Abstract
The interaction between trees’ water needs during drought and the signals that appear in their canopies is not fully understood. The first visually detectable signs, which we describe as early warning signals in tree canopies, are often not noticeable at first glance. When these signs become widely apparent, tree decline is already underway. In this study, we focus on identifying early visible signs of drought stress in the tree crowns, such as very small leaves, premature needle/leaf discolouration and abscission, and defoliation. We provide guidance on recognising initial signs, offer specific examples, and comprehensively analyse each signal. Our focus is on signs in the tree crowns that appear during intense and prolonged droughts, which we confirmed by calculating the Standardised Precipitation Evapotranspiration Index (SPEI). Our findings are based on 20 years (2004–2024) of continuous fieldwork and data collection from permanent sample plots in Serbia, which was conducted as part of the International Co-operative Programme on Assessment and Monitoring of Air Pollution Effects on Forests (ICP Forests). We also conducted a comprehensive review of the literature and key findings related to the early signs we address. This research was further motivated by the signs observed in the tree crowns during the summer of 2024 due to extreme climatic events, which classify this year as one of the hottest recorded in Serbia. However, we still cannot conclusively determine which specific trees will die back based solely on these early warning signals, as some trees manage to withstand severe drought conditions. Nonetheless, the widespread appearance of these indicators is a clear warning of significant ecosystem instability, potentially leading to the decline of individual trees or larger groups.
1. Introduction
One of the fundamental characteristics of plants is their elevated sensitivity to environmental changes compared to animal species, which can avoid or escape suboptimal conditions, for example, through migration [1]. Therefore, trees should be recognised as susceptible organisms due to their inability to evade unfavourable environmental conditions or their limited capacity for movement, which is too slow to keep pace with the rapidly increasing global temperatures accompanied by drought [2]. However, changes in climatic conditions should ideally prompt trees to acclimate [3]. When exposed to drought, trees are forced to adapt to the new conditions, sometimes leading to increased drought resistance. In contrast, if the drought is too severe and prolonged, it can result in permanent damage or even death [4]. Some signs that manifest in trees due to drought-induced stress are often initially undetected, but as they become more intense, they are impossible to disregard. This usually indicates that metabolic changes have occurred in the plants even before visible symptoms appear [5]. Trees respond to drought accompanied by high temperatures in various ways. Some of the earliest signs observed in canopies include premature leaf or needle discolouration [6], premature leaf abscission [7], leaves significantly smaller than usual [8], and defoliation, which is widely recognised as an indicator of a tree’s physiological condition [9]. Also, some studies have documented several previously mentioned signs in tree crowns during droughts [10,11,12,13]. As key crown components, leaves are the primary organs for transpiration and photosynthesis. Under drought conditions, leaf turgor pressure and the rate of photosynthesis decrease, leading to a reduction in leaf area; thus, this change will directly impact the plant itself [14]. Under these conditions of critical transpiration, trees must maintain a balance to survive.
Some studies suggest that drought may have a lesser impact on smaller trees located beneath the canopies of larger ones [15,16], as well as due to specific characteristics of the species and its water requirements [17]. They also note that competition and species diversity do not always influence a particular species’ resistance to drought [18,19], meaning that mixed-species composition within the stand will not always reduce desiccation in drought-intolerant species [20]. However, regardless of whether a tree grows within a community, such as a forest, or as an individual in an open area, it encounters a range of favourable and adverse conditions for its growth and development throughout its life. Each tree individually competes for space and the prevailing conditions in its environment. When these conditions significantly deviate from the norm of its habitat, the tree struggles for survival and sends out early warning signals. These conditions are most often related to climatic factors, such as high temperatures accompanied by prolonged periods of precipitation deficit, i.e., drought. Additionally, seasonally variable plant physiology determines whether the resulting soil moisture deficit is physiologically detrimental [21]. Furthermore, living conditions may be altered by biotic factors, such as pest outbreaks (e.g., defoliators and bark beetles), which can affect large forest areas but are most often associated with a specific tree species, i.e., the host plant. Consequently, they can cause even greater damage in pure stands. Abiotic factors, including sudden weather events, can cause ice, snow and windbreaks, further creating favourable conditions for the pest and fungi population outbreak. Anthropogenic impacts, like mechanical damage from tree cutting or soil contamination from hazardous spills, can also contribute. However, the abovementioned impacts are typically localised and may occur over smaller or moderately large areas. Conversely, drought can affect and often covers extensive areas (with various tree species) that may extend beyond the borders of an area, country, or region. Indeed, some signals are visually indistinguishable, but these are not the focus of this study. We concentrate exclusively on visible signs in tree canopies observed in the field, which, when detected, can be further confirmed using advanced technologies such as remote sensing over broader areas [22].
Drought can also have varying negative impacts depending on the tree species. Norway spruce (Picea abies (L) Karst.) is identified as the most drought-intolerant conifer species, in contrast to the highly tolerant Scots pine (Pinus sylvestris L.), while deciduous species such as beech (Fagus sylvatica L.) and oaks (Quercus petraea (Matt.) Liebl. and Quercus robur L.) have demonstrated greater tolerance [23]. However, during intense and prolonged droughts, which have occurred several times in recent decades, it has been established that almost all studied species exhibited negative symptoms due to drought [23,24,25]. The studied species represent some of Europe’s most widespread tree species [26], which is also the case in our observation area [27]. In contrast, drought can have even stronger effects if a particular species is at the edge of its range or distribution limits. In this context, its stability and vulnerability in such locations can be assessed [28].
In addition to the lack of precipitation and high temperatures, which are the primary indicators of drought, soil moisture availability plays a significant role in vegetation’s response to drought-induced stress. Although vegetation adaptation is often considered linked to atmospheric conditions, its ultimate fate in terms of desiccation will also depend on soil properties [29]. Drought can potentially degrade soil properties, particularly its physical characteristics in the surface layer [30], so root depth may also be one of the key factors influencing tree tolerance to drought [31].
Drought is not necessarily confined to summer, when air temperatures are higher, and precipitation is usually lower. It can also occur during periods when more significant amounts of precipitation are naturally expected, such as spring, autumn, or winter. However, the visible changes or signals discussed in this paper are only observable during the vegetation period when the assimilative organs are fully developed. Our focus is, therefore, exclusively on these signals. We are further motivated by the fact that 2024 will be one of the hottest years ever recorded (the warmest winter, spring, and the hottest and dry summer) in Serbia since meteorological measurements began [32]. The signs discussed in this study emerged as early as mid-summer 2024. This trend of record-high temperatures, accompanied by strong heat stress and drought, is also confirmed by data at the European level [33].
Our research had some limitations, primarily the short seasonal period of data collection (July to early September) and the limited number of locations and observed trees (ICP Forests methodology) [34]. However, on the other hand, the value of this study lies in the long-term data collection period (20 years) and the fact that each data point can be attributed to a specific tree, allowing for deeper analysis. Crucially, this study highlights the importance of continuous monitoring as a vital tool for identifying the early warning signs and triggers of tree decline.
In our literature review, we found numerous studies addressing early warning signs of tree desiccation caused by drought [35,36,37]. However, we did not find a source that consolidates all early warning signs detectable solely through visual inspection of tree canopies. Therefore, in this review, we analyse the currently available literature and the acquired knowledge and personal observations gathered over 20 years of field research on early signs of tree desiccation that can be observed visually, providing answers on how trees respond under intense and prolonged drought conditions.
2. Thematic Framework
First, we had to distinguish between periodic and continuous (constantly present) stress factors and between low-intensity and high-intensity stress events. While low-intensity stress events may lead to adaptation and acclimatisation, severe or chronic stress, such as drought that causes significant damage, can result in plant mortality [38]. Some authors also emphasise the importance of distinguishing whether stress develops gradually, increasing its impact over time, or occurs as a sudden event [39]. Therefore, it is crucial to differentiate between gradual stress impacts and tolerance thresholds (e.g., increasing drought and temperatures that exceed a plant’s tolerance threshold). It has long been noted that stress can also be viewed from two perspectives—it is most often destructive, but under certain circumstances, it can be constructive, serving as a driving force for the adaptation and evolution of plants [40,41]. Drought develops slowly and its main characteristics such as start, duration, and intensity are not easily and quickly visible [42]. Only after its manifestation and consequences are visible to the naked eye can we speak about its influence. Forest ecosystems can manage single-year droughts, but repeated exposure to stress through multi-year droughts can have a significant negative impact on their functioning and manifestation of consequences [43]. It is difficult to determine how severe and prolonged a drought must be for a tree to die, as some trees manage to withstand even very severe, intense, and prolonged droughts [44]. However, the severity of the drought will undoubtedly influence the physiological response of trees [45]. Additionally, comparing tree mortality rates following extreme droughts across different locations worldwide can be highly problematic, primarily due to significant differences in climatic conditions, as well as tree species composition, soil characteristics, and the history and intensity of the droughts themselves [46].
