Host-Specific Fungal Assemblages, Dominated by Ophiostomatoid Taxa, in Scots Pine Bark Beetles from Slovakia Revealed by Metabarcoding
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Bark Beetle Sampling
2.2. DNA Extraction, PCR, and Sequencing Analysis
2.3. Data Processing
2.4. Statistical Analysis
3. Results
3.1. Bark Beetle Identification
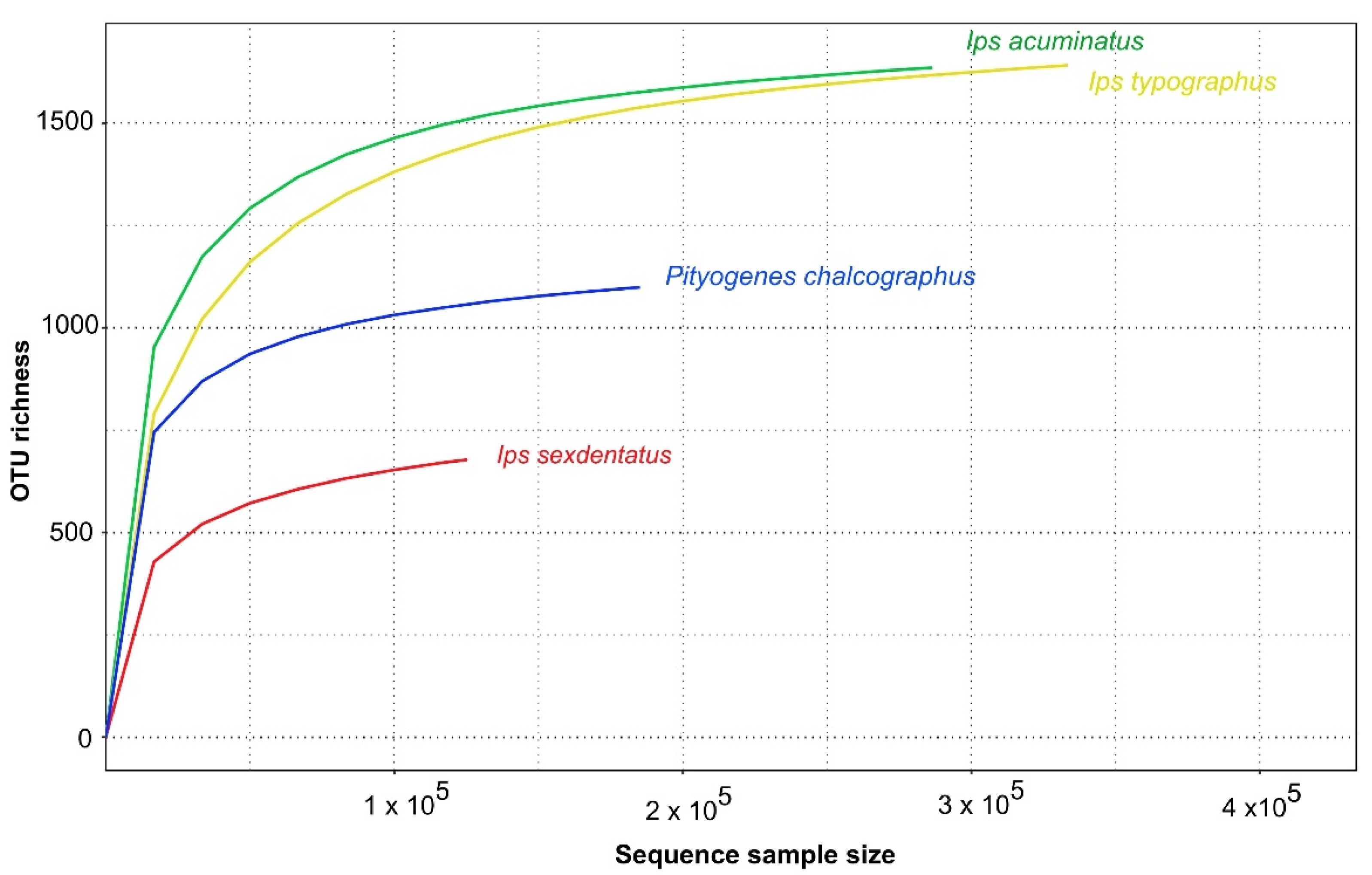
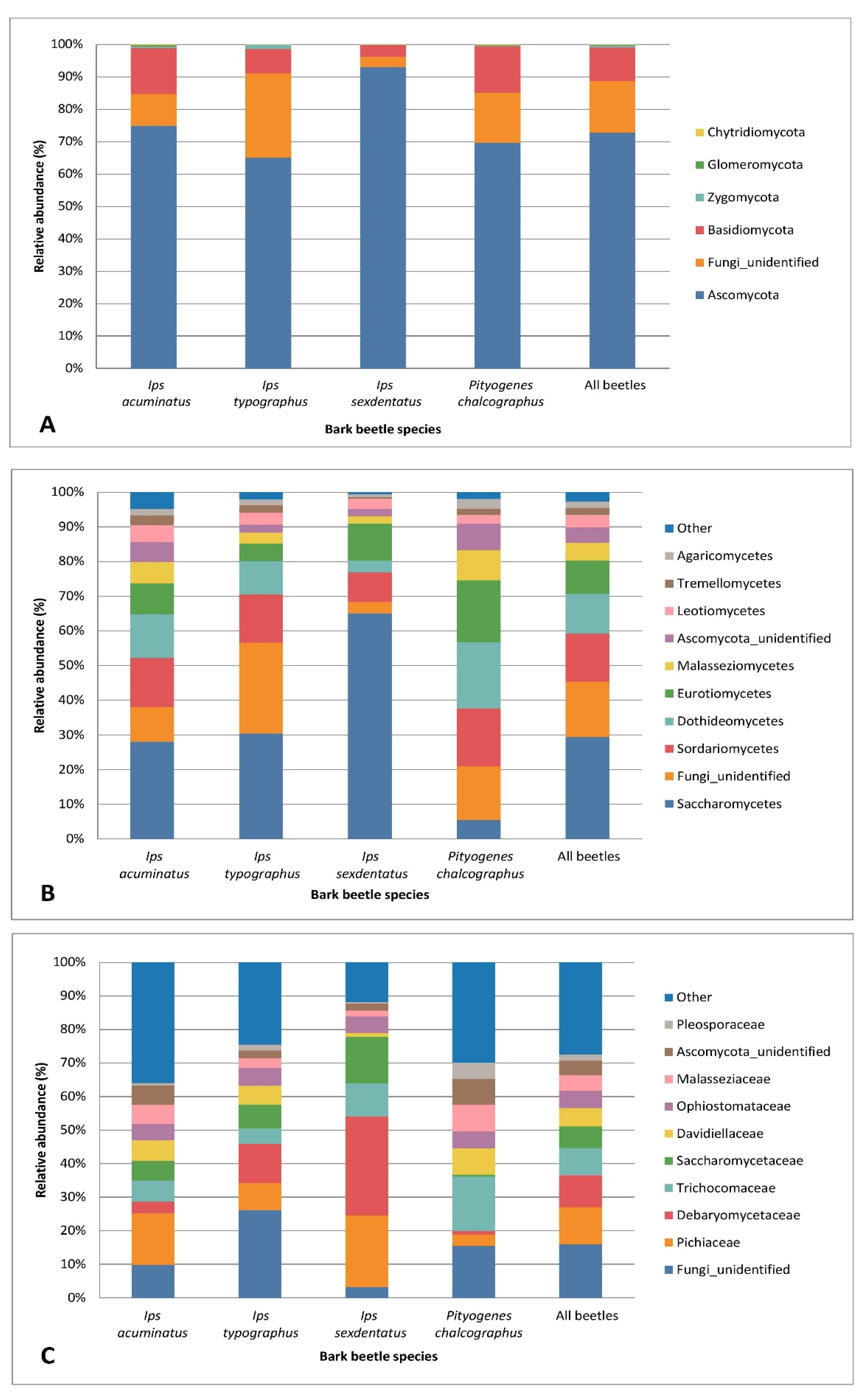
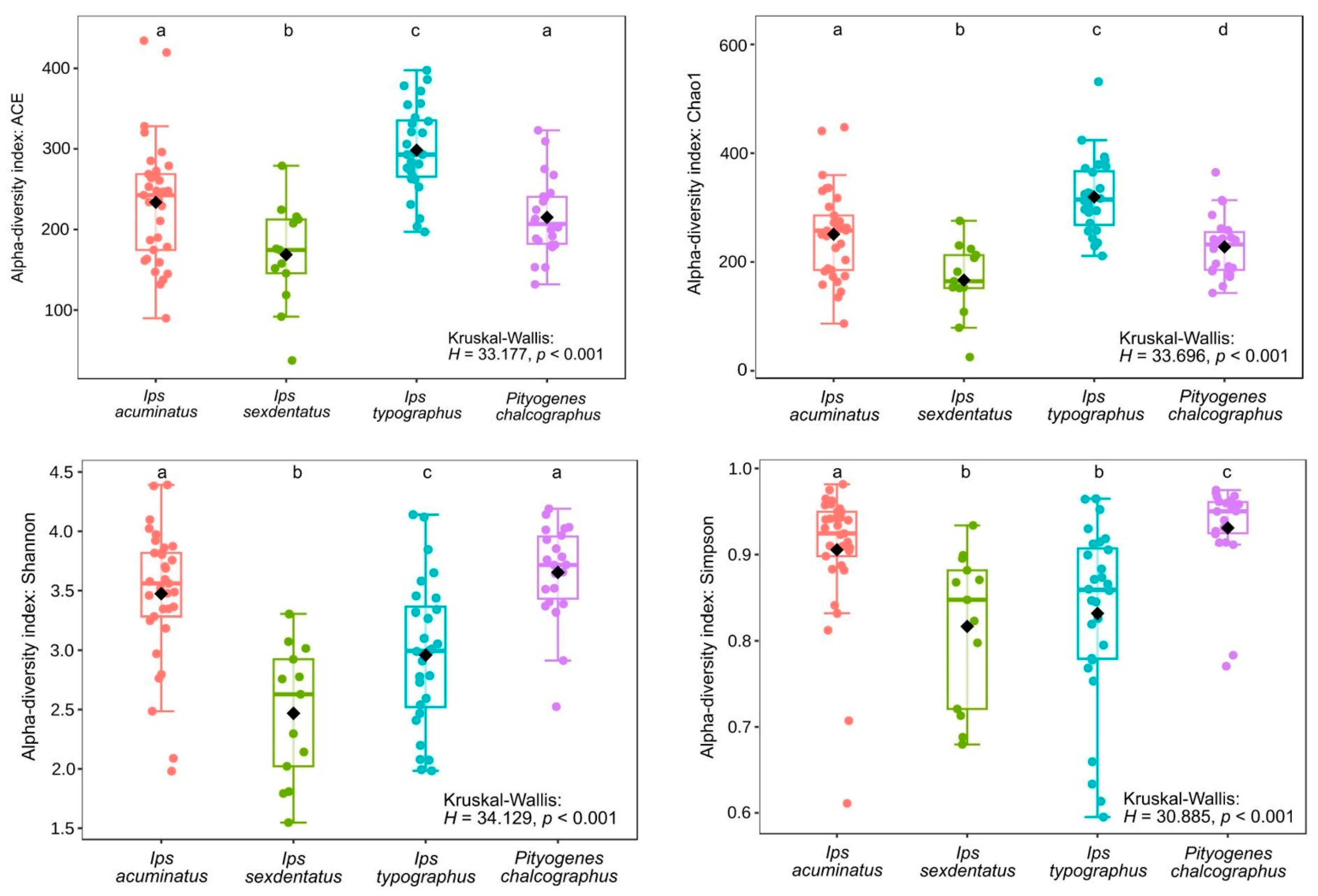
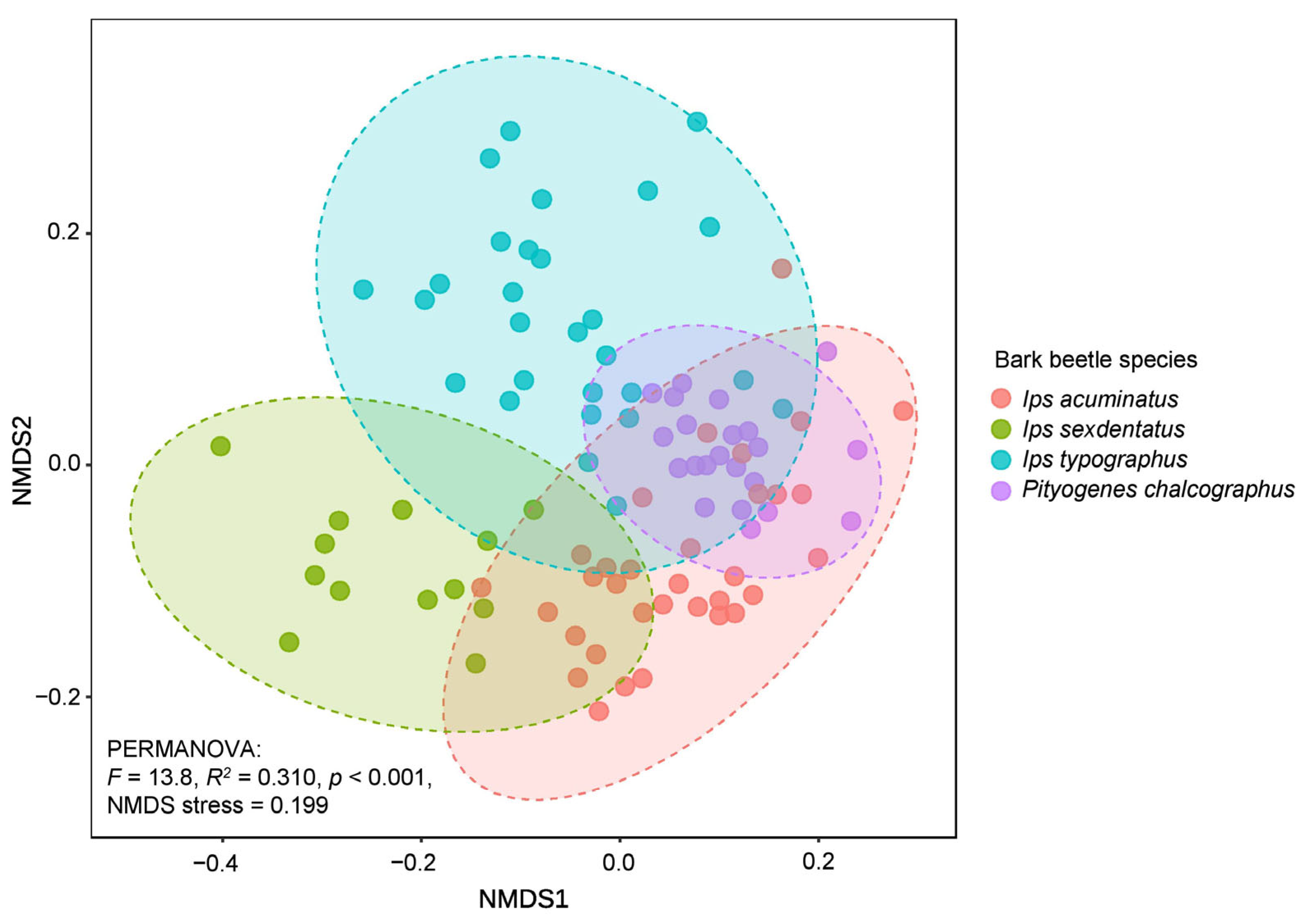
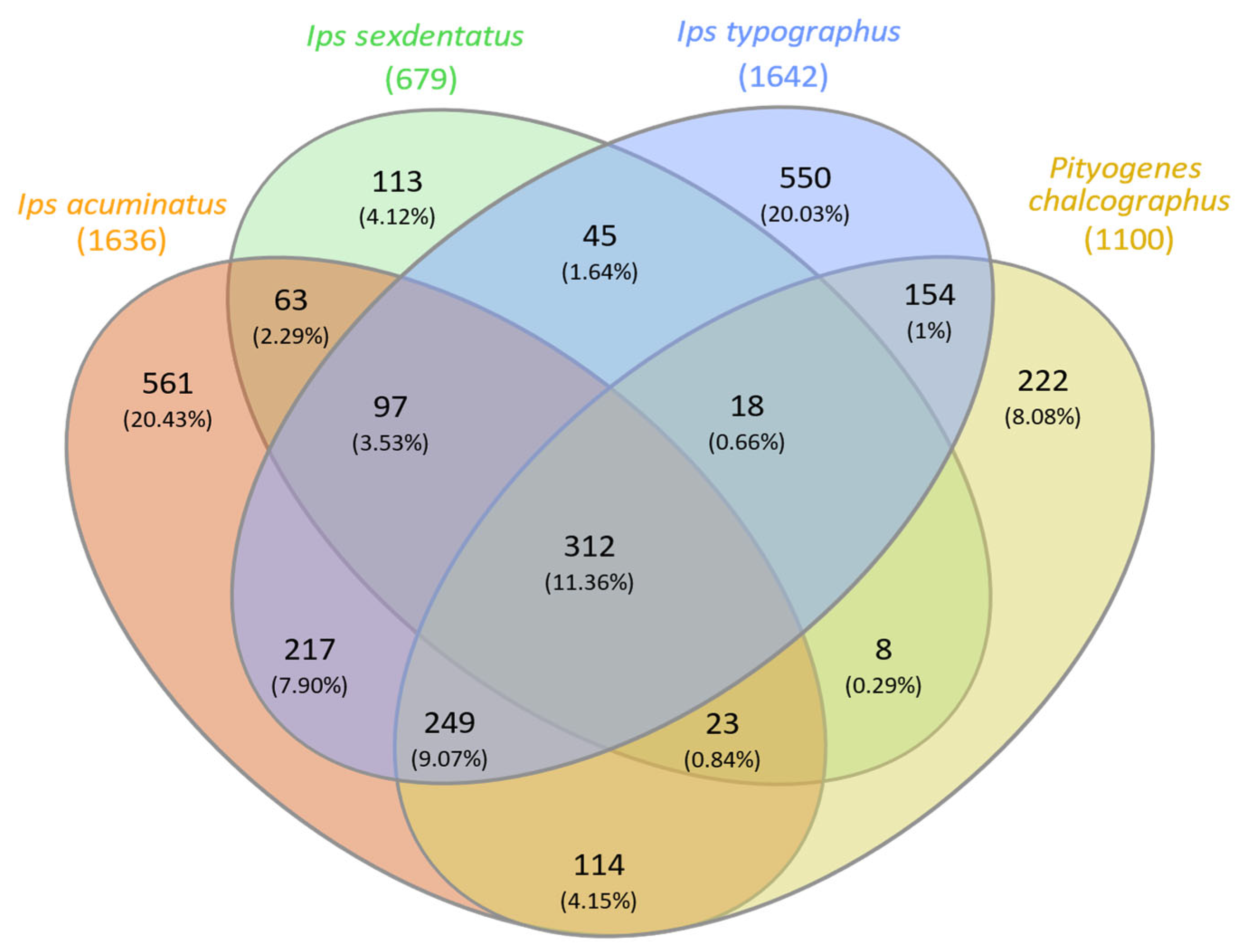
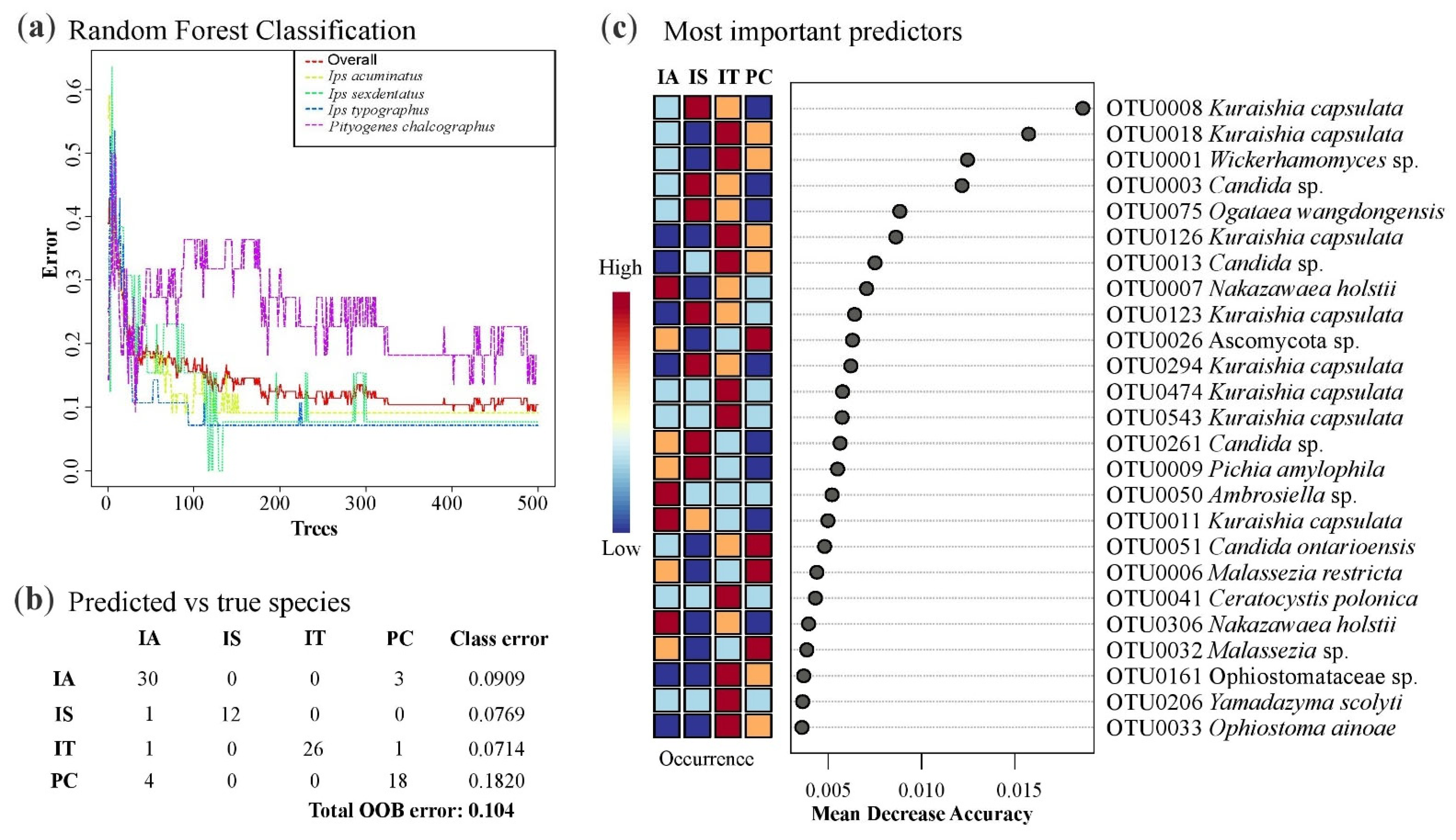
3.2. Bark Beetle Surface Mycobiome
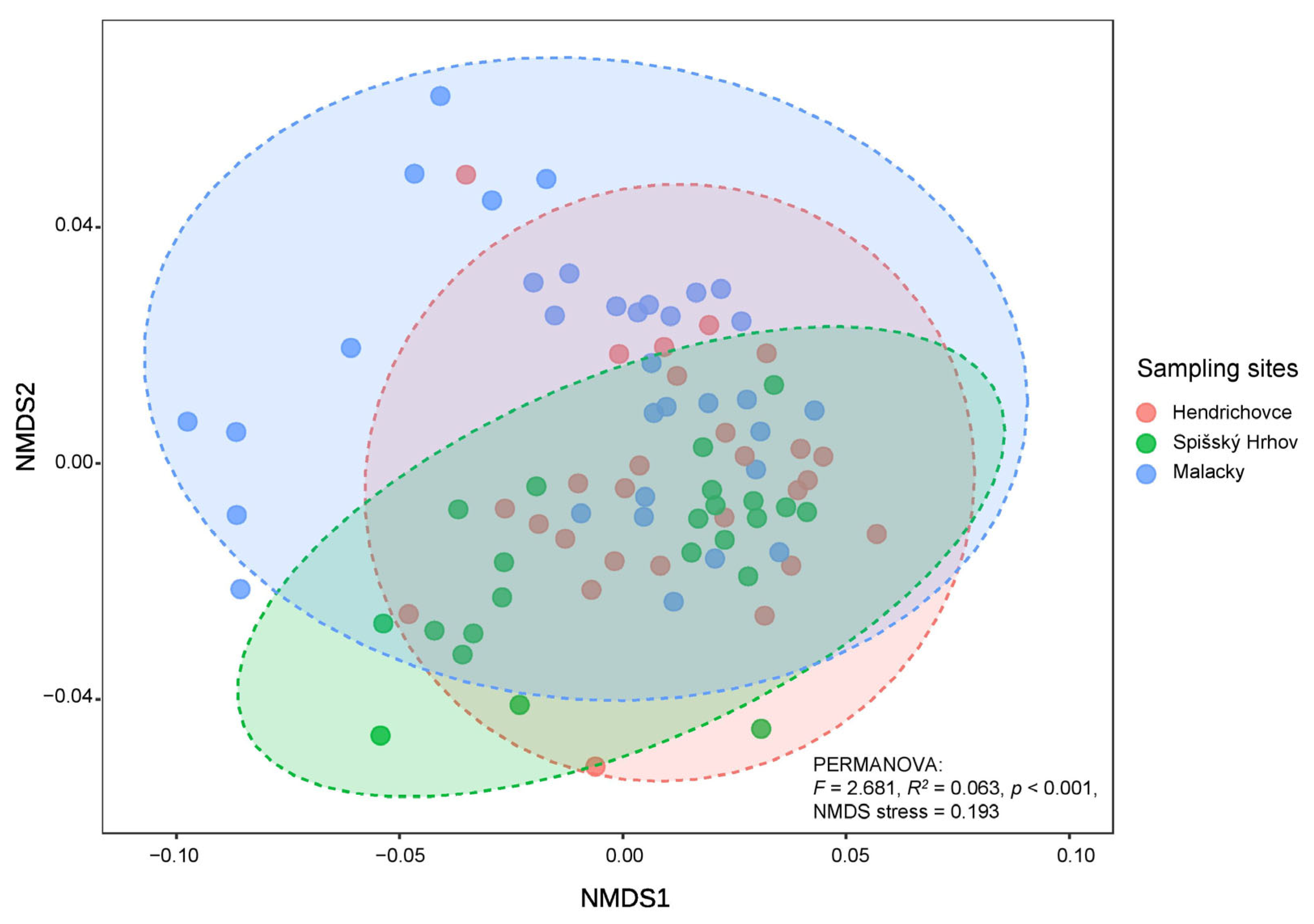
3.3. Habitat-Specific Fungal Communities
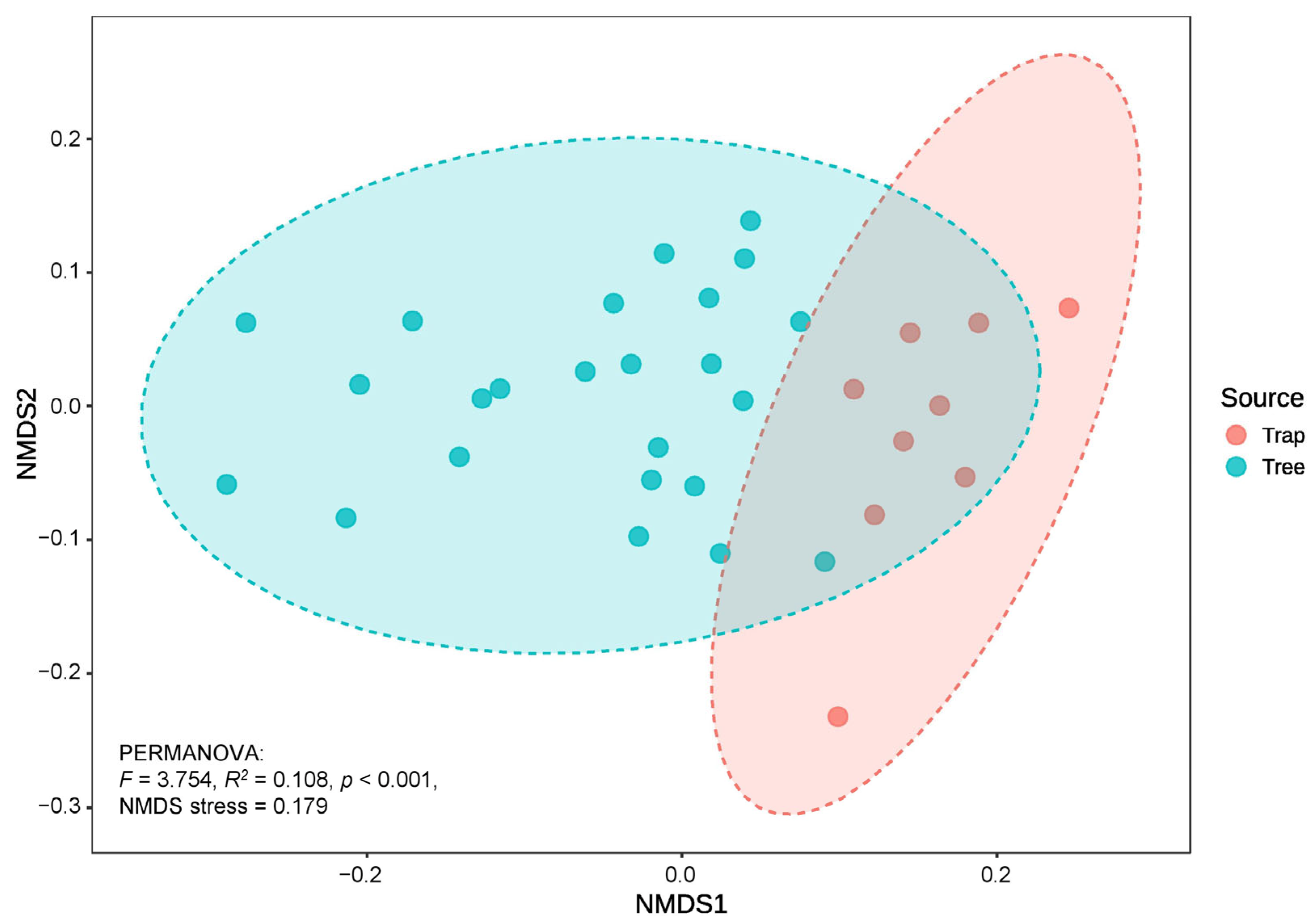
3.4. Impact of Bark Beetles Collection Method on Fungal Communities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegert, C.; Clay, N.; Pace, K.; Vissa, S.; Hofstetter, R.W.; Leverón, O.; Riggins, J.J. Bark beetle-driven community and biogeochemical impacts in forest ecosystems: A review. Ann. Entomol. Soc. Am. 2024, 117, 163–183. [Google Scholar] [CrossRef]
- Progar, R.A.; Eglitis, A.; Lundquist, J.E. Some Ecological, Economic, and Social Consequences of Bark Beetle Infestations. In The Western Bark Beetle Research Group: A Unique Collaboration with Forest Health Protection. In