Ontogenetic Stage Strongly and Differentially Influences Leaf Economic and Stomatal Traits Along Phyllotactic and Environmental Gradients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
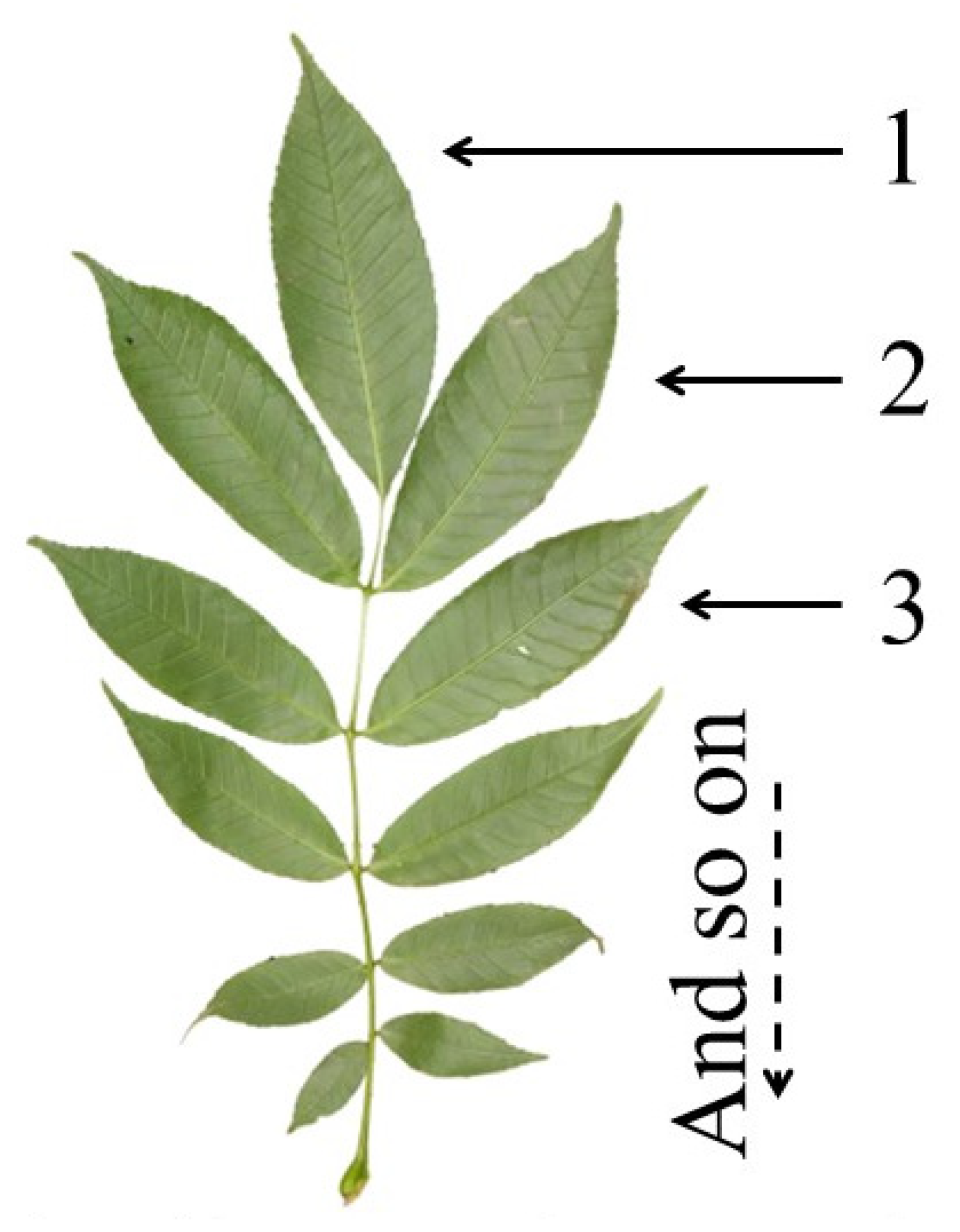
2.2. Sample Collection
2.3. Leaf Trait Measurements
2.4. Environmental Factors Measurements
2.5. Data Analysis
3. Results
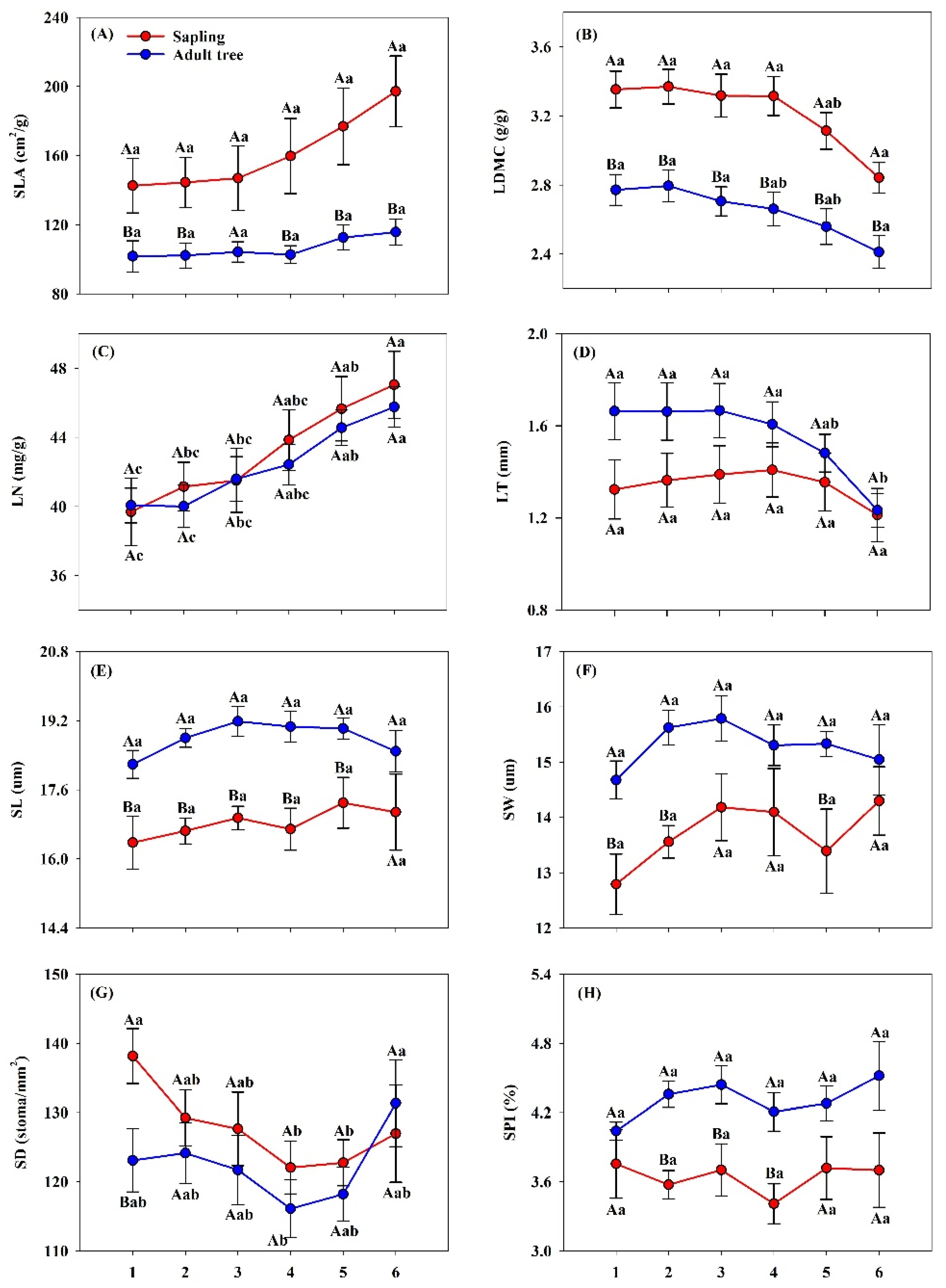
3.1. Variation in Leaf Economic and Stomatal Traits Across Phyllotaxy and Ontogenetic Stages
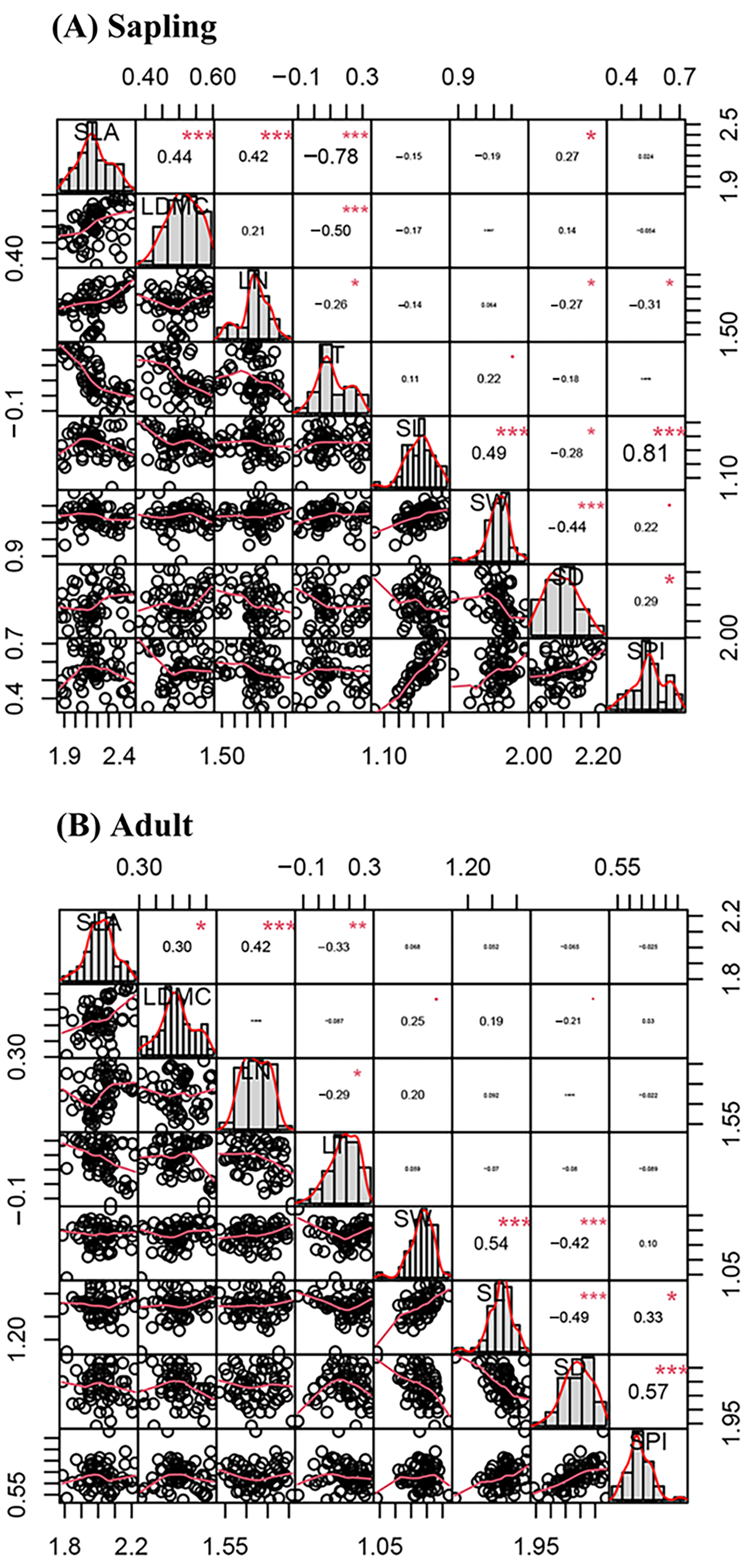
3.2. Correlations Among Leaf Traits Across Ontogenetic Stages
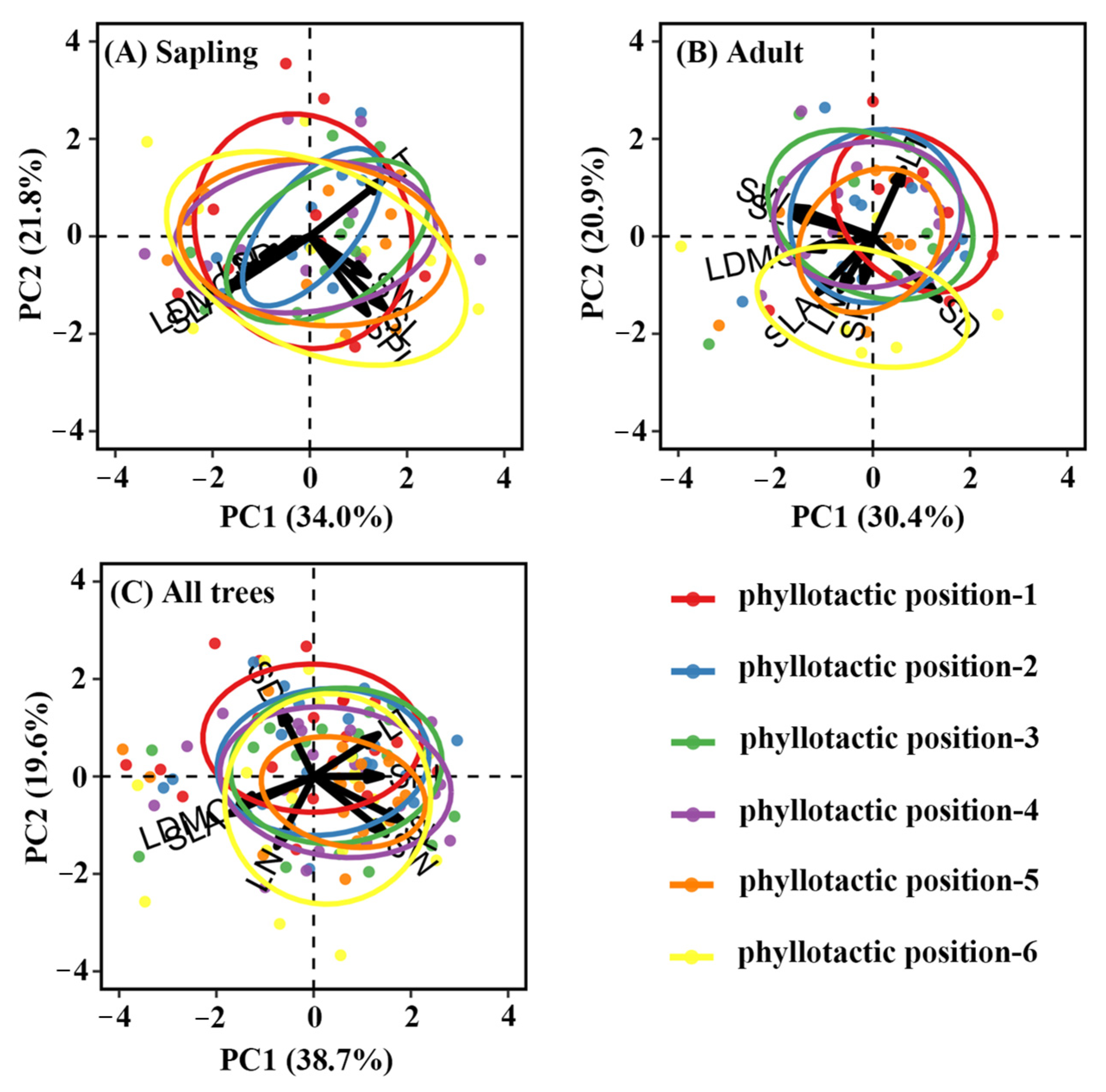
3.3. Effects of Phyllotaxy and Environmental Factors on Leaf Traits Across Ontogenetic Stages
4. Discussion
4.1. Economic Traits Are More Sensitive to Phyllotaxy than Stomatal Traits Across Ontogenetic Stages
4.2. Clear Separation of Phyllotaxy in Adults but Not in Saplings
4.3. Stage-Dependent Responses of Leaf Traits to Phyllotaxy and Environmental Factors
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SLA | Specific leaf area |
| LDMC | Leaf dry matter content |
| LN | Leaf nitrogen content |
| LT | Leaf thickness |
| SL | Stomatal length |
| SW | Stomatal width |
| SD | Stomatal density |
| SPI | Stomatal pore index |
| STN | soil total nitrogen |
| SWC | soil water content |
| CO | canopy openness |
Appendix A
| Phyllotaxy | LT | LDMC | SLA | LN | SW | SL | SD | SPI | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | LT | −0.45 | −0.36 | 0.25 | −0.03 | −0.31 | 0.24 | −0.11 | |
| LDMC | −0.65 | 0.48 | 0.03 | 0.04 | 0.46 | −0.5 | −0.1 | ||
| SLA | −0.87 | 0.87 | 0.54 | −0.52 | −0.15 | 0.05 | −0.26 | ||
| LN | −0.37 | 0.43 | 0.31 | −0.38 | −0.24 | 0.27 | 0.01 | ||
| SW | −0.16 | 0.34 | 0.16 | −0.02 | 0.27 | −0.29 | −0.02 | ||
| SL | −0.09 | 0.24 | 0.09 | −0.46 | 0.52 | −0.85 | 0.2 | ||
| SD | −0.1 | 0.26 | 0.25 | 0.02 | 0.32 | 0.01 | 0.34 | ||
| SPI | −0.05 | 0.29 | 0.11 | −0.43 | 0.57 | 0.93 | 0.37 | ||
| 2 | LT | −0.38 | −0.42 | −0.09 | 0.3 | 0.13 | −0.19 | −0.14 | |
| LDMC | −0.54 | 0.45 | 0.36 | 0.27 | 0.26 | −0.33 | −0.22 | ||
| SLA | −0.78 | 0.79 | 0.69 | 0.04 | 0.17 | −0.22 | −0.19 | ||
| LN | −0.12 | 0.5 | 0.35 | 0.36 | −0.07 | −0.13 | −0.26 | ||
| SW | 0.06 | 0.01 | −0.35 | 0.35 | 0.64 | −0.43 | −0.02 | ||
| SL | −0.54 | −0.01 | 0.11 | 0.12 | 0.38 | −0.66 | −0.01 | ||
| SD | −0.19 | 0.35 | 0.37 | −0.46 | −0.65 | −0.49 | 0.75 | ||
| SPI | −0.72 | 0.3 | 0.44 | −0.28 | −0.19 | 0.63 | 0.37 | ||
| 3 | LT | −0.3 | −0.43 | −0.29 | 0.31 | 0 | −0.15 | −0.24 | |
| LDMC | −0.78 | 0.82 | 0.32 | 0.33 | 0.42 | −0.01 | 0.45 | ||
| SLA | −0.67 | 0.78 | 0.45 | 0.33 | 0.22 | 0.06 | 0.32 | ||
| LN | 0.02 | 0.47 | 0.41 | 0.48 | 0.33 | −0.3 | 0.01 | ||
| SW | 0.03 | −0.1 | −0.3 | −0.07 | 0.46 | −0.46 | −0.09 | ||
| SL | 0.08 | −0.32 | −0.54 | −0.54 | 0.38 | −0.55 | 0.4 | ||
| SD | −0.16 | 0.29 | 0.28 | −0.05 | −0.36 | 0.35 | 0.54 | ||
