Productivity Dynamics in Chinese Fir Plantations: The Driving Role of Plant–Soil–Microbe Interactions in Northern Subtropical China
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Site Description
2.2. Experimental Design and Sample Collection
2.3. Analytical Methods
2.3.1. Soil Physicochemical Characterization
2.3.2. Determination of Soil Microbial Biomass and Enzyme Activities
2.3.3. Soil Microbial Sequencing Analysis
2.4. Data Statistical Analysis
2.4.1. Treevolume Calculation
2.4.2. Statistical Analysis
3. Results
3.1. Growth Characteristics of Chinese Fir Plantations with Different Stand Ages
3.2. Characteristics of Soil Nutrients and Microbial Biomass in Chinese Fir Plantations of Different Stand Ages
3.3. Soil Microbial Community Characteristics Shift with Stand Age in Chinese Fir Plantations
3.3.1. Dominant Soil Microbial Abundance at Phylum Levels Across Different Stand Ages
3.3.2. Variation in Soil Microbial Diversity with Different Stand Ages
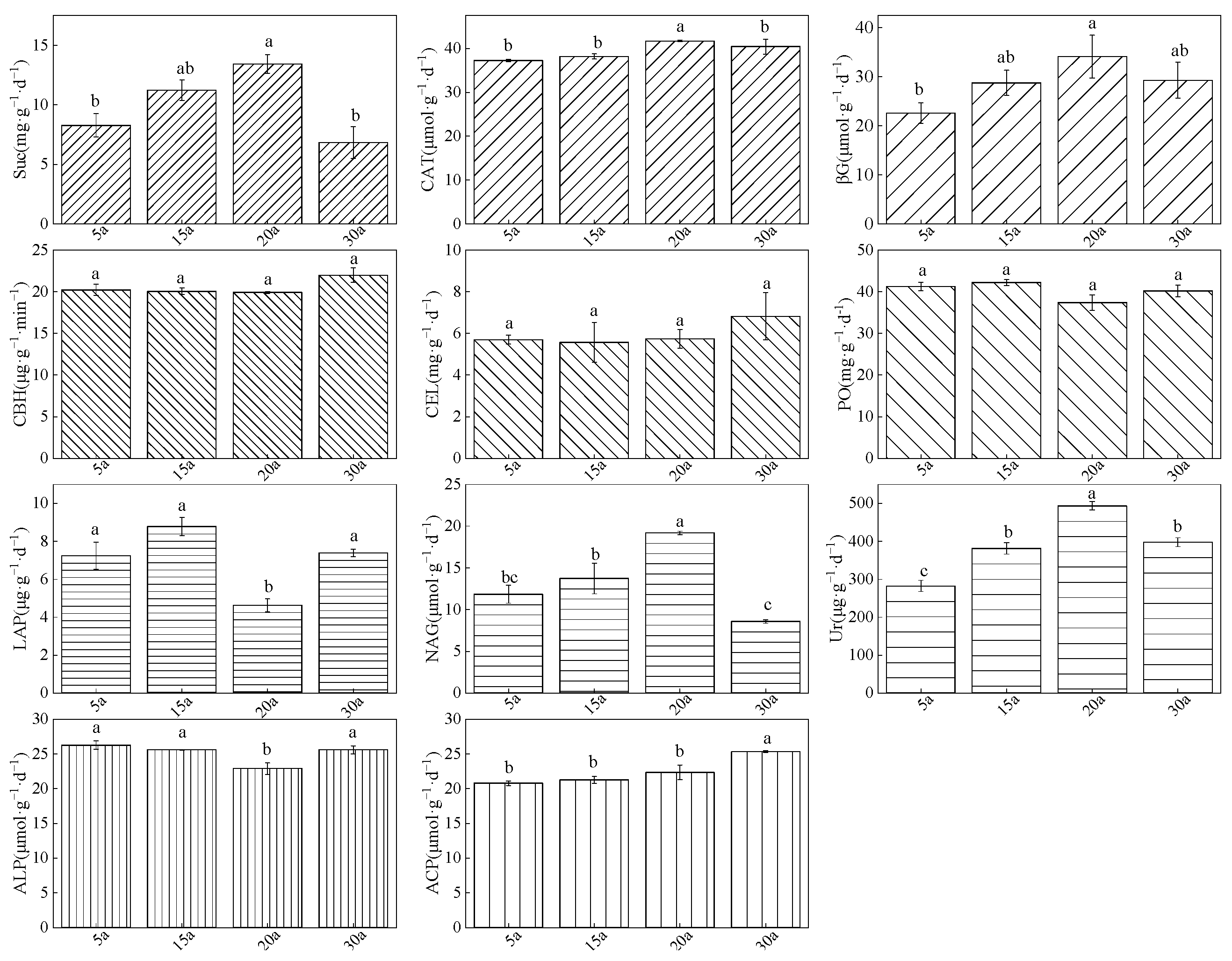
3.4. Variation Characteristics of Soil Enzyme Activities in Chinese Fir Plantations with Different Stand Ages
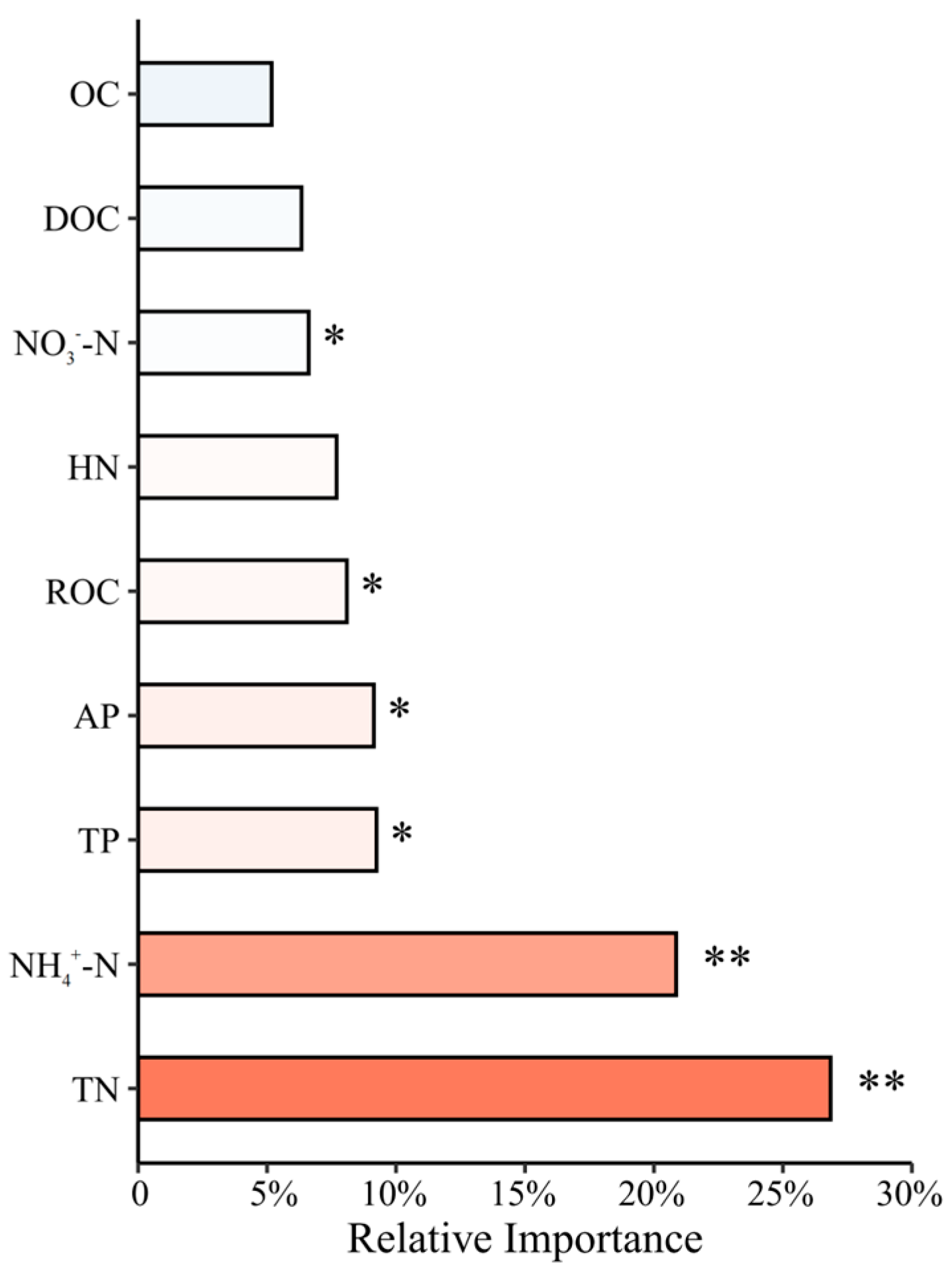
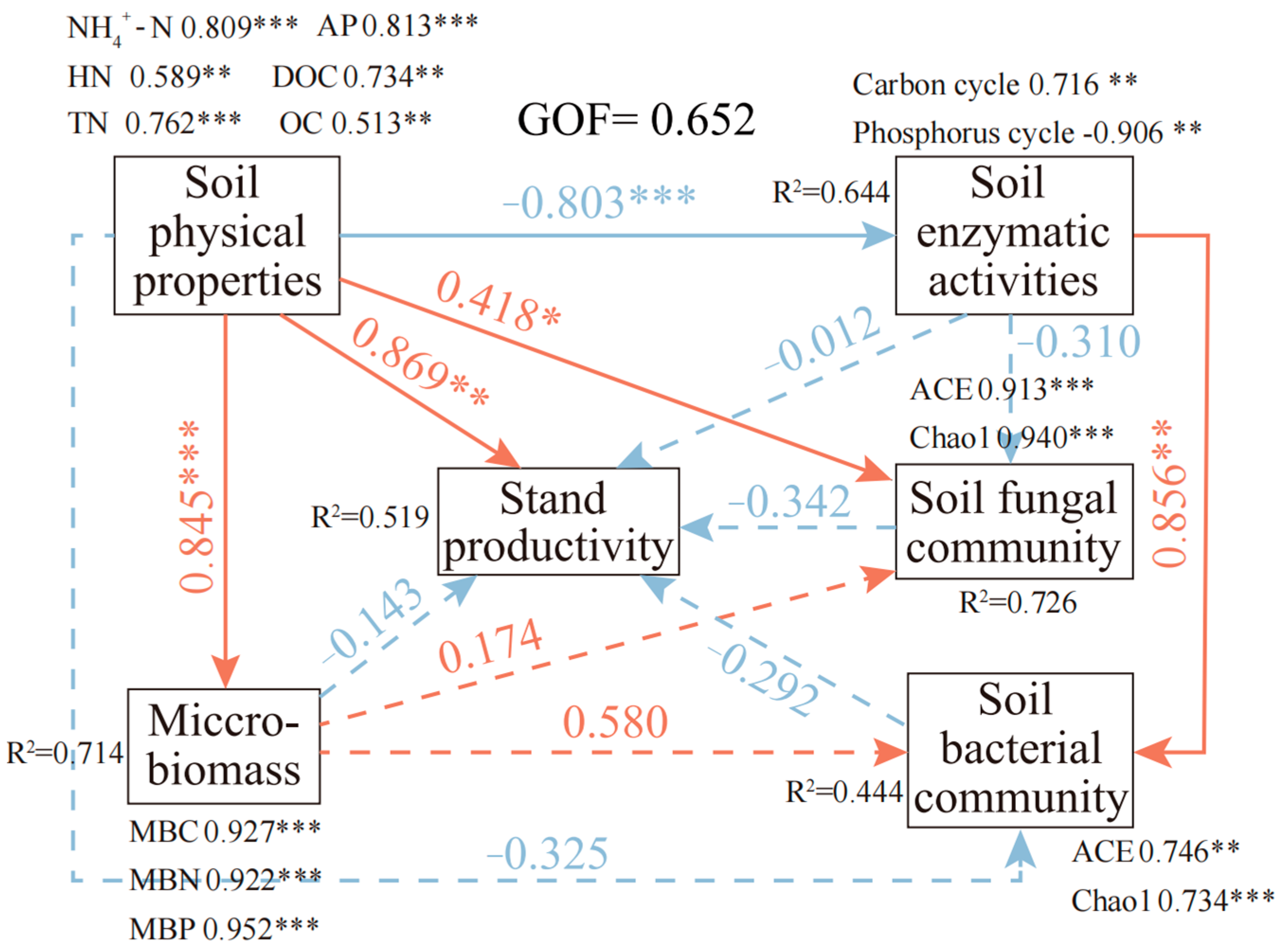
3.5. Drivers of Productivity: Linking Soil Nutrients, Microbial Communities, and Enzyme Activities
