Substrate and Fertilization Used in the Nursery Influence Biomass and Nutrient Allocation in Fagus sylvatica and Quercus robur Seedlings After the First Year of Growth in a Newly Established Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Substrate Composition and Seedling Production
2.3. Experimental Layout
2.4. Soil Sample Collection and Plantation Establishment
2.5. Parameter Assessment and Nutrient Analysis
2.6. Statistical Analysis
3. Results
3.1. Soil Properties
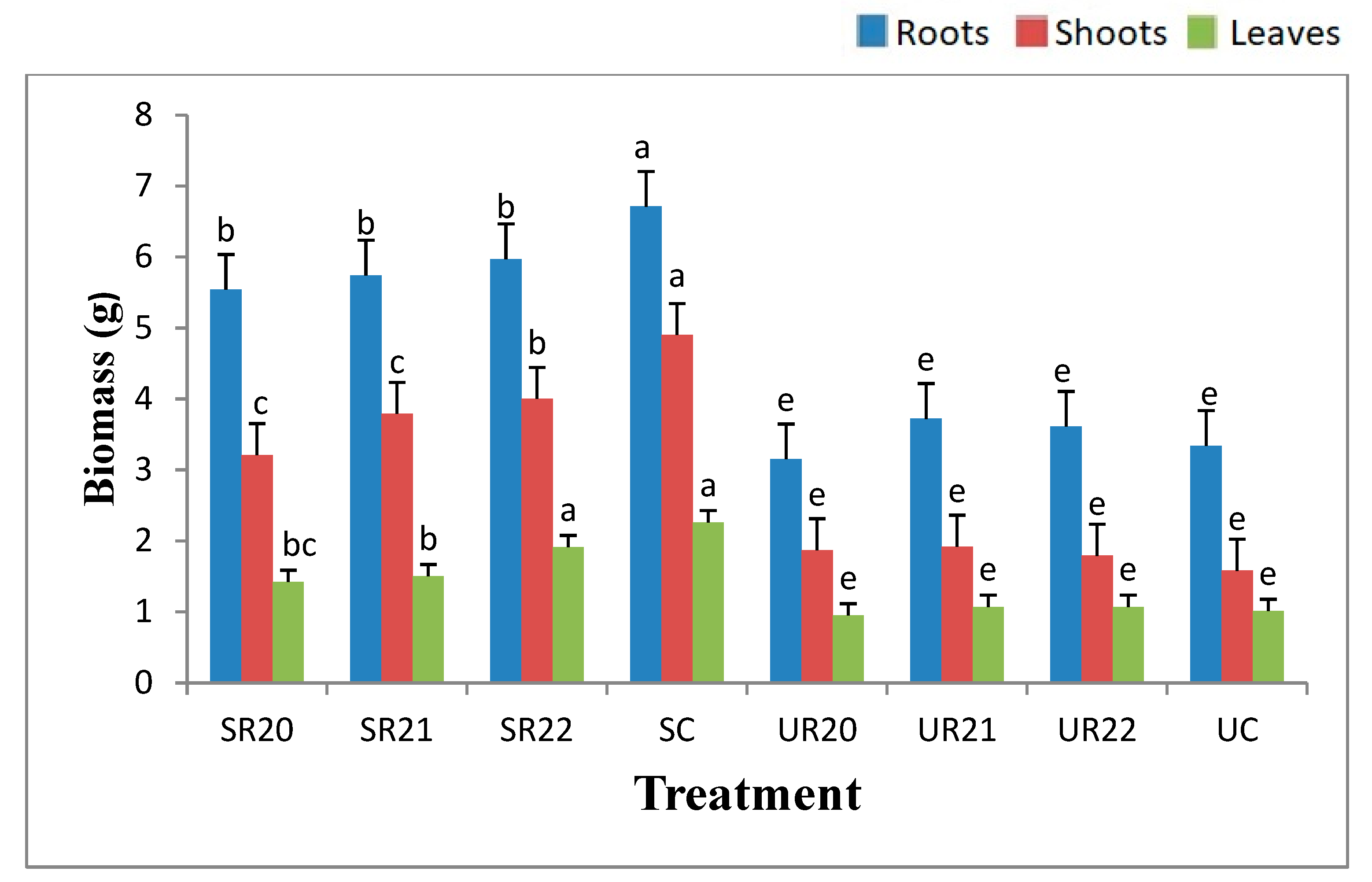
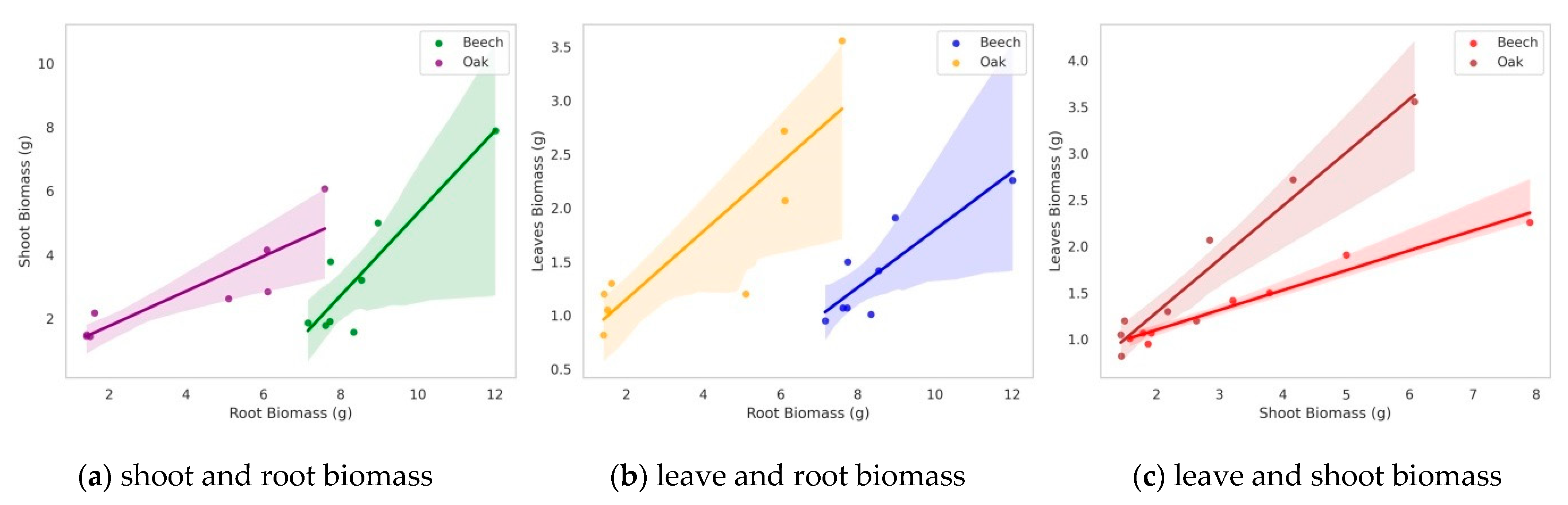
3.2. Biomass Allocation in Studied Organs of F. sylvatica and Q. robur Seedlings
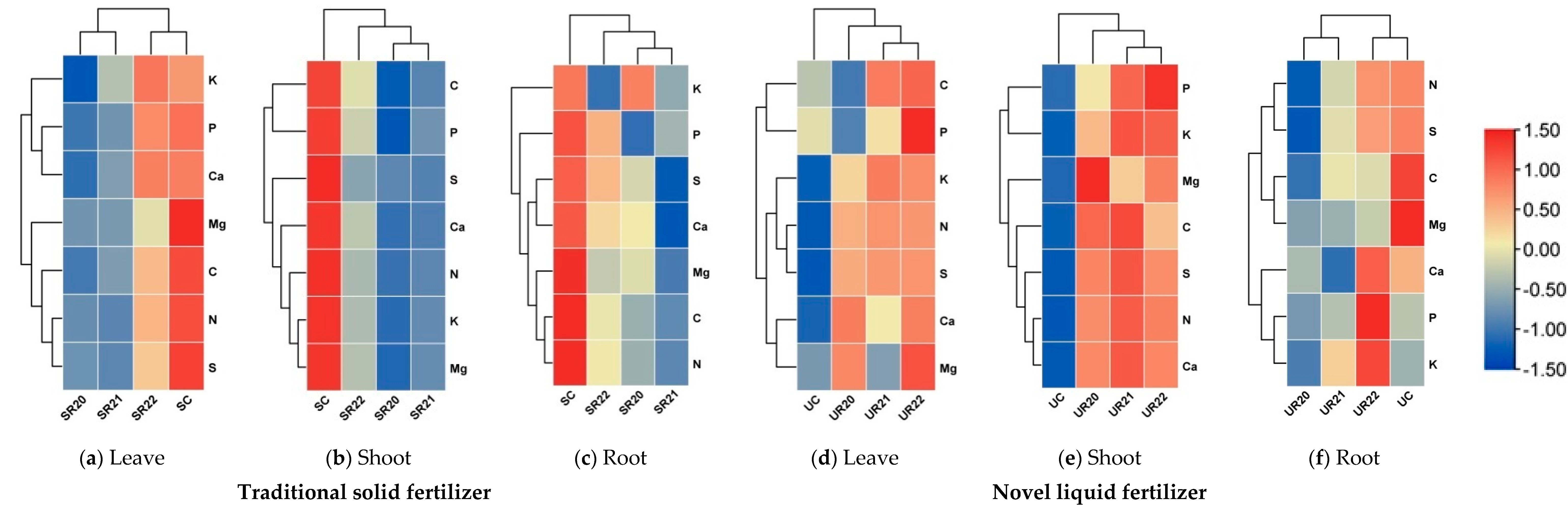
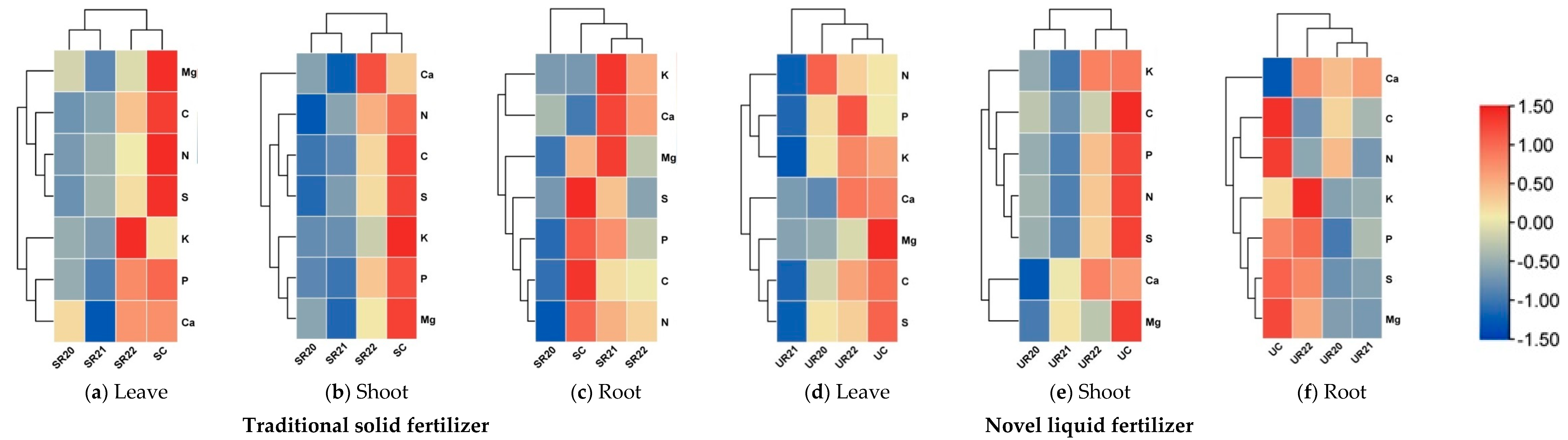
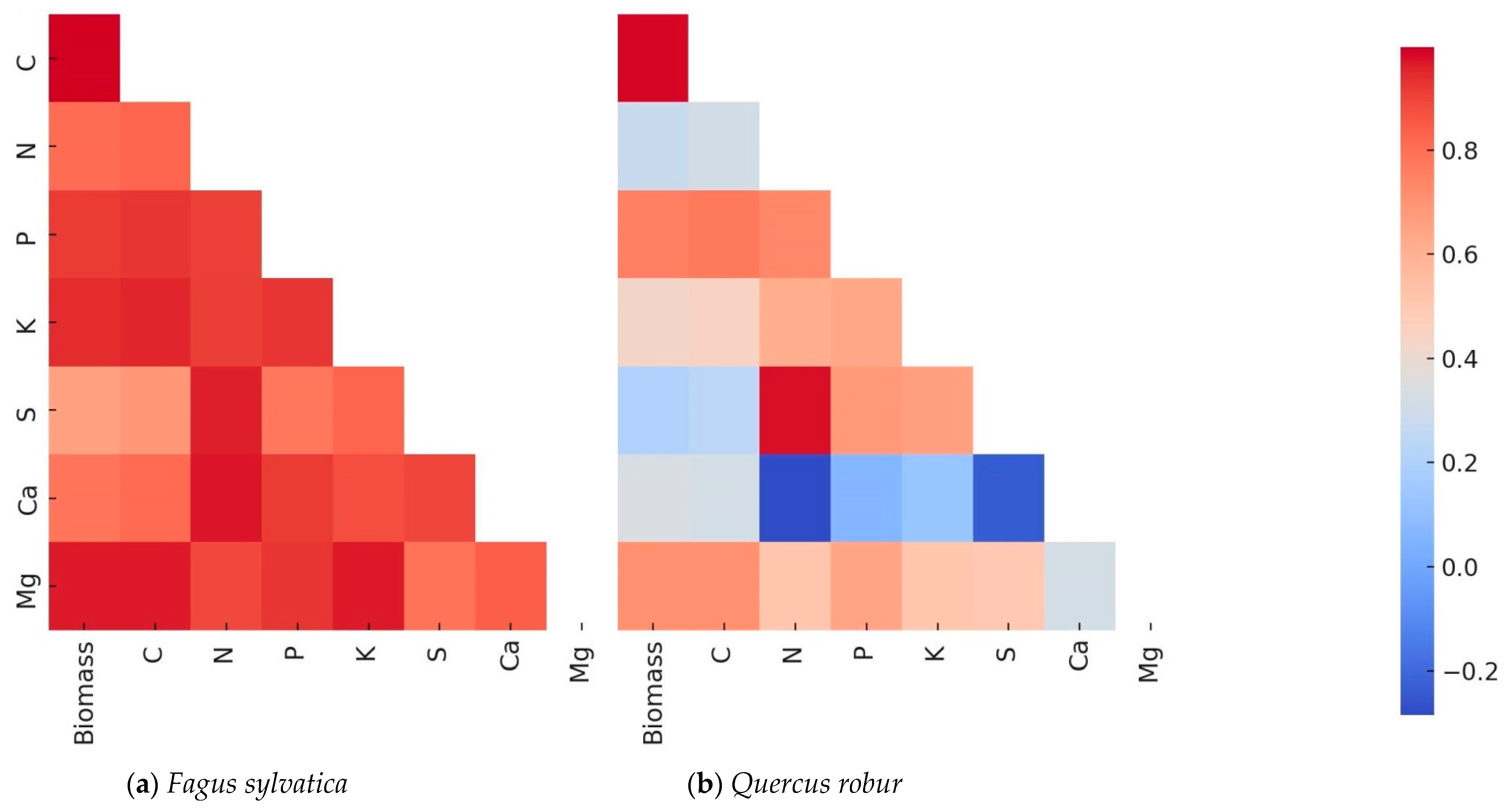
3.3. Allocation of Nutrients in F. sylvatica and Q. robur Seedlings One Year After Planting in the Forest
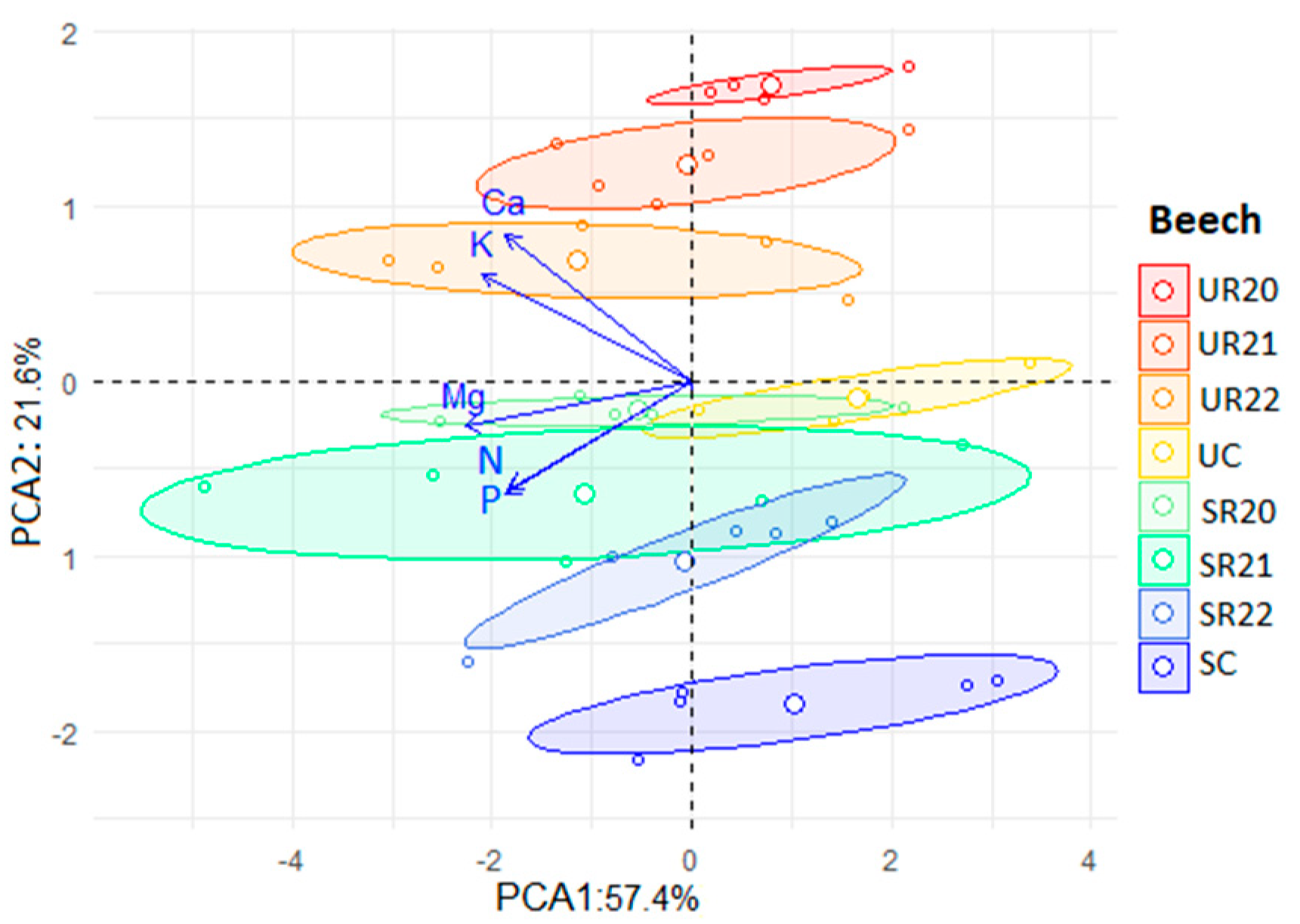
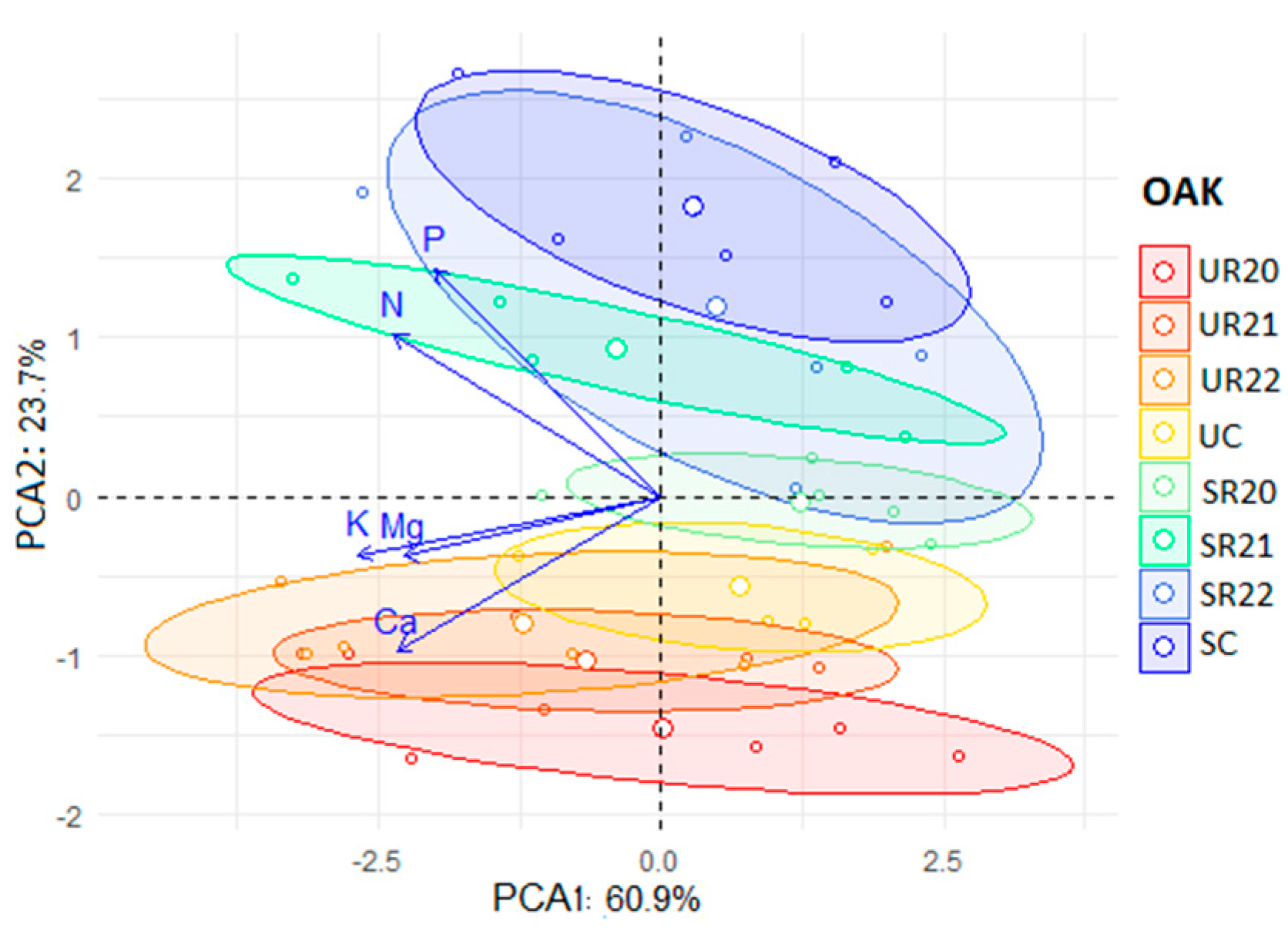
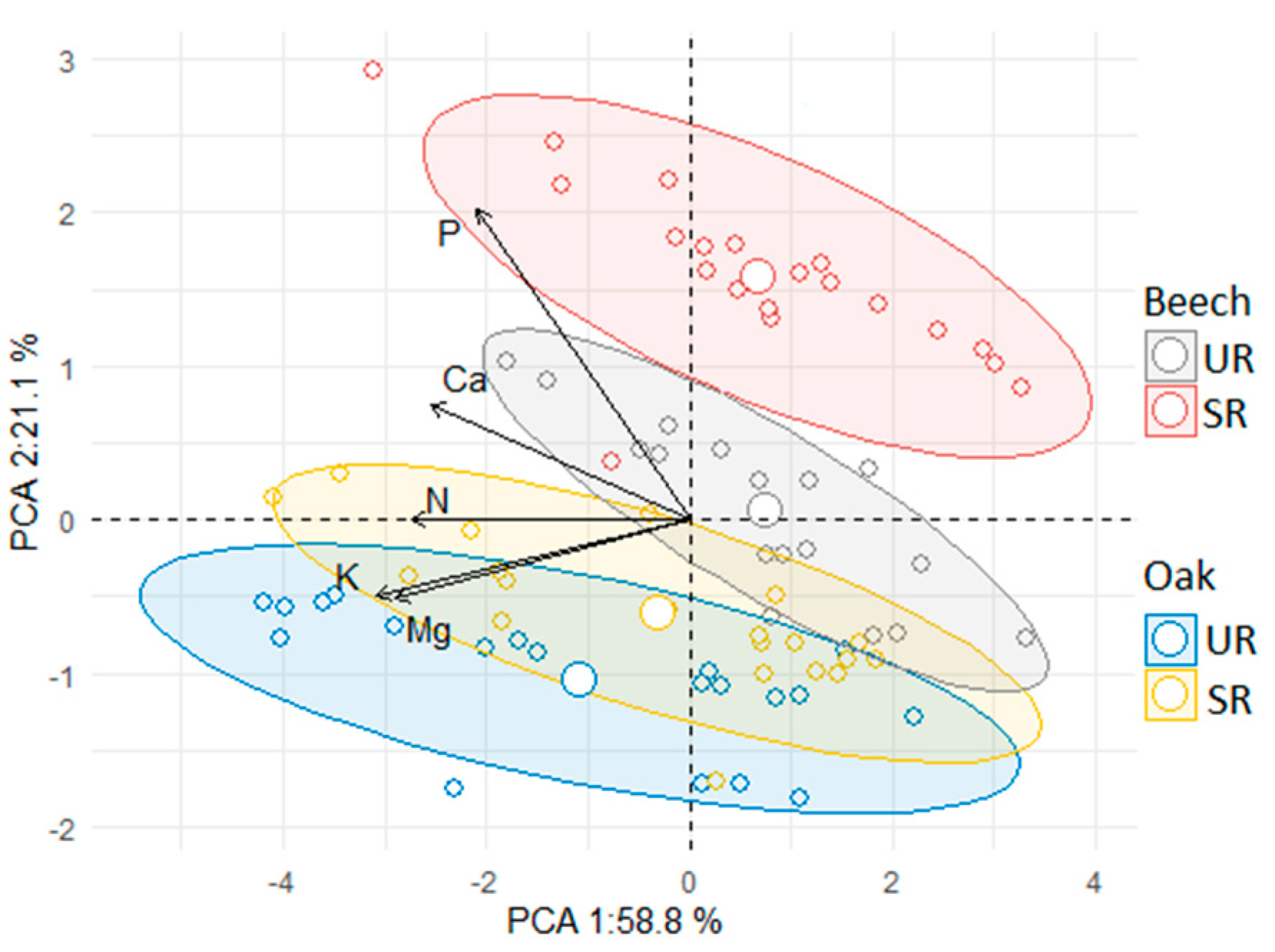
3.4. Principal Component Analysis (PCA) Results for F. sylvatica and Q. robur
4. Effects of Treatments on N and P Allocation in Beech and Oak Seedlings
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chambel, M.R.; Climent, J.; Alía, R. Divergence among species and populations of Mediterranean pines in biomass allocation of seedlings grown under two watering regimes. Ann. For. Sci. 2007, 64, 87–97. [Google Scholar] [CrossRef]
- Poorter, H.; Nagel, O. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: A quantitative review. Funct. Plant Biol. 2000, 27, 1191. [Google Scholar] [CrossRef]
- Sanchez-Gomez, D.; Valladares, F.; Zavala, M.A. Functional traits and plasticity in response to light in seedlings of four Iberian forest tree species. Tree Physiol. 2006, 26, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Ameray, A.; Bergeron, Y.; Valeria, O.; Montoro Girona, M.; Cavard, X. Forest carbon management: A review of silvicultural practices and management strategies across boreal, temperate and tropical forests. Curr. For. Rep. 2021, 7, 245–266. [Google Scholar] [CrossRef]
- Villar-Salvador, P.; Planelles, R.; Enrıquez, E.; Rubira, J.P. Nursery cultivation regimes, plant functional attributes, and field performance relationships in the Mediterranean oak Quercus ilex L. For. Ecol. Manag. 2004, 196, 257–266. [Google Scholar] [CrossRef]
- Grossnickle, S.C.; MacDonald, J.E. Why seedlings grow: Influence of plant attributes. New For. 2018, 49, 1–34. [Google Scholar] [CrossRef]
- Seva, J.P.; Valdecantos, A.; Vilagrosa, A.; Cortina, J.; Bellot, J.; Vallejo, V.R. Seedling morphology and survival in some Mediterranean tree shrub species. Mediterr. Desertif. Res. Results Policy Implic. 2000, 2, 397–406. [Google Scholar]
- Trubat, R.; Cortina, J.; Vilagrosa, A. Nutrient deprivation improves field performance of woody seedlings in a degraded semi-arid shrubland. Ecol. Eng. 2011, 37, 1164–1173. [Google Scholar] [CrossRef]
- Singh, D.K.; Sale, P.W. Growth and potential conductivity of white clover roots in dry soil with increasing phosphorus supply and defoliation frequency. Agron. J. 2000, 92, 868–874. [Google Scholar] [CrossRef]
