Abstract
This study explores the effects of afforestation in infertile mountainous areas on soil microbial communities and functional nutrient cycling genes in young Cyclobalanopsis gilva forests, aiming to provide a scientific basis for promoting C. gilva growth. Employing metagenomic sequencing coupled with integrative analyses of microbial community structure and functional genes, this study took 7-year-old C. gilva forest stands in infertile mountainous areas of Shouchang Forest Farm, Zhejiang Province as the research object, using adjacent 7-year-old C. gilva forest in woodland areas as a control, to analyze the differences in soil microbial community structure and nutrient cycling functional genes in the rhizosphere (SCG) and non-rhizosphere (SNR) of infertile mountainous areas, as well as from the rhizosphere (FCG) and non-rhizosphere (FNR) of control woodland areas, and further explore their relationships with the growth of C. gilva. The results indicated that the contents of soil organic carbon (SOC), total nitrogen (TN), total phosphorus (TP), and microbial biomass nitrogen (MBN) in SNR were significantly lower than those in FNR by 59.50%, 39.57%, 29.32%, and 53.13%, respectively. Bradyrhizobium and Trebonia were the dominant genera in both site conditions; however, the relative abundance of these genera was lower in infertile mountainous areas compared to the control. Notably, the Shannon and Simpson indices of SCG were significantly lower by 0.49 and 0.01 than those of SNR (p < 0.05), respectively. Additionally, the relative abundances of carbon fixation and nitrogen fixation of SCG were significantly higher than those of SNR. And the relative abundances of functional genes involved in carbon cycling (glyA, fdhA), nitrogen cycling (nasA, narfC, narC, and nirB), and phosphorus cycling (phoB) in infertile mountainous areas were significantly higher than those in the control. The nutrient cycling processes and the expression of functional genes in SCG are coordinately regulated by soil nutrients (SOC and TN) and microbial biomass [MBC (microbial biomass carbon) and MBN]. This work provides a mechanistic foundation for optimizing afforestation strategies and ecological restoration in nutrient-limited mountainous ecosystems, highlighting the critical role of microbial functional plasticity in overcoming edaphic constraints.
1. Introduction
Infertile mountainous areas are characterized by poor soil resources, thin soil layers, and limited water retention and nutrient-retention, which significantly complicates afforestation efforts [1]. While some studies have addressed afforestation in these regions, the majority have concentrated on plant growth and the stoichiometry of chemical elements [2]. Conversely, research on the soil microbial community structure and nutrient cycling post-afforestation remains relatively scarce. Soil microorganisms are crucial components of forest ecosystems, playing vital roles in litter decomposition and nutrient cycling [3]. Site conditions significantly influence soil microorganisms [4]; for instance, Ascomycetes exhibit higher relative abundance in nutrient-rich environments, whereas Actinomycetes are more prevalent in barren conditions [5]. Given the unique environmental conditions of infertile mountainous areas, soil microorganisms adapt through various mechanisms, including community structure reorganization, functional gene expression adjustments, and changes in symbiotic strategies [6]. Afforestation impacts soil physicochemical properties and enzyme activities, consequently altering soil microbial biomass and community structure [7]. Studies have shown that afforestation increased soil bacterial and fungal biomass by 36% and 49%, respectively, while the ratio of fungi to bacteria rises by 20% [8]. Additionally, the rhizosphere serves as a critical interface for interaction among plant roots, soil, and microorganisms [9]. As the most dynamic component of soil microbial communities, rhizospheric microorganisms influence microbial functions and nutrient cycling through unique plant exudates or microenvironments [10,11]. A meta-analysis on the effects of the rhizosphere on soil microbial communities revealed that compared to non-rhizosphere soil, the contents of carbon (C), nitrogen (N), and phosphorus (P) in rhizosphere soil increased by 37.5%, 20.4%, and 12.4%, respectively. Furthermore, microbial biomass, fungal diversity and bacterial diversity in the rhizosphere were 53.4%, 72.2%, and 46.5% higher than in non-rhizosphere soil, respectively [12].
Soil microorganism-mediated carbon, nitrogen, and phosphorus cycling plays a pivotal role in enhancing soil fertility and plant productivity [13]. This process involves carbon fixation genes (glyA), organic matter degradation genes (acdA), and methane metabolism genes (acs and mxaF) in carbon cycling [14]; nitrogen fixation genes (ureC) and denitrification genes (nirK) in nitrogen cycling [15]; and two-component system genes (phoB and phoR) and transporter genes (pstS) in phosphorus cycling [16]. These genes are critical for the degradation of organic matter and the transformation of nutrients into bioavailable forms. Additionally, the cycling of carbon, nitrogen, and phosphorus interacts and constrains one another, significantly influencing soil nutrient status and the acquisition of nutrients by plants [17]. For instance, carbon fixation is notably restricted by the availability of nitrogen and phosphorus in terrestrial ecosystems [18]. A comprehensive understanding of this intricate relationship can facilitate the development targeted strategies for the utilization of microbial fertilizers tailored to the conditions of infertile mountainous areas, thereby promoting improved growth of forest stands.
Cyclobalanopsis gilva, an evergreen tree species in the Fagaceae family, is recognized not only as a valuable timber species but also as an essential component for ecological restoration. It is widely cultivated in southern China [19]. This study focused on 7-year-old C. gilva forest stands located in infertile mountainous areas as research subjects, while 7-year-old C. gilva forest stands in woodland areas served as the control. The differences in microbial communities and nutrient cycling functional genes between the rhizosphere and non-rhizosphere soils in both the infertile mountainous areas and control woodland areas were explored utilizing metagenomic sequencing. The objective of this study was to clarify the mechanisms by which soil microorganisms influence the growth of C. gilva under the unique environmental conditions of infertile mountainous areas. This research aims to reveal the critical roles of both rhizospheric and non-rhizospheric soil microorganisms in nutrient cycling. Consequently, it seeks to provide theoretical foundations and practical guidance for scientific afforestation strategies, soil quality improvement, and sustainable ecosystem development during vegetation restoration in these challenging environments.
2. Materials and Methods
2.1. Study Area Overview
The study area is situated at Shouchang Forest Farm (119.22° N, 29.36° E), in Jiande City, Hangzhou, Zhejiang Province, China. The terrain primarily consists of low mountains and hills, with an average altitude of 102 m. The climate is classified as subtropical monsoon, characterized by abundant rainfall, distinct seasons, an average annual temperature of 16.9 °C, and an average total accumulated temperature of 6115 °C. The average annual precipitation is 1600 mm, with an average of 164 rainy days per year. The total annual sunshine hours amount to 1760 h, while the average annual frost-free period spans 261 days. The predominant soil types in the region are yellow and red soils, but they also contain incompletely weathered gravels. The forest exhibits a rich diversity of vegetation types, primarily characterized by subtropical evergreen broad-leaved forests. The main tree species include Castanopsis fargesii, Lithocarpus glaber and Cyclobalanopsis glauca along with other evergreen broad-leaved species. Additionally, coniferous species such as Pinus massoniana and Cunninghamia lanceolata are present, as well as rare and endangered species like Taxus chinensis.
2.2. Experimental Design
In March 2018, infertile mountainous areas characterized by shallow soil morphology (soil thickness ranging from 20 to 60 cm, with a sandy-loam texture and high content of incompletely weathered gravels derived from granite parent rock) were selected in Shouchang Forest to establish an experimental forest of C. gilva. Concurrently, woodland with deeper soil morphology (soil thickness ranging from 50 to 80 cm, with a finer texture and fewer rock fragments) and relatively better soil conditions in the same region was chosen to establish a control experimental forest of C. gilva. The parent rock type for both sites was granite, with partially weathered granite fragments prominent in the infertile mountainous areas. Both types of sites exhibited a sunny slope aspect with a slope gradient of approximately 30 degrees. The afforestation utilized 2-year-old containerized C. gilva seedlings, which had a height of 70 cm, and a ground diameter of 0.7 cm or more, planted at a spacing of 2 m × 2 m. Following afforestation, weeding and maintenance were conducted during the first two years, and once in the third year. Fertilization occurred annually from April to June during the second and third years, with 25–30 g of compound fertilizer applied per plant in an uphill circular furrow.
