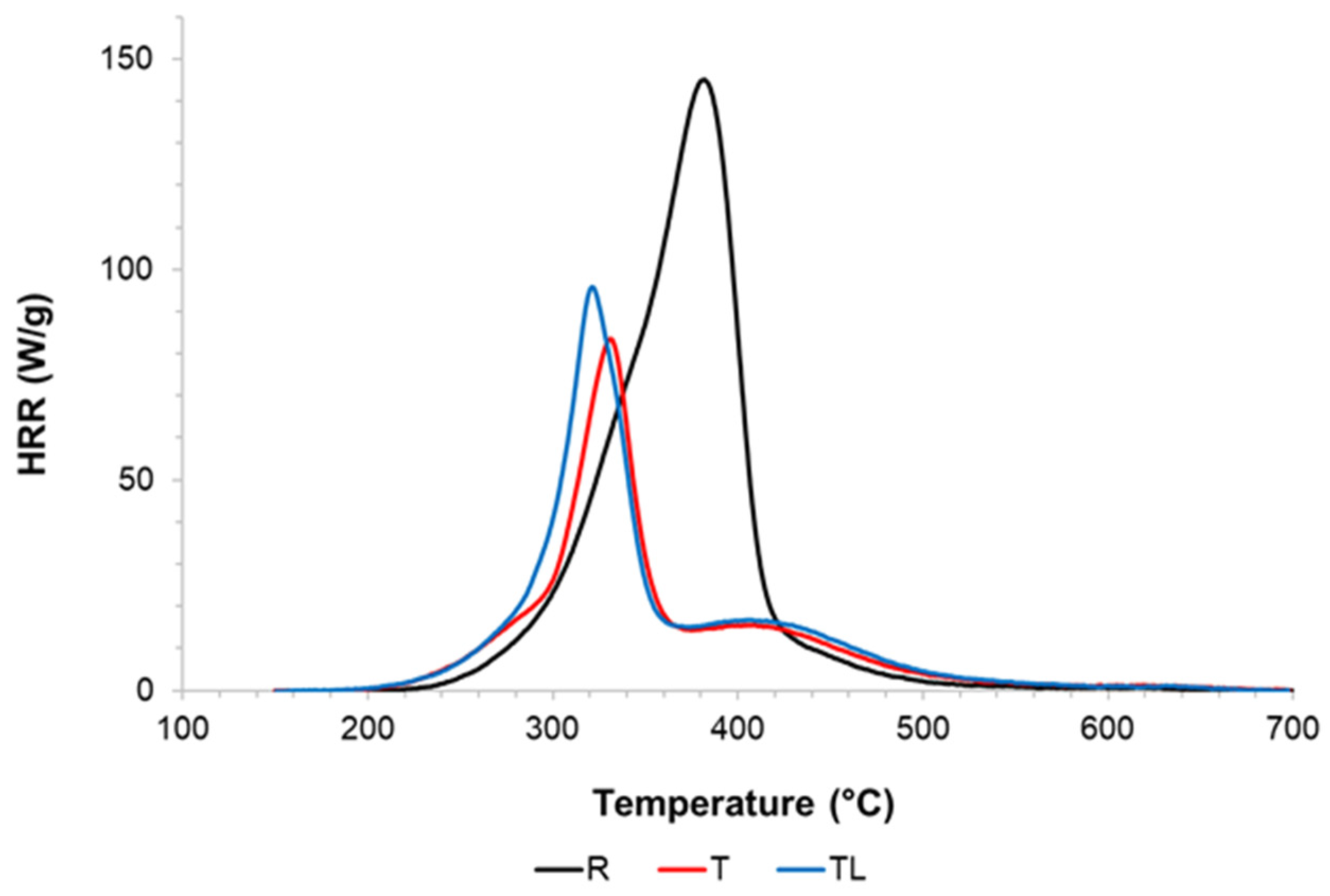
| Groups | MOE (GPa) | MOR (MPa) |
|---|---|---|
| R | 11.4 (1.99) | 83.8 (10.82) |
| RL | 10.2 (1.87) | 71.5 (10.32) |
| T | 9.9 (2.41) | 35.1 (13.83) |
| TL | 9.7 (1.98) | 36.2 (14.1) |
| Parameters | R | T | TL |
|---|---|---|---|
| THR (KJ/g) | 11.18 (0.1) | 6.1 (0.1) | 6.6 (0.2) |
| pHRR temperature (°C) | 385.1 (2.6) | 329.9 (1.1) | 324.2 (3.1) |
| pHRR (W/g) | 145.4 (4.6) | 85.68 (5.2) | 94.35 (6.5) |
| Residue (%) | 16.9 (1.9) | 29.4 (2.6) | 27.5 (1.9) |
| HRC (J/g∙K) | 148.4 (4.1) | 87.3 (5.4) | 96.5 (6.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, I.; Ross, L.; Alfredsen, G.; Karlsson, O.; Kaynak, E.; Das, O.; Jones, D.; Mantanis, G.I.; Sandberg, D. Enhancement of Biological Durability and Fire Safety in Wood Modified with Maleic Anhydride and Sodium Hypophosphite. Forests 2025, 16, 526. https://doi.org/10.3390/f16030526
Kim I, Ross L, Alfredsen G, Karlsson O, Kaynak E, Das O, Jones D, Mantanis GI, Sandberg D. Enhancement of Biological Durability and Fire Safety in Wood Modified with Maleic Anhydride and Sodium Hypophosphite. Forests. 2025; 16(3):526. https://doi.org/10.3390/f16030526
Chicago/Turabian StyleKim, Injeong, Lone Ross, Gry Alfredsen, Olov Karlsson, Elif Kaynak, Oisik Das, Dennis Jones, George I. Mantanis, and Dick Sandberg. 2025. "Enhancement of Biological Durability and Fire Safety in Wood Modified with Maleic Anhydride and Sodium Hypophosphite" Forests 16, no. 3: 526. https://doi.org/10.3390/f16030526
APA StyleKim, I., Ross, L., Alfredsen, G., Karlsson, O., Kaynak, E., Das, O., Jones, D., Mantanis, G. I., & Sandberg, D. (2025). Enhancement of Biological Durability and Fire Safety in Wood Modified with Maleic Anhydride and Sodium Hypophosphite. Forests, 16(3), 526. https://doi.org/10.3390/f16030526