Proceedings of the Symposium at the 2007 Society of American Foresters Conference, Portland, OR, USA, 23–28 October 2007; Hayes, J.L., Lundquist, J.E., Eds.; US Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2009; Volume 784, pp. 71–83. [Google Scholar]
- Hlásny, T.; Krokene, P.; Liebhold, A.; Montagné-Huck, C.; Müller, J.; Qin, H.; Raffa, K.; Schelhaas, M.J.; Seidl, R.; Svoboda, M.; et al. Living with Bark Beetles: Impacts, Outlook and Management Options. From Science to Policy 8; European Forest Institute: Joensuu, Finland, 2019; 50p. [Google Scholar] [CrossRef]
- Gandhi, K.J.K.; Miller, C.N.; Fornwalt, P.J.; Frank, J.M. Bark beetle outbreaks alter biotic components of forested ecosystems. In Bark Beetle Management, Ecology, and Climate Change, 1st ed.; Gandhi, K.J.K., Hofstetter, R.W., Eds.; Academic Press: Amsterdam, The Netherlands, 2022; pp. 227–259. [Google Scholar] [CrossRef]
- Schowalter, T.D. Ecology and management of bark beetles (Coleoptera: Curculionidae: Scolytinae) in southern pine forests. J. Integr. Pest Manag. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Holmes, T.; Koch, F. Bark Beetle Epidemics, Life Satisfaction, and Economic Well-Being. Forests 2019, 10, 696. [Google Scholar] [CrossRef]
