Formation- and Species-Level Responses of the Atlantic Forest to Climate Change
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Classification of Forest Formations
2.2. Occurrence Records of the Forest Formations
2.3. Environmental Layers
2.4. Habitat Suitability Models
2.5. Climatic Similarity of Species and Formation Models
3. Results
3.1. Performance of Habitat Suitability Models
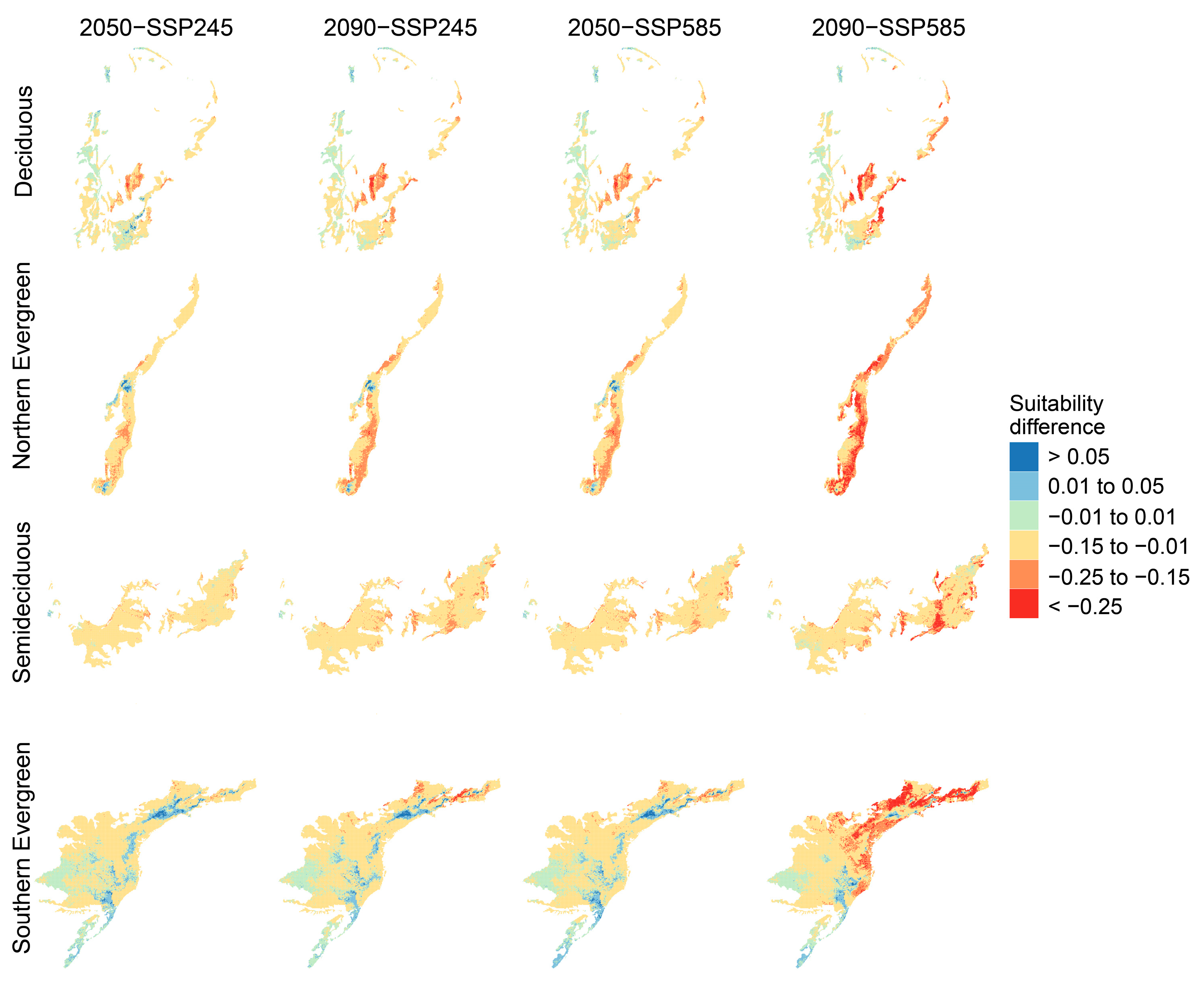
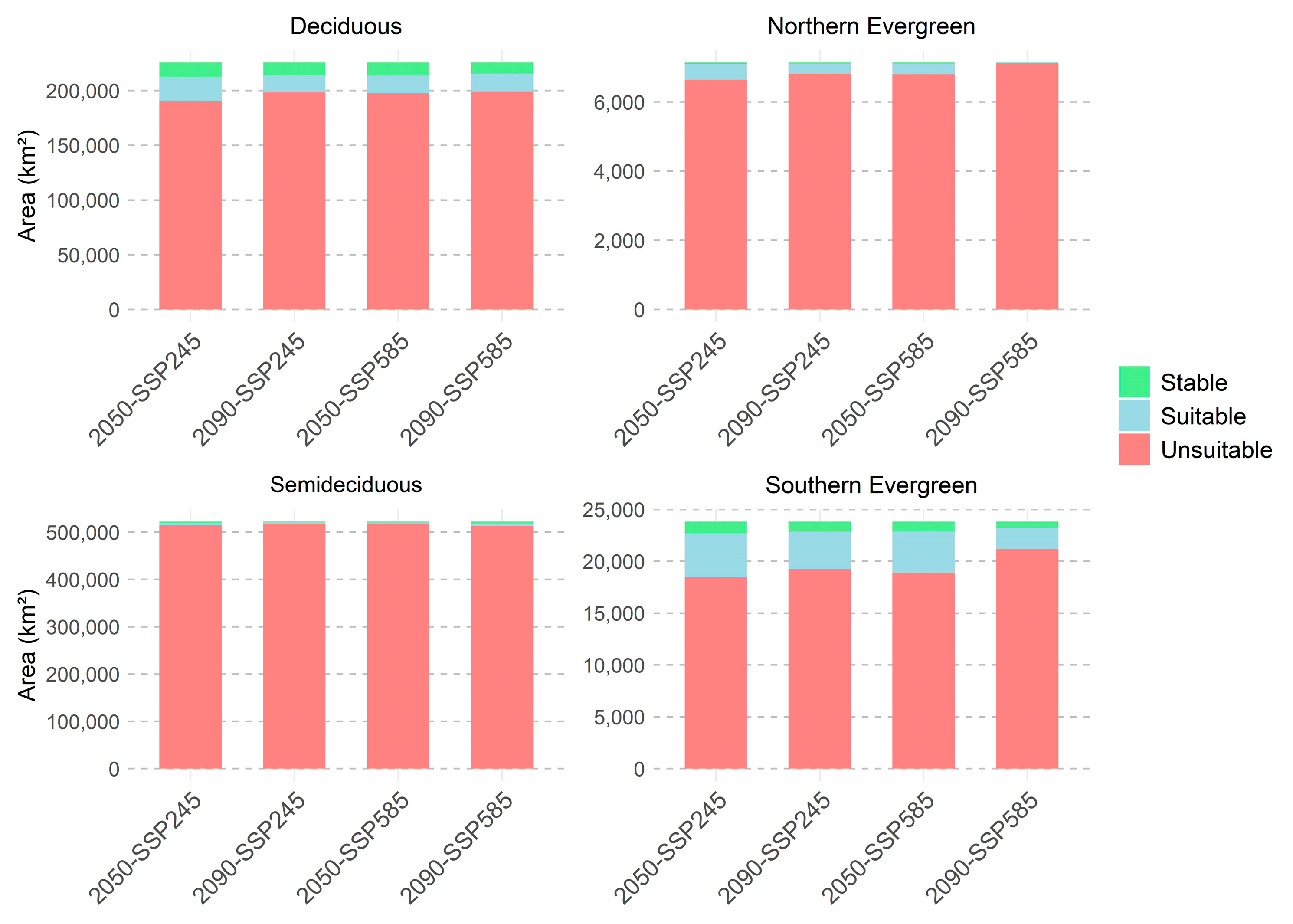
3.2. Future Formation Suitability from Separate Models
3.3. Future Formation Suitability from Joint Models
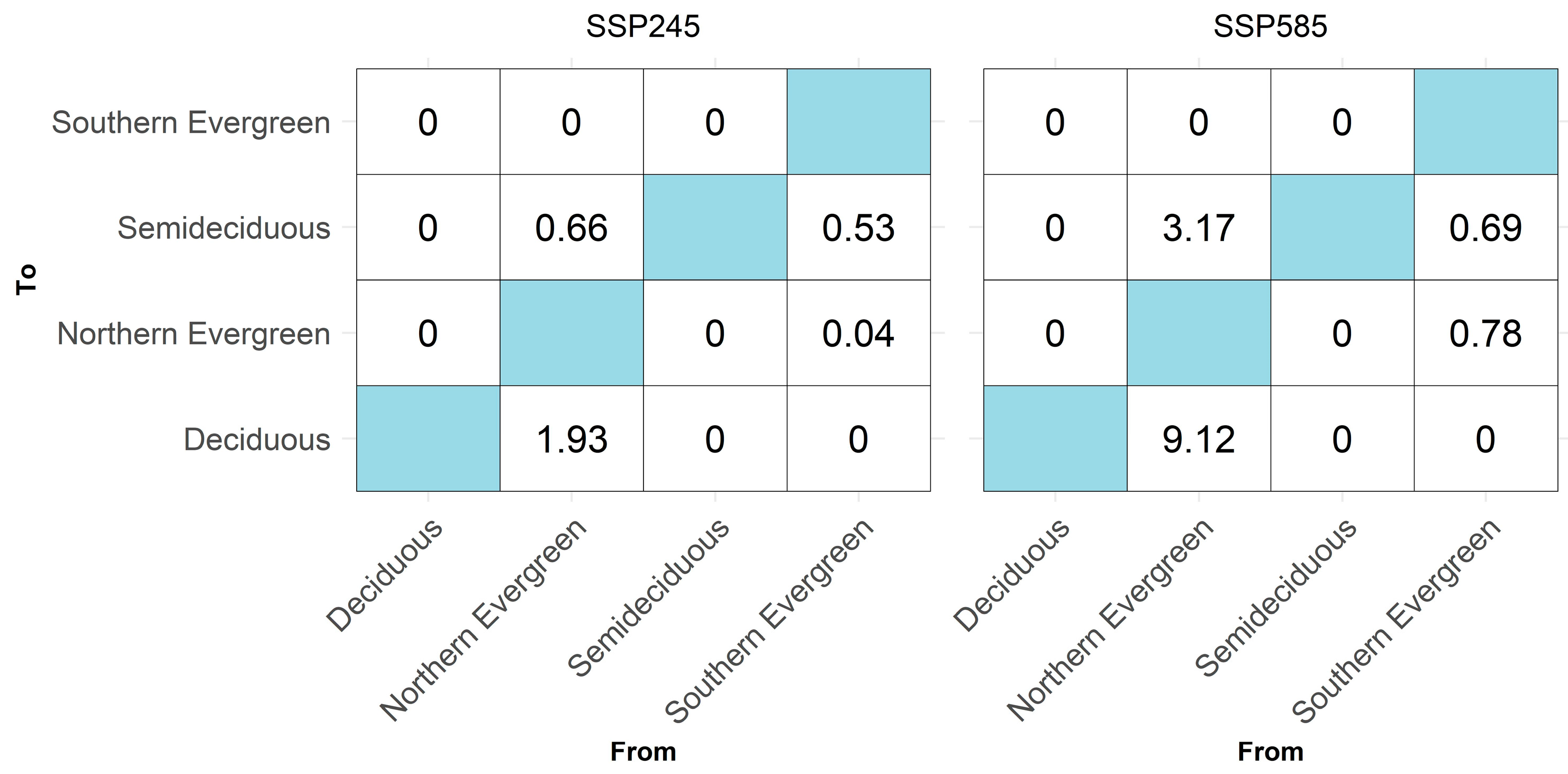
3.4. Climatic Similarity from Joint and Separated Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Clements, F.E. Plant Succession: An Analysis of the Development of Vegetation; Carnegie institution of Washington: Washington, DC, USA, 1916; p. 658. [Google Scholar]
- Gleason, H.A. The individualistic concept of the plant association. Bull. Torrey Bot. Club 1926, 53, 7–26. [Google Scholar] [CrossRef]
- Woodward, F.; Williams, B. Climate and plant distribution at global and local scales. Vegetatio 1987, 69, 189–197. [Google Scholar] [CrossRef]
- Wiens, J.J. The niche, biogeography and species interactions. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2336–2350. [Google Scholar] [CrossRef]
- Sutherland, J.P. Multiple stable points in natural communities. Am. Nat. 1974, 108, 859–873. [Google Scholar] [CrossRef]
- Hastings, A. Time scales, dynamics, and ecological understanding. Ecology 2010, 91, 3471–3480. [Google Scholar] [CrossRef]
- Walther, G.R. Plants in a warmer world. Perspect. Plant Ecol. Evol. Syst. 2003, 6, 169–185. [Google Scholar] [CrossRef]
- Koffel, T.; Daufresne, T.; Klausmeier, C.A. From competition to facilitation and mutualism: A general theory of the niche. Ecol. Monogr. 2021, 91, e01458. [Google Scholar] [CrossRef]
- Walther, G.R. Community ecosystem responses to recent climate change. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2019–2024. [Google Scholar] [CrossRef] [PubMed]
- Pucko, C.; Beckage, B.; Perkins, T.; Keeton, W.S. Species shifts in response to climate change: Individual or shared responses? J. Torrey Bot. Soc. 2011, 138, 156–176. [Google Scholar] [CrossRef]
- Beckage, B.; Osborne, B.; Gavin, D.G.; Pucko, C.; Siccama, T.; Perkins, T. A rapid upward shift of a forest ecotone during 40 years of warming in the Green Mountains of Vermont. Proc. Natl. Acad. Sci. USA 2008, 105, 4197–4202. [Google Scholar] [CrossRef] [PubMed]
- Felton, A.J.; Smith, M.D. Integrating plant ecological responses to climate extremes from individual to ecosystem levels. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160142. [Google Scholar] [CrossRef]
- Feeley, K.J. Distributional migrations, expansions, and contractions of tropical plant species as revealed in dated herbarium records. Glob. Change Biol. 2012, 18, 1335–1341. [Google Scholar] [CrossRef]