2.3. Sample Plot Survey
(1) Stand survey. In October 2023, three 20 m × 20 m plots were established in infertile mountainous areas and control woodlands, respectively, totaling 6 plots. For each C. gilva within these plots, tree height (TH), diameter at breast height (DBH), and crown width (CW) were measured (Table 1). The average TH, DBH, and CW of C. gilva in the infertile mountainous areas were found to be 70.41%, 82.85%, and 85.37% of those measured in the control woodland areas, respectively. Site conditions influenced the growth status of C. gilva (p < 0.05).

Table 1.
Characteristics of Cyclobalanopsis gilva in infertile mountainous areas and control woodland areas.
(2) Soil sample collection. Soil samples were collected from the rhizosphere (SCG) and non-rhizosphere (SNR) of infertile mountainous areas, as well as from the rhizosphere (FCG) and non-rhizosphere (FNR) of control woodland areas. In each plot, the locations for soil sampling were determined using the five-point sampling method. Prior to soil sampling, aboveground herbaceous plants and surface litter were removed. Soil samples were collected from the 0–60 cm soil profile, and a 100 cm3 core sampler was used to simultaneously determine soil water content and bulk density. After collection, the soil samples were placed in an ice chest and transported to the laboratory. Roots, stones, and other debris were removed, and the soil was sieved using a 2 mm mesh. Each sample was divided into three portions. One portion was air-dried naturally to determine the pH value, organic carbon, total nitrogen, total phosphorus, available phosphorus and ammonium nitrogen. Another portion was stored in a refrigerator at 4 °C for the determination of soil microbial biomass. The last portion was stored in a refrigerator at −80 °C for determination of the analysis of the composition and functions of soil microbial communities. For rhizosphere soil sampling, the rhizosphere soil was sampled within a depth range of 0–60 cm, and a distance of 1 m was maintained from the non-rhizosphere soil. Potential fine root zones were identified based on the growth status of the trees or the orientation of the surface roots. Fine roots (less than 2 mm in diameter) were located along the main roots, carefully extracted using sterile forceps and scissors, gently shaken to remove adhering loose soil, and placed into 50 mL centrifuge tubes. Approximately 20 to 30 segments of fine roots were collected around each tree to ensure an adequate amount of rhizosphere soil, and the root systems were stored in a −80 °C refrigerator for subsequent rhizosphere microbial analysis. For both rhizosphere soil and non-rhizosphere soil, three subsamples were collected from each plot. The collected subsamples of rhizosphere soil and those of non-rhizosphere soil were mixed thoroughly—yielding one composite rhizosphere soil sample and one composite bulk soil sample per plot, respectively—resulting in a total of 12 soil samples.
2.4. Soil Sample Analysis and Determination
(1) Determination of soil physicochemical properties. In accordance with the determination methods of soil agrochemical analysis [20], Soil water content (SWC) and bulk density (BD) were determined using the core method. Soil pH value was measured through the potentiometric method. Soil organic carbon (SOC) was analyzed using the Walkley-Black modified method (dichromate oxidation with external heating). Total nitrogen (TN) was determined by Kjeldahl method. Total phosphorus (TP) was measured via alkaline fusion. Available phosphorus (AP) was analyzed using the molybdenum-antimony anti-colorimetric method. Ammonium nitrogen (NH4+-N) was determined by KCl extraction followed by continuous flow analysis.
(2) Determination of soil microbial biomass. According to the determination methods for soil microbial biomass [21], Microbial biomass was determined using the chloroform fumigation-extraction method, Microbial biomass carbon (MBC) via TOC (Total Organic Carbon) analyzer, microbial biomass nitrogen (MBN) via Kjeldahl method, and microbial biomass phosphorus (MBP) via chloroform fumigation method.
(3) Metagenomic sequencing of soil microorganisms. DNA was extracted from soil samples using FastDNATM SPIN Kit for Soil (MP Biomedicals, Solon, OH, USA). The purity and integrity of DNA were evaluated through agarose gel electrophoresis (AGE), while its concentration was accurately quantified using a Qubit fluorometer. Qualified DNA samples were then fragmented into approximately 350 bp fragments using a Covaris ultrasonicator (Covaris, Waltham, MA, USA). This was followed by end repair, A-tailing, ligation of sequencing adapters, purification, and PCR amplification to complete the library preparation. The raw data generated by the NovaSeq sequencing platform were preprocessed using fastp (https://github.com/OpenGene/fastp, accessed on 10 September 2024) to obtain clean data for subsequent analysis. Metagenomic data were quality-controlled using Trimmomatic 0.39 from the KneaDdata pipeline to remove ambiguous bases (Ns) and low-quality reads, resulting in high-quality reads. These high-quality reads were then aligned with host DNA sequences using Bowtie2 from the KneaDdata pipeline, and reads exhibiting high similarity to host DNA were discarded [22,23]. Genes with read counts of ≤2 in any sample were filtered out, leading to the creation of a final gene catalog (unigenes) for subsequent analysis. Gene abundances in each sample were calculated based on the aligned read counts and gene lengths [24]. DNA sequences were aligned against the NR and KEGG databases using Diamond 2.1.8 (BLASTP with an E-value threshold of 1 × 10−5).
(4) Annotation of soil microbial functional genes. Based on the results of the comparison, the relative abundance of microbial composition and the functional level of the community at different levels were calculated. Additionally, C, N, and P cycling functional genes were identified based on the simultaneous presence of major pathways in metagenomic samples [25]. Furthermore, Diamond was used to annotate C-cycling-related genes utilizing CCycDB, N-cycling-related genes based on NCycDB [26], and P-cycling-related genes according to PCycDB [27]. The annotated functional genes were subsequently aligned with the NCBI_nr database and classified using the MEtaGenome Analyzer 6.25.10.
2.5. Data Processing and Analysis
Statistical analysis of the data was conducted using R version 4.2.3. Visualizations of soil physicochemical properties, microbial community stacked bar charts, and α-diversity indices were generated using the ggplot2 and vegan packages. A one-way analysis of variance (ANOVA) was performed to determine the significant differences in soil physicochemical properties, microbial biomass, nutrient cycling processes, and functional genes between rhizosphere and bulk soils under two site conditions. To analyze the differences in soil microbial diversity and functional gene abundances, the lme4 and lmerTest packages were used to perform a permutational multivariate analysis of variance (PERMANOVA). For the analysis of microbial β-diversity, the vegan, ade4, and ggplot2 packages were utilized to conduct an Adonis analysis based on Bray–Curtis matrix and to generate the corresponding visualizations. Additionally, correlation analysis was performed using Origin 2021 to analyze the correlations between microbial nutrient cycling, soil physicochemical properties, and growth characteristics of C. gilva.
3. Results
3.1. Soil Physicochemical Properties and Microbial Biomass
As demonstrated in Table 2, the soil quality in infertile mountainous areas is relatively poor, characterized by low nutrient content and weak water retention and storage capacities. The levels of SOC, TN, and TP in these areas were significantly lower than those in control regions, with reductions of 59.50%, 39.57%, and 29.32%, respectively. BD, SWC, and AP content were significantly lower in infertile mountainous areas compared to the control, while the pH value and NH4+-N content were higher in these areas. Furthermore, the contents of MBC, MBN, and MBP in infertile mountainous areas were all lower than in the control areas. Notably, the MBN content was significantly reduced by 53.13% compared to the control (p < 0.05). This indicates that the metabolic activity of soil microorganisms in infertile mountainous soils is weakened.

Table 2.
Soil physicochemical properties and microbial biomass in infertile mountainous areas and control woodland areas.
