Abstract
Cinnamomum bodinieri is a tree species highly valued for its superior-quality timber and ecological benefits. However, its large-scale propagation is hindered by the low efficiency of adventitious root (AR) formation. This study investigated the physiological and molecular mechanisms underlying AR formation in C. bodinieri. The results revealed that ARs originate from callus tissue, with the root primordium classified as a latent type. During AR formation, concentrations of soluble protein and soluble sugar decreased, while the activities of superoxide dismutase (SOD), peroxidase (POD), and indole-3-acetic acid oxidase (IAAO) peaked 20 days after cutting (CB2), with polyphenol oxidase (PPO) activity exhibiting an “N”-shaped trend. These findings indicate that substantial nutrient consumption is required for AR formation, with SOD, POD, PPO, and IAAO positively regulating the process. Indole-3-acetic acid (IAA) levels significantly decreased during the early stages of cutting but increased thereafter, whereas the concentration of abscisic acid (ABA) continuously rose. Similar trends were observed for zeatin riboside (ZR) and gibberellic acid (GA). Transcriptome analysis identified 28 key genes involved in plant hormone signal transduction pathways. Furthermore, weighted gene co-expression network analysis (WGCNA) pinpointed 14 hub genes, including CYP94B3 and NAC82, linked to hormone-associated traits. Furthermore, quantitative real-time PCR (qRT-PCR) confirmed the accuracy of the transcriptome sequencing results. This analysis uncovered critical interactions between hormonal signaling pathways and pivotal gene networks. Overall, the findings highlight the central regulatory role of endogenous hormones in AR formation, with IAA serving as the predominant regulator.
1. Introduction
Cinnamomum bodinieri is primarily distributed in the southeastern regions of China, where it is widely used for landscaping in rural courtyards and along urban roads [1]. This species holds significant economic value, as its leaves are rich in essential oils, making it a valuable industrial raw material. The essential oils extracted from Cinnamomum species have diverse applications, including uses in food, fragrances, pesticides, and health products [2,3]. Depending on the type of essential oil, C. bodinieri can be classified into several chemotypes, such as linalool, camphor, nerolidol, citral, and borneol types [4]. The progeny derived from the sexual reproduction of C. bodinieri exhibits substantial variation in both the content and composition of essential oils. In contrast, the clonal offspring produced through cutting propagation demonstrate a more stable profile and composition of essential oils that closely resemble those of the parent plant. However, the low rooting and survival rates of C. bodinieri cuttings represent a major challenge for large-scale propagation. Therefore, understanding the mechanisms underlying adventitious root (AR) formation in C. bodinieri cuttings is critical to overcoming these limitations and improving propagation efficiency.
AR formation is a critical process for the successful propagation of cuttings. However, AR formation is highly complex and influenced by both internal and external factors. Internal factors include the species of the mother tree [5], the position of the cutting [6], and the presence of leaves and buds on the cutting [7]. External factors include temperature [8], light [9], and the application of rooting hormones [10]. During AR formation, the rapid cell division and active metabolism create a high energy demand, necessitating significant consumption of carbohydrates such as sugars and starches [11]. Soluble proteins in the cuttings are also converted into essential substances required for root growth and development [12]. In addition, various oxidases within the plant, including peroxidase (POD), superoxide dismutase (SOD), and catalase (CAT), play crucial roles in the rooting process [13,14,15]. Studies have shown that exogenous substances can regulate the activity of these oxidases and thereby influence the rooting process. For example, the application of exogenous chitosan reduces the activity of indole-3-acetic acid oxidase (IAAO) and POD under NaCl stress in vegetable soybeans, mitigating IAA degradation [16]. Similarly, the addition of 0.1 mg/L graphene oxide significantly enhances the activities of SOD, POD, and CAT in apple plants, leading to an improved rooting rate [17]. However, while 0.2% graphene oxide has been found to promote root growth, higher concentrations can damage root structure, impair nutrient absorption, and ultimately inhibit root development in alfalfa [18].
The formation of ARs is closely associated with changes in plant hormone levels. Among these, auxin plays a central role in AR formation in most plant species [19,20,21]. The application of exogenous auxins, such as IAA, to cuttings has been shown to promote root primordium formation, enhance AR elongation, and improve the survival rate of cuttings [22]. Polar auxin transport (PAT) is essential for root development and is regulated by AUX1/LAX and PIN [23,24]. For instance, in apple cuttings, the MdPIN gene family mediates the anatomical and physiological changes that regulate AR formation. Specifically, MdPIN4, MdPIN5, and MdPIN8 promote lenticel dehiscence and AR protrusion during the elongation phase of AR development [25]. In addition, melatonin has been shown to regulate the root meristem in Arabidopsis thaliana by inhibiting auxin biosynthesis and PAT [26]. Cytokinin (CTK) is thought to have an antagonistic effect on auxin during AR formation [27]. Zeatin riboside (ZR), a type of CTK, has been shown to be the main inhibitor of AR formation in the hypocotyl of cucumber, as identified in the xylem sap of cucumber roots [28]. Other hormones, such as gibberellins (GAs) and abscisic acid (ABA), also play critical regulatory roles. High concentrations of ABA can inhibit root primordium formation, while GA suppresses AR development during its early stages [29,30,31].
Beyond changes in hormone homeostasis, recent studies suggest that hormone functions involve interactions with secondary metabolites, such as flavonoids [29], miRNAs [32], and downstream signaling pathways [33]. Transcriptome analysis has provided insights into the transcriptional regulation of AR formation triggered by auxin. Sequencing and annotation studies have identified several transcription factors (TFs), including MYB, APETALA2/ethylene response factor (AP2/ERF), and NAC, that play regulatory roles during AR formation [34,35,36]. For example, GA influences local auxin biosynthesis and PAT in rice seedling roots by modulating the expression of OsYUCCA6 and OsPIN. Flavonoids act as mediators in GA-regulated auxin biosynthesis and PAT [37]. Small RNA sequencing has further revealed 187 known and 117 novel miRNAs in grape tissue culture seedlings. Notably, the overexpression of Vvi-miR164b in A. thaliana increased lateral root numbers and enhanced root development [38]. Although significant progress has been made in elucidating AR formation mechanisms in model plants, research on C. bodinieri remains limited. To date, systematic studies addressing the molecular and physiological mechanisms of AR formation during cutting propagation in this economically important woody species are lacking.
With the continuous advancement of biological techniques, transcriptomics has become an essential tool for addressing diverse biological questions. RNA sequencing (RNA-seq), which sequences transcript cDNA, determines transcript abundance by quantifying the number of reads mapped to each transcript. This powerful technique has been greatly facilitated by the development of high-throughput sequencing technologies [39]. Weighted gene co-expression network analysis (WGCNA) is a complementary approach used to identify co-expression patterns among differentially expressed genes (DEGs), grouping highly correlated genes into distinct modules. This analysis enables further correlation studies to identify candidate key genes with potential regulatory roles [40]. In this study, C. bodinieri cuttings at four distinct stages of AR formation were used as the experimental materials. Key physiological indices, including soluble sugar, soluble protein, SOD, POD, IAAO, and PPO, were measured and analyzed. Additionally, RNA-seq was employed to investigate the differentially expressed genes at each stage. By integrating physiological and transcriptomic data, this study aimed to elucidate the underlying mechanisms of AR formation in C. bodinieri during cutting propagation.
2. Materials and Methods
2.1. Plant Materials
Cuttings for the propagation experiment were obtained from four-year-old clonal saplings of citral-chemotype C. bodinieri (Figure S1) maintained at the Camphor Tree Gene Resource Bank of the Jiangxi Academy of Forestry (27°53′ N, 116°47′ E). The experiment was conducted in July 2022. Healthy, pest-free, and vigorous one-year-old fully lignified sun-exposed branches with plump buds were selected. Cuttings measuring 6–8 cm in length, each with 2–3 buds and 1 retained leaf, were prepared. The basal ends of the cuttings were trimmed into a slanted surface and soaked in water for 30 min. Based on the developmental characteristics of AR formation, the rooting process was categorized into four stages: CB0 (Day 0), CB1 (Day 10), CB2 (Day 20), and CB3 (Day 30). Samples were collected at each stage, immediately frozen in liquid nitrogen, and stored at −80 °C until subsequent unified analysis.
2.2. Morphological and Anatomical Observation
The anatomical structures of the cuttings were examined using the traditional paraffin sectioning method. A 5 mm callus segment from the basal portion of the stem was excised, washed, and fixed in FAA solution for 48 h at 4 °C. The samples were then sequentially dehydrated, cleared, embedded in paraffin, and stained with safranin and fast green. Stained paraffin sections were mounted using neutral gum and observed under a Leica optical microscope (Leica Microsystems, Wetzlar, Germany). Representative anatomical structures were imaged, and key structural features were documented.
2.3. Physiological Index Measurement
The contents of soluble sugars and soluble proteins were quantified using the anthrone method [41] and the Coomassie Brilliant Blue method [42], respectively. The activity of peroxidase (POD) was measured using the guaiacol colorimetric method [43]. The polyphenol oxidase (PPO) activity was determined by the catechol colorimetric method [44]. The superoxide dismutase (SOD) and indole-3-acetic acid oxidase (IAAO) activities were measured using ELISA kits (Suzhou Greys Biological Technology Co., Ltd., Suzhou, China). The endogenous hormone levels, including IAA, GA, ABA, and ZR, in the phloem of the stem base were determined using high-performance liquid chromatography tandem mass spectrometry (HPLC-MS/MS) [45]. All experiments were conducted with six biological replicates to ensure statistical reliability and robustness.