3.6. Mechanism of Nutrient Cycling Impact on Stand Productivity in Chinese Fir Plantations
4. Discussion
4.1. Stage-Dependent Growth Dynamics and Productivity Responses of Chinese Fir Plantations to Stand Age Growth
4.2. Driving Mechanisms of Soil Nutrient Availability Across Stand-Age Gradients
4.3. Coordinated Succession of Soil Microbial Structure-Function and Shift in Ecological Strategies
4.4. Enzyme Activity Dynamics Reveal Nutrient Cycling Strategies of the Plantations
4.5. Integrated Effects of Multi-Factor Interactions on Productivity Change
4.6. Limitations and Future Perspectives
5. Conclusions
- (1)
- A belowground ecological strategy transition occurs during stand development: stand growth shifts from a resource-acquisition strategy to a nutrient-conservation strategy. This transition is closely associated with a shift from nitrogen to phosphorus limitation and concurrent microbial succession.
- (2)
- Microbial succession provides potential indicators: directional successional patterns in fungal and bacterial communities offer potential biomarkers for assessing ecosystem developmental status.
- (3)
- Enzyme activity acts as an early-warning signal: the sustained increase in phosphorus-cycle enzyme activity precedes detectable changes in soil available phosphorus, and thus may serve as an early-warning indicator of phosphorus limitation.
