Abstract
Eucalyptus plantations worldwide experience significant productivity losses due to herbivory caused by the weevil Gonipterus platensis (Coleoptera: Curculionidae. Marelli 1927); however, the role of leaf nutritional status in host preference remains poorly understood. In this study, we evaluated the incidence and severity of defoliation on two seed-propagated eucalypts—Eucalyptus globulus Labill. and Eucalyptus nitens Maiden, as well as two clonally propagated E. nitens × E. globulus hybrids—at a trial site in Mulchén, Chile. Sampling occurred after peak weevil activity (December 2022) and during austral autumn (May 2023). We determined foliar concentrations of nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), boron (B), carbon (C), and the carbon-to-nitrogen (C/N) ratio, and analyzed their relationships with herbivory using ANOVA, principal component analysis (PCA), and linear regression. Overall defoliation was low (<7%), but significantly higher on E. globulus, with hybrids exhibiting intermediate damage. Seasonally, N and Mg concentrations declined, while K and Ca levels increased, resulting in an elevated C/N ratio in autumn. A positive correlation was observed between leaf Ca concentration and both the incidence and severity of herbivory during peak activity in the susceptible E. globulus genotype (R2 = 0.96, p < 0.05). These findings suggest that calcium accumulation may influence weevil feeding preferences. Further research should explore nutrient-mediated resistance to guide selection and fertilization strategies for developing more resilient eucalyptus varieties.
1. Introduction
1.1. Eucalyptus Species Relevance
Eucalyptus spp. (Myrtaceae) are among the most widely utilized species for the establishment of managed plantations globally aimed at pulp production due to their rapid growth and adaptability to a diverse range of environments [1]. In Chile, Eucalyptus is the second most planted species following radiata pine, with 900,000 hectares in 2023, which accounts for 38% of the total planted area in the country [2]. Eucalyptus globulus Labill is the species with the largest established area within the genus, representing 25.1% of the planted forests in Chile [2], followed by E. nitens Maiden, which comprises 12.4% of the plantation area [1].
The limiting factors affecting Eucalyptus species in general include frost, drought, flooding, nutritional limitations, and susceptibility to pests and diseases. The development and utilization of hybrids in forestry, particularly within the Eucalyptus species, have emerged as a response to various needs and challenges related to overcoming the limitations of naturally occurring species while enhancing several desirable traits. In this context, hybridization among Eucalyptus species aims to combine economically valuable characteristics, such as rapid growth, disease and pest resistance, suitable structure, high wood density, and fiber length into a single entity [3].
1.2. Impact of Gonipterus platensis in Eucalyptus Plantations
One of the most significant and detrimental pests affecting Eucalyptus species worldwide is Gonipterus platensis Marelli (Coleoptera: Curculionidae) [4]. In Chile, the life cycle of G. platensis comprises two to three generations per year, depending on site conditions. Adults survive from six months to more than one year. They feed on young and mid-aged leaves, as well as on twigs and buds, and begin oviposition about one month after emergence. Larvae hatch within 10–15 days. Early instars (L1–L2) feed on the leaf epidermis, whereas later instars (L3–L4) consume entire leaves, shoots, and twigs. Mature larvae (L4) pupate in the soil, at a depth of 10–15 cm, for 30–50 days. After this period, adults emerge, completing the cycle and perpetuating defoliation damage [5].
Eucalyptus globulus is the most impacted species; however, other species such as E. viminalis, E. camalulensis, E. grandis, E. longuifolia, E. obliqua and E. robusta have also been identified as susceptible [4,6,7]. Both adult insects and larvae prefer to feed in the upper third of the tree crown, leading to severe defoliation [4]. Severe and repeated herbivory on new leaves, buds, and shoots not only diminishes tree growth and productivity but can also result in thinning the upper crown, tree deformation and tree decline [4,6,8].
Given the economic significance of the Eucalyptus species, various management strategies have been explored, including chemical, silvicultural, and biological control; the latter is one of the primary strategies for managing G. platensis, utilizing the egg parasitoid Anaphes nitens [9,10]. However, in several regions, particularly in the temperate zones of Portugal, Spain, and Chile, the parasitoid has not been as effective in mitigating the damage caused by the weevil as it is in its native range [4,6]. This limitation has been attributed to a mismatch in climate and/or host–parasitoid interactions in the aforementioned areas [11].