- Trubat, R.; Cortina, J.; Vilagrosa, A. Plant morphology and root hydraulics are altered by nutrient deficiency in Pistacia lentiscus (L.). Trees 2006, 20, 334–339. [Google Scholar] [CrossRef]
- Reinbott, T.M.; Blevins, D.G. Phosphorus nutritional effects on root hydraulic conductance, xylem water flow and flux of magnesium and calcium in squash plants. Plant Soil 1999, 209, 263–273. [Google Scholar] [CrossRef]
- Yan, Z.; Eziz, A.; Tian, D.; Li, X.; Hou, X.; Peng, H.; Han, W.; Guo, Y.; Fang, J. Biomass allocation in response to nitrogen and phosphorus availability: Insight from experimental manipulations of Arabidopsis thaliana. Front. Plant Sci. 2019, 10, 598. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, A. Silviculture Characteristics of Forest Trees and Shrubs; Universal Agricultural and Forestry Publishing House: Warszawa, Poland, 2019; Volume III, ISBN 978-83-09-01117-0. [Google Scholar]
- Romanov, A.A.; Tamarovskaya, A.N.; Gloor, E.; Brienen, R.; Gusev, B.A.; Leonenko, E.V.; Vasiliev, A.S.; Krikunov, E.E. Reassessment of carbon emissions from fires and a new estimate of net carbon uptake in Russian forests in 2001–2021. Sci. Total Environ. 2022, 846, 157322. [Google Scholar] [CrossRef] [PubMed]
- Rotowa, O.J.; Małek, S.; Pach, M. Forest Sustainability: A Force to Recon with in the Phase of Global Environmental Challenges. Am. J. Agric. For. 2023, 11, 58–66. [Google Scholar] [CrossRef]
- Crowley, K.F.; Lovett, G.M.; Arthur, M.A.; Weathers, K.C. Long-term effects of pest-induced tree species change on carbon and nitrogen cycling in northeastern US forests: A modeling analysis. For. Ecol. Manag. 2016, 372, 269–290. [Google Scholar] [CrossRef]
- Crowley, K.F.; Lovett, G.M. Effects of nitrogen deposition on nitrate leaching from forests of the northeastern United States will change with tree species composition. Can. J. For. Res. 2017, 47, 997–1009. [Google Scholar] [CrossRef]
- Yu, Q.; Ma, S.; Ni, X.; Jiang, L.; Zhou, Z.; Zhu, J.; Fang, J. A test of the mycorrhizal-associated nutrient economy framework in two types of tropical rainforests under nutrient enrichments. For. Ecosyst. 2023, 10, 100083. [Google Scholar] [CrossRef]
- Soman, C.; Li, D.; Wander, M.M.; Kent, A.D. Long-term fertilizer and crop-rotation treatments differentially affect soil bacterial community structure. Plant Soil 2017, 413, 145–159. [Google Scholar] [CrossRef]
- Hirschler, O.; Osterburg, B.; Weimar, H.; Glasenapp, S.; Ohmes, M.F. Peat Replacement in Horticultural Growing Media: Availability of Bio-Based Alternative Materials (No. 190); Thünen Working Paper; Johann Heinrich von Thünen-Institut: Braunschweig, Germany, 2022. [Google Scholar]
- Gruda, N.S.; Hirschler, O.; Stuart, J. Peat reduction in horticulture an overview of Europe. Acta Hortic. 2024, 1391, 545–560. [Google Scholar] [CrossRef]
- Gruda, N. Current and future perspective of growing media in Europe. Acta Hortic. 2012, 960, 37–43. [Google Scholar] [CrossRef]
- van Beek, R.; Quik, C.; van der Linden, M. Drowning landscapes revisited; Correlating peatland expansion, human habitation trends and vegetation dynamics in the Northwest European mainland. Quat. Sci. Rev. 2023, 312, 108170. [Google Scholar] [CrossRef]