- (4)
- Productivity is regulated by a multi-pathway network: soil properties govern productivity through a complex network involving both direct effects and indirect pathways mediated by microbial communities and enzyme activities.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5a | 5-year-old |
| 10a | 10-year-old |
| 20a | 20-year-old |
| 30a | 30-year-old |
| PLS-SEM | Partial Least Squares Structural Equation Modeling |
| SOC | Soil organic carbon |
| Mean vol. | Mean volume per tree |
| MAI | Mean annual increment |
| DBH | Diameter at breast height |
| ha | Hectare |
| C | Carbon |
| N | Nitrogen |
| P | Phosphorus |
| NO3−-N | Nitrate Nitrogen |
| NH4+-N | Ammonium Nitrogen |
| HN | Hydrolyzable Nitrogen |
| TN | Total Nitrogen |
| AP | Available Phosphorus |
| TP | Total Phosphorus |
| DOC | Dissolved Organic Carbon |
| ROC | Readily Oxidizable Carbon |
| MBC | Microbial Biomass Carbon |
| MBN | Microbial Biomass Nitrogen |
| MBP | Microbial Biomass Phosphorus |
| Suc | Sucrase |
| CAT | Catalase |
| βG | β-Glucosidase |
| CBH | Cellobiohydrolase |
| CEL | Cellulase |
| PO | Phenol Oxidase |
| LAP | Leucine Aminopeptidase |
| NAG | N-acetyl-β-D-glucosaminidase |
| Ur | Urease |
| ALP | Alkaline Phosphatase |
| ACP | Acid Phosphatase |
| NMDS | Non-metric multidimensional scaling |
References
- Michaletz, S.T.; Cheng, D.; Kerkhoff, A.J.; Enquist, B.J. Convergence of terrestrial plant production across global climate gradients. Nature 2014, 512, 39–43. [Google Scholar] [CrossRef]
- Doelman, J.C.; Stehfest, E.; van Vuuren, D.P.; Tabeau, A.; Hof, A.F.; Braakhekke, M.C.; Gernaat, D.E.; van den Berg, M.; van Zeist, W.J.; Daioglou, V. Afforestation for climate change mitigation: Potentials, risks and trade-offs. Glob. Change Biol. 2020, 26, 1576–1591. [Google Scholar] [CrossRef]
- Tang, S.; Ma, Q.; Marsden, K.A.; Chadwick, D.R.; Luo, Y.; Kuzyakov, Y.; Wu, L.; Jones, D.L. Microbial community succession in soil is mainly driven by carbon and nitrogen contents rather than phosphorus and sulphur contents. Soil Biol. Biochem. 2023, 180, 109019. [Google Scholar] [CrossRef]
- Selvaraj, S.; Duraisamy, V.; Huang, Z.; Guo, F.; Ma, X. Influence of long-term successive rotations and stand age of Chinese fir (Cunninghamia lanceolata) plantations on soil properties. Geoderma 2017, 306, 127–134. [Google Scholar] [CrossRef]
- Chen, J.; Deng, Z.; Jiang, Z.; Sun, J.; Meng, F.; Zuo, X.; Wu, L.; Cao, G.; Cao, S. Variations of rhizosphere and bulk soil microbial community in successive planting of Chinese fir (Cunninghamia lanceolata). Front. Plant Sci. 2022, 13, 954777. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Zhang, Y.; Wang, L.; Ge, S.; Zhang, Y.; Yang, Q.; Huang, H.; Tong, Z.; Zhang, J. Forest conversion from pure to mixed Cunninghamia lanceolata plantations enhances soil multifunctionality, stochastic processes, and stability of bacterial networks in subtropical southern China. Plant Soil 2023, 488, 411–429. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, D.H.; Wang, G.; Li, X.; Lin, G. Shifts in soil nitrogen availability and associated microbial drivers during stand development of Mongolian pine plantations. Land Degrad. Dev. 2023, 34, 3156–3169. [Google Scholar] [CrossRef]
- Deng, M.; Li, P.; Wang, Z.; Guo, L.; Wu, Y.; Huang, J.; Wang, X.; Liu, L. Drought and salinization stress induced by stand development alters mineral element cycling in a larch plantation. J. Geophys. Res.-Biogeosci. 2021, 126, e2020JG005906. [Google Scholar] [CrossRef]
- Chen, L.; Xiang, W.; Ouyang, S.; Wu, H.; Xia, Q.; Ma, J.; Zeng, Y.; Lei, P.; Xiao, W.; Li, S. Tight coupling of fungal community composition with soil quality in a Chinese fir plantation chronosequence. Land Degrad. Dev. 2021, 32, 1164–1178. [Google Scholar] [CrossRef]
- Lei, J.; Cao, Y.; Wang, J.; Chen, Y.; Peng, Y.; Shao, Q.; Dan, Q.; Xu, Y.; Chen, X.; Dang, P. Soil nutrients, enzyme activities, and microbial communities along a chronosequence of Chinese fir plantations in subtropical China. Plants 2023, 12, 1931. [Google Scholar] [CrossRef]
- Wang, T.; Dong, L.; Liu, Z. Stand structure is more important for forest productivity stability than tree, understory plant and soil biota species diversity. Front. For. Glob. Change 2024, 7, 1354508. [Google Scholar] [CrossRef]
- Shen, L.; Ye, S.; Liu, H.; Deng, X.; He, P.; Cheng, F. Linkage between Leaf–Litter–Soil, Microbial Resource Limitation, and Carbon-Use Efficiency in Successive Chinese Fir (Cunninghamia lanceolata) Plantations. Forests 2023, 14, 357. [Google Scholar] [CrossRef]
- Smal, H.; Ligęza, S.; Pranagal, J.; Urban, D.; Pietruczyk-Popławska, D. Changes in the stocks of soil organic carbon, total nitrogen and phosphorus following afforestation of post-arable soils: A chronosequence study. For. Ecol. Manag. 2019, 451, 117536. [Google Scholar] [CrossRef]
- Zheng, Y.; Ye, J.; Pei, J.; Fang, C.; Li, D.; Ke, W.; Song, X.; Sardans, J.; Peñuelas, J. Initial soil condition, stand age, and aridity alter the pathways for modifying the soil carbon under afforestation. Sci. Total Environ. 2024, 946, 174448. [Google Scholar] [CrossRef]
- Xu, Y.; Ge, X.; Zhou, B.; Lei, L.; Xiao, W. Variations in rhizosphere soil total phosphorus and bioavailable phosphorus with respect to the stand age in Pinus massoniana Lamb. Front. Plant Sci. 2022, 13, 939683. [Google Scholar] [CrossRef] [PubMed]
- Hou, E.; Luo, Y.; Kuang, Y.; Chen, C.; Lu, X.; Jiang, L.; Luo, X.; Wen, D. Global meta-analysis shows pervasive phosphorus limitation of aboveground plant production in natural terrestrial ecosystems. Nat. Commun. 2020, 11, 637. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.L.; Brenes-Arguedas, T.; Condit, R. Pervasive phosphorus limitation of tree species but not communities in tropical forests. Nature 2018, 555, 367–370. [Google Scholar] [CrossRef]
- Cao, J.; Yan, W.; Farooq, T.H.; Chen, X.; Wang, J.; Yuan, C.; Qi, Y.; Khan, K.A. Ecological stoichiometry of N and P across a chronosequence of Chinese fir plantation forests. Forests 2023, 14, 1685. [Google Scholar] [CrossRef]
- Wu, H.; Xiang, W.; Chen, L.; Ouyang, S.; Xiao, W.; Li, S.; Forrester, D.I.; Lei, P.; Zeng, Y.; Deng, X. Soil phosphorus bioavailability and recycling increased with stand age in Chinese fir plantations. Ecosystems 2020, 23, 973–988. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, J.; Zhang, W.; Zhang, Q.; Lu, D.; Zhang, Y.; Zheng, X.; Xu, S.; Wang, G.G. Litter decomposition and nutrient release from monospecific and mixed litters: Comparisons of litter quality, fauna and decomposition site effects. J. Ecol. 2022, 110, 1673–1686. [Google Scholar] [CrossRef]
- Abay, P.; Gong, L.; Chen, X.; Luo, Y.; Wu, X. Spatiotemporal variation and correlation of soil enzyme activities and soil physicochemical properties in canopy gaps of the Tianshan Mountains, Northwest China. J. Arid. Land 2022, 14, 824–836. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, Y.; Hu, Y.; Long, Q.; Chen, Y.; Duan, B.; Lu, X. Soil abiotic properties, not microbial composition or functional gene abundance, determine enzyme activities in alpine grassland soils. Appl. Soil Ecol. 2026, 217, 106645. [Google Scholar] [CrossRef]
- Hu, J.; Du, M.; Chen, J.; Tie, L.; Zhou, S.; Buckeridge, K.M.; Cornelissen, J.H.C.; Huang, C.; Kuzyakov, Y. Microbial necromass under global change and implications for soil organic matter. Glob. Change Biol. 2023, 29, 3503–3515. [Google Scholar] [CrossRef] [PubMed]
- Babur, E.; Dindaroğlu, T.; Uslu, Ö.S.; Gozukara, G.; Ozlu, E. Long-Term Effects of Land Use Conversion on Soil Microbial Biomass and Stoichiometric Indices in Eastern Mediterranean Karst Ecosystems (1981–2018). Land Degrad. Dev. 2025, 36, 5666–5680. [Google Scholar] [CrossRef]
- Jiang, W.; Gong, L.; Yang, L.; He, S.; Liu, X. Dynamics in C, N, and P stoichiometry and microbial biomass following soil depth and vegetation types in low mountain and hill region of China. Sci. Rep. 2021, 11, 19631. [Google Scholar] [CrossRef]
- Zhu, X.; Fang, X.; Wang, L.; Xiang, W.; Alharbi, H.A.; Lei, P.; Kuzyakov, Y. Regulation of soil phosphorus availability and composition during forest succession in subtropics. For. Ecol. Manag. 2021, 502, 119706. [Google Scholar] [CrossRef]
- Lladó, S.; López-Mondéjar, R.; Baldrian, P. Forest soil bacteria: Diversity, involvement in ecosystem processes, and response to global change. Microbiol. Mol. Biol. Rev. 2017, 81, e00063-16. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Zobel, M. How mycorrhizal associations drive plant population and community biology. Science 2020, 367, eaba1223. [Google Scholar] [CrossRef]
- Mooshammer, M.; Wanek, W.; Hämmerle, I.; Fuchslueger, L.; Hofhansl, F.; Knoltsch, A.; Schnecker, J.; Takriti, M.; Watzka, M.; Wild, B.; et al. Adjustment of microbial nitrogen use efficiency to carbon: Nitrogen imbalances regulates soil nitrogen cycling. Nat. Commun. 2014, 5, 3694. [Google Scholar] [CrossRef]
- Anderson-Teixeira, K.J.; Herrmann, V.; Morgan, R.B.; Bond-Lamberty, B.; Cook-Patton, S.C.; Ferson, A.E.; Muller-Landau, H.C.; Wang, M.M. Carbon cycling in mature and regrowth forests globally. Environ. Res. Lett. 2021, 16, 053009. [Google Scholar] [CrossRef]
- Chang, W.; Ma, W.; Song, L.; Tang, Y.; Long, Y.; Xu, G.; Yuan, J. Responses of soil N-cycle enzyme activities to vegetation degradation in a wet meadow on the Qinghai-Tibet Plateau. Front. Ecol. Evol. 2023, 11, 1210643. [Google Scholar] [CrossRef]
- Wang, Y.; Jiao, P.; Guo, W.; Du, D.; Hu, Y.; Tan, X.; Liu, X. Changes in bulk and rhizosphere soil microbial diversity and composition along an age gradient of Chinese fir (Cunninghamia lanceolate) plantations in subtropical China. Front. Microbiol. 2022, 12, 777862. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Liu, Y.; Chen, H.; Hu, Y. Response of bacterial and fungal soil communities to Chinese fir (Cunninghamia lanceolate) long-term monoculture plantations. Front. Microbiol. 2020, 11, 181. [Google Scholar] [CrossRef] [PubMed]
- ISO 10390:2021; Soil, Treated Biowaste and Sludge—Determination of pH. International Organization for Standardization: Geneva, Switzerland, 2021.
