Ophiostomatalean Fungi (Ascomycota, Ophiostomatales) Associated with Dendroctonus valens in Liaoning, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Fungi
2.2. Morphological and Growth Studies
2.3. PCR Amplification and Phylogenetic Analysis
3. Results
3.1. Isolation of Fungi
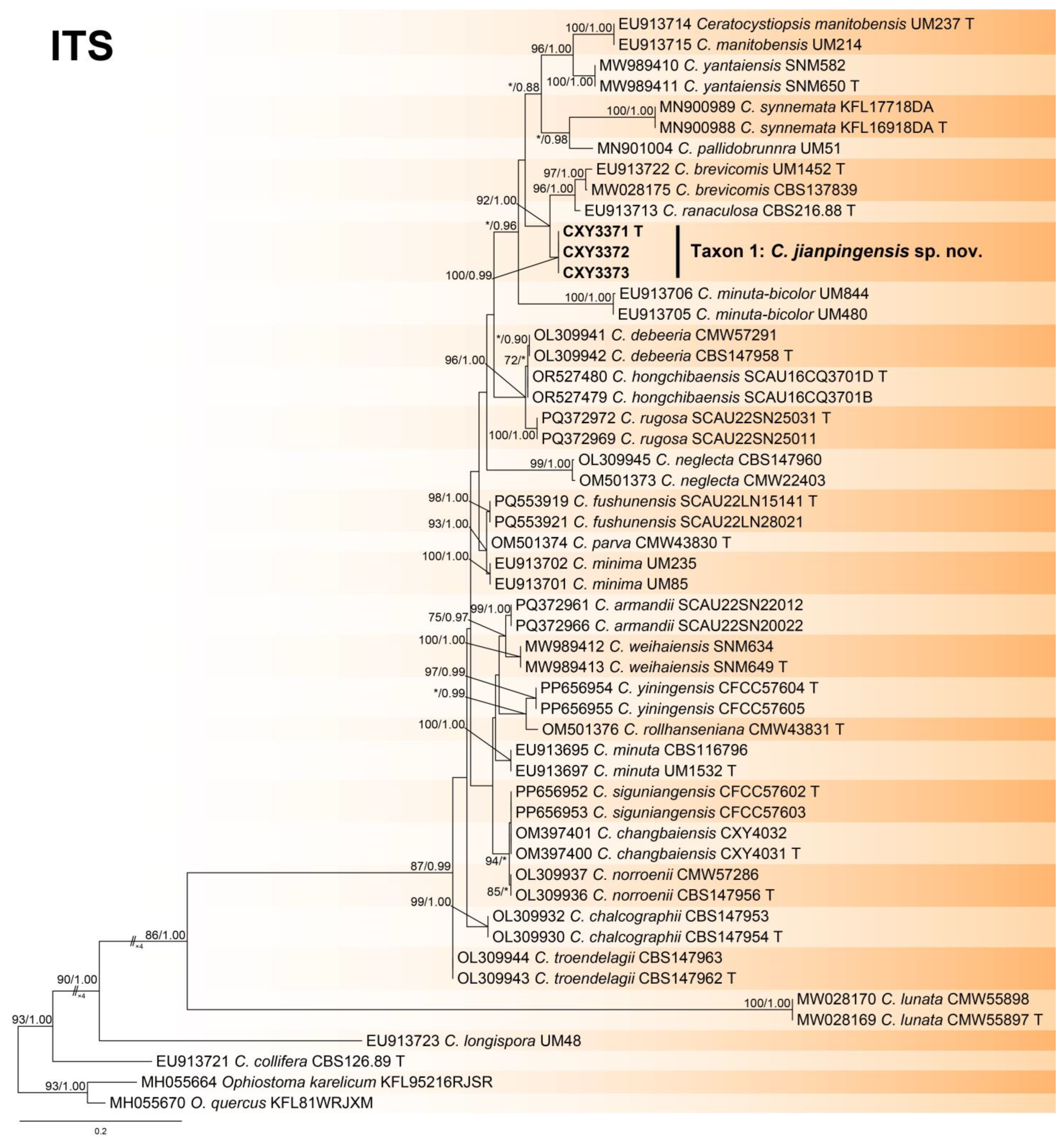
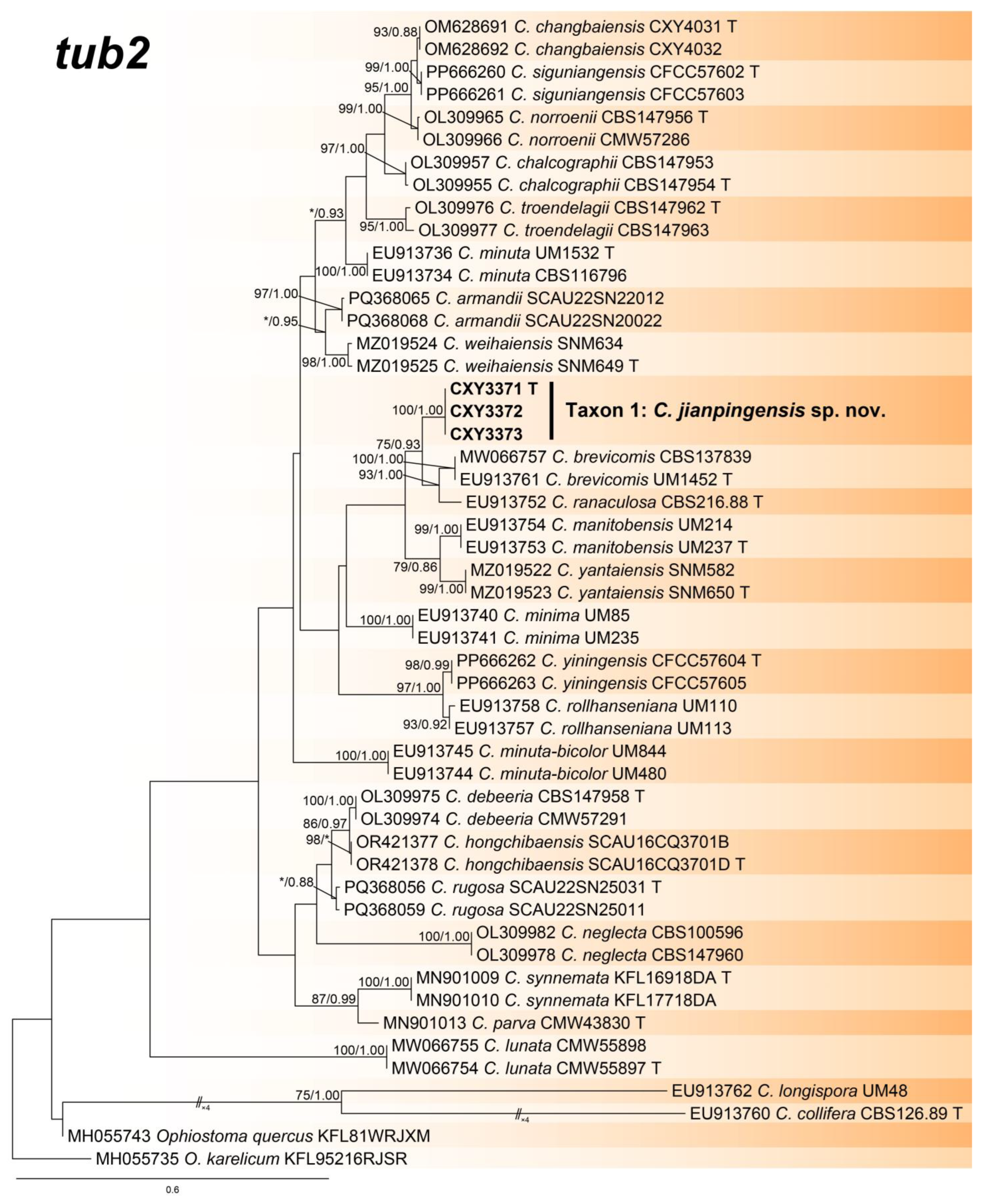
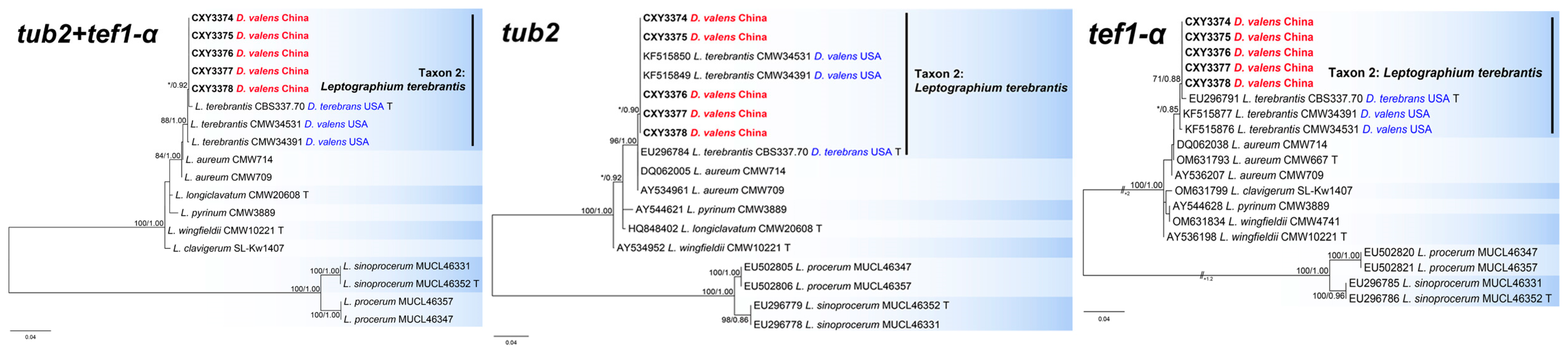
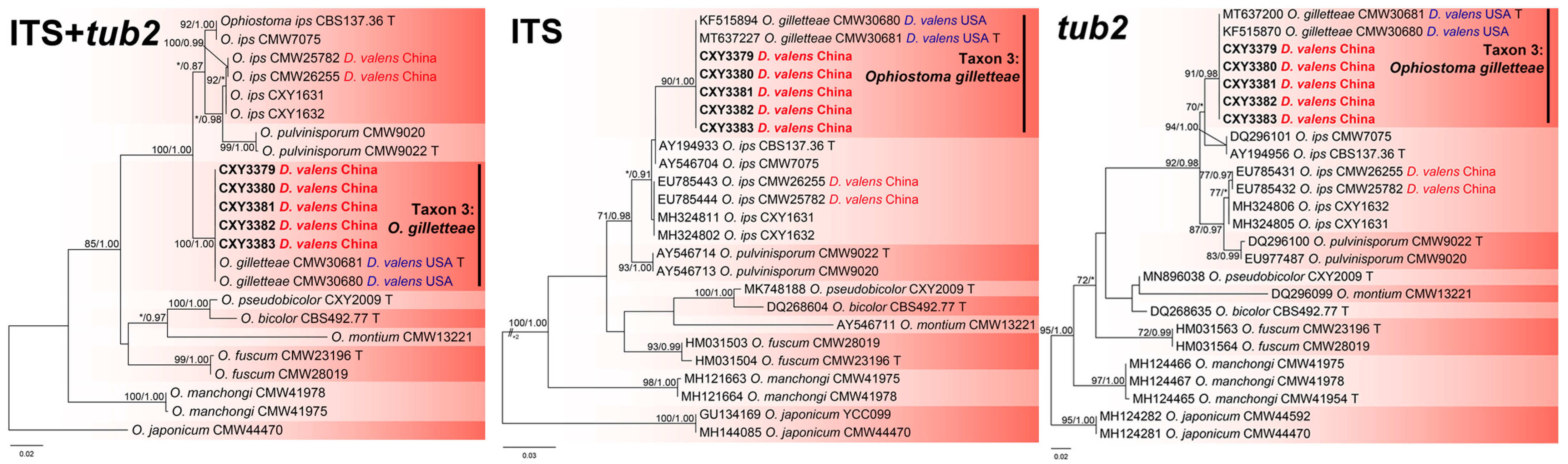
3.2. Phylogenetic Analysis
3.3. Taxonomy
- Ceratocystiopsis jianpingensis Z. Wang and Q. Lu, sp. nov. (Taxon 1, Figure 6).
- Mycobank: 856888
- Etymology: The name refers to Jianping, the county of Liaoning Province, where the fungus was collected.
