1. Introduction
With the increasing frequency of extreme weather events, waterlogging disasters have intensified globally, posing serious challenges to the production and application of landscape plants. A more in-depth understanding of plant response mechanisms to waterlogging stress is highly important for enhancing stress resistance and facilitating genetic improvement in horticultural species.
R. hainanense, an endemic species in China, is primarily distributed in the mountainous areas of southwestern Hainan Province, such as Limu Mountain, Diaoluo Mountain, and Jianfeng Ridge.
R. hainanense typically grows along streams at elevations between 200 and 800 m [
1] and is listed as a provincially protected plant in Hainan. This species is a low evergreen shrub, characterized by bright flower colors and dense flower clusters.
R. hainanense also shows strong heat-tolerance and wide adaptability and is distributed at a lower altitude. Its flowering period coincides with the New Year and the Spring Festival, so it has high ornamental value. In addition,
R. hainanense is well suited for use in landscape applications such as shaped bonsai, flower beds, group plantings, and specialized gardens [
2]. Furthermore, the species holds significant economic potential beyond horticulture, with prospects for development in botanical and traditional applications, which further supports its commercial value. Despite its significant ornamental, horticultural, medicinal, and economic potential, wild
R. hainanense predominantly grows in shady and humid environments, rendering it highly susceptible to waterlogging stress, which constitutes a major constraint to its domestication [
3]. Studies have shown that the AP2/ERF gene family is large and plays important roles in regulating gene expression during abiotic stresses [
4], particularly in response to waterlogging or flooding [
5,
6]. Therefore, research into the waterlogging tolerance mechanisms of plants is especially important.
AP2/ERF family belongs to one of the most conserved and important TF families in the plant kingdom [
7]. Transcription factor families engage in quite a lot of different activities in controlling biology and physiology, including responses to abiotic strain, growth and development, and plant formation [
8]. AP2/ERF TFs have important roles in plant growth and development and are grouped into five subfamilies based on amino acid sequence similarities and conserved domains: AP2, ERF, DREB, RAV, and Soloist. The AP2 subfamily, like the ERF and DREB subfamilies, have two defining AP2 domains. The RAV subfamily has a B3 domain, together with an AP2 domain. And the ERF family is divided into two subcategories, containing the DREB subfamily with the group B1-B6 and the ERF subfamily with the group A1-A6 [
7,
8,
9]. Also, different subfamilies also have different forms of structures. AP2 subfamily members control flowering time and floral organ development [
10,
11]. Members of the ERF and DREB subfamilies were mainly associated with responses to abiotic stress such as cold and waterlogging [
12,
13], while members of the RAV subfamily were mainly associated with the regulation of plant hormones such as ethylene and brassinosteroids [
14]. In contrast, the Soloist subfamily appears distinct, warranting further investigation to elucidate its functional role. The AP2/ERF family, meanwhile, has been extensively studied across diverse plant species, including strawberry (
Fragaria × ananassa), peach (
Prunus persica), grape (
Vitis vinifera), kiwifruit (
Actinidia valvata), and various citrus (
Citrus spp.) [
14,
15,
16,
17].
In ERF proteins, the 14th and 19th amino acid residue of the AP2 domain are Alanine and Aspartic acid, respectively, but in the DREB protein, Valine and Glutamic Acid, respectively, occupy the 14th and 19th position. The ERF family can also be classified into twelve groups [
18], which are shown to enhance plant waterlogging tolerance, in particular, group VII [
7]. In
Arabidopsis thaliana, five ERF-VII members (
HRE1,
HRE2,
RAP2.2,
RAP2.3, and
RAP2.12) are regarded as the major regulators for waterlogging and hypoxia tolerance [
19]. Two rice ERF-VII,
SNORKEL1 and
SNORKEL2, are involved in the escape strategy away from flooding and are transcriptionally activated under submergence in an ethylene-dependent manner [
6]. Another rice ERF-VII gene,
SUB1A, uses a quiescent survival strategy to limit growth and increase fermentative capacity [
5]. Also, overexpression of
ZmEREB180, which belongs to the Group V ethylene-responsive factor gene family in zeamaies, increases survival after an extended period of waterlogging, increases adventitious root formation, and regulates for antioxidants [
19].
TaERFVII.1 is a member of the ERF family in wheat (
Triticum aestivum), and it has been confirmed to improve waterlogging tolerance without affecting yield [
20].
Despite the limited knowledge of the AP2/ERF gene family in R. hainanense, this study performs a comprehensive, genome-wide identification and characterization of its members using bioinformatics approaches. These were employed together with RNA sequencing (RNA-seq) to screen for key waterlogged AP2/ERF genes. The purpose of this study is to provide a more detailed understanding about AP2/ERF family functions and to form the basis for later studies of R. hainanense waterlogging-tolerant gene functions.
3. Results
3.1. Identification and Physicochemical Characterization of the R. hainanense ERF Gene Family
Our research group sequenced the whole genome of R. hainanense. We first performed an alignment of the whole R. hainanense genome onto the HMM for the plant AP2/ERF domain (PF00847), with an extremely stringent E-value cut-off <1 × 10−20, and the protein sequences of the AtAP2/ERF family genes were searched from the Arabidopsis thaliana database and used as a query sequence to do Blast alignment against the full set of R. hainanense protein sequences. We regarded the genes at the intersection of the results from the two alignment approaches as candidate genes for the R. hainanense AP2/ERF gene family. To check the integrity of candidate R. hainanense proteins, the corresponding AP2/ERF conserved domain was checked by the NCBI-CDD and SMART databases. After thorough screening, 142 AP2/ERFs gene family members dispersed among 13 chromosomes were discovered; these genes were named RhERF1 to RhERF142 with respect to the order of genomic position.
We conducted bioinformatics analysis on the physicochemical properties of the analyzed RhAP2/ERF proteins (in
Supplementary File S2). In the results we can see a lot of diversity within the physiological members of the RhAP2/ERF family. Predicted properties show that in case of RhAP2/ERFs, amino acid length varies from 83 to 2143 aa, avg 292 aa. Their molecular weights were in the range of 9.12–241.11 kDa, with an average of 32.25, and their pH range was 4.13–11.74, with the average of 6.84. Out of these proteins, there were 54 RhAP2/ERF proteins with a pI > 7, which showed their basic character, and 88 RhAP2/ERF proteins were acidic, showing pI < 7. The instability index was 52.76 on average (range: 26.82~102.19). The number of proteins with an instability index larger than 40 was 125. These proteins are unstable proteins. Only 17 RhAP2/ERFs had an instability index lower than 40, which made them stable, showing that there were many unstable RhAP2/ERFs. The liphatic index was averaged at 62.26 (32.16–81.12), with a grand average hydropathicity (GRAVY) of −0.661 (−1.237–−0.039). All 142 RhAP2/ERFs have negative GRAVY values, which means that all the proteins are hydrophilic. Of all 90 RhAP2/ERFs, 90 RhAP2/ERFs were predicted to be in the nucleus, 45 in both the cytoplasm and the nucleus, 4 in the cytoplasm, 1 in both the chloroplast and the nucleus, 1 in both the chloroplast and the cytoplasm, and 1 in the cell membrane. The dominant nuclear localization agrees with the AP2/ERFs being transcription factors that operate within the nucleus to regulate the genes.
