Establishment, Survival, and Growth of Beech, Oak, and Spruce Seedlings During Unassisted Forest Recovery in Post-Mining Sites
Abstract
1. Introduction
2. Materials and Methods
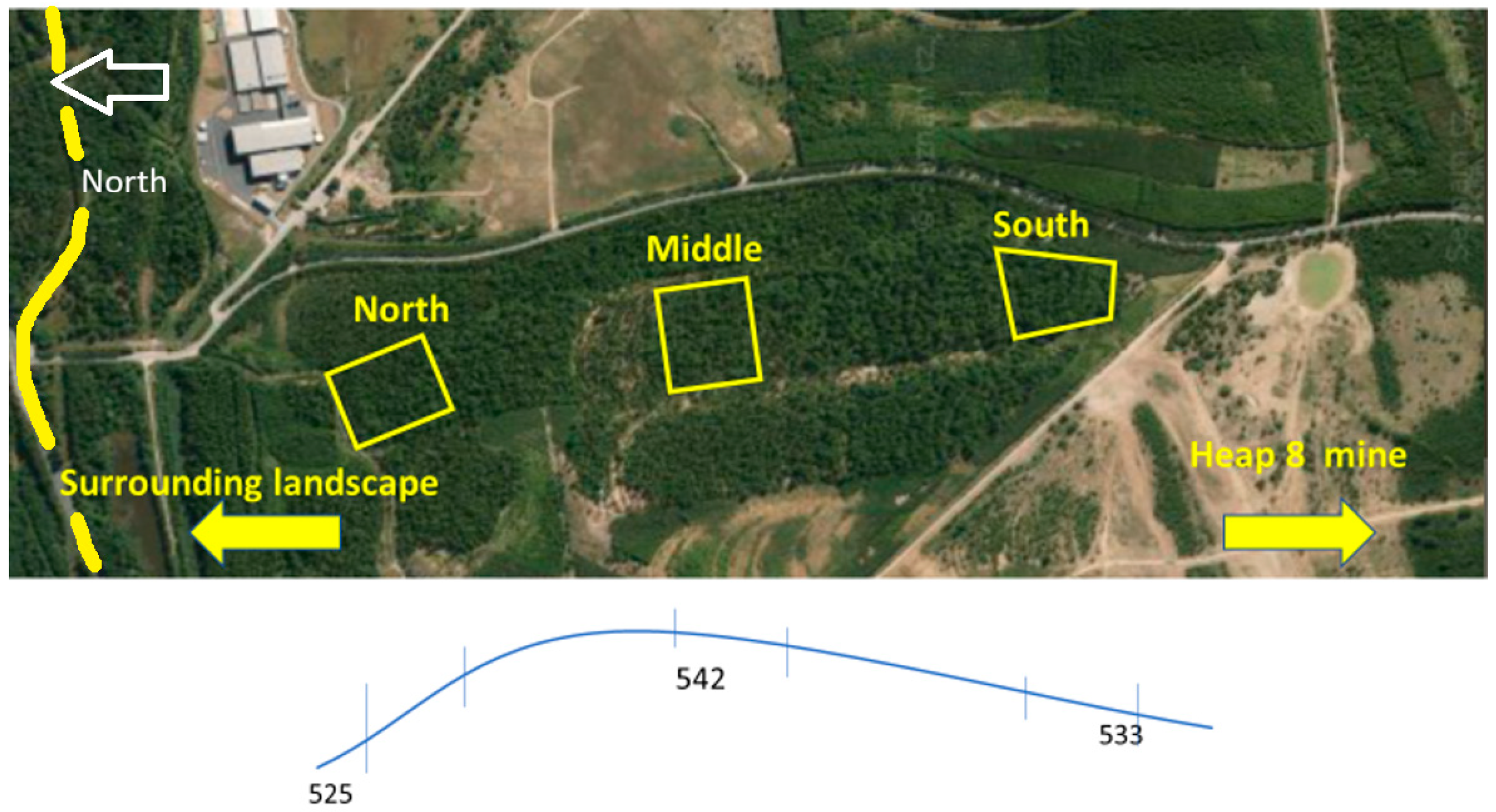
2.1. Study Site
2.2. Field Data Collection
2.3. Data Processing
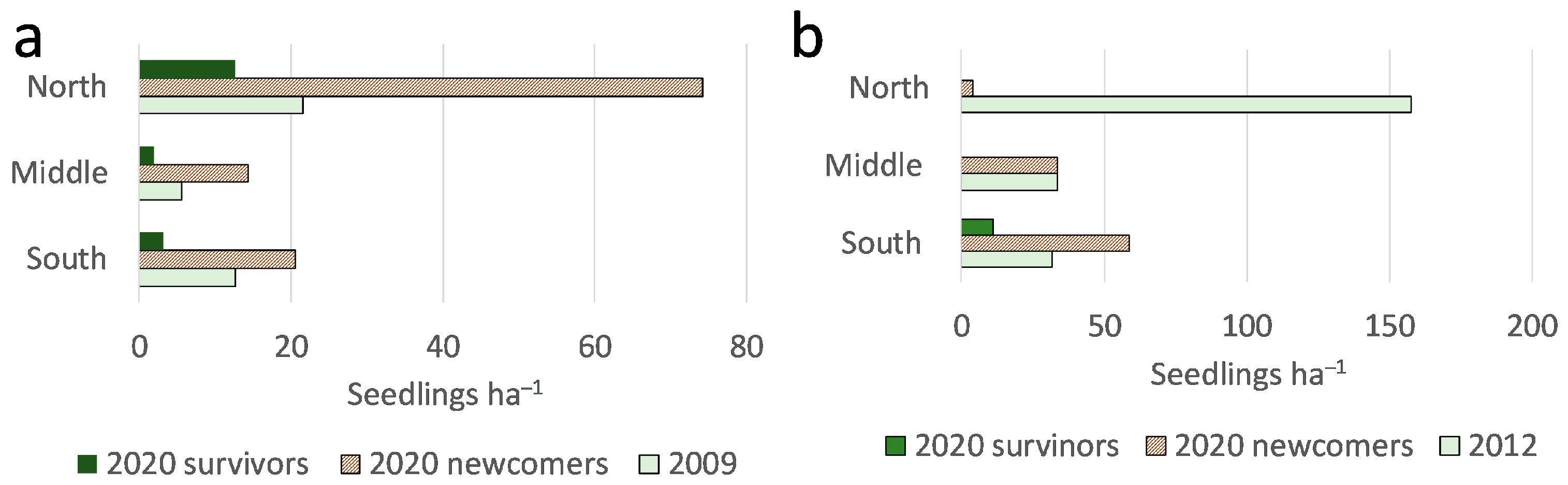
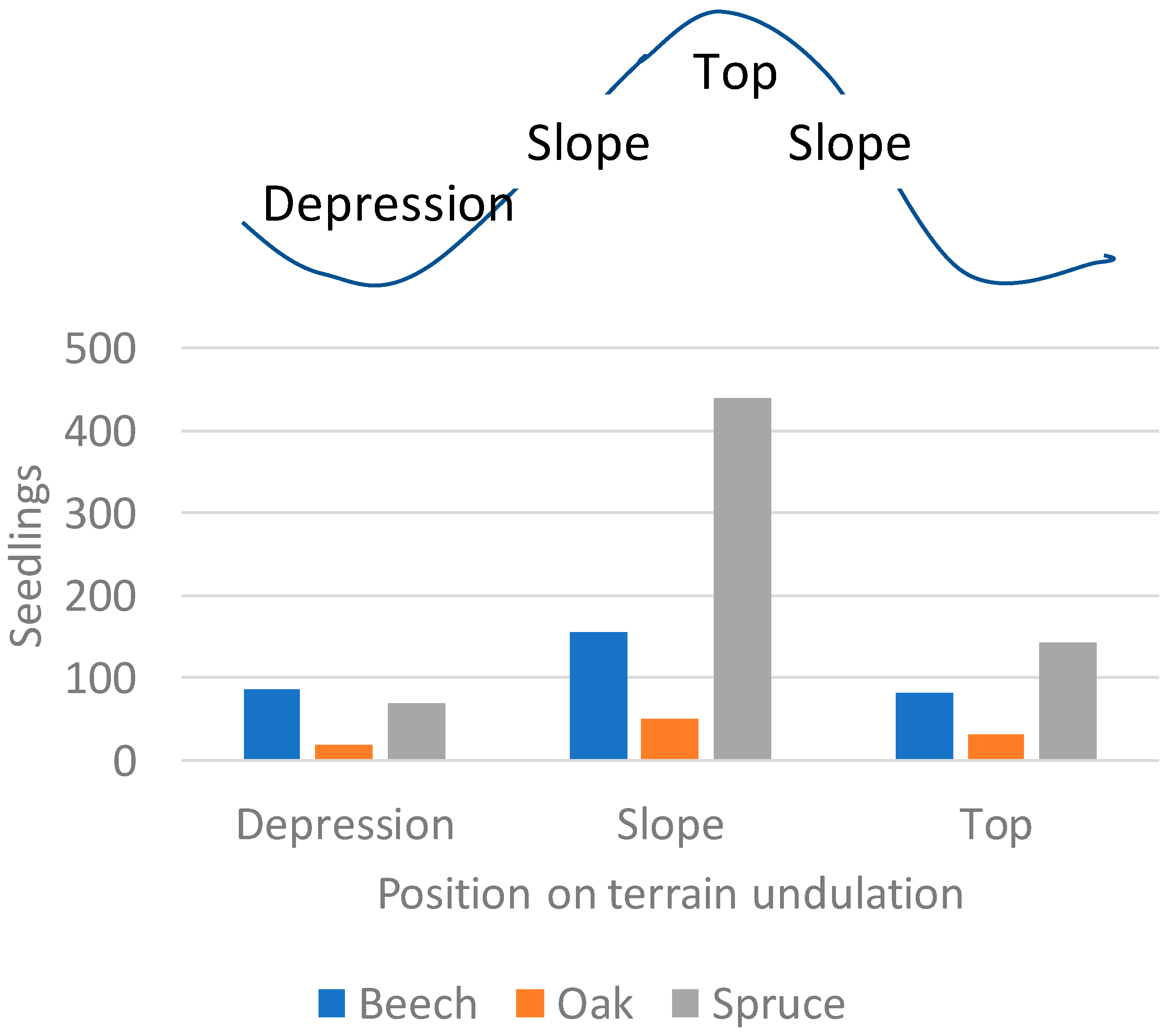
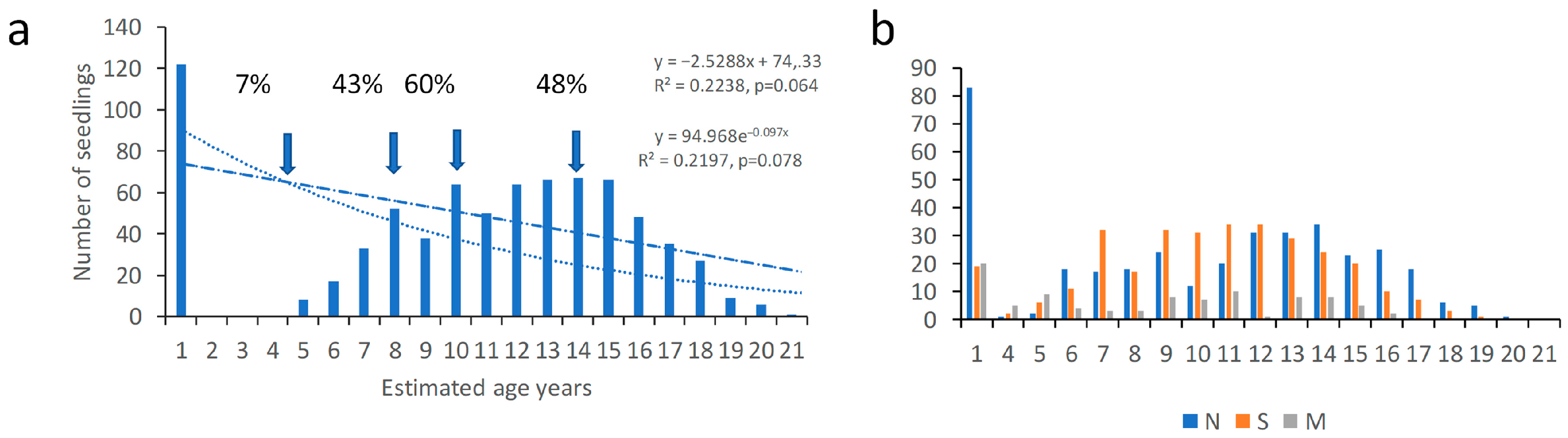
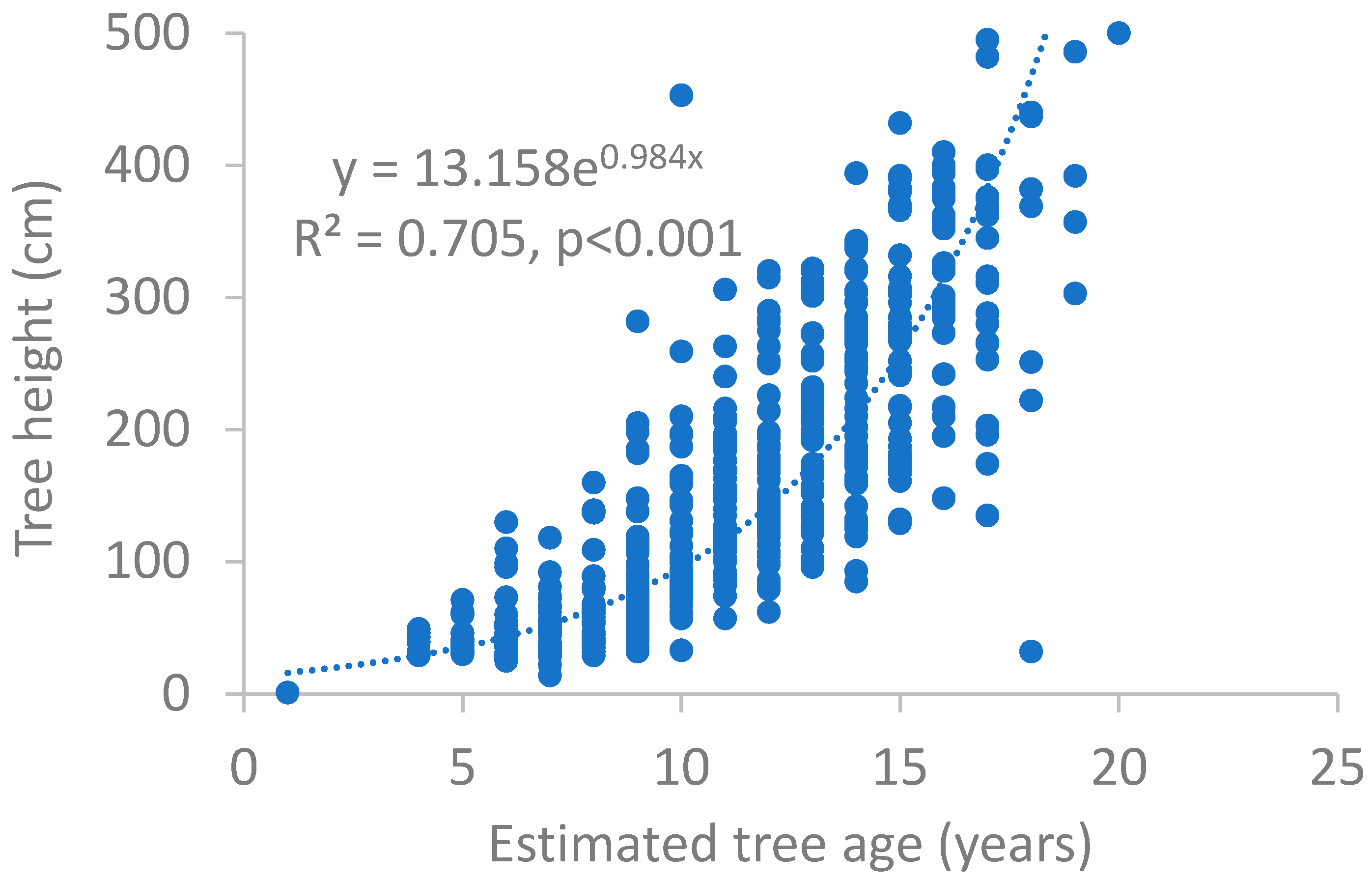
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bradshaw, A. Restoration of Mined Lands—Using Natural Processes. Ecol. Eng. 1997, 8, 255–269. [Google Scholar] [CrossRef]
- Worlanyo, A.S.; Jiangfeng, L. Evaluating the Environmental and Economic Impact of Mining for Post-Mined Land Restoration and Land-Use: A Review. J. Environ. Manag. 2021, 279, 111623. [Google Scholar] [CrossRef]
- Zine, H.; Hakkou, R.; Papazoglou, E.G.; Elmansour, A.; Abrar, F.; Benzaazoua, M. Revegetation and Ecosystem Reclamation of Post-Mined Land: Toward Sustainable Mining. Int. J. Environ. Sci. Technol. 2024, 21, 9775–9798. [Google Scholar] [CrossRef]
- Sena, K.; Barton, C.; Hall, S.; Angel, P.; Agouridis, C.; Warner, R. Influence of Spoil Type on Afforestation Success and Natural Vegetative Recolonization on a Surface Coal Mine in Appalachia, United States. Restor. Ecol. 2015, 23, 131–138. [Google Scholar] [CrossRef]
- Sonter, L.J.; Ali, S.H.; Watson, J.E.M. Mining and Biodiversity: Key Issues and Research Needs in Conservation Science. Proc. R. Soc. B 2018, 285, 20181926. [Google Scholar] [CrossRef]
- Sonter, L.J.; Dade, M.C.; Watson, J.E.M.; Valenta, R.K. Renewable Energy Production Will Exacerbate Mining Threats to Biodiversity. Nat. Commun. 2020, 11, 4174. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, J.; Chen, X.; Zheng, L. Analysis of the Source Apportionment and Pathways of Heavy Metals in Soil in a Coal Mining Area Based on Machine Learning and an APCS-MLR Model. Minerals 2023, 14, 54. [Google Scholar] [CrossRef]
- Da Silva, M.G.; Muniz, A.R.C.; Hoffmann, R.; Lisbôa, A.C.L. Impact of Greenhouse Gases on Surface Coal Mining in Brazil. J. Clean. Prod. 2018, 193, 206–216. [Google Scholar] [CrossRef]
