Sensitivity of First Leaf Date to Temperature Change for Typical Woody Plants in Guiyang, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Phenological and Temperature Data
2.3. Methods
3. Results
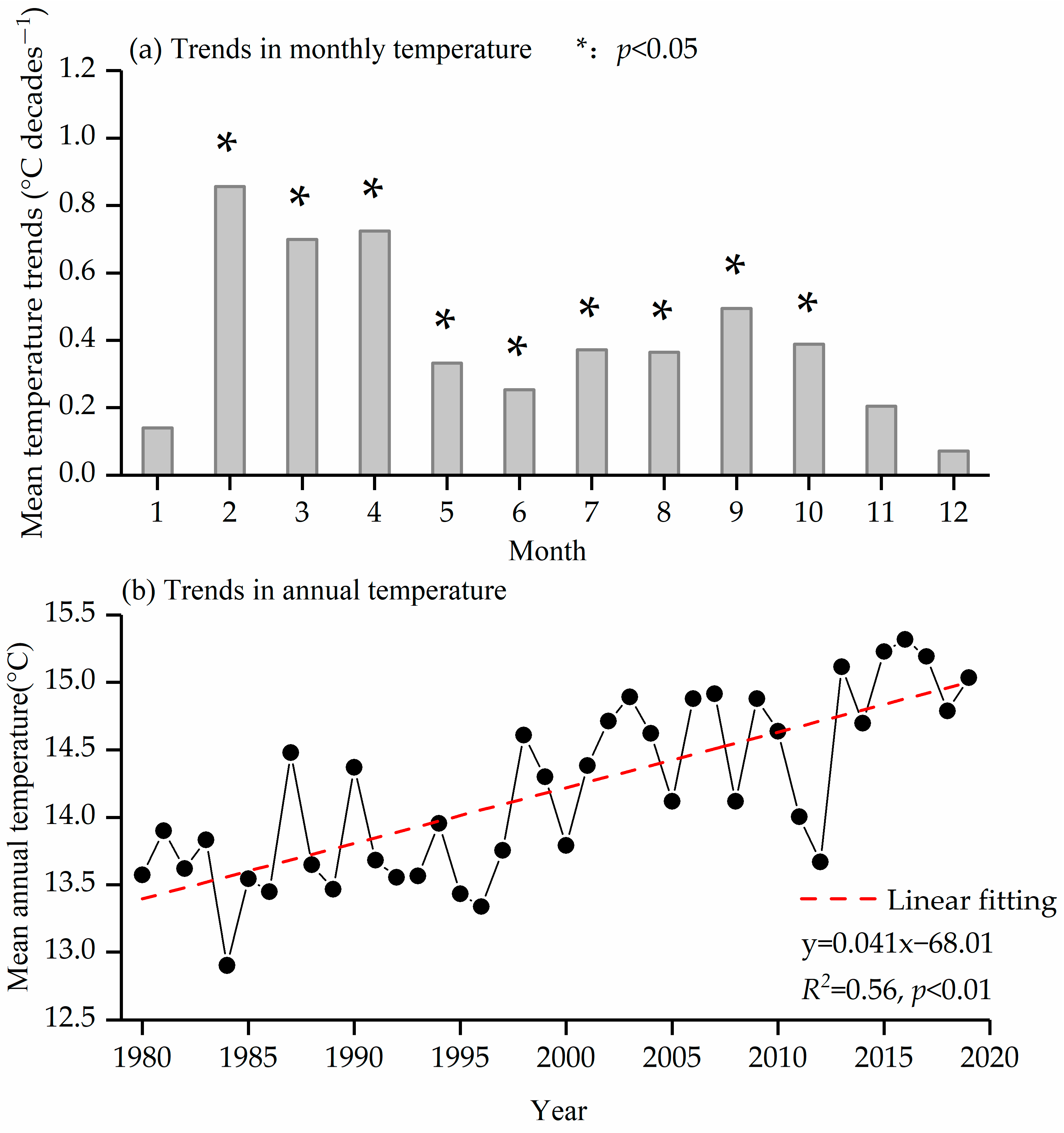
3.1. Temperature Trend
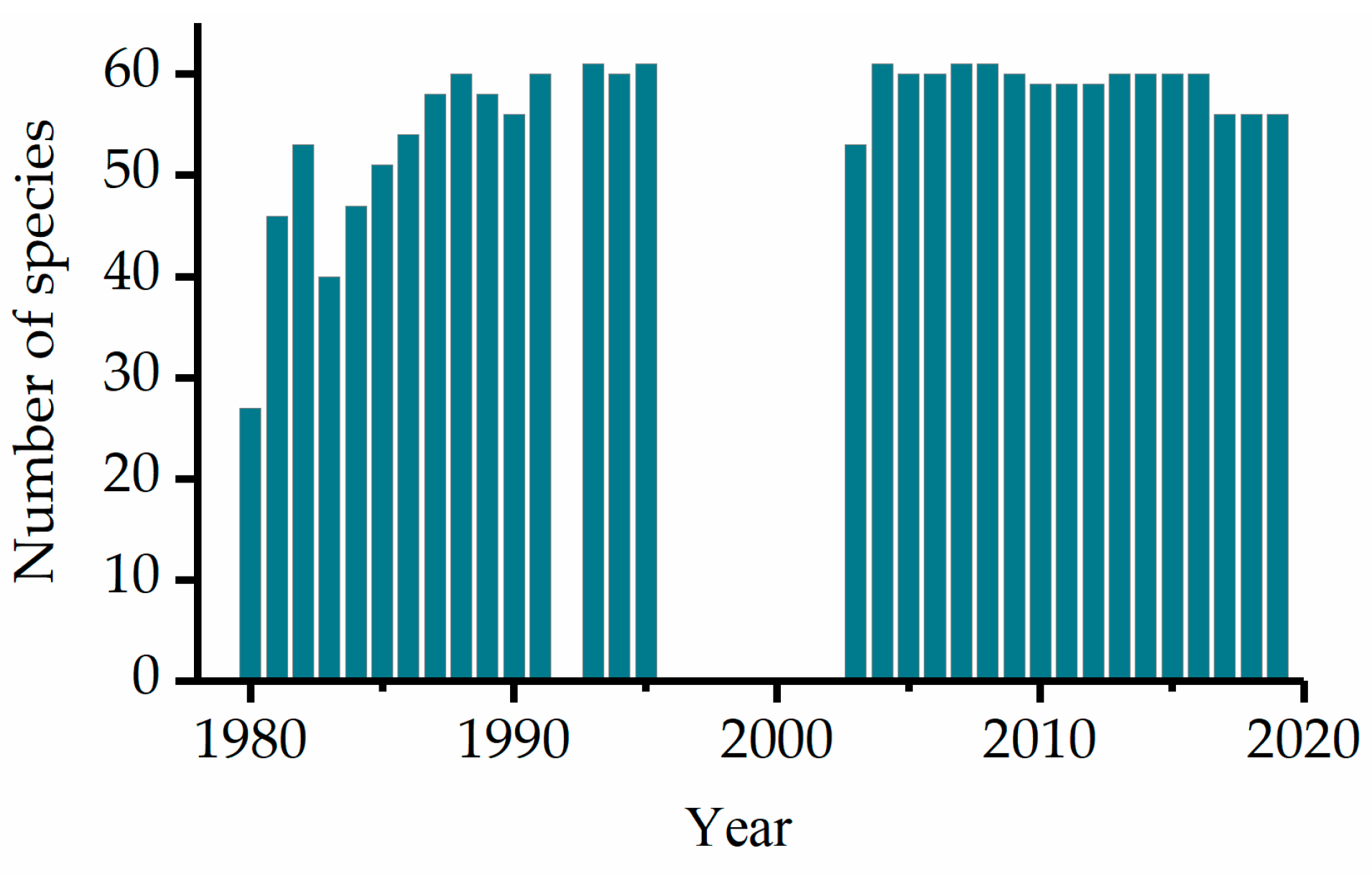
3.2. Leaf Unfolding Patterns and Changes Across Different Periods
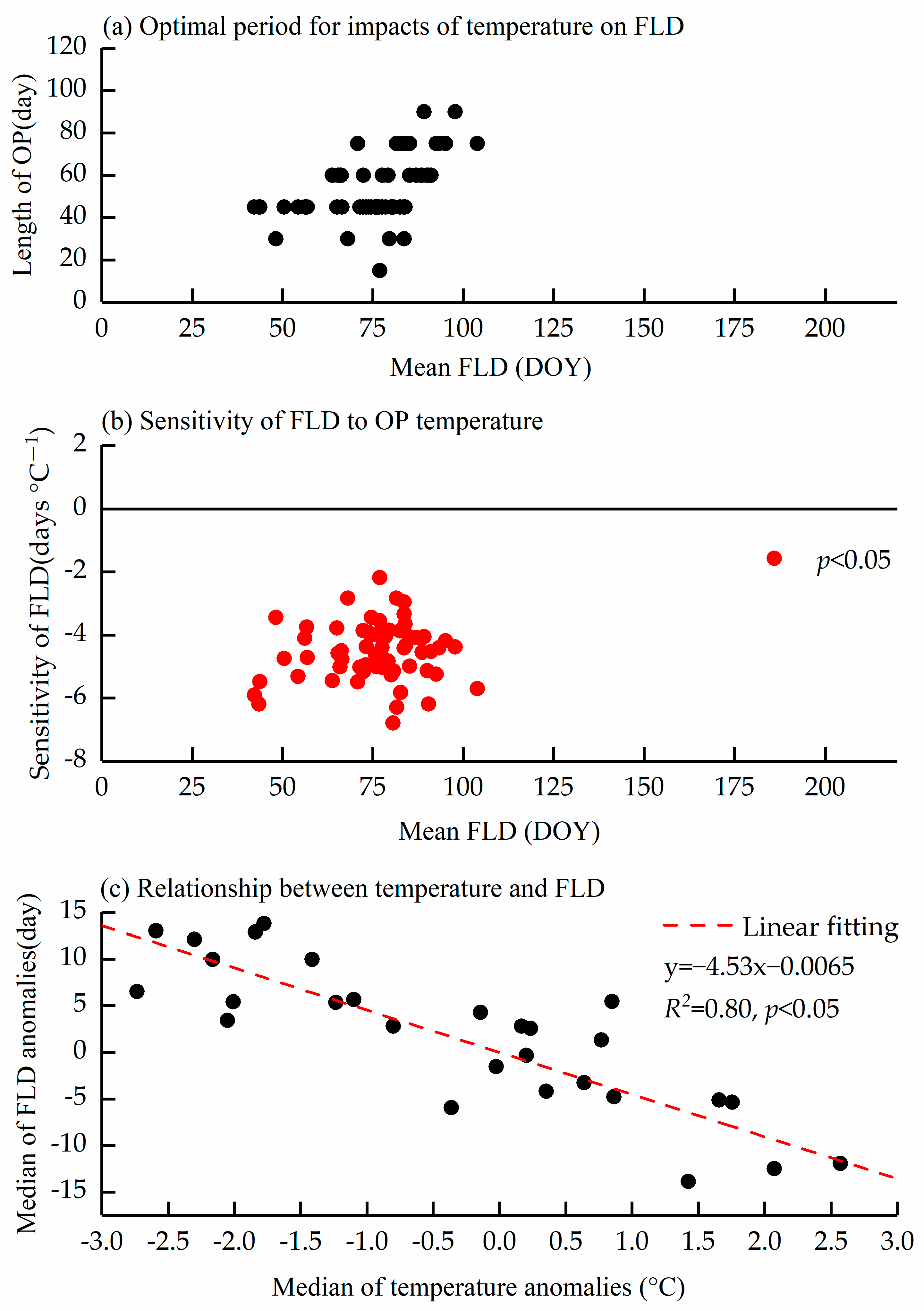
3.3. Relationship Between FLD and Temperature
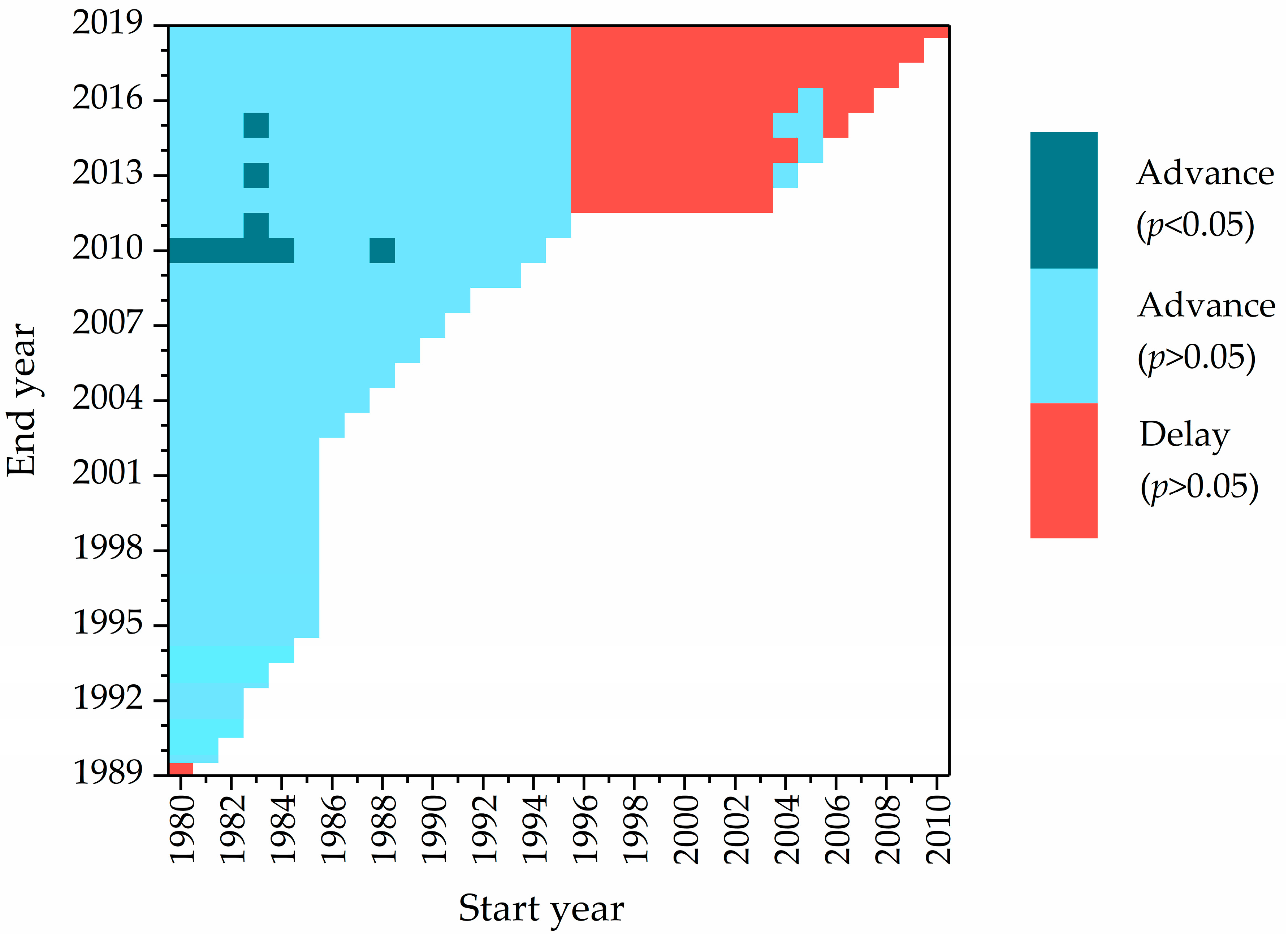
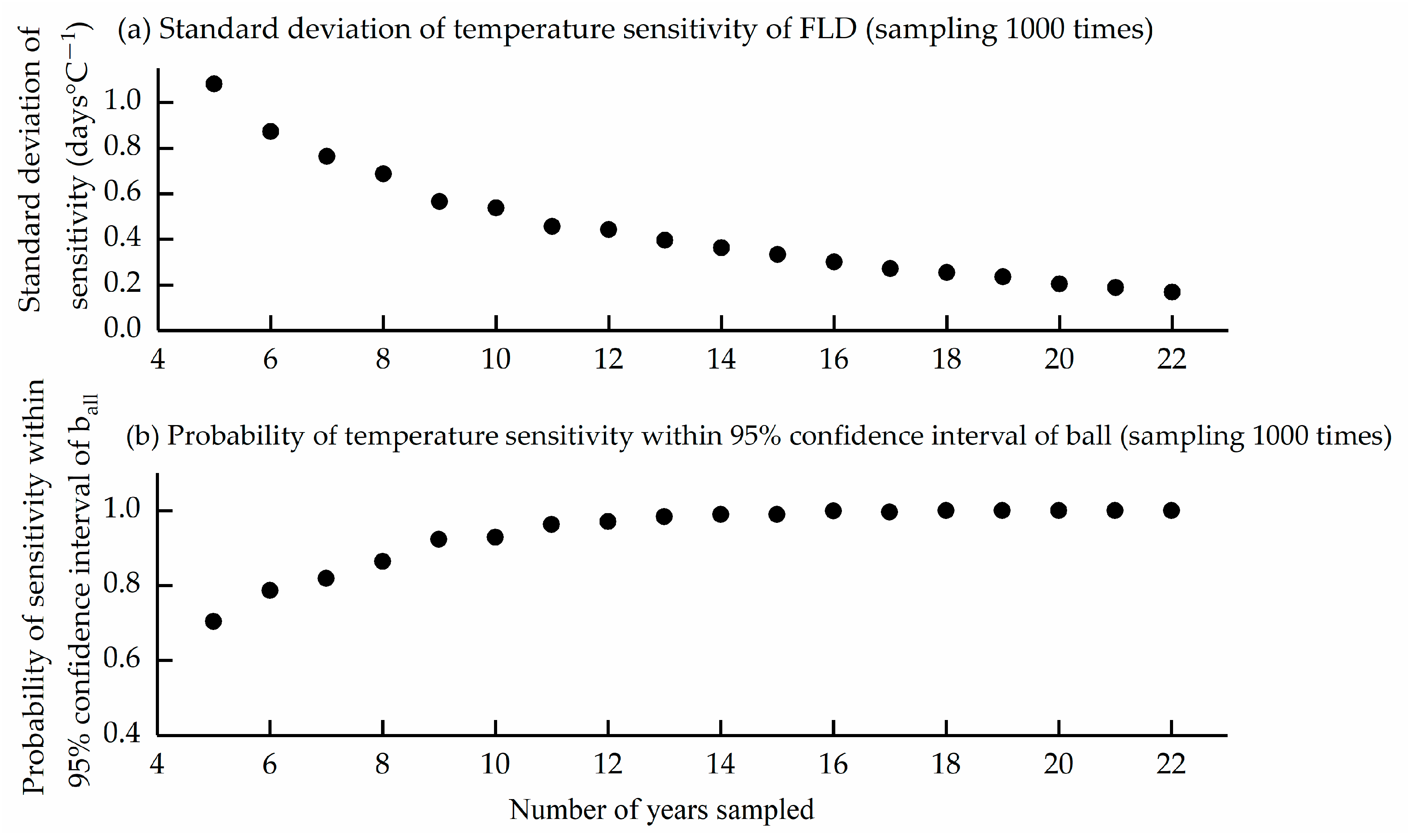
3.4. Impact of Sample Size on Sensitivity Estimation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Number | Species | Family | Number of Observation Years | Mean First Leaf Date | Trends (Days Decades−1) | Sensitivity (Days °C−1) |
|---|---|---|---|---|---|---|
| 1 | Chaenomeles cathayensis | Rosaceae | 26 | 11 February | −1.68 | −5.91 |
| 2 | Chaenomeles speciosa | Rosaceae | 28 | 12 February | −1.15 | −6.19 |
| 3 | Chaenomeles sinensis | Rosaceae | 32 | 12 February | −0.88 | −5.48 |
| 4 | Malus micromalus | Rosaceae | 23 | 17 February | −0.88 | −3.44 |
| 5 | Liriodendron chinense | Magnoliaceae | 23 | 17 February | 2.03 | −2.83 |
| 6 | Salix babylonica | Salicaceae | 30 | 19 February | −0.81 | −4.75 |
| 7 | Pyracantha fortuneana | Rosaceae | 27 | 23 February | −2.60 | −5.31 |
| 8 | Forsythia viridissima | Oleaceae | 28 | 25 February | −2.68 | −4.11 |
| 9 | Zanthoxylum simulans | Rutaceae | 26 | 25 February | −0.14 | −3.74 |
| 10 | Eriobotrya japonica | Rosaceae | 25 | 25 February | −3.49 | −4.72 |
| 11 | Swida paucinervis | Cornaceae | 31 | 3 March | −1.86 | −5.45 |
| 12 | Armeniaca vulgaris | Rosaceae | 30 | 5 March | −1.61 | −5.01 |
| 13 | Distylium dunnianum | Hamamelidaceae | 26 | 5 March | −3.43 | −3.78 |
| 14 | Ilex fargesiivar.angustifolia | Aquifoliaceae | 26 | 5 March | −2.86 | −4.58 |
| 15 | Cerasus subhirtella | Rosaceae | 30 | 6 March | −2.28 | −4.50 |