- Fascella, G. Growing substrates alternative to peat for ornamental plants. In Soilless Culture-Use of Substrates for the Production of Quality Horticultural Crops; BoD—Books on Demand: Norderstedt, Germany, 2015; pp. 47–67. [Google Scholar]
- Gavilanes-Terán, I.; Jara-Samaniego, J.; Idrovo-Novillo, J.; Bustamante, M.A.; Pérez-Murcia, M.D.; Pérez-Espinosa, A.; Paredes, C. Agroindustrial compost as a peat alternative in the horticultural industry of Ecuador. J. Environ. Manag. 2017, 186, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Haug, R. The Practical Handbook of Compost Engineering; Routledge: New York, NY, USA, 2018. [Google Scholar]
- Rotowa, O.J.; Malek, S.; Banach, J.; Pach, M. Effect of different innovative substrate mediums on roots characterization of European beech Fagus sylvatica L. and pedunculate oak Quercus robur L. seedlings. Sylwan 2023, 167, 535–548. [Google Scholar]
- Rotowa, O.J.; Małek, S.; Jasik, M.; Staszel-Szlachta, K. Effect of innovative peat-free organic growing media and fertilizer on nutrient allocation in pedunculate oak (Quercus robur L.) and European beech (Fagus sylvatica L.) seedlings. New For. 2024, 56, 1–22. [Google Scholar] [CrossRef]
- Szabla, K.; Pabian, R. Problems of seedling production of mountain admixture species in the Container Nursery of the Rudy Raciborskie Forest District. Scientific Papers of the Agricultural University in Krakow. Sci. Sess. 2009, 86, 169–176. [Google Scholar]
- Staszel, K.; Błońska, E.; Lasota, J. Slope aspect and altitude effect on selected soil organic matter characteristics in Beskid Mountains forest soils. Folia For. Pol. 2021, 63, 214–224. [Google Scholar] [CrossRef]
- Ostrowska, A.; Gawlinski, S.; Szczubialka, Z. Methods of Analysis and Assessment of Soil and Plant Properties; Institute of Environmental Protection: Warsaw, Poland, 1991. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Levene, H. Robust tests for the equality of variances. In Contributions to Probability and Statistics; Olkin, J., Ed.; Stanford University Press: Palo Alto, CA, USA, 1960. [Google Scholar]
- Taylor, R. Interpretation of the correlation coefficient: A basic review. J. Diagn. Med. Sonogr. 1990, 6, 35–39. [Google Scholar] [CrossRef]
- Trumbore, S. Age of soil organic matter and soil respiration: Radiocarbon constraints on belowground C dynamics. Ecol. Appl. 2000, 10, 399–411. [Google Scholar] [CrossRef]
- Reichstein, M.; Subke, J.A.; Angeli, A.C.; Tenhunen, J.D. Does the temperature sensitivity of decomposition of soil organic matter depend upon water content, soil horizon, or incubation time? Glob. Change Biol. 2005, 11, 1754–1767. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Debaeke, P.; Steinberg, C.; You, M.P.; Barbetti, M.J.; Aubertot, J.N. Abiotic and biotic factors affecting crop seed germination and seedling emergence: A conceptual framework. Plant Soil 2018, 432, 1–28. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef]
