Abstract
Camptotheca acuminata produces valuable camptothecin, a potent anticancer agent. To overcome the limitations of wild resources, we developed efficient in vitro regeneration and camptothecin production systems. Key findings include: Optimal sterilization of plant material was achieved using Plant Preservative Mixture (12 min). Axillary shoot induction was most effective on MS medium with 2.5 mg/L 6-BA and 0.25 mg/L NAA, while adventitious shoot regeneration showed a preference for 1 mg/L 6-BA and 0.1 mg/L NAA. Synchronous induction reached its peak at 83.45% using MS medium with 0.5 mg/L 6-BA, 0.05 mg/L NAA, and 0.5 mg/L GA3. Shoot elongation benefited from 5 mg/L phloroglucinol and 20 mg/L CaCl2. For biomass production, 2/3 MS macroelements yielded maximum adventitious shoot biomass (50.52 mg). Rooting reached 100% efficiency on 1/2 MS medium containing 1 mg/L IBA, 0.5 mg/L NAA, 5 mg/L phloroglucinol, and 2 g/L AC, averaging 10.50 roots per shoot. The four-stage camptothecin enrichment system produced shoots containing 795.10 µg/g DW camptothecin—fivefold higher than natural leaves. This breakthrough establishes: (1) the first in vitro camptothecin enrichment platform; (2) a sustainable alternative to wild harvesting; (3) a novel circular production model for endangered medicinal plants. The optimized protocols address the challenges of camptothecin supply while demonstrating remarkable productivity enhancements through controlled in vitro culture systems.
1. Introduction
Camptotheca acuminata, a species belonging to the Nyssaceae family and the Camptotheca genus, is a tall deciduous tree endemic to China and is classified as a second-class nationally protected wild plant. In 1966, Wall ME from the United States isolated camptothecin (CPT) from the bark of C. acuminata. Tumor trials demonstrated that this tryptophan-terpenoid alkaloid possessed anticancer activity [1], thereby attracting widespread attention. Given the inherent toxicity of CPT, there is potential for its development as a botanical pesticide for the control of field pests [2]. In addition to its medicinal value, C. acuminata also holds considerable economic value. As a fast-growing tree species, it can mature into a forest in approximately three years. Its high-yield characteristics make large-scale cultivation of C. acuminata an effective means to alleviate China’s timber shortage and meet the country’s timber demands [3]. However, current research on the production technology of CPT and the artificial cultivation of C. acuminata is relatively limited both domestically and internationally, primarily focusing on the artificial synthesis and pharmaceutical development of CPT. The chemical synthesis of CPT involves complex steps and an imperfect production system. The production of CPT through cultivation methods such as suspension cells and callus tissues is relatively simpler and more efficient. Over the past few decades, researchers have established regeneration systems for C. acuminata using explants such as shoot tips, stem segments, embryonic axes, and leaves. They have explored the effects of factors such as explant type, plant hormones, basic media, and additives on the regeneration of C. acuminata and the synthesis of CPT. Nevertheless, the CPT content in the products of C. acuminata tissue culture is currently not satisfactory [4]. Callus tissues induced on specific culture media exhibited detectable levels of camptothecin, albeit with relatively low overall content, ranging approximately from 0.0002% to 0.0004% (w/w). In contrast, camptothecin production in suspension cell cultures of C. acuminata varied between 0.001% and 0.02% (w/w). Furthermore, when Agrobacterium rhizogenes was employed to infect C. acuminata and induce hairy root formation, the resulting hairy roots demonstrated camptothecin accumulation levels in the range of approximately 0.2 mg/g to 1.0 mg/g dry weight [5,6,7,8,9,10,11].
From the current research status and trends both domestically and internationally, CPT and its analogs are primarily obtained through isolation from C. acuminata [12]. The development of the anticancer applications of CPT should be based on an adequate supply of C. acuminata resources. However, wild C. acuminata resources are severely limited, hindering the industrial and large-scale comprehensive utilization of C. acuminata resources. The bottleneck in the artificial cultivation of C. acuminata lies in the dormancy and low germination rate of its seeds. Moreover, in recent years, the issue of pests and diseases affecting C. acuminata has become increasingly prominent [13,14]. To rationally utilize, develop, and protect C. acuminata resources, tissue culture technology can be employed for the rapid propagation of C. acuminata seedlings, thereby addressing the shortage of C. acuminata resources.
This study established, for the first time, a whole-plant-based in vitro regeneration system for high-yield CPT accumulation by integrating dual-pathway strategies of organogenesis-driven regeneration and metabolic flux redirection. The system achieved scalable quantitative production of medicinal organs (shoots), overcoming the yield limitations of conventional cell suspension cultures and hairy root cultures. Notably, the entire shoot system demonstrated a CPT accumulation of 795.10 ± 5.02 μg/g dry weight (DW). By employing intact shoots as CPT accumulation carriers instead of cell/tissue fragments, this innovation resolved two critical industrialization bottlenecks: (1) the genetic instability inherent in long-term cell suspension cultures, and (2) the dependency on Agrobacterium rhizogenes-mediated transformation in hairy root systems. The developed production paradigm features a non-seasonal continuous harvesting system, enabling year-round raw material procurement with exceptional phenotypic consistency and biochemical uniformity.
2. Materials and Methods
2.1. Experimental Materials and Culture Conditions
The experimental samples were derived from 15-year-old mature Camptotheca acuminata trees, specifically young spring shoots with terminal shoots harvested in the current growing season. These explants were collected from C. acuminata specimens cultivated at Shanghai Chenshan Botanical Garden. To maintain favorable growth conditions, the culture media’s pH was set at 5.8. The incubation environment was kept at a constant temperature of 25 ± 2 °C. Additionally, the cultures were exposed to a light intensity of 33.6 μmol/m2/s under a 16/8 h light/dark cycle, the light source comes from LED.
2.2. Preparation of Sterile Materials
Young stem segments of Camptotheca acuminata were used as the initial explants. After rinsed under running tap water for 1 h, the explants were placed in a laminar flow hood. They were then treated with 75% (v/v) ethanol for 30 s, followed by immersion in a 50% (v/v) plant preservative mixture (PPM, add 500 mL of the PPM stock solution to an equal volume of deionized water to prepare a 50% (v/v) disinfectant solution). for 10, 12, 15, 17, or 20 min, respectively. Subsequently, the explants were rinsed 3–5 times with sterile distilled water. The treated explants were inoculated onto MS medium supplemented with 1 mg/L 6-benzylaminopurine (6-BA) and 0.1 mg/L α-naphthaleneacetic acid (NAA). Inoculate 50 explants for each treatment and repeat three times. After a 2-week culture period, the contamination and growth status of the materials under each treatment were recorded. The medium was fortified with 30 g/L sucrose and solidified using 5 g/L agar. The culture medium was sterilized by autoclaving (121 °C, 0.105 MPa) for 20 min. Sucrose, agar, and other medium components were procured from Hangzhou Lin’an Bottled Scientific Experimental Supplies Department in Hangzhou, China. For plant growth regulation, all phytohormones and supplements were provided by Hangzhou Morebert Biotechnology Co., Ltd., Hangzhou, China. Additionally, the plant preservative mixture was acquired from Yesen Biotechnology Co., Ltd., located in Shanghai, China. The high-pressure steam sterilization pot is sourced from Shanghai Shen’an Medical Machinery Factory (Shanghai, China), with the model LDZF-75L-I; The vertical laminar flow purification workbench is sourced from Shanghai Shangjing Purification Equipment Co., Ltd., Shanghai, China, model CA920-3.