2.4. RNA-Seq and Data Analysis
Samples of C. bodinieri cuttings from different stages of AR formation were sent to Bena Technology Co., Ltd. (Wuhan, China) for transcriptome sequencing, with three biological replicates for each stage. mRNA was enriched using oligo(dT) magnetic beads, followed by fragmentation of the enriched mRNA using fragmentation reagents to produce smaller fragments. First- and second-strand cDNA synthesis was performed following standard protocols. Library preparation included end repair, A-tailing, adapter ligation, purification, PCR amplification, and product circularization. After confirming library quality, sequencing was conducted on a DNBSEQ-T7 high-throughput sequencing platform by BGI. Raw sequencing data were processed using FASTP (version 0.21.0) to generate clean reads for subsequent analysis [29].
2.5. WGCNA
WGCNA was applied to DEGs to calculate weighted correlations among genes, following the scale-free topology criterion. This approach enabled the construction of a gene clustering tree and the grouping of highly correlated genes into distinct modules. Correlation analysis between these modules and endogenous hormone levels was performed to identify hub modules. Genes within hub modules were further filtered based on their weights using the R (version 4.4.1) package WGCNA [46], and a gene co-expression network was constructed and visualized using Cytoscape (version 3.9.0).
2.6. Validation of DEGs by qRT-PCR
Quantitative real-time PCR (qRT-PCR) was performed to validate the transcriptome sequencing results. Twelve genes closely associated with root formation were selected for validation, and specific primers were designed based on the RNA-seq data (Table S1). β-actin was used as the internal reference gene [47]. Total RNA was extracted from the samples and reverse-transcribed into cDNA. The qRT-PCR reactions were conducted using SYBR Green reagent (Mei 5 Biotechnology, Beijing, China) in a 20 µL reaction volume containing 10 µL of SYBR Green mix, 0.5 µL of each primer, 2 µL of cDNA template, and 7 µL of nuclease-free water. The following PCR conditions recommended by the manufacturer were used: 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s, and 60 °C for 1 min. qRT-PCR data were calibrated relative to the corresponding gene expression level at the CB0 stage for each treatment, following the 2−ΔΔCt method for relative quantification [48]. Each sample was analyzed in triplicate for both biological and technical replicates.
2.7. Statistical Analysis
One-way analysis of variance (ANOVA) with a significance threshold of p < 0.05 was performed, followed by the least significant difference (LSD) test, using SPSS software (version 26). Figures and graphical outputs were generated using GraphPad Prism (version 9.0).
3. Results
3.1. Morphological and Anatomical Changes During the Rooting Process
Morphological observations were conducted to examine AR formation in C. bodinieri cuttings. Following insertion, the basal region of the cuttings exhibited swelling, accompanied by the formation of callus tissue (Figure 1B). Subsequently, small, white, bud-like protrusions appeared on the surface of the callus (Figure 1C), which eventually elongated and developed into brown ARs (Figure 1D). These findings suggest that C. bodinieri cuttings follow a callus-mediated root formation pattern. Microscopic observations of the different stages of AR development are presented in Figure 1. At stage CB0, no latent root primordia were observed in the cross-sections of the cuttings (Figure 1a), indicating that the root primordium type was induced rather than performed. At stage CB1, densely packed callus cells with abundant cytoplasm and prominent nuclei were observed near the cambial region at the cutting base. Additionally, clump-like meristematic tissue began to appear within the callus (Figure 1b). At stage CB2, the clump-like meristematic tissue differentiated into well-defined root primordia structures (Figure 1c). By stage CB3, the root primordia continued to proliferate and elongate, eventually breaking through the cortex (Figure 1d). Based on the histological changes observed during AR development, the rooting process in C. bodinieri cuttings can be divided into three distinct stages: (1) callus induction (CB0–CB1), characterized by the initiation of callus tissue and meristematic activity; (2) root primordia induction (CB1–CB2), marked by the differentiation of root primordia within the callus; and (3) AR formation (CB2–CB3).
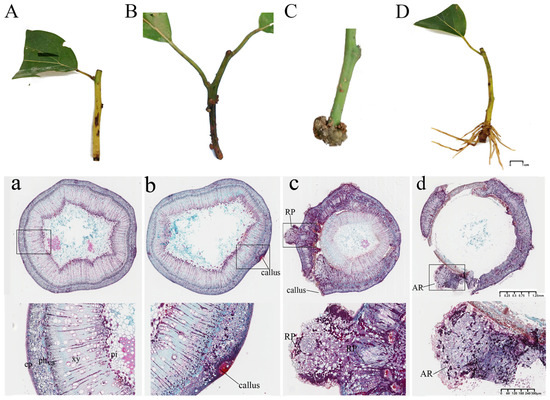
Figure 1.
Morphological and anatomical observations of the process of AR formation in C. bodinieri. Note: (A), (a) CB0. (B), (b) CB1. (C), (c) CB2. (D), (d) CB3. RP, root primordia; AR, adventitious root; ep, epidermis; ph, phloem; va, vascular; xy, xylem; pi, pith.
3.2. Variation in Nutrient and Enzyme Activity During Rooting Process
Significant changes in the soluble sugar and protein contents were observed during AR formation in the C. bodinieri cuttings (p < 0.05) (Figure 2). The soluble sugar content exhibited a fluctuating trend: it initially decreased, followed by an increase, and then declined again. This variation may be attributed to the rapid division of cells in the callus and root primordium, which requires large amounts of carbohydrates for energy and biosynthesis. The soluble protein content, on the other hand, increased sharply during callus formation, followed by a rapid and sustained decline throughout the AR formation stage. This indicates that substantial quantities of proteins are essential for the morphogenesis of AR structures. Enzyme activities also showed distinct trends across different stages of AR formation. SOD activity initially increased and then declined. A pronounced rise in SOD activity was observed during the callus and root primordium induction stages, followed by a decrease during the root primordium elongation stage. POD activity, however, exhibited a different pattern. POD activity decreased during the callus induction and AR elongation stages but significantly increased during root primordium formation, suggesting that POD inhibits callus formation and root elongation while promoting root primordium development. In contrast, polyphenol oxidase (PPO) activity displayed an inverse relationship to POD activity, following an “N”-shaped trend: it increased during the initial stage, decreased during the middle stage, and increased again in the final stage of AR formation. IAAO activity steadily increased throughout AR formation, peaking at CB2 before declining.
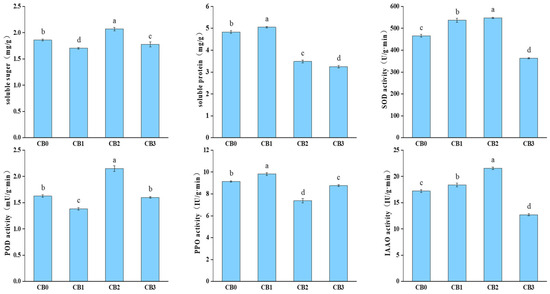
Figure 2.
Changes in nutrient content and enzyme activity in different stages. Note: different lowercase letters indicate significant differences (p < 0.05).
3.3. Changes in Endogenous Hormone Levels During AR Formation
Significant fluctuations in endogenous hormone levels were observed during the AR formation process (p < 0.05) (Figure 3). The content of IAA decreased during the CB0–CB1 stage, followed by a continuous increase. This trend suggests that AR formation relies on high concentrations of IAA to promote root initiation and elongation. The ZR and GA levels exhibited an “N”-shaped trend, increasing during the callus induction and AR formation stages but decreasing during root primordium induction. These patterns indicate that high concentrations of ZR and GA facilitate cell division but inhibit cell differentiation during the rooting process. In contrast, the content of ABA gradually increased throughout the AR formation process. This finding suggests that ABA plays a role in enhancing stress resistance during AR formation in C. bodinieri. The IAA/ZR ratio significantly decreased during the callus induction and AR formation stages but increased substantially during the root primordium induction stage. This trend indicates that a higher IAA/ZR ratio is favorable for root primordium formation. Similarly, the IAA/ABA ratio decreased significantly during callus induction, reaching its lowest value at CB1, and then gradually increased. This suggests that a higher IAA/ABA ratio promotes both root primordium formation and AR elongation during the rooting process in C. bodinieri.
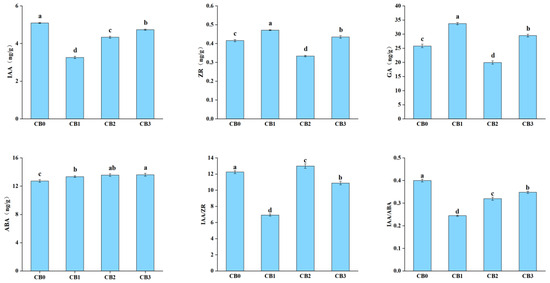
Figure 3.
Changes in endogenous hormone levels during AR formation. Note: different lowercase letters indicate significant differences (p < 0.05).
3.4. Transcriptomic Analysis of AR Formation
RNA-seq analysis was performed on samples collected from different developmental stages of AR formation, with three biological replicates per stage, resulting in a total of 12 libraries. Biological replicates from the same stage showed a high correlation, while samples from different stages exhibited a lower correlation. After filtering and quality control, the transcriptomic data yielded at least 4 million clean reads per sample, with Q20 values exceeding 97.5% (Table S2). The guanine (G) and cytosine (C) nucleotide contents (GC contents) were consistent across all samples, and the N50 length of the unigenes was 1610 bp (Figure S2). Functional annotation of the unigenes was conducted using seven databases. The Nr database provided the highest annotation rate, covering 31.48% of the unigenes, followed by the UniProt and Pfam databases, with annotation rates of 30.73% and 24.53%, respectively (Table S3).