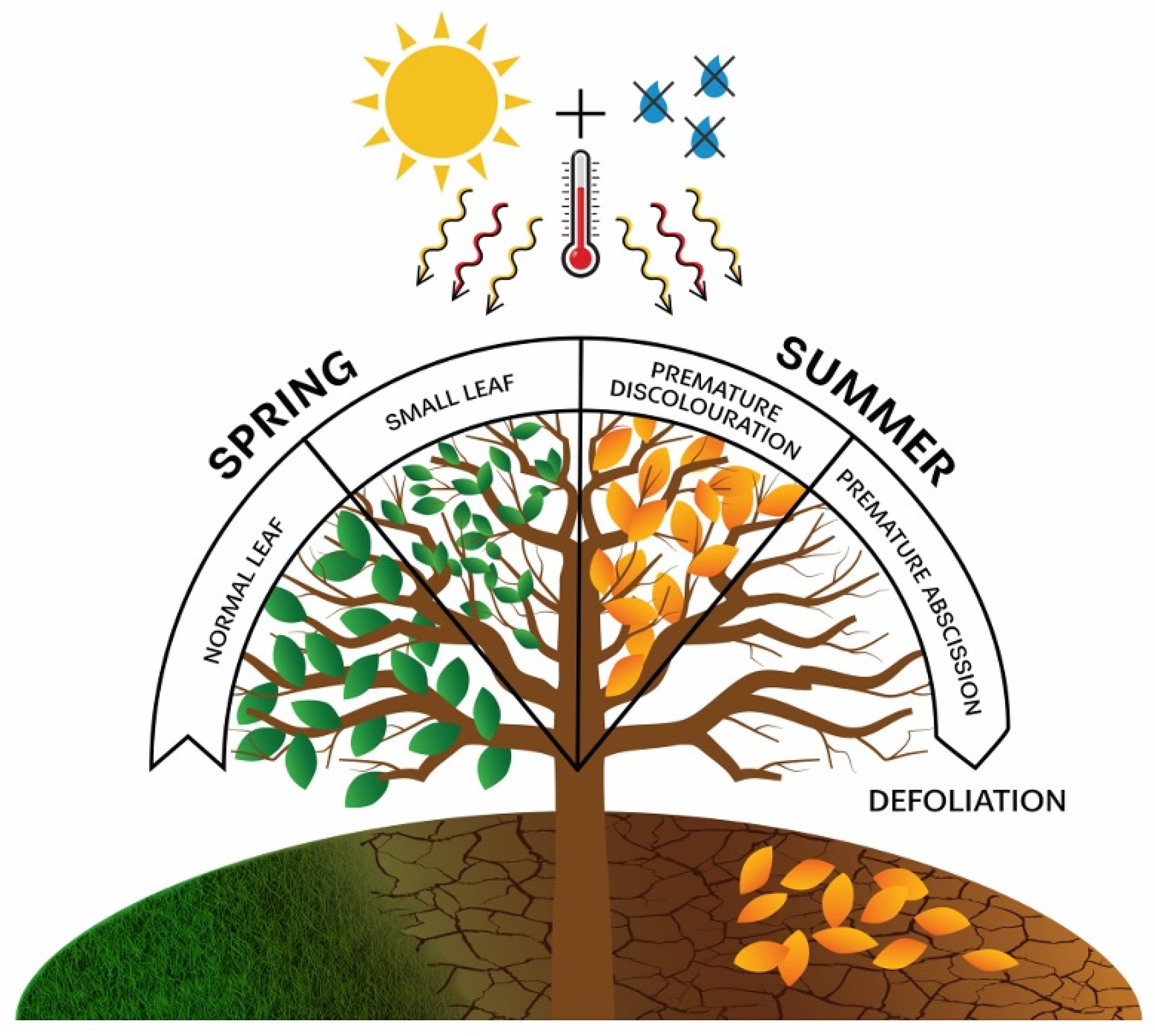
Based on the abovementioned considerations, our primary thematic focus was on prolonged, multi-year droughts of high intensity that gradually increased in severity. We included in the early warning signs all those that appear on tree canopies during the vegetation period when drought is present, noting that some signs could only be diagnosed after the drought had ended. Monitoring defoliation proved to be a valuable indicator, as it can gradually intensify over the years of severe drought and eventually lead to the death of a specific tree, or it can occur as a sudden event with a significant loss of foliage, also resulting in mortality [47]. Additionally, all visible changes in the canopy that are unusual for the time frame in which they are observed, such as premature leaf discolouration (premature ageing) and early leaf abscission or shedding well before the autumn, were considered. Finally, during severe droughts that begin as early as spring, some trees may develop a significantly smaller leaf area than usual, a phenomenon known as “small leaves” [48]. All the early warning signs mentioned above were highly dependent on the intensity of drought and commonly appeared during prolonged, multi-year droughts (Figure 1).
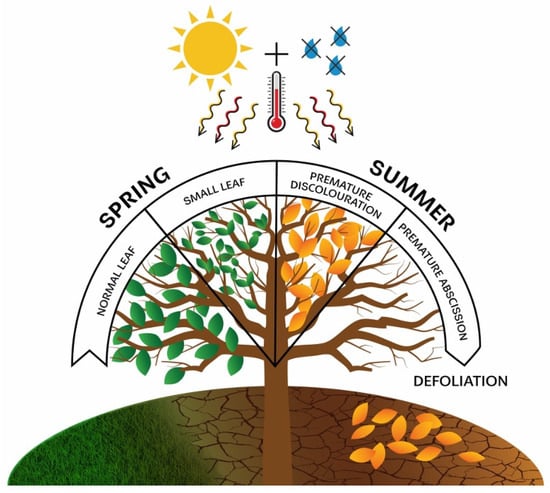
Figure 1.
Early warning signs in tree crowns as a response to the impact of drought.
3. Materials and Methods
3.1. Observation Area
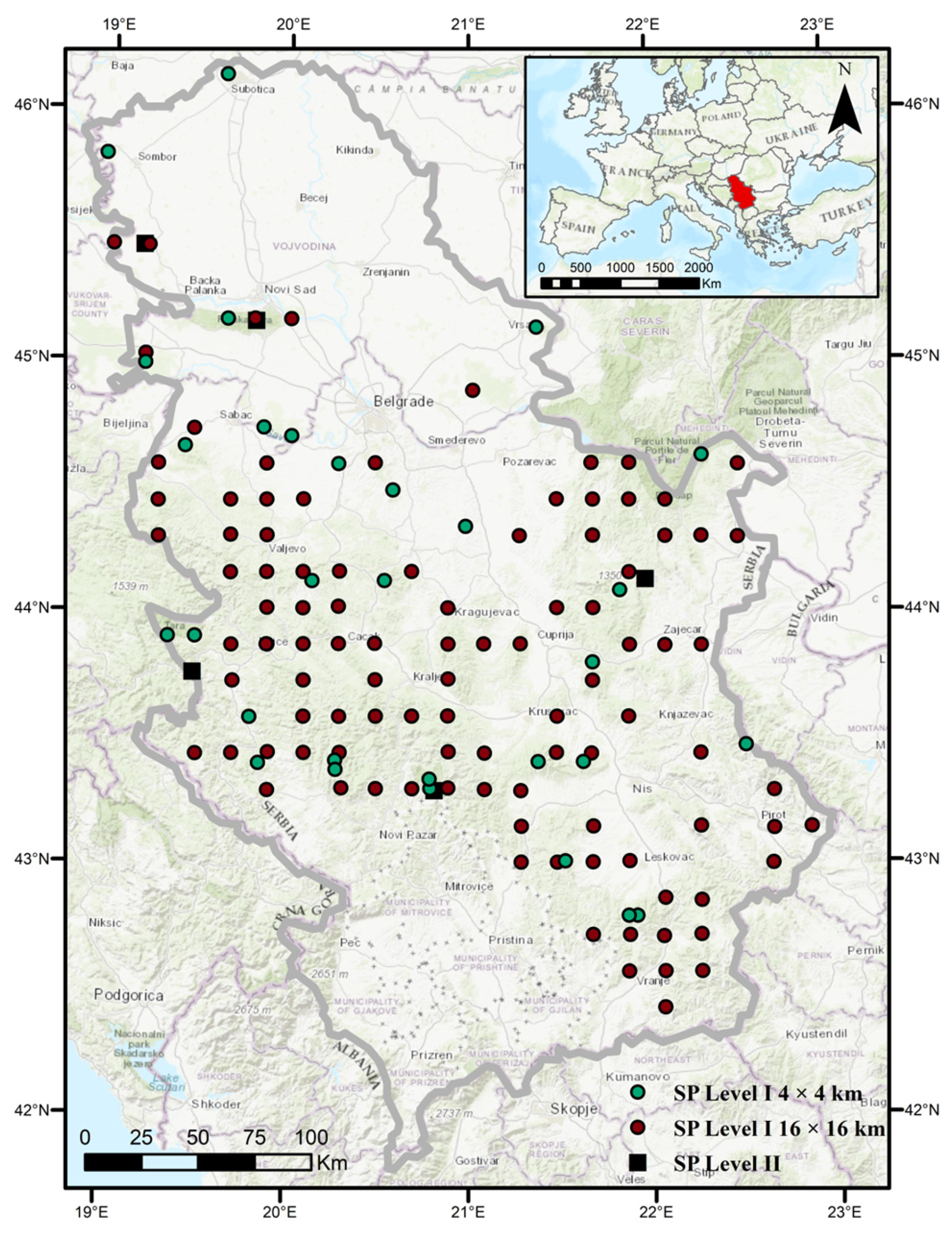
This study was conducted in Serbia (41°53′–46°11′ N, 18°49′–23°00′ E) on 130 permanent sample plots (Level I) arranged in a systematic transnational grid and 5 sample plots with intensive monitoring (Level II) following the ICP Forests Programme methodology [34] (Figure 2). In addition to the permanent sample plots, our observations were also based on forest areas in the immediate and broader vicinity of these plots. The most widespread deciduous tree species in Serbia is beech (Fagus sylvatica L.), followed by Turkey oak (Quercus cerris L.), sessile oak (Quercus petraea (Matt.) Liebl.), Hungarian oak (Quercus frainetto Ten.), hornbeam (Carpinus betulus L.), and others. Among conifers, Norway spruce (Picea abies (L) Karst.) is the most common species, followed by black pine (Pinus nigra Arn.), fir (Abies alba Mill.), and Scots pine (Pinus sylvestris L.) [27]. Most of Serbia belongs to a temperate climate zone, while the southwestern part lies at the border of the Mediterranean subtropical and continental climates [49]. According to the Köppen and Köppen–Geiger climate classification [50], two climate types are distinguished in Serbia: warm temperate and cold temperate [51]. The warm temperate climate dominates over 90% of the territory, except in areas at higher altitudes, where the cold temperate climate is present [51].