2.3. Efficient In Vitro Regeneration for Camptotheca acuminata
2.3.1. Induction of Axillary Shoots in Camptotheca acuminata
Sterilized C. acuminata explants were transferred to MS-based culture media enriched with 6-BA (1.5–3 mg/L) and NAA (0.15–0.3 mg/L). Inoculate 32 explants for each treatment and repeat three times. The inoculated cultures were incubated for 3–5 weeks, during which axillary shoot emergence time and germination frequency were monitored.
2.3.2. Induction of Adventitious Shoots in Camptotheca acuminata
For adventitious shoot induction, the previously generated axillary shoots were transferred to MS medium supplemented with various growth regulator combinations: 6-BA (0.5–1.5 mg/L) plus NAA (0.05–0.2 mg/L), or KT (0.1–0.5 mg/L) alone. Inoculate 32 explants for each treatment and repeat three times. After 3–5 weeks of culture, quantitative assessments included the percentage of explants producing shoots and the average shoot proliferation rate per explant.
2.3.3. Elongation of Camptotheca acuminata Adventitious Shoots
Adventitious shoots of C. acuminata induced in Section 2.3.2 were inoculated onto a synchronized elongation-inducing medium for adventitious shoots. The basal medium used was MS medium supplemented with 0.1–0.8 mg/L 6-BA, 0.01–0.08 mg/L NAA, and 0.1–0.8 mg/L GA3. Inoculate 25 explants for each treatment and repeat three times. After 3–5 weeks of culture, synchronized adventitious shoot clusters with uniform growth and development were obtained. The number of effective shoots with a height of approximately 2 cm, the high-frequency synchronization rate, and the uniformity were statistically analyzed.
2.4. In Vitro Drug Accumulation of Camptothecin
2.4.1. Stage I Culture for In Vitro CPT Enrichment
For stage I camptothecin enrichment, adventitious shoot clusters of C. acuminata showing synchronous elongation (>2 cm height) were cultured on two media formulations: (1) modified MS medium containing 0.5 mg/L 6-BA, 0.05 mg/L NAA, 0.5 mg/L GA3, and 0.005 mg/L Trp; and (2) control MS medium with identical components except Trp omission. Following a 3–5 week culture period, developed adventitious shoots were collected for subsequent camptothecin analysis.
2.4.2. Stage II Culture for In Vitro CPT Enrichment
Adventitious shoots obtained from Stage I culture of in vitro CPT enrichment were inoculated onto a Stage II culture medium for in vitro CPT enrichment. The basal medium used was MS, supplemented with 0.5 mg/L 6-BA, 0.05 mg/L NAA, 1–10 mg/L phloroglucinol (PG), and 0–40 mg/L calcium chloride (CaCl2). Inoculate 40 explants for each treatment and repeat three times. After 4–6 weeks of culture, the height and elongation rate of the adventitious shoots were statistically analyzed.
2.4.3. Stage III Culture for In Vitro CPT Enrichment
Adventitious shoots from Stage II CPT enrichment cultures were inoculated onto MS media containing modified macroelement concentrations (ranging from 1/3× to 4/3× standard strength). All media formulations included consistent supplements: 0.5 mg/L 6-BA, 0.05 mg/L NAA, 5 mg/L PG, and 20 mg/L CaCl2. Inoculate 20 explants for each treatment and repeat three times. Following a 4-week cultivation period, C. acuminata biomass production was quantitatively assessed.
2.4.4. Stage IV Culture for In Vitro CPT Enrichment
Adventitious shoots following Stage III culture for in vitro CPT enrichment were transferred to either a medium containing 1/2 MS + 0.5–1.5 mg/L IBA + 0.1–1 mg/L NAA + 5 mg/L PG + 2 g/L AC or a medium containing 1/2 MS + 0.5–2 mg/L IBA + 0.1–1 mg/L NAA + 5 mg/L PG. Inoculate 20 explants for each treatment and repeat three times. These shoots were then cultured for 4–8 weeks to induce rooting of C. acuminata adventitious shoots, with the aim of accumulating camptothecin in whole C. acuminata plants. The number of rooted adventitious shoots and the rooting rate were statistically analyzed.
2.5. Analysis of CPT Content
CPT analysis samples originated from four culture regimes: (i) Stage I control (MS + 0.5 mg/L 6-BA + 0.05 mg/L NAA + 0.5 mg/L GA3); (ii) Stage I with Trp supplementation (additional 0.005 mg/L Trp); (iii) Stage II (MS base with 0.5 mg/L 6-BA, 0.05 mg/L NAA, 5 mg/L PG, and 20 mg/L CaCl2); and (iv) Stage III using 2/3 MS medium with identical supplements to Stage II. The aerial parts of shootlets cultivated in 1/2 MS medium supplemented with 1 mg/L IBA, 0.5 mg/L NAA, 5 mg/L PG, and 2 g/L AC during the in vitro CPT enrichment culture of adventitious roots. The root systems of shootlets cultivated in 1/2 MS medium supplemented with 1 mg/L IBA, 0.5 mg/L NAA, 5 mg/L PG, and 2 g/L AC during the in vitro CPT enrichment culture of adventitious roots. Young leaves collected from mature C. acuminata trees. The CPT content in these samples was determined.
2.6. Statistical and Data Analysis
The mathematical expressions utilized for assessing various tissue culture regeneration parameters in the current study are as follows:
- Axillary shoot induction rate (%) = (Number of axillary shoots per explant)/(Initial number of explants) × 100%
- Adventitious shoot induction rate (%) = (Number of adventitious shoots per explant)/(Initial number of explants) × 100%
- High-frequency synchronization rate (%) = (Number of adventitious shoots showing high-frequency synchronous growth)/(Initial number of explants) × 100%
- Adventitious shoot elongation rate (%) = (Number of elongated shoots)/(Initial number of explants) × 100%
- Root induction rate (%) = (Number of plantlets with roots)/(Initial number of shoots) × 100%
- Data analysis involved one-way ANOVA with Duncan’s test (α = 0.05) using Excel 2020 and SPSS 27.