Differential expression analysis identified 4806 upregulated and 5004 downregulated genes in the CB1 vs. CB0 comparison (Figure 4A), 4317 upregulated and 4127 downregulated genes in the CB2 vs. CB0 comparison (Figure 4B), and 4904 upregulated and 4919 downregulated genes in the CB3 vs. CB0 comparison (Figure 4C). A Venn diagram comparing the DEGs across these three groups revealed 4695 common DEGs (Figure 4D).
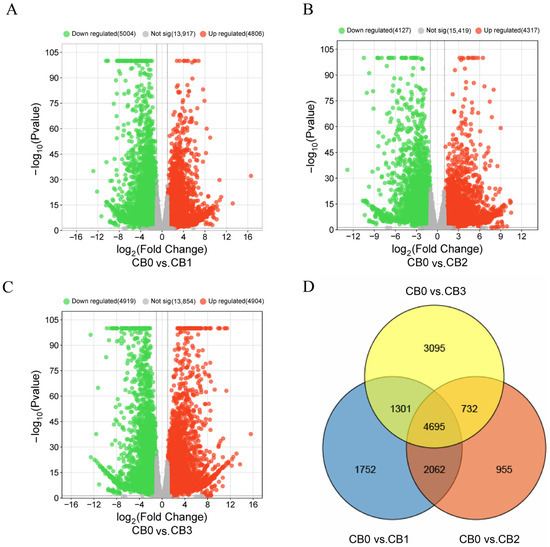
Figure 4.
Volcano plot and Venn diagram of DEGs. In the volcano plot, each point represents a unigene. The x-axis shows the log2 fold change, which indicates the log2 value of the fold change in expression levels of a unigene between two samples. The y-axis represents either the p-value or the false discovery rate (padj), with the y-value being the negative log10-transformed value of the p-value or padj.
3.5. Functional Annotation and Enrichment Analysis of DEGs
To investigate the biological functions of the DEGs during AR formation in C. bodinieri, GO enrichment analysis was performed. The top 20 significantly enriched GO terms from the biological process (BP), cellular component (CC), and molecular function (MF) categories were selected based on p-values for visualization (Figure 5). DEGs from the three comparison groups (CB0 vs. CB1, CB0 vs. CB2, and CB0 vs. CB3) were enriched in 483, 468, and 489 GO terms, respectively. For the CB0 vs. CB1 group, the most enriched biological process was the regulation of transcription, DNA-templated, which included 146 DEGs, followed by the carbohydrate metabolic process, with 114 DEGs—significantly more than the other processes. In the cellular component category, DEGs were primarily enriched in the plasma membrane and extracellular region, with additional enrichment observed in the cell wall and plastid. In the molecular function category, DNA-binding transcription factor activity was the most enriched term, with 213 annotated DEGs, followed by significant enrichment in enzyme activity (Figure 5A). For the CB0 vs. CB2 group, the most enriched biological process was the carbohydrate metabolic process, involving 100 DEGs, followed by methylation, which included 76 DEGs. In the cellular component category, the DEGs were predominantly enriched in the plasma membrane and extracellular region. In the molecular function category, DNA-binding transcription factor activity was again the most enriched term, with 198 DEGs, while enzyme activity was also significantly enriched (Figure 5B). For the CB0 vs. CB3 group, the most enriched biological process was the carbohydrate metabolic process, with 111 DEGs, followed by methylation, which included 85 DEGs. In the cellular component category, the DEGs were mainly enriched in the plasma membrane and extracellular region, with additional enrichment observed in the cell wall and plastid. Regarding molecular functions, DNA-binding transcription factor activity had the highest number of DEGs (190), with significant enrichment also observed for enzyme activity (Figure 5C). GO enrichment analysis revealed that these DEGs participate in critical biological processes, including transcriptional regulation, carbohydrate metabolism, and enzyme activity, as well as in key cellular components such as the plasma membrane and plastid. These findings provide insight into the biochemical and molecular processes associated with AR formation in C. bodinieri, shedding light onto the mechanisms underlying this process.
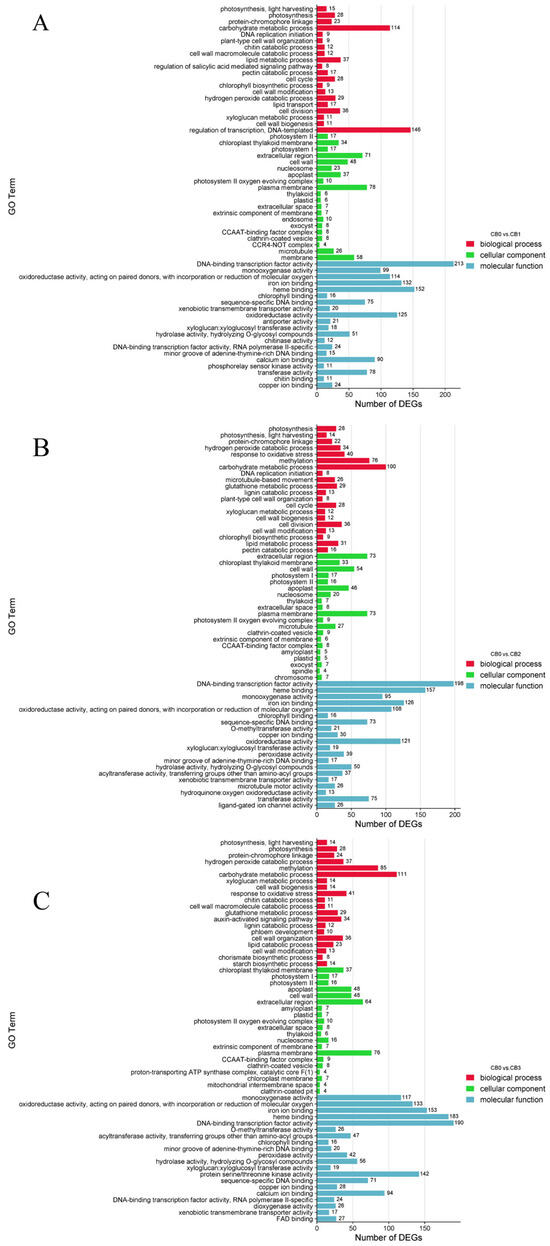
Figure 5.
GO enrichment analysis of DEGs from four time periods. (A) CB0 vs. CB1. (B) CB0 vs. CB2. (C) CB0 vs. CB3. In the figure above, the x-axis represents the GeneRatio (the proportion of differentially expressed genes in the given GO category relative to all DEGs), and the y-axis represents Gene Ontology terms.
To further elucidate the metabolic activities associated with the DEGs, KEGG pathway annotation and enrichment analysis were conducted. KEGG enrichment analysis identified 17, 22, and 32 significantly enriched pathways in the CB0 vs. CB1, CB0 vs. CB2, and CB0 vs. CB3 comparison groups, respectively. The top 20 enriched metabolic pathways were selected for visualization. In the CB0 vs. CB1 comparison, the plant hormone signal transduction pathway had the highest number of enriched DEGs, comprising 86 genes (Figure 6A). In the CB0 vs. CB2 comparison, the most significantly enriched pathways were the plant hormone signal transduction pathway and the phenylpropanoid biosynthesis pathway (Figure 6B). Similarly, in the CB0 vs. CB3 comparison, the plant hormone signal transduction pathway showed the most significant enrichment, followed by the phenylpropanoid biosynthesis pathway and the MAPK signaling pathway (Figure 6C). Overall, these results highlight the pivotal role of the plant hormone signal transduction pathway in the AR formation process in C. bodinieri.
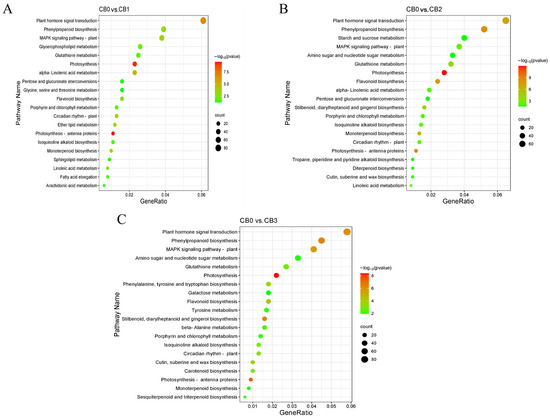
Figure 6.
KEGG enrichment analysis of DEGs from four time periods. (A) CB0 vs. CB1. (B) CB0 vs. CB2. (C) CB0 vs. CB3. In the figure above, the x-axis represents the number of differentially expressed genes, and the y-axis represents the KEGG pathways. The color of the dots indicates the p.adjust (p-value), with smaller p.adjust values indicating more significant enrichment results. The enrichment plot displays only the top 20 most significant pathways.
3.6. Analysis of DEGs Related to Plant Hormone Signal Transduction Pathway
KEGG analysis indicated that the plant hormone signal transduction pathway contained the highest number of enriched DEGs across all three comparisons. To explore the role of this pathway in the AR formation of C. bodinieri, enrichment and annotation analyses of the DEGs involved in this pathway were conducted (Figure 7). A total of 28 common DEGs were identified within the plant hormone signal transduction pathway. These genes were distributed among the auxin (17 genes), abscisic acid (5 genes), gibberellin (3 genes), and cytokinin (3 genes) signaling pathways. In the auxin signaling pathway, the DEGs were primarily involved in the synthesis of AUX/IAA proteins. Genes encoding SAUR proteins were upregulated, while most of the other auxin-related genes were downregulated during AR formation. In the cytokinin signaling pathway, DEGs encoding AHP, A-ARR, and B-ARR proteins were predominantly downregulated. For the gibberellin signaling pathway, three genes encoding GID1 and DELLA proteins were upregulated during AR formation. In the abscisic acid signaling pathway, genes encoding PYR/PYL receptors and the ABF protein (Unigene6838) were upregulated, while genes encoding PP2C and SnRK2 proteins were downregulated during AR development.