| SPI | −0.05 | 0.03 | −0.09 | −0.31 | −0.03 | 0.76 | 0.87 | ||
| 4 | LT | −0.49 | −0.63 | −0.39 | −0.12 | −0.01 | 0.24 | 0.2 | |
| LDMC | −0.82 | 0.88 | 0.36 | −0.09 | −0.32 | −0.03 | −0.29 | ||
| SLA | −0.63 | 0.82 | 0.45 | −0.02 | −0.21 | −0.3 | −0.49 | ||
| LN | −0.25 | 0.48 | 0.55 | 0.11 | −0.18 | 0.17 | −0.02 | ||
| SW | 0.4 | −0.32 | −0.38 | −0.2 | 0.78 | −0.52 | 0.23 | ||
| SL | 0.04 | −0.31 | −0.5 | −0.31 | 0.7 | −0.45 | 0.49 | ||
| SD | −0.09 | 0.27 | 0.58 | −0.04 | −0.38 | −0.5 | 0.55 | ||
| SPI | 0.06 | −0.23 | −0.21 | −0.38 | 0.57 | 0.82 | 0.09 | ||
| 5 | LT | −0.42 | −0.08 | 0.02 | −0.34 | −0.12 | −0.59 | −0.67 | |
| LDMC | −0.45 | 0.72 | 0.18 | 0.41 | 0.48 | 0.17 | 0.48 | ||
| SLA | −0.73 | 0.3 | 0.04 | 0.42 | 0.36 | −0.22 | 0.01 | ||
| LN | −0.24 | 0.52 | 0.35 | 0.38 | −0.07 | 0.23 | 0.16 | ||
| SW | 0.27 | 0.19 | −0.39 | −0.02 | 0.46 | −0.16 | 0.18 | ||
| SL | 0.3 | −0.57 | −0.42 | −0.09 | 0.3 | −0.31 | 0.42 | ||
| SD | 0.03 | −0.48 | 0.15 | −0.46 | −0.54 | 0.06 | 0.73 | ||
| SPI | 0.23 | −0.69 | −0.27 | −0.22 | 0.07 | 0.93 | 0.4 | ||
| 6 | LT | −0.61 | 0.29 | −0.57 | −0.43 | −0.32 | 0.7 | 0.31 | |
| LDMC | −0.72 | 0.39 | 0.47 | 0.73 | 0.64 | −0.55 | 0.08 | ||
| SLA | −0.63 | 0.58 | −0.32 | 0.54 | 0.52 | −0.26 | 0.24 | ||
| LN | −0.25 | 0.51 | 0.53 | 0.34 | −0.03 | −0.31 | −0.33 | ||
| SW | 0.46 | −0.23 | −0.26 | 0.01 | 0.72 | −0.72 | 0.05 | ||
| SL | 0.48 | −0.23 | −0.26 | 0.05 | 0.75 | −0.38 | 0.61 | ||
| SD | −0.21 | 0.04 | 0.39 | −0.42 | −0.53 | −0.53 | 0.49 | ||
| SPI | 0.43 | −0.31 | −0.08 | −0.23 | 0.53 | 0.85 | −0.01 |
| Ontogenetic Stage | Phyllotaxy | Environmental Factors | SLA | LDMC | LT | LN | SL | SW | SD | SPI |
|---|---|---|---|---|---|---|---|---|---|---|
| Sapling | 1 | STN | 0.32 | 0.11 | −0.58 | 0.41 | −0.31 | 0.28 | −0.22 | −0.43 |
| SWC | −0.22 | −0.34 | 0 | 0.06 | −0.16 | −0.08 | −0.57 | −0.37 | ||
| CO | −0.01 | 0.04 | 0.23 | −0.33 | 0.16 | 0.35 | 0.83 | 0.47 | ||
| 2 | STN | 0.53 | 0.28 | −0.55 | 0.43 | 0.26 | 0.02 | −0.24 | 0.05 | |
| SWC | −0.28 | −0.28 | −0.08 | 0.09 | 0.32 | 0.28 | −0.37 | 0.01 | ||
| CO | 0.13 | 0.04 | 0.1 | −0.43 | −0.44 | −0.4 | 0.54 | −0.02 | ||
| 3 | STN | 0.4 | 0.39 | −0.49 | 0.41 | −0.36 | 0.17 | −0.55 | −0.57 | |
| SWC | −0.44 | −0.23 | 0.04 | −0.04 | 0.38 | 0.53 | −0.59 | −0.21 | ||
| CO | 0.26 | −0.26 | 0.19 | −0.08 | −0.16 | −0.3 | 0.38 | 0.17 | ||
| 4 | STN | 0.43 | 0.5 | −0.62 | 0.54 | −0.03 | −0.21 | −0.42 | −0.33 | |
| SWC | −0.42 | −0.15 | −0.13 | 0.11 | 0.61 | 0.27 | −0.9 | 0.11 | ||
| CO | 0.16 | −0.32 | 0.33 | −0.2 | −0.2 | 0.08 | 0.64 | 0.2 | ||
| 5 | STN | 0.5 | 0.31 | −0.5 | 0.53 | 0.28 | 0.26 | −0.07 | 0.28 | |
| SWC | −0.16 | −0.21 | 0.03 | 0.07 | 0.79 | 0.5 | 0 | 0.74 | ||
| CO | −0.02 | −0.15 | 0.48 | −0.21 | −0.45 | −0.32 | 0.38 | −0.3 | ||
| 6 | STN | 0.46 | 0.74 | −0.5 | 0.55 | 0.27 | −0.06 | −0.23 | 0.16 | |
| SWC | −0.23 | 0.2 | −0.19 | 0.05 | 0.62 | 0.28 | −0.58 | 0.38 | ||