- Esquivel-Muelbert, A.; Baker, T.R.; Dexter, K.G.; Lewis, S.L.; Brienen, R.J.; Feldpausch, T.R.; Lloyd, J.; Monteagudo-Mendoza, A.; Arroyo, L.; Álvarez-Dávila, E.; et al. Compositional response of Amazon forests to climate change. Glob. Change Biol. 2019, 25, 39–56. [Google Scholar] [CrossRef]
- Bergamin, R.S.; Bastazini, V.A.G.; Esquivel-Muelbert, A.; Bordin, K.M.; Klipel, J.; Debastiani, V.J.; Vibrans, A.C.; Loyola, R.; Müller, S.C. Elevational shifts in tree community composition in the Brazilian Atlantic Forest related to climate change. J. Veg. Sci. 2024, 35, e13289. [Google Scholar] [CrossRef]
- D’Amato, G.; Vitale, C.; Rosario, N.; Neto, H.J.C.; Chong-Silva, D.C.; Mendonça, F.; Perini, J.; Landgraf, L.; Solé, D.; Sánchez-Borges, M.; et al. Climate change, allergy and asthma, and the role of tropical forests. WAO J. 2017, 10, 11. [Google Scholar] [CrossRef]
- Gatti, R.C.; Reich, P.B.; Gamarra, J.G.; Crowther, T.; Hui, C.; Morera, A.; Bastin, J.-F.; de-Miguel, S.; Nabuurs, G.-J.; Svenning, J.C.; et al. The number of tree species on Earth. Proc. Natl. Acad. Sci. USA 2022, 119, e2202784119. [Google Scholar]
- Brandão, D.O.; Barata, L.E.S.; Nobre, C.A. The effects of environmental changes on plant species and forest dependent communities in the Amazon region. Forests 2022, 13, 466. [Google Scholar] [CrossRef]
- Bennett, A.C.; Sousa, T.R.; Monteagudo-Mendoza, A.; Esquivel-Muelbert, A.; Morandi, P.S.; Souza, F.C.; Castro, W.; Duque, L.F.; Llampazo, G.F.; Santos, R.M. Sensitivity of South American tropical forests to an extreme climate anomaly. Nat. Clim. Chang. 2023, 13, 967–974. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; Metzger, J.P.; Martensen, A.C.; Ponzoni, F.J.; Hirota, M.M. The Brazilian Atlantic Forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biol. Conserv. 2009, 142, 1141–1153. [Google Scholar] [CrossRef]
- IBGE–Instituto Brasileiro de Geografia e Estatística. Províncias Estruturais, Compartimentos de Relevo, Tipos de Solos e Regiões Fitoecológicas; IBGE: Rio de Janeiro, Brazil, 2019; p. 179. [Google Scholar]
- Colombo, A.F.; Joly, C.A. Brazilian Atlantic Forest lato sensu: The most ancient Brazilian forest, and a biodiversity hotspot, is highly threatened by climate change. Braz. J. Biol. 2010, 70, 697–708. [Google Scholar] [CrossRef]
- Leão, T.C.; Reinhardt, J.R.; Nic Lughadha, E.; Reich, P.B. Projected impacts of climate and land use changes on the habitat of Atlantic Forest plants in Brazil. Glob. Ecol. Biogeogr. 2021, 30, 2016–2028. [Google Scholar] [CrossRef]
- Mata-Guel, E.O.; Soh, M.C.; Butler, C.W.; Morris, R.J.; Razgour, O.; Peh, K.S.H. Impacts of anthropogenic climate change on tropical montane forests: An appraisal of the evidence. Biol. Rev. 2023, 98, 1200–1224. [Google Scholar] [CrossRef]