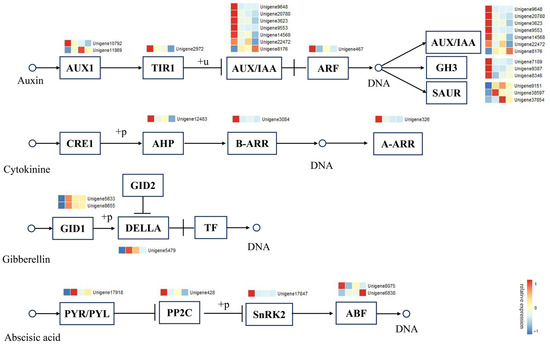
Figure 7.
Expression analysis of DEGs in plant hormone signal transduction pathways. The colored boxes represent the expression levels of the DEGs for one period. The color gradient from blue to red indicates expression levels ranging from −1 to 1.
3.7. Analysis of Association Between Modules and Endogenous Hormones
To better understand the regulatory network of endogenous hormones during AR formation, WGCNA was conducted on the DEGs obtained from transcriptome sequencing. Using the scale-free topology criterion, a soft threshold of β = 24 was selected for constructing the clustering tree (Figure 8B). The highly correlated modules were then merged using a one-step method, resulting in 15 modules (Figure 8A). Subsequently, correlation analysis was performed between all co-expression modules and the contents of the endogenous hormones, including IAA, GA, ABA, and ZR. A heatmap of hormone–module correlations was generated (Figure 8D). Three key hub modules were identified, and the gene expression patterns of these modules were analyzed across the different sampling stages (Figure 8C). The steelblue module showed a positive correlation with IAA content, with DEGs in this module upregulated during CB0 and CB2 and downregulated during CB1, reflecting the changes in the IAA levels. The sienna3 module exhibited a positive correlation with the ABA content, while the lightcyan1 module was strongly positively correlated with both the ZR and GA contents. The expression patterns of the DEGs in the sienna3 and lightcyan1 modules were similar, suggesting a potential synergistic role in hormone regulation.
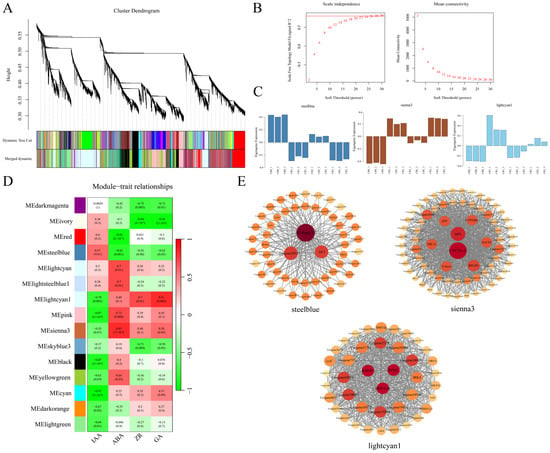
Figure 8.
Selection of hub modules and construction of their expression networks. (A) The appropriate power value was selected to construct the weighted co-expression network model. (B) The dynamic tree cut method is used in the lower part to identify modules, while merged dynamic represents the final modules obtained after merging similar ones. (C) Expression profiles of the hub modules most correlated with hormone levels at different periods. (D) Heatmap showing the correlation between the modules and hormone levels, with the values inside each colored block representing the correlation coefficient. (E) Co-expression network construction of the hub modules most correlated with hormone levels.
To identify the key genes within these modules, a co-expression network was constructed using the top 10% of module-weighted unigenes for each of the three hub modules (Figure 8E). Fourteen hub genes were identified, including three in the steelblue module: CbU-boxE3, Unigene20123, and CbAKT; eight hub genes were identified in the sienna3 module: CbCYCT1-3, CbtalA, CbCYP94B3, CbRPLP0, CbTBL33, CbF-box5, CbNAC82, and CbZFP; and three hub genes were identified in the lightcyan1 module: CbRPM1, CbRRP45, and CbMRP-L28.
3.8. Correlation Analysis Between Physiological Index and Candidate Genes
A correlation analysis was conducted between the 42 genes (including 28 key genes identified from the plant hormone signal transduction pathway and 14 hub genes identified via WGCNA) and the measured physiological index (Figure S3). The hub gene talA in the sinna3 module exhibited a significant correlation with IAAO and SOD activity. The key genes Unigene10792 (AUX1) and Unigene20780 (AUX/IAA), involved in the auxin signaling pathway, were highly correlated with ABA content, while Unigene38597 (SAUR) showed a significant correlation with IAA content and the IAA/ABA ratio.
3.9. Validation of Gene Expression
Twelve genes associated with root formation were selected from the DEGs for validation using qRT-PCR. The expression patterns of these genes, as determined by qRT-PCR, were consistent with those observed in the RNA-seq analysis, confirming the reliability and accuracy of the transcriptome data (Figure 9).
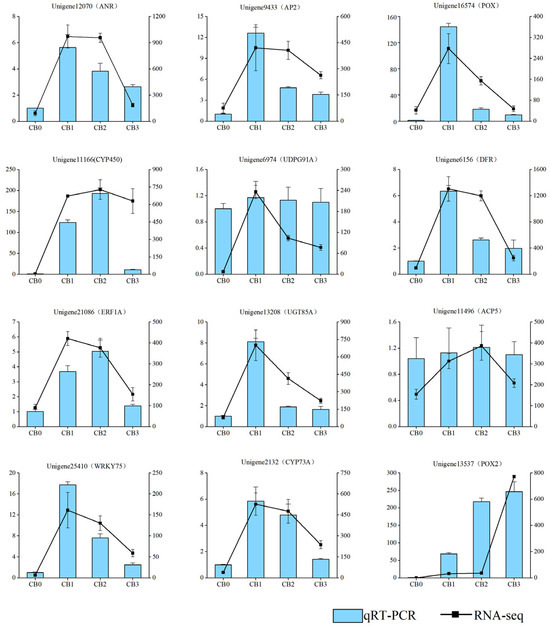
Figure 9.
Comparison analysis of qRT-PCR and RNA-seq results. The black line represents the RNA-seq FPKM, and the blue bars represent the relative expression levels from qRT-PCR.
4. Discussion
4.1. The Process of AR Formation in C. bodinieri
AR formation is a complex developmental process that is critical for the success of vegetative propagation in many plant species [49]. Root primordium formation is a pivotal stage in AR development, and these primordia can be classified into two types: latent and induced. Based on their origin, ARs can be categorized into three types: cortex rooting, callus rooting, and mixed rooting [50]. Anatomical observations of C. bodinieri cuttings revealed no latent root primordia during the early stages, indicating that the root primordia in this species are of the induced type. Notably, ARs primarily originate from callus tissue, which may explain the relatively low rooting and survival rates observed in C. bodinieri cuttings.
4.2. Significant Changes in Nutrient Content and Oxidase Activity
During the transition from the CB2 to the CB3 stage, we observed the upregulation of the genes encoding SUS and INV (Figure S4), while the soluble sugar content decreased. This suggests that substantial amounts of soluble sugars are consumed during AR formation. These findings align with previous studies on AR formation in Arabidopsis thaliana, where sugar consumption is also a critical factor in root development [51]. In addition, the soluble protein content decreased rapidly from the CB1 to CB2 stage, which was indicative of significant protein turnover during root primordium formation. Hydrogen peroxide (H2O2), known to act as a signaling molecule, plays a crucial role in auxin-induced AR formation. Plants maintain H2O2 homeostasis through the regulation of the enzymes that produce and degrade H2O2, such as SOD and POD [52,53]. In this study, we observed substantial variation in the POD activity, suggesting fluctuations in the H2O2 levels during AR formation. Additionally, the activities of PPO and IAAO, which are involved in the metabolism of auxin, increased during the callus formation stage [54]. These enzymes participate in the oxidation and degradation of auxin, influencing its availability and activity in the tissues. This trend corresponds closely with the observed changes in IAA levels, supporting the idea that these enzymes play an essential role in regulating hormonal balance during AR development.
4.3. IAA Signaling Pathway in the Regulation of AR Formation
Plant hormones are essential regulators of AR formation, as they respond to environmental changes, modulate internal signaling networks, and influence cell fate decisions [55]. Among these hormones, auxin plays a pivotal role in AR development, with auxin-related genes providing the molecular foundation for root formation [56,57]. In this study, the auxin levels decreased during the CB0 to CB1 stage, suggesting that low concentrations of IAA may promote callus formation. However, the auxin levels steadily increased thereafter, supporting the well-established idea that the accumulation of IAA is crucial for root primordium initiation and AR development [58]. Additionally, the plant hormone signal transduction pathway analysis revealed that the IAA signaling pathway had the highest number of DEGs, highlighting the central role of auxin in the regulation of AR formation.