- Jirošová, A.; Modlinger, R.; Hradecký, J.; Ramakrishnan, R.; Beránková, K.; Kandasamy, D. Ophiostomatoid fungi synergize attraction of the Eurasian spruce bark beetle, Ips typographus, to its aggregation pheromone in field traps. Front. Microbiol. 2022, 13, 980251. [Google Scholar] [CrossRef]
- Anonymous. Správa o Lesnom Hospodárstve v Slovenskej Republike za Rok 2023: Zelená Správa [Report on the Status of Forestry in the Slovak Republic of 2023, Green Report]; Ministry of Agriculture and Rural Development of the Slovak Republic: Bratislava, Slovakia, 2024; 78p.
- Vakula, J.; Gubka, A.; Galko, J.; Rell, S.; Úradník, M. Plošné odumieranie borovíc v Záhorskej nížine. In Aktuálne Problémy v Ochrane Lesa 2013; Kunca, A., Ed.; National Forest Centre: Zvolen, Slovakia, 2013; pp. 105–112. [Google Scholar]
- Chakraborty, A.; Modlinger, R.; Ashraf, M.Z.; Synek, J.; Schlyter, F.; Roy, A. Core Mycobiome and Their Ecological Relevance in the Gut of Five Ips Bark Beetles (Coleoptera: Curculionidae: Scolytinae). Front. Microbiol. 2020, 11, 568853. [Google Scholar] [CrossRef]
- Kirisits, T. Fungal Associates of European Bark Beetles with Special Emphasis on the Ophiostomatoid Fungi. In Bark and Wood Boring Insects in Living Trees in Europe, a Synthesis; Lieutier, F., Day, K.R., Battisti, A., Grégoire, J.C., Evans, H.F., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 181–236. [Google Scholar] [CrossRef]
