Abstract
Trunk sap flow is essential for assessing plant water use efficiency and adaptation, yet the mechanisms underlying drought resistance and water utilization strategies in dry and hot valleys remain poorly understood. This study investigates the sap flow dynamics of four tree species (Albizia kalkora, Diospyros dumetorum, Terminalia franchetii, and Acacia auriculiformis) in a dry and hot valley using Granier’s thermal diffusion probe method. The aims were to analyze interspecific differences and their response mechanisms to environmental factors using a fitted model of sap flow density and transpiration variables, supplemented by Pearson’s and Mantel’s tests. The results showed that (1) the trunk sap flow of each tree species is significantly higher in the wet season than in the dry season. (2) In the dry and wet seasons, the average trunk sap flow rates were in the order Albizia kalkora > Diospyros dumetorum > Terminalia franchetii > Acacia auriculiformis. (3) The correlation between environmental factors and trunk sap flow was in the order photosynthetically active radiation > atmospheric temperature > saturated water vapor pressure difference > relative humidity > wind speed. (4) Deciduous plants demonstrated stronger water-conducting capacities than evergreen plants and native plants exhibited better drought resistance than introduced plants. (5) Acacia auriculiformis and Albizia kalkora were identified as rainfall-sensitive plants, while Diospyros dumetorum and Terminalia franchetii were rainfall-insensitive. By optimizing species selection based on water use efficiency, rainfall sensitivity, and environmental conditions such as light and temperature, this research contributes to enhancing the stability and resilience of ecosystem restoration in arid regions.
1. Introduction
In the soil–plant–atmosphere continuum, plant transpiration plays a crucial role in water consumption. The water use efficiency of trees directly affects the water cycle of ecosystems and soil water retention capacity [1,2,3]. The water use efficiency of trees is generally studied from a xylem perspective due to its accuracy [4,5]. Many researchers use the thermal diffusion technique to determine the sap flow rates in the sapwood of tree trunks. This method effectively reflects the status of water utilization in the xylem and offers advantages such as continuous monitoring, high temporal resolution, and highly reliable data. The calibration and validation of the Granier thermal diffusion probe (TDP) method are critical to ensuring the reliability of sap flow measurements. In this study, the TDP probes were carefully calibrated before installation, and the calibration process followed standard procedures outlined in previous studies [6,7,8]. The data obtained through this technique can be employed to characterize the dynamics of water transport in plants and indicate plant responses to environmental factors [9]. Long-term and multi-species monitoring can accurately reflect the pattern of trunk sap flow across different seasons and climatic conditions. This approach is essential for assessing the water utilization dynamics and growth status of trees [10], and it is critical for determining plant spatial allocation and vegetation restoration [11].
Most studies on trunk sap flow focus on temperate and tropical forests, with relatively few long-term studies on plant water physiology in extreme environments, such as dry and hot valleys. These valleys experience high temperatures, low rainfall, and strong seasonal changes, which increase the complexity and difficulty of research. The long-term monitoring of trunk sap flow in these conditions faces significant challenges owing to extreme climatic conditions. Understanding trunk sap flow dynamics in these extreme conditions is crucial for addressing ecological degradation and vegetation restoration, as it directly relates to water use efficiency, tree survival, and adaptation to seasonal water scarcity. Therefore, previous studies in these areas primarily focus on seed germination, community characteristics, and vegetative growth. Few systematic long-term experiments on adult plants in the field and limited long-term analyses and comparisons of trunk sap flow across multiple species exist. In particular, research incorporating long-term meteorological observatories and water-use monitoring equipment in the field is lacking, and the mechanisms of drought adaptation in various tree species remain unclear [7,12,13,14].
In Asia, North America, and South America, dry and hot valleys are characterized by high temperatures, strong evapotranspiration, sparse vegetation, severe soil aridity and erosion. These areas represent fragile ecosystems, whose emergence and distribution indicate the degradation of natural zonal habitats [15,16,17,18]. Severe soil erosion in these regions and hot valleys has led to a decline in vegetation cover, creating a vicious cycle of environmental degradation and increased soil erosion. For example, 94.6% of the rainfall in the Jinsha River’s dry and hot valleys occurs during the wet season between June and October, leading to natural disasters such as flooding owing to the unequal distribution of water resources [19]. The dry and hot valleys in China, located in the upper reaches of major international rivers, including the Jinsha, Lancang, and Yuanjiang valleys, play a crucial role in national ecological security and economic development. These valleys are situated in the upper reaches of the Yangtze, Mekong, and Red Rivers, respectively, highlighting their significant geographical importance [20]. Recently, desertification trends in arid and rocky regions have intensified, resulting in severe degradation of native vegetation, loss of species diversity, and overall ecosystem decline [21,22,23]. In the extremely harsh conditions of dry and hot valley areas, factors such as high temperature, low humidity, low soil moisture content, and poor soil nutrients limit vegetation recovery and influence the selection of suitable tree species [17,24]. Vegetation in these regions adapts uniquely to extreme weather conditions through specific physiological and ecological mechanisms. Albizia kalkora (Roxb.) Prain, Diospyros dumetorum W. W. Smith, Terminalia franchetii Gagnep, and Acacia auriculiformis A. Cunn. ex Benth are key species for vegetation restoration in the dry and hot valleys of southwest China to adapt to the extreme climatic conditions. They show excellent drought and heat tolerance, and they effectively realize soil and water conservation and ecological restoration through nitrogen fixation, soil improvement, and a deep rooting system [25,26]. Trunk sap flow, a crucial pathway for water transport, directly influences water utilization and tree survival [27]. Therefore, studying the seasonal dynamics of trunk sap flow and its response to environmental factors of typical tree species in the valley, as well as exploring the drought resistance of major suitable silvicultural species, provides essential theoretical and practical support for the selection of tree species for vegetation restoration and protection of forest stand management in these valleys. In addition, the savanna ecosystem, found in Africa, South America, Oceania, and Asia, experiences dry and wet seasons. This ecosystem is characterized by sparse tree scrub and infertile soil [28,29,30,31]. The dry and hot valleys and savannas share significant similarities in climate, soil conditions, vegetation types, and water allocation [32,33]. Understanding vegetation water utilization in dry and hot valleys could provide scientific guidance for ecological restoration in extreme climatic regions and enhance the efficient water use of vegetation in the context of climate change [7,12,13,14]. Additionally, this study will provide valuable insights into the restoration and ecological management of special habitat flora in the savanna ecosystem, which is similarly marked by dry and wet season environments.
Therefore, in the present study, we aimed to investigate the diurnal rhythm and seasonal dynamics of sap flow in typical tree species in dry and hot valleys during key months of the dry and wet seasons. To the best of our knowledge, our study is the first to monitor, characterize, and systematically compare the drought tolerance and sap flow rates of multiple tree species in these valleys. We explore the effects of environmental factors and precipitation events on trunk sap flow in typical tree species, assess the water-conducting capacity and survival adaptability of native and introduced, deciduous, and evergreen tree species in this extreme environment, and reveal the adaptive mechanisms of plants.
2. Materials and Methods
2.1. Study Area
Given the typicality and representativeness of the Jinsha River valley among dry hot valleys [15,34], the study site is situated at the Experimental Site of the Yuanmou Desert Ecosystem Research Station, National Long-Term Scientific Research Base of comprehensive control, Jinsha River Dry and Hot Valley Region, China (25°58′8″ N,101°44′44″ E), at an elevation of 1575.58 m above sea level (Figure 1) [35]. The climate in this area is unusually dry and hot, with distinct wet and dry seasons. The average annual atmospheric temperature (Ta) is 21.90 °C, with the coldest month averaging 15.84 °C (December) and the hottest month being 27.1 °C (May). The highest Ta reaches 42 °C. The average annual precipitation is 500–600 mm, with the wet season lasting between June and October and accounting for >95% of the annual rainfall. The dry season extends between November and May of the following year [36,37]. The average annual relative humidity (RH) is 53.72%.