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis: Part 3 Chemical Methods; American Society of Agronomy: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Jiang, P.; Xu, Q.; Xu, Z.; Cao, Z. Seasonal changes in soil labile organic carbon pools within a Phyllostachys praecox stand under high rate fertilization and winter mulch in subtropical China. For. Ecol. Manag. 2006, 236, 30–36. [Google Scholar] [CrossRef]
- Blair, G.; Lefroy, R.; Lisle, L. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust. J. Agric. Res. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Foss Analytical AB. Manual for Kjeltec System 2300 Distilling and Titration Unit; Foss Analytical AB: Höganäs, Sweden, 2003. [Google Scholar]
- Kjeldahl, J. Neue methode zur bestimmung des stickstoffs in organischen körpern. Z. Anorg. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Khan, S.; Mulvaney, R.; Hoeft, R. A simple soil test for detecting sites that are nonresponsive to nitrogen fertilization. Soil Sci. Soc. Am. J. 2001, 65, 1751–1760. [Google Scholar] [CrossRef]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen—Total. In Methods of Soil Analysis. Part 2: Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Allen, S.E. Chemical Analysis of Ecologicalmaterials; Blackwell Scientific Publications: Oxford, UK, 1974. [Google Scholar]
- Lu, K. Analytical Methods of Soil and Agricultural Chemistry; China Agricultural Science and Technology Press: Beijing, China, 1999. [Google Scholar]
- Wu, J.; Xiao, H.; Tong, C. Methods and Applications of Soil Microbial Biomass Measurement; China Meteorological Press: Beijing, China, 2006. [Google Scholar]
- Guan, S. Soil Enzyme and Research Technology; Agriculture Press: Beijing, China, 1986. [Google Scholar]
- Bilgin, R.; Yalcin, M.S.; Yildirim, D. Optimization of covalent immobilization of Trichoderma reesei cellulase onto modified ReliZyme HA403 and Sepabeads EC-EP supports for cellulose hydrolysis, in buffer and ionic liquids/buffer media. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- German, D.P.; Weintraub, M.N.; Grandy, A.S.; Lauber, C.L.; Rinkes, Z.L.; Allison, S.D. Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol. Biochem. 2011, 43, 1387–1397. [Google Scholar] [CrossRef]
- Duan, H.; Cao, S.; Zheng, H.; Hu, D.; Lin, J.; Lin, H.; Hu, R.; Sun, Y.; Li, Y. Variation in the growth traits and wood properties of Chinese fir from six provinces of southern China. Forests 2016, 7, 192. [Google Scholar] [CrossRef]
- Trivedi, P.; Delgado-Baquerizo, M.; Trivedi, C.; Hu, H.; Anderson, I.C.; Jeffries, T.C.; Zhou, J.; Singh, B.K. Microbial regulation of the soil carbon cycle: Evidence from gene–enzyme relationships. ISME J. 2016, 10, 2593–2604. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Hair, J.F. A Primer on Partial Least Squares Structural Equation Modeling (PLS-SEM); Sage: Thousand Oaks, CA, USA, 2014. [Google Scholar]
- Wang, H.; Wang, Z.; Qin, Q.; Ke, Q.; Chen, L.; Song, X.; Chen, X.; Wu, L.; Cao, J. Successive monoculture of Eucalyptus spp. alters the structure and network connectivity, rather than the assembly pattern of rhizosphere and bulk soil bacteria. Appl. Soil Ecol. 2024, 203, 105678. [Google Scholar] [CrossRef]
- Qiao, L.; Guan, Z.; Ren, F.; Ma, T. Comparative analysis of rhizosphere microbial communities in monoculture and mixed oak-pine forests: Structural and functional insights. Front. Microbiol. 2025, 16, 1646535. [Google Scholar] [CrossRef]
- Rehling, F.; Sandner, T.M.; Matthies, D. Biomass partitioning in response to intraspecific competition depends on nutrients and species characteristics: A study of 43 plant species. J. Ecol. 2021, 109, 2219–2233. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, G.; Luo, G.; Yang, T.; Wu, Q. Community-level consequences of harsh environmental constraints based on spatial patterns analysis in karst primary forest of southwest China. For. Ecol. Manag. 2021, 488, 119021. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Hardrath, A.; Jin, H.; Van Kleunen, M. Increases in multiple resources promote competitive ability of naturalized non-native plants. Commun. Biol. 2022, 5, 1150. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lu, K.; Liu, X.; Zhu, Y.; Liu, C. Comparative functional genome analysis reveals the habitat adaptation and biocontrol characteristics of plant growth-promoting bacteria in NCBI databases. Microbiol. Spectr. 2023, 11, e05007–e05022. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Song, Y.; Zhao, L.; Chen, P.; Bu, C.; Liu, P.; Zhang, D. The genetic basis of phosphorus utilization efficiency in plants provide new insight into woody perennial plants improvement. Int. J. Mol. Sci. 2022, 23, 2353. [Google Scholar] [CrossRef]