The classical auxin signaling pathway, known as the TIR1/AFBs-Aux/IAAs-ARFs nuclear signaling cascade, is key to auxin’s transcriptional regulation. TIR1/AFB proteins, which are auxin receptors, belong to the F-box protein family and interact with the SCF (Skp, Cullin, F-box containing complex) ubiquitin ligase complex [59,60]. Auxin response factor (ARF) proteins, essential transcription factors in the auxin signaling pathway, regulate the expression of auxin-responsive genes by binding to auxin response elements (AuxREs, typically the TGTCTC sequence) within the promoters of the target genes [61]. Aux/IAA proteins act as transcriptional repressors in the presence of low auxin concentrations, preventing the activation of ARF-mediated gene expression. These proteins function by binding to ARFs, particularly through their conserved domains, III and IV, thereby inhibiting ARF activity [62,63]. As auxin concentrations rise, auxin molecules serve as molecular “glue”, promoting the interaction between TIR1/AFB and Aux/IAA proteins. This interaction facilitates the ubiquitination and degradation of Aux/IAA proteins [64]. Once Aux/IAA repression is lifted, ARF proteins are free to bind to auxin-responsive gene promoters, activating their expression. This leads to the rapid initiation of processes such as cellular division, expansion, and other physiological activities controlled by auxin [65]. Moreover, AUX1, a key protein involved in the polar transport of auxin between plant cells, regulates the expression of the genes involved in cell expansion and cell wall loosening, thereby promoting plant growth [66]. As shown in this study, the significant upregulation of AUX1 indicates that polar auxin transport is highly active during AR formation. Additionally, the GH3 family, which encodes IAA-amido synthetases, plays a crucial role in modulating auxin levels by conjugating IAA with amino acids, thus maintaining the auxin homeostasis within cells.
In this study, genes from the GH3 and ARF families were downregulated during the rooting process, which may have contributed to an increase in the levels of free auxin within the cells, thereby promoting AR formation. In contrast, the members of the SAUR family were significantly upregulated, highlighting their distinct and active role in the AR formation process. This observation is consistent with findings in Arabidopsis thaliana, where SAUR15 promotes the formation of lateral and ARs by accelerating cell division [67,68]. Furthermore, research suggests that PagSAUR36 enhances auxin accumulation by upregulating the genes involved in auxin biosynthesis [69]. This explains the consistent expression patterns of SAUR genes and the corresponding changes in IAA levels observed in this study. Additionally, the WGCNA identified a regulatory relationship between U-box E3, AKT, and IAA content. AKT proteins mediate potassium ion absorption from the soil to the roots in Arabidopsis and other plants [70]. Studies on AR formation in apples have shown that KCl promotes root development by enhancing the expression of genes involved in auxin signaling, sugar metabolism, and the cell cycle [27]. U-box E3 is a ubiquitin ligase that likely plays a role in the degradation of Aux/IAA proteins [71]. These findings suggest that both AKT and U-box E3 contribute to AR formation by directly or indirectly regulating the auxin signaling pathway. Therefore, IAA plays a pivotal role in AR formation, particularly during the CB1–CB3 stages, where its accumulation may promote root primordium differentiation and AR formation. Polar auxin transport and SAUR may further enhance cell division and elongation, while GH3 helps maintain auxin homeostasis.
4.4. The Role of ABA in AR Formation Through Stress Response Mechanisms
ABA is known to play an inhibitory role in AR formation [72,73]. ABA exerts its regulatory function through the PYR/PYL-PP2C-SnRK2 signaling pathway. In the absence of ABA, the PP2C protein remains highly active, preventing the accumulation of phosphorylated SnRK2 kinases. However, when ABA is present, it binds to PYR/PYL receptors, leading to their interaction with PP2C. This facilitates the phosphorylation of SnRK2 kinases, which, in turn, activates the expression of downstream target genes [74,75]. In this study, ABA levels exhibited a slight and continuous increase during AR formation, which contrasts the findings from many previous studies. The expression of PYR/PYL genes was continuously upregulated during callus formation, suggesting that ABA binds to PYR/PYL receptors, thereby activating the phosphorylation of ABA-responsive element-binding factors (ABFs/AREBs) and enhancing the stress tolerance of the cuttings [76]. Key hub genes such as CYCT1-3, CYP94B3, RPLP0, TBL33, F-box5, NAC82, and ZFP may play important roles in regulating ABA levels. RPLP0 is involved in protein translation regulation [77], while CYCT1-3 encodes the cyclin proteins that are critical for cell cycle regulation and transcriptional control [78]. TBL33 is crucial for the acetylation of plant cell walls, with TBL33 mutants in Arabidopsis thaliana exhibiting impaired growth [79]. Both NAC and ZFP transcription factor families are known to be involved in plant responses to abiotic stresses such as drought and salt stress [80,81]. Additionally, the overexpression of GmNAC109 in A. thaliana has been shown to promote lateral root formation [82], suggesting that the NAC82 in this study contributed to stress tolerance and AR formation through ABA signaling. F-box proteins, such as F-box5, are also key players in regulating the IAA signaling pathway via the ubiquitin–proteasome system [83]. During AR formation, F-box5 may serve as a link between the ABA and IAA signaling pathways. Additionally, correlation analysis between the candidate genes and physiological indices revealed that Unigene10792 (AUX1) and Unigene20780 (AUX/IAA), which are involved in the auxin signaling pathway, were highly correlated with ABA content. Furthermore, CYP94B3, a member of the cytochrome P450 family, regulates jasmonic acid (JA) signaling by oxidizing the C-12 position of JA-Ile, which affects plant defense responses and other physiological processes [84]. Overall, our results suggest that ABA enhances the stress tolerance of cuttings by interacting with the PYR/PYL-PP2C signaling pathway to activate the expression of hub genes such as NAC82 and ZFP. Additionally, ABA might influence the IAA signaling pathway through the regulation of F-box.
4.5. Impact of ZR on AR Formation: From Cytokinin Homeostasis to Feedback Regulation
The effect of cytokinins, including ZR, is closely linked to the balance of IAA. When the cytokinin-to-auxin ratio is high, cytokinins primarily promote shoot formation. In contrast, when the ratio is low, cytokinin favors root formation. While high concentrations of cytokinins strongly inhibit rooting, low concentrations can enhance AR formation [85]. In this study, the ZR content initially increased, followed by a decrease, and then a subsequent rise, while the IAA/ZR ratio increased during the CB1 to CB2 period. These findings suggest that ZR may stimulate the formation of root primordia by promoting cell division. This pattern aligns with previous studies on AR formation in Paeonia species [86]. Cytokinin signaling is mediated through a complex two-component system, which involves a series of phosphotransfer processes. This system includes histidine (His) protein kinases, histidine-containing phosphotransfer proteins (Hpts), and response regulators (RRs). These RRs are categorized into A-type and B-type ARR proteins [87]. B-type ARRs function as transcription factors that regulate the expression of downstream genes, thereby initiating the cytokinin response. On the other hand, A-type ARRs provide negative feedback, suppressing the prolonged activation of the pathway. In this study, the expression of both A-ARR and B-ARR genes was significantly downregulated during the callus formation stage. This suggests that an increase in ZR content activates the negative feedback regulation of A-ARR proteins, thereby maintaining the homeostasis of the signaling intensity and promoting callus formation. Additionally, key hub genes were identified that may be involved in various aspects of cellular function. For example, RPM1 encodes a plant disease resistance protein that plays a role in pathogen recognition and immune responses [88]. MRP-L28 may be involved in the protein synthesis within the mitochondria [89], while RRP45, a component of the exosome complex, is primarily responsible for RNA degradation, processing, and quality control [90].
4.6. GA Regulation of Callus and Root Primordium Formation
GA plays a key role in both callus formation and root primordium differentiation [91]. Callus formation, which is a crucial early step in rooting, requires significant levels of GA [92]. This aligns with the observed increase in GA levels during the initial phase of cutting propagation in our study. However, during the transition from CB1 to CB2, GA concentrations gradually decreased, suggesting that low levels of GA may promote root primordium differentiation. This observation is consistent with previous studies on root formation in Zizyphus jujuba cuttings [93]. GA exerts its effects through the GID1 receptor, which interacts with DELLA proteins. This interaction triggers the degradation of DELLA proteins via the proteasome pathway, thus relieving the suppression of GA signaling and promoting various downstream effects, including cell elongation and differentiation [94,95,96]. Furthermore, GA has been shown to influence the distribution of IAA by regulating flavonol biosynthesis through the DELLA-MYB12/111 module [97]. In our study, the upregulation of both GID1 and DELLA genes throughout the rooting process suggests that GA influences auxin distribution. Specifically, it likely promotes auxin accumulation at the root tip, thereby accelerating root formation.
5. Conclusions
In this study, we conducted detailed morphological and anatomical analyses of C. bodinieri and observed that AR primarily originated from callus tissue. Significant changes in nutrient levels, endogenous hormone content, and enzyme activities were observed during the AR formation process. Notably, root primordium formation was associated with the substantial consumption of soluble proteins. The enzymes IAAO and POD likely work synergistically to regulate AR formation, while PPO and IAAO appear to negatively influence auxin accumulation during the callus formation stage. KEGG pathway analysis identified 28 key genes enriched in the plant hormone signal transduction pathway, with 17 genes specifically enriched in the IAA signaling pathway. This finding underscores the central role of IAA as the primary hormonal regulator of AR formation. Additionally, both GA and ABA influence IAA signaling through the DELLA-MYB12/111 module and the F-box5 protein, respectively. ABA, in particular, enhances stress tolerance in cuttings by activating resistance-related genes, such as NAC82. Through WGCNA, we identified 14 hub genes, including CYP94B3 and F-box5, within hormone-associated modules. These hub genes represent promising targets for future studies aimed at further unraveling the complex regulatory mechanisms underlying hormone signaling networks during AR formation. Our findings offer valuable insights into the hormonal regulation of rooting processes, which could inform the development of strategies to improve plant rooting efficiency during cutting propagation. These insights have significant applications in sustainable forest resource management and root system enhancement, both of which are crucial for optimizing plant growth and propagation strategies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f16030509/s1, Figure S1: Clonal saplings of the citral chemotype of C. bodinieri; Figure S2: Length distribution of unigenes; Figure S3: Heatmap of the correlation between candidate genes and physiological index; Figure S4: Heatmap of gene expression associated with soluble proteins, soluble sugars, and antioxidant enzymes; Table S1: Primer sequences for qRT-PCR analysis of selected genes; Table S2: Statistical summary of sequencing data volume; Table S3: Statistical summary of sequencing data volume.