- Fleming-Muñoz, D.A.; Poruschi, L.; Measham, T.; Meyers, J.; Moglia, M. Economic Vulnerability and Regional Implications of a Low Carbon Emissions Future. Aust. J. Agric. Resour. Econ. 2020, 64, 575–604. [Google Scholar] [CrossRef]
- Doležalová, J.; Vojar, J.; Smolová, D.; Solský, M.; Kopecký, O. Technical Reclamation and Spontaneous Succession Produce Different Water Habitats: A Case Study from Czech Post-Mining Sites. Ecol. Eng. 2012, 43, 5–12. [Google Scholar] [CrossRef]
- Matýsek, D.; Jirásek, J. Mineralogy of the Coal Waste Dumps from the Czech Part of the Upper Silesian Basin: Emphasized Role of Halides for Element Mobility. Int. J. Coal Geol. 2022, 264, 104138. [Google Scholar] [CrossRef]
- Frantál, B.; Nováková, E.A. Curse of Coal? Exploring Unintended Regional Consequences of Coal Energy in the Czech Republic. Morav. Geogr. Rep. 2014, 22, 55–65. [Google Scholar] [CrossRef]
- Macdonald, S.E.; Landhäusser, S.M.; Skousen, J.; Franklin, J.; Frouz, J.; Hall, S.; Jacobs, D.F.; Quideau, S. Forest restoration following surface mining disturbance: Challenges and solutions. New For. 2015, 46, 703–732. [Google Scholar] [CrossRef]
- Woś, B.; Pietrzykowski, M.; Józefowska, A. Reclaimed Mine Soil Substrates and Tree Stands vs. Successional Forest Floor Vegetation: A Case Study of Developing Ecosystems on Afforested Mine Sites. Ecol. Eng. 2018, 120, 504–512. [Google Scholar] [CrossRef]
- Badraghi, A.; Krůček, M.; Král, K.; Reitschmiedová, E.; Šálek, V.; Kotápišová, M.; Novotná, B.; Frouz, J. Woody Species Succession and Spontaneous Forest Development in Post-Mining Sites after Coal Mining in the Czech Republic. Ecol. Eng. 2023, 194, 107051. [Google Scholar] [CrossRef]
- Frouz, J.; Vobořilová, V.; Janoušová, I.; Kadochová, Š.; Matějíček, L. Spontaneous Establishment of Late Successional Tree Species English Oak (Quercus robur) and European Beech (Fagus sylvatica) at Reclaimed Alder Plantation and Unreclaimed Post Mining Sites. Ecol. Eng. 2015, 77, 1–8. [Google Scholar] [CrossRef]