- Zwiener, V.P.; Lira--Noriega, A.; Grady, C.J.; Padial, A.A.; Vitule, J.R. Climate change as a driver of biotic homogenization of woody plants in the Atlantic Forest. Glob. Ecol. Biogeogr. 2018, 27, 298–309. [Google Scholar] [CrossRef]
- Esser, L.F.; Neves, D.M.; Jarenkow, J.A. Habitat—Specific impacts of climate change in the Mata Atlântica biodiversity hotspot. Divers. Distrib. 2019, 25, 1846–1856. [Google Scholar] [CrossRef]
- Stan, K.D.; Sanchez-Azofeifa, A.; Duran, S.M.; Hesketh, M.; Laakso, K.; Portillo-Quintero, C.; Rankine, C.; Doetterl, S. Tropical dry forest resilience and water use efficiency: An analysis of productivity under climate change. Environ. Res. Lett. 2021, 16, 054027. [Google Scholar] [CrossRef]
- Klipel, J.; Bergamin, R.S.; Esquivel-Muelbert, A.; Lima, R.A.; Oliveira, A.A.; Prado, P.I.; Müller, S.C. Climatic distribution of tree species in the Atlantic Forest. Biotropica 2022, 54, 1170–1181. [Google Scholar] [CrossRef]
- Bawa, K.S.; Dayanandan, S. Global climate change and tropical forest genetic resources. Clim. Change 1998, 39, 473–485. [Google Scholar] [CrossRef]
- Guedes, M.L.; Batista, M.D.A.; Ramalho, M.; Freitas, H.D.B.; Silva, E.D. Breve incursão sobre a biodiversidade da Mata Atlântica. In Mata Atlântica e Biodiversidade; Franke, C.R., Rocha, P.L.B., Klein, W., Gomes, S.L., Eds.; Edufba: Salvador, Brazil, 2005; pp. 39–92. [Google Scholar]
- Joly, C.A.; Metzger, J.P.; Tabarelli, M. Experiences from the Brazilian Atlantic Forest: Ecological findings and conservation initiatives. New Phytol. 2014, 204, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Arruda, D.M.; Fernandes-Filho, E.I.; Solar, R.R.C.; Schaefer, C.E.G.R. Combining climatic and soil properties better predicts covers of Brazilian biomes. Sci. Nat. 2017, 104, 32. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.M.C.; Casteleti, C.H.M. Status of the biodiversity of the Atlantic Forest of Brazil. In The Atlantic Forest of South America: Biodiversity Status, Threats, and Outlook; Galindo-Leal, C., Câmara, I.G., Eds.; CABS and Island Press: Washington, DC, USA, 2003; pp. 43–59. [Google Scholar]
- Sobral-Souza, T.; Lima-Ribeiro, M.S.; Solferini, V.N. Biogeography of Neotropical Rainforests: Past connections between Amazon and Atlantic Forest detected by ecological niche modeling. Evol. Ecol. 2015, 29, 643–655. [Google Scholar] [CrossRef]
- Maitner, B.S.; Boyle, B.; Casler, N.; Condit, R.; Donoghue, J.; Durán, S.M.; Guaderrama, D.; Hinchliff, C.R.; Jørgensen, P.M.; Kraft, N.J.B.; et al. The bien r package: A tool to access the Botanical Information and Ecology Network (BIEN) database. Methods Ecol. Evol. 2018, 9, 373–379. [Google Scholar] [CrossRef]
- Boria, R.A.; Olson, L.E.; Goodman, S.M.; Anderson, R.P. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Model. 2014, 275, 73–77. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W.; Zimmermann, N.E. Habitat Suitability and Distribution Models: With Applications in R; Cambridge University Press: Cambridge, UK, 2017; p. 478. [Google Scholar]