| 16 | Acanthopanax trifoliatus | Araliaceae | 29 | 6 March | −4.64 | −4.78 |
| 17 | Pterocarya stenoptera | Juglandaceae | 32 | 8 March | −1.50 | −2.83 |
| 18 | Prunus salicina | Rosaceae | 21 | 10 March | −2.18 | −5.49 |
| 19 | Caesalpinia decapetala | Leguminosae | 29 | 11 March | −4.53 | −5.02 |
| 20 | Cercis chinensis | Leguminosae | 31 | 12 March | −1.70 | −3.86 |
| 21 | Camellia oleifera | Theaceae | 30 | 12 March | −3.32 | −5.17 |
| 22 | Pyrus pyrifolia | Rosaceae | 21 | 13 March | −6.50 | −4.37 |
| 23 | Fraxinus chinensis | Oleaceae | 30 | 13 March | −2.49 | −3.92 |
| 24 | Hibiscus syriacus | Malvaceae | 27 | 13 March | −3.87 | −4.95 |
| 25 | Ulmus pumila | Ulmaceae | 30 | 14 March | −0.77 | −3.44 |
| 26 | Populus yunnanensis | Salicaceae | 29 | 15 March | −2.98 | −3.99 |
| 27 | Cedrus deodara | Pinaceae | 31 | 15 March | −4.08 | −4.62 |
| 28 | Populus adenopoda | Salicaceae | 25 | 16 March | −1.58 | −3.55 |
| 29 | Nerium indicum | Apocynaceae | 26 | 16 March | −1.06 | −5.01 |
| 30 | Serissa japonica | Rubiaceae | 27 | 16 March | −1.13 | −4.69 |
| 31 | Prunus persica | Rosaceae | 24 | 16 March | −0.06 | −2.18 |
| 32 | Salix chaenomeloides | Salicaceae | 30 | 17 March | −3.18 | −4.40 |
| 33 | Quercus acutissima | Fagaceae | 30 | 17 March | −0.51 | −3.83 |
| 34 | Cinnamomum camphora | Lauraceae | 29 | 17 March | −3.11 | −5.03 |
| 35 | Metasequoia glyptostroboides | Cupressaceae | 32 | 18 March | −2.98 | −4.04 |
| 36 | Cinnamomum bodinieri | Lauraceae | 32 | 19 March | −2.53 | −3.85 |
| 37 | Pistacia chinensis | Anacardiaceae | 27 | 19 March | −0.05 | −4.83 |
| 38 | Camptotheca acuminata | Nyssaceae | 32 | 20 March | −1.86 | −5.14 |
| 39 | Hibiscus mutabilis | Malvaceae | 29 | 20 March | −5.74 | −6.79 |
| 40 | Celtis sinensis | Cannabaceae | 31 | 20 March | −3.18 | −5.26 |
| 41 | Catalpa bungei | Bignoniaceae | 31 | 21 March | −2.88 | −6.29 |
| 42 | Lindera communis | Lauraceae | 31 | 22 March | −2.21 | −3.87 |
| 43 | Punica granatum | Punicaceae | 32 | 22 March | −2.66 | −5.83 |