- Šálek, M.; Hendrychová, M.; Řehoř, M. Breeding Habitat of Sparrowhawks, Accipiter Nisus on 22. Spoil Heaps after Coal Mining. Acta Oecologica 2010, 36, 197–201. [Google Scholar] [CrossRef]
- Wiegleb, G.; Felinks, B. Primary Succession in Post-Mining Landscapes of Lower Lusatia—Chance or Necessity. Ecol. Eng. 2001, 17, 199–217. [Google Scholar] [CrossRef]
- Frouz, J.; Dvorščík, P.; Vávrová, A.; Doušová, O.; Kadochová, Š.; Matějíček, L. Development of Canopy Cover and Woody Vegetation Biomass on Reclaimed and Unreclaimed Post-Mining Sites. Ecol. Eng. 2015, 84, 233–239. [Google Scholar] [CrossRef]
- Mihók, B.; Kenderes, K.; Kirby, K.J.; Paviour-Smith, K.; Elbourn, C.A. Forty-Year Changes in the Canopy and the Understorey in Wytham Woods. Forestry 2009, 82, 515–527. [Google Scholar] [CrossRef]
- Trap, J.; Laval, K.; Akpa-Vinceslas, M.; Gangneux, C.; Bureau, F.; Decaëns, T.; Aubert, M. Humus Macro-Morphology and Soil Microbial Community Changes along a 130-Yr-Old Fagus sylvatica Chronosequence. Soil. Biol. Biochem. 2011, 43, 1553–1562. [Google Scholar] [CrossRef]
- Baumbach, L.; Niamir, A.; Hickler, T.; Yousefpour, R. Regional adaptation of European beech (Fagus sylvatica) to drought in Central European conditions considering environmental suitability and economic implications. Reg. Environ. Change 2019, 19, 1159–1174. [Google Scholar] [CrossRef]
- Leuschner, C.; Meier, I.C.; Hertel, D. On the Niche Breadth of Fagus Sylvatica: Soil Nutrient Status in 50 Central European Beech Stands on a Broad Range of Bedrock Types. Ann. For. Sci. 2006, 63, 355–368. [Google Scholar] [CrossRef]
- Ellenberg, H. Vegetation Ecology of Central Europe; Cambridge University Press: Cambridge, UK, 1988; ISBN 0521236428. [Google Scholar]
- Ducousso, A.; Bordacs, S. EUFORGEN Technical Guidelines for Genetic Conservation and Use for Pedunculate and Sessile Oaks (Quercus robur) and (Quercus petraea); Bioversity International: Rome, Italy, 2003; ISBN 9290436603. [Google Scholar]