| CO | 0.01 | −0.39 | 0.63 | −0.21 | 0.1 | 0.34 | 0.36 | 0.32 | ||
| Adult tree | 1 | STA | −0.45 | −0.07 | −0.05 | −0.06 | 0.05 | 0.01 | 0.09 | 0.35 |
| SWC | −0.01 | −0.5 | −0.41 | −0.36 | −0.27 | −0.23 | 0.34 | 0.13 | ||
| CO | −0.05 | −0.03 | 0.79 | 0.57 | −0.16 | 0.08 | 0.09 | −0.14 | ||
| 2 | STN | −0.38 | −0.08 | −0.09 | 0 | −0.19 | −0.05 | 0.35 | 0.35 | |
| SWC | −0.21 | −0.62 | −0.25 | −0.5 | −0.42 | −0.61 | 0.35 | 0.13 | ||
| CO | 0.1 | 0.14 | 0.67 | 0.55 | 0.33 | 0.63 | −0.38 | −0.14 | ||
| 3 | STN | −0.34 | 0.02 | −0.06 | 0.3 | 0.3 | 0.07 | 0 | 0.29 | |
| SWC | −0.33 | −0.59 | −0.38 | −0.37 | −0.49 | −0.58 | 0.47 | 0.02 | ||
| CO | 0.03 | 0.17 | 0.74 | 0.31 | 0.27 | 0.48 | −0.28 | −0.08 | ||
| 4 | STN | −0.16 | 0.09 | 0.1 | 0.22 | 0 | 0.06 | 0.19 | 0.22 | |
| SWC | −0.49 | −0.49 | −0.21 | −0.32 | −0.19 | −0.36 | 0.24 | 0.06 | ||
| CO | 0.12 | 0.08 | 0.59 | 0.28 | −0.01 | 0.01 | −0.04 | −0.08 | ||
| 5 | STN | −0.06 | −0.1 | 0.03 | 0.23 | 0.04 | 0.06 | −0.04 | 0.01 | |
| SWC | −0.47 | −0.34 | −0.44 | −0.39 | −0.5 | −0.51 | 0.31 | −0.03 | ||
| CO | 0.11 | −0.07 | 0.7 | 0.44 | 0.03 | 0.33 | −0.45 | −0.44 | ||
| 6 | STN | −0.08 | 0.05 | −0.1 | 0.08 | 0.16 | 0.16 | 0.12 | 0.23 | |
| SWC | −0.45 | −0.35 | 0.05 | −0.34 | −0.25 | −0.59 | 0.3 | 0.08 | ||
| CO | 0.1 | −0.09 | 0.28 | 0.27 | −0.12 | 0.23 | 0.14 | −0.04 |
References
- Sanczuk, P.; Govaert, S.; Meeussen, C.; De Pauw, K.; Vanneste, T.; Depauw, L.; Moreira, X.; Schoelynck, J.; De Boevre, M.; De Saeger, S.; et al. Small-scale environmental variation modulates plant defence syndromes of understorey plants in deciduous forests of Europe. Glob. Ecol. Biogeogr. 2020, 30, 205–219. [Google Scholar] [CrossRef]
- Liu, Y.H.; Zhao, Y.F.; Li, Q.S.; Tan, Z.J.; Zhang, Z.G.; Liu, Y.X.; Wang, J.M.; Liu, C.C.; Xiao, C.W. Variation in leaf construction cost and environmental drivers in China. J. Plant Ecol. 2025, 18, rtaf012. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Cornelissen, J.H.C.; Falster, D.S.; Groom, P.K.; Hikosaka, K.; Lee, W.; Lusk, C.H.; Niinemets, Ü.; Oleksyn, J.; et al. Modulation of leaf economic traits and trait relationships by climate. Glob. Ecol. Biogeogr. 2005, 14, 411–421. [Google Scholar] [CrossRef]
- Liu, H.D.; Zhang, G.Q.; Li, T.Q.; Ren, S.Y.; Chen, B.H.; Feng, K.B.; Wang, S.S.; Zhao, X.Q.; Rong, X.X.; Qin, P.Y.; et al. Unraveling key environmental drivers of spatial variation in plant functional traits: Insights from Dacrydium pectinatum de Laub. in natural communities on Hainan Island, China. Glob. Ecol. Conserv. 2024, 56, e0326. [Google Scholar] [CrossRef]
- Jin, M.Y.; Jin, G.Z.; Guo, Q.X.; Liu, Z.L. Responses of economic and anatomical leaf traits to soil fertility factors in eight coexisting broadleaf species in temperate forests. Front. For. Glob. Change 2023, 6, 1232333. [Google Scholar] [CrossRef]