- Barrow, N.J.; Hartemink, A.E. The effects of pH on nutrient availability depend on both soils and plants. Plant Soil 2023, 487, 21–37. [Google Scholar] [CrossRef]
- Yang, M.; He, X.; Liang, J.; Liu, Q.; Fu, L.; Cui, X.; Huang, S.; Luan, H. Linkage of living microbial biomass, function, and necromass to soil organic carbon storage along a chronosequence of Larix principis-rupprechtii plantation in North China. Front. Microbiol. 2025, 16, 1588030. [Google Scholar] [CrossRef] [PubMed]
- Villarino, S.H.; Pinto, P.; Jackson, R.B.; Piñeiro, G. Plant rhizodeposition: A key factor for soil organic matter formation in stable fractions. Sci. Adv. 2021, 7, eabd3176. [Google Scholar] [CrossRef]
- Tao, F.; Huang, Y.; Hungate, B.A.; Manzoni, S.; Frey, S.D.; Schmidt, M.W.; Reichstein, M.; Carvalhais, N.; Ciais, P.; Jiang, L. Microbial carbon use efficiency promotes global soil carbon storage. Nature 2023, 618, 981–985. [Google Scholar] [CrossRef]
- Tischer, A.; Blagodatskaya, E.; Hamer, U. Microbial community structure and resource availability drive the catalytic efficiency of soil enzymes under land-use change conditions. Soil Biol. Biochem. 2015, 89, 226–237. [Google Scholar] [CrossRef]
- Li, J.; Dong, L.; Fan, M.; Shangguan, Z. Long-term vegetation restoration promotes lignin phenol preservation and microbial anabolism in forest plantations: Implications for soil organic carbon dynamics. Sci. Total Environ. 2024, 928, 172635. [Google Scholar] [CrossRef]
- Witzgall, K.; Vidal, A.; Schubert, D.I.; Höschen, C.; Schweizer, S.A.; Buegger, F.; Pouteau, V.; Chenu, C.; Mueller, C.W. Particulate organic matter as a functional soil component for persistent soil organic carbon. Nat. Commun. 2021, 12, 4115. [Google Scholar] [CrossRef]
- Ma, J.; Han, H.; Cheng, X. Soil temperatures and active carbon components as key drivers of C stock dynamics between two different stand ages of Larix principis-rupprechtii plantation. PeerJ 2020, 8, e8384. [Google Scholar] [CrossRef]
- Penuelas, J. Decreasing efficiency and slowdown of the increase in terrestrial carbon-sink activity. One Earth 2023, 6, 591–594. [Google Scholar] [CrossRef]
- Zhang, H.; Du, Y.; Pan, H.; Li, M.; Hu, S.; Tang, Y.; Wang, H.; Liu, X.; Hu, B.; Bao, W. Deforestation for terraced orchards declined microbial carbon use efficiency in arid valley soils. Ecol. Process. 2025, 14, 83. [Google Scholar] [CrossRef]
- Janusz, G.; Mazur, A.; Pawlik, A.; Kołodyńska, D.; Jaroszewicz, B.; Marzec-Grządziel, A.; Koper, P. Metagenomic Analysis of the Composition of Microbial Consortia Involved in Spruce Degradation over Time in Białowieża Natural Forest. Biomolecules 2023, 13, 1466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, Z.; Li, X.; Wu, J.; Li, Z.; Yang, Z.; Wan, H.Y.; Pan, D.; Jiang, S.; Yue, X. Soil phosphorus availability is enhanced by nitrogen and litter addition during the growing season. Plant Soil 2024, 504, 847–859. [Google Scholar] [CrossRef]
- Pfülb, L.; Elsgaard, L.; Dörsch, P.; Fuß, R.; Well, R. Impact of liming and maize residues on N2O and N2 fluxes in agricultural soils: An incubation study. Biol. Fertil. Soils 2025, 61, 575–594. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, X.; Terrer, C.; Choi, W.J.; Chen, J.; Luo, Y.; Ciais, P. Global evidence for joint effects of multiple natural and anthropogenic drivers on soil nitrogen cycling. Glob. Change Biol. 2024, 30, e17309. [Google Scholar] [CrossRef] [PubMed]