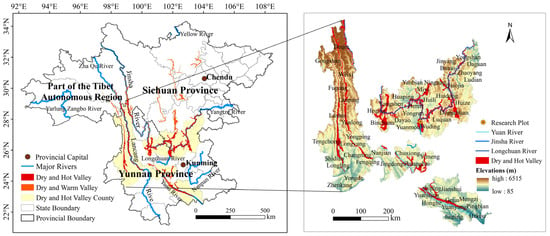
Figure 1.
Location, elevation, and relative position of the research plot within the dry and hot valleys in southwest China.
2.2. Test Time
We applied the thermal diffusion probe method to continuously monitor trunk sap flow over one year, from June 2023 to June 2024. This period was chosen to investigate the seasonal dynamics of trunk sap flow in representative tree species during typical months of the dry and wet seasons.
2.3. Test Material
Typical tree species in the dry and hot valleys of the Jinsha River were selected as the research materials, including Albizia kalkora (Roxb.) Prain, Diospyros dumetorum W. W. Smith, Terminalia franchetii Gagnep, and Acacia auriculiformis A. Cunn. ex Benth. We selected well-grown adult trees with straight trunks that were not damaged. For each tree species, four plants with similar growth were selected for measurement (Table 1). All four species are prevalent in dry and hot valleys of the Jinsha River. Albizia kalkora, Diospyros dumetorum, and Terminalia franchetii are characteristic native species of southwestern China [26,38]. Native tree species have developed strong drought-resistant adaptability through long-term natural selection, making them excellent choices for vegetation restoration [39]. Recent research focuses on screening and rapid propagation techniques for stress-resistant plants in the dry and hot valleys. Acacia auriculiformis introduced from Australia shows good adaptability to the high temperatures, drought, and infertile soils of this region. It has played a pivotal role in consolidating soil, retaining water, and preserving nutrients in the restoration of planted forests [40]. In dry and hot valley regions, the unique climatic conditions of higher and drier temperatures can affect the growth cycle and defoliation time of tree species. Trees may enter dormancy earlier to decrease water evaporation and cope with drought. Owing to these conditions, deciduous tree species often concentrate their leaf fall in the dry season between November and May of the following year. Albizia kalkora and Terminalia franchetii shed their leaves from January to February and from December to May of the following year, respectively [38,41].

Table 1.
Mean values of basic information of sample trees.
2.4. Test Methods
2.4.1. Measurement of Trunk Sap Flow
The Granier thermal diffusion probe (TDP) method was adopted using the SF-G Ecomatik sap flow sensor (Campbell Scientific Inc., Logan, UT, USA). To avoid thermal effects, the sensor was uniformly installed on the north side of the test tree trunk, 1.5 m above the ground. The data collector was connected to the probe, recording data every 15 min. Data were collected for each species at multiple representative trees within the study sites. Based on the principle that the temperature difference between the upper and lower probes of the TDP is closely related to the trunk sap flow rate, Granier establishes the following empirical equations to calculate the sap flow rate (Equations (1) and (2)) [7,42,43,44,45,46].
where U is the sap flow density (mL·cm−2·min−1), ΔTmax is the maximum temperature difference between the upper and lower probes during day and night (°C), ΔT is the instantaneous temperature difference (°C), F is the sap flow rate (mL·h−1), SA is the area of sapwood layer (cm2), and 60 is the unit conversion factor, indicating that I set the unit of F to mL·h−1 instead of mL·min−1.
2.4.2. Meteorological Data Monitoring
A WS1000X standard ground-based meteorological station (Campbell Scientific Inc., Logan, UT, USA) was deployed at the experimental site to monitor environmental conditions, including the photosynthetically active radiation (PAR) (µmol·m−2·s−1), Ta (°C), RH (%), wind speed (WS) (mL·s−1). Data were recorded every 30 min.
To analyze the response of trunk sap flow rate to environmental factors for four tree species, we introduced the saturated water vapor pressure difference (VPD) (kPa) (Equation (3)) [47,48] and transpiration variables (V, kPa·(µmol·m−2·s−1)1/2) (Equation (4)), which simultaneously reflect synergistic changes in PAR, Ta, and RH. An equation reflecting the relationship between sap flow density and transpiration variables was also introduced (Equation (5)) [49,50]. The equations were calculated as follows:
In Equation (5), U is the sap flow density (mL·cm−2·h−1), parameter a represents the degree of curve shifts; a larger value indicates greater sensitivity of sap flow density to environmental factors. Parameter b represents the slope of the curve; a larger value indicates a stronger ability of the tree to conduct water [50].
2.5. Data Processing
Excel 2016 was used for data organization and calculation, while SPSS 22.0, Origin 2022, and ArcMap 10.8.1 were utilized for graphing and data analysis.
3. Results
3.1. Daily Dynamics of Trunk Sap Flow Rates of Typical Tree Species
In this experiment, in order to illustrate the daily dynamics of trunk sap flow rates patterns of each typical tree species, we selected the average of the monthly hourly sap flow data from the monitoring data of the wet and dry seasons for graphical analysis. Graphical analysis is applied to examine the daily dynamics of trunk sap flow rates in typical tree species of the dry and hot valleys during the characteristic months of both the wet and dry seasons (Figure 2). It shows that the trunk sap flow of the four tree species exhibited clear diurnal variation patterns during dry and wet seasons. These patterns featured a “daytime high and nighttime low” single-peak curve, with the trunk sap flow rates peaking between 10:00 and 18:00. During the daytime, the sap flow rate varied significantly, contributing to the majority of the daily sap flow. In contrast, the nighttime sap flow rate was considerably lower and more stable while still present to a certain extent.
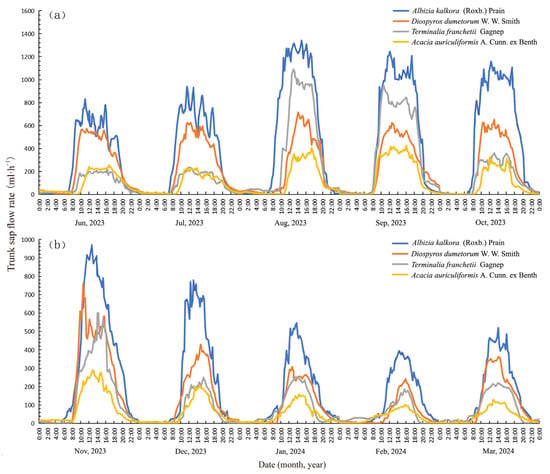
Figure 2.
Daily dynamics of trunk sap flow rates for the four study species: (a) wet season and (b) dry season.
The monthly hourly averages of environmental factors are plotted (Figure 3). Figure 3 shows that the RH in a typical month in the wet season was significantly higher than that of a typical month in the dry season. As rainfall increased between June and October during the wet season, the RH gradually rose, and the rate of sap flow of the four plant species showed a corresponding upward trend. August had the highest RH, which corresponded to the peak values of trunk sap flow for the four plant species. Conversely, as rainfall sharply decreased between November and March during the dry season, the rate of trunk sap flow for each tree species significantly declined. The lowest RH was observed between February and March of the following year, corresponding to the minimum value of trunk sap flow during the dry season. The VPD showed an inverse relationship with RH. Seasonally, when the VPD reached its maximum and minimum values, it corresponded to the minimum and maximum values of the sap flow rate of each tree species, respectively.