| 44 | Platanus acerifolia | Platanaceae | 16 | 23 March | −2.85 | −3.34 |
| 45 | Robinia pseudoacacia | Leguminosae | 32 | 23 March | −1.55 | −4.40 |
| 46 | Gleditsia sinensis | Leguminosae | 31 | 23 March | −0.72 | −3.65 |
| 47 | Alangium chinense | Alangiaceae | 27 | 23 March | −1.53 | −3.82 |
| 48 | Ginkgo biloba | Ginkgoaceae | 30 | 23 March | −0.98 | −2.95 |
| 49 | Juniperus chinensis | Cupressaceae | 26 | 24 March | 1.55 | −4.33 |
| 50 | Cerasus yedoensis | Rosaceae | 31 | 25 March | −1.01 | −4.06 |
| 51 | Broussonetia papyifera | Moraceae | 31 | 25 March | −0.95 | −4.99 |
| 52 | Clerodendrum trichotomum | Verbenaceae | 32 | 27 March | −1.89 | −4.09 |
| 53 | Wisteria sinensis | Leguminosae | 26 | 28 March | −2.36 | −4.55 |
| 54 | Ligustrum lucidum | Oleaceae | 31 | 28 March | −0.27 | −4.11 |
| 55 | Sophora japonica | Leguminosae | 31 | 29 March | 0.96 | −4.05 |
| 56 | Melia azedarach | Meliaceae | 21 | 30 March | −5.11 | −6.19 |
| 57 | Hovenia acerba | Rhamnaceae | 25 | 30 March | −4.09 | −5.13 |
| 58 | Lagerstroemia indica | Lythraceae | 31 | 31 March | −2.02 | −4.52 |
| 59 | Sapium sebiferum | Euphorbiaceae | 29 | 1 April | −2.29 | −5.25 |
| 60 | Catalpa ovata | Bignoniaceae | 31 | 2 April | −1.33 | −4.41 |
| 61 | Firmiana simples | Sterculiaceae | 32 | 4 April | −1.69 | −4.19 |
| 62 | Ziziphusjujuba | Rhamnaceae | 31 | 6 April | −0.42 | −4.38 |
| 63 | Pinus massoniana | Pinaceae | 30 | 12 April | −4.11 | −5.70 |
Appendix B
References
- Lambert, F. Why the timing of climate assessments matters. Nature 2024, 634, 9. [Google Scholar] [CrossRef] [PubMed]
- Masson-Delmotte, V.P.; Zhai, A.; Pirani, S.L.; Connors, C.; Péan, S.; Berger, N.; Caud, Y.; Chen, L.; Goldfarb, M.I.; Gomis, M.; et al. (Eds.) IPCC, 2021: Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2023. [Google Scholar] [CrossRef]
- Menzel, A.; Yuan, Y.; Matiu, M.; Sparks, T.; Scheifinger, H. Climate change fingerprints in recent European plant phenology. Glob. Change Biol. 2020, 26, 2599–2612. [Google Scholar] [CrossRef]