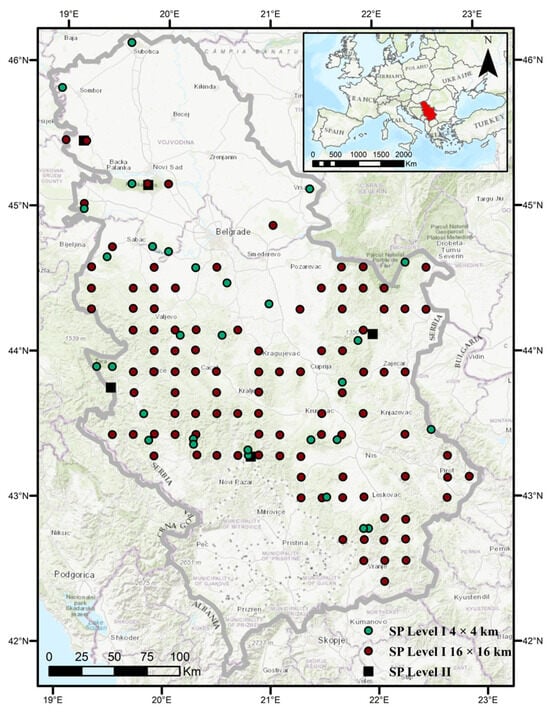
Figure 2.
Observation area with permanent sample plots Level I and Level II.
3.2. Primary Data Source
Our observations and data were collected from 130 permanent sample plots (Level I) in Serbia, where approximately 2900 trees were observed annually on average from 2004 to 2024. Additionally, data were collected from 5 sample plots with intensive monitoring (Level II), where 889 trees were selected and monitored. The monitoring period for these plots was slightly shorter due to their later establishment (2009–2024). The sampled population included 33 tree species, of which 29 were deciduous and 4 were coniferous. The most represented or dominant tree species accounted for 84% of the entire sample, reflecting the distribution of the main species in the observation area.
It is essential to describe the basic methodology and data collection process based on the International Co-operative Programme on Assessment and Monitoring of Air Pollution Effects on Forests [34] to clarify the method used to identify specific early warning signs. Each year, research is conducted on permanent sample plots (Level I) during the full vegetation period (July–September) when the assimilative organs are fully developed. The timing for visiting a particular sample plot is approximately the same date each year as in previous years. Expert teams responsible for data collection visit the same sample plots annually to gain a comprehensive understanding of the sample plot’s immediate and broader surroundings, aiming to draw more accurate conclusions regarding any observed changes in and around the locality. The primary rule of this methodology is to observe and record every noticeable change in the entire tree and identify the cause of the damage. For most species, the optimal time for crown analysis is from the full development of leaves or needles (early summer) until late summer or the onset of autumn senescence. During crown assessment, specific considerations were made in cases where the crown was influenced by competition (e.g., shading). In such instances, only the portion of the crown unaffected by shading was evaluated. Additionally, the assessment of the tree’s current condition included recently desiccated branches but excluded those that had been dead for many years and no longer influenced the tree’s vitality. Thus, only active processes in the crown were considered in the evaluation. Furthermore, a tree was classified as desiccated if it lacked assimilation organs, meaning it exhibited 100% defoliation for two consecutive years. In such cases, the previous year was recorded as the year of desiccation. Defoliation was assessed by comparing the observed tree with a reference tree in the closest vicinity, which represented typical crown morphology and stand age within the plot. The defoliation assessment followed a scale ranging from 5% to 100% [52]. As key evidence to support the evaluation of early warning signs in tree crowns, photographic documentation of trees from the monitored sample plots was used as well from broader area.
The signals highlighted in this review pertain exclusively to the condition of the tree canopy, which is the most commonly used indicator for assessing the health and vitality of forests [52]. As an additional data source, insights gained from monitoring forest conditions on permanent sample plots (Level II) within the ICP Forests Programme, where continuous monitoring is conducted throughout the year, were also utilised. Research on these plots aims to explain cause-and-effect relationships in forest ecosystems further [52].
3.3. Literature Search Strategies and Selection Criteria
We conducted a systematic literature review using the Scopus, Web of Science, and Google Scholar databases to supplement and confirm our observations of early warning signs in tree crowns caused by drought and drought periods. We applied four levels of keyword searches based on the topic of this study as follows:
The first search focused on studies addressing the impact of drought on leaf size. It included the following keywords: “small leaf” OR “very small leaf” OR “leaf traits” OR “drought effects on leaf” OR “leaf morphological traits” OR “variation in leaf size” OR “effects of drought on leaf”.
The second search focused on studies addressing the impact of drought and drought periods on premature discolouration of leaves and needles. It included the following keywords: “premature discolouration” OR “discolouration” OR “leaf reaction to drought” OR “leaf phenology”.
The third search focused on studies addressing the impact of drought and drought periods on premature abscission of leaves and needles. It included the following keywords: “premature abscission of leaves” OR “effects of drought on leaf” OR “leaf senescence” OR “early abscission of leaves”.
The fourth search focused on studies emphasising the impact of drought and drought periods on defoliation. It included the following keywords: “tree defoliation” OR “ICP Forests” OR “tree canopy dieback” OR “Level I” OR “Level II” OR “forest monitoring” OR “tree response to drought”.
The search was conducted based on the keywords mentioned above and their combinations. However, the authors also applied the snowball method to expand the range of relevant literature sources [53]. This method allowed us to use the references of already identified sources to locate additional relevant studies until sufficient information was obtained. The search was limited to papers published in English, except for cases where the results directly addressed our research topic and were identified using the snowball method. We also focused on the most recent studies on this topic. While there were no strict geographical limitations, we prioritised studies related to the European region due to its specific climatic conditions and the comparability of the dominant tree species inhabiting it. Thus, a systematic approach to the literature review was applied, ensuring transparency and reproducibility.
3.4. Long-Term Drought Analysis
When transpiration reaches a critical point due to drought, trees may begin to experience stress, which can affect their metabolism and overall health. To illustrate the impact of drought on forest ecosystems in the observation area and the signs we diagnosed, which appeared extensively in tree crowns, we conducted an analysis. We used the Standardised Precipitation Evapotranspiration Index (SPEI) to present the drought period, which incorporates both precipitation and temperature data [54]. This index allows for a detailed assessment of water stress conditions that can affect vegetation on both global and regional scales. It also allows for the comparison of drought severity over time and space across a wide range of climatic conditions and can measure drought intensity and duration. For this analysis, we used data from the global SPEI database [55], calculating time series for the study region based on coordinates (upper left: 42.25, 23.25; lower right: 46.25, 18.75). We focused on the hottest and driest years within the study period (2004–2024). SPEI calculation is based on the Thornthwaite equation for estimating potential evapotranspiration (PET) [56] and moisture conditions were assessed according to the SPEI categorisation [54] in which drought begins when values are equal to or below −1.0 and ends when these values become positive. We opted to calculate SPEI on an annual scale (12 months) as it provides a good representation of drought periods and drought characteristics (frequency, duration, severity, and intensity). However, since this study examines the impact of drought on the appearance of early signs in tree crowns that are visible exclusively during the growing season, we also calculated SPEI for six months, corresponding to the vegetation season.