3.2. Analysis of Gene Structure and Conserved Motifs in the R. hainanense ERF Family
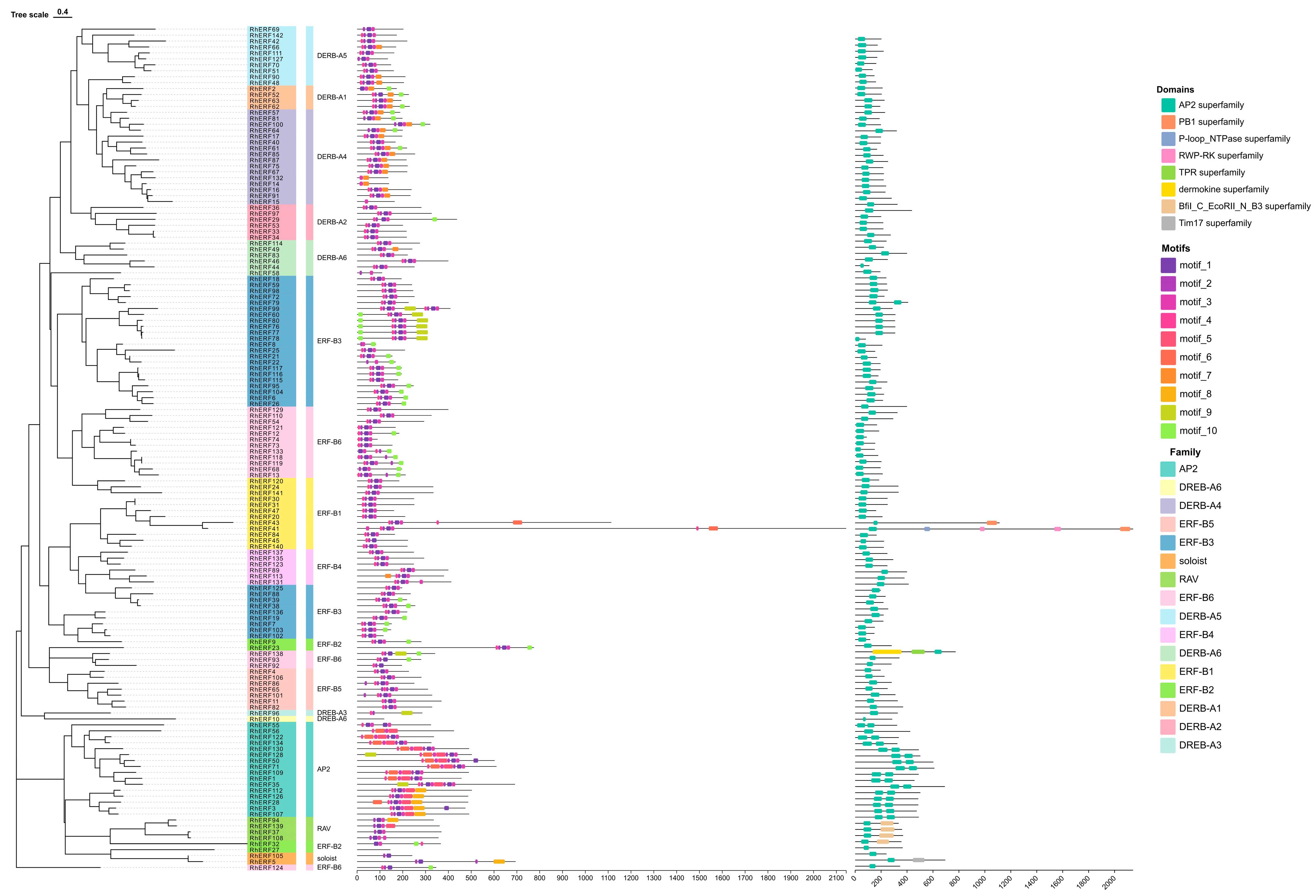
Furthermore, we studied the phylogenetic relationship and structural characteristics of AP2/ERF in
R. hainanense by analyzing its gene structures and conserved motifs (
Figure 1): there is an AP2 in each gene. From the analysis we can see that there is a big variation in the number of motifs that are present in AP2/ERF genes—motif 1–motif 10. In particular, the AP2 subfamily had seven motifs (motifs 1, 2, 3, 4, 5, 6, and 8), members of this subfamily contained motifs (1, 2, 3, 4, and 5), the ERF subfamily had seven motifs (motif 1, 2, 3, 4, 7, 9, and 10), and the DREB subfamily had six motifs (motif 1, 2, 3, 4, 6, and 10). The Soloist subfamily contained only one motif, and the RAV subfamily contained 1, 2, and 3. Interestingly, the Soloist and RAV subfamilies shared motif 1, which may correspond to the AP2 domain.