- Hysen, L.; Nayeri, D.; Cushman, S.; Wan, H.Y. Background sampling for multi-scale ensemble habitat selection modeling: Does the number of points matter? Ecol. Inform. 2022, 72, 101914. [Google Scholar] [CrossRef]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J.; Hijmans, M.R.J. Package ‘dismo’ v1.3-16. CRAN: Contributed Packages. 2024. Available online: https://cran.r-project.org/web/packages/dismo/index.html (accessed on 31 August 2025).
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1—km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- ISRIC SoilGrids250m version 2.0. Available online: https://soilgrids.org/ (accessed on 13 October 2024).
- Del Río, S.; Canas, R.; Cano, E.; Cano-Ortiz, A.; Musarella, C.; Pinto-Gomes, C.; Penas, Á. Modelling the impacts of climate change on habitat suitability and vulnerability in deciduous forests in Spain. Ecol. Indic. 2021, 131, 108202. [Google Scholar] [CrossRef]
- Casalegno, S.; Amatulli, G.; Bastrup-Birk, A.; Durrant, T.; Pekkarinen, A. Modelling and mapping the suitability of European forest formations at 1-km resolution. Eur. J. For. Res. 2011, 130, 971–981. [Google Scholar] [CrossRef]
- Bergamin, R.S.; Debastiani, V.; Joner, D.C.; Lemes, P.; Guimarães, T.; Loyola, R.D.; Müller, S.C. Loss of suitable climatic areas for Araucaria forests over time. Plant Ecol. Divers. 2019, 12, 115–126. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Araújo, M.B.; New, M. Ensemble forecasting of species distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef]
- Ovaskainen, O.; Abrego, N. Joint Species Distribution Modelling: With Applications in R; Cambridge University Press: Cambridge, UK, 2020; p. 372. [Google Scholar]
- Norberg, A.; Abrego, N.; Blanchet, F.G.; Adler, F.R.; Anderson, B.J.; Anttila, J.; Araújo, M.B.; Dallas, T.; Dunson, D.; Elith, J.; et al. A comprehensive evaluation of predictive performance of 33 species distribution models at species and community levels. Ecol. Monogr. 2019, 89, e01370. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Ortega, G.; Arias, P.A.; Villegas, J.C.; Marquet, P.A.; Nobre, P. Present--day and future climate over central and South America according to CMIP5/CMIP6 models. Int. J. Clim. 2021, 41, 6713–6735. [Google Scholar] [CrossRef]
- Zhang, K.; Yao, L.; Meng, J.; Tao, J. Maxent modeling for predicting the potential geographical distribution of two peony species under climate change. Sci. Total Environ. 2018, 634, 1326–1334. [Google Scholar] [CrossRef]
- Van Vuuren, D.P.; Riahi, K.; Calvin, K.; Dellink, R.; Emmerling, J.; Fujimori, S.; KC, S.; Kriegler, E.; O’Neill, B. The Shared Socio-economic Pathways: Trajectories for human development and global environmental change. Glob. Environ. Change 2017, 42, 148–152. [Google Scholar] [CrossRef]
- Araújo, M.B.; Peterson, A.T. Uses and misuses of bioclimatic envelope modeling. Ecology 2012, 93, 1527–1539. [Google Scholar] [CrossRef]