4. Results and Discussion
4.1. Droughts Analysis
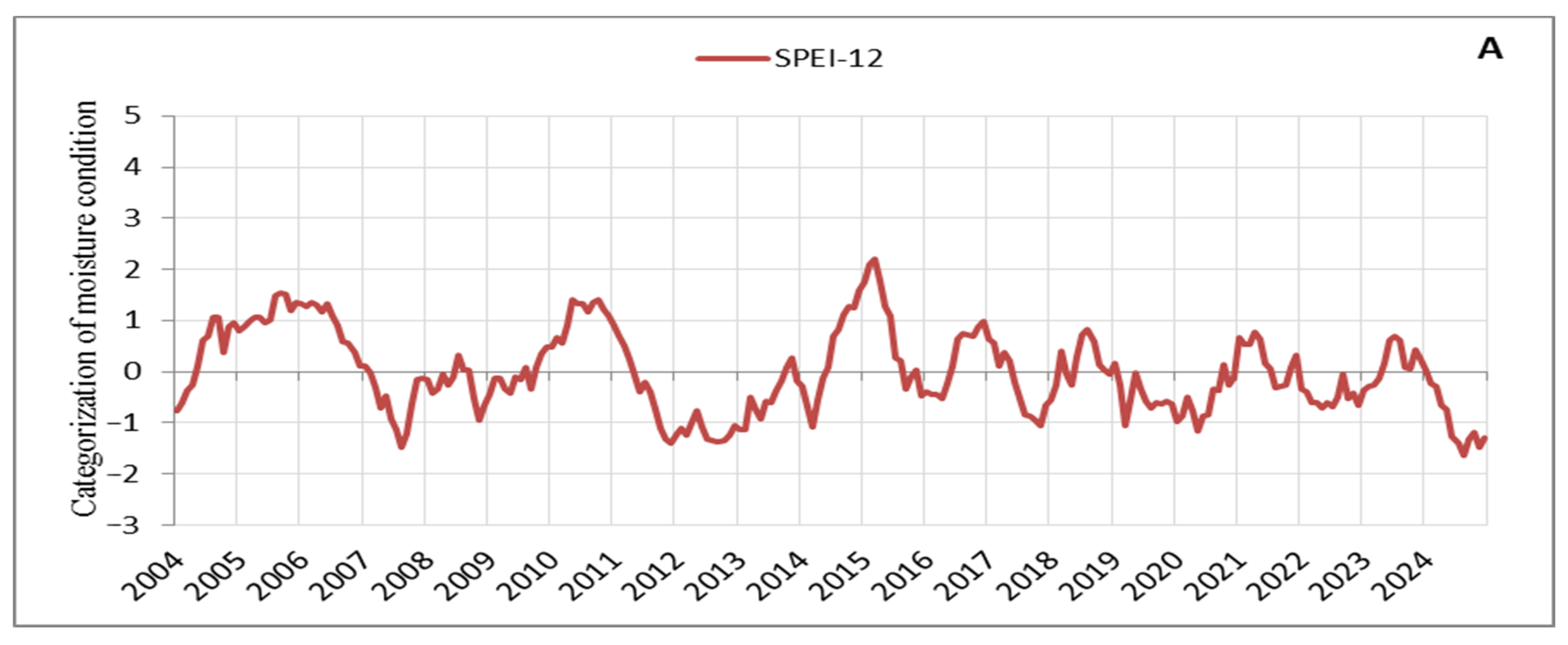
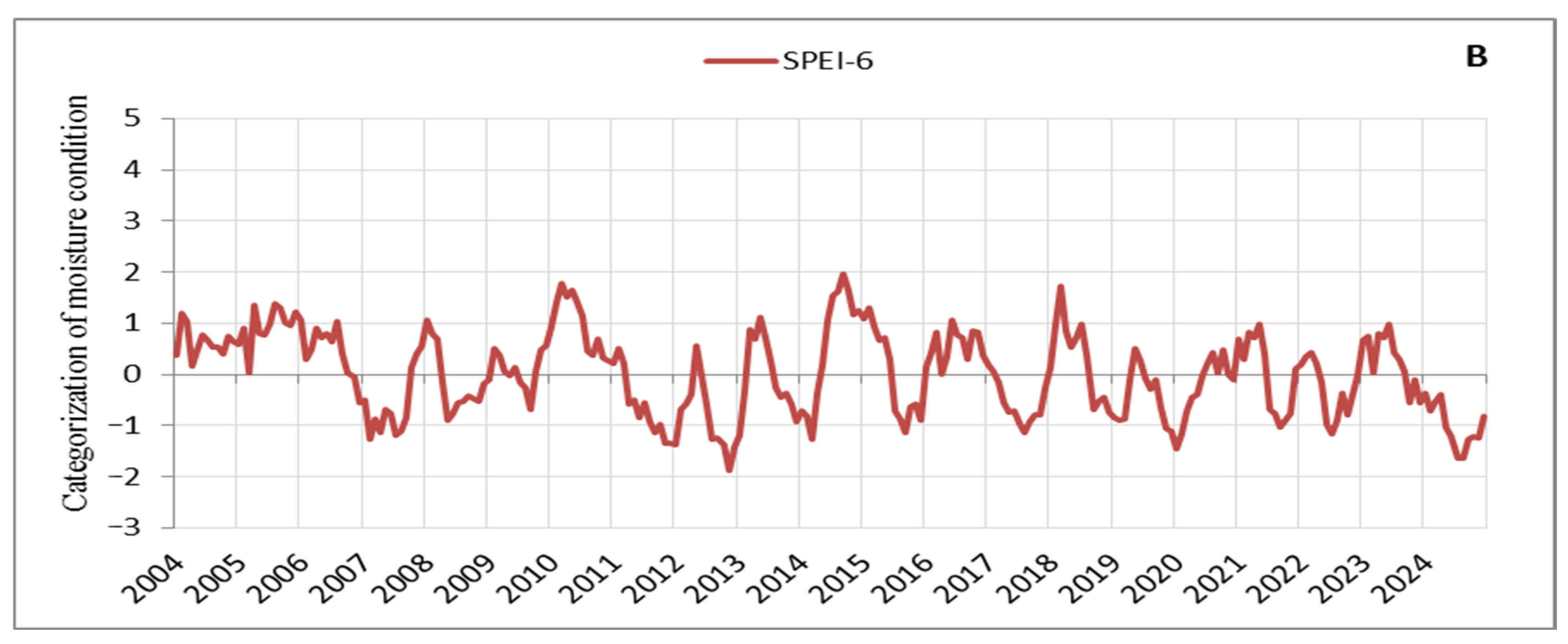
Based on the processed SPEI values on an annual scale, droughts with varying periods and characteristics can be identified in the observation area (Figure 3). Since we focus on the signs that manifested in tree crowns during intense and prolonged droughts, we carefully tracked its progression and distinguished between periodic and continuous drought periods, as well as between low- and high-intensity stress events. It can be observed that several drought periods occurred, during which we also identified and recorded early warning signs in tree crowns, which are discussed here. These drought periods impacted vegetation and aligned with droughts in other parts of Europe, with their effects being particularly intense in recent years [57]. The drought from 2011 to 2013 was the most severe in terms of duration and intensity in our observation area, with 29 consecutive months of negative SPEI values (Figure 3A). It was followed by the droughts of 2019–2020 with 20 consecutive months of negative SPEI values and 2022–2023 with 16 months (Figure 3A). However, while the drought events in recent years (2019–2020, 2022–2023, and 2024) were less intense compared to the 2011–2013 drought, their consistency and duration are noticeable (Figure 3). Additionally, the periods reflecting moisture are significantly shorter and of very low intensity, further emphasising the drought events in recent years (Figure 3A,B). Thus, of the droughts considered here, the 2011–2013 drought placed significant stress on a large number of trees, many of which died in 2014 (Table S1). A notable characteristic of the 2011–2013 drought was the extremely low temperatures during the winter of 2012 (the third coldest winter in the last 20 years) [30], followed by a continued drought period in 2013. In contrast, in recent years (2019–2024), there have been consistently high temperatures with record-breaking heat observed year after year, adding a unique specificity to this period (Figure 3A,B). The tendency is for tree responses to drought to intensify with the severity and duration of the drought [58]. The impact of the multi-year drought from 2011 to 2013, combined with the nearly constant record-breaking high temperatures in recent years (2019–2024) and the particularly intense drought in 2024, has resulted in similar stress responses in the form of signs observed in tree crowns, such as small leaves, premature discolouration of leaves, premature abscission of leaves, and defoliation. Following the 2011–2013 drought, tree mortality increased [47], as evidenced by the rise in the number of dead trees after the drought of 2022 (Table S1). Indications suggest that this trend will continue beyond 2024, a year characterised by the severity and intensity of droughts far exceeding all previously observed years (Figure 3A,B).
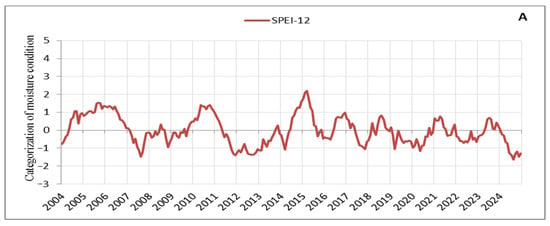
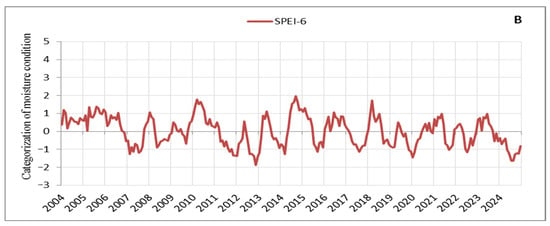
Figure 3.
Moisture conditions in the observation area: (A) SPEI-12, (B) SPEI-6.
4.2. Stress Response Under Extreme Events
4.2.1. Small Leaf
The studies listed in Table 1 have investigated the impact of drought on leaf size. However, there is still an insufficient number of studies addressing the effects of drought on leaf morphology [59]. The morphological characteristics of leaves are often considered indicators of plant functioning, as leaves play a crucial role in the exchange of water and air with the environment [60]. As such, they enable the plant to adapt and acclimate to environmental conditions, such as high temperatures and water availability [61]. Taking beech (Fagus sylvatica L.) as an example, one of the most important and widespread deciduous tree species in Europe [62], as well as being at the core of its natural habitat within our observation area, we can observe that the morphological characteristics of its leaves exhibit high variability within the population [63], and consequently, a high potential for acclimatisation [64]. It has been proven that climatic conditions, specifically early summer droughts, reduce the number of buds, while late summer droughts affect leaf size in the following year [65]. Moreover, severe multi-year droughts result in smaller leaves [8] and significantly reduce the canopy’s overall leaf mass [66]. Thus, as a response to drought, trees reduce the size of their leaves during growth to decrease the surface area available for photosynthesis and transpiration, thereby minimising water loss during drought [67]. This phenomenon can also be an indicator of already initiated and irreversible processes of gradual tree desiccation. However, as previously noted, it is important to consider the variability of leaves, including variability based on their location within the canopy (shade vs. sun leaves), as leaf size can vary significantly [68,69]. Additionally, apart from leaf size, their age will also influence drought resistance, as younger leaves often have a lower water retention capacity and may be more susceptible to damage during drought periods [70]. It is also important to emphasise that leaf size should not be compared between the same species on different habitat types, as elevation can influence their variability [69], while drought may have varying effects depending on the species’ geographic location [71]. Thus, leaf characteristics are essential for ecosystem functioning, and another good indicator of a plant’s functioning is leaf mass (leaf thickness). It has been proven that species with greater leaf mass exhibit higher drought resistance [72]. However, visually observing that the leaves on an entire tree are significantly smaller than the nearest trees of the same species nearby is a clear sign of some disturbance (Figure 4). If these signs persist in subsequent years and are observed in a more significant number of trees within the stand, irreversible desiccation processes will likely occur. Visual observation of these changes is most evident in forests dominated by a single tree species, making comparisons and conclusions about possible causes more manageable and straightforward. Specifically, for beech, smaller leaf size can be used as an early indication of severe drought stress effects, as this phenomenon can be observed on various geological substrates and soil types inhabited by this species [73].

Table 1.
Studies addressing the impact of drought on leaf size.

Figure 4.
Individual Beech tree (Fagus sylvatica L.): (A) with small leaves (May 2024), (B) and with the final outcome of desiccation during the drought period of the same year (July 2024), (C) the observed tree on the left with small leaves compared to the tree on the right with normal leaf size (May 2024).