3.2. Soil Microbial Community Composition and Diversity Analysis
The taxonomic annotation results reveal the top 10 genera with the highest relative abundance and their corresponding diversity indices in both the rhizosphere and non-rhizosphere soils of the two forest stands, as illustrated in Figure 1. Bradyrhizobium and Trebonia were the dominant genera in both rhizosphere and non-rhizosphere soils across both stands (relative abundances > 5%). The average relative abundances were higher in the control areas compared to the infertile mountainous areas, and were also greater in the rhizosphere soil than in the non-rhizosphere soil (Figure 1a). Furthermore, site conditions and soil types exerted a more pronounced effect on the relative abundance of microbial genera than on the composition of the microbial community structure. Principal coordinates analysis (PCoA) based on Bray–Curtis distances indicated that PCoA1 and PCoA2 accounted for 87.30% and 5.17% of the variation in soil microbial community structure, respectively (Figure 1b). Furthermore, the permutational multivariate analysis of variance (PERMANOVA) demonstrated significant differences in microbial community structure across various soil types (p < 0.01). Compared with site conditions, the rhizosphere environment has a more significant impact on the soil microbial community structure, which indicates that root exudates have a greater impact on the soil microbial community structure. The differences in Shannon and Simpson indices between the rhizosphere and non-rhizosphere in infertile mountainous areas were significantly greater than those in the control (Figure 1c). The Shannon and Simpson indices of SCG were significantly lower by 0.49 and 0.01 than those of SNR (p < 0.05), respectively. Additionally, soil types and their interaction with site conditions had significant effects on diversity indices (p < 0.01). This finding suggests that soil types exert a more pronounced influence on microbial diversity indices, whereas the impact of site conditions is comparatively less significant.
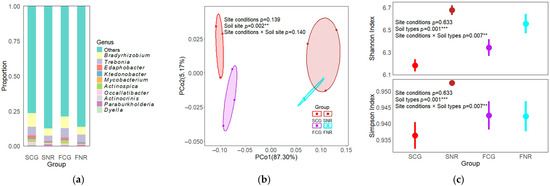
Figure 1.
Soil microbial community composition and diversity in infertile mountainous areas and control woodland areas. (a) Stacked bar charts showing the top 10 microbial genera in rhizosphere and non-rhizosphere soils of both site conditions. (b) Principal coordinates analysis (PCoA) of microbial community structure in rhizosphere and non-rhizosphere soils of both site conditions. (c) Shannon and Simpson diversity indices in rhizosphere and non-rhizosphere soils of both site conditions. SCG: Rhizosphere of infertile mountainous areas, SNR: Non-rhizosphere of infertile mountainous areas, FCG: Rhizosphere of control woodland areas, FNR: Non-rhizosphere of control woodland areas. ** p < 0.01, *** p < 0.001.
3.3. Soil Microbial Nutrient Cycling Processes
The organic matter degradation and methane metabolism within carbon cycling, along with the process of organic matter degradation and synthesis, assimilatory and dissimilatory nitrate in nitrogen cycling, purine and pyrimidine metabolism, two-component systems, and transporters in phosphorus cycling, exhibited higher relative abundances (Figure 2). Compared to control woodland areas, carbon fixation, dissimilatory nitrate reduction, nitrogen fixation, and pyruvate metabolism exhibited higher relative abundances in infertile mountainous areas. In contrast, organic matter degradation and synthesis, and denitrification were observed at lower levels. The rhizosphere conditions of both forest stands strengthened processes such as carbon fixation, dissimilatory nitrate reduction, nitrogen fixation, transporter, and phosphonate and phosphinate metabolism, while diminishing processes including methane metabolism, purine and pyrimidine metabolism, and two-component systems. Overall, site conditions and soil types can influence the relative abundances of soil microbial nutrient cycling processes. However, they do not alter the overall ranking of these relative abundances; that is, the dominant nutrient cycling processes of microorganisms remain unchanged.
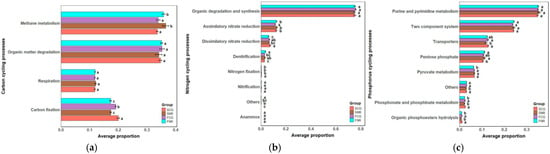
Figure 2.
Bar charts of relative abundance of soil microbial nutrient cycling processes in infertile mountainous areas and control woodland areas. (a) Bar charts of relative abundance of soil microbial carbon cycling processes in infertile mountainous areas and control woodland areas. (b) Bar charts of relative abundance of soil microbial nitrogen cycling processes in infertile mountainous areas and control woodland areas. (c) Bar charts of relative abundance of soil microbial phosphorus cycling processes in infertile mountainous areas and control woodland areas. The lowercase letters indicate significant differences (p < 0.05) in the relative abundances of nutrient cycling processes between infertile mountainous areas and control woodland areas of both site conditions. SCG: Rhizosphere of infertile mountainous areas, SNR: Non-rhizosphere of infertile mountainous areas, FCG: Rhizosphere of control woodland areas, FNR: Non-rhizosphere of control woodland areas.
3.4. Soil Microbial Nutrient Cycling Functional Genes
3.4.1. Soil Microbial Carbon Cycling Functional Genes
The analysis of soil microbial carbon cycling functional genes with relative abundances exceeding 1% (Figure 3a) indicates that site conditions and the rhizosphere environment significantly influence functional genes associated with carbon fixation and methane metabolism. In contrast, the impact on genes related to respiration is comparatively minor. Compared to control woodland areas, the relative abundances of genes such as glyA, fdhA, and fdhA-K00148 were found to be upregulated in both the rhizosphere and non-rhizosphere of infertile mountainous areas, whereas mxaF and fdwB exhibited downregulation. Additionally, the relative abundances of most organic matter degradation genes in infertile mountainous areas were lower than those in the control. The rhizosphere conditions of both forest stands enhanced the relative abundances of genes such as glyA, folA, and serB, while simultaneously reducing the relative abundances of genes such as serA, mdh-K00024, and acs.
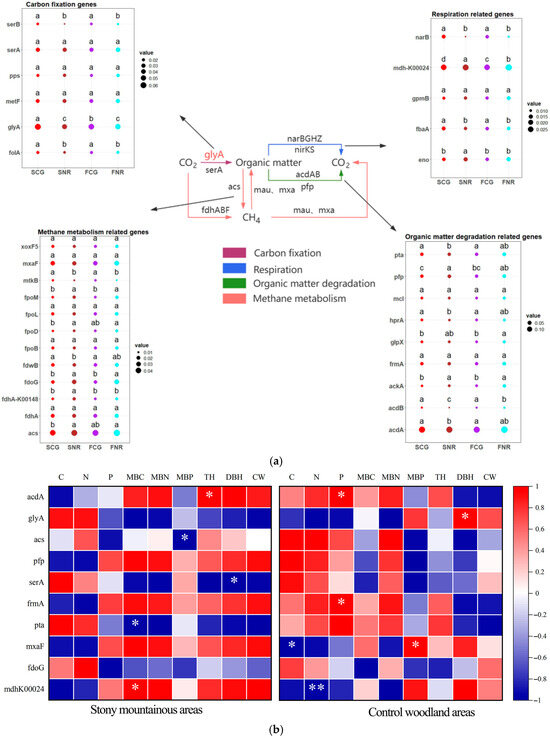
Figure 3.
Analysis of soil microbial carbon cycling functional genes in infertile mountainous areas and control woodland areas. (a) Flow chart of soil microbial carbon cycling and diagram of relative abundances of functional genes in each process. In nutrient cycling flowcharts, red text indicates functional genes showed significant differences in soil types, black text indicated functional genes with no significant differences. In diagram of relative abundances of functional genes in each process, lowercase letters indicate significant differences in nutrient cycling functional genes between rhizosphere and non-rhizosphere of both site conditions at p < 0.05. Dots of different colors represent different treatments, while the size of the dots represents the relative abundance of functional genes. (b) Correlation analysis of the top 10 most relative abundance carbon cycling functional genes with soil nutrient, microbial biomass, and growth characteristics of C. gilva. Red indicated positive correlation, and blue indicated negative correlation. * p < 0.05, ** p < 0.01.
The correlation analysis (Figure 3b) of the top 10 carbon cycling functional genes based on the relative abundance of soil microorganisms in rhizosphere soil indicates that the correlation between carbon cycling functional genes in infertile mountainous areas and soil nutrients is weaker compared to the control. Conversely, the correlation with microbial biomass and plant growth characteristics is stronger than that of the control. Additionally, mxaF exhibited a significant positive correlation with MBP, while glyA demonstrated a significant positive correlation with DBH. These findings suggest that the functional genes involved in carbon cycling in infertile mountain soils are jointly influenced by microbial biomass and plant growth characteristics. In contrast, in control woodlands, these genes are primarily affected by soil nutrient availability. Meanwhile, when carbon cycling functional genes in infertile mountainous areas exhibited a positive correlation with soil nutrients, while demonstrating a negative correlation with microbial biomass, and vice versa.