- Naimi, B.; Araújo, M.B. sdm: A reproducible and extensible R platform for species distribution modelling. Ecography 2016, 39, 368–375. [Google Scholar] [CrossRef]
- Zuur, A.F.; Leno, E.N.; Smith, G.M. Analysing Ecological Data; Springer: New York, NY, USA, 2007; p. 698. [Google Scholar]
- Zhang, Q.; Canosa, R.L. A comparison of histogram distance metrics for content-based image retrieval. SPIE 2014, 9027, 154–162. [Google Scholar]
- Wada, A.; Sumiyoshi, C.; Yoshimura, N.; Hashimoto, R.; Matsumoto, J.; Stickley, A.; Yamada, Y.; Kikuchi, A.; Kubota, R.; Matsui, M.; et al. Semantic memory disorganization linked to social functioning in patients with schizophrenia. Schizophrenia 2025, 11, 61. [Google Scholar] [CrossRef]
- Irimatsugawa, T.; Shimizu, Y.; Okubo, S.; Inaba, H. Cosine similarity for quantitatively evaluating the degree of change in an optical frequency comb spectra. Opt. Express 2021, 29, 35613–35622. [Google Scholar] [CrossRef]
- Fan, Y.; Rui, X.; Poslad, S.; Zhang, G.; Yu, T.; Xu, X.; Song, X. A better way to monitor haze through image based upon the adjusted LeNet-5 CNN model. Signal Image Video P 2020, 14, 455–463. [Google Scholar] [CrossRef]
- Dray, S.; Dufour, A.B. The ade4 package: Implementing the duality diagram for ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef]
- Stan, K.; Sanchez-Azofeifa, A. Tropical dry forest diversity, climatic response, and resilience in a changing climate. Forests 2019, 10, 443. [Google Scholar] [CrossRef]
- Chevalier, M.; Broennimann, O.; Guisan, A. Climate change may reveal currently unavailable parts of species’ ecological niches. Nat. Ecol. Evol. 2024, 8, 1298–1310. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Ossola, A.; Beaumont, L. Records of urban occurrences expand estimates of the climate niches in tree species. Glob. Ecol. Biogeogr. 2024, 33, e13809. [Google Scholar] [CrossRef]
- Grinder, R.; Wiens, J. Niche width predicts extinction from climate change and vulnerability of tropical species. Glob. Change Biol. 2022, 29, 618–630. [Google Scholar] [CrossRef]
- Vincent, H.; Bornand, C.; Kempel, A.; Fischer, M. Rare species perform worse than widespread species under changed climate. Biol. Conserv. 2020, 246, 108586. [Google Scholar] [CrossRef]
- Esperón-Rodríguez, M.; Tjoelker, M.; Lenoir, J.; Laugier, B.; Gallagher, R. Wide climatic niche breadth and traits associated with climatic tolerance facilitate eucalypt occurrence in cities worldwide. Glob. Ecol. Biogeogr. 2024, 33, e13833. [Google Scholar] [CrossRef]
- Bazzaz, F.A. Tropical forests in a future climate: Changes in biological diversity and impact on the global carbon cycle. Clim. Change 1998, 39, 317–336. [Google Scholar] [CrossRef]
- Taylor, P.; Cleveland, C.; Wieder, W.; Sullivan, B.; Doughty, C.; Dobrowski, S.; Townsend, A. Temperature and rainfall interact to control carbon cycling in tropical forests. Ecol. Lett. 2017, 206, 779–788. [Google Scholar] [CrossRef]