- Westerband, A.C.; Funk, J.L.; Barton, K.E. Intraspecific trait variation in plants: A renewed focus on its role in ecological processes. Ann. Bot. 2021, 127, 397–410. [Google Scholar] [CrossRef]
- Guo, X.H.; Zhang, J.S.; Gu, J.C.; Li, Z.Y.; Wang, Y. Variations and coordination of leaflet and petiole functional traits within compound leaves in three hardwood species. Forests 2025, 16, 139. [Google Scholar] [CrossRef]
- Umaña, M.N.; Needham, J.; Fortunel, C. From seedlings to adults: Linking survival and leaf functional traits over ontogeny. Ecology 2024, 106, e4469. [Google Scholar] [CrossRef]
- Liu, Z.L.; Hikosaka, K.; Li, F.R.; Jin, G.Z. Coordination of intra- and inter-species leaf traits according to leaf phenology and plant age for three temperate broadleaf species with different shade tolerances. For. Ecol. Manag. 2019, 434, 63–75. [Google Scholar] [CrossRef]
- Scalon, M.C.; Bohn, A.; Coelho, G.C.; Alves, R.F.; Secco, R.T.; Meister, L.; Zwiener, V.P.; Marcilio-Silva, V.; Trindade, W.C.F.; Marques, M.C.M. Relationship between growth trajectories and functional traits along tree ontogeny. Front. For. Glob. Change 2022, 5, 754656. [Google Scholar] [CrossRef]
- Caringella, M.A.; Bobich, B.A.; Stanfield, R.C.; Ewers, M.M.; Bobich, E.G.; Ewers, F.W. Effects of phyllotaxy on biomechanical properties of stems of Cercis occidentalis (Fabaceae). Am. J. Bot. 2014, 101, 206–210. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.F.; Wang, R.R.; Liu, M.L.; Ji, X.M.; He, Y.X.; Zhao, B.L.; Li, W.J.; Mo, X.Y.; Zhang, X.J.; et al. Control of compound leaf patterning by MULTI-PINNATE LEAF. Nat. Commun. 2023, 14, 7985. [Google Scholar] [CrossRef]
- He, L.L.; Liu, Y.; He, H.; Qi, J.F.; Zhang, X.J.; Li, Y.H.; Mao, Y.W.; Zhou, S.L.; Zheng, X.L.; Bai, Q.Z.; et al. A molecular framework underlying the compound leaf pattern of Medicago truncatula. Nat. Plants 2020, 6, 511–521. [Google Scholar] [CrossRef]
- Koch, G.; Rolland, G.; Dauzat, M.; Bédiée, A.; Baldazzi, V.; Bertin, N.; Guédon, Y.; Godin, C. Are compound leaves more complex than simple ones? A multi-scale analysis. Ann. Bot. 2018, 122, 1173–1185. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.Z.; Wang, Q.; Wang, C.J. Biomass and nitrogen content of petiole and rachis predict leaflet trait variation in compound pinnate leaves of plants. Flora 2023, 298, 152207. [Google Scholar] [CrossRef]
- Jin, M.Y.; Diao, Y.F.; Wang, Y.L.; Zhang, M.K.; Wang, T.Y.; Ren, Y.J.; Zhong, M.; Cheng, W.T.; Wang, C.D.; Teng, H.H. Phyllotaxy and environmental factors influence on leaf trait dimensions in Fraxinus mandshurica: A multidimensional approach within temperate forests. Front. Plant Sci. 2025, 16, 1626579. [Google Scholar] [CrossRef] [PubMed]
- Barton, K.E. The ontogenetic dimension of plant functional ecology. Funct. Ecol. 2024, 38, 98–113. [Google Scholar] [CrossRef]
- Thurner, M.; Yu, K.; Manzoni, S.; Prokushkin, A.; Thurner, M.A.; Wang, Z.; Hickler, T. Nitrogen concentrations in boreal and temperate tree organs and their drivers. Biogeosciences 2025, 22, 1475–1499. [Google Scholar] [CrossRef]