- Von Wuhlisch, G. European Beech (Fagus sylvatica). In EUFORGEN Technical Guidelines for Genetic Conservation and Use; Bioversity International: Rome, Italy, 2008. [Google Scholar]
- Norby, R.J.; Loader, N.J.; Mayoral, C.; Ullah, S.; Curioni, G.; Smith, A.R.; Reay, M.K.; Van Wijngaarden, K.; Amjad, M.S.; Brettle, D. Enhanced Woody Biomass Production in a Mature Temperate Forest Under Elevated CO2. Nat. Clim. Change 2024, 14, 983–988. [Google Scholar] [CrossRef]
- Jansone, D.; Liepiņa, A.A.; Barone, I.; Elferts, D.; Lībiete, Z.; Matisons, R. The Older, the Richer? A Comparative Study of Tree-Related Microhabitats and Epiphytes on Champion and Planted Mature Oaks. Diversity 2025, 17, 484. [Google Scholar] [CrossRef]
- Emborg, J. Understorey Light Conditions and Regeneration with Respect to the Structural Dynamics of a Near-Natural Temperate Deciduous Forest in Denmark. For. Ecol. Manag. 1998, 106, 83–95. [Google Scholar] [CrossRef]
- Collet, C.; Lanter, O.; Pardos, M. Effects of Canopy Opening on Height and Diameter Growth in Naturally Regenerated Beech Seedlings. Ann. For. Sci. 2001, 58, 127–134. [Google Scholar] [CrossRef]
- Brunet, J.; Fritz, Ö.; Richnau, G. Biodiversity in European Beech Forests—A Review with Recommendations for Sustainable Forest Management. Ecol. Bull. 2010, 53, 77–94. [Google Scholar]
- Blanc-Jolivet, C.; Liesebach, M. Tracing the Origin and Species Identity of Quercus robur and Quercus petraea in Europe: A Review. Silvae Genet. 2015, 64, 182–193. [Google Scholar] [CrossRef]
- Cambi, M.; Mariotti, B.; Fabiano, F.; Maltoni, A.; Tani, A.; Foderi, C.; Laschi, A.; Marchi, E. Early Response of Quercus robur Seedlings to Soil Compaction Following Germination. Land Degrad. Dev. 2018, 29, 916–925. [Google Scholar] [CrossRef]
- Fuchs, Z.; Vacek, Z.; Vacek, S.; Cukor, J.; Šimůnek, V.; Štefančík, I.; Brabec, P.; Králíček, I. European Beech (Fagus sylvatica L.): A Promising Candidate for Future Forest Ecosystems in Central Europe amid Climate Change. Cent. Eur. For. J. 2024, 70, 62–76. [Google Scholar] [CrossRef]