- Rocha, S.; Torres, C.; Villanova, P.; Schettini, B.; Jacovine, L.; Leite, H.; Gelcer, E.; Reis, L.; Neves, K.; Comini, I.; et al. Drought effects on carbon dynamics of trees in a secondary Atlantic Forest. For. Ecol. Manag. 2020, 465, 118097. [Google Scholar] [CrossRef]
- Lyra, A.; Imbach, P.; Rodriguez, D.; Chou, S.C.; Georgiou, S.; Garofolo, L. Projections of climate change impacts on central America tropical rainforest. Clim. Change 2017, 141, 093–105. [Google Scholar] [CrossRef]
- Crous, K.Y. Plant responses to climate warming: Physiological adjustments and implications for plant functioning in a future, warmer world. Am. J. Bot. 2019, 106, 1049. [Google Scholar] [CrossRef]
- Ferreira, P.; Boscolo, D.; Lopes, L.; Carvalheiro, L.; Biesmeijer, J.; Da Rocha, P.; Viana, B. Forest and connectivity loss simplify tropical pollination networks. Oecologia 2020, 192, 577–590. [Google Scholar] [CrossRef]
- IPCC-Intergovernmental Panel on Climate Change. Climate Change: The Physical Science Basis (Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021; p. 1300. [Google Scholar]
- Cubino, J.P.; Vilà-Cabrera, A.; Retana, J. Tree species abundance changes at the edges of their climatic distribution: An interplay between climate change, plant traits and forest management. J. Ecol. 2024, 112, 2785–2797. [Google Scholar] [CrossRef]
- Loarie, S.R.; Carter, B.E.; Hayhoe, K.; McMahon, S.; Moe, R.; Knight, C.A.; Ackerly, D.D. Climate change and the future of California’s endemic flora. PLoS ONE 2008, 3, e2502. [Google Scholar] [CrossRef]
- Kramer, R.D.; Ishii, H.R.; Carter, K.R.; Miyazaki, Y.; Cavaleri, M.A.; Araki, M.G.; Azuma, W.A.; Inoue, Y.; Hara, C. Predicting effects of climate change on productivity and persistence of forest trees. Ecol. Res. 2020, 35, 562–574. [Google Scholar] [CrossRef]
- Feng, X.; Uriarte, M.; González, G.; Reed, S.; Thompson, J.; Zimmerman, J.K.; Murphy, L. Improving predictions of tropical forest response to climate change through integration of field studies and ecosystem modeling. Glob. Change Biol. 2018, 24, 213–232. [Google Scholar] [CrossRef] [PubMed]
- Colwell, R.K.; Brehm, G.; Cardelús, C.L.; Gilman, A.C.; Longino, J.T. Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science 2008, 322, 258–261. [Google Scholar] [CrossRef]
- Sharma, M.; Ram, B.; Chawla, A. Ensemble modelling under multiple climate change scenarios predicts reduction in highly suitable range of habitats of Dactylorhiza hatagirea (D.Don) Soo in Himachal Pradesh, western Himalaya. S. Afr. J. 2023, 154, 203–218. [Google Scholar] [CrossRef]
- Bakkenes, M.; Alkemade, J.R.M.; Ihle, F.; Leemans, R.; Latour, J.B. Assessing effects of forecasted climate change on the diversity and distribution of European higher plants for 2050. Glob. Change Biol. 2002, 8, 390–407. [Google Scholar] [CrossRef]