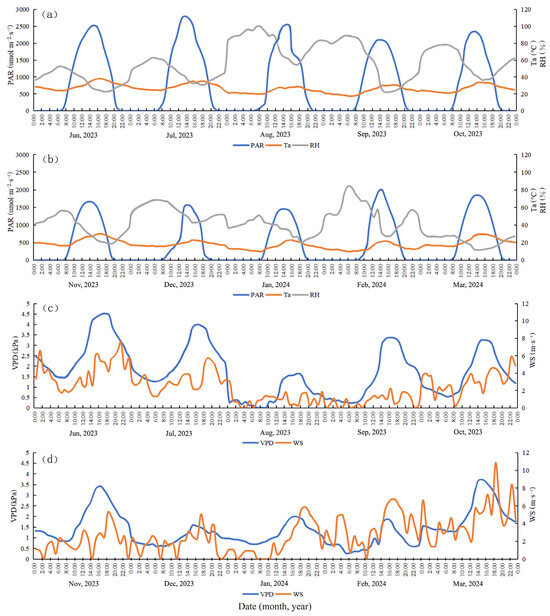
Figure 3.
Daily dynamics of environmental factors including (a) PAR (photosynthetically active radiation), Ta (atmospheric temperature), and RH (relative humidity) during the wet season. (b) PAR, Ta, and RH during the dry season. (c) VPD (saturated water vapor pressure difference) and WS (wind speed) during the wet season, and (d) VPD and WS during the dry season.
3.2. Seasonal Dynamics of Trunk Sap Flow Rates of Typical Tree Species
The monitored data were analyzed to compare the maximum and average values of the trunk sap flow rates of four typical tree species in the dry and hot valleys across different months, along with the timing of the maximum sap flow rate (Table 2). The mean values of sap flow rates for Albizia kalkora, Diospyros dumetorum, Terminalia franchetii, and Acacia auriculiformis in the wet season were 4.20 × 102, 2.27 × 102, 2.04 × 102, and 1.10 × 102 mL·h−1, respectively. In the dry season, the mean values were 1.93 × 102, 1.18 × 102, 8.88 × 101, and 5.48 × 101 mL·h−1, respectively. The mean values of the sap flow rates in the wet season were approximately twice as high as those in the dry season. Moreover, the mean and peak values of the sap flow rates in November were generally higher than those in the other months of the dry season, owing to the rainfall retention from early-season precipitation. In addition, comparing the mean values of the four species in dry and wet seasons, the hierarchy was as follows: Albizia kalkora > Diospyros dumetorum > Terminalia franchetii > Acacia auriculiformis. In the wet season, the peak sap flow rates of Albizia kalkora and Terminalia franchetii were observed from 10:45 to 16:30, which were slightly delayed to 13:15 to 16:45 in the dry season. The peak sap flow rates of Diospyros dumetorum and Acacia auriculiformis fluctuated from 10:30 to 17:30 in the two seasons, with no significant delay during the dry season despite changes in rainfall. In the wet season, the tree trunk sap flow rate of all four species reached their peak within specific periods, forming multiple small peaks known as a “peak platform”. In the dry season, the peak intervals were shorter than in the wet season and the daily variation showed a “sharp upward and wide downward” trend.

Table 2.
Seasonal dynamics of trunk sap flow rate.
3.3. Correlation Analysis Between Dynamic Characteristics of Trunk Sap Flow Rate of Typical Tree Species and Environmental Factors Specific
The rate of trunk sap flow is influenced by the physiological condition of plants and environmental factors such as PAR, Ta, RH, VPD, and WS [51,52,53]. Interactions between meteorological factors and plant physiological conditions also play a role, with the primary factors affecting changes in sap flow varying by region. To visualize the changes in sap flow and meteorological factors for the four typical tree species, sap flow data from five days (5–9 August 2023 during the wet season, and 5–9 January 2024 during the dry season) were selected for synergistic analysis. These data were plotted alongside synchronized meteorological data for the monitoring periods of dry and wet seasons (Figure 4 and Figure 5), respectively. In the wet and dry seasons, the maximum PAR was 2766 and 1472 μmol·m−2·s−1, maximum Ta was 32.0 °C and 22.9 °C, minimum Ta was 18.8 °C and 8.8 °C, maximum RH was 99.9% and 54.5%, minimum RH was 47.3% and 15.4%, maximum VPD was 2.511 and 2.218 kPa, minimum VPD was 0.002 and 0.538 kPa, and maximum WS was 4.06 and 6.87 m·s−1, respectively. In the dry season, the peak sap flow rate of the typical tree species was approximately 3 h earlier than the corresponding maxima of Ta and VPD.
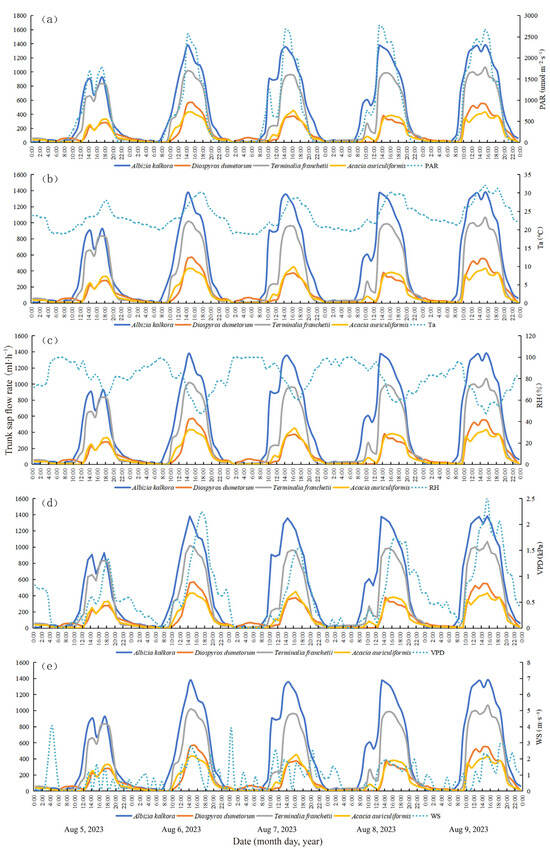
Figure 4.
Dynamic characteristics of trunk sap flow rate and relationship with environmental factors in the wet season (a–e).
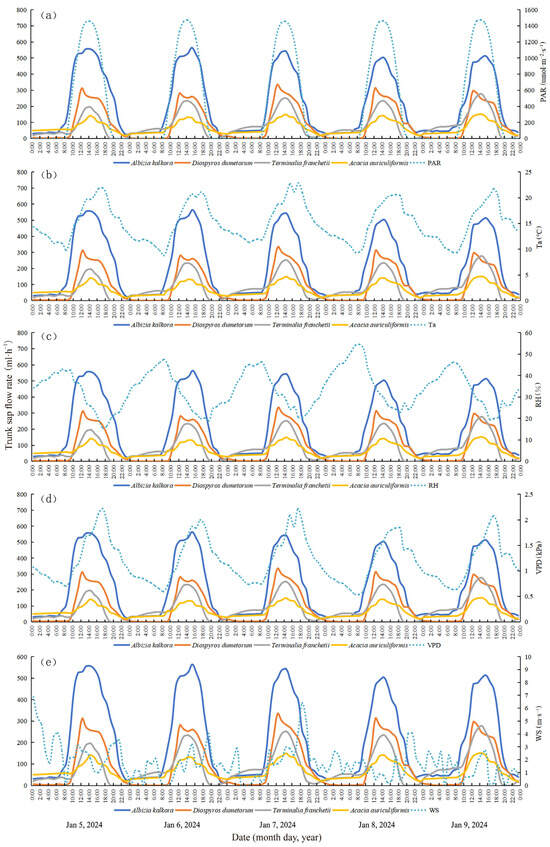
Figure 5.
Dynamic characteristics of trunk sap flow rate and relationship with environmental factors in the dry season (a–e).