3.4.2. Soil Microbial Nitrogen Cycling Functional Genes
The analysis of soil microbial nitrogen cycling functional genes with relative abundances exceeding 1% (Figure 4a). Compared to the control, the relative abundances of nirB, nasA, and gs_k00284 were significantly upregulated in both rhizosphere and non-rhizosphere of infertile mountainous areas, while the abundances of glsA significantly decreased. Furthermore, the rhizosphere conditions of both forest stands significantly enhanced the relative abundances of gs_K00265, nmo and nirB, while concurrently leading to a significant reduction in the relative abundances of NR, nirK and asnB. Overall, site conditions significantly influence functional genes related to organic matter degradation and synthesis, assimilatory nitrate reduction, and denitrification. Additionally, rhizosphere conditions have a profound impact on the functional genes involved in all processes of nitrogen cycling.
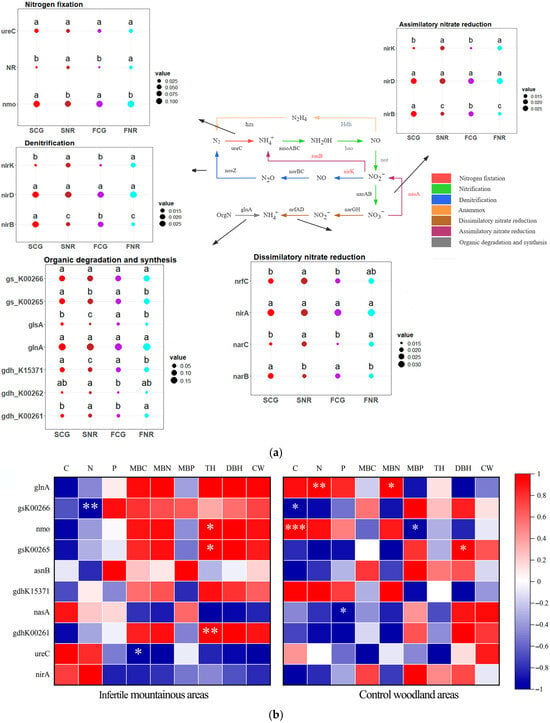
Figure 4.
Analysis of soil microbial nitrogen cycling functional genes in infertile mountainous areas and control woodland areas. (a) Flow chart of soil microbial nitrogen cycling and diagram of relative abundances of functional genes in each process. In nutrient cycling flowcharts, red text indicated functional genes showing significant differences in soil types, black text indicated functional genes with no significant differences, gray text indicated undetected functional genes. In diagram of relative abundances of functional genes in each process, lowercase letters indicated significant differences in nitrogen cycling functional genes between rhizosphere and non-rhizosphere of both site conditions at p < 0.05. Dots of different colors represent different treatments, while the size of the dots represents the relative abundance of functional genes. (b) Correlation analysis of the top 10 most relative abundance nitrogen cycling functional genes with soil nutrient, microbial biomass, and growth characteristics. Red indicated positive correlation, and blue indicated negative correlation. * p < 0.05, ** p < 0.01, *** p < 0.001.
The correlation analysis (Figure 4b) of the top 10 nitrogen cycling functional genes based on the relative abundance of soil microorganisms in rhizosphere soil indicates that the correlations between nitrogen cycling functional genes in infertile mountainous areas and soil nutrients, as well as microbial biomass are weaker than those observed in the control. Conversely, the correlations with the growth characteristics of C. gilva are stronger than those observed in the control. In infertile mountainous areas, a significant negative correlation was observed between gs_K00266 and soil P content, as well as between ureC and MBC content. Conversely, a significant positive correlation was found between nmo, gs_K00265, gs_K00261 and TH. In control woodlands, nitrogen cycling functional genes exhibited significant correlations with the content of C, N, P, MBN and MBP. However, these genes only showed a significant correlation with the DBH of C. gilva in infertile mountainous areas. Compared to the control, nitrogen cycling functional genes in infertile mountains are less influenced by soil nutrients and microbial biomass, but are more affected by growth characteristics.
3.4.3. Soil Microbial Phosphorus Cycling Functional Genes
The analysis of soil microbial phosphorus cycling functional genes with relative abundances exceeding 1% (Figure 5a). In comparison to the control, the relative abundances of phoB and thyA in the rhizosphere of infertile mountainous areas were significantly upregulated. Additionally, the relative abundances of adk, pyk and pckG in the non-rhizosphere of infertile mountainous areas were also significantly increased, whereas the relative abundance of guaB decreased. The rhizosphere conditions of both forest stands significantly reduced the relative abundances of phosphorus cycle functional genes including PhoR, tmk, ack, gnd, and pstS, while only significantly upregulating the relative abundance of prsA. Overall, site conditions can significantly alter the relative abundances of functional genes associated with two-component systems, purine and pyrimidine metabolism, and pyruvate metabolism. However, they do not significantly affect the functional genes related to pentose phosphate and transporters. Additionally, rhizosphere conditions markedly reduce the relative abundances of most functional genes involved in phosphorus cycling.
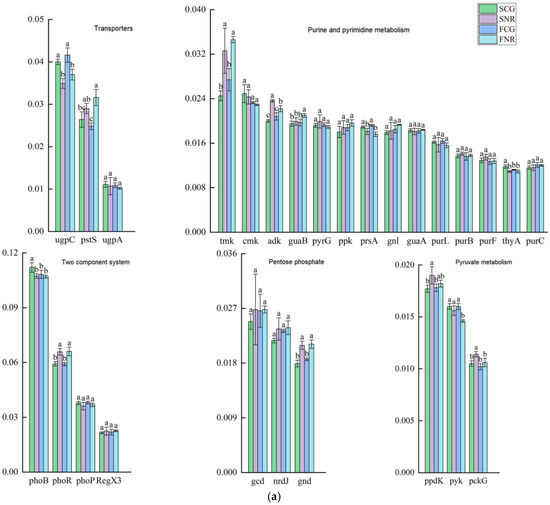
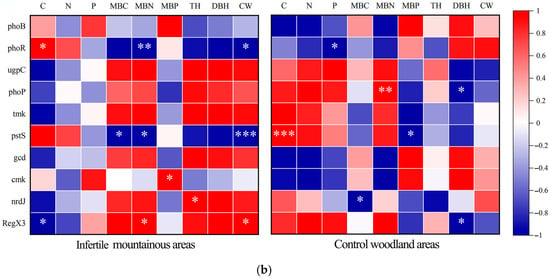
Figure 5.
Analysis of soil microbial phosphorus cycling functional genes in infertile mountainous areas and control woodland areas. (a) Diagram of relative abundances of functional genes in each process, lowercase letters indicated significant differences in phosphorus cycling functional genes between rhizosphere and non-rhizosphere of both site conditions at p < 0.05. (b) Correlation analysis of the top 10 most relative abundances phosphorus cycling functional genes with soil nutrient, microbial biomass, and growth characteristics. Red indicated positive correlation, and blue indicated negative correlation. * p < 0.05, ** p < 0.01, *** p < 0.001.
The correlation analysis (Figure 5b) of the top 10 phosphorus cycling functional genes based on the relative abundance of soil microorganisms in rhizosphere soil indicates that the correlation between phosphorus cycling functional genes in infertile mountainous areas and soil nutrients is weaker than that observed in the control. In contrast, the correlation with microbial biomass and plant growth characteristics is stronger compared to the control. In infertile mountainous areas, nrdJ demonstrated a significant positive correlation with TH, while RegX3 exhibited a significant positive correlation with CW. Conversely, both phoR and pstS displayed significant negative correlations with CW. In control woodland, both phoP and RegX3 exhibited significant negative correlations with DBH. In both site conditions, phosphorus cycling functional genes displayed significant correlations with soil nutrients and microbial biomass, indicating that soil nutrients and microbial biomass have strong regulatory effects on phosphorus cycling functional genes across both site conditions.