3. Results
3.1. Effects of PPM on the Preparation of Camptotheca acuminata Explants
Healthy and juvenile shoots of C. acuminata were harvested, and stem segments with apical shoots were selected. These segments were subjected to rinsing under running water for 1 h and then transferred to a laminar flow hood. They were treated with 75% ethanol for 30 s and subsequently immersed in 50%(v/v) PPM for a duration ranging from 10 to 20 min (Table 1). Following this, the segments were inoculated onto MS culture media supplemented with 1 mg/L 6-BA and 0.1 mg/L NAA. After 2 weeks of culture, the contamination and growth status of the materials under each treatment were statistically analyzed. The results indicated that as the PPM treatment duration increased, the sterilization rate gradually elevated, reaching 100% with a 20 min PPM treatment. However, the survival rate progressively declined from 100% to 83.54%. Prolonged PPM treatment could lead to the inactivation or growth retardation of some explants. The optimal sterilization treatment was identified as a 12 min PPM treatment, which achieved a sterilization rate of 98.5% and a survival rate of 100%.

Table 1.
Effects of PPM on the sterilization efficacy of starting materials.
3.2. Effects of PGRs on Axillary Shoot Induction
Surface-sterilized C. acuminata explants were cultured on MS medium containing 1.5–3 mg/L 6-BA and 0.15–0.3 mg/L NAA for 3–5 weeks. Axillary shoot emergence timing and frequency were quantitatively analyzed. As presented in Table 2, both parameters exhibited a unimodal response to phytohormone concentrations, with initial enhancement followed by decline at higher levels. Maximum shoot induction efficiency occurred at 2.5 mg/L 6-BA combined with 0.25 mg/L NAA, achieving peak performance with 95.37% shoot emergence frequency and the shortest emergence time (17.42 days) (Figure 1).

Table 2.
Effects of different combinations of PGRs on axillary shoot germination.

Figure 1.
Induction of axillary shoots in Camptotheca acuminata. (A) Initial explant; (B) Germinated axillary shoots 2 weeks after inoculation; (C) Axillary shoots 5 weeks after inoculation. The culture medium used was MS + 2.5 mg/L 6-BA + 0.25 mg/L NAA. Bar = 1 cm.
3.3. Effects of PGRs on Adventitious Shoots Induction
The induced axillary shoots were subsequently transferred to MS-based regeneration media formulated with either: 0.5–1.5 mg/L 6-BA + 0.05–0.2 mg/L NAA, or 0.5–1.5 mg/L 6-BA + 0.1–0.5 mg/L KT. After a 3–5 week culture period, quantitative analysis demonstrated superior performance in terms of adventitious shoot formation in NAA-containing media compared to the media supplemented with KT (Table 3). The optimal hormonal combination (1 mg/L 6-BA + 0.1 mg/L NAA) yielded remarkable regeneration efficiency, resulting in the production of 22.64 shoots per explant at a 92.75% induction frequency (Figure 2B).

Table 3.
Effects of different combinations of PGRs on adventitious shoot induction in Camptotheca acuminata.
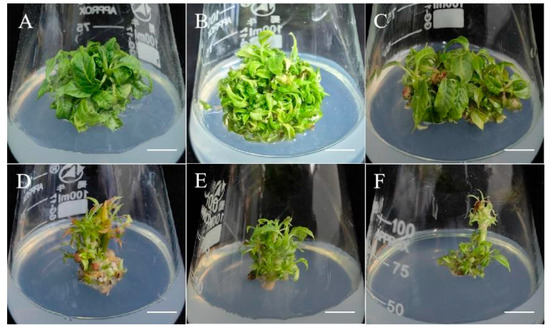
Figure 2.
The effect of PGRs combinations on the induction of adventitious shoots in Camptotheca acuminata. (A) 0.5 mg/L 6-BA + 0.05 mg/L NAA; (B) 1.0 mg/L 6-BA + 0.1 mg/L NAA; (C) 1.5 mg/L 6-BA + 0.2 mg/L NAA; (D) 0.5 mg/L 6-BA + 0.1 mg/L KT; (E) 1.0 mg/L 6-BA + 0.3 mg/L KT; (F) 1.5 mg/L 6-BA + 0.5 mg/L KT. Bar = 0.9 cm.
3.4. Effects of Exogenous Additive Combinations on High-Frequency Synchronous Adventitious Shoots Induction
The adventitious shoot clusters obtained from the preceding step were transferred to a culture medium for Stage I of in vitro CPT enrichment, which was composed of MS basal medium supplemented with 0.1–0.8 mg/L 6-BA, 0.01–0.08 mg/L NAA, 0.1–0.8 mg/L GA3. After 3–5 weeks of culture, synchronously developing adventitious shoot clusters were acquired. The quantity of effective shoots with a height exceeding 1 cm, the high-frequency synchronization rate, and the uniformity of growth were statistically documented. As presented in Table 4, the treatment comprising MS basal medium supplemented with 0.5 mg/L 6-BA, 0.05 mg/L NAA, and 0.5 mg/L GA3 demonstrated superior performance in synchronous induction, attaining peak efficiency (83.45%) among all tested combinations. Figure 3A,B depict the process of high-frequency synchronized elongation of C. acuminata adventitious shoots, while Figure 3C presents the case where the adventitious shoots failed to undergo synchronized elongation.

Table 4.
Effects of exogenous additive combinations on high-frequency synchronous adventitious shoot induction.

Figure 3.
High-frequency synchronized elongation and non-high-frequency synchronized elongation of Camptotheca acuminata adventitious shoots. (A) Early stage of high-frequency synchronous elongation culture; (B) Cultivate high-frequency synchronous elongation for 4 weeks; (C) Cultivate non high-frequency synchronous elongation for 4 weeks. Bar = 1 cm.
3.5. Effects of Exogenous Additives on the Elongation of Camptotheca acuminata Adventitious Shoots
Figure 3 weeks of Stage I CPT enrichment, adventitious shoots were transferred to Stage II culture medium was composed of MS basal salts supplemented with 0.5 mg/L 6-BA, 0.05 mg/L NAA, and varying concentrations of PG (1–10 mg/L) and CaCl2 (0–40 mg/L). After a culture period of 4–6 weeks, shoot elongation parameters were quantified. The results indicated that both PG and CaCl2 significantly enhanced shoot elongation, though the effects were concentration-dependent (Table 5). Shoot growth response exhibited a bell-shaped curve, with optimal performance observed at 5 mg/L PG combined with 20 mg/L CaCl2. This treatment produced the highest elongation rate (77.04%) and mean shoot length (64.16 mm), along with excellent morphological characteristics (Figure 4A). However, supraoptimal concentrations (>7 mg/L PG or >30 mg/L CaCl2) induced symptoms of phytotoxicity, including leaf chlorosis and shoot mortality, despite retaining some growth-promoting activity (Figure 4B).

Table 5.
Effects of exogenous additives on the elongation of Camptotheca acuminata adventitious shoots.

Figure 4.
Elongation of Camptotheca acuminata adventitious shoots. (A) MS + 0.5 mg/L 6-BA + 0.05 mg/L NAA + 5 mg/L PG + 20 mg/L CaCl2; (B) MS + 0.5 mg/L 6-BA + 0.05 mg/L NAA + 10 mg/L PG + 40 mg/L CaCl2. Cultured for 5 weeks, bar = 1 cm.