To explore the relationship between trunk sap flow rate and rainfall in four typical tree species, changes in sap flow density were simulated using an evapotranspiration variable that incorporates solar radiation and VPD [49,50]. In the short term, Ta, RH, and solar radiation intensity are the primary factors influencing vegetation transpiration. The transpiration variable captures the combined effects of solar radiation and VPD (Figure 6). Trunk sap flow data for the four tree species were collected on three typical days during the dry and wet seasons (10–12 August 2023, in the wet season, and 12 January 2024, in the dry season). The average rainfall from 10–12 August, considered to be moderate, was 12.65 mm. Trunk sap flow density and transpiration variables were calculated for each species before and after rainfall. These measurements were used to analyze the response of trunk sap flow to environmental factors and to examine the synergistic effects of variables such as PAR, Ta, and RH on the sap flow of each tree species.
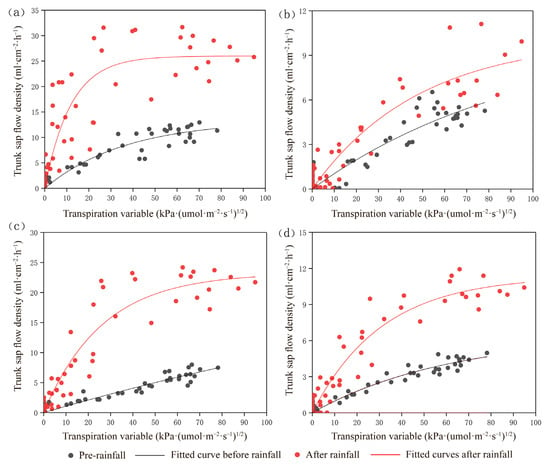
Figure 6.
Relationship between trunk sap density and transpiration variables before and after rainfall for: (a) Albizia kalkora, (b) Diospyros dumetorum, (c) Terminalia franchetii, and (d) Acacia auriculiformis.
Monitoring sap flow density and environmental factors of the four tree species during the study period showed that the sap flow rates were significantly higher after rainfall than before. Each tree species exhibited a positive exponential relationship between sap flow density and transpiration variables before and after rainfall (Figure 6 and Table 3). The fitting parameters (a and b) of sap flow density to transpiration variables differed significantly before and after rainfall (Table 3). Parameters a and b increased significantly for Acacia auriculiformis and Albizia kalkora after rainfall, suggesting that the sensitivity of their sap flow density to the environment and their hydraulic conductivity increased after rainfall. For Diospyros dumetorum and Terminalia franchetii, the sensitivity of sap flow density to the environment decreased slightly, while their hydraulic conductivity showed an increasing trend. Compared to Diospyros dumetorum and Terminalia franchetii, the sap flow density of Acacia auriculiformis and Albizia kalkora was more strongly affected by rainfall. Comparing the increase in the fitted parameter b, the hydraulic conductivity of the tree hydraulic system was ranked as follows: Terminalia franchetii > Albizia kalkora > Acacia auriculiformis > Diospyros dumetorum. When water is effectively replenished after drought, plants with strong bolting and refilling abilities are more suited for survival [54]. The comprehensive results of the study showed that Acacia auriculiformis and Albizia kalkora are rainfall-sensitive plants, while Diospyros dumetorum and Terminalia franchetii are rainfall-insensitive. The water conductivity of deciduous tree species was greater than that of evergreen tree species, and tree species with longer deciduous periods had stronger water conductivity [55]. We analyzed the correlation heatmap of the Pearson correlation coefficient and Mantel test results (Figure 7) and found that the trunk sap flow of different tree species, which is significantly correlated with various environmental factors. The trunk sap flow rates of all four species showed highly significant positive correlations with PAR, Ta, and VPD (p ≤ 0.001) and highly significant negative correlations with RH (p ≤ 0.001). Albizia kalkora exhibited non-significant correlations with WS (p > 0.05), while Diospyros dumetorum showed significant correlations with WS (0.001 < p ≤ 0.01). Terminalia franchetii and Acacia auriculiformis showed lower significance with RH (0.01 < p ≤ 0.05). The r values of the Mantel test indicated that the strongest correlations of trunk sap flow were with PAR for all four species, followed by Ta, with the smallest correlations observed with WS. Overall, the hierarchy of correlations between environmental factors and trunk sap flow rates was PAR > Ta > VPD > RH > WS. Within a single day, RH showed highly significant negative correlations with Ta and VPD (r = −0.96, r = −0.97), consistent with the formula for calculating VPD. Ta exhibited a highly significant positive correlation with VPD (r = 0.98), indicating a significant increase in VPD with rising Ta.

Table 3.
Fitting equations for sap flow density and transpiration variables before and after rainfall for typical tree species. Parameter a represents the degree of curve shifts. Parameter b represents the slope of the curve.
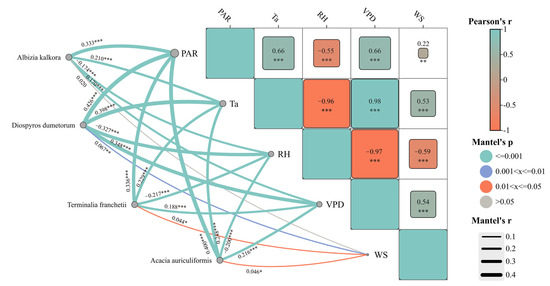
Figure 7.
Correlation heat map of Pearson correlation coefficient and Mantel tests. Note: * p < 0.05, ** p < 0.01 and *** p < 0.001.
4. Discussion
4.1. Circadian and Seasonal Rhythms in Trunk Sap Flow of Various Tree Species
Trunk sap flow reflects the water utilization strategies of plants in a day seasonally. In dry and wet seasons, the sap flow rates for all tree species consistently followed a trend of “daytime high and nighttime low”. Sap flow began with sunrise and a rise in air temperature, reaching its highest in the late afternoon before gradually declining. The significant daytime increase and nocturnal reduction in sap flow at night may be attributed to the excessive transpirational losses during the day, which resulted in a water deficit in the trees. To meet their physiological needs, trees continue water uptake at night through root pressure [7,56,57]. The peak sap flow varied among tree species throughout the day but generally followed similar trends, primarily influenced by light, temperature, and plant characteristics [58]. During the wet season, abundant water supply saturated the soil, enabling trees to maximize water use for photosynthesis and growth. Conversely, in the dry season, water scarcity induces water stress, prompting trees to reduce sap flow to minimize water loss. Studies report that the sap flow rate of each tree species is significantly higher in the wet season than in the dry season [25,59]. The peak sap flow rate occurred earlier in the wet season than in the dry season for the same species. In the morning of the wet season, ample soil moisture and moderate air temperature may enhance the conduit activity, boosting the efficiency of water transport. This allows trees to absorb and transport water quickly, reaching the peak sap flow rates sooner. In contrast, during the dry season, insufficient soil moisture causes trees to take longer to absorb limited water more slowly and possibly reduce the activity of conduits or numbers to prevent water loss, resulting in delayed peak sap flow rates. Different tree species showed varying sensitivity to seasonal rainfall. For instance, Acacia auriculiformis and Albizia kalkora significantly increased their sap flow rates during the wet season, indicating a robust capacity for rapid water utilization. In contrast, Diospyros dumetorum and Terminalia franchetii exhibited a weaker response, suggesting stronger drought adaptability. In conserving dry and hot valley ecosystems, selecting tree species with higher water utilization efficiency and stronger adaptability can enhance the stability and resilience of the ecosystem. Additionally, improving the growing conditions and reducing water stress in the dry season is crucial. Measures such as planting artificial vegetation to enhance the physical and chemical properties of the soil can be employed to achieve this.