- Kržišnik, D.; Lesar, B.; Thaler, N.; Humar, M. Performance of bark beetle damaged Norway spruce wood against water and fungal decay. Bioresources 2018, 13, 3473–3486. [Google Scholar] [CrossRef]
- Bosela, M.; Tumajer, J.; Cienciala, E.; Dobor, L.; Kulla, L.; Marčiš, P.; Popa, I.; Sedmák, R.; Sedmáková, D.; Sitko, R. Climate warming induced synchronous growth decline in Norway spruce populations across biogeographical gradients since 2000. Sci. Total Environ. 2021, 752, 141794. [Google Scholar] [CrossRef]
- Szwagrzyk, J.; Szewczyk, J.; Bodziarczyk, J. Dynamics of seedling banks in beech forest: Results of a 10-year study on germination, growth and survival. For. Ecol. Manag. 2001, 141, 237–250. [Google Scholar] [CrossRef]
- Dobrovolny, L. Density and spatial distribution of beech (Fagus sylvatica L.) regeneration in Norway spruce (Picea abies (L.) Karsten) stands in the central part of the Czech Republic. iForest-Biogeosci. For. 2016, 9, 666. [Google Scholar] [CrossRef]
- Van Diggelen, R. Landscape: Spatial interactions. In Restoration Ecology: The New Frontier; Blackwell Publishing: Oxford, UK, 2006; pp. 31–44. [Google Scholar]
- Clark, D.A.; Clark, D.B. Spacing dynamics of a tropical rainforest tree: Evaluation of the Janzen-Connell model. Am. Nat. 1984, 124, 769–788. [Google Scholar] [CrossRef]
- Comita, L.S.; Queenborough, S.A.; Murphy, S.J.; Eck, J.L.; Xu, K.; Krishnadas, M.; Beckman, N.; Zhu, Y. Testing predictions of the Janzen–Connell hypothesis: A meta-analysis of experimental evidence for distance-and density-dependent seed and seedling survival. J. Ecol. 2014, 102, 845–856. [Google Scholar] [CrossRef]
- Deniau, M.; Jung, V.; Le Lann, C.; Morra, T.; Murray, P.J.; Prinzing, A. Janzen–Connell patterns are not the result of Janzen–Connell process: Oak recruitment in temperate forests. Perspect. Plant Ecol. Evol. Syst. 2017, 24, 72–79. [Google Scholar] [CrossRef]
- Millerón, M.; López de Heredia, U.; Lorenzo, Z.; Alonso, J.; Dounavi, A.; Gil, L.; Nanos, N. Assessment of spatial discordance of primary and effective seed dispersal of European beech (Fagus sylvatica L.) by ecological and genetic methods. Mol. Ecol. 2013, 22, 1531–1545. [Google Scholar] [CrossRef]