3.6. Effect of Basic Media on the Biomass of Camptotheca acuminata
Adventitious shoots from preceding stages were cultured on MS-based media standard MS concentration (ranging from 1/3× to 4/3× standard MS concentration), along with consistent supplements: 0.5 mg/L 6-BA, 0.05 mg/L NAA, 5 mg/L PG, and 20 mg/L CaCl2. After a 4-week cultivation period, biomass accumulation was quantified (Table 6). The 2/3× MS formulation demonstrated optimal performance for biomass production, achieving a maximum biomass yield of 50.52 mg per explant. Additionally, plants grown under these conditions exhibited vigorous growth with healthy, green foliage.

Table 6.
Effects of basic media on the biomass amplification of Camptotheca acuminata adventitious shoots.
3.7. Rooting of Camptotheca acuminata Shootlets
The elongated adventitious shoots obtained from the preceding procedures were transferred to one of the following media for rooting induction, with the objective of accumulating CPT in whole C. acuminata plants: 1/2 MS medium supplemented with 0.5–1.5 mg/L IBA, 0.1–1 mg/L NAA, 5 mg/L PG, and 2 g/L AC; or 1/2 MS medium supplemented with 0.5–2 mg/L IBA, 0.1–1 mg/L NAA, and 5 mg/L PG. The cultures were incubated for 4–8 weeks. The rooting rate and the quantity of roots per shootlet were recorded for each treatment. The experimental results are presented in Table 7 and Figure 5. All treatments promoted rooting in the aseptic shootlets of C. acuminata. The 1/2 MS medium supplemented with 1 mg/L IBA, 0.5 mg/L NAA, 5 mg/L PG, and 2 g/L AC demonstrated superior adventitious root induction capacity, producing fully developed roots with a 100% regeneration frequency and an average of 10.50 roots per explant (Figure 5H).

Table 7.
Effects of different culture formulations on the rooting of Camptotheca acuminata shootlets.

Figure 5.
Effects of different culture formulations on the rooting of Camptotheca acuminata shootlets. (A) Medium with activated carbon, bar = 1 cm; (B) Medium without the addition of activated charcoal, bar = 1 cm; (C) Rooted C. acuminata adventitious shoots, bar = 2 cm; (D) 0.5 mg/L IBA + 0.1 mg/L NAA + 5 mg/L PG, bar = 1 cm; (E) 1 mg/L IBA + 0.5 mg/L NAA + 5 mg/L PG, bar = 1 cm; (F) 1.5 mg/L IBA + 1 mg/L NAA + 5 mg/L PG, bar = 1 cm; (G) 0.5 mg/L IBA + 0.5 mg/L NAA + 5 mg/L PG + 2 g/L activated charcoal, bar = 1 cm; (H) 1 mg/L IBA + 0.5 mg/L NAA + 5 mg/L PG + 2 g/L AC, bar = 1 cm; (I) 1.5 mg/L IBA + 1.0 mg/L NAA + 5 mg/L PG + 2 g/L AC, bar = 1 cm.
3.8. Analysis of Camptothecin Content
Samples for camptothecin content analysis were collected from the following treatments:
- Treatment 1: Shootlets cultivated in MS medium supplemented with 0.5 mg/L 6-BA, 0.05 mg/L NAA, 0.5 mg/L GA3, and 0 mg/L tryptophan during the first stage of in vitro camptothecin accumulation.
- Treatment 2: Shootlets cultivated in MS medium supplemented with 0.5 mg/L 6-BA, 0.05 mg/L NAA, 0.5 mg/L GA3, and 0.005 mg/L tryptophan during the first stage of in vitro camptothecin accumulation.
- Treatment 3: Shootlets cultivated in MS medium supplemented with 0.5 mg/L 6-BA, 0.05 mg/L NAA, 5 mg/L PG, and 20 mg/L CaCl2 during the second stage of in vitro camptothecin accumulation.
- Treatment 4: Shootlets cultivated in 2/3 MS medium supplemented with 0.5 mg/L 6-BA, 0.05 mg/L NAA, 5 mg/L PG, and 20 mg/L CaCl2 during the third stage of in vitro camptothecin accumulation.
- Treatment 5: The aerial parts of shootlets cultivated in 1/2 MS medium supplemented with 1 mg/L IBA, 0.5 mg/L NAA, 5 mg/L PG, and 2 g/L AC for the in vitro enrichment of camptothecin in adventitious roots.
- Treatment 6: The root systems of shootlets cultivated in the same 1/2 MS medium formulation as in Treatment 5.
- Treatment 7: Tender leaves collected from mature adult trees.
The camptothecin content in each of these samples was subsequently determined.
The quantification of camptothecin was performed employing liquid chromatography-mass spectrometry (LC-MS). Detailed Detection Protocol:
- Sample PreprocessingFresh tissue samples were lyophilized to obtain dry-weight specimens. An appropriate mass of plant material was accurately weighed and dissolved in methanol (MeOH) to prepare a sample of suitable concentration. The mixture was subjected to ultrasonic extraction for 30 min, followed by centrifugation at 15,000 rpm for 10 min. The supernatant was collected and diluted with a MeOH/H2O (1:1, v/v) mixture prior to liquid chromatography-mass spectrometry (LC-MS) analysis.
- Standard Solution PreparationCamptothecin reference standard was precisely weighed and dissolved in dimethyl sulfoxide (DMSO) to obtain a 1 mg/mL stock solution. This stock solution was diluted with a MeOH/H2O (1:1, v/v) mixture to prepare a 1 μg/mL external standard working solution (L6). Serial dilutions were performed as follows:L5: 500 μL of L6 was mixed with 500 μL of MeOH/H2O (1:1, v/v).L4: 200 μL of L5 was mixed with 800 μL of MeOH/H2O (1:1, v/v).L3: 500 μL of L4 was mixed with 500 μL of MeOH/H2O (1:1, v/v).L2: 200 μL of L3 was mixed with 800 μL of MeOH/H2O (1:1, v/v).L1: 500 μL of L2 was mixed with 500 μL of MeOH/H2O (1:1, v/v).The resulting standard solutions (L1–L6) were used for the quantification of camptothecin in plant samples.
- LC-MS AnalysisInstrument: XEVO-TQS WAA499 mass spectrometer (Waters Technology (Shanghai) Co., Ltd., Shanghai, China)Chromatographic Column: ACQUITY UPLC BEH C18 (1.7 μm particle size, 2.1 mm × 50 mm) (Waters Technology (Shanghai) Co., Ltd., Shanghai, China)Mobile Phases:Phase A: 0.1% formic acid (FA) in water (H2O)Phase B: 0.1% FA in acetonitrile (ACN)Flow Rate: 0.5 mL/minInjection Volume: 0.5 μLMass Spectrometry Conditions: Electrospray ionization positive mode (ES+).