4.2.2. Premature Discolouration (Ageing) of Needles and Leaves
Several studies presented in Table 2 have reported premature discolouration of leaves and needles due to drought. Premature discolouration of leaves is defined as “any deviation from the usual colour of the living foliage for the assessed tree species” [50]. Prolonged droughts during the growing season induce tree stress, which may accelerate the premature discolouration (ageing) of leaves/needles [71,74,75], followed by premature abscission compared to the typical autumn timing [10,12]. In dry years, leaves tend to discolour and abscise faster compared to years with higher precipitation levels [76]. Trees respond in this way to enter the dormancy phase sooner and reduce the process of photosynthesis, which is essential for growth, functioning, and the overall condition of the plant [77]. Additionally, some studies have shown that global temperature changes have also contributed to earlier leaf development [78]. These events, such as leaf discolouration and leaf abscission in autumn, blooming, and leaf-out in spring, are part of phenology. This term encompasses all biological activities of trees and the climate, including the dormancy period and the growing season. Discolouration or ageing of leaves/needles is a phenomenon experienced by all trees. The primary driver of discolouration is the preparation of trees for the dormancy period influenced by shorter day lengths, the angle of sunlight, and the slower production of chlorophyll, which eventually ceases entirely [79]. Undoubtedly, the local climate (microclimate) can also impact the progression of leaf discolouration, and variations can increase with altitude (higher altitude means lower air temperatures). However, in years of intense drought and with rising temperatures and dry summers, premature discolouration of leaves/needles was noted (Figure 3). During intense and prolonged droughts, these signals have also been observed across Europe [11,13,80]. Depending on the prevalence of tree species, the premature discolouration or premature ageing of leaves is most easily observed in the most prevalent deciduous species such as beech (Figure 5A), while in coniferous species, this change has been most noticeable in Fir and Spruce (Figure 5B,C). The reason for referencing these species is to facilitate comparison of the same tree species across multiple localities and correlate them with climatic conditions. This term refers exclusively to the significant discolouration of leaves/needles before the usual autumn period. It is triggered by a dominant stress factor, such as high temperatures, followed by a period without precipitation (drought). If these signs are observed over a wider area (Figure 6), the impact of drought cannot be ruled out, and the likely desiccation of individual trees may occur in the coming period.

Table 2.
Studies addressing the impact of drought on the premature discolouration of leaves and needles.

Figure 5.
Examples of premature discolouration (ageing) of leaves and needles induced by drought during the growing season. (A) Fagus sylvatica L. (August 2024), (B) Abies alba Mill. (August 2013), (C) Picea abies (L.) H. Karst. (July 2023).

Figure 6.
The impact of drought on leaf discolouration at a broader area level (August 2024).
4.2.3. Premature Abscission of Needles and Leaves
The premature abscission or shedding of needles and leaves will also occur after their premature discolouration, as described in the studies listed in Table 3. Deciduous trees may shed their leaves during a drought to reduce water loss through transpiration, thereby increasing their chances of surviving the drought period [80]. This process is less noticeable in coniferous species and requires much more attention than in deciduous species. It is known that some conifers retain needles of various ages for several years. Additionally, a specific trait of conifers is that they can retain dried needles on branches after they have desiccated, thus hindering the timely identification of this process [47]. However, this premature needle abscission in conifers can be a good indicator of drought impact, as the defoliation process in conifers is often recorded with a one-year delay [81]. Although conifers inhabit areas where both drought and low temperatures may occur during the year, the impact of drought is significantly stronger on them than the impact of freezing [82,83]. Therefore, the difference in the effects of these two phenomena can be confirmed through continuous monitoring of defoliation and its correlation with climatic events (Table S1). In the climate of Serbia, located in the Northern Hemisphere, the typical phenological phases for leaf abscission are from October to December [84]. However, moving from the north to the south of Europe, the typical time for leaf abscission in Serbia is November, i.e., leaves usually begin to abscise at the end of October and finish by the end of November [85]. This missing leaf mass on a tree is considered the term defoliation. However, premature leaf/needle abscission cannot be exclusively seen as defoliation because it refers to the moment when trees start shedding leaves significantly before the usual autumn. A drought can trigger this premature leaf abscission during the vegetation period (summer) as the trees attempt to defend themselves by reducing the process of photosynthesis, i.e., reducing water loss [12,13,86]. Additionally, the response of premature abscission of leaves during years of intense drought will largely depend on the soil water deficit [10,87], as well as the soil type itself [88]. However, some studies suggest that leaf abscission during extreme droughts must also be considered a symptom of tree vulnerability, not just protection against excessive water loss [89,90]. This process also differs from the general concept of defoliation because it is accompanied by leaf discolouration, making the trees appear as if late autumn has arrived or, for example, an outbreak of defoliators (Figure 7).

Table 3.
Studies addressing the impact of drought on premature abscission of leaves and needles.

Figure 7.
Examples of premature leaf abscission/shedding caused by drought during the vegetative period. Fagus sylvatica L.: (A) (August 2013), (B) (beginning of September 2024), (C) (beginning of September 2024).
As we have already noted, we must be aware of the phenological phases of trees to better understand the concept of premature abscission of leaves. Knowing the usual time for leaf abscission, and if it begins to be noticed during the vegetation period, it is a clear sign of environmental disturbance. The signal that indicates this fact is the dried leaves that can be observed on the branches, especially for species this is not typical, such as beech (Figure 5), unlike some oaks, which can retain their dried leaves on their branches long after their desiccation. Additionally, as we have already mentioned, premature leaf abscission must be considered beyond the concept of defoliation since it intensely manifests due to the impact of some stressful event and at an unusual time for this occurrence. Experts dealing with this topic note that one year of early leaf abscission due to drought will have minimal impact on most tree species, but if there are several consecutive years, it can be very stressful [91]. As with leaf discolouration, if signs of premature leaf abscission occur over a wider area, it will be a clear indicator of drought impact (Figure 8).

Figure 8.
The impact of drought on premature leaf abscission at a broader area level (August 2024).
4.2.4. Defoliation
Several studies presented in Table 4 have reported defoliation as a consequence of drought. Defoliation was recognised as a primary indicator of the health status of individual trees and forests as early as the 1980s [52], and many researchers have used this metric in their studies [92,93,94]. Defoliation, a term referring to the missing leaf mass, aims to signal changes in tree metabolism [95,96], which can over time serve as an excellent health record of a tree [47]. We can detect early signs of tree desiccation by continuously monitoring defoliation [52] in individual trees and associating them with drought periods [97,98]. Through a detailed chronology, we can precisely identify the “trigger”, i.e., the onset of increasing defoliation over the years of observation (Table S1). To make this scenario more straightforward to understand, we provide a graphical representation in Table S2. Therefore, as an initial early sign of desiccation in individual trees or larger forest areas, an increased occurrence of defoliation can be registered.
Because of the effects of drought, symptoms of defoliation in trees can be quite variable and manifest differently depending on factors such as the specific type of tree (deciduous or coniferous) [81], as well as the intensity of the water deficit [99]. However, symptoms of defoliation in trees due to drought are often not immediately noticeable at the onset of the drought. Still, they can be diagnosed sometime after the event, even up to one or two years later, which can complicate the accurate diagnosis [47,48]. This delay in symptom visibility is also supported by other results from long-term monitoring of canopy conditions [81,100].

Table 4.
Studies highlighting the impact of drought on defoliation.
Table 4.
Studies highlighting the impact of drought on defoliation.
| References | Country/ Region | Species | Main Findings/Period |
|---|---|---|---|
| [11] | Italy (Tuscany) | Many species | Drought affected premature discolouration of leaves, premature abscission of leaves, and defoliation (2017–2018). |
| [20] | Serbia (Tara NP) | Several species | During drought periods, tree species diversity will not significantly reduce defoliation and mortality, especially for species considered drought-intolerant (2004–2021). |
| [47] | Serbia (country) | Many species | Drought increases defoliation and the progression of mortality, which may continue even after the drought ends (2004–2018). |
| [81] | Germany (country) | Several species | Significant correlations were found between defoliation and deviations from long-term average temperatures in all examined tree species (1990–2004). |
| [92] | Europe | Several species | Severe climate change and drought are the main drivers of increased defoliation in certain species (2001, 2006, and 2011). |
| [93] | Europe | Several species | The spatial distribution of defoliation trends shows a clear pattern of significant deterioration due to meteorological differences (1986–1995). |
| [94] | Italy (country) | Several species | The recurrence of extreme heatwaves and droughts can increase forest vulnerability, with increased tree mortality expected in the future (1997–2020). |
| [95] | Europe | Many species | The complex events of hot summers and drought years have caused tree mortality across Europe, with a continuous increase detected in southern and eastern Europe (1993–2013). |
| [96] | Europe | Many species | Mortality patterns in European forests show a concerning upward trend that could be further accelerated by droughts (1995–2020). |
| [97] | Slovakia (country) | Many species | Drought significantly impacted defoliation in the studied year (2022). |
| [100] | Croatia (country) | Fagus sylvatica | Drought from the previous year affects beech defoliation in the following year (1996–2017). |
Through continuous monitoring of selected trees over the last 20 years, we have identified three distinct groups of defoliation, all categorised under the final outcome of desiccation due to the following drought effects [47]:
- Defoliation that gradually increased during drought periods, followed by tree desiccation several years later (Table 5 and Table S1—Group I);
 Table 5. Selected example of monitoring defoliation in individual trees (Fagus sylvatica L.) over several years, with the final outcome of desiccation during a multi-year intense drought.
Table 5. Selected example of monitoring defoliation in individual trees (Fagus sylvatica L.) over several years, with the final outcome of desiccation during a multi-year intense drought.
These three distinct groups of defoliation aim to demonstrate the varied effects of drought periods on trees. Each tree is an individual entity and reacts based on its current condition, which often relates to its ability to absorb moisture from the soil during drought periods. If the final outcome of desiccation occurs simultaneously across the groups, we can conclude that the drought initiated and concluded this phenomenon (Table 1 and Table S1). This indicates that dieback can occur in all three identified groups of trees due to drought, even in Group II (Table 1 and Table S1), which did not exhibit any prior signs of defoliation.
Symptoms of drought stress may be somewhat visible during the drought, but due to the delay in manifestation and detection, they often continue to develop in subsequent seasons. Accordingly, special attention should be given to coniferous species when assessing defoliation, as it may be delayed [81]. Conifers tend to retain their needles on branches much longer after desiccating, so this process can be masked by the later needle drop, thereby delaying detection. It is important to note that defoliation represents an active process in the crown and is assessed based solely on the current state, without accounting for, for example, old dry branches that reflect the historical mortality of crown parts. A potential issue in assessing defoliation for certain trees can be poor visibility of their crowns. Such trees are not excluded from the sample; instead, information about the visibility of individual tree crowns is used to aid in interpreting data from those trees. Specifically, the use of an objective defoliation assessment means that excluding such trees could lead to bias in the results [52].