- Piao, S.; Wang, X.; Park, T.; Chen, C.; Lian, X. Characteristics, drivers and feedbacks of global greening. Nat. Rev. Earth Environ. 2019, 1, 14–27. [Google Scholar] [CrossRef]
- Fu, Y.H.; Piao, S.; Vitasse, Y.; Zhao, H. Increased heat requirement for leaf flushing delays spring phenology. Glob. Chang. Biol. 2015, 21, 2687–2697. [Google Scholar] [CrossRef] [PubMed]
- Guralnick, R.; Crimmins, T.; Grady, E.; Campbell, L. Phenological response to climatic change depends on spring warming velocity. Commun. Earth Environ. 2024, 5, 634. [Google Scholar] [CrossRef]
- Sun, M.; Li, P.; Sun, M.; Li, P.; Zhang, C.; Zhou, X.; Peng, C. Divergent response of vegetation phenology to extreme temperatures and precipitation of different intensities on the Tibetan Plateau. Sci. China Earth Sci. 2023, 66, 2200–2210. [Google Scholar] [CrossRef]
- Yu, H.; Wang, L.; Yang, Z.; Guo, L. Divergent response of leaf unfolding to climate warming in subtropical and temperate zones. Agric. For. Meteorol. 2023, 15, 103625. [Google Scholar] [CrossRef]
- Cao, L.; Lin, S.; Liu, W.; Gao, C.; Bai, W.; Zhu, M.; Hao, Y.; Hao, X.; Dai, J. Shifts in Plant Phenology and Its Responses to Climate Warming in Three Temperate Cities of China during 1963–2020. Forests 2024, 15, 1712. [Google Scholar] [CrossRef]
- Richardson, A.D.; Hufkens, K.; Milliman, T.; Aubrecht, D.M.; Chen, M. Tracking vegetation phenology across diverse North American biomes using PhenoCam imagery. Sci. Data 2018, 5, 180028. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Parada, T.H.; Park, I.W.; Record, S.; Davis, C.C.; Ellison, A.M.; Mazer, S.J. Plasticity and not adaptation is the primary source of temperature-mediated variation in flowering phenology in North America. Nat. Ecol. Evol. 2024, 8, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhou, W.; Chen, J.; Xu, X.; Wang, Y.; Zhuang, J.; Chi, Y. Land surface phenology detections from multi-source remote sensing indices capturing canopy photosynthesis phenology across major land cover types in the Northern Hemisphere. Ecol. Indic. 