- Wingfield, M.J.; Brockerhoff, E.G.; Wingfield, B.D.; Slippers, B. Planted Forest Health: The Need for a Global Strategy. Science 2015, 349, 832–836. [Google Scholar] [CrossRef]
- Jankowiak, R.; Kacprzyk, M.; Młynarczyk, M. Diversity of ophiostomatoid fungi associated with bark beetles (Coleoptera: Scolytidae) colonizing branches of Norway spruce (Picea abies) in southern Poland. Biologia 2009, 64, 1170–1177. [Google Scholar] [CrossRef]
- Repe, A.; Kirisits, T.; Piškur, B.; de Groot, M.; Kump, B.; Jurc, M. Ophiostomatoid fungi associated with three spruce-infesting bark beetles in Slovenia. Ann. For. Sci. 2013, 70, 717–727. [Google Scholar] [CrossRef]
- Jankowiak, R. Fungi associated with Tomicus minor on Pinus sylvestris in Poland and their succession into the sapwood of beetle-infested windblown trees. Can. J. For. Res. 2008, 38, 2579–2588. [Google Scholar] [CrossRef]
- Jankowiak, R. Ophiostomatoid fungi associated with Ips sexdentatus on Pinus sylvestris in Poland. Dendrobiology 2012, 68, 43–54. [Google Scholar] [CrossRef]
- Pastirčáková, K.; Adamčíková, K.; Pastirčák, M.; Zach, P.; Galko, J.; Kováč, M.; Laco, J. Two blue-stain fungi colonizing Scots pine (Pinus sylvestris) trees infested by bark beetles in Slovakia, Central Europe. Biologia 2018, 73, 1053–1066. [Google Scholar] [CrossRef]
- Bochníček, O.; Kajaba, P. Air temperature changes in the period 1991–2020. Meteorol. Čas. 2023, 26, 73–89. [Google Scholar]