5. Conclusions
Early warning signs can serve as valuable indicators of the current condition of individual trees and entire forest ecosystems during droughts. It is essential to evaluate all early signs in the context of their immediate surroundings, considering whether they are also observed on other trees concerning the constantly monitored trees and how frequently they occur. During prolonged drought periods, the previously described early signs can appear simultaneously, overlap, and vary in visibility. However, the more extensively these signs are observed, the more confident we can be that tree desiccation will occur. If trees show a progression of defoliation, or sudden and complete defoliation, leaf/needle discolouration and drying out start to happen, and leaf/needle abscission appears before the usual autumn period, these are strong indicators of potential problems. Noticing multiple dry branches within a single canopy may suggest the tree is stressed. Significant deviations in leaf size within the canopy can also indicate underlying issues. The extent of the observed early warning signs can help predict the scope of future tree desiccation. Our review of the visually observable early warning signs in tree canopies offers a general analysis. It should serve as an important resource for future research on the impact of drought on trees. Continuous monitoring of phenological phases in relation to air temperature and precipitation in the spring and autumn months can further enhance our understanding of early warning signs in trees affected by drought, mainly as they manifest in tree canopies. Additionally, for future research, it will be important to examine how the climatic events of 2024 and the early signs described in this paper and observed in the tree crowns will impact forests in the coming years.
In conclusion, we suggest that the existing methodology should be adapted or improved to enable the continued monitoring of widespread early warning signs in tree crowns in the future, with the aim of understanding the ultimate consequences of their occurrence as a result of intense and prolonged droughts.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f16030405/s1, Table S1: trends in defoliation on individual trees during the years of research with the final outcome of dying; Table S2: graphical representation of defoliation monitoring on the same tree over several years, with the final outcome of desiccation due to drought effects.
Author Contributions
Conceptualisation, G.Č.; Data Curation, G.Č. and I.Đ.; Formal Analysis, G.Č. and S.E.; Investigation, G.Č., M.M. and R.G.S.; Methodology, G.Č.; Project Administration, G.Č.; Resources, G.Č., M.M. and R.G.S.; Validation, G.Č., I.Đ., S.E., M.M., R.G.S., A.L. and N.Č.; Visualisation, G.Č., I.Đ., S.E., M.M., R.G.S., A.L. and N.Č.; Writing—Original Draft, G.Č.; Writing—Review and Editing, G.Č. and N.Č. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ministry of Science, Technological Development, and Innovation (Contract No. 451-03-136/2025-03/200027), and the Ministry of Agriculture, Forestry, and Water Management of the Republic of Serbia’s Forest Directorate within the project “Monitoring and Assessment of Air Pollution Impacts and its Effects on Forest Ecosystems in Republic of Serbia—Forest Condition Monitoring”.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Valcu, C.M. Proteome Changes Following Biotic and Abiotic Stress in Forest Trees. Ph.D. Disertation, Technischen Universität München, Fachgebiet Forstgenetik, München, Germany, 2007. [Google Scholar]
- Feeley, K.J.; Bernal-Escobar, M.; Fortier, R.; Kullberg, A.T. Tropical Trees Will Need to Acclimate to Rising Temperatures—But Can They? Plants 2023, 12, 3142. [Google Scholar] [CrossRef] [PubMed]
- Nicotra, A.B.; Atkin, O.K.; Bonser, S.P.; Davidson, A.M.; Finnegan, E.J.; Mathesius, U.; Poot, P.; Purugganen, M.D.; Richards, C.L.; Valladares, F.; et al. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought. New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Jardine, K.J.; Dewhirst, R.A.; Som, S.; Lei, J.; Tucker, E.; Young, R.P.; Portillo-Estrada, M.; Gao, Y.; Su, L.; Fares, S. Cell wall ester modifications and volatile emission signatures of plant response to abiotic stress. Plant Cell Environ. 2022, 45, 3429–3444. [Google Scholar] [CrossRef] [PubMed]
- Bigler, C.; Vitasse, Y. Premature leaf discoloration of European deciduous trees is caused by drought and heat in late spring and cold spells in early fall. Agric. For. Meteorol. 2021, 307, 108492. [Google Scholar] [CrossRef]
- Mediavilla, S.; Martínez-Ortega, M.; Andrés, S.; Bobo, J.; Escudero, A. Premature losses of leaf area in response to drought and insect herbivory through a leaf lifespan gradient. J. For. Res. 2021, 33, 39–50. [Google Scholar] [CrossRef]
- Weithmann, G.; Schuldt, B.; Link, R.M.; Heil, D.; Hoeber, S.; John, H.; Müller-Haubold, H.; Schüller, L.-M.; Schumann, K.; Leuschner, C. Leaf trait modification in European beech trees in response to climatic and edaphic drought. Plant Biol. 2021, 24, 1272–1286. [Google Scholar] [CrossRef]
- Eichhorn, J.; Roskams, P. Chapter 8—Assessment of Tree Condition. Dev. Environ. Sci. 2013, 12, 139–167. [Google Scholar] [CrossRef]
- Bréda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef]
- Pollastrini, M.; Puletti, N.; Selvi, F.; Iacopetti, G.; Bussotti, F. Widespread Crown Defoliation After a Drought and Heat Wave in the Forests of Tuscany (Central Italy) and Their Recovery—A Case Study from Summer 2017. Front. For. Glob. Change 2019, 2, 74. [Google Scholar] [CrossRef]
- Rigling, A.; Etzold, S.; Bebi, P.; Brang, P.; Ferretti, M.; Forrester, D.; Gärtner, H.; Gessler, A.; Ginzler, C.; Moser, B.; et al. Wie viel Trockenheit ertragen unsere Wälder? Lehren aus extremen Trockenjahren. Forum Wissen 2019, 78, 39–51. [Google Scholar] [CrossRef]
- Schuldt, B.; Buras, A.; Arend, M.; Vitasse, Y.; Beierkuhnlein, C.; Damm, A.; Gharun, M.; Grams, T.E.E.; Hauck, M.; Hajek, P.; et al. A first assessment of the impact of the extreme 2018 summer drought on Central European forests. Basic Appl. Ecol. 2020, 45, 86–103. [Google Scholar] [CrossRef]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response Mechanism of Plants to Drought Stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Bennett, A.; McDowell, N.; Allen, C.; Anderson-Teixeira, K.J. Larger trees suffer most during drought in forests worldwide. Nat. Plants 2015, 1, 15139. [Google Scholar] [CrossRef] [PubMed]
- Mathes, T.; Seidel, D.; Klemmt, H.J.; Thom, D.; Annighöfer, P. The effect of forest structure on drought stress in beech forests (Fagus sylvatica L.). For. Ecol. Manag. 2024, 554, 121667. [Google Scholar] [CrossRef]
- Pretzsch, H.; Schütze, G.; Biber, P. Drought can favour the growth of small in relation to tall trees in mature stands of Norway spruce and European beech. For. Ecosyst. 2018, 5, 20. [Google Scholar] [CrossRef]
- Gillerot, L.; Forrester, D.I.; Bottero, A.; Rigling, A.; Lévesque, M. Tree Neighbourhood Diversity Has Negligible Effects on Drought Resilience of European Beech, Silver Fir and Norway Spruce. Ecosystems 2021, 24, 20–36. [Google Scholar] [CrossRef]
- de Sauvage, J.C.; Bugmann, H.; Bigler, C.; Lévesque, M. Species diversity and competition have minor effects on the growth response of silver fir, European larch and Douglas fir to drought. Agr. For. Meteorol. 2023, 341, 109664. [Google Scholar] [CrossRef]
- Češljar, G.; Čule, N.; Đorđević, I.; Eremija, S.; Momirović, N.; Tomić, M.; Jovanović, F. Can the desiccation of forests in Tara National Park (Serbia) be attributed to the effects of a drought period? J. For. Res. 2024, 35, 96. [Google Scholar] [CrossRef]
- Anderegg, L.D.L.; Anderegg, W.R.L.; Berry, J.A. Not all droughts are created equal: Translating meteorological drought into woody plant mortality. Tree Physiol. 2023, 33, 672–683. [Google Scholar] [CrossRef]
- Buras, A.; Rammig, A.; Zang, C.S. The European Forest Condition Monitor: Using Remotely Sensed Forest Greenness to Identify Hot Spots of Forest Decline. Front. Plant Sci. 2021, 12, 689220. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Marchand, W.; Rydval, M.; Matula, R.; Janda, P.; Begović, K.; Thom, D.; Fruleux, A.; Buechling, A.; Pavlin, J.; et al. Drought resistance of major tree species in the Czech Republic. Agric. For. Meteorol. 2024, 348, 109933. [Google Scholar] [CrossRef]
- Bose, A.K.; Scherrer, D.; Camarero, J.J.; Ziche, D.; Babst, F.; Bigler, C.; Bolte, A.; Dorado-Liñán, I.; Etzold, S.; Fonti, P.; et al. Climate sensitivity and drought seasonality determine post-drought growth recovery of Quercus petraea and Quercus robur in Europe. Sci. Total Environ. 2021, 784, 147222. [Google Scholar] [CrossRef] [PubMed]
- Leuschner, C. Drought response of European beech (Fagus sylvatica L.)—A review. Perspect. Plant Ecol. Evol. Syst. 2020, 47, 125576. [Google Scholar] [CrossRef]
- FISE. Forest Information System for Europe 2024. Available online: https://forest.eea.europa.eu/topics/forest-management/tree-species-selection (accessed on 28 November 2024).
- NFI. National Forest Inventory of the Republic of Serbia 2023. Available online: https://upravazasume.gov.rs/oglasna-tabla/naredbu-o-proglasenju-prirodne-nepogode-i-merama-zastite-i-sanacije-suma-ostecenih-vetrolomima-i-vetroizvalama-2/ (accessed on 28 November 2024).