- Goulding, K. Soil acidification and the importance of liming agricultural soils with particular reference to the United Kingdom. Soil Use Manag. 2016, 32, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Chen, Q.; Lei, P.; Xiang, W.; Ouyang, S.; Chen, L. Soil fungal communities and enzyme activities along local tree species diversity gradient in subtropical evergreen forest. Forests 2021, 12, 1321. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, G.; Luo, X.; Hou, E.; Zheng, M.; Zhang, L.; He, X.; Shen, W.; Wen, D. Mycorrhizal fungi and phosphatase involvement in rhizosphere phosphorus transformations improves plant nutrition during subtropical forest succession. Soil Biol. Biochem. 2021, 153, 108099. [Google Scholar] [CrossRef]
- Jian, Z.; Zeng, L.; Lei, L.; Frey, B.; Liu, C.; Shen, Y.; Zhang, J.; Xiao, W.; Li, M.-H. Fungi stimulate organic phosphorus fraction transformation in subtropical masson pine plantation soils after nine years of thinning and understory removal. Ecol. Process. 2025, 14, 23. [Google Scholar] [CrossRef]
- Yan, T.; Lü, X.-T.; Zhu, J.-J.; Yang, K.; Yu, L.-Z.; Gao, T. Changes in nitrogen and phosphorus cycling suggest a transition to phosphorus limitation with the stand development of larch plantations. Plant Soil 2018, 422, 385–396. [Google Scholar] [CrossRef]
- Du, E.; Terrer, C.; Pellegrini, A.F.; Ahlström, A.; van Lissa, C.J.; Zhao, X.; Xia, N.; Wu, X.; Jackson, R.B. Global patterns of terrestrial nitrogen and phosphorus limitation. Nat. Geosci. 2020, 13, 221–226. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Reich, P.B.; Trivedi, C.; Eldridge, D.J.; Abades, S.; Alfaro, F.D.; Bastida, F.; Berhe, A.A.; Cutler, N.A.; Gallardo, A. Multiple elements of soil biodiversity drive ecosystem functions across biomes. Nat. Ecol. Evol. 2020, 4, 210–220. [Google Scholar] [CrossRef]
- Tian, K.; Chen, S.; Ye, R.; Xie, Y.; Yao, L.; Lin, H. Initial microbiome and tree root status structured the soil microbial community discrepancy of the subtropical pine-oak forest in a large urban forest park. Front. Microbiol. 2024, 15, 1391863. [Google Scholar] [CrossRef] [PubMed]
- Eaton, W.D.; Hamilton, D.A. Increasing Ages of Inga punctata Tree Soils Facilitate Greater Fungal Community Abundance and Successional Development, and Efficiency of Microbial Organic Carbon Utilization. Microorganisms 2024, 12, 1996. [Google Scholar] [CrossRef]
- Qu, Q.; Zhang, Z.; Peijnenburg, W.; Liu, W.; Lu, T.; Hu, B.; Chen, J.; Chen, J.; Lin, Z.; Qian, H. Rhizosphere microbiome assembly and its impact on plant growth. J. Agric. Food Chem. 2020, 68, 5024–5038. [Google Scholar] [CrossRef]
- He, B.; Zhang, P.; Bai, X.; Li, W.; Zou, S. Compartment-specific dynamics of soil microbiota along a Pinus armandii plantation chronosequence in Karst mountain ecosystems. Front. Microbiol. 2025, 16, 1626892. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, N.; Zhang, S.; Zhu, X.; Wang, H.; Xiu, W.; Zhao, J.; Liu, H.; Zhang, H.; Yang, D. Soil bacterial community composition is altered more by soil nutrient availability than pH following long-term nutrient addition in a temperate steppe. Front. Microbiol. 2024, 15, 1455891. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, B.; Wu, L.; Liu, S.; Yue, L.; Wu, J.; Chen, D. Fifty-year habitat subdivision enhances soil microbial biomass and diversity across subtropical land-bridge islands. Front. Microbiol. 2022, 13, 1063340. [Google Scholar] [CrossRef]
- Tian, Y.; Shi, C.; Malo, C.; Kwatcho Kengdo, S.; Heinzle, J.; Inselsbacher, E.; Ottner, F.; Borken, W.; Michel, K.; Schindlbacher, A. Long-term soil warming decreases microbial phosphorus utilization by increasing abiotic phosphorus sorption and phosphorus losses. Nat. Commun. 2023, 14, 864. [Google Scholar] [CrossRef]
- Na, S.; Hayden, H.L.; He, J.Z.; He, Z.Y.; Ghaderi, R.; Bi, L.; Hu, H.W. Bacterivorous Nematodes Drive Ammonification and Bacterial Community Growth in a Strongly Acidic Soil. J. Sustain. Agric. Environ. 2025, 4, e70057. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Z.; Liu, Y.; Han, Y.; Wang, Y.; Wang, Q.; Liu, T. Nematodes and their bacterial prey improve phosphorus acquisition by wheat. New Phytol. 2023, 237, 974–986. [Google Scholar] [CrossRef] [PubMed]