3.3. Phylogenetic Analysis of the R. hainanense ERF Family
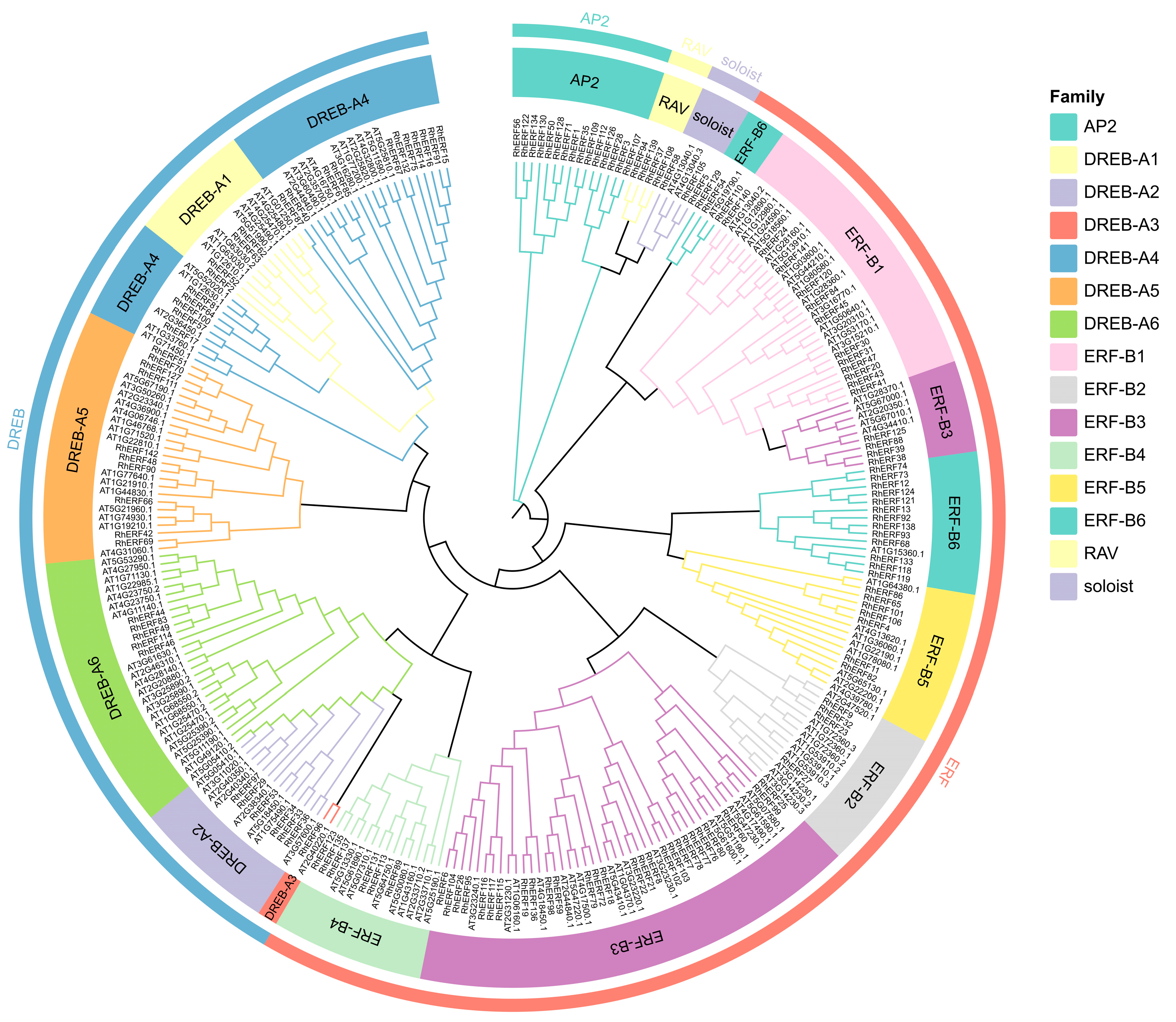
To elucidate the evolutionary relationships within the AP2/ERF gene family of
R. hainanense, a phylogenetic tree was generated using the full-length protein sequences of these genes from both
R. hainanense (RhAP2/ERFs) and
Arabidopsis thaliana (AtAP2/ERFs) (
Figure 2). Following the established classification system for
A. thaliana, the RhAP2/ERF genes were grouped into five subfamilies: RhAP2, RhERF, RhDREB, RhRAV, and RhSoloist. The RhERF subfamily, comprising 76 members, was the most extensive and was further divided into six subgroups (B1–B6). Subgroup B3 constituted the largest group with 31 genes, whereas B2 was the smallest, containing only four members. The RhDREB subfamily included 43 genes distributed across six subgroups (A1–A6), of which A4 was the largest (15 members) and A3 the smallest (1 member). Additionally, the RhAP2 subfamily contained 15 members. Collectively, the ERF, DREB, and AP2 subfamilies represented 95.7% of all RhAP2/ERF genes. The remaining members belonged to the Soloist and RAV subfamilies, with two and four genes, respectively.
3.4. Chromosomal Localization and Cis-Acting Element Analysis of R. hainanense ERF Genes
Chromosomal localization is often a critical factor influencing gene function and evolution. On the basis of the general feature format (GFF) file of the
R. hainanense genome, the chromosomal locations of the ERF gene family were extracted and mapped (
Figure 3). The results revealed that all 142 RhAP2/ERF genes were distributed across all 13 chromosomes of
R. hainanense. The distribution is as follows:
RhERF1–
RhERF9 are located on chromosome 1 (6.34% of the total),
RhERF10–
RhERF22 on chromosome 2 (9.15%),
RhERF23–
RhERF32 on chromosome 3 (7.04%),
RhERF33–
RhERF43 on chromosome 4 (7.74%),
RhERF44–
RhERF53 on chromosome 5 (7.04%),
RhERF54–
RhERF69 on chromosome 6 (11.27%),
RhERF70–
RhERF87 on chromosome 7 (12.68%),
RhERF88–
RhERF90 on chromosome 8 (2.12%),
RhERF91–
RhERF101 on chromosome 9 (7.74%),
RhERF102–
RhERF110 on chromosome 10 (6.34%),
RhERF111–
RhERF117 on chromosome 11 (4.94%),
RhERF118–
RhERF131 on chromosome 12 (9.86%), and
RhERF132–
RhERF142 on chromosome 13 (7.74%).
RhAP2/ERF genes are distributed across all chromosomes. Among them, Chr2, Chr6, Chr7, and Chr12 contain the greatest number of AP2/ERF family members, with 13, 16, 18, and 14 members, respectively. In contrast, Chr8 had the fewest members, with only three. Notably, although Chr1 is the longest chromosome, it does not harbor the greatest number of AP2/ERF members, indicating a lack of significant correlation between chromosome length and the distribution of AP2/ERF genes.
Transcription factors are key to coordinate plants’ response to biotic and abiotic stresses. In living things,
cis-regulatory parts change how genes work by making places where different proteins can attach to them [
29]. Therefore, it can be concluded that the role of
cis-regulatory elements is determined by the specific binding sites for the
cis-regulatory element and whether they are interacting with different kinds of functional proteins [
30]. We obtained a 2000 bp promoter region of the AP2/ERF gene upstream and conducted the analysis of
cis-elements using the online tool PlantCARE (in
Supplementary File S3) (
Figure 4). AP2/ERF is a very important gene for plant growth and development. And they show great functional differences in response to different psychotropics and environmental stresses as well. We can also observe that there are a large amount of cis-acting elements on the promoter region of AP2/ERF, including the response element of plant hormones, light, stress, biotic stimuli and abiotic stimuli.
Analysis of the RhAP2/ERF promoter sequences identified common core elements, including CAAT-boxes and TATA-boxes, along with various cis-elements associated with environmental stress, hormone signaling, and growth and development. In them, the hormone-responding components (eight types) are ABRE (abscisic acid-responding portion), TGA-part (auxin-responding element), CGTCA-motif (methyl jasmonate-responding component), TGACG-motif (methyl jasmonate-responding element) P-box (gibberellin-responding component), TCA-portion (salicylic acid-responding portion) AUXRR-core (auxin-responding portion), and TATC-box (gibberellin-responding portion). Light-responsive elements (five types) include G-box, I-box, MRE, SP1, and Box II. The stress-responsive (four types) are LTR (low-temperature response element), ARE (anaerobic induction element), MBS (drought response element), and TC-rich repeat (defense and stress response element). The growth and development elements (two sorts) are CAT-box (Meristem-expression related element) and O2-site (Zein metabolism-independent element).