- Labudová, L.; Ivaňáková, G.; Faško, P.; Kajaba, P.; Labuda, M. Changes in drought occurrence and intensity in the context of climate change in Slovakia. Theor. Appl. Climatol. 2024, 155, 4009–4022. [Google Scholar] [CrossRef]
- Slovenský Hydrometeorologický Ústav (SHMÚ). Bulletin Meteorológia a Klimatológia Slovenská Republika 2023, 29, Issues 5–7. Available online: https://www.shmu.sk/sk/index.php?page=1613 (accessed on 20 October 2025).
- Pfeffer, A. Zentral- Und Westpaläarktische Borken- Und Kernkäfer (Coleoptera: Scolytidae, Platypodidae); Pro Entomologia, c/o Naturhistorisches Museum: Basel, Switzerland, 1995; 310p. [Google Scholar]
- Ihrmark, K.; Bödeker, I.T.M.; Cruz-Martinez, K.; Friberg, H.; Kubartova, A.; Schenck, J.; Strid, Y.; Stenlid, J.; Brandström-Durling, M.; Clemmensen, K.E.; et al. New primers to amplify the fungal ITS2 region—Evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol. Ecol. 2012, 82, 666–677. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications, 1st ed.; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Vetrovský, T.; Baldrian, P.; Morais, D. SEED 2: A user-friendly platform for amplicon high-throughput sequencing data analyses. Bioinformatics 2018, 34, 2292–2294. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 27 August 2025).