- Holotype: CXY3371
- Description: Teleomorph unknown. Anamorphs: sporothrix-like and hyalorhinocladiella-like. Conidiophores macronematous or micronematous, simple or branched, erect, occasionally serpentine, arising from the mycelium; conidiogenous cells rough or smooth, hyaline, (5.7–)8.1–13.2(–14.6) × 1.3–1.6(–1.7) μm. Conidia hyaline, smooth, aseptate, obovoid with upper part swollen, (3.1–)3.3–3.9(–4.5) × (1.7–)1.9–2.4(–2.9) μm.
- Culture features: Colonies grew slowly and reached 42.9 mm in 20 days, pure white, occasionally faint yellow. Abundant aerial mycelia, which were either clumpy or spiny, developed on the inoculated plugs. Colonies were dense with irregular margins. Colonies grew fastest at 25 °C and slowest at 5 °C. No growth was observed at 35 °C.
- Associated insects: Dendroctonus valens.
- Hosts: Pinus tabuliformis.
- Material examined: CHINA, Liaoning Province, Chaoyang City, Jianping County, from Dendroctonus valens infesting Pinus tabuliformis, July 2024, Z. Wang and Q. Lu, holotype: CXY3371, ex-type culture CFCC72301, ibid. CFCC72302, and CFCC72303.
- Notes: Ceratocystiopsis jianpingensis was a phylogenetic sister to C. brevicomis and C. ranaculosa (Figure 1, Figure 2 and Figure 3) [26]. Although all three species produce a sporothrix-like asexual morph, C. jianpingensis also produces a hyalorhinocladiella-like asexual morph. Furthermore, C. jianpingensis grew slower than C. brevicomis at 25 °C on 2% MEA (42.9 mm in 20 days vs. 40–50 mm in 13 days). Ceratocystiopsis jianpingensis, C. brevicomis, and C. ranaculosa are associated with pine-infesting Dendroctonus. However, C. jianpingensis is an associate of D. valens in China, and C. brevicomis and C. ranaculosa are associated with D. brevicomis and D. frontalis in North America, respectively [26].
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, J.H.; Lu, M.; Gillette, N.E.; Wingfield, M.J. Red turpentine beetle: Innocuous native becomes invasive tree killer in China. Annu. Rev. Entomol. 2013, 58, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Zhang, S.; Du, R.; Wang, H. Occurrence status of main forestry invasive species in China and their research trends. Plant Prot. 2022, 48, 21–38. (In Chinese) [Google Scholar]
- Lu, M.; Hulcr, J.; Sun, J. The role of symbiotic microbes in insect invasions. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 487–505. [Google Scholar] [CrossRef]
- De Beer, Z.W.; Procter, M.; Wingfield, M.J.; Marincowitz, S.; Duong, T.A. Generic boundaries in the Ophiostomatales reconsidered and revised. Stud. Mycol. 2022, 101, 57–120. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, K.; Wingfield, M.J. Leptographium Species: Tree Pathogens, Insect Associates and Agents of Bluestain; APS Press: St Paul, MN, USA, 2001. [Google Scholar]
- Eckhardt, L.G. Blackstain root disease and other Leptographium diseases. In Infectious Forest Diseases; Gonthier, P., Nicolotti, G., Eds.; CAB International: Wallingford, UK, 2013; pp. 283–297. [Google Scholar]
- Lu, M.; Wingfield, M.J.; Gillette, N.E.; Mori, S.R.; Sun, J.H. Complex interactions among host pines and fungi vectored by an invasive bark beetle. New Phytol. 2010, 187, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Wingfield, M.J.; Gillette, N.; Sun, J.H. Do novel genotypes drive the success of an invasive bark beetle–fungus complex? Implications for potential reinvasion. Ecology 2011, 92, 2013–2019. [Google Scholar] [CrossRef] [PubMed]