Author Contributions
Conceptualization, W.Y. and Y.L.; methodology, W.Y., Y.Z. (Yueting Zhang) and C.L.; software, Y.L., Y.Z. (Yongjie Zheng) and T.Z.; data curation, W.Y., C.F. and X.L.; writing—original draft preparation, W.Y., Y.L. and X.L.; writing—review and editing, W.Y. and Y.L.; visualization, Y.L.; supervision, X.L.; project administration, T.Z. and X.L.; funding acquisition, T.Z. and X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key Research and Development Program of China (Project No. 2024YFD2200105), Youth Project of Jiangxi Academy of Forestry (Project No. 2023522705), Natural Science Foundation of Jiangxi Province (Project No. 20224BAB205030), and Doctoral project of Jiangxi Academy of Forestry (Project No. 2022522702).
Data Availability Statement
The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive (Genomics, Proteomics & Bioinformatics 2021) in National Genomics Data Center (Nucleic Acids Res 2024), China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences (CRA019609), which are publicly accessible at https://bigd.big.ac.cn/gsa/browse/CRA019609, accessed on 14 October 2024.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fu, C.; Liu, X.; Liu, Q.; Qiu, F.; Yan, J.; Zhang, Y.; Zhang, T.; Li, J. Variations in essential oils from the leaves of Cinnamomum bodinieri in China. Molecules 2023, 28, 3659. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhou, H.; Yang, L.; Jiang, L.; Chen, D.; Qiu, D.; Yang, Y. Advances in biosynthesis and pharmacological effects of Cinnamomum camphora essential oil. Forests 2022, 13, 1020. [Google Scholar] [CrossRef]
- Yang, Y.; Isman, M.B.; Tak, J.H. Insecticidal activity of 28 essential oils and a commercial product containing Cinnamomum cassia bark essential oil against Sitophilus zeamais Motschulsky. Insects 2020, 11, 474. [Google Scholar] [CrossRef] [PubMed]
- Ling, Q.; Zhang, B.; Wang, Y.; Xiao, Z.; Hou, J.; Xiao, C.; Liu, Y.; Jin, Z. Chemical composition and antioxidant activity of the essential oils of citral-rich chemotype Cinnamomum camphora and Cinnamomum bodinieri. Molecules 2022, 27, 7356. [Google Scholar] [CrossRef]
- Tate, H.T.; Page, T. Cutting propagation of santalum austrocaledonicum: The effect of genotype, cutting source, cutting size, propagation medium, IBA and irradiance. New For. 2018, 49, 551–570. [Google Scholar] [CrossRef]
- Cavalcante, U.R.; Megguer, C.A.; Vieira, J.S.; Dionísio, F.; Vilarinho, M.S. Influence of different parts of cuttings and substrates on vegetative propagation of Pereskia aculeata miller. Biosci. J. 2019, 35, 691–699. [Google Scholar] [CrossRef]
- Daskalakis, I.; Biniari, K.; Bouza, D.; Stavrakaki, M. The effect that indolebutyric acid (IBA) and position of cane segment have on the rooting of cuttings from grapevine rootstocks and from Cabernet franc (Vitis vinifera L.) under conditions of a hydroponic culture system. Sci. Hortic. 2018, 227, 79–84. [Google Scholar] [CrossRef]
- Dale, A.; Galić, D.; Willenborg, C. Repetitive vegetative propagation of first-year sea buckthorn (Hippophae rhamnoides L.) cuttings. Can. J. Plant Sci. 2018, 98, 609–615. [Google Scholar] [CrossRef]
- Cho, K.H.; Laux, V.Y.; Wallace-Springer, N.; Clark, D.G.; Folta, K.M.; Colquhoun, T.A. Effects of light quality on vegetative cutting and in vitro propagation of coleus (Plectranthus scutellarioides). HortScience 2019, 54, 926–935. [Google Scholar] [CrossRef]
- Costa, E.d.S., Jr.; Barbosa, M.S.; Silva, C.M.; Silva, R.C.; Kiill, L.H.; Beckmann-Cavalcante, M.Z. Vegetative propagation of Rhaphiodon echinus Schauer (Lamiaceae): Effects of the period of cutting in rooting, cuttings arrangement and IBA concentrations for seedlings production. Ornam. Hortic. 2018, 24, 238–247. [Google Scholar] [CrossRef]
- Gonin, M.; Bergougnoux, V.; Nguyen, T.D.; Gantet, P.; Champion, A.J.P. What makes adventitious roots? Plants 2019, 8, 240. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wang, C.; Wang, N.; Wei, L.; Li, W.; Yao, Y.; Liao, W. Roles of small-molecule compounds in plant adventitious root development. Biomolecules 2019, 9, 420. [Google Scholar] [CrossRef] [PubMed]
- Castro Rivera, J.A.; Baquero Duarte, L.E.; Narváez Cuenca, C.E. Catalasa, peroxidasa y polifenoloxidasa de pitahaya amarilla (Acanthocereus pitajaya). Rev. Colomb. Quím. 2006, 35, 91–100. [Google Scholar]
- Qian, J.; Li, Y.; Xu, H.; Wang, X.; Qin, A.; Ren, J.; Wang, L.; Yu, H. Rooting, anatomical analysis and changes of enzyme activity of softwood cuttings of Quercus variabilis at different ages. J. Zhejiang A&F Univ. 2023, 40, 107–114. (In Chinese) [Google Scholar]
- Meng, X.; Wang, Z.; He, S.; Shi, L.; Song, Y.; Lou, X.; He, D. Endogenous hormone levels and activities of IAA-modifying enzymes during adventitious rooting of tree peony cuttings and grafted scions. Horticult. Environ. Biotechnol. 2019, 60, 187–197. [Google Scholar] [CrossRef]
- Hao, J.; Meng, F.; Li, X.; Wang, C. Chitosan promotes root hair growth and endogenous IAA accumulation in root tips of vegetable soybean under NaCl stress. J. Plant Nutr. Fert. 2023, 29, 1689–1699. [Google Scholar]
- Li, F.; Sun, C.; Li, X.; Yu, X.; Luo, C.; Shen, Y.; Qu, S. The effect of graphene oxide on adventitious root formation and growth in apple. Plant Physiol. Biochem. 2018, 129, 122–129. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, W.; Chen, X.; Gao, Y.; Wu, X.; Ding, M.; Duo, L. Graphene oxide affected root growth, anatomy, and nutrient uptake in alfalfa. Ecotoxicol. Environ. Saf. 2023, 250, 114483. [Google Scholar] [CrossRef]
- Roychoudhry, S.; Kepinski, S. Auxin in root development. Cold Spring Harb. Perspect. Biol. 2022, 14, a039933. [Google Scholar] [CrossRef]
- Saini, S.; Sharma, I.; Kaur, N.; Pati, P.K. Auxin: A master regulator in plant root development. Plant Cell Rep. 2013, 32, 741–757. [Google Scholar] [CrossRef]
- Zhao, X.; Zheng, H.; Li, S.; Yang, C.; Jiang, J.; Liu, G. The rooting of poplar cuttings: A review. New For. 2014, 45, 21–34. [Google Scholar] [CrossRef]
- Li, H.; Liu, T.; Zhang, H.; Yang, Y.; Yang, S. Research progress in rooting mechanism of plant cuttings. World For. Res. 2014, 27, 23–28. [Google Scholar]
- Guan, L.; Tayengwa, R.; Cheng, Z.; Peer, W.A.; Murphy, A.S.; Zhao, M. Auxin regulates adventitious root formation in tomato cuttings. BMC Plant Biol. 2019, 19, 435. [Google Scholar] [CrossRef]
- Ugartechea-Chirino, Y.; Swarup, R.; Swarup, K.; Péret, B.; Whitworth, M.; Bennett, M.; Bougourd, S. The AUX1 LAX family of auxin influx carriers is required for the establishment of embryonic root cell organization in Arabidopsis thaliana. Ann. Bot. 2009, 105, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Li, Y.; Huang, K.; Cheng, Z. Auxin regulation and MdPIN expression during adventitious root initiation in apple cuttings. Horticult. Res. 2020, 7, 143. [Google Scholar] [CrossRef]
- Wang, Q.; An, B.; Wei, Y.; Reiter, R.J.; Shi, H.; Luo, H.; He, C. Melatonin Regulates Root Meristem by Repressing Auxin Synthesis and Polar Auxin Transport in Arabidopsis. Front. Plant Sci. 2016, 7, 1882. [Google Scholar] [CrossRef]
- Tahir, M.M.; Mao, J.; Li, S.; Li, K.; Liu, Y.; Shao, Y.; Zhang, D.; Zhang, X. Insights into factors controlling adventitious root formation in apples. Horticulturae 2022, 8, 276. [Google Scholar] [CrossRef]
- Kuroha, T.; Kato, H.; Asami, T.; Yoshida, S.; Kamada, H.; Satoh, S. A trans-zeatin riboside in root xylem sap negatively regulates adventitious root formation on cucumber hypocotyls. J. Exp. Bot. 2002, 53, 2193–2200. [Google Scholar] [CrossRef]