Due to the economic losses resulting from decreased productivity attributed to the weevil, alternative management strategies have been explored. These include biological control using different species, such as A. inexpectatus and A. tasmanidae, as well as the utilization of eucalyptus species or hybrids that exhibit lower susceptibility to G. platensis [4,11].
Variations in herbivory among Eucalyptus species have been strongly associated with differences in foliar chemical defense, particularly phenolic compounds, tannins, and essential oils that deter insect feeding [12,13]. These defensive metabolites, both constitutive and inducible, play a key role in modulating insect preference and susceptibility across species. Recent studies comparing E. globulus and E. nitens indicate that the feeding preferences of G. plantesis are largely determined by the constitutive stilbenes and hydrolysable tannins. Because E. globulus is unable to produce these metabolites constitutively, nor are they induced after herbivory, this species is more susceptible to attack by G. platensis [1].
1.3. Nutritional Quality of Eucalyptus Foliage and Its Influence on G. platensis Herbivory
Regarding insect–plant interactions, the nutritional quality of the host is a crucial factor that influences the specialization of phytophagous insects [14]. This quality serves as a key determinant of their fertility. Components such as carbon, nitrogen, and metabolites associated with defense responses significantly impact fertility and, concurrently, influence the selection of hosts that can provide optimal nutritional quality [15,16].
It has also been observed that the consumption of different Eucalyptus species can significantly influence the final weight of Gonipterus pulverulentum larvae. Specifically, larvae that fed on leaves of E. maidenii nearly doubled their weight compared to those that consumed E. camaldulensis. This study establishes that both E. camaldulensis and E. globulus are among the most palatable species, in contrast to E. viminalis. These findings suggest that implementing alternatives, such as using resistant or less palatable host species, is a viable strategy when biological control measures are insufficient [17].
Nitrogen (N) has been identified as the leaf component that most significantly influences the growth of Eucalyptus defoliators. Since the availability of nitrogen on the leaves can vary with the age of the foliage in Eucalyptus, insects must consume large quantities of leaves to support their development [18]. Other studies have established a correlation between the nitrogen concentrations available in the leaves and the performance of insect larvae, as measured by their dry weight. The larvae must ingest substantial amounts of foliage to meet their nitrogen requirements [19]. Additionally, it has been found that the total consumption of dry matter by larvae increases as the concentration of nitrogen in Eucalyptus leaves decreases [20]. This phenomenon is related to the concept of which posits that when phytophagous insects encounter food that is low in nutritional value, they compensate for the suboptimal diet by increasing their intake to maximize growth rates [21].
Another important element in insect–plant interactions is calcium, specifically in the form of calcium oxalate crystals. Migacz et al. (2018) have reported these crystals in E. globulus and proposed them as a microscopic characteristic for distinguishing between species within the genus [22]. In other plant species, it has been demonstrated that these crystals may serve as a defensive function or act as a response to herbivory, as evidenced by their increased concentrations following consumption [23]. For instance, in Sida rhombifolia (Malvaceae), higher levels of these crystals were observed when the plant was subjected to artificial herbivory [24]. Additionally, some herbivores tend to avoid varieties with elevated levels of these crystals [25].
1.4. Nutrients in the Foliage of Eucalyptus Species
There are notable differences in the levels of macro and microelements among various Eucalyptus species, with significant variations observed in some cases. A study comparing the nutritional status of the foliage of nine Eucalyptus species established in the same plot identified substantial differences in nutrient levels between species. For instance, nitrogen concentrations in E. globulus (1.5%) and E. nitens (1.4%) differed significantly from those in seven of the nine species, placing them at intermediate levels within the overall observed range. Conversely, the calcium and potassium content were significantly higher in E. globulus (1.10% Ca and 0.49% K) compared to E. nitens (0.80% Ca and 0.39% K) [26]. In a separate study conducted at three locations in northern Tasmania, where plantations had identical site preparations and fertilization, it was found that calcium concentrations in the leaves were significantly higher in E. globulus. Differences in nitrogen, phosphorus, potassium, and magnesium levels between E. globulus and E. nitens were minor and not statistically significant, depending on the site (with the exception of magnesium). Additionally, the nitrogen content in the leaves of E. nitens exceeded that of E. globulus [27].
There is evidence that certain Eucalyptus varieties are more susceptible to attacks by G. platensis than others, prompting research into the defense mechanisms that differentiate these species. However, insufficient information exists regarding the relationship between the nutritional status of the foliage and specific elements—such as nitrogen, phosphorus, potassium, boron, calcium, magnesium, and carbon—in relation to the palatability or preferences of G. platensis for various Eucalyptus species.