- Marincowitz, S.; Duong, T.A.; Taerum, S.J.; De Beer, Z.W.; Wingfield, M.J. Fungal associates of an invasive pine-infesting bark beetle, Dendroctonus valens, including seven new ophiostomatalean fungi. Persoonia 2020, 45, 177–195. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Decock, C.; Zhang, X.Y.; Maraite, H. Leptographium sinoprocerum sp. nov., an undescribed species associated with Pinus tabuliformis-Dendroctonus valens in northern China. Mycologia 2008, 100, 275–290. [Google Scholar] [CrossRef]
- Lu, Q.; Decock, C.; Zhang, X.Y.; Maraite, H. Ophiostomatoid fungi (Ascomycota) associated with Pinus tabuliformis infested by Dendroctonus valens (Coleoptera) in northern China and an assessment of their pathogenicity on mature trees. Antonie Leeuwenhoek 2009, 96, 275–293. [Google Scholar] [CrossRef]
- Lu, M.; Zhou, X.; De Beer, Z.W.; Wingfield, M.J.; Sun, J.H. Ophiostomatoid fungi associated with the invasive pine-infesting bark beetle, Dendroctonus valens, in China. Fungal Divers. 2009, 38, 133–145. [Google Scholar]
- Taerum, S.J.; Duong, T.A.; De Beer, Z.W.; Gillette, N.; Sun, J.H.; Owen, D.R.; Wingfield, M.J. Large shift in symbiont assemblage in the invasive red turpentine beetle. PLoS ONE 2013, 8, e78126. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, D.; Luo, Z.; He, S.; Jiang, S.; Song, Y. Disaster risk analysis of Dendroctonus valens in Northeast China. For. Pest. Dis. 2022, 41, 22–28. (In Chinese) [Google Scholar]
- Huang, F.; Lu, J. The Classification Outline of Scolytidae from China; Tongji University Press: Shanghai, China, 2015; pp. 37–38. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.J.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press: Cambridge, MA, USA, 1990; Volume 18, pp. 315–322. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous Ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef]
- Jacobs, K.; Bergdahl, D.R.; Wingfield, M.J.; Halik, S.; Seifert, K.A.; Bright, D.E.; Wingfield, B.D. Leptographium wingfieldii introduced into North America and found associated with exotic Tomicus piniperda and native bark beetles. Mycol. Res. 2004, 108, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Stamatakis, A. RaxML Version 8: A tool phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Hsiau, P.T.W.; Harrington, T.C. Ceratocystiopsis brevicomi sp. nov., a mycangial fungus from Dendroctonus brevicomis (Coleoptera: Scolytidae). Mycologia 1997, 89, 661–669. [Google Scholar]
- Taerum, S.J.; Hoareau, T.B.; Duong, T.A.; De Beer, Z.W.; Jankowiak, R.; Wingfield, M.J. Putative origins of the fungus Leptographium procerum. Fungal Biol. 2017, 121, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.H.; Wang, B.; Clarke, S.R.; Sun, J.H. Effect of associated fungi on the immunocompetence of red turpentine beetle larvae, Dendroctonus valens (Coleoptera: Curculionidae: Scolytinae). Insect Sci. 2012, 19, 579–584. [Google Scholar]
- Koski, T.M.; Zhang, B.; Wickham, J.D.; Bushley, K.E.; Blanchette, R.A.; Kang, L.; Sun, J. Chemical interactions under the bark: Bark-, ambrosia-, and wood-boring beetles and their microbial associates. Rev. Environ. Sci. Bio Technol. 2024, 23, 932–948. [Google Scholar]
- Liu, F.; Ye, F.; Yang, Y.; Kang, Z.; Liu, Y.; Chen, W.; Wang, S.; Kou, H.; Kang, L.; Sun, J. Gut bacteria are essential for development of an invasive bark beetle by regulating glucose transport. Proc. Natl. Acad. Sci. USA 2024, 121, e2410889121. [Google Scholar] [CrossRef]
- Bi, P.; Yu, L.; Zhou, Q.; Kuang, J.; Tang, R.; Ren, L.; Luo, Y. Early detection of Dendroctonus valens infestation with UAV-based thermal and hyperspectral images. Remote Sens. 2024, 16, 3840. [Google Scholar] [CrossRef]