4.2. Interspecific Differences in Trunk Sap Flow Among Tree Species
The trunk sap flow rate is a critical indicator for evaluating the ability of trees to absorb, transport, and utilize water. Significant interspecific differences exist in the mean sap flow rates of various tree species during dry and wet seasons under identical stand conditions. These differences arise from a combination of environmental and biological factors [60]. The mean sap flow rate of tree trunks differed significantly among species, reflecting differences in water use efficiency and adaptability to wet and dry environments. Albizia kalkora, Diospyros dumetorum, Terminalia franchetii, and Acacia auriculiformis may have evolved distinct water management mechanisms to cope with severe habitat challenges. Across the dry and wet seasons, the order of mean trunk sap flow rates was Albizia kalkora > Diospyros dumetorum > Terminalia franchetii > Acacia auriculiformis. Despite a one-month defoliation period, Albizia kalkora maintained the most efficient transpiration, about twice that of Diospyros dumetorum, indicating its superior water transport capacity in dry and wet seasons. This may be attributed to its root system structure, leaf transpiration efficiency and conduit diameter [55]. Albizia kalkora might have a larger conduit diameter and higher water conductivity, maintaining high transpiration levels to support rapid growth and high productivity. This enables it to maintain efficient water transport in dry and wet seasons. Comparatively, Acacia auriculiformis had the lowest sap flow rate, about one-fourth that of Albizia kalkora, which may indicate smaller conduits or less efficient water transport. Plants may adapt to drier conditions by reducing transpiration to minimize water loss. This difference in strategy reflects the varied choices made by tree species for resource use and survival [61]. The consistent order of mean trunk sap flow rates in dry and wet seasons suggests that these species maintain water transport capacities and strategies despite seasonal moisture changes. Tree species with higher sap flow rates typically grow faster, form larger canopies, and increase vegetation cover more rapidly [62]. This provides more habitats and food resources, enhances water absorption and transport capacity, effectively utilizes water resources in the soil, reduces soil and wind erosion, and improves soil stability, especially in the restoration and management of vegetation in arid or semiarid areas. Selecting tree species with higher trunk sap flow rates for planting can effectively maintain the water cycle in fragile ecosystems and enhance the efficiency of water resource utilization.
4.3. Comparison of Water Use Strategies Among Tree Species
Two evergreens (Diospyros dumetorum and Acacia auriculiformis) and deciduous tree species (Albizia kalkora and Terminalia franchetii) were selected to study trunk sap flow density and transpiration variables. Terminalia franchetii was the strongest water conductor, followed by Albizia kalkora, with Acacia auriculiformis and Diospyros dumetorum being the weakest. This may be because deciduous tree species reduce transpiration in the dry season by shedding their leaves, retaining more water for conductivity. Deciduous species generally have a stronger hydraulic system for water conduction, transmission, and drought resistance than evergreen species [55]. Canopy species supply water to their leaves for transpiration and photosynthesis by increasing the transpiration pull per unit cross-sectional area, offsetting the resistance to water transport due to tree height. Moreover, deciduous trees accumulate more energy during the growing season and have more stomata per leaf area [63], resulting in higher trunk sap flow density than in evergreen tree species. In the process of adapting to the natural environment, deciduous tree species develop ecophysiological behaviors suited for growth and drought resistance. In dry and hot valleys, where drought persists for nearly half the year, deciduous tree species have a greater survival advantage. Therefore, to restore ecosystems of the dry and wet seasons in the dry and hot valley and Savanna, considering the community ratios of deciduous and evergreen tree species and the vertical forest structure is crucial. A multi-level and multi-structured forest ensures that deciduous species can grow rapidly and provide biomass and shade during the wet season, while evergreen tree species maintain the ecological functions and stability of the ecosystem during the dry season. Integrating deciduous and evergreen tree species leverages their respective advantages, facilitating the restoration and management of ecosystems in areas with distinct dry and wet seasons and promoting long-term ecological health and stability.
4.4. Correlative Analysis Between Environmental Factors and Trunk Sap Flow
As an essential component of the water and energy balance, trunk sap flow reflects the water use strategy of plants and how environmental factors regulate plant water utilization. Trunk sap flow results from several environmental factors, with PAR, Ta, RH, vapor pressure deficit, and WS being the primary factors influences. In our study, the significant positive correlation between trunk sap flow rate and PAR indicates that light is the most crucial factor affecting sap flow [64,65]. Ta and VPD follow in importance, while RH shows a negative correlation because, in environments with low humidity, dryer air increases the transpiration pull, prompting plants to transport more water through the trunk to replenish water evaporated from leaves [57,58]. WS has the least effect but remains a non-negligible factor in arid environments [66]. Following wet season rainfall, the soil moisture is replenished, making plant transpiration more responsive to environmental factors. Xylem hydraulic conductivity increases, and stomata become more sensitive to solar radiation. Conversely, during the dry season, insufficient soil moisture causes leaf stomata to close, leading to a decrease in their responsiveness to solar radiation. In our results, regardless of the season, the trunk sap flow in typical tree species of the dry and hot valleys generally precedes changes in VPD, Ta, and RH by 60 to 120 min, indicating a time lag effect and environmental factors. Environmental factors significantly drive trunk sap flow, suggesting that when studying the response of physiological parameters, including canopy stomatal conductance and transpiration, care needs to be taken to consider the time lag effects to avoid large errors [7,67]. However, the time lag between trunk sap flow and environmental factors varies slightly among tree species, which may be due to the differences in canopy and xylem structure and root water absorption capacity [68]. Further in-depth studies are needed on these time lag effects in dry and hot valley species. Additionally, topographic factors such as slope create significant light stress and regional differences between shady and sunny slopes. These variations affect how tree species respond to environmental factors. Future studies on the interaction between trunk sap flow and environmental factors should use methodologies such as geographic detectors to analyze topography effects and multi-regional and multi-angle interaction [69].
4.5. Differences in Drought Adaptation Between Native and Introduced Tree Species
The fragile ecological environment of arid regions in southwest China faces challenges such as serious soil erosion, low ground cover, and a single vegetation structure. These factors, along with significant differences in the vegetation of various watersheds, limit the recovery of vegetation. Most of the areas must also contend with high temperatures, drought, and intense light, raising the requirements for selecting land-suitable tree species. In the study, the mean trunk sap flow rates of Albizia kalkora, Diospyros dumetorum, and Terminalia franchetii were higher than those of Acacia auriculiformis in dry and wet seasons. Albizia kalkora, Diospyros dumetorum, and Terminalia franchetii, as the native species of southwest China, showed superior trunk sap flow differences than that of Acacia auriculiformis, which is native to Australia. The high sap flow rates of Albizia kalkora, Diospyros dumetorum, and Terminalia franchetii indicate their efficient water utilization and ability to maintain physiological activities under water-limited conditions. Terminalia franchetii and Albizia kalkora exhibit strong hydraulic conductivity, enabling them to transport water efficiently and maintain the water balance under drought conditions. The rainfall insensitivity of Diospyros dumetorum and Terminalia franchetii suggests they stabilize sap flow rates during dry seasons, coping well with long-term water deficits. In contrast, the rapid response of Albizia kalkora to rainfall helps to restore water balance quickly in the short term. This difference in sensitivity to rainfall may be related to physiological or morphological traits, such as root depth and water storage capacity, and future research could focus on examining how these traits allow Albizia kalkora to respond rapidly to water availability. Native tree species can respond sensitively to changes in environmental factors, such as PAR and Ta, adjusting their sap flow rates to enhance survivability in variable environments [70]. These traits enable native tree species to thrive in arid environments and provide a valuable basis for selecting tree species for ecosystem restoration and management in arid zones. The order of the hydraulic capacity was as follows: Terminalia franchetii > Albizia kalkora > Acacia auriculiformis > Diospyros dumetorum, indicating Acacia auriculiformis had a better hydraulic capacity of the tree hydraulic system. Overall, the drought resistance of native tree species in the dry and hot valleys was superior to that of the introduced Acacia auriculiformis. However, Acacia auriculiformis still showed good water conductivity, drought resistance, and water retention, even though its adaptive capacity to the dry and hot valley was lower than that of the native tree species.