- Ren, J.; Fang, S.; Wang, Q.W.; Liu, H.Y.; Lin, F.; Ye, J.; Hao, Z.Q.; Wang, X.G.; Fortunel, C. Ontogeny influences tree growth response to soil fertility in a subtropical forest. Ann. Bot. 2023, 132, 599–611. [Google Scholar]
- Winkler, D.E.; Garbowski, M.; Kožić, K.; Larson, J.; Martin, S.; Rosche, C.; Slate, M.L.; Korell, L. Facilitating comparable research in seedling functional ecology. Methods Ecol. Evol. 2024, 15, 464–476. [Google Scholar] [CrossRef]
- Querejeta, J.I.; Prieto, I.; Armas, C.; Casanoves, F.; Diouf, M.; Yossi, H.; Kaya, B.; Pugnaire, F.I.; Rusch, G.M. Higher leaf nitrogen content is linked to tighter stomatal regulation of transpiration and more efficient water use across dryland trees. New Phytol. 2022, 235, 1351–1364. [Google Scholar] [CrossRef]
- Bardy, L.R.; Debiasi, T.V.; Sanada, K.; Rondina, A.B.L.; Torezan, J.M.D.; Stolf-Moreira, R.; Bianchini, E.; Oliveira, H.C. Effect of nitrogen addition to the soil on Atlantic Forest tree seedlings. Forests 2023, 14, 1111. [Google Scholar] [CrossRef]
- Houter, N.C.; Pons, T.L. Ontogenetic changes in leaf traits of tropical rainforest trees differing in juvenile light requirement. Oecologia 2012, 169, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Kenzo, T.; Inoue, Y.; Ichie, T. Leaf toughness increases with tree height and is associated with bundle sheath extension in tropical rainforest trees. Front. For. Glob. Change 2022, 5, 1002472. [Google Scholar] [CrossRef]
- Kühnhammer, K.; von Hardenberg, J.; Kübert, A.; Bailey, K.; Hu, J.; Ladd, N.; Meredith, L.; Werner, C.; Beyer, M. Deep roots mitigate drought impacts on tropical trees despite limited quantitative contribution to transpiration. Sci. Total Environ. 2023, 893, 164763. [Google Scholar] [CrossRef] [PubMed]
- Coble, A.P.; Cavaleri, M.A. Light acclimation optimizes leaf functional traits despite height-related constraints in a canopy shading experiment. Oecologia 2015, 177, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Perez-Martinez, L.V.; Medlyn, B.E.; Nicolle, D.; Colin, A.C.; Choat, B.; Tissue, D.T.; Rymer, P.D. Drought sensitivity is climate-adapted and consistently influenced by wood density and maximum height in eucalypts. Funct. Ecol. 2025, 39, 2905–2924. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; ter Steege, H.; Morgan, H.D.; van der Heijden, M.; et al. Handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef]
- Liu, Z.L.; Hikosaka, K.; Li, F.R.; Jin, G.Z. Variations in leaf economics spectrum traits for an evergreen coniferous species: Tree size dominates over environmental factors. Funct. Ecol. 2020, 34, 458–467. [Google Scholar] [CrossRef]
- Liu, C.C.; Li, Y.; Xu, L.; Chen, Z.; He, N.P. Variation in leaf morphological, stomatal, and anatomical traits and their relationships in temperate and subtropical forests. Sci. Rep. 2019, 9, 5803. [Google Scholar] [CrossRef]