- Eaton, E.; Caudullo, G.; Oliveira, S.; de Rigo, D. Quercus robur and Quercus petraea in Europe. In European Atlas of Forest Tree Species; Publication Official EU: Luxembourg, 2016; pp. 160–163. [Google Scholar]
- Antonucci, S.; Santopuoli, G.; Marchetti, M.; Tognetti, R.; Chiavetta, U.; Garfì, V. What is known about the management of European beech forests facing climate change? A review. Curr. For. Rep. 2021, 7, 321–333. [Google Scholar] [CrossRef]
- Wilkens, J.F.; Schlicht, R.; Wagner, S. Resource-Based Growth Models Reveal Opportunities to Mitigate Climate Change Effects on Beech Regeneration by Silvicultural Measures. For. Ecol. Manag. 2023, 532, 120815. [Google Scholar] [CrossRef]
- Van de Peer, T.; Verheyen, K.; Kint, V.; Van Cleemput, E.; Muys, B. Plasticity of Tree Architecture through Interspecific and Intraspecific Competition in a Young Experimental Plantation. For. Ecol. Manag. 2017, 385, 1–9. [Google Scholar] [CrossRef]
- Annighöfer, P.; Beckschäfer, P.; Vor, T.; Ammer, C. Regeneration Patterns of European Oak Species (Quercus petraea (Matt.) Liebl., Quercus robur L.) in Dependence of Environment and Neighborhood. PLoS ONE 2015, 10, e0134935. [Google Scholar] [CrossRef] [PubMed]
- Plomion, C.; Aury, J.; Amselem, J.; Alaeitabar, T.; Barbe, V.; Belser, C.; Bergès, H.; Bodénès, C.; Boudet, N.; Boury, C. Decoding the Oak Genome: Public Release of Sequence Data, Assembly, Annotation and Publication Strategies. Mol. Ecol. Resour. 2016, 16, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Collet, C.; Madsen, P.; Nakashizuka, T.; Nyland, R.D.; Sagheb-Talebi, K. Beech Regeneration Research: From Ecological to Silvicultural Aspects. For. Ecol. Manag. 2010, 259, 2172–2182. [Google Scholar] [CrossRef]
- Brom, J.; Nedbal, V.; Procházka, J.; Pecharová, E. Changes in Vegetation Cover, Moisture Properties and Surface Temperature of a Brown Coal Dump from 1984 to 2009 Using Satellite Data Analysis. Ecol. Eng. 2012, 43, 45–52. [Google Scholar] [CrossRef]