- Rumpf, S.; Nagel, J.; Schmidt, M. Biomass Treasure Functions of Spruce, Pine, Beech, Oak and Douglas Fir for Northwest Germany, Research Project: Possibilities and Limits of Whole Tree Use; Report on results (FKZ: 22015407); Agency for Renewable Resources of the Federal Ministry of Food; Agriculture and Consumer Protection, Ed.; Agriculture and Consumer Protection: Göttingen, Germany, 2011. [Google Scholar]
- Meiwes, K.J.; Rumpf, S.; Klinck, U.; Meesenburg, H.; Nagel, J. Whole tree use: Results of investigations on nutrient element export in northwest Germany and approaches to local assessment. In German Association of Forest Research Institutes Section Yield Science Annual Conference Ottenstein; DVFFA: Göttingen, Germany, 2012; pp. 88–92. [Google Scholar]
- Freschet, G.T.; Kichenin, E.; Wardle, D.A. Explaining within-community variation in plant biomass allocation: A balance between organ biomass and morphology above vs below ground? J. Veg. Sci. 2015, 26, 431–440. [Google Scholar] [CrossRef]
- Husmann, K.; Rumpf, S.; Nagel, J. Biomass functions and nutrient contents of European beech, oak, sycamore maple and ash and their meaning for the biomass supply chain. J. Clean. Prod. 2018, 172, 4044–4056. [Google Scholar] [CrossRef]
- Klimešová, J.; Martínková, J.; Ottaviani, G. Belowground plant functional ecology: Towards an integrated perspective. Funct. Ecol. 2018, 32, 2115–2126. [Google Scholar] [CrossRef]
- Yue, K.; Fornara, D.A.; Li, W.; Ni, X.; Peng, Y.; Liao, S.; Tan, S.; Wang, D.; Wu, F.; Yang, Y. Nitrogen addition affects plant biomass allocation but not allometric relationships among different organs across the globe. J. Plant Ecol. 2021, 14, 361–371. [Google Scholar] [CrossRef]
- Grotkopp, E.; Rejmánek, M.; Rost, T.L. Toward a causal explanation of plant invasiveness: Seedling growth and life-history strategies of 29 pine (Pinus) species. Am. Nat. 2002, 159, 396–419. [Google Scholar] [CrossRef]
- Climent, J.; Chambel, M.R.; Pardos, M.; Lario, F.; Villar-Salvador, P. Biomass allocation and foliage heteroblasty in hard pine species respond differentially to reduction in rooting volume. Eur. J. For. Res. 2011, 130, 841–850. [Google Scholar] [CrossRef]
- Laliberté, E.; Turner, B.L.; Costes, T.; Pearse, S.J.; Wyrwoll, K.H.; Zemunik, G.; Lambers, H. Experimental assessment of nutrient limitation along a 2-million-year dune chronosequence in the south-western Australia biodiversity hotspot. J. Ecol. 2012, 100, 631–642. [Google Scholar] [CrossRef]
- Minden, V.; Kleyer, M. Internal and external regulation of plant organ stoichiometry. Plant Biol. 2014, 16, 897–907. [Google Scholar] [CrossRef]
- Tang, Z.; Xu, W.; Zhou, G.; Bai, Y.; Li, J.; Tang, X.; Chen, D.; Liu, Q.; Ma, W.; Xiong, G.; et al. Patterns of plant carbon, nitrogen, and phosphorus concentration in relation to productivity in China’s terrestrial ecosystems. Proc. Natl. Acad.Sci. USA 2018, 115, 4033–4038. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, H.; Vandana Sharma, S.; Pandey, R. Phosphorus nutrition: Plant growth in response to deficiency and excess. In Plant Nutrients and Abiotic Stress Tolerance; Springer: Singapore, 2018; pp. 171–190. [Google Scholar]