2022, 135, 108579. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, J.; Wang, Y.; Zhang, H.; Zhang, Z.; Guo, X. Comparison of land surface phenology in the Northern Hemisphere based on AVHRR GIMMS3g and MODIS datasets. ISPRS J. Photogramm. Remote Sens. 2020, 169, 1–16. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, H.; Xu, F.; Bento, V.A.; Zhang, R.; Wu, X.; Guo, P. Understanding vegetation phenology responses to easily ignored climate factors in China’s mid-high latitudes. Sci. Rep. 2024, 14, 8773. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bai, W.; Hu, Z.; Lin, S.; Ge, Q. Effectiveness of freezing temperatures on dormancy release of temperate woody species. Ann. Bot. 2024, 134, 615–630. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dai, J.; Peñuelas, J.; Ge, Q.; Fu, Y.; Wu, C. Winter warming offsets one half of the spring warming effects on leaf unfolding. Glob. Chang. Biol. 2022, 28, 6033–6049. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Wang, H.; Lin, S. Magnitude and direction of green-up date in response to drought depend on background climate over Mongolian grassland. Sci. Total Environ. 2023, 902, 166051. [Google Scholar] [CrossRef] [PubMed]
- Bock, A.; Sparks, T.H.; Estrella, N.; Jee, N.; Casebow, A.; Schunk, C.; Leuchner, M.; Menzel, A. Changes in first flowering dates and flowering duration of 232 plant species on the island of Guernsey. Glob. Chang. Biol. 2014, 20, 3508–3519. [Google Scholar] [CrossRef]
- Gonsamo, A.; Chen, J.M.; Wu, C. Citizen science: Linking the recent rapid advances of plant flowering in Canada with climate variability. Sci. Rep. 2013, 3, 2239. [Google Scholar] [CrossRef]
- Menzel, A.; Sparks, T.H.; Estrella, N.; Koch, E.; Aasa, A.; Ahas, R.; Alm-Kübler, K.; Bissolli, P.; Braslavsk, O.; Briede, A.; et al. European phenological response to climate change matches the warming pattern. Glob. Change Biol. 2006, 12, 1969–1976. [Google Scholar] [CrossRef]