- Price, D.T.; Alfaro, R.I.; Brown, K.J.; Flannigan, M.D.; Fleming, R.A.; Hogg, E.H.; Girardin, M.P.; Lakusta, T.; Johnston, M.; McKenney, D.W.; et al. Anticipating the consequences of climate change for Canada’s boreal forest ecosystems. Environ. Rev. 2013, 21, 322–365. [Google Scholar] [CrossRef]
- Anjos, L.; De Toledo, P. Measuring resilience and assessing vulnerability of terrestrial ecosystems to climate change in South America. PLoS ONE 2018, 13, e0194654. [Google Scholar] [CrossRef]
- Dyderski, M.; Paź, S.; Frelich, L.; Jagodziński, A. How much does climate change threaten European forest tree species distributions? Glob. Change Biol. 2018, 24, 1150–1163. [Google Scholar] [CrossRef]
- Ali, F.; Khan, N.; Khan, A.; Ali, K.; Abbas, F. Species distribution modelling of Monotheca buxifolia (Falc.) A. DC.: Present distribution and impacts of potential climate change. Heliyon 2023, 9, e13417. [Google Scholar] [CrossRef] [PubMed]
- Salazar, L.F.; Nobre, C.A. Climate change and thresholds of biome shifts in Amazonia. Geophys. Res. Lett. 2010, 37, 1–5. [Google Scholar] [CrossRef]
- Bussotti, F.; Pollastrini, M.; Holland, V.; Brüggemann, W. Functional traits and adaptive capacity of European forests to climate change. Environ. Exp. Bot. 2015, 111, 91–113. [Google Scholar] [CrossRef]
- Shen, Y.; Tu, Z.; Zhang, Y.; Zhong, W.; Xia, H.; Hao, Z.; Zhang, C.; Li, H. Predicting the impact of climate change on the distribution of two relict Liriodendron species by coupling the MaxEnt model and actual physiological indicators in relation to stress tolerance. J. Environ. Manag. 2022, 322, 116024. [Google Scholar] [CrossRef]
- Fadrique, B.; Báez, S.; Duque, Á.; Malizia, A.; Blundo, C.; Carilla, J.; Osinaga-Acosta, O.; Malizia, L.; Silman, M.; Farfán-Ríos, W.; et al. Widespread but heterogeneous responses of Andean forests to climate change. Nature 2018, 564, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; He, H.S.; Thompson, F.R., III; Spetich, M.A.; Fraser, J.S. Effects of species biological traits and environmental heterogeneity on simulated tree species distribution shifts under climate change. Sci. Total Environ. 2018, 634, 1214–1221. [Google Scholar] [CrossRef]
- Bond, N.; Thomson, J.; Reich, P.; Stein, J. Using species distribution models to infer potential climate change-induced range shifts of freshwater fish in south-eastern Australia. Mar. Freshw. Res. 2011, 62, 1043–1061. [Google Scholar] [CrossRef]
- Dollinger, C.; Rammer, W.; Suzuki, K.F.; Braziunas, K.H.; Keller, T.T.; Kobayashi, Y.; Mohr, J.; Mori, A.S.; Turner, M.G.; Seidl, R. Beyond resilience: Responses to changing climate and disturbance regimes in temperate forest landscapes across the Northern Hemisphere. Glob. Change Biol. 2024, 30, e17468. [Google Scholar] [CrossRef]