3.5. Synteny Analysis of AP2/ERF Genes
To investigate gene duplication events within the RhAP2/ERF family, MCScanX (v2.119) was used to analyze the 142 members located across all 13 chromosomes (
Figure 5). A total of 16 pairs of duplicate gene pairs were identified, namely,
RhERF3 & RhERF107,
RhERF5 & RhERF105,
RhERF6 & RhERF104,
RhERF8 & RhERF103,
RhERF11 & RhERF82,
RhERF50 & RhERF71,
RhERF51 & RhERF70,
RhERF59 & RhERF98,
RhERF65 & RhERF101,
RhERF81 & RhERF100,
RhERF86 & RhERF101,
RhERF118 & RhERF133,
RhERF122 & RhERF134,
RhERF123 & RhERF135,
RhERF127 & RhERF51, and
RhERF139 & RhERF94 (in
Supplementary File S4). Notably, all duplicated gene pairs belonged to the same subgroup, and chromosomes 1 and 12 presented a relatively high frequency of segmental duplication events. To explore the evolutionary constraints acting on these duplicates, the Ka/Ks Calculator (v1.0) was employed to compute nonsynonymous (Ka) and synonymous (Ks) substitution rates. The Ka/Ks values for all duplicated segments were less than 1, indicating that the RhAP2/ERF genes have evolved under purifying selection, which may act as an important evolutionary force in maintaining functional constraint (
Table 1).
A comparative synteny analysis was performed to identify conserved AP2/ERF orthologs in
Arabidopsis thaliana and
Rhododendron simsii (
Figure 6). The results demonstrated that the collinear gene pairs have experienced lineage-specific expansion. Furthermore, the highest level of syntenic conservation was observed in
R. simsii, while a comparatively lower degree was found in
A. thaliana. Notably, chromosome 7 harbored the most significant concentration of these orthologous genes among all species examined. Overall, the greatest number of syntenic orthologues was identified between
A. thaliana, suggesting that AP2/ERF genes are evolutionarily conserved and likely share a common ancestral origin, despite instances of gene duplication or loss. Throughout evolution,
R. hainanense and
R. simsii, both of which are classified within the genus
Rhododendron, exhibited a closer evolutionary relationship, as reflected by their higher syntenic conservation.
3.6. Visualization of AP2/ERF Protein Structures and Interaction Networks
Protein function is determined by its three-dimensional structure, and different structural conformations can lead to distinct functional outcomes [
31]. AlphaFold2 was used to predict the structure of AP2/ERF proteins, and confidence (pLDDT) and expected errors were extracted from the model output; among them, the dark blue territory represents high pLDDT, the blue territory represents medium pLDDT, and the gray territory represents low pLDDT. Conserved residues are marked in yellow, and the green territory on the right indicates the credibility of the prediction. The results reveal structural differences between different subfamilies, and closely related subfamilies show similar structures, thus having similar biological functions (
Figure 7). For example, ERF B-1, B-2, B-3, B-4, B-5, and B-6, which are members of the ERF subfamily, share highly similar structures. Similarly, DREB A-1, A-2, A-3, A-4, A-5, and A-6, which constitute the DREB subfamily, also exhibited consistent structural features. Notably, the RAV subfamily showed pronounced central symmetry. Three-dimensional modeling of these proteins and structural predictions across subfamilies provide a foundational basis for exploring their functional mechanisms.
To elucidate the functions of the RhAP2/ERF proteins, a protein interaction network was predicted based on their homologous genes in
Arabidopsis thaliana (
Figure 8). The resulting network consisted of 77 nodes and interactions. Furthermore, the majority of AP2/ERF proteins were predicted to interact with other proteins, suggesting their involvement in complex regulatory mechanisms.