- Hauer-Jákli, M.; Tränkner, M. Critical leaf magnesium thresholds and the impact of magnesium on plant growth and photo-oxidative defense: A systematic review and meta-analysis from 70 years of research. Front. Plant Sci. 2019, 10, 437187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xiong, G.; Li, J.; Lu, Z.; Li, Y.; Xu, W.; Wang, Y.; Zhao, C.; Tang, Z.; Xie, Z. Nitrogen and phosphorus concentrations and allocation strategies among shrub organs: The effects of plant growth forms and nitrogen-fixation types. Plant Soil 2018, 427, 305–319. [Google Scholar] [CrossRef]
- Yang, S.; Shi, Z.; Zhang, M.; Li, Y.; Gao, J.; Wang, X.; Liu, D. Stoichiometry of carbon, nitrogen and phosphorus in shrub organs linked closely with Mycorrhizal strategy in Northern China. Front. Plant Sci. 2021, 12, 687347. [Google Scholar] [CrossRef] [PubMed]
- Ingestad, T.; Agren, G.I. The influence of plant nutrition on biomass allocation. Ecol. Appl. 1991, 1, 168–174. [Google Scholar] [CrossRef]
- Hernández, E.I.; Vilagrosa, A.; Luis, V.C.; Llorca, M.; Chirino, E.; Vallejo, V.R. Root hydraulic conductance, gas exchange and leaf water potential in seedlings of Pistacia lentiscus L. and Quercus suber L. grown under different fertilization and light regimes. Environ. Exp. Bot. 2009, 67, 269–276. [Google Scholar] [CrossRef]
- Corcuera, L.; Camarero, J.J.; Gil-Pelegrín, E. Functional groups in Quercus species derived from the analysis of pressure–volume curves. Trees 2002, 16, 465–472. [Google Scholar] [CrossRef]
- Egbewole, Z.T.; Rotowa, O.J.; Kuje, E.D.; Ogundana, O.A.; Mairafi, H.H.; Yohanna, I. Early germination, growth and establishment of Khaya senegalensis (DESR.) A. Juss in Middle-Belt of Nigeria. J. Energy Nat. Resour. 2018, 7, 75–82. [Google Scholar] [CrossRef]
- Gratani, L.; Bombelli, A. Differences in leaf traits among Mediterranean broad-leaved evergreen shrubs. In Annales Botanici Fennici; Finnish Zoological and Botanical Publishing Board: Helsinki, Finland, 2001; pp. 15–24. [Google Scholar]
- Roca, G.; Alcoverro, T.; Krause-Jensen, D.; Balsby, T.; van Katwijk, M.; Marbà, N.; Santos, R.; Arthur, R.; Mascaró, O.; Fernández-Torquemada, Y.; et al. Response of seagrass indicators to shifts in environmental stressors: A global review and management synthesis. Ecol. Indic. 2016, 63, 310–323. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, X.; Wilson, A.M.; Silander, J.A., Jr. Predicting autumn phenology: How deciduous tree species respond to weather stressors. Agric. For. Meteorol. 2018, 250, 127–137. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B. Biomass allocation to leaves shoots and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- André, F.; Jonard, M.; Ponette, Q. Biomass and nutrient content of sessile oak (Quercus petraea (Matt.) Liebl.) and beech (Fagus sylvatica L.) stem and branches in a mixed stand in southern Belgium. Sci. Total Environ. 2010, 408, 2285–2294. [Google Scholar] [CrossRef] [PubMed]
- Pretzsch, H.; Block, J.; Dieler, J.; Gauer, J.G. Export of nutrients from forest ecosystems by harvesting timber and biomass. Part 1: Functions for estimating tree biomass and nutrient content and their application for scenario analyses. Allg. Forst-Und Jagdztg. 2014, 185, 261–285. [Google Scholar]