The analytical results, presented in Table 8 and Figure 6, reveal a marked upward trend in camptothecin content within C. acuminata aseptic seedlings following in vitro accumulation culture. Notably, the camptothecin extracted from tissue-cultured C. acuminata shoots exhibited no compositional discrepancies compared to that obtained from natural plant materials, as confirmed by chromatographic analysis (Figure 6). In media supplemented with tryptophan, the mean camptothecin content in aseptic shootlets increased by 39.20 µg/g dry weight (DW). However, at this stage, the camptothecin content in in vitro-grown shootlets was still lower than that in tender leaves from natural materials. Compared with the first stage of camptothecin accumulation, the addition of appropriate concentrations of phloroglucinol and calcium chloride during the second stage significantly promoted camptothecin accumulation, resulting in a 2.6-fold increase in camptothecin content. A moderate reduction in the nitrogen concentration in the culture medium was conducive to both biomass expansion and camptothecin accumulation in aseptic shootlets. Compared with the second stage of camptothecin accumulation, a 1.8-fold increase in camptothecin content was achieved in the third stage after reducing the nitrogen content in the medium.Adventitious shoots of C. acuminata also continued to accumulate camptothecin during the rooting stage. The camptothecin content in the aerial parts of shootlets reached up to 795.10 µg/g DW, which was five times that in tender leaves from natural materials. Moreover, the camptothecin content in the adventitious roots was only marginally lower than that in tender leaves from natural materials.

Table 8.
Camptothecin content.
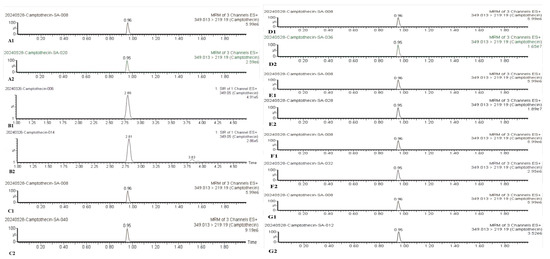
Figure 6.
Test spectrum of camptothecin sample. (A1,A2) processing 1; (B1,B2) processing 2; (C1,C2) processing 3; (D1,D2) processing 4; (E1,E2) processing 5; (F1,F2) processing 6; (G1,G2) processing 7.
4. Discussion
The wild resources of C. acuminata in China are quite limited, and the extracted camptothecin is insufficient to meet the current demand [4]. To achieve rational utilization, development, and conservation of C. acuminata resources, tissue culture technology provides an effective approach for the rapid propagation of C. acuminata seedlings. This technology enables the accumulation of camptothecin throughout the entire plant under in vitro conditions, thereby offering a viable solution to the problems of limited resources and insufficient medicinal sources of C. acuminata.
During the process of plant tissue culture, PGRs represent one of the critical factors influencing morphogenesis. Research has demonstrated that signaling molecules derived from plant hormones, such as 6-benzylaminopurine (6-BA), salicylic acid (SA), indole-3-butyric acid (IBA), naphthaleneacetic acid (NAA), indole-3-acetic acid (IAA), and abscisic acid (ABA), can effectively regulate the biosynthesis of camptothecin in C. acuminata [15,16,17]. Liu revealed that exogenous SA treatment of C. acuminata tissue-cultured seedlings could promote the expression of relevant genes and enhance camptothecin synthesis [16]. The research conducted by Lü Y and others revealed that a low concentration of exogenous 6-BA was beneficial for the growth of C. acuminata tissue-cultured seedlings [18]. It promotes camptothecin synthesis by enhancing the expression of key enzyme genes. When 0.1 mg/L of 6-BA was applied to the culture medium, the camptothecin content in the callus tissue of C. acuminata reached 0.75 μg/mg fresh weight (FW) [18]. The study by Yang et al. showed that 6-BA can induce the transcription of RESPONSE REGULATOR 1 (StRR1) at wounds of potato tubers, and overexpression of StRR1 can induce NbPAL, Nb4CL, and NbCAD10, thereby promoting the accumulation of phenolic acids and lignin monomers [19]. Recent studies have indicated that gibberellic acid (GA) and ABA can modulate primary and secondary metabolism in grapevines, thereby alleviating biotic and abiotic stresses [20]. In Ophiorrhiza pumila hairy roots, the transcription factor OpWRKY3 responds to GA signals to regulate camptothecin biosynthesis [17]. Research by Huo et al. showed that under the stimulation of GA and methyl jasmonate (MeJA), the expression levels of CaTDC1, CaSTR, Ca7DLGT, and CaG8O, which are involved in the camptothecin synthesis pathway in C. acuminata suspension cells, were increased [21]. Although numerous studies have confirmed that plant hormones (including MeJA, ABA, GA, and SA) can regulate camptothecin biosynthesis, the specific mechanisms underlying this regulation remain unclear.
The biosynthesis pathway of camptothecin can be divided into the upstream and downstream pathways. Currently, the specific synthesis processes in the downstream pathway remain unclear. The upstream biosynthesis of camptothecininvolves three main pathways: the shikimate pathway, the mevalonate (MVA) pathway, and the methylerythritol phosphate (MEP) pathway. During the synthesis of camptothecin, strictosidine synthase (STR), tryptophan synthase (TSB), andtryptophan decarboxylase (TDC) in the shikimate pathway are key enzymes. Specifically, TDC catalyzes the conversion of tryptophan into tryptamine, which isessential for forming the indole ring in camptothecin and its derivatives. Tryptamine is derived from tryptophan, which is synthesized from serine and subsequently decarboxylated, thereby providing the necessary precursor amino acid forcamptothecin synthesis [22,23]. Some studies have demonstrated that adding a certain amount of the camptothecin precursor, tryptophan, to the B5 medium can effectively increase the camptothecin content in cultured cells of C. acuminata, with the camptothecin content reaching 0.115 g/kg FW [24]. Ourfindings demonstrate that C. acuminata adventitious shoots cultured in medium containing 0.005 mg/L tryptophan accumulated significantly higher camptothecinlevels than non-supplemented controls. These findings are supported by previous experimental evidence.
Calcium (Ca), an essential micronutrient among the 17 vital elements for plant physiology, serves critical biological functions. In response to abiotic stresses, calcium mitigates the detrimental effects of low temperature, high temperature, drought, high salinity, and pests and diseases by activating protective enzyme systems and improving photosynthesis [25]. Calcium facilitates the conversion of carbohydrates and the absorption and metabolism of other nutrients, thus promoting plant growth and development. Both calcium deficiency and excess can adversely affect plant growth [26]. Prolonged calcium deficiency or excess can inhibit plant growth [27]. Calcium, while essential for basic plant physiology, also exerts considerable influence on secondary metabolite accumulation. Research has established that optimal calcium ion concentrations enhance flavonoid biosynthesis across multiple medicinal species, including Glycyrrhiza uralensis (licorice) seedlings [28], Silybum marianum (milk thistle) cell cultures [29], Atractylodes lancea rhizomes [30], and G. uralensis [31]. It can also increase the alkaloid content in medicinal plants including Corydalis yanhusuo W. T. Wang (corydalis), Sophora tonkinensis Gagnep. (sophora root), Fritillaria thunbergii Miq. (Zhejiang fritillary bulb), and Pinellia ternata (Thunb.) Ten. ex Breitenb. (pinellia tuber) [32,33,34,35]. Calcium chloride (25 mmol/L) functioned as an effective elicitor, boosting camptothecin biosynthesis in N. nimmoniana calli [36]. During Stage II of in vitro camptothecin accumulation, culture media were supplemented with optimized CaCl2 concentrations. It was observed that the camptothecin content in C. acuminata test-tube seedlings significantly increased after the addition of CaCl2, the observed effects are congruent with previous reports.