- Wang, Q.; Cole, J.R. Updated RDP taxonomy and RDP Classifier for more accurate taxonomic classification. Microbiol. Resour. Announc. 2024, 13, e01063-23. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhou, G.; Ewald, J.; Pang, Z.; Shiri, T.; Xia, J. MicrobiomeAnalyst 2.0: Comprehensive statistical, functional and integrative analysis of microbiome data. Nucleic Acids Res. 2023, 51, W310–W318. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Menkis, A.; Vasiliauskas, R.; Taylor, A.F.S.; Stenlid, J.; Finlay, R. Fungal Communities in Mycorrhizal Roots of Conifer Seedlings in Forest Nurseries under Different Cultivation Systems, Assessed by Morphotyping, Direct Sequencing and Mycelial Isolation. Mycorhiza 2005, 16, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Abarenkov, K.; Zirk, A.; Piirmann, T.; Pöhönen, R.; Ivanov, F.; Nilsson, R.H.; Kõljalg, U. Full UNITE+INSD Dataset for Eukaryotes, Version 10.05.2021; UNITE Community, 2021; Available online: https://doi.plutof.ut.ee/doi/10.15156/BIO/1281567 (accessed on 27 August 2025).
- Six, D.L.; Wingfield, M.J. The Role of Phytopathogenicity in Bark Beetle–Fungus Symbioses: A Challenge to the Classic Paradigm. Annu. Rev. Entomol. 2011, 56, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Davydenko, K.; Baturkin, D.; Dyshko, V.; Lazarević, J.; Marčiulynas, A.; Elfstrand, M.; Vasaitis, R.; Menkis, A. Fungal Pathogens Associated with Tomicus Species in European Forests: Regional Variations and Impacts on Forest Health. Insects 2025, 16, 277. [Google Scholar] [CrossRef] [PubMed]
- Kolařík, M.; Jankowiak, R. Vector Affinity and Diversity of Geosmithia Fungi Living on Subcortical Insects Inhabiting Pinaceae Species in Central and Northeastern Europe. Microb. Ecol. 2013, 66, 682–700. [Google Scholar] [CrossRef]
- Six, D.L.; Klepzig, K.D. Context Dependency in Bark Beetle–Fungus Mutualisms Revisited: Assessing Potential Shifts in Interaction Outcomes Against Varied Genetic, Ecological, and Evolutionary Backgrounds. Front. Microbiol. 2021, 12, 682187. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Larsson, K.H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE Database for Molecular Identification of Fungi: Handling Dark Taxa and Parallel Taxonomic Classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef]
- Hofstetter, R.W.; Dinkins-Bookwalter, J.; Davis, T.S.; Klepzig, K.D. Symbiotic Associations of Bark Beetles. In Bark Beetles—Biology and Ecology of Native and Invasive Species; Vega, F.E., Hofstetter, R.W., Eds.; Academic Press: Amsterdam, The Netherlands, 2015; pp. 209–245. [Google Scholar] [CrossRef]
- Davis, T.S. The Ecology of Yeasts in the Bark Beetle Holobiont: A Century of Research Revisited. Microb. Ecol. 2015, 69, 723–732. [Google Scholar] [CrossRef]
- Hulcr, J.; Stelinski, L.L. The Ambrosia Symbiosis: From Evolutionary Ecology to Practical Management. Annu. Rev. Entomol. 2017, 62, 285–303. [Google Scholar] [CrossRef]
- Cheng, T.; Veselská, T.; Křížková, B.; Švec, K.; Havlíček, V.; Stadler, M.; Kolařík, M. Insight into the genomes of dominant yeast symbionts of European spruce bark beetle, Ips typographus. Front. Microbiol. 2023, 14, 1108975. [Google Scholar] [CrossRef]
- Botella, L.; Diez, J.J. Phylogenic diversity of fungal endophytes in Spanish stands of Pinus halepensis. Fungal Divers. 2011, 47, 9–18. [Google Scholar] [CrossRef]
- Giordano, L.; Garbelotto, M.; Nicolotti, G.; Gonthier, P. Characterization of fungal communities associated with the bark beetle Ips typographus varies depending on detection method, location, and beetle population levels. Mycol. Progr. 2013, 12, 127–140. [Google Scholar] [CrossRef]
- Barta, M.; Kautmanová, I.; Čičková, H.; Ferenčík, J.; Florián, Š.; Novotný, J.; Kozánek, M. Hypocrealean fungi associated with populations of Ips typographus in West Carpathians and selection of local Beauveria strains for effective bark beetle control. Biologia 2018, 73, 53–65. [Google Scholar] [CrossRef]
- Barta, M.; Takov, D.; Pilarska, D.; Doychev, D.; Kádasi Horáková, M. Entomopathogenic fungi of the genus Beauveria and their pathogenicity to Ips typographus (Coleoptera: Curculionidae) in the Vitosha National Park, Bulgaria. J. For. Sci. 2020, 66, 420–435. [Google Scholar] [CrossRef]
- Hyblerová, S.; Medo, J.; Barta, M. Diversity and prevalence of entomopathogenic fungi (Ascomycota, Hypocreales) in epidemic populations of bark beetles (Coleoptera, Scolytinae) in spruce forests of the Tatra National Park in Slovakia. Ann. For. Res. 2021, 64, 129–145. [Google Scholar] [CrossRef]
- Milosavljević, M.; Tabaković-Tošić, M.; Radulović, Z.; Marković, M.; Rindoš, M. Isolation, identification and phylogenetic position of entomopathogenic fungus Beauveria bassiana from Ips typographus in Serbia. Fresenius Environ. Bull. 2021, 30, 9443–9448. [Google Scholar]
- Vakula, J.; Nikolov, C.; Lalík, M.; Kádasi-Horáková, M.; Rell, S.; Galko, J.; Gubka, A.; Zúbrik, M.; Kunca, A.; Barta, M. Selection, application, and pathogenicity of naturally occurring Beauveria bassiana strains against Ips duplicatus (Coleoptera: Curculionidae, Scolytinae). Biol. Control 2025, 204, 105740. [Google Scholar] [CrossRef]
- Khan, S.; Guo, L.; Maimaiti, Y.; Mijit, M.; Qiu, D. Entomopathogenic fungi as microbial biocontrol agents. Mol. Plant Breed. 2012, 3, 63–79. [Google Scholar] [CrossRef]
- Vega, F.E.; Meyling, N.V.; Luangsa-ard, J.J.; Blackwell, M. Chapter 6—Fungal Entomopathogens. In Insect Pathology, 2nd ed.; Vega, F.E., Kaya, H.K., Eds.; Academic Press: San Diego, CA, USA, 2012; pp. 172–220. [Google Scholar] [CrossRef]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef]
- Jakuš, R.; Blaženec, M. Treatment of bark beetle attacked trees with entomopathogenic fungus Beauveria bassiana (Balsamo) Vuillemin. Folia For. Pol. Ser. A For. 2011, 53, 150–155. [Google Scholar]