3.5. Correlation Analysis of Soil Microbial Nutrient Cycling Processes with Soil Nutrient and Microbial Biomass
To further clarify the differences in rhizosphere effects, a correlation analysis was conducted using the relative abundances of nutrient cycling processes of soil microorganisms in the rhizosphere soil of two forest stands as well as the nutrients and microbial biomass present in non-rhizosphere soil. As illustrated in Figure 6, in infertile mountainous areas, both carbon fixation and respiration exhibit a highly significant negative correlation with MBC content. Conversely, assimilatory nitrate reduction in these areas shows a significant positive correlation with C content, while it demonstrates a significant negative correlation with MBN content. Nitrogen fixation exhibited a significant negative correlation with P content. However, no significant correlations were observed between the phosphorus cycling process and soil nutrients as well as microbial biomass. This suggests that in infertile mountainous areas, soil nutrients and microbial biomass exert a more substantial regulatory influence on carbon and nitrogen cycling processes, while their regulatory effect on phosphorus cycling is comparatively weaker. In the control woodland, only carbon fixation and respiration demonstrated a highly significant negative correlation with P content (p < 0.01). This finding suggests that soil microbial carbon cycling in the control may be influenced by phosphorus limitation. Overall, the correlations between nutrient cycling processes of soil microorganisms in rhizosphere soil of infertile mountainous areas and the nutrients and microbial biomass of non-rhizosphere soil are stronger than those observed in control woodland areas. Additionally, the contents of C and N in infertile mountainous areas were positively correlated with nutrient fixation process, while they exhibited a negative correlation with organic matter degradation process. Conversely, the contents of MBC and MBN displayed an opposite trend. Notably, this pattern was not observed in control woodland areas. These findings suggest that microbial nutrient cycling processes including nutrient synthesis and organic matter degradation in infertile mountainous areas are jointly regulated by soil nutrients and microbial biomass.
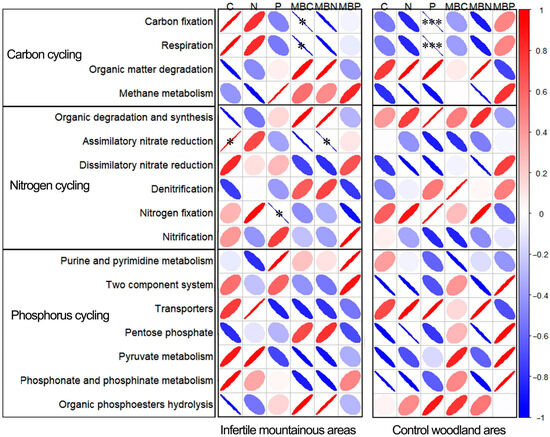
Figure 6.
Correlation analysis of soil microbial nutrient cycling processes with soil nutrient and microbial biomass. The greater the narrowness of the ellipse, the stronger the correlation it indicates, and simultaneously, a deeper color also denotes a stronger correlation. Red indicates positive correlation, and blue indicates negative correlation. * p < 0.05, *** p < 0.001.
4. Discussion
4.1. Effects of Site Conditions and Soil Types on Soil Microbial Communities
This study found that Bradyrhizobium and Trebonia were the dominant genera in both rhizosphere and non-rhizosphere soils across different site conditions. This indicates that while site conditions and rhizosphere environments do not affect the composition of dominant genera, they do influence their relative abundance [28,29]. Infertile mountainous areas exhibit compact soil structures and inadequate aeration, creating favorable conditions for the proliferation of anaerobic microorganisms [30]. These anaerobic microorganisms facilitate the hydrolysis of macromolecular substances such as lignin and cellulose in the soils of infertile mountainous areas [31]. Furthermore, the low status of soil nutrients in infertile mountainous areas restricts microbial nutrient uptake and metabolism. This limitation adversely affects microbial proliferation and metabolism, leading to a relatively low abundance of microorganisms involved in nutrient cycling [32]. The relative abundance of dominant microbial genera in the rhizosphere was significantly higher compared to the non-rhizosphere. Allelochemicals present in the rhizosphere, such as organic acids, significantly influence the composition and function of microbial communities by altering the microenvironmental conditions for soil microorganisms [33]. Furthermore, rhizosphere roots indirectly influence microbial diversity and abundance through soil nutrient uptake processes [34].
The microbial diversity index indicates that the differences in the Shannon and Simpson indices of soil microorganisms between the rhizosphere and non-rhizosphere of infertile mountainous areas are more pronounced than in the control. This observation is attributed to the limited soil nutrient conditions in infertile mountainous areas, which amplify the impact of rhizosphere exudates on the microbial community. Principal coordinate analysis revealed that site conditions and soil types significantly influenced the composition of soil microbial communities. Microorganisms under varying site conditions exhibited distinct response patterns to disturbance stress, thereby affecting the functional diversity of microbial communities [35]. Overall, soil types significantly impact the composition of soil microbial communities. This is likely because there are fundamental differences in the physical, chemical, and biological properties between rhizosphere and non-rhizosphere soils [36]. The effects of root exudates and rhizosphere sediments on shaping the microbial community in rhizosphere soil are more direct and intense [37]. Consequently, the influence of the rhizosphere environment on the soil microbial community is stronger than that of site conditions.
4.2. Effects of Site Conditions and Soil Types on Functional Genes Involved in Soil Microbial Carbon, Nitrogen, and Phosphorus Cycling
Carbon, nitrogen, and phosphorus are essential elements in the soil nutrient cycling process, playing a critical role in ecosystem functions as well as the growth and development of plants [38]. This study demonstrates that nutrient cycling processes including carbon fixation and nitrogen fixation are enhanced in infertile mountainous areas, whereas processes such as organic matter degradation are diminished. This phenomenon can be attributed to the poor soil nutrient conditions prevalent in these regions. To optimize the utilization of existing resources, soil microorganisms facilitate nutrient synthesis while mitigating the degradation of organic matter, thereby reducing nutrient consumption. Furthermore, functional genes involved in nutrient cycling exhibited high compositional consistency in both rhizosphere and non-rhizospheric soils across various site conditions; however, significant differences were observed in the relative abundances of dominant functional genes. Compared to the control, the metabolic activity of microorganisms in infertile mountainous areas is weaker, and the stability and structure of the soil microbial community are compromised [39]. Additionally, the low nutrient status in these areas restricts the nutrient uptake processes of soil microorganisms, thereby affecting the structure of the microbial community [40]. Overall, microorganisms develop complex and ordered microbial community structures through mechanisms such as niche construction, competition, or complementarity [41,42].
Methane metabolism (fdhA) and carbon fixation (glyA) exhibited higher relative abundances in both the rhizosphere and non-rhizosphere of infertile mountainous areas compared to the control. This phenomenon can be attributed to poor aeration, which creates anaerobic conditions that promote methane metabolic processes [43]. FdhA plays a crucial role in formate metabolism by encoding formate dehydrogenase (FDH) [44]. Additionally, soil microorganisms enhance nutrient synthesis by upregulating carbon fixation genes (glyA) in infertile mountainous areas [45]. Most organic matter degradation genes (acdA) exhibited higher relative abundance in woodland areas compared to infertile mountainous areas. This discrepancy can be attributed to the poor nutrient conditions prevalent in infertile mountainous areas, which inhibit the expression of functional genes responsible for organic matter degradation to a certain extent. In contrast, a high nutrient status in woodland soils promotes microbial metabolism and proliferation [46]. This enhancement facilitates the secretion of extracellular enzymes involved in organic matter degradation, thereby accelerating the involvement of genes (acdA) in degradation processes [47]. Respiration exhibited minimal variation across different site conditions, as it is a fundamental metabolic process that microorganisms require to generate energy and sustain essential life processes, irrespective of these conditions [48]. The rhizosphere environment significantly alters the relative abundances of most carbon cycling functional genes due to marked differences in environmental conditions between rhizosphere and non-rhizosphere areas, as well as differing nutrient requirements. These factors collectively influence the expression of carbon cycling functional genes in soil microorganisms.