- Kane, J.L.; Kotcon, J.B.; Freedman, Z.B.; Morrissey, E.M. Fungivorous nematodes drive microbial diversity and carbon cycling in soil. Ecology 2023, 104, e3844. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.; Orlando, V.; Collett, R.L.; Moreira, D.; Costa, S.R.; Inácio, M.L. Linking nematode communities and soil health under climate change. Sustainability 2023, 15, 11747. [Google Scholar] [CrossRef]
- Li, X.; Liu, T.; Li, H.; Geisen, S.; Hu, F.; Liu, M. Management effects on soil nematode abundance differ among functional groups and land-use types at a global scale. J. Anim. Ecol. 2022, 91, 1770–1780. [Google Scholar] [CrossRef]
- Pan, C.; Sun, C.; Qu, X.; Yu, W.; Guo, J.; Yu, Y.; Li, X. Microbial community interactions determine the mineralization of soil organic phosphorus in subtropical forest ecosystems. Microbiol. Spectr. 2024, 12, e01355-23. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, J.; Hui, D.; Wang, Y.; Li, J.; Chen, J.; Chen, G.; Zhu, Y.; Zhang, L.; Zhang, D. Mycorrhizal fungi alleviate acidification-induced phosphorus limitation: Evidence from a decade-long field experiment of simulated acid deposition in a tropical forest in south China. Glob. Change Biol. 2022, 28, 3605–3619. [Google Scholar] [CrossRef]
- Liao, X.; Zhao, J.; Magura, T.; Zhang, W.; Pan, F.; Hu, P.; Xiao, D.; Li, J.; Wang, K. Multitrophic biodiversity drives soil phosphorus mobilization in subtropical ecosystems. J. Adv. Res. 2025, in press. [Google Scholar] [CrossRef]
- Chen, H.; Gou, M.; Hu, J.; Lei, L.; Zhu, S.; Hu, R.; Zhao, H.; Xiao, W.; Liu, C. Seasonal Variations in Soil Enzyme Activity and Nutrient Limitations of Differently Aged Pinus massoniana Plantation. Microorganisms 2024, 12, 2314. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Chen, F.; Huang, R.; Deng, X.; Jiang, Y. Seasonal dynamics of soil microbial biomass C and N of Keteleeria fortunei var. cyclolepis forests with different ages. J. For. Res. 2020, 31, 2377–2384. [Google Scholar] [CrossRef]
- Li, J.; Luo, D.; Ma, G.; Jia, L.; Xu, J.; Huang, H.; Tong, Z.; Lu, Y.-Q. Response of Chinese fir seedlings to low phosphorus stress and analysis of gene expression differences. J. For. Res. 2019, 30, 183–192. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Q.; Wang, D.; Zhang, Z. Soil microbial community, soil quality, and productivity along a chronosequence of Larix principis-rupprechtii forests. Plants 2023, 12, 2913. [Google Scholar] [CrossRef]
- Della Mónica, I.F.; Godoy, M.S.; Godeas, A.M.; Scervino, J.M. Fungal extracellular phosphatases: Their role in P cycling under different pH and P sources availability. J. Appl. Microbiol. 2018, 124, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yang, H. Utilization of soil residual phosphorus and internal reuse of phosphorus by crops. PeerJ 2021, 9, e11704. [Google Scholar] [CrossRef]
- Oliverio, A.M.; Bissett, A.; McGuire, K.; Saltonstall, K.; Turner, B.L.; Fierer, N. The role of phosphorus limitation in shaping soil bacterial communities and their metabolic capabilities. MBio 2020, 11, e01718-20. [Google Scholar] [CrossRef]
- Schaap, K.J.; Fuchslueger, L.; Quesada, C.A.; Hofhansl, F.; Valverde-Barrantes, O.; Camargo, P.B.; Hoosbeek, M.R. Seasonal fluctuations of extracellular enzyme activities are related to the biogeochemical cycling of C, N and P in a tropical terra-firme forest. Biogeochemistry 2023, 163, 1–15. [Google Scholar] [CrossRef]
- Huang, W.J.; Zhou, G.Y.; Liu, J.X. Nitrogen and phosphorus status and their influence on aboveground production under increasing nitrogen deposition in three successional forests. Acta Oecol. 2012, 44, 20–27. [Google Scholar] [CrossRef]
- Li, Z.; Mao, C.; Wu, Q.; Peng, Y.; Wang, J.; Zhang, B.; Zhang, S.; Liang, X.; Yan, W.; Chen, X. Temporal Variations in Aboveground Biomass, Nutrient Content, and Ecological Stoichiometry in Young and Middle-Aged Stands of Chinese Fir Forests. Plants 2024, 13, 1877. [Google Scholar] [CrossRef] [PubMed]
- Moretti, S.; Goddard, M.-L.; Puca, A.; Lalevée, J.; Di Marco, S.; Mugnai, L.; Gelhaye, E.; Goodell, B.; Bertsch, C.; Farine, S. First description of non-enzymatic radical-generating mechanisms adopted by Fomitiporia mediterranea: An unexplored pathway of the white rot agent of the esca complex of diseases. J. Fungi 2023, 9, 498. [Google Scholar] [CrossRef] [PubMed]