- Gutierrez, L.; Mongelard, G.; Floková, K.; Păcurar, D.I.; Novák, O.; Staswick, P.; Kowalczyk, M.; Păcurar, M.; Demailly, H.; Geiss, G. Auxin controls Arabidopsis adventitious root initiation by regulating jasmonic acid homeostasis. Plant Cell 2012, 24, 2515–2527. [Google Scholar] [CrossRef]
- Mauriat, M.; Petterle, A.; Bellini, C.; Moritz, T. Gibberellins inhibit adventitious rooting in hybrid aspen and Arabidopsis by affecting auxin transport. Plant J. 2014, 78, 372–384. [Google Scholar] [CrossRef]
- Niu, S.; Li, Z.; Yuan, H.; Fang, P.; Chen, X.; Li, W. Proper gibberellin localization in vascular tissue is required to regulate adventitious root development in tobacco. J. Exp. Bot. 2013, 64, 3411–3424. [Google Scholar] [CrossRef] [PubMed]
- Teale, W.D.; Pasternak, T.; Dal Bosco, C.; Dovzhenko, A.; Kratzat, K.; Bildl, W.; Schwörer, M.; Falk, T.; Ruperti, B.; V Schaefer, J. Flavonol-mediated stabilization of PIN efflux complexes regulates polar auxin transport. EMBO J. 2021, 40, e104416. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Li, Y.; Zhang, M.; Jin, F.; Li, J. A Novel Arabidopsis microRNA promotes IAA biosynthesis via the Indole-3-acetaldoxime Pathway by suppressing SUPERROOT1. Plant Cell Physiol. 2015, 56, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Rigal, A.; Yordanov, Y.S.; Perrone, I.; Karlberg, A.; Tisserant, E.; Bellini, C.; Busov, V.B.; Martin, F.; Kohler, A.; Bhalerao, R.; et al. The AINTEGUMENTA LIKE1 Homeotic Transcription Factor PtAIL1 Controls the Formation of Adventitious Root Primordia in Poplar. Plant Physiol. 2012, 160, 1996–2006. [Google Scholar] [CrossRef]
- Li, M.; Chen, R.; Jiang, Q.; Sun, X.; Zhang, H.; Hu, Z. GmNAC06, a NAC domain transcription factor enhances salt stress tolerance in soybean. Plant Mol. Biol. 2021, 105, 333–345. [Google Scholar] [CrossRef]
- Iwase, A.; Ohme-Takagi, M.; Sugimoto, K. WIND1:A key molecular switch for plant cell dediffrentiation. Plant Signal. Behav. 2011, 6, 1943–1945. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.; Chai, M.; Ren, M.; Yuan, J.; Yang, W.; Dong, Y.; Liu, B.; Jian, Q.; Wang, S.; et al. Gibberellins modulate local auxin biosynthesis and polar auxin transport by negatively affecting flavonoid biosynthesis in the root tips of rice. Plant Sci. 2020, 298, 110545. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Q.; Liu, J.; Dou, F.; Wang, H.; Song, Y.; Ren, Y.; He, J.; Wang, L.; Zhang, C.; et al. Identification of grape miRNA revealed Vvi-miR164b involved in auxin induced root development. Sci. Hortic. 2022, 295, 110804. [Google Scholar] [CrossRef]
- Lowe, R.; Shirley, N.; Bleackley, M.; Dolan, S.; Shafee, T. Transcriptomics technologies. PLoS Comput. Biol. 2017, 13, e1005457. [Google Scholar] [CrossRef]
- Yin, L.; Cai, Z.; Zhu, B.; Xu, C. Identification of key pathways and genes in the dynamic progression of HCC based on WGCNA. Genes 2018, 9, 92. [Google Scholar] [CrossRef]
- Grandy, A.S.; Erich, M.S.; Porter, G.A. Suitability of the anthrone–sulfuric acid reagent for determining water soluble carbohydrates in soil water extracts. Soil. Biol. Biochem. 2000, 32, 725–727. [Google Scholar] [CrossRef]
- Noble, J.E.; Bailey, M.J. Quantitation of protein. Methods Enzymol. 2009, 463, 73–95. [Google Scholar] [PubMed]
- Thongsook, T.; Barrett, D.M. Purification and partial characterization of broccoli (Brassica oleracea Var. Italica) peroxidases. J. Agric. Food Chem. 2005, 53, 3206–3214. [Google Scholar] [CrossRef] [PubMed]
- Aquino-Bolaños, E.N.; Mercado-Silva, E. Effects of polyphenol oxidase and peroxidase activity, phenolics and lignin content on the browning of cut jicama. Postharvest Biol. Technol. 2004, 33, 275–283. [Google Scholar] [CrossRef]
- Yu, J.; Meng, Q.; Liu, W.; Lu, Y.; Ren, X. Analysis of acidic endogenous phytohormones in grapes by using online solid-phase extraction coupled with LC–MS/MS. Chromatogr. Sci. 2014, 52, 1145–1149. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Dai, F.; Zhao, X.; Tang, C.; Wang, Z.; Kuang, Z.; Li, Z.; Huang, J.; Luo, G. Identification and validation of reference genes for qRT-PCR analysis in mulberry (Morus alba L.). PLoS ONE 2018, 13, e0194129. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Díaz-Sala, C. A perspective on adventitious root formation in tree species. Plants 2020, 9, 1789. [Google Scholar] [CrossRef]
- Sheng, L.; Chen, Y.; Wang, N.; Xu, C.; Cao, F. Histological observation of somatic embryogenesis and adventitious buds induction from Ginkgo biloba L. different expalnts in vitro culture. J. Cent. South. Univ. For. Technol. 2012, 32, 153–158. (In Chinese) [Google Scholar]
- Takahashi, F.; Sato-Nara, K.; Kobayashi, K.; Suzuki, M.; Suzuki, H. Sugar-induced adventitious roots in Arabidopsis seedlings. J. Plant Res. 2003, 116, 83–91. [Google Scholar] [CrossRef]
- Li, S.; Xue, L.; Xu, S.; Feng, H.; An, L. Hydrogen peroxide involvement in formation and development of adventitious roots in cucumber. Plant Growth Regul. 2007, 52, 173–180. [Google Scholar] [CrossRef]
- Li, S.; Xue, L.; Xu, S.; Feng, H.; An, L. IBA-induced changes in antioxidant enzymes during adventitious rooting in mung bean seedlings: The role of H2O2. Environ. Exp. Bot. 2009, 66, 442–450. [Google Scholar] [CrossRef]
- Zhu, Y.; Liao, W.; Wang, M.; Niu, L.; Xu, Q.; Jin, X. Nitric oxide is required for hydrogen gas-induced adventitious root formation in cucumber. J. Plant Physiol. 2016, 195, 50–58. [Google Scholar] [CrossRef]
- Druege, U.; Franken, P.; Hajirezaei, M.R. Plant hormone homeostasis, signaling, and function during adventitious root formation in cuttings. Front. Plant Sci. 2016, 7, 381. [Google Scholar] [CrossRef]
- Li, S. Molecular bases for the regulation of adventitious root generation in plants. Front. Plant Sci. 2021, 12, 614072. [Google Scholar] [CrossRef]
- Vidoz, M.L.; Loreti, E.; Mensuali, A.; Alpi, A.; Perata, P. Hormonal interplay during adventitious root formation in flooded tomato plants. Plant J. 2010, 63, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Saha, K.; Choudhuri, M.A. Role of auxin and polyamines in adventitious root formation in relation to changes in compounds involved in rooting. J. Plant Growth Regul. 2001, 20, 182–194. [Google Scholar] [CrossRef]
- Dubey, S.M.; Han, S.; Stutzman, N.; Prigge, M.J.; Medvecká, E.; Platre, M.P.; Busch, W.; Fendrych, M.; Estelle, M. The AFB1 auxin receptor controls the cytoplasmic auxin response pathway in Arabidopsis thaliana. Mol. Plant 2023, 16, 1120–1130. [Google Scholar] [CrossRef]
- Li, S.; Xue, L.; Xu, S.; Feng, H.; An, L. Mediators, Genes and Signaling in Adventitious Rooting. Bot. Rev. 2009, 75, 230–247. [Google Scholar] [CrossRef]
- Gutierrez, L.; Bussell, J.D.; Păcurar, D.I.; Schwambach, J.L.; Păcurar, M.; Bellini, C. Phenotypic plasticity of adventitious rooting in Arabidopsis is controlled by complex regulation of AUXIN RESPONSE FACTOR transcripts and microRNA abundance. Plant Cell 2009, 21, 3119–3132. [Google Scholar] [CrossRef] [PubMed]
- Dharmasiri, N.; Dharmasiri, S.; Estelle, M. The F-box protein TIR1 is an auxin receptor. Nature 2005, 435, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Yang, H.; Li, C.; Zheng, M.; Song, H.; Zou, X.; Chen, X.; Zhang, J. Genome-Wide identification and expression analysis of the Aux/IAA Gene family of the drumstick tree (Moringa oleifera Lam.) reveals regulatory effects on shoot regeneration. Int. J. Mol. Sci. 2022, 23, 15729. [Google Scholar] [CrossRef]