- Dai, J.; Wang, H.; Ge, Q. Multiple phenological responses to climate change among 42 plant species in Xi’an, China. Int. J. Biometeorol. 2013, 57, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Piao, S.; Fu, Y.H.; Gao, M.; Peñuelas, J.; Janssens, I.A. Climatic Warming Increases Spatial Synchrony in Spring Vegetation Phenology Across the Northern Hemisphere. Geophys. Res. Lett. 2019, 46, 1641–1650. [Google Scholar] [CrossRef]
- Peñuelas, J.; Rutishauser, T.; Filella, I. Phenology Feedbacks on Climate Change. Science 2009, 324, 887–888. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, W.; Du, Y. Effects of Temperature, Precipitation, and CO2 on Plant Phenology in China: A Circular Regression Approach. Forests 2023, 14, 1844. [Google Scholar] [CrossRef]
- Bigler, C.; Vitasse, Y. Daily Maximum Temperatures Induce Lagged Effects on Leaf Unfolding in Temperate Woody Species Across Large Elevational Gradients. Front. Plant Sci. 2019, 10, 398. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ge, Q.; Dai, J.; Wang, H. Variations in temperature sensitivity of leaf unfolding date and their influencing factors for typical woody plants in China over the past 50 years. Acta Ecol. Sin. 2019, 39, 8135–8143. [Google Scholar]
- Wang, H.; Gao, C.; Ge, Q. Low temperature and short daylength interact to affect the leaf senescence of two temperate tree species. Tree Physiol. 2022, 42, 2252–2265. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Dai, J.; Ge, Q.; Wang, H.; Tao, Z. Comparison of chilling and heat requirements for leaf unfolding in deciduous woody species in temperate and subtropical China. Int. J. Biometeorol. 2021, 65, 393–403. [Google Scholar] [CrossRef]
- Atsuko, M.-K.; Han, Q.; Keiko, K.; Akiko, S. Impacts of climate change on the transcriptional dynamics and timing of bud dormancy release in Yoshino-cherry tree. New Phytol. 2024, 6, 1505–1521. [Google Scholar]