- García-Martín, A.B.; Rodríguez, J.; Molina-Guijarro, J.M.; Fajardo, C.; Domínguez, G.; Hernández, M.; Guillén, F. Induction of extracellular hydroxyl radicals production in the white-rot fungus pleurotus eryngii for dyes degradation: An advanced bio-oxidation process. J. Fungi 2024, 10, 52. [Google Scholar] [CrossRef]
- Cao, T.; Luo, Y.; Shi, M.; Tian, X.; Kuzyakov, Y. Microbial interactions for nutrient acquisition in soil: Miners, scavengers, and carriers. Soil Biol. Biochem. 2024, 188, 109215. [Google Scholar] [CrossRef]
- Liu, H.; Li, S.; Qiang, R.; Lu, E.; Li, C.; Zhang, J.; Gao, Q. Response of soil microbial community structure to phosphate fertilizer reduction and combinations of microbial fertilizer. Front. Environ. Sci. 2022, 10, 899727. [Google Scholar] [CrossRef]
- Deng, J.; Hu, J.; Huang, Y.; Wang, S.; Ye, S. Mixed planting of subtropical Chinese fir in South China improves microbial carbon source metabolism and functional diversity through the accumulation of nutrients from soil aggregates. Front. Microbiol. 2024, 15, 1404428. [Google Scholar] [CrossRef] [PubMed]
- Onet, A.; Grenni, P.; Onet, C.; Stoian, V.; Crisan, V. Forest soil microbiomes: A review of key research from 2003 to 2023. Forests 2025, 16, 148. [Google Scholar] [CrossRef]
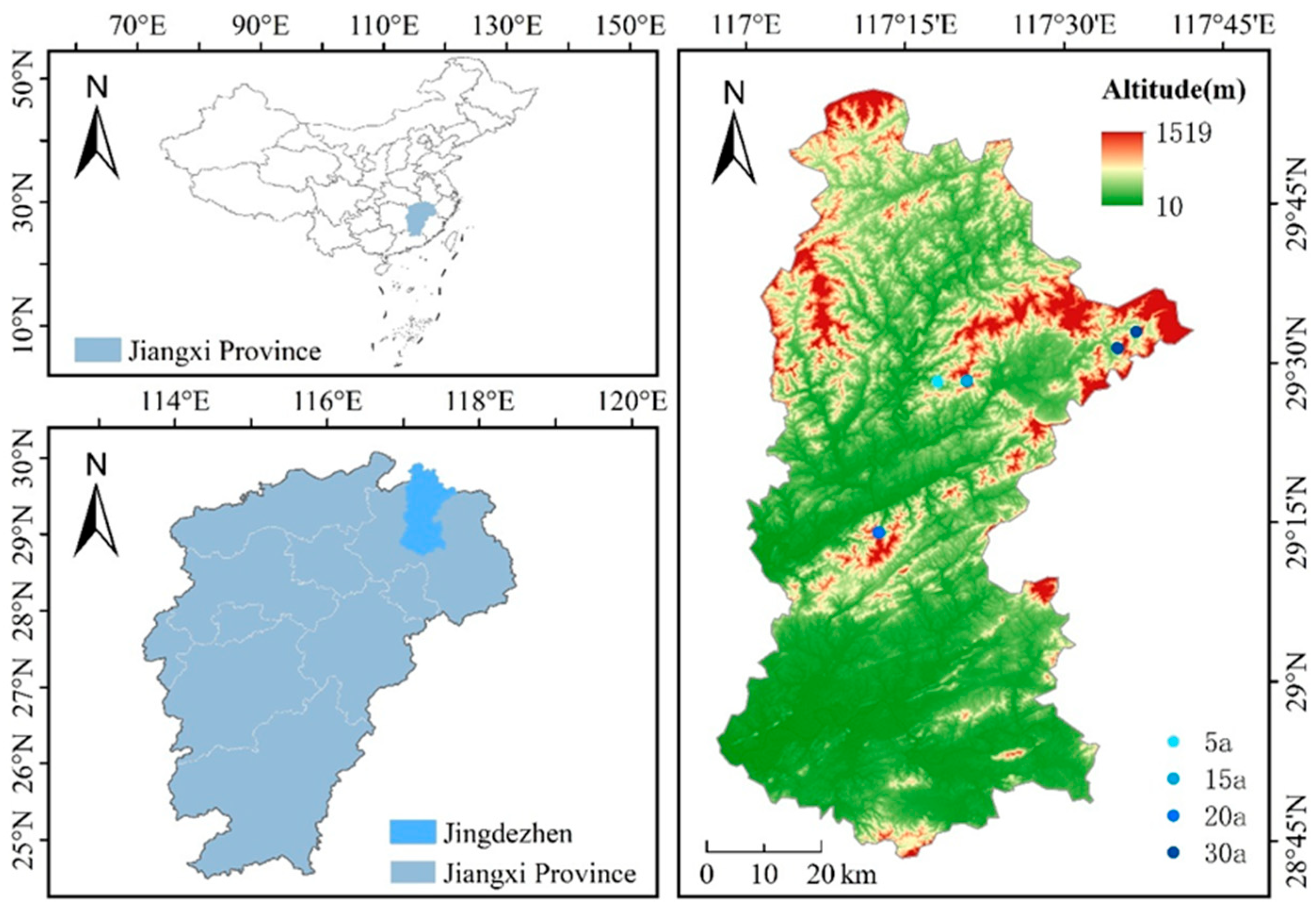
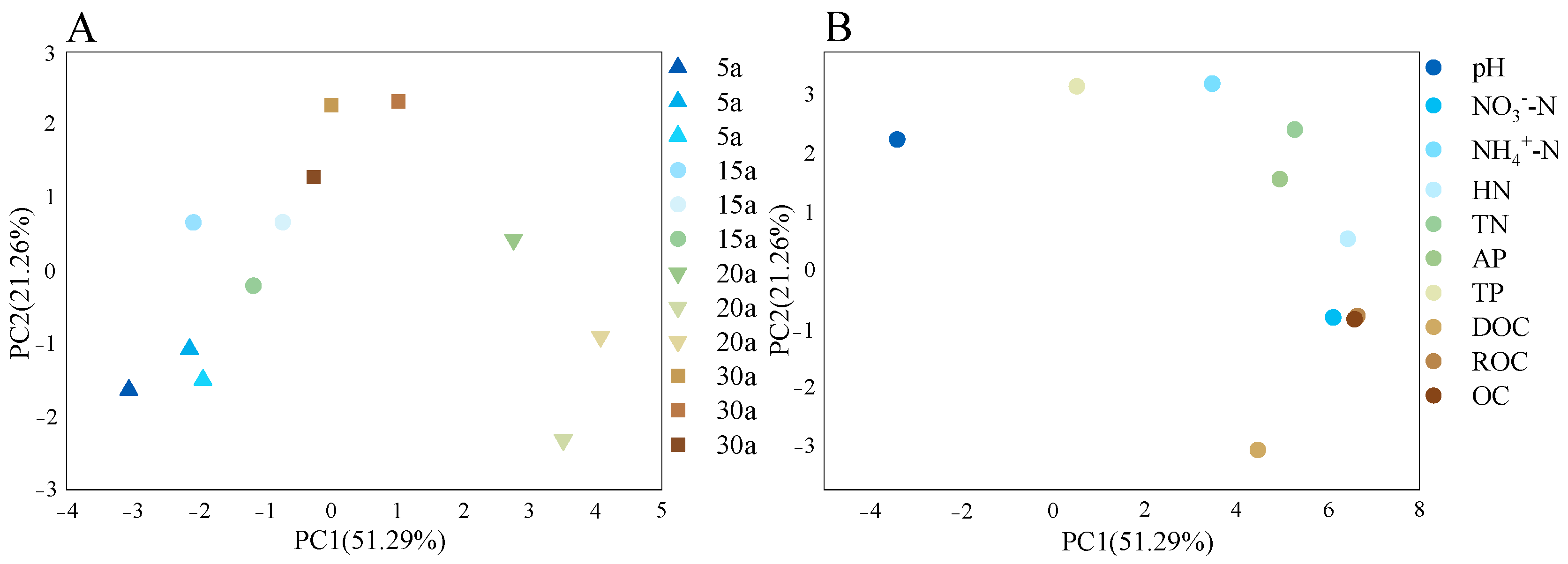


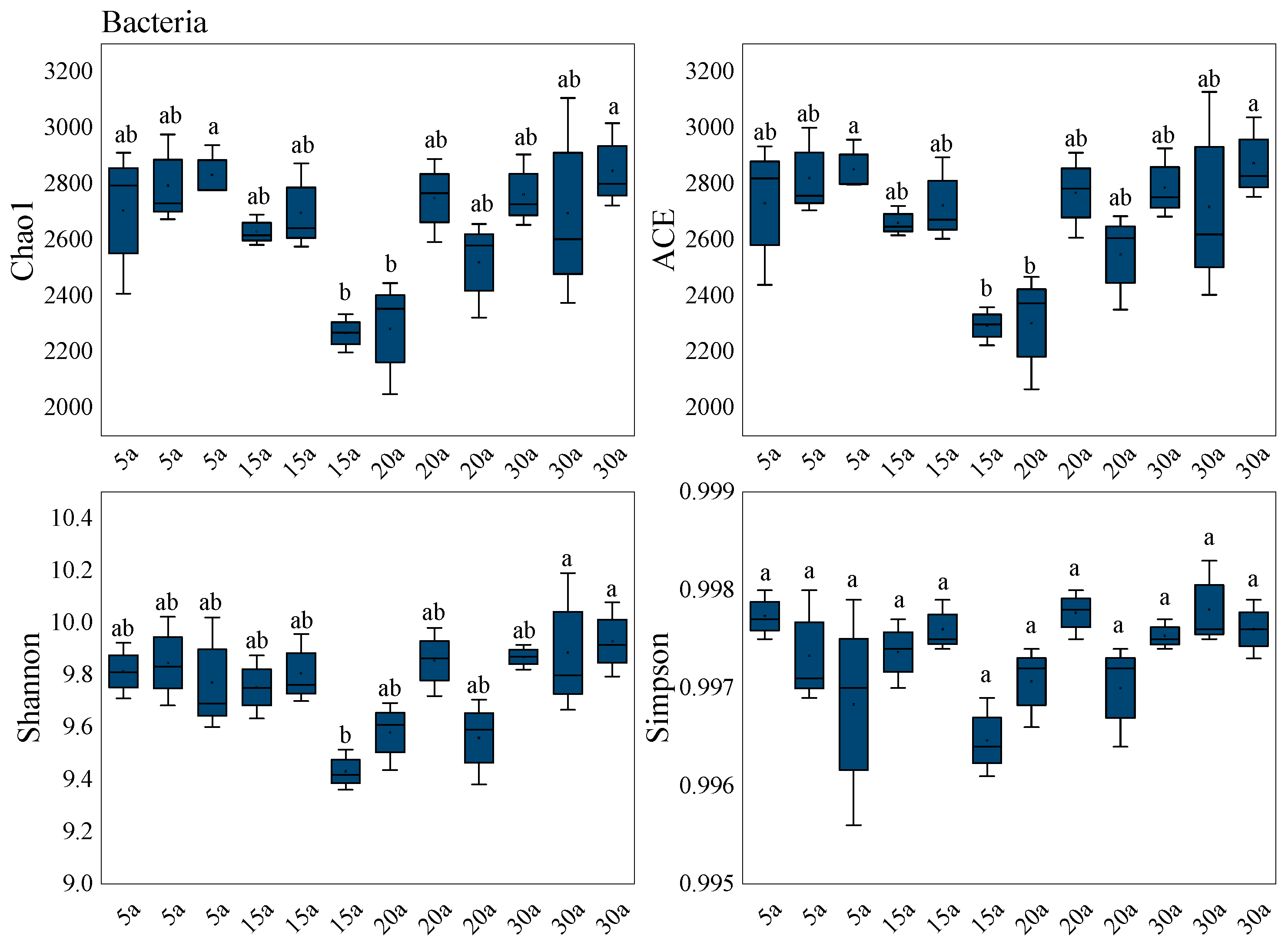
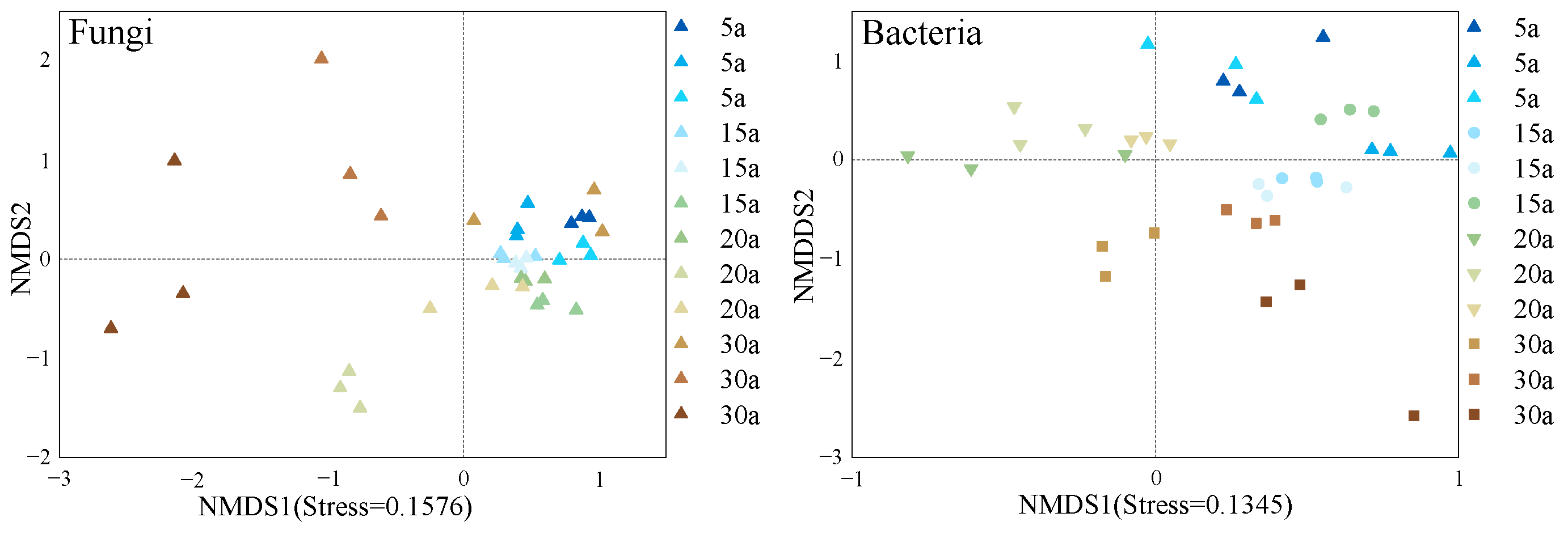
| Stand Age | MTV | MAI | Mean DBH | Mean Height |
|---|---|---|---|---|
| (Year) | (m3) | (m3·year−1) | (cm) | (m) |
| 5 | 0.002 ± 0.001 c | 0.02 ± 0.01 b | 3.9 ± 0.3 b | 3.2 ± 0.2 b |
| 15 | 0.051 ± 0.009 bc | 0.84 ± 0.19 a | 11.0 ± 0.7 a | 8.5 ± 0.5 a |
| 20 | 0.098 ± 0.021 b | 0.33 ± 0.14 ab | 13.7 ± 1.2 a | 10.2 ± 0.4 a |
| 30 | 0.115 ± 0.028 a | 0.53 ± 0.04 ab | 14.1 ± 1.2 a | 11.1 ± 1.5 a |
| Stand Age | pH | OC | DOC | ROC | TN | NO3−-N | NH4+-N | HN | TP | AP |
|---|---|---|---|---|---|---|---|---|---|---|
| (Year) | (g·kg−1) | (mg·kg−1) | (g·kg−1) | (g·kg−1) | (mg·kg−1) | (mg·kg−1) | (mg·kg−1) | (g·kg−1) | (mg·kg−1) | |
| 5 | 5.04 ± 0.04 a | 22.96 ± 1.94 b | 33.47 ± 1.53 b | 8.02 ± 0.16 b | 1.83 ± 0.05 b | 1.02 ± 0.41 b | 21.19 ± 2.89 b | 142.75 ± 4.05 b | 0.41 ± 0.03 b | 2.74 ± 0.21 a |
| 15 | 5.41 ± 0.12 a | 22.13 ± 0.52 b | 33.32 ± 0.61 b | 9.46 ± 0.49 b | 2.12 ± 0.06 ab | 1.35 ± 0.64 b | 35.52 ± 0.72 a | 165.83 ± 7.39 ab | 0.38 ± 0.04 b | 3.12 ± 0.33 a |
| 20 | 4.88 ± 0.10 a | 32.87 ± 1.75 a | 42.73 ± 2.22 a | 18.56 ± 0.54 a | 2.51 ± 0.15 a | 7.50 ± 0.47 a | 36.36 ± 6.27 a | 200.58 ± 8.05 a | 0.42 ± 0.02 b | 3.60 ± 0.11 a |
| 30 | 5.14 ± 0.14 a | 26.43 ± 1.28 b | 27.01 ± 2.33 b | 10.35 ± 0.96 b | 2.46 ± 0.23 a | 3.10 ± 1.42 b | 37.82 ± 0.71 a | 174.00 ± 10.88 ab | 0.70 ± 0.13 a | 3.72 ± 0.23 a |
| Stand Age | MBC | MBN | MBP |
|---|---|---|---|
| (Year) | (mg·kg−1) | (mg·kg−1) | (mg·kg−1) |
| 5 | 218.83 ± 4.12 c | 32.90 ± 6.00 a | 9.52 ± 2.85 b |
| 15 | 212.36 ± 13.91 c | 35.38 ± 5.10 a | 18.02 ± 0.97 ab |
| 20 | 487.33 ± 22.09 a | 60.00 ± 5.10 a | 25.65 ± 2.81 a |
| 30 | 374.50 ± 0.29 b | 39.49 ± 7.57 a | 21.19 ± 4.13 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Sun, H.; Zhang, J.; Dong, L. Productivity Dynamics in Chinese Fir Plantations: The Driving Role of Plant–Soil–Microbe Interactions in Northern Subtropical China. Forests 2025, 16, 1854. https://doi.org/10.3390/f16121854
Wang L, Sun H, Zhang J, Dong L. Productivity Dynamics in Chinese Fir Plantations: The Driving Role of Plant–Soil–Microbe Interactions in Northern Subtropical China. Forests. 2025; 16(12):1854. https://doi.org/10.3390/f16121854
Chicago/Turabian StyleWang, Lijie, Honggang Sun, Jianfeng Zhang, and Linshui Dong. 2025. "Productivity Dynamics in Chinese Fir Plantations: The Driving Role of Plant–Soil–Microbe Interactions in Northern Subtropical China" Forests 16, no. 12: 1854. https://doi.org/10.3390/f16121854
APA StyleWang, L., Sun, H., Zhang, J., & Dong, L. (2025). Productivity Dynamics in Chinese Fir Plantations: The Driving Role of Plant–Soil–Microbe Interactions in Northern Subtropical China. Forests, 16(12), 1854. https://doi.org/10.3390/f16121854