- Pająk, K.; Małek, S.; Kormanek, M.; Jasik, M.; Banach, J. Macronutrient content in European Beech (Fagus sylvatica L.) seedlings grown in differently compacted peat substrates in a container nursery. Forests 2022, 13, 1793. [Google Scholar] [CrossRef]
- Johnson, R.; Vishwakarma, K.; Hossen, M.S.; Kumar, V.; Shackira, A.M.; Puthur, J.T.; Abdi, G.; Sarraf, M.; Hasanuzzaman, M. Potassium in plants: Growth regulation, signaling, and environmental stress tolerance. Plant Physiol. Biochem. 2022, 172, 56–69. [Google Scholar] [CrossRef]
- Agathokleous, E.; Kitao, M.; Komatsu, M.; Tamai, Y.; Harayama, H.; Koike, T. Single and combined effects of fertilization, ectomycorrhizal inoculation, and drought on container-grown Japanese larch seedlings. J. For. Res. 2023, 34, 1077–1094. [Google Scholar] [CrossRef]
- Qu, L.; Quoreshi, A.M.; Koike, T. Root growth characteristics, biomass and nutrient dynamics of seedlings of two larch species raised under different fertilization regimes. In Roots: The Dynamic Interface between Plants and the Earth: The 6th Symposium of the International Society of Root Research, 11–15 November 2001, Nagoya, Japan; Springer: Heidelberg, Germany, 2003; pp. 293–302. [Google Scholar]
- Rotowa, O.J.; Ugonma, D.A.; Egbewole, Z.T.; Bhadmus, H.B. Growth Response of Moringa oleifera Lam. to Organic and Mineral Fertilizers Treatment. Int. J. Appl. Res. Technol. 2017, 6, 51–56. [Google Scholar]
- James, R.O.; Akorede, A.A.; Abimbola, A.I.; Omoh, O.P. Effect of organic manure and potting media on germination and early growth of Eucalyptus torelliana F. Muell. Am. J. Agric. For. 2020, 8, 100–107. [Google Scholar]
- Magh, R.-K.; Yang, F.; Rehschuh, S.; Burger, M.; Dannenmann, M.; Pena, R.; Burzlaff, T.; Ivanković, M.; Rennenberg, H. Nitrogen nutrition of European beech is maintained at sufficient water supply in mixed beech-fir stands. Forests 2018, 9, 733. [Google Scholar] [CrossRef]
- Bzdyk, R.M.; Olchowik, J.; Studnicki, M.; Oszako, T.; Sikora, K.; Szmidla, H.; Hilszczańska, D. The impact of effective microorganisms (EM) and organic and mineral fertilizers on the growth and mycorrhizal colonization of Fagus sylvatica and Quercus robur seedlings in a bare-root nursery experiment. Forests 2018, 9, 597. [Google Scholar] [CrossRef]
- Banach, J.; Małek, S.; Kormanek, M.; Durło, G. Growth of Fagus sylvatica L. and Picea abies (L.) Karst. seedlings grown in Hiko containers in the first year after planting. Sustainability 2020, 12, 7155. [Google Scholar] [CrossRef]
- Ferrini, F.; Giuntoli, A.; Nicese, F.P.; Pellegrini, S.; Vignozzi, N. Effect of Fertilization and Backfill Amendments on Soil Characteristics, Growth, and Leaf Gas Exchange of English Oak (Quercus robus L.). J. Arboric. 2005, 31, 182. [Google Scholar]
- Chu, X.; Wang, X.; Zhang, D.; Wu, X.; Zhou, Z. Effects of fertilization and container-type on nutrient uptake and utilization by four subtropical tree seedlings. J. For. Res. 2020, 31, 1201–1213. [Google Scholar] [CrossRef]
- Khasa, P.D.; Sigler, L.; Chakravarty, P.; Dancik, B.P.; Erickson, L.; Mc Curdy, D. Effect of fertilization on growth and ectomycorrhizal development of container-grown and bare-root nursery conifer seedlings. New For. 2001, 22, 179–197. [Google Scholar] [CrossRef]