To interpret the functional significance of this protein interaction network, we studied the topological property analysis centered on hub genes and functional modules. The study found that multiple RhAP2/ERF proteins form core hubs with high connectivity, especially the homologous proteins of AtRAP2.2 (AT3G14230), AtRAP2.12 (AT1G53910), and AtERF73/HRE1 (AT1G72360). In
Arabidopsis thaliana, these hub genes are recognized as core regulatory factors of hypoxic response, and the core signaling modules formed by them can integrate ethylene and reactive oxygen species signals, thereby activating the anaerobic fermentation pathway and other adaptability genes [
13,
19]. The core position of these homologous genes in the network strongly indicates that the RhAP2/ERF protein family plays a conserved and crucial role in waterlogging stress of
R. hainanense. In addition, the network shows clear functional clustering characteristics. One of the main modules is closely associated with ethylene signaling components, containing homologous proteins of AtERF1 and AtERF2 that are known to regulate ethylene-responsive gene expression, and this module may support the rapid transcription triggered by ethylene accumulation during waterlogging stress. Another part is associated with genes related to osmotic stress and ABA signaling, which may coordinately regulate the maintenance of water balance in hypoxic environments. Prediction results show that there are associations between different subgroups of RhAP2/ERF proteins (such as ERF-VII and DREB members), indicating the existence of a complex signal crosstalk mechanism that can integrate multiple stress signals such as hypoxia, osmosis, and oxidation, and ultimately form a harmonized transcriptional output, thereby enhancing the overall stress resistance of plants to waterlogging stress.
3.7. Analysis of Expression Patterns in R. hainanense Under Waterlogging Stress
3.7.1. Analysis of AP2/ERF Gene Expression Patterns Under Waterlogging Stress
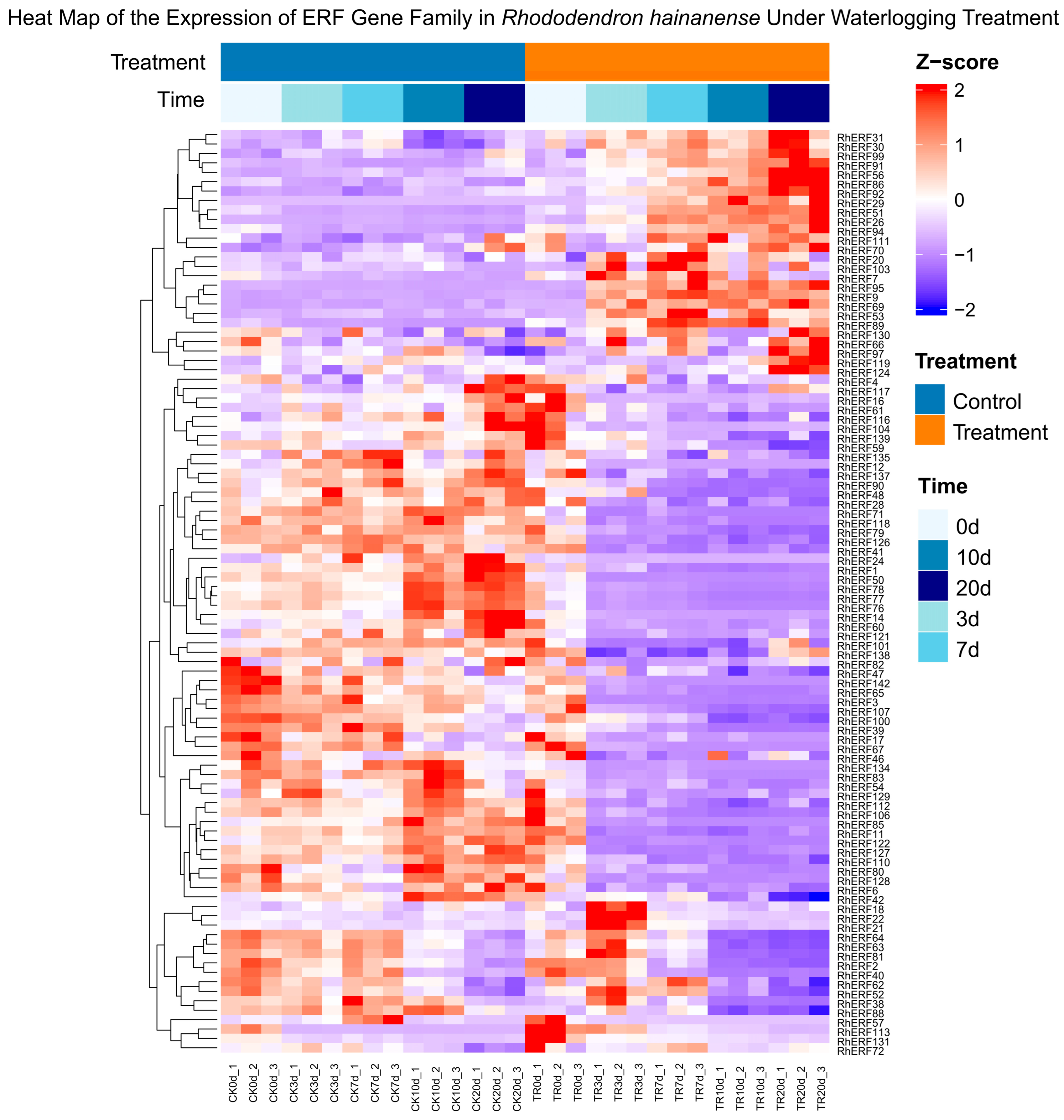
To investigate the dynamic response of the RhAP2/ERF gene family to waterlogging stress,
R. hainanense plants were subjected to waterlogging treatment for different durations (0 d, 3 d, 7 d, 10 d, and 20 d), with control groups maintained under normal conditions. Transcriptomic analysis was performed to compare gene expression profiles between treated and control samples. We collected the fpkm values of 98 RhERFs in the transcriptome under waterlogging treatment and used the TBtools Software to draw a heat map. The results showed that among the 98 ERF sequences, 6 RhERFs showed high expression levels after waterlogging stress treatment (
RhERF9,
RhERF95,
RhERF31,
RhERF69,
RhERF94, and
RhERF51) (
Figure 9). The results revealed differential expression of six RhAP2/ERF members following waterlogging treatment. Specifically, among all the observed genes, the expression of
RhERF9 was the highest; it significantly increased after 3 d of treatment. The expression of
RhERF51,
RhERF31, and
RhERF69 peaked at 20 d, whereas that of
RhERF95 and
RhERF94 was high at 7 d. The expression of the remaining RhAP2/ERF genes did not significantly differ in response to waterlogging.
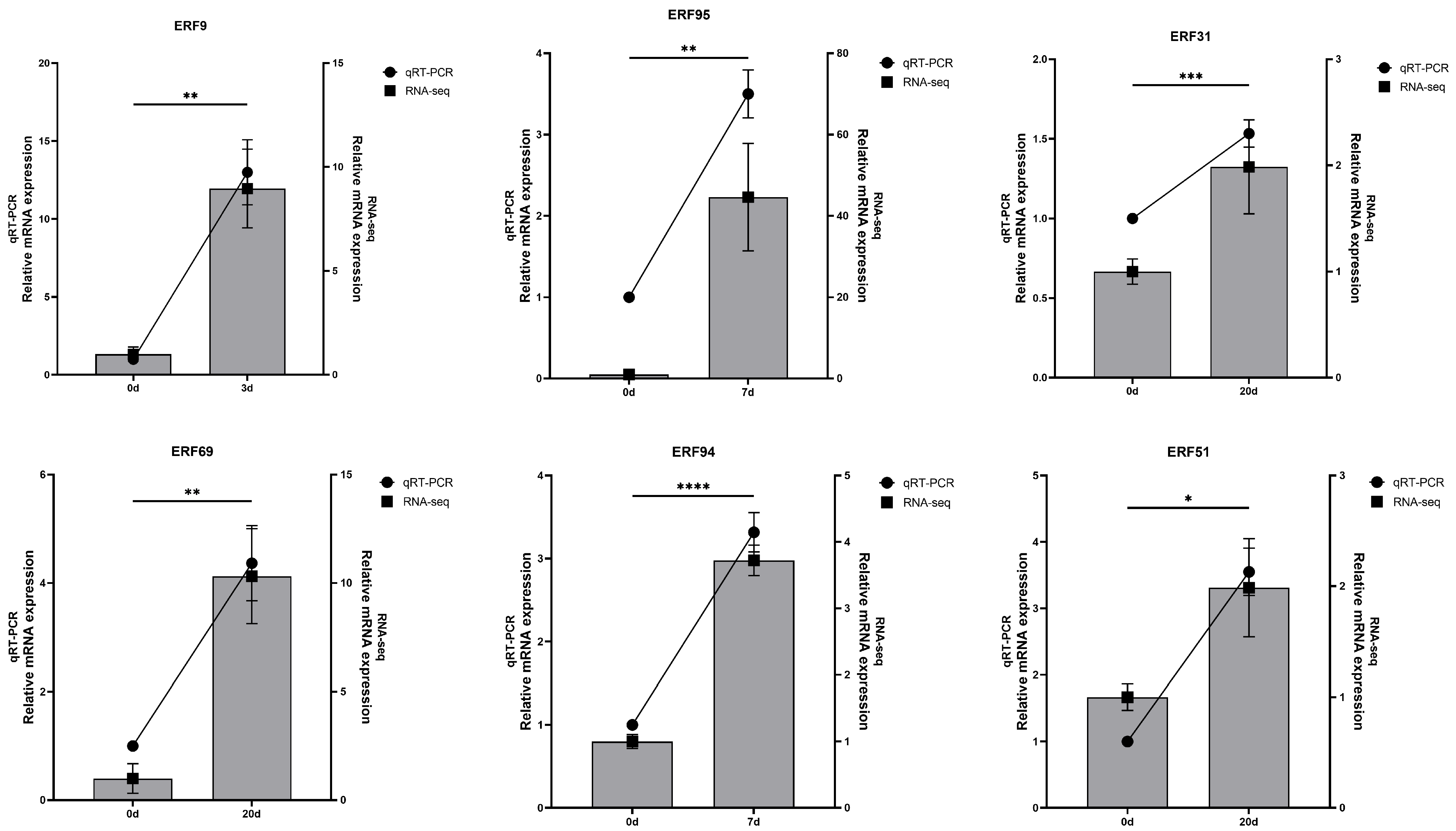
3.7.2. Validation of RhERF Gene Expression Using qRT-PCR
To verify the accuracy of RNA-seq for the ERF gene, we selected six up-regulated RhERF genes for qPCR analysis. The results showed that their expression trends were consistent with those of RNA-seq (
p < 0.05), which attested to the accuracy of the data (
Figure 10). The results showed that
RhERF31,
RhERF51, and
RhERF69 were highly expressed at 20 days of waterlogging treatment;
RhERF95 and
RhERF94 exhibited high expression at 7 days; and
RhERF9 showed the highest expression level at 3 days. These findings suggest that RhERF9 may play an important early regulatory role in the response to waterlogging stress.