Previous studies have demonstrated that phenolic compounds can react with indole-3-acetic acid (IAA) in the presence of polyphenol oxidase to form an “IAA-phenolic acid complex”. This complex can serve as an auxiliary factor for rooting and promotes the initiation of adventitious roots [37,38]. Additionally, phenolic substances can influence the rooting rate of cuttings by affecting the content or activity of certain enzymes, such as polyphenol oxidase (PPO) and peroxidase (POD) [39,40]. Research conducted by Balakrishnamurthy et al. and Hatmanr evealed that high concentrations of phenolic compounds accumulated in plant shoots can facilitate the formation of rooting-promoting substances [41,42]. Phloroglucinol supplementation was initiated during Stage II of in vitro camptothecin accumulation, with concentration optimized through preliminary trials. It was observed that phloroglucinol, in interaction with auxins, promoted the initiation of adventitious roots in C. acuminata.
Nitrogen (N) is one of the most essential mineral nutrients required by plants in large quantities, playing a pivotal role in promoting biomass accumulation [43,44]. The critical role of nitrogen in regulating the production and deposition of secondary metabolites in plants has been well-documented in multiple investigations. Alkaloids are basic organic compounds found in nature, distributed across approximately 20% of plant species. Most alkaloids are derived from amino acids or adenosine monophosphate [45]. Among the most clinically representative plant alkaloids are CPT, taxol, berberine, L-ephedrine, morphine (a pain-relieving drug), and codeine (an opium alkaloid) [46,47]. Research has indicated that appropriately increasing the application rates of nitrogen [48,49,50,51,52], phosphorus [53,54], or potassium can promote the growth of tissue-cultured hairy roots and the accumulation of their secondary metabolites, alkaloids [55]. Evidence indicates that the interactive effects of N, P and K nutrients significantly enhance biomass production and alkaloid content in medicinal plants [56]. Camptothecin is an alkaloid derived from amino acids. Nitrogen is a crucial element for amino acid synthesis and also constitutes part of the molecular structure of camptothecin [57]. Research on C. acuminata reveals that leaf camptothecin levels exhibit a biphasic response to nitrogen fertilization, rising initially before declining at higher concentrations. Low nitrogen levels are conducive to promoting the synthesis and accumulation of camptothecin [58,59,60]. Pan et al. conducted a study on the cultivation of C. acuminata suspension cells and found that the maximum camptothecin yield, reaching 0.36 mg/g, was achieved when the total nitrogen content in the culture medium was 40 mM [61]. During the final accumulation phase, 2/3-strength MS medium supported optimal shoot growth, as evidenced by biomass measurements. Quantitative analysis revealed maximal camptothecin content in axenic shoots grown in 1/2-strength MS medium, aligning with established literature.
With the ever-increasing incidence of cancer, the demand for camptothecin-based anticancer drugs has been rising annually, and the clinical supply has emerged as a critical issue that demands urgent resolution. Leveraging modern biotechnology to enhance CPT production holds immense application prospects. In this study, through tissue culture techniques, we achieved, for the first time, whole-plant enrichment of CPT under in vitro conditions. The produced camptothecin exhibits no compositional differences from natural CPT, and there are no toxic or harmful by-products. Moreover, this production method is not constrained by seasonal limitations, enabling the year-round provision of C. acuminata germplasm and CPT drug sources. Subsequently, we will further optimize the established system, conduct molecular mechanistic studies on key regulatory genes involved in camptothecin biosynthesis, perform a comprehensive life cycle cost analysis, and proceed with pilot-scale production to advance toward industrial-scale manufacturing.
5. Conclusions
Taken together, this study presents an efficient in vitro regeneration and CPT accumulation framework utilizing C. acuminata stem explants (Figure 7). The system we developed facilitates the quantitative production of CPT-rich adventitious shoots of C. acuminata, promoting vigorous development of its medicinal organs and shortening the growth cycle. Moreover, the harvesting of raw materials is not constrained by seasonal factors, allowing for the continuous supply of CPT-enriched pharmaceutical base materials with stable properties and consistent quality to the market, thereby addressing the issue of CPT raw material sources. Optimal axillary shoot germination in C. acuminata necessitates supplementation with elevated concentrations of 6-BA and NAA. The induction efficiency of adventitious shoots is significantly enhanced with judicious reduction in 6-BA and NAA levels. An adequate amount of GA3 can promote the high-frequency synchronized emergence of adventitious shoots in C. acuminata. The inclusion of activated charcoal in the culture medium simplifies to induce rooting of adventitious shoots. Appropriate concentrations of tryptophan, PG, and CaCl2 can facilitate the accumulation of CPT in axenic seedlings of C. acuminata, and a similar promoting effect can be achieved by appropriately decreasing the nitrogen concentration in the culture medium. After undergoing in vitro CPT enrichment from Stage I to Stage IV, the CPT content in the aerial parts of the adventitious shoots of C. acuminata reached up to 795.10 µg/g DW, which is five times the CPT content in the tender leaves of natural plant materials.

Figure 7.
In vitro camptothecin accumulation system in Camptotheca acuminata. (A) Germination of axillaryshoots; (B) Induction of adventitious shoots; (C) High-frequency synchronized occurrence of adventitious shoots and Stage I of in vitro CPT accumulation; (D) Elongation of adventitious shoots and Stage II of in vitro CPT accumulation; (E) Biomass expansion of C. acuminata and Stage III of in vitro CPT accumulation; (F) Induction of adventitious roots and in vitro CPT accumulation in adventitious roots.
Author Contributions
Responsible for experimental execution, data analysis, and drafting the initial manuscript, H.Z.; collaborated on data analysis, K.Z. and Y.X.; oversaw manuscript review and finalization of the publication-ready version, W.Z. (Wenqing Zhang) and W.Z. (Weizhong Zhang); led experimental design, manuscript revision, and approval of the final publication-ready draft, M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (2023YFD2200604) and Special Fund for Scientific Research of Building National Botanical Garden (XM04-10).
Data Availability Statement
Data will be made available on request.
Acknowledgments
The Chinese Academy of Sciences—Zhihong Xu—provided supervision for the research, which the authors gratefully acknowledge. The authors gratefully acknowledge experimental material support from Shanghai Chenshan Botanical Garden.