Dissimilatory nitrate reduction genes (nrfC) and nitrogen fixation genes (nmo) exhibited a significantly higher relative abundance in both the rhizosphere and non-rhizosphere of infertile mountainous areas compared to woodland areas. Due to the limited availability of nitrogen in infertile mountainous soils, soil microorganisms enhance their nitrate reduction capabilities by upregulating nrfC, which facilitates the conversion of insoluble nitrogen into nitrite for their own utilization [49]. At the same time, the efficient expression of nmo suggests that soil microorganisms can effectively fix limited nitrogen in the soil of infertile mountainous areas to satisfy various metabolic requirements [15]. However, denitrification (nirK) in the rhizosphere and non-rhizosphere of infertile mountainous areas is lower than that in woodland areas. This difference can be attributed to the reduced expression of functional genes related to denitrification in infertile mountainous regions, which serves to minimize nitrogen loss. In contrast, woodlands exhibit better soil aeration, allowing microorganisms to effectively utilize denitrification genes (nirK) under partial anaerobic conditions to maintain soil nitrogen balance [50]. Furthermore, the regulatory influence of the rhizosphere environment on functional genes involved in nitrogen cycling is significantly stronger than that of site conditions. The relative abundance of nitrogen fixation (nmo) in rhizosphere soil is notably high due to the presence of rhizosphere exudates, which create a microenvironment characterized by local nitrogen richness. This condition stimulates the metabolic activities of soil microorganisms, thereby enhancing the nitrogen synthesis process. Concurrently, the rhizosphere has a significant demand for nitrogen, which promotes the efficient expression of nitrogen fixation genes such as nmo [51]. In contrast, the relative abundance of assimilatory nitrate reduction (asnB) is higher in non-rhizosphere soil. This phenomenon can be attributed to the relatively stable nitrogen content and metabolism in non-rhizosphere environments, leading microorganisms to preferentially regulate nitrogen metabolism through the assimilatory nitrate reduction pathway [52].
PhoB and PhoR form a two-component system that plays a critical role in phosphorus cycling, jointly regulating the acquisition and utilization of phosphorus by microorganisms [53]. Two-component system genes (phoB) exhibited a higher relative abundance in both the rhizosphere and non-rhizosphere of infertile mountainous areas. This gene is essential in phosphorus cycling as it perceives changes in external phosphorus concentrations and regulates the expression of other related genes to adapt to low phosphorus conditions [54]. Due to the low availability of phosphorus in infertile mountainous soils, microorganisms activate phosphorus acquisition mechanisms by upregulating the expression of phoB [55]. The relative abundance of rhizosphere transporters (pstS) in infertile mountainous areas is higher than that in control woodlands. This phenomenon can primarily be attributed to the low phosphorus content in the soils of infertile mountainous areas, coupled with the high demand for phosphorus in the rhizosphere. PstS may exhibit a high affinity and transport rate, facilitating microorganisms in acquiring limited phosphorus resources [27]. ThyA is a crucial gene involved in nucleotide metabolism and phosphorus utilization. By catalyzing the conversion of deoxyuridine monophosphate (dUMP) to deoxythymidine monophosphate (dTMP), it plays a significant role in the salvage pathway of DNA synthesis, thereby minimizing nutrient loss during the cycling process [56]. Additionally, adk maintains the stability of microbial metabolism by catalyzing the mutual conversion between ADP and ATP, thereby enhancing the utilization efficiency of limited phosphorus elements [57]. PckG plays a crucial role in replenishing intermediate products in the tricarboxylic acid cycle, sustaining energy metabolism, and improving the utilization of external nutrients by effectively utilizing limited organic substances [58]. The rhizosphere environment exhibits reduced relative abundances of most phosphorus cycling functional genes. This phenomenon occurs because the phosphorus content in the rhizosphere is more abundant, which subsequently decreases the expression of phosphorus cycling functional genes compared to non-rhizosphere environments.
4.3. Correlation Analysis of Growth of Cyclobalanopsis gilva, Soil Nutrient, and Microbial Nutrient Cycling
Site conditions mediate soil microbial nutrient cycling by influencing soil nutrient status [59]. Correlation analysis revealed that most nutrient cycling functional genes in infertile mountainous areas exhibited positive correlations with plant growth characteristics. This phenomenon can be attributed to the fact that poor nutrient conditions in infertile mountainous areas impede plant growth, while nutrient cycling functional genes enhance soil nutrient availability by regulating nutrient cycling processes, thereby promoting plant growth [60].
Correlation analysis of nutrient cycling processes with soil nutrients and microbial biomass revealed that an increase in nutrient contents such as C and N in infertile mountainous areas promotes nutrient synthesis processes including carbon fixation and nitrogen fixation while inhibiting organic matter degradation in nutrient cycling. On one hand, soil nutrients serve as substrates for microbial nutrient cycling processes, thereby facilitating microbial nutrient synthesis and establishing a positive feedback mechanism [61]. On the other hand, the availability of soil nutrients reduces the microbial demand for organic matter degradation to some extent. When nutrient availability is sufficient, soil microorganisms preferentially utilize existing nutrients rather than degrading organic matter to fulfill their nutrient requirements [62]. The contents of MBC and MBN exhibited an inverse correlation with the levels of C and N. Specifically, an increase in MBC and MBN content inhibits nutrient synthesis processes such as carbon fixation and nitrogen fixation, while simultaneously promoting the degradation of organic matter. High microbial biomass indicates a greater demand for nutrients for growth and reproduction, promoting the degradation of organic matter to release more nutrients for microbial uptake and utilization [63]. In general, in infertile mountainous areas, available soil nutrients are utilized to promote nutrient synthesis, while soil microorganisms are engaged to accelerate the degradation of organic matter, thereby facilitating the release and utilization of nutrients. These two aspects coordinately regulate the soil microbial nutrient cycling process.
5. Conclusions
The poor nutrient status of soils in infertile mountainous areas, with SOC, TN, TP, and MBN contents significantly lower than those in control woodland areas by 59.50%, 39.57%, 29.32%, and 53.13%, respectively, restricts the reproduction and metabolism of soil microorganisms, leading to a reduction in the relative abundances of dominant genera such as Bradyrhizobium and Trebonia, and increases the differences in soil microbial diversity indices between the rhizosphere and non-rhizosphere. The environmental conditions in infertile mountainous areas do not alter the main processes of nutrient cycling in soil microorganisms, while soil microorganisms have upregulated the abundances of functional genes related to carbon fixation (glyA and fdhA), dissimilatory nitrate reduction (narC and nirB), nitrogen fixation (nasA), purine and pyrimidine metabolism (purB and purF) and two-component systems (phoB), thereby developing metabolic strategies to adapt to the poor nutrient condition. The nutrient cycling functional genes in the rhizosphere exhibited a strong correlation with the growth characteristics of C. gilva. Furthermore, the nutrient cycling processes of the rhizosphere and the expression of functional genes in infertile mountainous areas are coordinately regulated by soil nutrients (SOC and TN) as well as microbial biomass (MBC and MBN). This interaction results in a distinct rhizosphere effect. The research results provide a theoretical basis for the tending of afforestation tree species in difficult sites, soil nutrient cycling, and the improvement of soil nutrient use efficiency strategies.
Author Contributions
W.Y.: Writing—original draft, Visualization, Formal analysis. S.H.: Validation, Formal analysis. Y.D.: Software, Investigation. Y.L.: Software, Investigation. Y.X.: Project administration, Resources. J.F.: Project administration, Resources. Z.Z.: Writing—review and editing. Z.Y.: Conceptualization, Writing—review and editing. B.W.: Writing—original draft, Funding acquisition, Methodology. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from Key Scientific and Technological Grant of Zhejiang for Breeding New Agricultural Varieties (2021C02070-9).