In future vegetation restoration efforts, beyond cultivating and applying native tree species, introduced species with specific advantages should be considered. Planting a mix of Acacia auriculiformis and native tree species can enable dynamic and diversified vegetation restoration strategies. In addition, utilizing water conservation measures such as mulching and soil improvement can enhance the water retention capacity of the soil and provide a stable water supply for all vegetation. Evaluating the relationship between the dynamic traits and environmental factors and analyzing drought resistance performance in the dry and hot valleys will provide a scientific basis for selecting regional vegetation. This approach is significant for coping with increased drought frequency that may be exacerbated by global warming and for offering a diverse selection of species suitable for extreme climate regions, thereby enhancing the adaptive capacity of ecosystems in seasonal arid zones.
The scarcity of vegetation in the valley has resulted in fewer consistent plant samples within areas affected by identical environmental factors. Future studies should combine remote sensing technology with ground observations to gather comprehensive spatial information, optimize the layout of sample sites, and improve collection strategies. This will address the challenges of vegetation scarcity and the limited number of samples, facilitating more in-depth and comprehensive progress in restoring the dry and hot valley and similar savanna ecosystems.
4.6. Limitations and Future Directions
Despite the valuable insights gained from this study, there are several limitations that should be acknowledged. While we aimed to select representative species from the dry and hot valley region, a larger sample size would provide a more robust and comprehensive understanding of interspecific differences in trunk sap flow and water use strategies. The scarcity of vegetation in the study area, due to the harsh environmental conditions, constrained our ability to gather more consistent and varied plant samples across different ecological zones. Future research should aim to increase the sample size by expanding the number of individuals per species and including additional species to strengthen the conclusions drawn.
Secondly, while environmental factors such as photosynthetically active radiation (PAR), temperature (Ta), relative humidity (RH), wind speed (WS), and vapor pressure deficit (VPD) were extensively measured and analyzed in this study, other potential environmental variables, such as soil moisture and root-zone conditions, were not directly monitored. These factors may influence trunk sap flow dynamics and integrating them into future studies would provide a more holistic view of the ecological processes at play. Additionally, investigating the time lag effects between environmental changes and sap flow responses across different species could further enhance our understanding of water use strategies in relation to environmental cues.
5. Conclusions
The results of the study show that: (1) there were significant seasonal variations in trunk sap flow rates, with each tree species exhibiting significantly higher sap flow in the wet season compared to the dry season. (2) In both dry and wet seasons, the average trunk sap flow rates followed the order: Albizia kalkora > Diospyros dumetorum > Terminalia franchetii > Acacia auriculiformis. (3) The correlation between environmental factors and trunk sap flow was ranked as follows: photosynthetically active radiation > atmospheric temperature > saturated water vapor pressure difference > relative humidity > wind speed. (4) Deciduous plants demonstrated stronger water-conducting capacities than evergreen plants, with tree species having longer defoliation periods showing greater water-conducting capacity. The overall drought resistance of native tree species in the dry and hot valley environment was better than that of introduced tree species. However, the introduced species Acacia auriculiformis exhibited better water-conducting ability and still shows good adaptability in the dry and hot valley. (5) Acacia auriculiformis and Albizia kalkora were identified as rainfall-sensitive plants, while Diospyros dumetorum and Terminalia franchetii were rainfall-insensitive. These findings contribute a valuable scientific understanding of plant water balance mechanisms in challenging environmental conditions. The results provide practical insights for tree species selection in dry and hot valley and savanna ecosystems, offering potential guidance for ecological management and restoration strategies in similar climatic contexts.
Author Contributions
Conceptualization, L.P. and Y.S.; methodology, L.P. and Y.S.; software, L.P.; validation, Y.S. and Z.O.; formal analysis, L.P.; investigation, L.P., Z.H. and Z.L.; resources, Y.S.; data curation, L.P. and S.Z.; writing—original draft preparation, L.P.; writing—review and editing, L.P., Y.S. and Z.O.; visualization, L.P.; supervision, X.L.; project administration, Y.S.; funding acquisition, Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation Project of China, “The Mechanism and Prevention Measures of Soil Water Erosion of Sloping Farmland in Alpine Canyon Areas of Yunnan Province” (Grant No. U24A20581), and the project topic of China’s National Key Research and Development Program, “Experimental demonstration of comprehensive ecological management and agro-industrial development technology in the dam area of the Jinsha River Dry and Hot Valley” (Grant No. 2017YFC0505102), and “National Forestry and Grassland Administration Forestry Ecological Station Monitoring and Operation Project” (Grant No. 2024132133). As well as “Yunnan Provincial Natural Ecological Monitoring Network Project” (Grant No. 2024-YN-07).
Data Availability Statement
The dry and hot valley extent, county maps, digital elevation model data, and river system vector data were obtained from the Ministry of Natural Resources of the People’s Republic of China (https://www.mnr.gov.cn/sj/, accessed on 26 June 2024), Landsat series satellite data from Geospatial Data Cloud Platform (https://www.gscloud.cn, accessed on 26 June 2024), and OpenStreetMap (www.openstreetmap.org, accessed on 27 June 2024). Correlation heat map analysis was conducted using the ChiPlot platform (https://www.chiplot.online, accessed on 29 June 2024).
Acknowledgments
The authors would like to thank Yongyu Sun for his careful guidance and assistance during the design and implementation of the experiment and the writing of the paper, and Zhaorong Ou for her guidance and valuable comments during the writing of the paper. We are grateful to the Yuanmou Desert Ecosystem Research Station and the National Long-Term Scientific Research Base of Comprehensive Control for providing the experimental site.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fassio, C.; Heath, R.; Arpaia, M.L.; Castro, M. Sap flow in ’Hass’ avocado trees on two clonal rootstocks in relation to xylem anatomy. Sci. Hortic. 2009, 120, 8–13. [Google Scholar] [CrossRef]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M.; Hacke, U.G. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Y.; Xu, X.; Zhang, J. Study on the dynamics of stem sap flow in minqin wind and sand control haloxylon Ammodendron forest, China. Sustainability 2023, 15, 609. [Google Scholar] [CrossRef]
- Gansert, D. Xylem sap flow as a major pathway for oxygen supply to the sapwood of birch (Betula pubescens Ehr.). Plant Cell Environ. 2003, 26, 1803–1814. [Google Scholar] [CrossRef]