- Kormanek, M.; Małek, S.; Banach, J.; Durło, G. Effect of Changing Substrate Density and Water Application Method on Substrate Physical Properties and Container-Grown Seedling Growth. Forests 2023, 14, 1490. [Google Scholar] [CrossRef]
- Banach, J.; Kormanek, M.; Malek, S.; Durlo, G.; Skrzyszewska, K. Effect of the changing seedlings density of Quercus robur L. grown in nursery containers on their morphological traits and planting suitability. Sylwan 2023, 167, 1–12. [Google Scholar]
- Kalcsits, L.A.; Min, X.; Guy, R.D. Interspecific variation in leaf–root differences in δ 5 N among three tree species grown with either nitrate or ammonium. Trees 2015, 29, 1069–1078. [Google Scholar] [CrossRef]
- Mediavilla, S.; Escudero, A. Stomatal responses to drought at a Mediterranean site: A comparative study of co-occurring woody species differing in leaf longevity. Tree Physiol. 2003, 23, 987–996. [Google Scholar] [CrossRef]
- Aerts, R.; Chapin, F.S., III. The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns. In Advances in Ecological Research; Academic Press: Cambridge, MA, USA, 2000; Volume 30, pp. 1–67. [Google Scholar]
- Aerts, R.; Cornelissen, J.H.C.; Dorrepaal, E. Plant performance in a warmer world: General responses of plants from cold, northern biomes and the importance of winter and spring events. Plants Clim. Change 2006, 2006, 65–78. [Google Scholar]
- Villar-Salvador, P.; Heredia, N.; Millard, P. Remobilization of acorn nitrogen for seedling growth in holm oak (Quercus ilex), cultivated with contrasting nutrient availability. Tree Physiol. 2010, 30, 257–263. [Google Scholar] [CrossRef]
- Vadeboncoeur, M.A. Meta-analysis of fertilization experiments indicates multiple limiting nutrients in northeastern deciduous forests. Can. J. For. Res. 2010, 40, 1766–1780. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen–phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef]
- Martini, F.; Xia, S.W.; Zou, C.; Goodale, U.M. Seedling growth and survival responses to multiple soil properties in subtropical forests of south China. For. Ecol. Manag. 2020, 474, 118382. [Google Scholar] [CrossRef]
- Holste, E.K.; Kobe, R.K.; Vriesendorp, C.F. Seedling growth responses to soil resources in the understory of a wet tropical forest. Ecology 2011, 92, 1828–1838. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, B.; Davis, M.; Sardans, J.; Peñuelas, J.; Billings, S. Long-term nitrogen deposition linked to reduced water use efficiency in forests with low phosphorus availability. New Phytol. 2016, 210, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tian, D.; Yang, H.; Niu, S. Size-dependent nutrient limitation of tree growth from subtropical to cold temperate forests. Funct. Ecol. 2018, 32, 95–105. [Google Scholar] [CrossRef]
- Timmer, V. Exponential nutrient loading: A new fertilization technique to improve seedling performance on competitive sites. New For. 1997, 13, 279–299. [Google Scholar] [CrossRef]
- Landis, T.D.; Haase, D.L.; Dumroese, R.K. Plant Nutrient Testing and Analysis in Forest and Conservation Nurseries USDA Forest Service Proceedings RMRS-P-35; Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2005.