- Dos Santos Maraschin, F.; Memelink, J.; Offringa, R. Auxin-induced, SCFTIR1-mediated poly-ubiquitination marks AUX/IAA proteins for degradation. Plant J. 2009, 59, 100–109. [Google Scholar] [CrossRef]
- Da Costa, C.T.; Gaeta, M.L.; de Araujo Mariath, J.E.; Offringa, R.; Fett-Neto, A.G. Comparative adventitious root development in pre-etiolated and flooded Arabidopsis hypocotyls exposed to different auxins. Plant Physiol. Biochem. 2018, 127, 161–168. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Loosening of plant cell walls by expansins. Nature 2000, 407, 321–326. [Google Scholar] [CrossRef]
- Spartz, A.K.; Lee, S.H.; Wenger, J.P.; Gonzalez, N.; Itoh, H.; Inze, D.; Peer, W.A.; Murphy, A.S.; Overvoorde, P.J.; Gray, W.M. The SAUR19 subfamily of SMALL AUXIN UP RNA genes promote cell expansion. Plant J. 2012, 70, 978–990. [Google Scholar] [CrossRef]
- Yin, H.; Li, M.; Lv, M.; Hepworth, S.R.; Li, D.; Ma, C.; Li, J.; Wang, S.-M. SAUR15 promotes lateral and adventitious root development via activating H+-ATPases and auxin biosynthesis. Plant Physiol. 2020, 184, 837–851. [Google Scholar] [CrossRef]
- Liu, R.; Wen, S.; Sun, T.; Wang, R.; Zuo, W.; Yang, T.; Wang, C.; Hu, J.; Lu, M.; Wang, L. PagWOX11/12a positively regulates the PagSAUR36 gene that enhances adventitious root development in poplar. J. Exp. Bot. 2022, 73, 7298–7311. [Google Scholar] [CrossRef]
- Ren, X.; Qi, G.; Feng, Q.; Zhao, S.; Zhao, S.; Wang, Y.; Wu, W. Calcineurin B-like protein CBL10 directly interacts with AKT1 and modulates K+ homeostasis in Arabidopsis. Plant J. 2013, 74, 258–266. [Google Scholar] [CrossRef]
- Zeng, L.; Park, C.; Venu, R.; Gough, J.; Wang, G. Classification, expression pattern, and E3 Ligase activity assay of rice U-Box-containing proteins. Mol. Plant 2008, 1, 800–815. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Murphy, A.S.; Peer, W.A.; Gan, L.; Li, Y.; Cheng, Z. Physiological and molecular regulation of adventitious root formation. Crit. Rev. Plant Sci. 2015, 34, 506–521. [Google Scholar] [CrossRef]
- Zeng, Y.; Verstraeten, I.; Trinh, H.K.; Heugebaert, T.; Stevens, C.V.; Garcia-Maquilon, I.; Rodriguez, P.L.; Vanneste, S.; Geelen, D. Arabidopsis hypocotyl adventitious root formation is suppressed by ABA signaling. Genes 2021, 12, 1141. [Google Scholar] [CrossRef]
- Weiner, J.J.; Peterson, F.C.; Volkman, B.F.; Cutler, S.R. Structural and functional insights into core ABA signaling. Curr. Opin. Plant Biol. 2010, 13, 495–502. [Google Scholar] [CrossRef]
- Raghavendra, A.S.; Gonugunta, V.K.; Christmann, A.; Grill, E. ABA perception and signalling. Trends Plant Sci. 2010, 15, 395–401. [Google Scholar] [CrossRef]
- Fidler, J.; Graska, J.; Gietler, M.; Nykiel, M.; Prabucka, B.; Rybarczyk-Płońska, A.; Muszyńska, E.; Morkunas, I.; Labudda, M. PYR/PYL/RCAR receptors play a vital role in the abscisic-acid-dependent responses of plants to external or internal stimuli. Cells 2022, 11, 1352. [Google Scholar] [CrossRef]
- Liu, C.; Lu, R.; Guo, G.; He, T.; Li, Y.; Xu, H.; Gao, R.; Chen, Z.; Huang, J. Transcriptome analysis reveals translational regulation in barley microspore-derived embryogenic callus under salt stress. Plant Cell Rep. 2016, 35, 1719–1728. [Google Scholar] [CrossRef]
- Liu, H.; Rice, A.P. Isolation and characterization of the human cyclin T1 promoter. Gene 2000, 252, 39–49. [Google Scholar] [CrossRef]
- Yuan, Y.; Teng, Q.; Zhong, R.; Haghighat, M.; Richardson, E.A.; Ye, Z. Mutations of arabidopsis TBL32 and TBL33 affect xylan acetylation and secondary wall deposition. PLoS ONE 2016, 11, e0146460. [Google Scholar] [CrossRef]
- Li, W.; He, M.; Wang, J.; Wang, Y. Zinc finger protein (ZFP) in plants-a review. Plant Omics 2013, 6, 474–480. [Google Scholar]
- Hu, Y.; Lacroix, B.; Citovsky, V. Modulation of plant DNA damage response gene expression during Agrobacterium infection. Biochem. Biophys. Res. Commun. 2021, 554, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Kim, M.Y.; Ha, J.; Lee, S.-H. Overexpression of the soybean NAC gene GmNAC109 increases lateral root formation and abiotic stress tolerance in transgenic Arabidopsis plants. Front. Plant Sci. 2019, 10, 1036. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gonzalez-Carranza, Z.H.; Zhang, S.; Miao, Y.; Liu, C.; Roberts, J.A. F-Box proteins in plants. Annu. Plant Rev. 2019, 2, 307–328. [Google Scholar]
- Kitaoka, N.; Kawaide, H.; Amano, N.; Matsubara, T.; Nabeta, K.; Takahashi, K.; Matsuura, H. CYP94B3 activity against jasmonic acid amino acid conjugates and the elucidation of 12-O-β-glucopyranosyl-jasmonoyl-l-isoleucine as an additional metabolite. Phytochemistry 2014, 99, 6–13. [Google Scholar] [CrossRef]
- Arya, A.; Sharma, V.; Tyagi, P.K.; Gola, D.; Husen, A. Role of cytokinins in adventitious root formation. In Environmental, Physiological and Chemical Controls of Adventitious Rooting in Cuttings; Academic Press: Cambridge, MA, USA, 2022; pp. 239–249. [Google Scholar]
- Wen, S.; Miao, D.; Cui, H.; Li, S.; Gu, Y.; Jia, R.; Leng, Y. Physiology and transcriptomic analysis of endogenous hormones regulating in vitro adventitious root formation in tree peony. Sci. Hortic. 2023, 318, 112122. [Google Scholar] [CrossRef]
- Hwang, I.; Chen, H.; Sheen, J. Two-component signal transduction pathways in Arabidopsis. Plant Physiol. 2002, 129, 500–515. [Google Scholar] [CrossRef]
- Boyes, D.C.; Nam, J.; Dangl, J.L. The Arabidopsis thaliana RPM1 disease resistance gene product is a peripheral plasma membrane protein that is degraded coincident with the hypersensitive response. Proc. Natl. Acad. Sci. USA 1998, 95, 15849–15854. [Google Scholar] [CrossRef]
- Robles, P.; Quesada, V. Emerging roles of mitochondrial ribosomal proteins in plant development. Int. J. Mol. Sci. 2017, 18, 2595. [Google Scholar] [CrossRef]
- Makino, D.L.; Baumgärtner, M.; Conti, E. Crystal structure of an RNA-bound 11-subunit eukaryotic exosome complex. Nature 2013, 495, 70–75. [Google Scholar] [CrossRef]
- Liu, G.; Yang, C.; Qu, G.; You, X. Dynamic changes of four endogenous hormones in the larch hybrid during cuttings rooting. J. Northeast For. Univ. 2001, 29, 1–3. (In Chinese) [Google Scholar]
- Haddon, L.; Northcote, D.H. The influence of gibberellic acid and abscisic acid on cell and tissue differentiation of bean callus. J. Cell Sci. 1976, 20, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Shao, F.; Wang, S.; Huang, W.; Liu, Z. Effects of IBA on the rooting of branch cuttings of chinese jujube (Zizyphus jujuba Mill.) and changes to nutrients and endogenous hormones. J. For. Res. 2018, 29, 1557–1567. [Google Scholar] [CrossRef]
- Shimada, A.; Ueguchi-Tanaka, M.; Nakatsu, T.; Nakajima, M.; Naoe, Y.; Ohmiya, H.; Kato, H.; Matsuoka, M. Structural basis for gibberellin recognition by its receptor GID1. Nature 2008, 456, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Ueguchi-Tanaka, M.; Ashikari, M.; Nakajima, M.; Itoh, H.; Katoh, E.; Kobayashi, M.; Chow, T.; Hsing, Y.C.; Kitano, H.; Yamaguchi, I.; et al. GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature 2005, 437, 693–698. [Google Scholar] [CrossRef]
- Ubeda-Tomas, S.; Federici, F.; Casimiro, I.; Beemster, G.T.; Bhalerao, R.; Swarup, R.; Doerner, P.; Haseloff, J.; Bennett, M.J. Gibberellin signaling in the endodermis controls Arabidopsis root meristem size. Curr. Biol. 2009, 19, 1194–1199. [Google Scholar] [CrossRef]
- Tan, H.; Man, C.; Xie, Y.; Yan, J.; Chu, J.; Huang, J. A crucial role of GA-regulated flavonol biosynthesis in root growth of Arabidopsis. Mol. Plant 2019, 12, 521–537. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).