- Etzold, S.; Zweifel, R.; Ruehr, N.K.; Eugster, W.; Buchmann, N. Long-term stem CO2 concentration measurements in Norway spruce in relation to biotic and abiotic factors. New Phytol. 2013, 197, 1173–1184. [Google Scholar] [CrossRef]
- Kluitenberg, G.J.; Ham, J.M. Improved theory for calculating sap flow with the heat pulse method. Agric. For. Meteorol. 2004, 126, 169–173. [Google Scholar] [CrossRef]
- Bowman, W.P.; Barbour, M.M.; Turnbull, M.H.; Tissue, D.T.; Whitehead, D.; Griffin, K.L. Sap flow rates and sapwood density are critical factors in within-and between-tree variation in CO2 efflux from stems of mature Dacrydium cupressinum trees. New Phytol. 2005, 167, 815–828. [Google Scholar] [CrossRef]
- Clearwater, M.J.; Luo, Z.; Mazzeo, M.; Dichio, B. An external heat pulse method for measurement of sap flow through fruit pedicels, leaf petioles and other small-diameter stems. Plant Cell Environ. 2009, 32, 1652–1663. [Google Scholar] [CrossRef]
- Chabot, R.; Bouarfa, S.; Zimmer, D.; Chaumont, C.; Moreau, S. Evaluation of the sap flow determined with a heat balance method to measure the transpiration of a sugarcane canopy. Agric. Water Manag. 2005, 75, 10–24. [Google Scholar] [CrossRef]
- Poyatos, R.; Granda, V.; Molowny, R.; Mencuccini, M.; Steppe, K.; Martínez, J. Sapfluxnet: Towards a global database of sap flow measurements. Tree Physiol. 2016, 36, 1449–1455. [Google Scholar] [CrossRef]
- Patakas, A.; Noitsakis, B.; Chouzouri, A. Optimization of irrigation water use in grapevines using the relationship between transpiration and plant water status. Agric. Ecosyst. Environ. 2005, 106, 253–259. [Google Scholar] [CrossRef]
- Steppe, K.; Vandegehuchte, M.W.; Tognetti, R.; Mencuccini, M. Sap flow as a key trait in the understanding of plant hydraulic functioning. Tree Physiol. 2015, 35, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zheng, Y.; Zhang, C. Tree Introduction and Domestication for Vegetation Restoration at Harsh Site Conditions; China Forestry Press: Beijing, China, 2016; pp. 1–33. [Google Scholar]
- Xue, J.; Gui, D.; Lei, J.; Sun, H.; Zeng, F.; Mao, D.; Jin, Q.; Liu, Y. Oasification: An unable evasive process in fighting against desertification for the sustainable development of arid and semiarid regions of China. Catena 2019, 179, 197–209. [Google Scholar] [CrossRef]
- The Comprehensive Scientific Expedition to The Qinghai–Tibetan Plateau, Chinese Academy of Sciences. The Dry Valleys of the Hengduan Mountains Region; Science Publishing House: Beijing, China, 1992; pp. 7–75. [Google Scholar]
- Zhong, X. Degradation of ecosystem and ways of its rehabilitation and reconstruction in dry and hot valley-Take representative area of Jinsha River, Yunnan Province as an example. Resour. Environ. Yangtze Basin 2000, 9, 376–383. [Google Scholar] [CrossRef]
- Fan, Z.; Bräuning, A.; Thomas, A.; Li, J.; Cao, K. Spatial and temporal temperature trends on the Yunnan Plateau (Southwest China) during 1961–2004. Int. J. Climatol. 2011, 31, 2078–2090. [Google Scholar] [CrossRef]
- Zong, H.; Sun, J.; Zhou, L.; Bao, F.; Zheng, X. Effect of altitude and climatic parameters on shrub-meadow community composition and diversity in the dry valley region of the eastern Hengduan Mountains. China. J. Mt. Sci. 2022, 19, 1139–1155. [Google Scholar] [CrossRef]
- Dong, Y.; Xiong, D.; Li, J.; Yang, D.; Shi, L.; Liu, G. The distribution of and factors influencing the vegetation in a gully in the Dry-hot Valley of southwest China. Catena 2014, 116, 60–67. [Google Scholar] [CrossRef]
- Xiong, D.; Zhou, H.; Yang, Z.; Zhang, X. Slope lithologic property, soil moisture condition and revegetation in dry-hot valley of Jinsha River. Chin. Geogr. Sci. 2005, 15, 186–192. [Google Scholar] [CrossRef]
- Zheng, J.; Feng, W.; Wang, F.; Yuan, D.; Gong, X.; Huang, Y. Spatial definition and its range variation of arid valley in the upper reaches of Minjiang River. Arid Land Geogr. 2017, 40, 541–548. [Google Scholar] [CrossRef]
- Li, K.; Zhang, M.; Li, Y.; Xing, X.; Fan, S.; Cao, Y.; Dong, L.; Chen, D. Karren habitat as the key in influencing plant distribution and species diversity in Shilin Geopark, southwest China. Sustainability 2020, 12, 5808. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, X.; Li, Z.; Xu, C.; Luo, W. Improvements in soil quality with vegetation succession in subtropical China karst. Sci. Total Environ. 2021, 775, 145876. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, Z.; Hu, J.; Han, J.; Yang, J.; Ying, L. Protection and utilization of plant biodiversity resources in dry valleys of Southwest China. Biodivers. Sci. 2016, 24, 475. [Google Scholar] [CrossRef]
- Duan, A. Characteristics of Water Consumption Through Transpiration and Evaluation of Adaptability Mechanism of Main Trees for Vegetation Restoration in the Dry-Hot River Valley. Ph.D. Thesis, Chinese Academy of Forestry Sciences, Beijing, China, 2008. [Google Scholar]
- Peng, L.; He, Z.; Luo, Z.; Ou, Z.; Sun, Y. The photosynthetic and water physiological characteristics of wild Diospyros dumetorum in Southwest China. J. Northeast Univ. 2024, 6, 33–42. [Google Scholar] [CrossRef]
- Duan, A.; Zhang, J.; Zhang, S.; Zhang, J.; Wang, J.; He, C.; Li, Y. Transpiration of tree species for vegetation restoration in dry-hot river valleys. Acta Ecol. Sin. 2009, 29, 6691–6701. [Google Scholar] [CrossRef]
- Moustakas, A.; Sakkos, K.; Wiegand, K.; Ward, D.; Meyer, K.M.; Eisinger, D. Are savannas patch-dynamic systems? a landscape model. Ecol. Model. 2009, 220, 3576–3588. [Google Scholar] [CrossRef]
- Julier, A.C.; Jardine, P.E.; Adu-Bredu, S.; Coe, A.L.; Duah-Gyamfi, A.; Fraser, W.T.; Lomax, B.H.; Malhi, Y.; Moore, S.; Owusu-Afriyie, K. The modern pollen–vegetation relationships of a tropical forest–savannah mosaic landscape, Ghana, West Africa. Palynology 2018, 42, 324–338. [Google Scholar] [CrossRef]
- Muumbe, T.P.; Baade, J.; Singh, J.; Schmullius, C.; Thau, C. Terrestrial laser scanning for vegetation analyses with a special focus on savannas. Remote Sens. 2021, 13, 507. [Google Scholar] [CrossRef]
- Lewis, K.; Barros, F.D.V.; Moonlight, P.W.; Hill, T.C.; Oliveira, R.S.; Schmidt, I.B.; Sampaio, A.B.; Pennington, R.T.; Rowland, L. Identifying hotspots for ecosystem restoration across heterogeneous tropical savannah-dominated regions. Philos. Trans. Soc. B. 2023, 378, 20210075. [Google Scholar] [CrossRef] [PubMed]
- Poilecot, P.; Gaidet, N. A quantitative study of the grass and woody layers of a Mopane (Colophospermum mopane) savannah in the mid-Zambezi Valley, Zimbabwe. Afr. J. Ecol. 2011, 49, 150–164. [Google Scholar] [CrossRef]
- Zhu, H.; Tan, Y.; Yan, L.; Liu, F. Flora of the savanna-like vegetation in hot dry valleys, southwestern China with implications to their origin and evolution. Bot. Review 2020, 86, 281–297. [Google Scholar] [CrossRef]
- Li, K.; Zeng, J. A study on transpiration of some tree species planted in hot and arid valley of Jinsha River. Forest Res. 1999, 12, 244. [Google Scholar] [CrossRef]
- Fan, J.; Yang, C.; Bao, W.; Liu, J.; Li, X. Distribution scope and district statistical analysis of dry valleys in southwest China. Mt Res. 2020, 38, 303–313. [Google Scholar] [CrossRef]
- Ma, H. Silviculture in Dry-Hot Valley; Yunnan Science and Technology Press: Kunming, China, 2001; pp. 2–16. [Google Scholar]
- Duan, A.; Zhang, J.G.; Zhang, J.P.; He, C. Dynamics of water-use efficiency of tree species for vegetation restoration in dry-hot river valleys. J. Beijing For. Univ. 2010, 32, 13–19. [Google Scholar] [CrossRef]