Conflicts of Interest
Author Weizhong Zhang was employed by the Yunnan Xinkexing Pharmaceutical Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Wall, M.E.; Wani, M.C.; CookKeith, C.E. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from Camptotheca acuminata. J. Am. Chem. Soc. 1966, 88, 3888–3890. [Google Scholar] [CrossRef]
- Liu, Z.M.; Cui, Y.D.; Bin, S.Y. Biotechnology in Moderniation of the Development in the Research on Camptotheca acuminata. Guangdong Chem. Ind. 2006, 33, 67–70+52. [Google Scholar]
- Feng, J.C.; Zhang, Y.J.; Tan, Y.D. The Development in Research on Camptotheca acuminata and Uilization of Camptothecin. For. Sci. 2000, 36, 100–108. [Google Scholar]
- Zhang, Y.B.; Zhang, X.J.; Xiao, L. Research Progress on Tissue Culture and Rapid Propagation of Camptotheca acuminata Decne. Hebei Agric. Sci. 2015, 19, 67–70. [Google Scholar]
- Zhang, D.Y.; Zhao, X.Q.; Yu, F. Callus induction of Camptotheca acumianta and camptothecin production. J. Northeast. Norm. Univ. (Nat. Sci. Ed.) 2002, 34, 45–48. [Google Scholar]
- Wang, L.L.; Liu, W.Z. Induction and Effects of Elicitor on Camptothecin Content in Camptotheca acuminata Callus. Bull. Bot. Res. 2015, 35, 704–709+715. [Google Scholar]
- Pan, X.W.; Liu, X.; Lv, Y.T. Optimization of cell suspension cultures in hypocotyls of Camptotheca acuminata and camptothecin accumulation. Chin. Tradit. Herb. Drugs 2005, 36, 1221–1225. [Google Scholar]
- Liu, W.Z. Screening of Cell Lines with High Camptothecin Content via Tissue Culture. Acta Biol. Exp. Sin. 2003, 36, 275–278. [Google Scholar]
- Liu, Z.M.; Cui, Y.D. Study on the Induction of Hairy Roots in Camptotheca acuminata and Their Camptothecin Production. Chem. Reag. 2007, 29, 499–502. [Google Scholar]
- Liu, Z.M.; Cui, Y.D. Study on Camptothecin Production in Hairy Root Cultures of Camptotheca acuminata Induced by Ri Plasmid. Ecol. Environ. Sci. 2007, 16, 1266–1270. [Google Scholar]
- Lorence, A.; Medina-Bolivar, F.; Nessler, C.L. Camptothecin and 10-hydroxycamptothecin from Camptotheca acuminata hairy roots. Plant Cell Rep. 2004, 22, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.Y. Greening and medicinal functions of Camptotheca acuminata. Yunnan For. 2008, 29, 37. [Google Scholar]
- Zeng, M.; Yu, F.; Wang, L.Y. Research progress on cultivation techniques of camptothecin and accumulation of camptothecin. Anhui Agric. Sci. 2009, 37, 16849–16850. [Google Scholar]
- Cai, Y.F. Study on Infection Route of the Camptotheca acuminata Witches’-broom Phytoplasma in the Empoasca Paraparvipenis Zhang and Liu–CA. Master’s Thesis, Yunnan Agricultural University, Kunming, China, 2023. [Google Scholar]
- Hu, Y.Y.; Yu, W.W.; Song, L.L. Effects of light on production of camptothecin and expression of key enzyme genes in seedlings of Camptotheca acuminate Decne. Acta Physiol. Plant. 2016, 38, 1–9. [Google Scholar] [CrossRef]
- Liu, Z.W.; Qiong, W.; Ya, L. Effects of exogenous salicylic acid on accumulation of camptothecin and gene expression in Camptotheca acuminata. Can. J. For. Res. 2019, 49, 104–110. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, L.Y.; Chen, X.X. Sl HY5 integrates temperature, light, and hormone signal ing to balance plant growth and cold tolerance. Plant Physiol. 2019, 179, 749–760. [Google Scholar] [CrossRef]
- Lü, Y.; Song, T.; Li, L. Effects of 6-BA on Tissue Culture and Camptothecin Biosynthesis in Camptotheca acuminata Decne. Genom. Appl. Biol. 2021, 40, 3648–3654. [Google Scholar]
- Yang, R.R.; Wang, Q.H.; Wang, Y. Cytokinin signaling pathway involved in 6-BA-induced wound healing in potato tubers. Postharvest Biol. Technol. 2025, 226, 113536. [Google Scholar] [CrossRef]
- Murcia, G.; Fontana, A.; Pontin, M. ABA and GA3 regulate the synthesis of primary and secondary metabolites related to alleviation from biotic and abiotic stresses in grapevine. Phytochemistry 2017, 135, 34–52. [Google Scholar] [CrossRef]
- Huo, X.L.; Yang, B.R.; Wang, Y.Y. Effects of Plant Hormones on Key Genes of Camptothecin Biosynthesis in Camtoptheca acuminat. Mol. Plant Breed. 2023, 21, 1570–1575. [Google Scholar]
- Wang, L.; Shen, W.J.; Liao, L. Camptothecin: A biosynthetic pathway with associated enzymes and genes. J. Zhejiang For. Coll. 2008, 25, 791–797. [Google Scholar]
- Shen, S.H.; Liu, J.Y.; Hu, J.Q. Advances in studies on biosynthetic pathways of camptothecin and their synthases. Chin. Tradit. Herb. Drugs 2011, 42, 1862–1868. [Google Scholar]
- Gu, Q.; Song, D.F. Effects of L-tryptophan on Camptothecin acumulation in Camptotheca acuminate cell line. Acta Agric. Zhejiangensis 2007, 19, 169–173. [Google Scholar]
- Chen, D.W.; Tang, Y.H.; Shi, W.B. Progress in the Regulation of Calcium Growth and Development. Mol. Plant Breed. 2019, 17, 3593–3601. [Google Scholar]
- Liu, W.P.; Chen, R.Y.; Sun, G.W. The effect of different calcium levels on the growth and quality of scallions. Chin. J. Veg. 2008, 2, 25–27. [Google Scholar]
- Zhang, L.X.; Peng, J.M.; Ma, J. Study Progress on Nutrient Deficiency of Plants. Chin. Agric. Sci. Bull. 2010, 26, 157–163. [Google Scholar]
- Tong, X.C.; Cao, A.P.; Wang, F. Calcium-dependent protein kinase genes in Glycyrrhiza uralensis appear to be involved in promoting the biosynthesis of glycyrrhizic acid and flavonoids under salt stress. Molecules 2019, 24, 1837. [Google Scholar] [CrossRef]
- Maria, A.; Jorge, F.; Purificacion, C. Enhanced silymarin accumulation is related to calcium deprivation in cell suspension cultures of Silybum marianum (L.) Gaertn. Plant Physiol. 2005, 162, 1177–1182. [Google Scholar]
- He, W.; Yan, L.; Gao, X.C. Effects of exogenous calcium on physiological characteristics of Atracyodes rhizoma seedlings. Subtrop. Agric. Res. 2022, 18, 246–251. [Google Scholar]
- Zhang, Q.Y.; Wang, N.J.; Yang, C.X. Effects of Different Calcium Fertilizers Application Amount on Yield and Quality of Ural Licorice Glycyrrhiza Uralensis. Bull. Soil Water Conserv. 2015, 35, 107–117. [Google Scholar]