- Morelli, T.L.; Daly, C.; Dobrowski, S.Z.; Dulen, D.M.; Ebersole, J.L.; Jackson, S.T.; Lundquist, J.D.; Millar, C.I.; Maher, S.P.; Monahan, W.B.; et al. Managing climate change refugia for climate adaptation. PLoS ONE 2016, 11, e0159909. [Google Scholar] [CrossRef]
- Fremout, T.; Thomas, E.; Taedoumg, H.; Briers, S.; Gutiérrez-Miranda, C.E.; Alcázar-Caicedo, C.; Lindau, A.; Kpoumie, H.M.; Vincet, B.; Kettle, C.; et al. Diversity for Restoration (D4R): Guiding the selection of tree species and seed sources for climate—Resilient restoration of tropical forest landscapes. J. Appl. Ecol. 2022, 59, 664–679. [Google Scholar] [CrossRef]
- Antongiovanni, M.; Venticinque, E.M.; Tambosi, L.R.; Matsumoto, M.; Metzger, J.P.; Fonseca, C.R. Restoration priorities for Caatinga dry forests: Landscape resilience, connectivity and biodiversity value. J. Appl. Ecol. 2022, 59, 2287–2298. [Google Scholar] [CrossRef]
- Law, B.E.; Hudiburg, T.W.; Berner, L.T.; Kent, J.J.; Buotte, P.C.; Harmon, M.E. Land use strategies to mitigate climate change in carbon dense temperate forests. Proc. Natl. Acad. Sci. USA 2018, 115, 3663–3668. [Google Scholar] [CrossRef]
- Ferreira, I.J.M.; Campanharo, W.A.; Fonseca, M.G.; Escada, M.I.S.; Nascimento, M.T.; Villela, D.M.; Brancalion, P.; Magnago, L.F.S.; Anderson, L.O.; Nagy, L.; et al. Potential aboveground biomass increase in Brazilian Atlantic Forest fragments with climate change. Glob. Change Biol. 2023, 29, 3098–3113. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]



| SSP245 | SSP585 | |||||
|---|---|---|---|---|---|---|
| Forest Formation | 2050 | 2050–2090 | 2090 | 2050 | 2050–2090 | 2090 |
| Deciduous | +1.99 | +1.71 | +3.70 | +3.74 | +6.98 | +10.72 |
| Northern Evergreen | −10.49 | −3.52 | −14.01 | −17.94 | −12.47 | −30.41 |
| Semideciduous | +4.37 | +0.46 | +4.83 | +3.22 | +3.41 | +6.63 |
| Southern Evergreen | −5.32 | −2.20 | −7.52 | −5.35 | −7.01 | −12.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, E.V.S.; Vieira, C.D.C.; Zárate-Salazar, J.R.; Correia, W.d.J.; Pinto, A.d.S.; Gouveia, S.F. Formation- and Species-Level Responses of the Atlantic Forest to Climate Change. Forests 2025, 16, 1674. https://doi.org/10.3390/f16111674
Oliveira EVS, Vieira CDC, Zárate-Salazar JR, Correia WdJ, Pinto AdS, Gouveia SF. Formation- and Species-Level Responses of the Atlantic Forest to Climate Change. Forests. 2025; 16(11):1674. https://doi.org/10.3390/f16111674
Chicago/Turabian StyleOliveira, Eduardo Vinícius S., Carla Diele Cabral Vieira, Jhonatan Rafael Zárate-Salazar, Wadson de Jesus Correia, Alexandre de Siqueira Pinto, and Sidney F. Gouveia. 2025. "Formation- and Species-Level Responses of the Atlantic Forest to Climate Change" Forests 16, no. 11: 1674. https://doi.org/10.3390/f16111674
APA StyleOliveira, E. V. S., Vieira, C. D. C., Zárate-Salazar, J. R., Correia, W. d. J., Pinto, A. d. S., & Gouveia, S. F. (2025). Formation- and Species-Level Responses of the Atlantic Forest to Climate Change. Forests, 16(11), 1674. https://doi.org/10.3390/f16111674