- Barta, M.; Kautmanová, I.; Čičková, H.; Ferenčík, J.; Florián, Š.; Novotný, J.; Kozánek, M. The potential of Beauveria bassiana inoculum formulated into a polymeric matrix for a microbial control of spruce bark beetle. Biocontrol Sci. Technol. 2018, 28, 718–735. [Google Scholar] [CrossRef]
- Ortiz-Urquiza, A.; Keyhani, N.O. Action on the Surface: Entomopathogenic Fungi versus the Insect Cuticle. Insects 2013, 4, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Mann, A.J.; Davis, T.S. Entomopathogenic fungi to control bark beetles: A review of ecological recommendations. Pest Manag. Sci. 2021, 77, 3841–3846. [Google Scholar] [CrossRef]
- Wegensteiner, R.; Tkaczuk, C.; Bałazy, S.; Griesser, S.; Rouffaud, M.A.; Stradner, A.; Steinwender, B.M.; Hager, H.; Papierok, B. Occurrence of pathogens in populations of Ips typographus, Ips sexdentatus (Coleoptera, Curculionidae, Scolytinae) and Hylobius spp. (Coleoptera, Curculionidae, Curculioninae) from Austria, Poland and France. Acta Protozool. 2015, 54, 219–232. [Google Scholar] [CrossRef]
- Mann, A.J.; Davis, T.S. Plant secondary metabolites and low temperature are the major limiting factors for Beauveria bassiana (Bals.-Criv.) Vuill. (Ascomycota: Hypocreales) growth and virulence in a bark beetle system. Biol. Control 2020, 141, 104130. [Google Scholar] [CrossRef]
- Davis, T.S.; Stewart, J.E.; Mann, A.; Bradley, C.; Hofstetter, R.W. Evidence for multiple ecological roles of Leptographium abietinum, a symbiotic fungus associated with the North American spruce beetle. Fungal Ecol. 2019, 38, 62–70. [Google Scholar] [CrossRef]
- Draganova, S.; Takov, D.; Doychev, D. Naturally-occurring entomopathogenic fungi on three bark beetle species (Coleoptera: Curculionidae) in Bulgaria. Pestic. Fitomed. 2010, 25, 59–63. [Google Scholar] [CrossRef]
- Ramanenka, M.O.; Panteleev, S.V.; Sazonov, A.A.; Ivashchanka, L.O. Mycobiota of Ips sexdentatus (Borner, 1776) (Coleoptera, Curculionidae: Scolytinae) in Belarus. Entomol. Rev. 2023, 103, 545–556. [Google Scholar] [CrossRef]
- Takov, D.; Belilov, S.; Doychev, D.; Pilarska, D.; Barta, M. Occurrence of parasites and pathogens in the six-toothed bark beetle, Ips sexdentatus (Curculionidae: Scolytinae) in Bulgarian pine stands. In Proceedings of the 33 International Scientific Conference "Management and Quality“—Ecology and management of forest ecosystems, Sofia, Bulgaria, 9–10 October 2024; pp. 18–24. [Google Scholar]
- Six, D.L. Ecological and Evolutionary Determinants of Bark Beetle–Fungus Symbioses. Insects 2012, 3, 339–366. [Google Scholar] [CrossRef] [PubMed]
- Linnakoski, R.; de Beer, Z.W.; Niemelä, P.; Wingfield, M.J. Associations of Conifer-Infesting Bark Beetles and Fungi in Fennoscandia. Insects 2012, 3, 200–227. [Google Scholar] [CrossRef] [PubMed]
- Klepzig, K.D.; Six, D.L. Bark Beetle–Fungal Symbiosis: Context Dependency in Complex Associations. Symbiosis 2004, 37, 189–205. [Google Scholar]
- Lieutier, F.; Yart, A.; Salle, A. Stimulation of Tree Defences by Ophiostomatoid Fungi Can Explain Attack Success of Bark Beetles on Conifers. Ann. For. Sci. 2009, 66, 801. [Google Scholar] [CrossRef]
- Miller, K.E.; Hopkins, K.; Inward, D.J.G.; Vogler, A.P. Metabarcoding of fungal communities associated with bark beetles. Ecol. Evol. 2016, 6, 1590–1600. [Google Scholar] [CrossRef]
- Ceballos-Escalera, A.; Richards, J.; Arias, M.B.; Inward, D.J.G.; Vogler, A.P. Metabarcoding of insect-associated fungal communities: A comparison of internal transcribed spacer (ITS) and large-subunit (LSU) rRNA markers. MycoKeys 2022, 88, 1–33. [Google Scholar] [CrossRef]
- Linnakoski, R.; de Beer, Z.W.; Ahtiainen, J.; Sidorov, E.; Niemelä, P.; Pappinen, A.; Wingfield, M.J. Ophiostoma spp. associated with pine- and spruce-infesting bark beetles in Finland and Russia. Persoonia 2010, 25, 72–93. [Google Scholar] [CrossRef] [PubMed]
- Eisenhofer, R.; Minich, J.J.; Marotz, C.; Cooper, A.; Knight, R.; Weyrich, L.S. Contamination in Low Microbial Biomass Microbiome Studies: Issues and Recommendations. Trends Microbiol. 2019, 27, 105–117. [Google Scholar] [CrossRef]
- Rahimlou, S.; Amend, A.S.; James, T.Y. Malassezia in environmental studies is derived from human inputs. mBio 2025, 16, e01142-25. [Google Scholar] [CrossRef]
- Jacobs, K.; Bergdahl, D.R.; Wingfield, M.J.; Halik, S.; Seifert, K.A.; Bright, D.E.; Wingfield, B.D. Leptographium wingfieldii introduced into North America and found associated with exotic Tomicus piniperda and native bark beetles. Mycol. Res. 2004, 108, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-H.; Schlyter, F. Olfactory recognition and behavioral avoidance of angiosperm nonhost volatiles by conifer-inhabiting bark beetles. Agric. Forest Entomol. 2004, 6, 1–19. [Google Scholar] [CrossRef]
| Bark Beetles | Collection Method 1 | Sampling Sites | Total | ||
|---|---|---|---|---|---|
| Hendrichovce | Spišský Hrhov | Malacky | |||
| Ips acuminatus | 24/9 | 11 | 4 | 18 | 33 |
| Ips sexdentatus | 13/0 | 0 | 0 | 13 | 13 |
| Ips typographus | 0/28 | 10 | 10 | 8 | 28 |
| Pityogenes chalcographus | 0/22 | 6 | 9 | 7 | 22 |
| Total | 37/59 | 27 | 23 | 46 | 96 |
| I. sexdentatus | I. acuminatus | I. typographus | P. chalcographus | |
|---|---|---|---|---|
| Ips sexdentatus | – | 0.001 | 0.001 | 0.001 |
| Ips acuminatus | 0.195 | – | 0.001 | 0.001 |
| Ips typographus | 0.200 | 0.130 | – | 0.001 |
| Pityogenes chalcographus | 0.315 | 0.077 | 0.143 | – |
| Fungal OTUs Identified to Genus/Species Level | Ips acuminatus | Ips sexdentatus | Ips typographus | Pityogenes chalcographus | All Beetles |
|---|---|---|---|---|---|
| Alternaria eichhorniae Nag Raj & Ponnappa | 0.545 | 0.538 | 0.0 | 0.0 | 0.292 |
| Beauveria bassiana (Bals.-Criv.) Vuill. | 0.394 | 0.0 | 0.0 | 0.591 | 0.312 |
| Candida sp. | 0.485 | 0.231 | 0.286 | 0.773 | 0.458 |
| Davidiella tassiana (De Not.) Crous & U. Braun | 0.970 | 0.231 | 0.821 | 0.955 | 0.823 |