Data Availability Statement
The data are available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to thank the editor and anonymous reviewers for their support in improving this manuscript.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- He, J.; Cai, Q.; Li, G.; Wang, Z. Integrated erosion control measures and environmental effects in rocky mountainous areas in northern China. Int. J. Sediment. Res. 2010, 25, 294–303. [Google Scholar] [CrossRef]
- Huang, L.; Bao, W.; Kuzyakov, Y.; Hu, H.; Zhang, H.; Li, F. Enzyme stoichiometry reveals microbial nitrogen limitation in stony soils. Sci. Total Environ. 2024, 946, 174124. [Google Scholar] [CrossRef] [PubMed]
- Che, Y.; Jin, G. Plant-soil microbial diversity and structural attributes jointly dominate the multifunctionality of the temperate forest. Forests 2024, 166, 11282. [Google Scholar] [CrossRef]
- Yoon, J.-H.; Adhikari, M.; Jeong, S.S.; Lee, S.P.; Kim, H.S.; Lee, G.S.; Park, D.H.; Kim, H.; Yang, J.E. Microbial diversity of soils under different land use and chemical conditions. Appl. Biol. Chem. 2024, 67, 111. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, Z.; Zong, Y.; Li, W.; Chen, F.; Wang, G.G.; Li, J.; Fang, X. Mixing with coniferous tree species alleviates rhizosphere soil phosphorus limitation of broad-leaved trees in subtropical plantations. Soil Biol. Biochem. 2023, 175, 108853. [Google Scholar] [CrossRef]
- Yang, Y.; Qiu, K.; Xie, Y.; Li, X.; Zhang, S.; Liu, W.; Huang, Y.; Cui, L.; Wang, S.; Bao, P. Geographical, climatic, and soil factors control the altitudinal pattern of rhizosphere microbial diversity and its driving effect on root zone soil multifunctionality in mountain ecosystems. Sci. Total Environ. 2023, 904, 166932. [Google Scholar] [CrossRef]
- Hu, M.; Wang, Y.; Li, H.; Hu, L.; Liu, Q.; Zhou, F.; Yang, A.; Yu, F.; Ouyang, X. The impacts of tree species on soil properties in afforested areas: A case study in central subtropical China. Forests 2024, 15, 895. [Google Scholar] [CrossRef]
- Luo, X.; Hou, E.; Zhang, L.; Kuang, Y.; Wen, D. Altered soil microbial properties and functions after afforestation increase soil carbon and nitrogen but not phosphorus accumulation. Biol. Fert. Soils 2023, 59, 645–658. [Google Scholar] [CrossRef]
- Liu, L.; Ma, L.; Zhu, M.; Liu, B.; Liu, X.; Shi, Y. Rhizosphere microbial community assembly and association networks strongly differ based on vegetation type at a local environment scale. Front Microbiol. 2023, 14, 1129471. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ma, L.; Zuo, X.; Ye, X.; Wang, R.; Huang, Z.; Liu, G.; Cornelissen, J.H.C. Plant diversity has stronger linkage with soil fungal diversity than with bacterial diversity across grasslands of northern China. Glob. Ecol. Biogeogr. 2022, 31, 886–900. [Google Scholar] [CrossRef]
- Xu, Z.; Hu, Z.; Jiao, S.; Bell, S.M.; Xu, Q.; Ma, L.; Chen, J. Depth-dependent effects of tree species identity on soil microbial community characteristics and multifunctionality. Sci. Total Environ. 2023, 878, 162972. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Wang, C.; Cai, A.; Zhou, Z. Global magnitude of rhizosphere effects on soil microbial communities and carbon cycling in natural terrestrial ecosystems. Sci. Total Environ. 2023, 856, 158961. [Google Scholar] [CrossRef]
- Luo, G.; Xue, C.; Jiang, Q.; Xiao, Y.; Zhang, F.; Guo, S.; Shen, Q.; Ling, N. Soil carbon, nitrogen, and phosphorus cycling microbial populations and their resistance to global change depend on soil C:N:P stoichiometry. mSystems 2020, 5, 10–1128. [Google Scholar] [CrossRef]
- Huang, Q.; Huang, Y.; Wang, B.; Dippold, M.A.; Li, H.; Li, N.; Jia, P.; Zhang, H.; An, S.; Kuzyakov, Y. Metabolic pathways of CO2 fixing microorganisms determined C-fixation rates in grassland soils along the precipitation gradient. Soil Biol. Biochem. 2022, 172, 108764. [Google Scholar] [CrossRef]
- Kuypers, M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Zhao, J.; Yi, Q.; Li, J.; Li, Z.; Wu, S.; Zhang, W.; Wang, K. Metagenomic insights into the effects of organic and inorganic agricultural managements on soil phosphorus cycling. Agric. Ecosyst. Environ. 2023, 343, 108281. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, S.; Zhuang, W.; Li, K.; Wang, F.; Li, T.; Chen, D.; Fan, Q.; Zhang, Z.; Tudi, M.; et al. Reforestation significantly enriches soil microbial carbon, nitrogen, and phosphorus cycling genes but simplifies their co-occurrence network. Appl. Soil Ecol. 2025, 207, 105935. [Google Scholar] [CrossRef]
- Du, E.; Terrer, C.; Pellegrini, A.F.; Ahlström, A.; van Lissa, C.J.; Zhao, X.; Xia, N.; Wu, X.; Jackson, R.B. Global patterns of terrestrial nitrogen and phosphorus limitation. Nat. Geosci. 2020, 13, 221–226. [Google Scholar] [CrossRef]
- Liu, B.; Li, Y.; Zhao, J.; Weng, H.; Ye, X.; Liu, S.; Zhao, Z.; Ahmad, S.; Zhan, C. The potential habitat response of Cyclobalanopsis gilva to climate change. Plants 2024, 13, 2336. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agrochemical Analysis, 3rd ed.; China Agricultural Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Li, W.; Godzik, A. Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Fåk, F.; Nookaew, I.; Tremaroli, V.; Fagerberg, B.; Petranovic, D.; Bäckhed, F.; Nielsen, J. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat. Commun. 2012, 3, 1245. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jia, H.; Cai, X.; Zhong, H.; Feng, Q.; Sunagawa, S.; Arumugam, M.; Kultima, J.R.; Prifti, E.; Nielsen, T.; et al. An integrated catalog of reference genes in the human gut microbiome. Nat. Biotechnol. 2014, 32, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Tu, Q.; Lin, L.; Cheng, L.; Deng, Y.; He, Z. NCycDB: A curated integrative database for fast and accurate metagenomic profiling of nitrogen cycling genes. Bioinformatics 2019, 35, 1040–1048. [Google Scholar] [CrossRef]
- Zeng, J.; Tu, Q.; Yu, X.; Qian, L.; Wang, C.; Shu, L.; Liu, F.; Liu, S.; Huang, Z.; He, J.; et al. PCycDB: A comprehensive and accurate database for fast analysis of phosphorus cycling genes. Microbiome 2022, 10, 101. [Google Scholar] [CrossRef]
- Huang, R.; Li, W.; Qiu, S.; Long, Y.; Zeng, Z.; Tang, J.; Huang, Q. Impact of land use types on soil microbial community structure and functional structure in Baihualing Village, China. Glob. Ecol. Conserv. 2025, 57, e03379. [Google Scholar] [CrossRef]
- Xu, H.; Du, H.; Zeng, F.; Song, T.; Peng, W. Diminished rhizosphere and bulk soil microbial abundance and diversity across succession stages in Karst area, southwest China. Appl. Soil Ecol. 2021, 158, 103799. [Google Scholar] [CrossRef]
- Zhao, S.; Su, X.; Xu, C.; Gao, X.; Lu, S. Microbial adaptation and genetic modifications for enhanced remediation in low-permeability soils. Sci. Total Environ. 2025, 958, 177916. [Google Scholar] [CrossRef]
- Menzel, T.; Neubauer, P.; Junne, S. Role of microbial hydrolysis in anaerobic digestion. Energies 2020, 13, 5555. [Google Scholar] [CrossRef]
- Coonan, E.C.; Kirkby, C.A.; Kirkegaard, J.A.; Amidy, M.R.; Strong, C.L.; Richardson, A.E. Microorganisms and nutrient stoichiometry as mediators of soil organic matter dynamics. Nutr. Cycl. Agroecosyst. 2020, 117, 273–298. [Google Scholar] [CrossRef]