- Dulias, R. Landscape Planning in Areas of Sand Extraction in the Silesian Upland, Poland. Landsc. Urban. Plan. 2010, 95, 91–104. [Google Scholar] [CrossRef]
- Brabec, P.; Cukor, J.; Vacek, Z.; Vacek, S.; Skoták, V.; Ševčík, R.; Fuchs, Z. Wildlife Damage to Forest Stands in the Context of Climate Change—A Review of Current Knowledge in the Czech Republic. Cent. Eur. For. J. 2024, 70, 207–221. [Google Scholar] [CrossRef]
- Woś, B.; Nezhad, M.T.K.; Mustafa, A.; Pietrzykowski, M.; Frouz, J. Soil Carbon Storage in Unreclaimed Post Mining Sites Estimated by a Chronosequence Approach and Comparison with Historical Data. Catena 2023, 220, 106664. [Google Scholar] [CrossRef]
- Janoušová, I. Faktory Ovlivňující Šíření Dubu Letního Na Výsypce Po Těžbě Uhlí. Master’s Thesis, Univerzita Karlova, Prague, Czech Republic, 2013. [Google Scholar]
- Antwi, E.K.; Krawczynski, R.; Wiegleb, G. Detecting the Effect of Disturbance on Habitat Diversity and Land Cover Change in a Post-Mining Area Using GIS. Landsc. Urban Plan. 2008, 87, 22–32. [Google Scholar] [CrossRef]
- Hendrychová, M.; Kabrna, M. An Analysis of 200-Year-Long Changes in a Landscape Affected by Large-Scale Surface Coal Mining: History, Present and Future. Appl. Geogr. 2016, 74, 151–159. [Google Scholar] [CrossRef]
- Kompała-Bąba, A.; Sierka, E.; Dyderski, M.K.; Bierza, W.; Magurno, F.; Besenyei, L.; Błońska, A.; Ryś, K.; Jagodziński, A.M.; Woźniak, G. Do the Dominant Plant Species Impact the Substrate and Vegetation Composition of Post-Coal Mining Spoil Heaps? Ecol. Eng. 2020, 143, 105685. [Google Scholar] [CrossRef]
- Vojar, J.; Doležalová, J.; Solský, M.; Smolova, D.; Kopecký, O.; Kadlec, T.; Knapp, M. Spontaneous Succession on Spoil Banks Supports Amphibian Diversity and Abundance. Ecol. Eng. 2016, 90, 278–284. [Google Scholar] [CrossRef]
| Parameter-Year | 2014 | 2024 | p-Value |
|---|---|---|---|
| Betula pendula cover % | 3.0 ± 7.8 | 9.4 ± 17.7 | 0.366 |
| Populus tremula cover % | 0.4 ± 1.3 | 7.8 ± 8.9 | 0.048 |
| Salix caprea cover % | 49.4 ± 26.9 | 29.4 ± 12.3 | 0.082 |
| Picea abies cover % | 0.0 ± 0.0 | 11.0 ± 9.3 | 0.010 |