| Diplodia allocellula Jami, Gryzenh., Slippers & M.J. Wingf. | 0.0 | 0.0 | 0.893 | 0.227 | 0.323 |
| Geosmithia pallida (G. Sm.) M. Kolařík, Kubátová & Pažoutová | 0.242 | 0.231 | 0.500 | 0.500 | 0.375 |
| Kuraishia capsulata (Wick.) Y. Yamada, K. Maeda & Mikata | 0.667 | 0.385 | 0.464 | 0.864 | 0.615 |
| Malassezia restricta E. Guého, J. Guillot & Midgley | 0.939 | 0.308 | 0.464 | 0.955 | 0.719 |
| Malassezia sp. | 0.303 | 0.0 | 0.0 | 0.636 | 0.292 |
| Nakazawaea holstii (Wick.) Y. Yamada, K. Maeda & Mikata | 0.455 | 0.0 | 0.0 | 0.591 | 0.354 |
| Ophiostoma distortum (R.W. Davidson) de Hoog & R.J. Scheff. | 0.455 | 0.0 | 0.0 | 0.682 | 0.354 |
| Ophiostoma minus (Hedgc.) Syd. & P. Syd. | 0.485 | 1.000 | 0.536 | 0.0 | 0.458 |
| Penicillium bialowiezense K.W. Zaleski | 0.879 | 0.308 | 0.536 | 0.909 | 0.708 |
| Penicillium citreonigrum Dierckx | 0.0 | 0.0 | 0.214 | 0.386 | 0.191 |
| Yamadazyma scolyti (Phaff & Yoney.) Billon-Grand | 0.394 | 0.0 | 0.250 | 0.682 | 0.385 |
| Taxa | Family | Frequency (%) | ||||
|---|---|---|---|---|---|---|
| IA (n = 33) | IS (n = 13) | IT (n = 28) | PC (n = 22) | All Beetles (n = 96) | ||
| Ambrosiella sp. 3PG4P A2 | Ceratocystidaceae | 75.76 | 7.69 | 57.14 | 45.45 | 54.17 |
| Ceratocystidaceae sp. | Ceratocystidaceae | 18.18 | – | – | – | 6.25 |
| Ceratocystiopsis minuta (Siemaszko) H.P. Upadhyay & W.B. Kendr. | Ophiostomataceae | 18.18 | 30.77 | 64.29 | 18.18 | 33.33 |
| Ceratocystis polonica (Siemaszko) C. Moreau | Ceratocystidaceae | 3.03 | – | 67.86 | 4.55 | 21.88 |
| Ceratocystis rufipenni M.J. Wingf., T.C. Harr. & H. Solheim | Ceratocystidaceae | – | – | 7.14 | – | 2.08 |
| Ceratocystis sp. CspXger3 | Ceratocystidaceae | 21.21 | 23.08 | 7.14 | 9.09 | 14.58 |
| Geosmithia langdonii M. Kolařík, Kubátová & Pažoutová | Bionectriaceae | 9.09 | – | 14.29 | 13.64 | 10.42 |
| Geosmithia pallida (G. Sm.) M. Kolařík, Kubátová & Pažoutová | Bionectriaceae | 100.00 | 76.92 | 100.00 | 100.00 | 96.88 |
| Geosmithia rufescens M. Kolařík | Bionectriaceae | 3.03 | 7.69 | 3.57 | 4.55 | 4.17 |
| Geosmithia sp. CCF3645 | Bionectriaceae | – | – | – | 4.55 | 1.04 |
| Geosmithia sp. RJ0258 | Bionectriaceae | – | – | – | 4.55 | 1.04 |
| Gondwanamyces sp. JAL 2011b | Ceratocystidaceae | – | – | 3.57 | – | 1.04 |
| Graphium fimbriasporum (M. Morelet) K. Jacobs, Kirisits & M.J. Wingf. | Microascaceae | 9.09 | – | 57.14 | 9.09 | 21.88 |
| Graphium pseudormiticum M. Mouton & M.J. Wingf. | Microascaceae | 6.06 | 7.69 | 32.14 | – | 12.50 |
| Graphium sp. JJK 2009a | Microascaceae | – | – | 3.57 | – | 1.04 |
| Hyalorhinocladiella sp. 2YT4P H2 | Ophiostomataceae | – | 7.69 | 21.43 | – | 7.29 |
| Leptographium cf. truncatum CMW 22857 | Ophiostomataceae | 3.03 | 46.15 | – | 4.55 | 8.33 |
| Ophiostoma ainoae H. Solheim | Ophiostomataceae | 9.09 | 7.69 | 71.43 | 18.18 | 29.17 |
| Ophiostoma bicolor R.W. Davidson & D.E. Wells | Ophiostomataceae | 6.06 | – | 50.00 | 4.55 | 17.71 |
| Ophiostoma brunneociliatum Math.-Käärik | Ophiostomataceae | 30.30 | 76.92 | 46.43 | 22.73 | 39.58 |
| Ophiostoma distortum (R.W. Davidson) de Hoog & R.J. Scheff. | Ophiostomataceae | 96.97 | 92.31 | 100.00 | 100.00 | 97.92 |
| Ophiostoma floccosum Math.-Käärik | Ophiostomataceae | 3.03 | 7.69 | 3.57 | – | 3.13 |
| Ophiostoma ips (Rumbold) Nannf. | Ophiostomataceae | 18.18 | 69.23 | 14.29 | 9.09 | 21.88 |
| Ophiostoma minus (Hedgc.) Syd. & P. Syd. | Ophiostomataceae | 93.94 | 76.92 | 100.00 | 100.00 | 94.79 |
| Ophiostoma piliferum (Fr.) Syd. & P. Syd. | Ophiostomataceae | – | – | 3.57 | – | 1.04 |
| Ophiostoma pluriannulatum (Hedgc.) Syd. & P. Syd. | Ophiostomataceae | 3.03 | 7.69 | – | 18.18 | 6.25 |
| Ophiostoma rectangulosporium Ohtaka, Masuya & Yamaoka | Ophiostomataceae | – | – | 3.57 | – | 1.04 |
| Ophiostoma cf. rectangulosporium 1039RJP | Ophiostomataceae | 51.52 | 15.38 | 42.86 | 45.45 | 42.71 |
| Ophiostoma cf. rectangulosporium CMW 26258 | Ophiostomataceae | 54.55 | – | 17.86 | 22.73 | 29.17 |
| Ophiostoma saponiodorum Linnak., Z.W. de Beer & M.J. Wingf. | Ophiostomataceae | – | 23.08 | – | – | 3.13 |
| Ophiostoma tapionis Linnak., Z.W. de Beer & M.J. Wingf. | Ophiostomataceae | – | – | 7.14 | – | 2.08 |
| Ophiostomataceae sp. | Ophiostomataceae | 45.45 | 23.08 | 78.57 | 63.64 | 56.25 |
| Pesotum australi Kamg. Nkuek., K. Jacobs & M.J. Wingf. | Ophiostomataceae | 18.18 | 15.38 | 14.29 | 27.27 | 18.75 |
| Sporothrix sp. 2 ML 2011 | Ophiostomataceae | – | – | 7.14 | 4.55 | 3.13 |
| Thielaviopsis basicola (Berk. & Broome) Ferraris | Ceratocystidaceae | – | – | – | 4.55 | 1.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barta, M.; Artimová, R.; Medo, J.; Kádasi Horáková, M.; Strmisková, M.; Pastirčáková, K. Host-Specific Fungal Assemblages, Dominated by Ophiostomatoid Taxa, in Scots Pine Bark Beetles from Slovakia Revealed by Metabarcoding. Forests 2025, 16, 1690. https://doi.org/10.3390/f16111690
Barta M, Artimová R, Medo J, Kádasi Horáková M, Strmisková M, Pastirčáková K. Host-Specific Fungal Assemblages, Dominated by Ophiostomatoid Taxa, in Scots Pine Bark Beetles from Slovakia Revealed by Metabarcoding. Forests. 2025; 16(11):1690. https://doi.org/10.3390/f16111690
Chicago/Turabian StyleBarta, Marek, Renata Artimová, Juraj Medo, Miriam Kádasi Horáková, Michaela Strmisková, and Katarína Pastirčáková. 2025. "Host-Specific Fungal Assemblages, Dominated by Ophiostomatoid Taxa, in Scots Pine Bark Beetles from Slovakia Revealed by Metabarcoding" Forests 16, no. 11: 1690. https://doi.org/10.3390/f16111690
APA StyleBarta, M., Artimová, R., Medo, J., Kádasi Horáková, M., Strmisková, M., & Pastirčáková, K. (2025). Host-Specific Fungal Assemblages, Dominated by Ophiostomatoid Taxa, in Scots Pine Bark Beetles from Slovakia Revealed by Metabarcoding. Forests, 16(11), 1690. https://doi.org/10.3390/f16111690