- Frazer, G.W.; Canham, C.; Lertzman, K. Gap light analyzer (GLA), version 2.0. Bull. Ecol. Soc. Am. 2000, 81, 191–197. [Google Scholar]
- Sun, L.; Yang, G.J.; Liu, X.; Zhang, Y.; Qin, S.Q.; Zheng, P.M.; Wang, R.Q. Leaf functional traits of two species affected by nitrogen addition rate and period, not nitrogen compound type, in a meadow grassland. Front. Plant Sci. 2022, 13, 841464. [Google Scholar] [CrossRef]
- Coble, A.P.; Cavaleri, M.A. Vertical leaf mass per area gradient of mature sugar maple reflects both height-driven increases in vascular tissue and light-driven increases in palisade layer thickness. Tree Physiol. 2017, 37, 1337–1351. [Google Scholar] [CrossRef]
- Kitao, M.; Kitaoka, S.; Harayama, H.; Tobita, H.; Agathokleous, E.; Utsugi, H. Canopy nitrogen distribution is optimized to prevent photoinhibition throughout the canopy during sunflecks. Sci. Rep. 2018, 8, 503. [Google Scholar] [CrossRef]
- Schmiege, S.C.; Griffin, K.L.; Boelman, N.T.; Vierling, L.A.; Bruner, S.G.; Min, E.; Maguire, A.J.; Jensen, J.; Eitel, J.U.H. Vertical gradients in photosynthetic physiology diverge at the latitudinal range extremes of white spruce. Plant Cell Environ. 2023, 46, 45–63. [Google Scholar] [CrossRef]
- Cheng, Y.K.; Liu, X.; Song, Z.P.; Ma, M.J.; Zhou, S.R.; Allan, E. Divergent trait responses to nitrogen addition in tall and short species. J. Ecol. 2023, 111, 1443–1454. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Huang, H.; Sardans, J.; Niinemets, Ü.; Niklas, K.J.; Wang, H.; Peñuelas, J.; Li, Y.; Xie, J.; Wright, I.J. Leaf water content contributes to global leaf trait relationships. Nat. Commun. 2022, 13, 5519. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, R.X.; Harrison, S.P.; Prentice, I.C. Leaf morphological traits as adaptations to multiple climate gradients. J. Ecol. 2022, 110, 1344–1355. [Google Scholar] [CrossRef]
- Drake, P.L.; Froend, R.H.; Franks, P.J. Smaller, faster stomata: Scaling of stomatal size, rate of response, and stomatal conductance. Proc. Natl. Acad. Sci. USA 2013, 110, 10135–10140. [Google Scholar] [CrossRef]
- Wang, R.L.; Yu, G.R.; He, N.P.; Wang, Q.F.; Zhao, N.; Xu, Z.W.; Ge, J.P. Latitudinal variation of leaf stomatal traits from species to community level in forests: Linkage with ecosystem productivity. Sci. Rep. 2015, 5, 14454. [Google Scholar] [CrossRef]
- Sun, M.L.; Niinemets, Ü.; Li, Q.Y.; Jiao, Y.B.; Yao, W.H.; Shi, P.J. An inverse scaling relationship between stomatal density and mean nearest neighbor distance: Evidence from a Photinia hybrid and one of its parents. Plants 2023, 12, 3701. [Google Scholar] [CrossRef]
- Liu, C.C.; Sack, L.; Li, Y.; Zhang, J.H.; Yu, K.L.; Zhang, Q.Y.; He, N.P.; Yu, G.R. Relationships of stomatal morphology to the environment across plant communities. Nat. Commun. 2023, 14, 6629. [Google Scholar] [CrossRef]