| Herb cover % | 22.4 ± 18.8 | 13.3 ± 14.3 | 0.292 |
| Shrub cover % | 2.8 ± 7.9 | 12.1 ± 13.9 | 0.123 |
| Tree cover % | 51.1 ± 27.1 | 47.8 ± 17.7 | 0.775 |
| Number of plant species | 20.6 ± 5.7 | 19.3 ± 6.9 | 0.706 |
| Factor/Species | Beech | Oak | Spruce |
|---|---|---|---|
| Position on the heap | |||
| North | 61.5 ± 86.6 | 26.3 ± 19.7 | 196.2 ± 63.9 |
| Central | 41.6 ± 42.6 | 13.4 ± 3.7 | 280.1 ± 0.7 |
| South | 89.0 ± 104.0 | 14.8 ± 24.1 | 178.0 ± 51.5 |
| Position in terrain undulation | |||
| Bottom | 130.2 ± 140.2 | 16.2 ± 10.2 | 205.2 ± 72.0 |
| Slope | 51.8 ± 61.6 | 13.6 ± 10.8 | 203.9 ± 57.3 |
| Top | 72.7 ± 78.2 | 19.1 ± 38.8 | 167.1 ± 67.1 |
| Survival | |||
| Newcomers | 81.6 ± 96.9 | 16.0 ± 26.4 | n/a |
| Survivors | 84.5 ± 107.7 | 15.1 ± 5.6 | n/a |
| ANOVA F p values | |||
| Position on heap | 12.0, 0.1479 | 1.6, 0.8271 | 10.7, 0.0001 |
| Position on terrain undulation | 1.8, 0.0001 | 0.6, 0.4777 | 0.1, 0.9084 |
| Newcomers-survivors t-test, p | 0.5471 | 0.8927 | n/a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Černý, J.; Daňková, T.; Mudrák, O.; Spurná, V.; Frouz, J. Establishment, Survival, and Growth of Beech, Oak, and Spruce Seedlings During Unassisted Forest Recovery in Post-Mining Sites. Forests 2025, 16, 1651. https://doi.org/10.3390/f16111651
Černý J, Daňková T, Mudrák O, Spurná V, Frouz J. Establishment, Survival, and Growth of Beech, Oak, and Spruce Seedlings During Unassisted Forest Recovery in Post-Mining Sites. Forests. 2025; 16(11):1651. https://doi.org/10.3390/f16111651
Chicago/Turabian StyleČerný, Jakub, Tereza Daňková, Ondřej Mudrák, Veronika Spurná, and Jan Frouz. 2025. "Establishment, Survival, and Growth of Beech, Oak, and Spruce Seedlings During Unassisted Forest Recovery in Post-Mining Sites" Forests 16, no. 11: 1651. https://doi.org/10.3390/f16111651
APA StyleČerný, J., Daňková, T., Mudrák, O., Spurná, V., & Frouz, J. (2025). Establishment, Survival, and Growth of Beech, Oak, and Spruce Seedlings During Unassisted Forest Recovery in Post-Mining Sites. Forests, 16(11), 1651. https://doi.org/10.3390/f16111651