- Singewar, K.; Fladung, M. Double-stranded RNA (dsRNA) technology to control forest insect pests and fungal pathogens: Challenges and opportunities. Funct. Integr. Genom. 2023, 23, 185. [Google Scholar]
- Joga, M.R.; Mogilicherla, K.; Smagghe, G.; Roy, A. RNA interference-based forest protection products (FPPs) against wood-boring coleopterans: Hope or hype? Front. Plant Sci. 2021, 12, 733608. [Google Scholar]
- Kyre, B.R.; Rodrigues, T.B.; Rieske, L.K. RNA interference and validation of reference genes for gene expression analyses using qPCR in southern pine beetle, Dendroctonus frontalis. Sci. Rep. 2019, 9, 5640. [Google Scholar]
- Xue, Q.; Avila dos Santos, É.; Smagghe, G.; Zotti, M.J.; Taning, N.T.C. Engineering strategies for insect viruses and microbes for dsRNA production and delivery to insects for targeted gene silencing. Entomol. Gen. 2023, 43, 31–53. [Google Scholar] [CrossRef]


| Locus | Primer Pairs | PCR Amplification Procedures | References |
|---|---|---|---|
| ITS | ITS1-F/ITS4 | initial denaturation: 94 °C for 3 min; thermal cycles: (94 °C: 1 min, 55 °C: 45 s, and 72 °C: 1 min) × 35 cycles; final chain elongation: 72 °C for 8 min | [16,17] |
| ITS2-LSU | ITS3/LR3 | initial denaturation: 95 °C for 2 min; thermal cycles: (95 °C: 30 s, 54 °C: 30 s, and 72 °C: 1 min) × 35 cycles; final chain elongation: 72 °C for 8 min | [16,18] |
| tub2 | Bt2a/Bt2b | initial denaturation: 95 °C for 2 min; thermal cycles: (95 °C: 30 s, 56 °C: 30 s, and 72 °C: 1 min) × 35 cycles; final chain elongation: 72 °C for 8 min | [19] |
| tef1-α | EF1F/EF2R | initial denaturation: 95 °C for 2 min; thermal cycles: (95 °C: 30 s, 56 °C: 30 s, and 72 °C: 1 min) × 35 cycles; final chain elongation: 72 °C for 8 min | [20] |
| Species | Taxa | Isolates | Other Numbers | GenBank Accession Numbers | ||
|---|---|---|---|---|---|---|
| ITS/ITS2-LSU | tub2 | tef1-α | ||||
| Ceratocystiopsis jianpingensis sp. nov. | 1 | CFCC72301 | CXY3371 | PQ685080 | PQ687506 | - |
| CFCC72302 | CXY3372 | PQ685081 | PQ687507 | - | ||
| CFCC72303 | CXY3373 | PQ685082 | PQ687508 | - | ||
| Leptographium terebrantis | 2 | CFCC72304 | CXY3374 | PQ685083 | PQ687509 | PQ687519 |
| CFCC72305 | CXY3375 | PQ685084 | PQ687510 | PQ687520 | ||
| CFCC72306 | CXY3376 | PQ685085 | PQ687511 | PQ687521 | ||
| CFCC72307 | CXY3377 | PQ685086 | PQ687512 | PQ687522 | ||
| CFCC72308 | CXY3378 | PQ685087 | PQ687513 | PQ687523 | ||
| Ophiostoma gilletteae | 3 | CFCC72309 | CXY3379 | PQ686155 | PQ687514 | - |
| CFCC72310 | CXY3380 | PQ686156 | PQ687515 | - | ||
| CFCC72311 | CXY3381 | PQ686157 | PQ687516 | - | ||
| CFCC72312 | CXY3382 | PQ686158 | PQ687517 | - | ||
| CFCC72313 | CXY3383 | PQ686159 | PQ687518 | - | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Liang, L.; Yan, S.; Wang, H.; Lu, Q. Ophiostomatalean Fungi (Ascomycota, Ophiostomatales) Associated with Dendroctonus valens in Liaoning, China. Forests 2025, 16, 299. https://doi.org/10.3390/f16020299
Wang Z, Liang L, Yan S, Wang H, Lu Q. Ophiostomatalean Fungi (Ascomycota, Ophiostomatales) Associated with Dendroctonus valens in Liaoning, China. Forests. 2025; 16(2):299. https://doi.org/10.3390/f16020299
Chicago/Turabian StyleWang, Zheng, Lingyu Liang, Shuo Yan, Huimin Wang, and Quan Lu. 2025. "Ophiostomatalean Fungi (Ascomycota, Ophiostomatales) Associated with Dendroctonus valens in Liaoning, China" Forests 16, no. 2: 299. https://doi.org/10.3390/f16020299
APA StyleWang, Z., Liang, L., Yan, S., Wang, H., & Lu, Q. (2025). Ophiostomatalean Fungi (Ascomycota, Ophiostomatales) Associated with Dendroctonus valens in Liaoning, China. Forests, 16(2), 299. https://doi.org/10.3390/f16020299