- Liu, F.; Li, K.; Chen, M. Primary Color Atlas of Typical Plants in the Dry and Hot Valley of the Jinsha River; Science Publishing House: Beijing, China, 2016; pp. 35–65. [Google Scholar]
- Song, L.; Zhu, J.; Zhang, J.; Wang, K.; Lü, L.; Wang, F.; Wang, G. Divergent growth responses to warming and drying climates between native and non-native tree species in Northeast China. Trees 2019, 33, 1143–1155. [Google Scholar] [CrossRef]
- Gao, C.; Li, K.; Tang, G.; Zhang, C.; Li, B. Nutrient accumulation and cycling in pure and mixed plantations of Azadirachta indica and Acacia auriculiformis in a dry-hot valley, Yunnan Province, southwest China. J. Appl. Ecol. 2014, 25, 1889–1897. [Google Scholar] [CrossRef]
- Liu, F.Y.; Wang, X.Q.; Chen, M. Flowering phenology and breeding system of Terminalia franchetii (Combretaceae) in the dry-hot valley of the Jinsha River. China. Acta Ecol. Sin. 2015, 35, 7043–7051. [Google Scholar] [CrossRef]
- Granier, A. Une nouvelle méthode pour la mesure du flux de sève brute dans le tronc des arbres. Ann Sci For. 1985, 42, 193–200. [Google Scholar] [CrossRef]
- Granier, A. Evaluation of transpiration in a Douglas-fir stand by means of sap flow measurements. Tree Physiol. 1987, 3, 309–320. [Google Scholar] [CrossRef]
- Fuchs, S.; Leuschner, C.; Link, R.; Coners, H.; Schuldt, B. Calibration and comparison of thermal dissipation, heat ratio and heat field deformation sap flow probes for diffuse-porous trees. Agric. Meteorol. 2017, 244, 151–161. [Google Scholar] [CrossRef]
- Dix, M.J.; Aubrey, D.P. Calibration approach and range of observed sap flow influences transpiration estimates from thermal dissipation sensors. Agric. Meteorol. 2021, 307, 108534. [Google Scholar] [CrossRef]
- Chang, L.; Liu, M.; Lyu, J.; Sheng, D. Characteristics of soil moisture limitation and non-limitation in the response of sap flow to transpiration driving factors. J. Appl. Ecol. 2024, 35, 1064–1072. [Google Scholar] [CrossRef]
- Campbell, G.; Norman, J. An introduction to environmental physics. Biol. Plant. 1977, 21, 104. [Google Scholar] [CrossRef]
- Wu, F.; Chen, Y.; Yu, Z. Growing season sap-flow dynamics of Robinia pseudoacacia plantation in the semi-arid region of Loess Plateau, China. Chin J Plant Ecol. 2010, 4, 469–476. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Zhao, P.; Zheng, H.; Ren, Y.; Gao, F.; Ouyang, Z. Transpiration rates of urban trees, Aesculus chinensis. J. Environ. Sci. 2012, 24, 1278–1287. [Google Scholar] [CrossRef]
- Huang, Y.; Wei, W.; Chen, S. Sap flow characteristics of Platycladus orientalis and Caragana korshinskii and its response to environmental factors in the loess plateau. Ecol. Environ. 2024, 33, 389. [Google Scholar] [CrossRef]
- Lei, H.; Zhi, Z.; Xin, L. Sap flow of Artemisia ordosica and the influence of environmental factors in a revegetated desert area: Tengger Desert, China. Hydrol. Process. Int. J. 2010, 24, 1248–1253. [Google Scholar] [CrossRef]
- Xu, T.; Niu, X.; Wang, B.; Song, Q.; Wang, N.; Sun, J.; Liu, R. Responses of sap flow characteristics under different Chinese fir provenances to meteorological factors under different soil moisture conditions. Sci. Soil Water Conserv. 2023, 5, 99–105. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, J.; Jia, G.; Lei, Z.; Zhang, L.; Liu, R.; Lu, X.; Dai, Y. Effects of precipitation variations on characteristics of sap flow and water source of Platycladus orientalis. Chin. J. Plant Ecol. 2023, 47, 1585–1599. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, J.; Liao, T.; Wang, Y.; Guo, L.; Yao, Y.; Cao, J. Histological dissection of cutting-inducible adventitious rooting in Platycladus orientalis reveals developmental endogenous hormonal homeostasis. Ind. Crop. Prod. 2021, 170, 113817. [Google Scholar] [CrossRef]
- Choat, B.; Ball, M.C.; Luly, J.G.; Holtum, J.A.M. Hydraulic architecture of deciduous and evergreen dry rainforest tree species from north-eastern Australia. Trees. 2005, 19, 305–311. [Google Scholar] [CrossRef]
- Nakai, T.; Abe, H.; Muramoto, T.; Nakao, T. The relationship between sap flow rate and diurnal change of tangential strain on inner bark in Cryptomeria japonica saplings. J. Wood Sci. 2005, 51, 441–447. [Google Scholar] [CrossRef]
- Liu, F.; You, Q.; Xue, X.; Zhang, L. Stem sap flow variation of Tamarix ramosissima in oasis-desert ecotone and its response to environmental factors. J. Arid Land Res. Environ. 2024, 3, 112–122. [Google Scholar] [CrossRef]
- Ma, J.; Chen, Y.; Li, W.; Huang, X.; Zhu, C.; Ma, X. Sap flow characteristics of four typical species in desert shelter forest and their responses to environmental factors. Environ. Earth Sci. 2012, 67, 151–160. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Y.; Li, K.; Zhang, C.; Li, B. Seasonal dynamics of Albizia kalkora stem sap flow in Yunmou dry hot valley of Southwest China. Chin. J. Ecol. 2013, 32, 597. [Google Scholar] [CrossRef]
- Pataki, D.E.; Oren, R.; Smith, W.K. Sap flux of co-occurring species in a western subalpine forest during seasonal soil drought. Ecology. 2000, 81, 2557–2566. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Mangirón, M.; Ogaya, R.; Sauret, M.; Serrano, L.; Peñuelas, J.; Piñol, J. Sap flow of three co-occurring Mediterranean woody species under varying atmospheric and soil water conditions. Tree Physiol. 2003, 23, 747–758. [Google Scholar] [CrossRef]
- Wang, A.; Lu, Y.; Cui, H.; Liu, S.; Li, S.; Hao, G. Xylem hydraulics of two temperate tree species with contrasting growth rates. Plants 2024, 13, 3575. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Q.; Cao, K. Inter-species variation of photosynthetic and xylem hydraulic traits in the deciduous and evergreen Euphorbiaceae tree species from a seasonally tropical forest in south-western China. Ecol. Res. 2009, 24, 65–73. [Google Scholar] [CrossRef]
- Stöhr, A.; Lösch, R. Xylem sap flow and drought stress of Fraxinus excelsior saplings. Tree Physiol. 2004, 24, 169–180. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Y.; Li, K.; Zhang, C.; Li, B. Stem sap flow characteristics of Acacia auriculaeformis in dry-hot valley and their relations to meteorological factors. For. Res. 2013, 26, 145–150. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, P.; Niu, J.; Ni, G.; Zhu, L.; Gao, J.; Zhao, X.; Zhang, Z.; Zhou, J. Seasonal variations of sap flow and transpiration water consumption of introduced tree species Acacia auriculaeformis and Eucalyptus citriodora. Chin. J. Ecol. 2014, 33, 2588. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Du, S. Research progress in the characteristics and driving factors of time lags in stem sap flow. Chin. J. Appl. Environ. Biol. 2023, 2, 507–514. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Y.; Li, K.; Zhang, C.; Li, B.; Hou, R. Time lag characteristics of stem sap flow of main afforestation tree species during their growth season in Yuanmou dry-hot valley of southwest China. Acta Agric Univ. Jiang. 2013, 35, 462–467. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, S.; Zhang, J.; Sun, Y.; Yang, X.; Ou, Z. Topographic effect of county-level land-use landscape pattern in the dry-hot valley of Jinsha River, Yunnan Province. Chin. J. Ecol. 2023, 42, 1982. [Google Scholar] [CrossRef]
- Lu, S.; Chen, Y.; Sardans, J.; Peñuelas, J. Water and nutrient use efficiency of three tree species in monoculture and mixed stands and potential drivers in the Loess Hilly Region, China. Plant Soil. 2024, 496, 657–675. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).