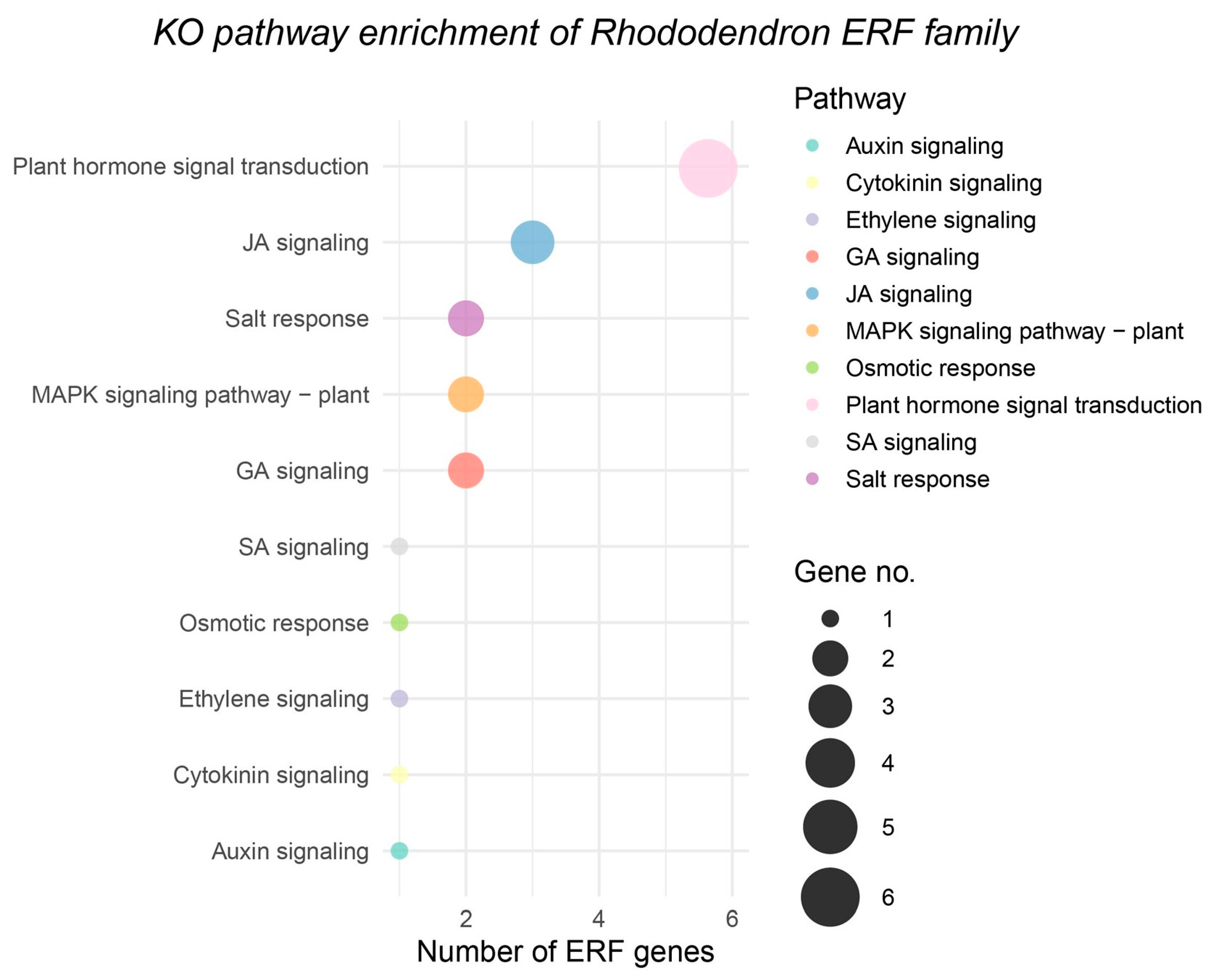
3.7.3. KEGG and GO Enrichment Analysis of Six Up-Regulated RhERF Genes in R. hainanense Under Waterlogging Stress
In the transcriptome analysis of
R. hainanense under waterlogging stress treatment, we identified six RhERF genes with significantly up-regulated expression. To further explore their potential core functions in the adaptation of
R. hainanense to waterlogging stress, we conducted KEGG and GO enrichment analyses on this gene set. The results of KEGG pathway enrichment (
Figure 11) showed that these genes were significantly enriched in two key pathways: “Plant hormone signal transduction” and “MAPK signaling pathway—plant”. Specifically, these up-regulated RhERF genes were precisely localized to the signal transduction pathways of ethylene, jasmonic acid (JA), and salicylic acid (SA), and were also closely related to the responses to osmotic stress and salt stress. This enrichment pattern is highly consistent with the typical physiological characteristics of waterlogging stress: lack of oxygen in soil leads to massive accumulation of ethylene, and a sharp increase in reactive oxygen species (ROS) leads to cell osmotic loss of balance. The coordinated up-regulation of these genes strongly indicates that they are the core transcription regulatory elements of
R. hainanense in response to waterlogging disasters. In addition, their close association with the MAPK signaling pathway suggests that they may serve as key downstream nodes integrating multiple stress signals and hormone signals, thereby harmonizing the start-up of adaptive gene expression procedures. The results of GO enrichment analysis are mutually confirmed with KEGG analysis, and these genes are significantly enriched in biological processes such as “response to ethylene”, “response to reactive oxygen species”, “response to osmotic stress”, and “regulation of jasmonic acid-mediated signaling pathway”. In terms of molecular function, it specifically enriches “DNA binding transcription factor activity” and “sequence-specific DNA binding”, clarifying its molecular foundation as a transcription regulatory factor. Based on the above viewpoints, the enrichment pathways and functional articles of these six RhERF genes specifically up-regulated under waterlogging stress together outline a relatively clear regulatory network. They are most likely the key regulatory factors for the waterlogging stress adaptability of
R. hainanense and play a core role in the physiological processes of maintaining redox balance and osmotic homeostasis by mediating the crosstalk of ethylene, JA, and SA signals and integrating the MAPK signaling pathway.
3.8. Tissue-Specific Expression Analysis of RhERF9
We used cDNA templates of different tissue development stages of
Rhododendron hai-nanense including young leaves, mature leaves, young stems, mature stems, young roots, and mature roots to analyze the relative expression levels of the
RhERF9 gene in various tissues via qRT-PCR (
Figure 12). The results show that the
RhERP9 gene was highly ex-pressed in mature stems, moderately expressed in mature leaves and young stems, and little express in young roots, mature roots and young leaves. It is worthy of note that the expression level of mature stems was about 125 times that of young leaves.