- Camarero, J.J.; Gazol, A.; Sangüesa-Barreda, G.; Vergarechea, M.; Alfaro-Sánchez, R.; Cattaneo, N.; Vicente-Serrano, S.M. Tree growth is more limited by drought in rear-edge forests most of the times. For. Ecosyst. 2021, 8, 25. [Google Scholar] [CrossRef]
- Wankmüller, F.J.P.; Delval, L.; Lehmann, P.; Baur, M.J.; Cecere, A.; Wolf, S.; Or, D.; Javaux, M.; Carminati, A. Global influence of soil texture on ecosystem water limitation. Nature 2024, 635, 631–638. [Google Scholar] [CrossRef]
- Zhang, Q.; Shao, M.; Jia, X.; Wei, X. Changes in soil physical and chemical properties after short drought stress in semi-humid forests. Geoderma 2019, 338, 170–177. [Google Scholar] [CrossRef]
- Lindh, M.; Zhang, L.; Falster, D.; Franklin, O.; Brännström, A. Plant diversity and drought: The role of deep roots. Ecol. Model. 2014, 290, 85–93. [Google Scholar] [CrossRef]
- RHSS. Republic Hydrometeorological Service of Serbia. Seasonal Climate Characteristics for the Territory of Serbia. Republic Hydrometeorological Service of Serbia, Belgrade. Available online: https://www.hidmet.gov.rs/eng/meteorologija/klimatologija_produkti.php (accessed on 20 October 2024).
- CCS. Climate Change Service. Record ‘Warm Daytimes’ in Southeastern Europe: C3S Seasonal Lookback: Summer 2024. Available online: https://climate.copernicus.eu/c3s-seasonal-lookback-summer-2024 (accessed on 25 October 2024).
- ICP Forests. International Co-Operative Programme on Assessment and Monitoring of Air Pollution Effects on Forests. Available online: http://icp-forests.net/ (accessed on 25 October 2024).
- Camarero, J.J.; Gazol, A.; Sangüesa-Barreda, G.; Oliva, J.; Vicente-Serrano, S. To die or not to die: Early-warning signals of dieback in response to a severe drought. J. Ecol. 2015, 103, 44–57. [Google Scholar] [CrossRef]
- Liu, Y.; Kumar, M.; Katul, G.G.; Porporato, A. Reduced resilience as an early warning signal of forest mortality. Nat. Clim. Change 2019, 9, 880–885. [Google Scholar] [CrossRef]
- Sangüesa-Barreda, G.; Gazol, A.; Camarero, J.J. Drops in needle production are early-warning signals of drought-triggered dieback in Scots pine. Trees 2023, 37, 1137–1151. [Google Scholar] [CrossRef]
- Georgieva, M.; Vassileva, V. Stress Management in Plants: Examining Provisional and Unique Dose-Dependent Responses. Int. J. Mol. Sci. 2023, 24, 5105. [Google Scholar] [CrossRef] [PubMed]
- Nikolaus, A.; Klaus, A.; Sacha, B.; Nina, B.; Markus, G.; Felix, K.; Christian, K.; Enrico, M.; Lutz, M.; Christine, M.; et al. Plant Response to Stress. Zurich-Basel Plant Sciences Center; PSC: Basel, Switzerland, 2012; pp. 8–153. [Google Scholar]
- Levitt, J. Responses of Plants to Environmental Stresses; Academic Press: Cambridge, MA, USA, 1980; Volume 1, p. 496. [Google Scholar]
- Lichtenthaler, H.K. Vegetation Stress: An Introduction to the Stress Concept in Plants. J. Plant Physiol. 1996, 148, 4–14. [Google Scholar] [CrossRef]
- Dai, A. Drought under global warming: A review Wiley Interdiscipl. Rev. Clim. Change 2011, 2, 45–65. [Google Scholar] [CrossRef]
- Moravec, V.; Markonis, Y.; Rakovec, O.; Svoboda, M.; Trnka, M.; Kumar, R.; Hanel, M. Europe under multi-year droughts: How severe was the 2014–2018 drought period? Environ. Res. Lett. 2021, 16, 034062. [Google Scholar] [CrossRef]
- Trugman, A.T.; Anderegg, L.D.L.; Anderegg, W.R.L.; Das, A.J.; Stephenson, N.L. Why is Tree Drought Mortality so Hard to Predict? Trends Ecol. Evol. 2021, 36, 520–532. [Google Scholar] [CrossRef]
- Ryan, M.G. Tree responses to drought. Tree Physiol. 2011, 31, 237–239. [Google Scholar] [CrossRef]
- Bonal, D.; Burban, B.; Stahl, C.; Wagner, F.; Hérault, B. The response of tropical rainforests to drought—Lessons from recent research and future prospects. Ann. For. Sci. 2016, 73, 27–44. [Google Scholar] [CrossRef]
- Češljar, G.; Jovanović, F.; Brašanac-Bosanac, L.; Đorđević, I.; Mitrović, S.; Eremija, S.; Ćirković-Mitrović, T.; Lučić, A. Impact of an Extremely Dry Period on Tree Defoliation and Tree Mortality in Serbia. Plants 2022, 11, 1286. [Google Scholar] [CrossRef]
- Češljar, G.; Đorđević, I.; Brašanac-Bosanac, L.; Eremija, S.; Mitrović, S.; Ćirković-Mitrović, T.; Lučić, A. Determination of forest decline due to the action of dominant stress factor through monitoring of defoliation—Case study of Maljen, Serbia. Agric. For. 2021, 67, 211–226. [Google Scholar] [CrossRef]
- RHSS. Republic Hydrometeorological Service of Serbia. Basic Climate Characteristics for the Territory of Serbia. Republic Hydrometeorological Service of Serbia, Belgrade. Available online: https://www.hidmet.gov.rs/eng/meteorologija/klimatologija_srbije.php (accessed on 5 December 2024).
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Koppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Mihajlović, J. Application of Recent Climate Classifications for the Climate Regionalization of Serbia. Ph.D. Dissertation, Faculty of Geography, University of Belgrade, Belgrade, Serbia, 2018; pp. 1–368. Available online: https://nardus.mpn.gov.rs/handle/123456789/10657 (accessed on 30 January 2025). (In Serbian).
- Eichhorn, J.; Roskams, P.; Potočić, N.; Timmermann, V.; Ferretti, M.; Mues, V.; Szepesi, A.; Durrant, D.; Seletković, I.; Schröck, H.-W.; et al. ICP Forests Manual Part IV. Visual Assessment of Crown Condition and Damaging Agents. Version 2020-3. 2020; pp. 5–54. Available online: https://storage.ning.com/topology/rest/1.0/file/get/9995547265?profile=original (accessed on 20 October 2024).
- Greenhalgh, T.; Peacock, R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: Audit of primary sources. BMJ 2005, 331, 1064. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A Multi-scalar drought index sensitive to global warming: The Standardized Precipitation Evapotranspiration Index—SPEI. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef]
- SPEI. Standardized Precipitation Evapotranspiration Index Database. Available online: http://sac.csic.es/spei/database.html (accessed on 25 November 2024).
- Thornthwaite, C.W. An approach toward a rational classification of climate. Geogr. Rev. 1948, 38, 55–94. [Google Scholar] [CrossRef]
- EEA. European Environment Agency. Drought Impact on Ecosystems in Europe. Available online: https://www.eea.europa.eu/en/analysis/indicators/drought-impact-on-ecosystems-in-europe (accessed on 5 December 2024).
- Stovall, A.E.L.; Shugart, H.; Yang, X. Tree height explains mortality risk during an intense drought. Nat. Commun. 2019, 10, 4385. [Google Scholar] [CrossRef]
- Zhu, J.; Thimonier, A.; Etzold, S.; Meusburger, K.; Waldner, P.; Schmitt, M.; Schleppi, P.; Schaub, M.; Thormann, J.J.; Lehmann, M.M. Variation in Leaf Morphological Traits of European Beech and Norway Spruce Over Two Decades in Switzerland. Frontiers 2022, 4, 2021. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef]
- Sanginés de Cárcer, P.; Signarbieux, C.; Schlaepfer, R.; Buttler, A.; Vollenweider, P. Responses of antinomic foliar traits to experimental climate forcing in beech and spruce saplings. Environ. Exp. Bot. 2017, 140, 128–140. [Google Scholar] [CrossRef]
- Caudullo, G.; De Rigo, D.; Mauri, A.; Houston, D.T.; San-Miguel-Ayanz, J. European Atlas of Forest Tree Species; Caudullo, G., De Rigo, D., Mauri, A., Houston Durrant, T., San-Miguel-Ayanz, J., Eds.; European Commission, Joint Research Centre, Publications Office of the European Union: Brussels, Belgium, 2016; Available online: https://data.europa.eu/doi/10.2760/776635 (accessed on 28 November 2024).