- Voges, M.J.; Bai, Y.; Schulze-Lefert, P.; Sattely, E.S. Plant-derived coumarins shape the composition of an Arabidopsis synthetic root microbiome. Proc Natl. Acad. Sci. USA 2019, 116, 12558–12565. [Google Scholar] [CrossRef]
- Zhu, G.; Yan, G.; Liu, G.; Xing, Y.; Wang, Q. Nitrogen deposition changes the root nutrient uptake strategies by affecting microbial diversity of the rhizosphere. Appl. Soil Ecol. 2025, 205, 105773. [Google Scholar] [CrossRef]
- Hernandez, D.J.; David, A.S.; Menges, E.S.; Searcy, C.A.; Afkhami, M.E. Environmental stress destabilizes microbial networks. ISME J. 2021, 15, 1722–1734. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Lv, J.; He, X.; Wang, J.; Teng, D.; Jiang, L.; Wang, H.; Lv, G. Rhizosphere effect alters the soil microbiome composition and C, N transformation in an arid ecosystem. Appl. Soil Ecol. 2022, 170, 104296. [Google Scholar] [CrossRef]
- Sui, B.; Wang, L.; Wang, H.; Zhao, X.; Jin, F.; Wang, H.; Guo, J.; Xu, Q. Deep tillage inhibits microbial species interactions and exhibits contrasting roles in bacterial and fungal assembly. Agric. Ecosyst Environ. 2023, 357, 108679. [Google Scholar] [CrossRef]
- Fang, W.; Tian, W.; Yan, D.; Li, Y.; Cao, A.; Wang, Q. Linkages between soil nutrient turnover and above-ground crop nutrient metabolism: The role of soil microbes. iMetaOmics 2025, 2, e55. [Google Scholar] [CrossRef]
- Kang, H.; Xue, Y.; Cui, Y.; Moorhead, D.L.; Lambers, H.; Wang, D. Nutrient limitation mediates soil microbial community structure and stability in forest restoration. Sci. Total Environ. 2024, 935, 173266. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Wen, D.; Bates, C.T.; Wu, L.; Guo, X.; Liu, S.; Su, Y.; Lei, J.; Zhou, J.; Yang, Y. Nutrient supply controls the linkage between species abundance and ecological interactions in marine bacterial communities. Nat. Commun. 2022, 13, 175. [Google Scholar] [CrossRef]
- Muchane, M.N.; Sileshi, G.W.; Gripenberg, S.; Jonsson, M.; Pumariño, L.; Barrios, E. Agroforestry boosts soil health in the humid and sub-humid tropics: A meta-analysis. Agric. Ecosyst. Environ. 2020, 295, 106899. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, L.; Yang, T.; Qian, Z.; Xu, C.; Tian, D.; Tang, L. Poplar agroforestry systems in eastern China enhance the spatiotemporal stability of soil microbial community structure and metabolism. Land Degrad. Dev. 2022, 33, 916–930. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, J.-C.; Sun, S.; Rehman, M.M.U.; He, S.; Zhou, W. Dissimilatory nitrate reduction processes and corresponding nitrogen loss in tidal flow constructed wetlands. J. Clean Prod. 2021, 295, 126429. [Google Scholar] [CrossRef]
- Moon, M.; Park, G.W.; Lee, J.-p.; Lee, J.-S.; Min, K. Recent progress in formate dehydrogenase (FDH) as a non-photosynthetic CO2 utilizing enzyme: A short review. J. CO2 Util. 2020, 42, 101353. [Google Scholar] [CrossRef]
- Yue, X.-L.; Xu, L.; Cui, L.; Fu, G.-Y.; Xu, X.-W. Metagenome-based analysis of carbon-fixing microorganisms and their carbon-fixing pathways in deep-sea sediments of the southwestern Indian Ocean. Mar. Genom. 2023, 70, 101045. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lin, H.; Li, J. Are there links between nutrient inputs and the response of microbial carbon use efficiency or soil organic carbon? A meta-analysis. Soil Biol. Biochem. 2025, 201, 109656. [Google Scholar] [CrossRef]
- Luo, L.; Meng, H.; Gu, J.-D. Microbial extracellular enzymes in biogeochemical cycling of ecosystems. J. Environ. Manag. 2017, 197, 539–549. [Google Scholar] [CrossRef]
- Wang, C.; Kuzyakov, Y. Energy use efficiency of soil microorganisms: Driven by carbon recycling and reduction. Glob. Change Biol. 2023, 29, 6170–6187. [Google Scholar] [CrossRef]
- Kraft, B.; Strous, M.; Tegetmeyer, H.E. Microbial nitrate respiration–genes, enzymes and environmental distribution. J. Biotechnol. 2011, 155, 104–117. [Google Scholar] [CrossRef]
- Pan, B.; Xia, L.; Lam, S.K.; Wang, E.; Zhang, Y.; Mosier, A.; Chen, D. A global synthesis of soil denitrification: Driving factors and mitigation strategies. Agric. Ecosyst. Environ. 2022, 327, 107850. [Google Scholar] [CrossRef]
- Henneron, L.; Kardol, P.; Wardle, D.A.; Cros, C.; Fontaine, S. Rhizosphere control of soil nitrogen cycling: A key component of plant economic strategies. New Phytol. 2020, 228, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Meng, X.; Li, Q.; Ho, S.-H. Nitrogen metabolic responses of non-rhizosphere and rhizosphere microbial communities in constructed wetlands under nanoplastics disturbance. J. Hazard. Mater. 2025, 484, 136777. [Google Scholar] [CrossRef] [PubMed]
- Rang, J.; He, H.; Chen, J.; Hu, J.; Tang, J.; Liu, Z.; Xia, Z.; Ding, X.; Zhang, Y.; Xia, L. SenX3-RegX3, an important two-component system, regulates strain growth and butenyl-spinosyn biosynthesis in Saccharopolyspora pogona. iScience 2020, 23, 101398. [Google Scholar] [CrossRef]
- Santos-Beneit, F. The Pho regulon: A huge regulatory network in bacteria. Front. Microbiol. 2015, 6, 402. [Google Scholar] [CrossRef]
- Oliverio, A.M.; Bissett, A.; McGuire, K.; Saltonstall, K.; Turner, B.L.; Fierer, N. The role of phosphorus limitation in shaping soil bacterial communities and their metabolic capabilities. MBio. 2020, 11, 1718–1720. [Google Scholar] [CrossRef]
- Gorelova, V.; De Lepeleire, J.; Van Daele, J.; Pluim, D.; Meï, C.; Cuypers, A.; Leroux, O.; Rébeillé, F.; Schellens, J.H.; Blancquaert, D.; et al. Dihydrofolate reductase/thymidylate synthase fine-tunes the folate status and controls redox homeostasis in plants. Plant Cell 2017, 29, 2831–2853. [Google Scholar] [CrossRef] [PubMed]
- Duhamel, S. The microbial phosphorus cycle in aquatic ecosystems. Nat. Rev. Microbiol. 2025, 23, 239–255. [Google Scholar] [CrossRef]
- Liu, F.; Zeng, J.; Ding, J.; Wang, C.; He, Z.; Liu, Z.; Shu, L. Microbially-driven phosphorus cycling and its coupling mechanisms with nitrogen cycling in mangrove sediments. Sci. Total Environ. 2025, 958, 178118. [Google Scholar] [CrossRef]
- Wu, X.; Rensing, C.; Han, D.; Xiao, K.-Q.; Dai, Y.; Tang, Z.; Liesack, W.; Peng, J.; Cui, Z.; Zhang, F. Genome-resolved metagenomics reveals distinct phosphorus acquisition strategies between soil microbiomes. mSystems 2022, 7, e01107–e01121. [Google Scholar] [CrossRef]
- Liu, L.; Gao, Y.; Yang, W.; Liu, J.; Wang, Z. Community metagenomics reveals the processes of nutrient cycling regulated by microbial functions in soils with P fertilizer input. Plant Soil. 2024, 499, 139–154. [Google Scholar] [CrossRef]
- Hopkins, D.W.; Dungait, J.A. Soil microbiology and nutrient cycling. In Soil Microbiology and Sustainable Crop Production; Springer: Dordrecht, The Netherlands, 2010; pp. 59–80. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Priming effects: Interactions between living and dead organic matter. Soil Biol. Biochem. 2010, 42, 1363–1371. [Google Scholar] [CrossRef]
- Kouno, K.; Chowdhury, M.; Nagaoka, T.; Ando, T. Nutrient requirements (N, P, S) of microbial biomass formation in a regosol of Japan. In Plant Nutrition: Food Security and Sustainability of Agro-Ecosystems Through Basic and Applied Research; Springer: Dordrecht, The Netherlands, 2001; pp. 622–623. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).