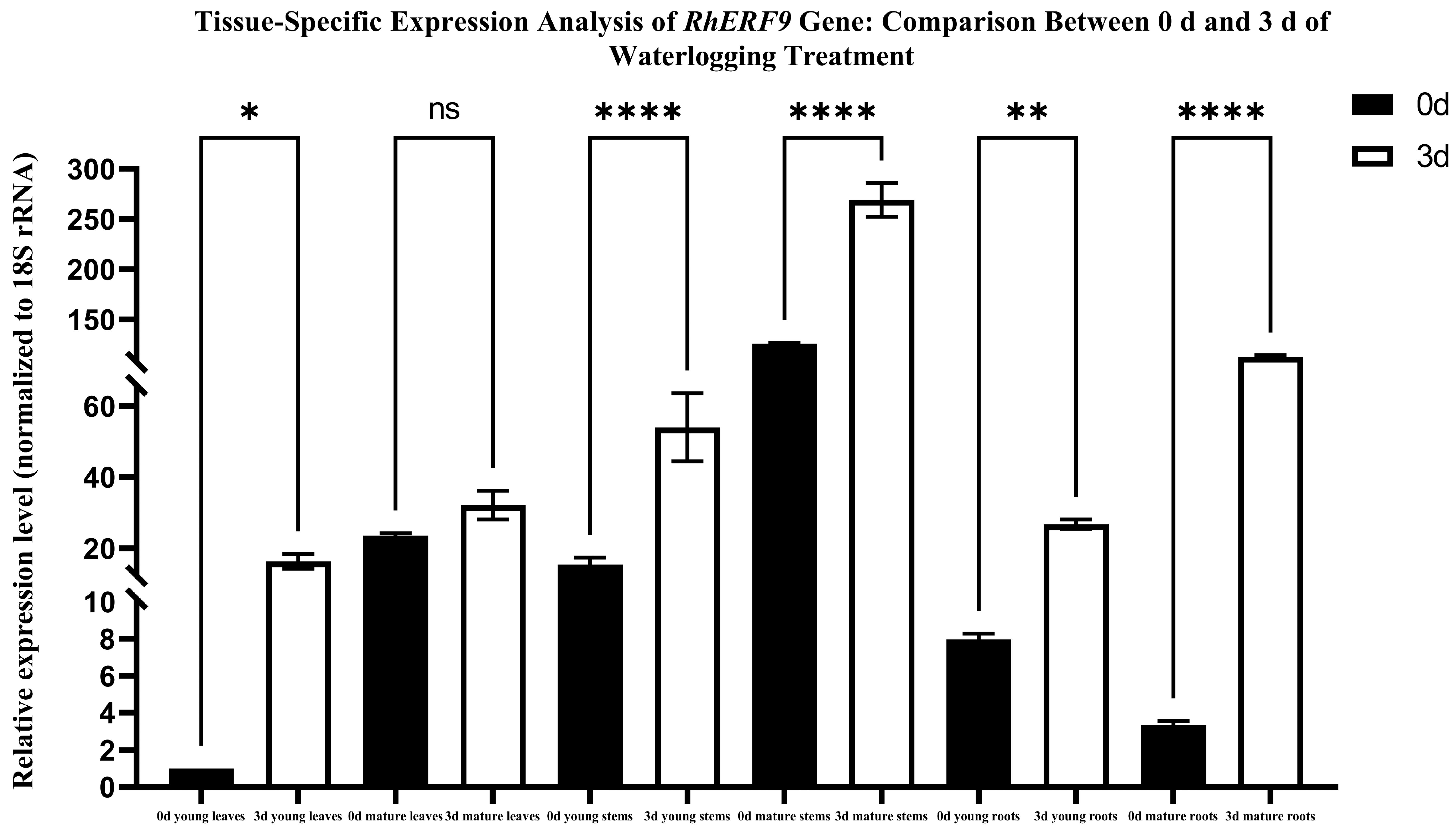
To determine the tissue-specific expression of the RhERF9 gene subjected to water-logging stress, we conducted 0 d and 3 d of waterlogging treatment on R. hainanense plants. We prepared cDNA templates using young leaves, mature leaves, young stems, mature stems, young roots, and mature root samples, collected before and after the treatment. Relative expression levels of RhERF9 among those different tissues were evaluated by way of qRT-PCR; attention was given to changes in expression following 3 days of waterlogging. In response to waterlogging stress, RhERF9 shows marked spacetime heterogeneity, as seen from the qRT-PCR analysis, which reports a highly significant overexpression of RhERF9 in roots. So, RhERF9 seems to play an important role in the development of root aerenchyma and the regulation of hypoxia adaptation. In the case of the young stem tissue, however, a less pronounced but notable induction was seen, which hints at an involvement with the formation process of initiation of lateral roots and the elongation found in the plant stem. These kinds of findings show us that RhERF9 is helping to create a group of regulations where waterlogging is the main factor, especially from inside the roots, then also in the stem, and then the leaf seems to hold it down, which provides us with some clues about how plants deal with waterlogging in their systems.
4. Discussion
Adverse environmental conditions (low temperature [
32], high temperature [
24], drought [
33], high salinity [
33], waterlogging [
34], etc.) will exert negative impacts on plants’ growth and development [
35]. AP2/ERF has only one AP2 conserved domain, widely distributed in the plant abiotic stress response, which regulates the expression of many stress response genes [
8]. Most of the
Rhododendron varieties are extremely waterlogging-sensitive [
36], mainly because of their shallow and sparse roots. But
R. hainanense does have very good waterlogging endurance as far as we know, so perhaps there are some specific genes in the AP2/ERF family. The ERF family genes play various roles in plant defense and abiotic stress. For example,
MBERR11 from Malus baccate can improves the expression level of
Arabidopsis’s cold and salt stress through improving ROS scavenging [
37]. Another ERF gene is
MdERF38 in apple, which promotes anthocyanin biosynthesis due to drought stress [
38]. The functional characterization of the ERF family in
R. hainanense remains limited. To address this, we identified its ERF members and investigated their expression patterns under waterlogging stress to explore the underlying molecular mechanisms. Transcriptome sequencing provided a cost-effective and reliable approach to obtain the necessary expression data for this analysis [
39]. Transcriptomic data of
R. hainanense was obtained from our previous group work. We extracted AP2/ERF genes and performed bioinformatics analysis with respect to the physicochemical properties, phylogenic relationship, gene structure, conserved motifs,
cis-acting elements, syntenic region, and protein properties. Gene expression with respect to waterlogging stress was carried out, and the gene expression at specific plant parts was carried out.
Therefore, there were 142 AP2/ERF family members with a complete domain found in the entire genome of
R. hainanense. Depending on their location on the chromosome, they were organized under the names
RhERF 1,
RhERF 2,
RhERF 3, and so on, up to
RhERF 142. This is unlike other species with respect to the number of RhERFs: for example,
Arabidopsis thaliana has 122 ERF genes [
18], soybean (Glycine max) has 47 ERF genes [
40], and potatoes (Solanum tuberosum) have 155 ERF genes [
41]. ERF numbers may be different on different plants as they have their own evolution. According to previous research,
A. thaliana has an AP2/ERF protein that can be classified into the following five subfamilies: AP2, ERF, DREB, RAV, and Soloist. Based on this classification, a phylogenetic tree of the AP2/ERF family protein sequences of
R. hainanensis was constructed and analyzed. The RhAP2/ERF gene family was classified into the following subfamilies: AP2 (15 members), ERF (76 members), DREB (43 members), RAV (4 members), and Soloist (2 members).