- Šijačić-Nikolić, M.; Milovanović, J.; Nonić, M.; Knežević, R.; Stanković, D. Leaf morphometric characteristics variability of different beech provenances in juvenile development stage. Genetika 2013, 45, 369–380. [Google Scholar] [CrossRef]
- Bresson, C.C.; Vitasse, Y.; Kremer, A.; Delzon, S. To what extent is altitudinal variation of functional traits driven by genetic adaptation in European oak and beech? Tree Physiol. 2011, 31, 1164–1174. [Google Scholar] [CrossRef]
- Roloff, A. Morphology of Crown Development of Fagus sylvatica L. (Beech) in Consideration of New Modifications: I. Morphogenetic Cycle, Abnormalities Specific to Proleptic Shoots and Leaf Fall. Flora 1987, 179, 355–378. [Google Scholar] [CrossRef]
- Arend, M.; Link, R.M.; Zahnd, C.; Hoch, G.; Schuldt, B.; Kahmen, A. Lack of hydraulic recovery as a cause of post-drought foliage reduction and canopy decline in European beech. New Phytol. 2022, 234, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Maréchaux, I.; Saint-André, L.; Bartlett, M.K.; Sack, L.; Chave, J. Leaf drought tolerance cannot be inferred from classic leaf traits in a tropical rainforest. J. Ecol. 2020, 108, 1030–1045. [Google Scholar] [CrossRef]
- Boutsios, S.; Vidalis, A.; Adamidis, G.C.; Hatziskakis, S.; Varsamis, G.; Tsiripidis, I.; Karanikola, P.; Papageorgiou, A.C. Diversity in Shade and Light Leaf Morphology in Beech Populations of South Rodopi Mountains. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 91, 53–61. [Google Scholar] [CrossRef]
- Adamidis, G.C.; Georgios, V.; Ioannis, T.; Panayiotis, G.D.; Aristotelis, C.P. Patterns of Leaf Morphological Traits of Beech (Fagus sylvatica L.) along an Altitudinal Gradient. Forests 2021, 12, 1297. [Google Scholar] [CrossRef]
- Polle, A.; Schwanz, P.; Rudolf, C. Developmental and seasonal changes of stress responsiveness in beech leaves (Fagus sylvatica L.). Plant Cell Environ. 2001, 24, 821–829. [Google Scholar] [CrossRef]
- Rukh, S.; Sanders, T.G.M.; Krüger, I.; Schad, T.; Bolte, A. Distinct Responses of European Beech (Fagus sylvatica L.) to Drought Intensity and Length—A Review of the Impacts of the 2003 and 2018–2019 Drought Events in Central Europe. Forests 2023, 14, 248. [Google Scholar] [CrossRef]
- De la Riva, E.G.; Olmo, M.; Poorter, H.; Ubera, J.L.; Villar, R. Leaf Mass per Area (LMA) and Its Relationship with Leaf Structure and Anatomy in 34 Mediterranean Woody Species along a Water Availability Gradient. PLoS ONE 2016, 11, e0148788. [Google Scholar] [CrossRef]
- Thomas, F.M.; Preusser, S.; Backes, B.; Werner, W. Leaf traits of Central-European beech (Fagus sylvatica) and oaks (Quercus petraea/robur): Effects of severe drought and long-term dynamics. For. Ecol. Manag. 2024, 559, 121823. [Google Scholar] [CrossRef]
- Wu, C.; Peng, J.; Ciais, P.; Peñuelas, J.; Wang, H.; Beguería, S.; Black, T.A.; Jassal, R.S.; Zhang, X.; Yuan, W.; et al. Increased drought effects on the phenology of autumn leaf senescence. Nat. Clim. Change 2022, 12, 943–949. [Google Scholar] [CrossRef]
- Ma, C.; Wang, X.; Wu, C. Early leaf senescence under drought conditions in the Northern hemisphere. Agric. For. Meteorol. 2024, 358, 110231. [Google Scholar] [CrossRef]
- Polgar, C.A.; Primack, R.B. Leaf-out phenology of temperate woody plants: From trees to ecosystems. New Phytol. 2011, 191, 926–941. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Singh Sidhu, G.P.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K.; et al. Photosynthetic Response of Plants Under Different Abiotic Stresses: A Review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Penuelas, J.; Rutishauser, T.; Filella, I. Phenology feedbacks on climate change. Science 2009, 324, 887–888. [Google Scholar] [CrossRef] [PubMed]
- Keskitalo, J.G.; Bergquist, P.; Gardeström, S.; Jansson, S. A cellular timetable of autumn senescence. Plant Physiol. 2005, 139, 1635–1648. [Google Scholar] [CrossRef]
- Frei, E.R.; Gossner, M.M.; Vitasse, Y.; Queloz, V.; Dubach, V.; Gessler, A.; Ginzler, C.; Hagedorn, F.; Meusburger, K.; Moor, M.; et al. European beech dieback after premature leaf senescence during the 2018 drought in northern Switzerland. Plant Biol. 2022, 24, 1132–1145. [Google Scholar] [CrossRef]
- Seidling, W. Signals of summer drought in crown condition data from the German Level I network. Eur. J. Forest. Res. 2007, 126, 529–544. [Google Scholar] [CrossRef]
- McCulloh, K.A.; Augustine, S.P.; Goke, A.; Jordan, R.; Krieg, C.P.; O’Keefe, K.; Smith, D.D. At least it is a dry cold: The global distribution of freeze–thaw and drought stress and the traits that may impart poly-tolerance in conifers. Tree Physiol. 2023, 43, 1–15. [Google Scholar] [CrossRef]
- Song, Y.; Sass-Klaassen, U.; Sterck, F.; Goudzwaard, L.; Akhmetzyanov, L.; Poorter, L. Growth of 19 conifer species is highly sensitive to winter warming, spring frost and summer drought. Ann. Bot. 2021, 128, 545–557. [Google Scholar] [CrossRef]
- Charrier, G.; Martin-StPaul, N.; Damesin, C.; Deloierre, N.; Hänninen, H.; Torres-Ruiz, J.M.; Davi, H. Interaction of drought and frost in tree ecophysiology: Rethinking the timing of risks. Ann. For. Sci. 2021, 78, 40. [Google Scholar] [CrossRef]
- Pekeč, S.; Drekić, M.; Milović, M.; Karaklić, V. Phenological phases of leaf unfolding and leaf fall of sessile oak (Quercus petraea Matt./Liebl.) at the second level bioindication point in Fruška gora. Šumarstvo 2020, 1–2, 119–126. (In Serbian) [Google Scholar]
- Descals, A.; Verger, A.; Yin, G.; Filella, I.; Peñuelas, J. Widespread drought-induced leaf shedding and legacy effects on productivity in European deciduous forests. Remote Sens. Ecol. Conserv. 2022, 9, 76–89. [Google Scholar] [CrossRef]
- Massonnet, C.; Chuste, P.-A.; Levillain, J.; Gérémia, F.; Silva, D.E.; Maillard, P.; Dreyer, E.; Dupouey, J.-L.; Bréda, N. Leafy season length is reduced by a prolonged soil water deficit but not by repeated defoliation in beech trees (Fagus sylvatica L.): Comparison of response among regional populations grown in a common garden. Agric. For. Meteorol. 2021, 297, 108228. [Google Scholar] [CrossRef]
- Arend, M.; Gessler, A.; Schaub, M. The influence of the soil on spring and autumn phenology in European beech. Tree Physiol. 2016, 36, 78–85. [Google Scholar] [CrossRef][Green Version]
- Wolfe, B.T.; Sperry, J.S.; Kursar, T.A. Does leaf shedding protect stems from cavitation during seasonal droughts? A test of the hydraulic fuse hypothesis. New Phytol. 2016, 212, 1007–1018. [Google Scholar] [CrossRef]
- Walthert, L.; Ganthaler, A.; Mayr, S.; Saurer, M.; Waldner, P.; Walser, M.; Zweifel, R.; von Arx, G. From the comfort zone to crown dieback: Sequence of physiological stress thresholds in mature European beech trees across progressive drought. Sci. Total Environ. 2021, 753, 141792. [Google Scholar] [CrossRef] [PubMed]
- Savage, J. Expert Alert: The Drought’s Impact on Autumn Colors. University of Minnesota Duluth. 2021. Available online: https://news.d.umn.edu/articles/expert-alert-drought-and-fall-colors (accessed on 5 August 2024).
- De Marco, A.; Proietti, C.; Cionni, I.; Fischer, R.; Screpanti, A.; Vitale, M. Future impacts of nitrogen deposition and climate change scenarios on forest crown defoliation. Environ. Pollut. 2014, 194, 171–180. [Google Scholar] [CrossRef] [PubMed]
- De Vries, W.; Klap, J.M.; Erisman, J.W. Effects of environmental stress on forest crown condition in Europe. Part I: Hypotheses and approach to the study. Water Air Soil Pollut. 2000, 119, 317–333. [Google Scholar] [CrossRef]
- Bussotti, F.; Papitto, G.; Di Martino, D.; Cocciufa, C.; Cindolo, C.; Cenni, E.; Bettini, D.; Iacopetti, G.; Pollastrini, M. Defoliation, Recovery and Increasing Mortality in Italian Forests: Levels, Patterns and Possible Consequences for Forest Multifunctionality. Forests 2021, 12, 1476. [Google Scholar] [CrossRef]
- Gazol, A.; Camarero, J.J. Compound climate events increase tree drought mortality across European forests. Sci. Total Environ. 2022, 816, 151604. [Google Scholar] [CrossRef]
- George, J.-P.; Bürkner, P.-C.; Sanders, T.G.M.; Neumann, M.; Cammalleri, C.; Vogt, J.V.; Lang, M. Long-term forest monitoring reveals constant mortality rise in European forests. Plant Biol. 2022, 24, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Bucha, T.; Pavlenda, P.; Konôpka, B.; Tomaštík, J.; Chudá, J.; Surový, P. Satellite Assessment of Forest Health in Drought Conditions: A Novel Approach Combining Defoliation and Discolouration. Forests 2024, 15, 1567. [Google Scholar] [CrossRef]
- Gottardini, E.; Cristofolini, F.; Cristofori, A.; Pollastrini, M.; Camin, F.; Ferretti, M. A multi-proxy approach reveals common and species-specific features associated with tree defoliation in broadleaved species. For. Ecol. Manag. 2020, 467, 118151. [Google Scholar] [CrossRef]
- Balducci, L.; Fierravanti, A.; Rossi, S.; Delzon, S.; De Grandpré, L.; Kneeshaw, D.D.; Deslauriers, A. The paradox of defoliation: Declining tree water status with increasing soil water content. Agric. For. Meteorol. 2020, 290, 108025. [Google Scholar] [CrossRef]
- Ognjenović, M.; Seletković, I.; Potočić, N.; Marušić, M.; Tadić, M.P.; Jonard, M.; Rautio, P.; Timmermann, V.; Lovreškov, L.; Ugarković, D. Defoliation Change of European Beech (Fagus sylvatica L.) Depends on Previous Year Drought. Plants 2022, 11, 730. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).