- Hsu, H.-W.; Yun, K.; Kim, S.-H. Variable warming effects on flowering phenology of cherry trees across a latitudinal gradient in Japan. Agric. For. Meteorol. 2023, 339, 109571. [Google Scholar] [CrossRef]
- Kim, J.; Jeong, S.; Park, C.-E.; Park, H.; Son, S.; Kim, S.Y. Regional and Species Variations in Spring and Autumn Phenology of 25 Temperate Species in South Korea. Asia-Pac. J. Atmos. Sci. 2022, 58, 181–195. [Google Scholar] [CrossRef]
- Wittich, K.; Liedtke, M. Shifts in plant phenology: A look at the sensitivity of seasonal phenophases to temperature in Germany. Int. J. Climatol. 2015, 35, 3991–4000. [Google Scholar] [CrossRef]
- Munson, S.M.; Long, A.L. Climate drives shifts in grass reproductive phenology across the western USA. New Phytol. 2017, 213, 1945–1955. [Google Scholar] [CrossRef]
- Wan, M.; Liu, X. Zhong Guo Wu Hou Guan Ce Fang Fa[M]; Science Press: Beijing, China, 1979. [Google Scholar]
- Bolmgren, K.; Vanhoenacker, D.; Miller-Rushing, A.J. One man, 73 years, and 25 species. Evaluating phenological responses using a lifelong study of first flowering dates. Int. J. Biometeorol. 2013, 57, 367–375. [Google Scholar] [CrossRef]
- Ge, Q.; Wang, H.; Rutishauser, T.; Dai, J. Phenological response to climate change in China: A meta-analysis. Glob. Change Biol. 2015, 21, 265–274. [Google Scholar] [CrossRef]
- Lu, C.; van Groenigen, K.J.; Gillespie MA, K.; Hollister, R.D.; Post, E.; Cooper, E.J.; Welker, J.M.; Huang, Y.; Min, X.; Chen, J.; et al. Diminishing warming effects on plant phenology over time. New Phytol. 2024, 245, 523–533. [Google Scholar] [CrossRef]
- Rosenzweig, C.; Karoly, D.; Vicarelli, M.; Neofotis, P.; Wu, Q.; Casassa, G.; Menzel, A.; Root, T.L.; Estrella, N.; Seguin, B.; et al. Attributing physical and biological impacts to anthropogenic climate change. Nature 2008, 453, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Wang, H.; Zheng, J.; This, R.; Dai, J. A 170 year spring phenology index of plants in eastern China. J. Geophys. Res. Biogeosci. 2014, 119, 301–311. [Google Scholar] [CrossRef]