- Ishida, A.; Yazaki, K.; Hoe, A.L. Ontogenetic transition of leaf physiology and anatomy from seedlings to mature trees of a rain forest pioneer tree, Macaranga gigantea. Tree Physiol. 2005, 25, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Liang, M.X.; Tong, S.; Qiao, X.T.; Li, B.H.; Yang, Q.; Chen, T.; Hu, P.; Yu, S.X. Response of leaf functional traits to soil moisture seasonality in a wet–dry tropical forest. Front. Plant Sci. 2023, 14, 1236607. [Google Scholar] [CrossRef] [PubMed]
- Cavender-Bares, J.; Bazzaz, F.A. Changes in drought response strategies with ontogeny in Quercus rubra: Implications for scaling from seedlings to mature trees. Oecologia 2000, 124, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.L.; Arend, M.; Zahnd, C.; Hoch, G.; Arndt, S.K.; Cernusak, L.A.; Poyatos, R.; Zhorzel, T.; Kahmen, A. Uniform regulation of stomatal closure across temperate trees. Tree Physiol. 2025, 45, 725–730. [Google Scholar]
- Ye, X.M.; Bu, W.S.; Hu, X.F.; Wang, F.C.; Sun, R.X.; He, P.C.; Liang, X.Y.; Chen, F.S. Are small trees more responsive to nutrient addition than large trees in an evergreen broadleaved forest? For. Ecol. Manag. 2023, 543, 121129. [Google Scholar] [CrossRef]
| Ontogenetic Stage | Leaf Traits | Abbreviation | Units | Min | Max | Mean | SE | CV (%) |
|---|---|---|---|---|---|---|---|---|
| Sapling | Specific leaf area | SLA | cm2/g | 72.685 | 331.003 | 201.844 | 8.281 | 0.780 |
| Leaf dry matter content | LDMC | g/g | 2.397 | 3.938 | 3.168 | 0.050 | 0.391 | |
| Leaf nitrogen content | LN | mg/g | 30.271 | 58.533 | 44.402 | 0.816 | 0.483 | |
| Leaf thickness | LT | mm | 0.767 | 2.167 | 1.467 | 0.051 | 0.646 | |
| Stomatal length | SL | μm | 11.642 | 20.586 | 16.114 | 0.235 | 0.434 | |
| Stomatal width | SW | μm | 7.390 | 18.915 | 13.153 | 0.266 | 0.609 | |
| Stomatal density | SD | stoma/mm2 | 101.423 | 164.375 | 132.899 | 2.072 | 0.383 | |
| Stomatal pore index | SPI | % | 2.181 | 5.176 | 3.678 | 0.102 | 0.579 | |
| Adult tree | Specific leaf area | SLA | cm2/g | 61.158 | 166.230 | 113.694 | 2.999 | 0.632 |
| Leaf dry matter content | LDMC | g/g | 2.019 | 3.328 | 2.673 | 0.042 | 0.393 | |
| Leaf nitrogen content | LN | mg/g | 34.390 | 51.002 | 42.696 | 0.552 | 0.326 | |
| Leaf thickness | LT | mm | 0.733 | 2.183 | 1.458 | 0.048 | 0.664 | |
| Stomatal length | SL | μm | 14.916 | 21.018 | 17.967 | 0.147 | 0.290 | |
| Stomatal width | SW | μm | 10.955 | 18.670 | 14.812 | 0.173 | 0.413 | |
| Stomatal density | SD | stoma/mm2 | 87.433 | 153.883 | 120.658 | 2.064 | 0.432 | |
| Stomatal pore index | SPI | % | 3.361 | 6.749 | 5.055 | 0.075 | 0.502 |
| Ontogenetic Stages | Leaf Trait | Phyllotaxy | Soil Total Nitrogen | Soil Water Content | Canopy Openness | Intercept | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate Std. | p Value | Estimate Std. | p Value | Estimate Std. | p Value | Estimate Std. | p Value | Estimate Std. | p Value | ||