- Nilozhijie Zhang, J.; Qi, J.S. The effect of calcium, magnesium, and sulfur dosage on the yield and quality of Corydalis yanhusuo. Sci. Consult. (Technol. Manag.) 2021, 19, 46–47. [Google Scholar]
- Liang, Y.; Li, L.X.; Cai, J.Y. Effect of exogenous Ca2+ on growth and accumulation of major components in tissue culture seedlings of Sophora tonkinensis Gagnep. Pharmacogn. Mag. 2020, 16, 386–392. [Google Scholar]
- Wang, D.; Wu, X.H.; Zhou, N. Effects of Calcium, Magnesium and Sulfur on the Growth, Yield and Quality of Fritillaria thunbergii Miq. Chin. Wild Plant Resour. 2022, 41, 51–58, 64. [Google Scholar]
- Yang, W.X.; Hei, G.G.; Li, L.L. The effect of calcium on the quality and yield of Pinellia ternata under daytime sub high temperature stress. J. Southwest Univ. (Nat. Sci. Ed.) 2014, 36, 20–26. [Google Scholar]
- Isah, T.; Masood, S.; Umar, S. Biomass and camptothecin production in the calcium chloride elicited and liquid medium overlayed Nothapodytes nimmoniana (J. Graham) Mabberly callus cultures. Vegetos 2021, 35, 104–114. [Google Scholar] [CrossRef]
- Leopold, A.C.; Plummer, T.H. Auxin-phenol complexes. Plant Physiol. 1961, 36, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Haissing, B.E. Metabolic Proeesses in adventitious rooting of cuttings. In New Root Formation in Plant and Cuttings; Jackson, M.B., Ed.; Springer: Berlin/Heidelberg, Germany, 1986; pp. 141–189. [Google Scholar]
- Song, J.L.; He, W.L.; Li, S.B. Analysis of physicochemical properties related to rooting of Populus tomentosa mosaic cuttings. For. Sci. 2001, 137, 64–67. [Google Scholar]
- Li, M.; Huang, Z.L.; Tan, S.M. Comparative study on the activities and isoenzymes of polyphenol oxidase and indole-3-acetic acid oxidase in difficult to root eucalyptus trees. For. Sci. Res. 2000, 13, 493–500. [Google Scholar]
- Balakrishnamurthy, O.; Madhava, R.V.N. Changes in phenols during rhizogenesis in rose(Rosa bourboniana Desp). Curr. Sci. 1988, 57, 960–962. [Google Scholar]
- Hartman, T.; Kester, D.E. Plant Propagation-Priciple and Practices, 3rd ed.; Prentice Hall of India: New Delhi, India, 1976; pp. 47–86. [Google Scholar]
- Lynch, J. Root Architecture and Plant Productivity. Plant Physiol. 1995, 109, 7–13. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; Deforest, J.L.; Burton, A.J. Fine root architecture of nine North American trees. Ecol. Monogr. 2002, 72, 293–309. [Google Scholar] [CrossRef]
- Hashimoto, T.; Yamada, Y. New Genes in Alkaloid Metabolism and Transport. Curr. Opin. Biotechnol. 2003, 14, 163–168. [Google Scholar] [CrossRef]
- Kuang, X.J.; Wang, C.X.; Zou, L.Z. Recent advances in biosynthetic pathway and synthetic biology of taxol. Chin. J. Tradit. Chin. Med. 2016, 44, 4144–4149. [Google Scholar]
- Zhu, R. Content Determination of Vincristine Sulfate for Injection by HPLC. China Pharm. 2016, 27, 407–409. [Google Scholar]
- Kuang, X.J.; Wang, C.X.; Zou, L.Z. Advance in biosynthesis of terpenoid indole alkaloids and its regulation in Catharanthus roseus. Chin. J. Tradit. Chin. Med. 2016, 44, 4129–4137. [Google Scholar]
- Engmann, O.; Labonte, B.; Mitchell, A. Cocaine-induced Chromatin Modifications are Associated with Increased Gene Expression and Dna-Dna Interactions of Auts2. FASEB J. 2016, 30, 1186. [Google Scholar] [CrossRef]
- Gershenzon, J. Phytochemical Adaptations to Stress; Springer: Berlin/Heidelberg, Germany, 1984; pp. 273–320. [Google Scholar]
- Khan, M.; Harborne, J. Effect of Nitrogen on Alkaloid Production in Atropa acuminata. Planta Medica 1990, 56, 605–606. [Google Scholar] [CrossRef]
- Zhang, L.P.; Chen, Z.; Ma, X.J. Effects of nitrogen sources on the growth of Huanglian plants and the content of berberine in roots and rhizomes. Chin. Herb. Med. 1995, 26, 387–388. [Google Scholar]
- Wang, Y.B.; Zhu, C.F.; Yang, H.F. Phosphate Fertilizer Affected Rhizospheric Soils: Speciation of Cadmium and Phytoremediation by Chlorophytum comosum. Environ. Sci. Pollut. Res. 2017, 24, 3934–3939. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Luo, X.Q.; Ju, G.H. Increased Accumulation of the Cardio-cerebrovascular Disease Treatment Drug Tanshinone in Salvia Miltiorrhiza Hairy Roots by the Enzymes 3-hydroxy-3-methylglutaryl Coa Reductase and 1-deoxy-d-xylulose 5-phosphate Reductoisomerase. Funct. Integr. Genom. 2014, 14, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Alaghemand, A.; Ghorbanpour, M.; Moghaddasian, B. Tropane Alkaloids Elicitation of Black Henbane Parts with Calcium and Nitrogen Application Under Hydroponics Culture. J. Hortic. For. Biotechnol. 2013, 17, 107–113. [Google Scholar]
- Zhang, Y.; Wang, W.Q.; Du, S.X. Effects of nitrogen, phosphorus, and potassium on seedling growth and stachydrine and total alkaloid from Leonurus japonicus. Chin. Tradit. Herb. Drugs 2007, 38, 1881–1884. [Google Scholar]
- Bian, S.N. Effects of Different Nitrogen Forms on Growth and Camptothecin Content of Camptotheca acuminata. Master’s Thesis, Zhejiang Agriculture & Forestry University, Hangzhou, China, 2019. [Google Scholar]
- Ying, Y.Q.; Liu, P.; Wang, X.H. Effect of Drought Stress on the Seedling Growth from Different Provenances and Camptothecin Yield in Leaves of Camptotheca acuminata. Sci. Silvea Sin. 2012, 48, 30–35. [Google Scholar]
- Xing, J.H. Effects of Nitrogen Nutrition on Growth and Camptothecin Concentration in leaves of Camptotheca acuminate Seedlings. Ph.D. Thesis, Northeast Forestry University, Harbin, China, 2003. [Google Scholar]
- Sun, S.Q.; Yan, X.F. The effect of nitrogen level on camptothecin content in camptothecin seedlings. Chin. J. Tradit. Chin. Med. 2008, 33, 356–359. [Google Scholar]
- Pan, X.W.; Xu, H.H.; Gao, X. Improvement of Growth and Camptothecin Yield by Altering Nitrogen Source Supply in Cell Suspension Cultures of Camptotheca acuminata. Biotechnol. Lett. 2004, 26, 1745–1748. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).