- Cleland, E.E.; Allen, J.M.; Crimmins, T.M.; Dunne, J.A.; Pau, S.; Travers, S.E.; Zavaleta, E.S.; Wolkovich, E.M. Phenological tracking enables positive species responses to climate change. Ecology 2012, 93, 1765–1771. [Google Scholar] [CrossRef]
- Willis, C.G.; Ruhfel, B.; Primack, R.B.; Miller-Rushing, A.J.; Davis, C.C. Phylogenetic patterns of species loss in Thoreau’s woods are driven by climate change. Proc. Natl. Acad. Sci. USA 2008, 105, 17029–17033. [Google Scholar] [CrossRef]
- Willis, C.G.; Ruhfel, B.R.; Primack, R.B.; Miller-Rushing, A.J.; Losos, J.B.; Davis, C.C. Favorable Climate Change Response Explains Non-Native Species’ Success in Thoreau’s Woods. PLoS ONE 2010, 5, e8878. [Google Scholar] [CrossRef]
- Wolkovich, E.M.; Cook, B.I.; Allen, J.M.; Crimmins, T.M.; Betancourt, J.L.; Travers, S.E.; Pau, S.; Regetz, J.; Davies, T.J.; Kraft, N.J.B.; et al. Warming experiments underpredict plant phenological responses to climate change. Nature 2012, 485, 494–497. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.; Cao, L.; Dai, J. Sensitivity of First Leaf Date to Temperature Change for Typical Woody Plants in Guiyang, China. Forests 2025, 16, 300. https://doi.org/10.3390/f16020300
Huang W, Cao L, Dai J. Sensitivity of First Leaf Date to Temperature Change for Typical Woody Plants in Guiyang, China. Forests. 2025; 16(2):300. https://doi.org/10.3390/f16020300
Chicago/Turabian StyleHuang, Wenjie, Lijuan Cao, and Junhu Dai. 2025. "Sensitivity of First Leaf Date to Temperature Change for Typical Woody Plants in Guiyang, China" Forests 16, no. 2: 300. https://doi.org/10.3390/f16020300
APA StyleHuang, W., Cao, L., & Dai, J. (2025). Sensitivity of First Leaf Date to Temperature Change for Typical Woody Plants in Guiyang, China. Forests, 16(2), 300. https://doi.org/10.3390/f16020300