In the
R. hainanense AP2/ERF gene family duplication events, there are 16 duplicated gene pairs found in 13 chromosomes, and they belong to the same subfamily. Also, Ka and Ks substitution rates were estimated on such duplicated pairs [
42]. The Ka/Ks ratio is widely recognized as an important indicator for evaluating selective pressure on protein-coding genes and estimating the approximate timing of duplication events. All duplicated pairs exhibited Ka/Ks values of less than 1, indicating that the family has undergone purifying selection during evolution, resulting in functional conservation. The comparative synteny analysis (
Figure 6) yielded insights into the evolutionary history of the AP2/ERF family. The observed high degree of synteny with
Rhododendron simsii, a closely related congener, underscores a strong evolutionary conservation of this gene family within the
Rhododendron lineage. In contrast, the fewer collinear gene pairs identified with the distantly related model plant
Arabidopsis thaliana reflect the expected lineage-specific divergence and gene loss over a longer evolutionary timescale. Notably, the presence of species-specific syntenic blocks, particularly on chromosome 7 of
R. hainanense, suggests potential lineage-specific expansion events. These expansions may have furnished
R. hainanense with a unique genetic repertoire that contributes to its adaptation to distinct ecological niches, such as the streamside habitats prone to waterlogging.
In promoters,
cis-acting elements have an important role on controlling gene expression [
43]. The 2000 bp promoter regions upstream of the AP2/ERF genes were analyzed, revealing the presence of various functional elements, including those responsive to plant hormones, light, and stress. Among the stress-responsive elements, the GC-motif is associated with hypoxia response, the LTR element is involved in low-temperature responsiveness [
44], and MBS participates in drought response. One of the most significant findings in this study is the widespread presence of anaerobic response elements in the promoters of RhAP2/ERF genes. Of 142 genes, 118 (83%) were observed to contain ARE, indicating that the vast majority of members of the transcription factor family may directly respond to the core signal of waterlogging stress—anaerobiosis. ARE is a well-characterized
cis-acting element that can recruit transcription factors to activate the expression of key genes involved in anaerobic metabolism. The extremely high prevalence of ARE suggests that the RhAP2/ERF family plays a role as a master regulatory switch in
R. hainanense, reconstructing energy homeostasis under hypoxic conditions through the harmonization of comprehensive transcriptional reprogramming. This finding provides a strong molecular interpretation for the significant waterlogging tolerance of
R. hainanense. The genome-wide enrichment of ARE suggests that this plant has highly sensitive and robust hypoxia sensing. When waterlogging occurs, the rapid accumulation of ethylene and its signal transduction can activate these ARE-containing RhAP2/ERF proteins, thereby driving the expression of downstream adaptability genes. This mechanism gives it an adaptive advantage in natural streamside habitats often affected by waterlogging. The remaining 24 RhAP2/ERF genes lacked the ARE element, indicating functional diversity within the family in regulating plant growth and environmental adaptation. Protein interaction analysis, based on homology with
A. thaliana, suggested that RhERF9 may interact with proteins such as RAP2.12, playing a central role in signal transduction following hypoxia perception. Moreover, the varying positions and connectivity of different subfamily members within the interaction network imply functional divergence, with some genes potentially acting as core hubs integrating upstream signals, while others may specifically regulate certain downstream pathways.
Our KEGG and GO enrichment analyses of these six key RhERF genes provided deeper insights into their functions. A prominent finding was their significant enrichment in the plant hormone signal transduction pathway, specifically associated with ethylene, jasmonic acid, and salicylic acid signaling. This strongly suggests that they form a specialized transcriptional module for integrating core hormone signals during waterlogging stress [
5,
13]—where ethylene accumulates rapidly, while JA and SA regulate subsequent defense mechanisms. Concurrently, their enrichment in the MAPK signaling pathway indicates that these transcription factors likely act as signaling hubs, transducing perceived stress signals, such as rising ROS levels and osmotic imbalance, into coordinated transcriptional responses. This inference is strongly supported by the GO analysis, which directly linked them to key biological processes including response to ethylene, response to reactive oxygen species, and response to osmotic stress. In summary, these six RhERF genes function as central regulatory components in the waterlogging tolerance of
R. hainanense, orchestrating the plant’s adaptive survival by synchronizing hormone signaling, mitigating oxidative damage, and maintaining osmotic homeostasis.
The interaction between promoter binding sites and transcription factors is crucial for transcription regulation [
18]. The conserved motifs in the same RhERF group probably do well together because they join things that happen again, like how two proteins hold hands to start a gene’s work or head to the cell’s center. This also supports the functional category for the ERF family. Genes within the same subgroup usually have the same role. For instance, there is evidence that each member—
AtCBF1,
AtCBF2,
AtCBF3, and
AtCBF4— from the four-member A1 subfamily of A. thaliana plays important roles in cold and drought stress [
45], and there are also roles for the five-member AtERF-VII subfamily—including AtERF71-75—in waterlogging and hypoxia responses [
46]. Interestingly, most genes strongly induced under waterlogging stress belong to Group VII. Notably,
RhERF9 is a homolog of
AtERF73 and also belongs to Group VII. Studies have shown that the AP2/ERF transcription factor
AtERF73 regulates ethylene responses during hypoxia, significantly enhancing plant waterlogging tolerance [
13]. It is thus plausible that RhERF9 may play a similar role in improving waterlogging resistance. To validate this, expression patterns of RhAP2/ERF genes under waterlogging treatment at different time points were analyzed using heatmaps and qRT-PCR. The results revealed significant induction of several genes, including
RhERF9,
RhERF31,
RhERF51,
RhERF69,
RhERF94, and
RhERF95, suggesting temporal specialization and functional complementarity within the AP2/ERF family during waterlogging response. Specifically,
RhERF31,
RhERF51, and
RhERF69 were highly expressed at 20 days of treatment;
RhERF95 and
RhERF94 showed peak expression at 7 days; and
RhERF9 exhibited the highest expression at 3 days. Collectively, these findings support the involvement of these genes in the waterlogging stress response.
Given the pronounced up-regulation of RhERF9 at 3 days of waterlogging, tissue-specific expression analysis was conducted comparing treated and untreated plants. The results showed a significant induction of RhERF9 in root tissues, suggesting a core role in the formation of root aerenchyma and the regulation of hypoxia adaptation. In conclusion, this study provides a theoretical basis and valuable insights into the functional characterization of the AP2/ERF gene family, noting that RhERF9 and its related transcription network constitute a key waterlogging tolerance regulation module, and observing the impact on the stress resistance of R. hainanense. These findings establish a solid basis for further elucidating the molecular mechanisms of waterlogging tolerance in this species and offer important candidate gene resources and theoretical support for future genetic engineering efforts aimed at enhancing stress resistance in horticultural plants.