| Sapling | SLA | 0.028 | 0.002 ** | 0.062 | <0.001 *** | −0.740 | <0.001 *** | −0.012 | 0.625 | 2.075 | <0.001 *** |
| LDMC | −0.013 | <0.001 *** | 0.013 | <0.001 *** | −0.245 | <0.001 *** | −0.026 | 0.002 ** | 0.638 | <0.001 *** | |
| LN | 0.015 | <0.001 *** | 0.015 | <0.001 *** | −0.184 | 0.005 ** | −0.023 | 0.038 | 1.610 | <0.001 *** | |
| LT | −0.005 | 0.432 | −0.037 | <0.001 *** | 0.522 | <0.001 *** | 0.071 | <0.001 *** | −0.026 | 0.778 | |
| SL | 0.003 | 0.322 | −0.004 | 0.144 | 0.228 | <0.001 *** | 0.018 | 0.048 | 1.086 | <0.001 *** | |
| SW | 0.005 | 0.257 | −0.002 | 0.612 | 0.239 | 0.004 ** | 0.024 | 0.091 | 0.954 | <0.001 *** | |
| SD | −0.008 | 0.021 * | 0.0003 | 0.911 | −0.110 | 0.056 | 0.016 | 0.109 | 2.161 | <0.001 *** | |
| SPI | −0.001 | 0.782 | −0.008 | 0.196 | 0.343 | 0.003 ** | 0.052 | 0.009 ** | 0.334 | <0.001 *** | |
| Adult tree | SLA | 0.012 | 0.051 | −0.012 | 0.015 * | −0.517 | <0.001 *** | −0.089 | 0.014 * | 2.545 | <0.001 *** |
| LDMC | −0.012 | <0.001 *** | −0.001 | 0.481 | −0.471 | <0.001 *** | −0.087 | <0.001 *** | 0.906 | <0.001 *** | |
| LN | 0.012 | <0.001 *** | 0.002 | 0.476 | −0.049 | 0.434 | 0.021 | 0.181 | 1.565 | <0.001 *** | |
| LT | −0.021 | <0.001 *** | −0.001 | 0.756 | 0.532 | <0.001 *** | 0.228 | <0.001 *** | −0.418 | 0.004 ** | |
| SL | 0.001 | 0.505 | 0.0004 | 0.777 | −0.165 | <0.001 *** | −0.031 | 0.004 ** | 1.415 | <0.001 *** | |
| SW | 0.001 | 0.976 | 0.001 | 0.981 | −2.097 | 0.001 ** | −2.055 | 0.186 | 1.345 | <0.001 *** | |
| SD | 0.001 | 0.753 | 0.004 | 0.171 | 0.276 | 0.005 ** | 0.037 | 0.115 | 1.808 | <0.001 *** | |
| SPI | 0.004 | 0.301 | 0.005 | 0.101 | −0.051 | 0.593 | −0.024 | 0.303 | 0.636 | <0.001 *** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wang, Y.; Mao, Q.; Cheng, W.; Cao, M.; Teng, H.; Diao, Y.; Jin, M.; Fei, N. Ontogenetic Stage Strongly and Differentially Influences Leaf Economic and Stomatal Traits Along Phyllotactic and Environmental Gradients. Forests 2025, 16, 1624. https://doi.org/10.3390/f16111624
Li J, Wang Y, Mao Q, Cheng W, Cao M, Teng H, Diao Y, Jin M, Fei N. Ontogenetic Stage Strongly and Differentially Influences Leaf Economic and Stomatal Traits Along Phyllotactic and Environmental Gradients. Forests. 2025; 16(11):1624. https://doi.org/10.3390/f16111624
Chicago/Turabian StyleLi, Jian, Yunlong Wang, Qingxin Mao, Wanting Cheng, Mingyang Cao, Honghui Teng, Yunfei Diao, Mingyue Jin, and Nuoya Fei. 2025. "Ontogenetic Stage Strongly and Differentially Influences Leaf Economic and Stomatal Traits Along Phyllotactic and Environmental Gradients" Forests 16, no. 11: 1624. https://doi.org/10.3390/f16111624
APA StyleLi, J., Wang, Y., Mao, Q., Cheng, W., Cao, M., Teng, H., Diao, Y., Jin, M., & Fei, N. (2025). Ontogenetic Stage Strongly and Differentially Influences Leaf Economic and Stomatal Traits Along Phyllotactic and Environmental Gradients. Forests, 16(11), 1624. https://doi.org/10.3390/f16111624