- Villar-Salvador, P.; Puértolas, J.; Cuesta, B.; Peñuelas, J.L.; Uscola, M.; Heredia-Guerrero, N.; Rey Benayas, J.M. Increase in size and nitrogen concentration enhances seedling survival in Mediterranean plantations. Insights from an ecophysiological conceptual model of plant survival. New For. 2012, 43, 755–770. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, H.; Chen, F.; Zhang, Y.; Liu, J.; Tigabu, M.; Ma, X.; Li, M. Nitrogen addition modulates adaptive responses of Chinese fir roots to phosphorus deficiency and promotes nutrient absorption efficiency. New For. 2024, 55, 1687–1705. [Google Scholar] [CrossRef]


| Substrate | Sawdust | Wood Bark | Perlite | Core Wood | Mixed Silage | Wood Chips | Straw |
|---|---|---|---|---|---|---|---|
| R20 | 73 | 10 | 4 | 2 | 1 | 10 | - |
| R21 | 20 | 10 | 4 | 2 | 1 | 63 | - |
| R22 | 50 | 33 | 4 | 2 | 1 | - | 10 |
| Substrate | Water Capacity (%) | Water Outflow Rate (L/min) | Bulk Density (g/cm3) | Solid Density (g/cm3) | Air Capacity (%) | Porosity (%) |
|---|---|---|---|---|---|---|
| R20 | 40.5 ± 2.9 b | 0.595 ± 0.150 b | 0.115 ± 0.009 a | 0.64 ± 0.08 a | 52.1 ± 3.19 c | 92.6 ± 0.60 d |
| R21 | 33.1 ± 2.5 d | 0.781 ± 0.114 a | 0.098 ± 0.014 c | 1.74 ± 0.07 a | 60.8 ± 3.06 a | 93.6 ± 0.87 c |
| R22 | 37.8 ± 5.1 c | 0.594 ± 0.150 b | 0.104 ± 0.020 b | 1.66 ± 0.11 a | 55.8 ± 5.58 b | 93.9 ± 0.98 b |
| Control | 57.7 ± 5.4 a | 0.417 ± 0.145 c | 0.085 ± 0.007 d | 1.69 ± 0.14 a | 37.0 ± 5.72 d | 94.7 ± 0.42 a |
| F | 387.45 | 56.32 | 65.81 | 1.0717 | 295.79 | 76.48 |
| p | 0.0000 | 0.0000 | 0.0000 | 0.3870 | 0.0000 | 0.0000 |
| Exchangeable Cations | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Soil Uptake Level (cm) | pH (H2O) | N | C | P2O5 | C/N | Ca | K | Mg | Na |
| Fagus sylvatica L. site | |||||||||
| 0–10 | 5.21 ± 0.55 | 0.36 ± 0.10 | 2.41 ± 0.21 | 3.14 ± 0.21 | 13.78 ± 0.32 | 5.51 ± 0.52 | 0.18 ± 0.01 | 0.57 ± 0.06 | 0.70 ± 0.08 |
| 10–20 | 5.10 ± 0.57 | 0.34 ± 0.09 | 1.94 ± 0.15 | 2.87 ± 0.14 | 12.90 ± 0.32 | 4.81 ± 0.39 | 0.16 ± 0.01 | 0.42 ± 0.03 | 0.63 ± 0.06 |
| Total | 5.15 ± 0.56 | 0.35 ± 0.10 | 2.16 ± 0.12 | 2.99 ± 0.12 | 13.30 ± 0.23 | 4.22 ± 0.32 | 0.15 ± 0.01 | 0.49 ± 0.03 | 0.55 ± 0.07 |
| p-value | 0.187 ns | 0.170 ns | 0.061 ns | 0.286 ns | 0.065 ns | 0.064 ns | 0.062 ns | 0.062 ns | 0.062 ns |
| Quercus robur L. site | |||||||||
| 0–10 | 5.14 ± 0.59 | 0.45 ± 0.95 | 2.14 ± 0.22 | 4.70 ± 0.45 | 13.31 ± 0.33 | 5.11 ± 0.53 | 0.23 ± 0.02 | 0.55 ± 0.06 | 0.81 ± 0.26 |
| 10–20 | 5.03 ± 0.61 | 0.44 ± 0.09 | 1.83 ± 0.14 | 4.22 ± 0.40 | 12.23 ± 0.31 | 4.65 ± 0.36 | 0.21 ± 0.02 | 0.46 ± 0.03 | 0.82 ± 0.27 |
| Total | 5.08 ± 0.59 | 0.45 ± 0.10 | 1.55 ± 0.13 | 4.44 ± 0.30 | 12.73 ± 0.23 | 3.32 ± 0.32 | 0.22 ± 0.01 | 0.38 ± 0.03 | 0.82 ± 0.29 |
| p-value | 0.208 ns | 0.945 ns | 0.061 ns | 0.427 ns | 0.069 ns | 0.061 ns | 0.363 ns | 0.061 ns | 0.989 ns |
| Fagus sylvatica | Quercus robur | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | Nutrient | Treatment/Organ | Estimate (mg g−1) | Std. Error | Lower CI | Upper CI | Estimate (mg g−1) | Std. Error | Lower CI | Upper CI |
| Beech | N | SR20 | −22.03 | 3.29 | −29.09 | −14.98 | −24.37 | 4.33 | −33.66 | −15.08 |
| SR21 | −21.47 | 3.29 | −28.52 | −14.41 | −20.13 | 4.33 | −29.42 | −10.84 | ||
| SR22 | −12.70 | 3.29 | −19.76 | −5.65 | −14.53 | 4.33 | −23.82 | −5.24 | ||
| UC | −38.17 | 3.29 | −45.22 | −31.11 | −37.10 | 4.33 | −46.39 | −27.81 | ||
| UR20 | −41.80 | 3.29 | −48.86 | −34.75 | −39.70 | 4.33 | −48.99 | −30.41 | ||
| UR21 | −41.20 | 3.29 | −48.26 | −34.15 | −42.57 | 4.33 | −51.86 | −33.28 | ||
| UR22 | −42.87 | 3.29 | −49.92 | −35.81 | −39.43 | 4.33 | −48.72 | −30.14 | ||
| Leaf | −27.26 | 2.01 | −31.58 | −22.94 | 7.25 | 2.65 | 1.56 | 12.94 | ||
| Stem | −21.33 | 2.01 | −25.65 | −17.01 | −10.36 | 2.65 | −16.05 | −4.67 | ||
| Beech | P | SR20 | −2.90 | 0.76 | −4.54 | −1.26 | −3.03 | 1.46 | −6.17 | 0.10 |
| SR21 | −2.40 | 0.76 | −4.04 | −0.76 | −3.00 | 1.46 | −6.14 | 0.14 | ||
| SR22 | −1.33 | 0.76 | −2.97 | 0.30 | −0.97 | 1.46 | −4.10 | 2.17 | ||
| UC | −6.00 | 0.76 | −7.64 | −4.36 | −6.67 | 1.46 | −9.80 | −3.53 | ||
| UR20 | −6.57 | 0.76 | −8.20 | −4.93 | −7.07 | 1.46 | −10.20 | −3.93 | ||
| UR21 | −6.47 | 0.76 | −8.10 | −4.83 | −7.30 | 1.46 | −10.44 | −4.16 | ||
| UR22 | −6.43 | 0.76 | −8.07 | −4.80 | −6.70 | 1.46 | −9.84 | −3.56 | ||
| Leaf | −5.40 | 0.47 | −6.40 | −4.40 | −1.96 | 0.90 | −3.88 | −0.04 | ||
| Stem | −3.46 | 0.47 | −4.47 | −2.46 | −3.31 | 0.90 | −5.23 | −1.39 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotowa, O.J.; Małek, S.; Jasik, M.; Staszel-Szlachta, K. Substrate and Fertilization Used in the Nursery Influence Biomass and Nutrient Allocation in Fagus sylvatica and Quercus robur Seedlings After the First Year of Growth in a Newly Established Forest. Forests 2025, 16, 511. https://doi.org/10.3390/f16030511
Rotowa OJ, Małek S, Jasik M, Staszel-Szlachta K. Substrate and Fertilization Used in the Nursery Influence Biomass and Nutrient Allocation in Fagus sylvatica and Quercus robur Seedlings After the First Year of Growth in a Newly Established Forest. Forests. 2025; 16(3):511. https://doi.org/10.3390/f16030511
Chicago/Turabian StyleRotowa, Odunayo James, Stanisław Małek, Michał Jasik, and Karolina Staszel-Szlachta. 2025. "Substrate and Fertilization Used in the Nursery Influence Biomass and Nutrient Allocation in Fagus sylvatica and Quercus robur Seedlings After the First Year of Growth in a Newly Established Forest" Forests 16, no. 3: 511. https://doi.org/10.3390/f16030511
APA StyleRotowa, O. J., Małek, S., Jasik, M., & Staszel-Szlachta, K. (2025). Substrate and Fertilization Used in the Nursery Influence Biomass and Nutrient Allocation in Fagus sylvatica and Quercus robur Seedlings After the First Year of Growth in a Newly Established Forest. Forests, 16(3), 511. https://doi.org/10.3390/f16030511