Given the information presented, we hypothesized that the defoliation potential of G. platensis for the species E. globulus, E. nitens, and their hybrids are linked to the nutritional status of each species.
2. Materials and Methods
2.1. Study Area and Location
The study was conducted on a 3.2 hectares trial located in the Mulchén commune of the Biobío region, in south-central Chile (37°37′49.3″ S, 72°24′09.8″ W). The trial was established in July 2018 by the Chilean forest company CMPC SpA. and comprised several plots containing 49 trees each. From these plots, four different treatments, corresponding to different genotypes of E. globulus, E. nitens and two different hybrids of E. nitens × E. globulus were selected. Each treatment included four replicates arranged randomly.
The general climate of the study area is classified as a temperate Mediterranean climate, characterized by distinct dry and rainy seasons. Summers are hot and dry, with maximum temperatures ranging from 25 °C to 30 °C, while winter maximum temperatures hover around 15 °C. The annual average rainfall can exceed 1000 mm [28].
According to Stolpe [29], soils are classified as red clays (Collipulli series), which are derived from ancient volcanic ash. These soils display a reddish hue with a granular surface structure and subangular blocks at greater depths. When wet, they are plastic and adhesive, while they become hard and cracked when dry. Their texture varies from silty clay to clay.
2.2. Foliar Samples
Two field samplings were conducted: the first on 19 and 20 December 2022, and the second on 18 and 19 May 2023 (abbreviated as M1 and M2, respectively). The purpose of these samplings was to investigate how summer influences the interactions between G. platensis and Eucalypts, as well as how seasonality affects foliar nutrient concentration. During each sampling event, defoliation was assessed, and leaf samples were collected for nutritional analysis. To collect the samples, leaves were collected from the upper third section of each tree, at a height of 6 to 8 m. Only fully expanded leaves were sampled, discarding any discolored, necrotic or otherwise unusual materials.
2.3. Eucalypts and Hybrids Species
Two operational clones named CH1 and CH2 (hybrid clones 1 and 2, respectively), which are hybrids between the species E. nitens and E. globulus, and two seed-propagated operational materials of E. globulus and E. nitens, named SG and SN, respectively, were included in the study. For SG, two categories after nutritional analysis of foliage were established, SGs (susceptible genotypes) and SGr (resistant genotypes), based on direct observation of the most affected and less affected trees, respectively. The categories are described in Section 2.5 (Nutritional Analysis of Foliage).
2.4. Evaluation of Defoliation Caused by Gonipterus platensis
To assess the damage caused by G. platensis, E. globulus, E. nitens and the two hybrids were included, with four repetitions (plots) for each. Each plot consisted of a configuration of 7 × 7 trees, and the evaluations were carried on the 5 × 5 central trees, representing a total of 25 trees per repetition.
The evaluation was focused on the incidence and severity of attacks in the upper third of the crown. For incidence, the number of trees exhibiting signs of defoliation was recorded in relation to the total number of trees assessed in each plot. The calculation was then performed as follows:
Incidence (%) = (Number of trees exhibiting defoliation signs/Total number of evaluated trees) × 100
For the assessment of severity, each tree was assigned a damage category. The categories and their corresponding percentage ranges of defoliation were established based on the criteria defined by the Consorcio Protección Fitosanitaria Forestal S.A. (CPF) and are detailed below: (0) no defoliation (0%–4%), (1) mild defoliation (5%–30%), (2) moderate defoliation (31%–60%), and (3) severe defoliation (>60%).
To calculate the severity for each plot, the number of trees in each category is multiplied by the corresponding index (0, 1, 2, or 3) and then divided by the total number of trees evaluated.
Additionally, during the second field evaluation (M2), we assessed the percentage of damage for each tree to evaluate the severity at the plot level by calculating a simple average.
Severity = (N° (Number in category 0 × 0) + (N° (Number in category × 1) + (N° (Number in category × 2) + (N° (Number in category × 3))/N° 3))/Number of trees evaluated.
2.5. Foliar Nutritional Analysis
To assess the nutritional status of the foliage, the protocol established by the Forest Productivity Cooperative (FCP) was followed (Forest Productivity Cooperative, s.f.) [30]. This protocol involves extracting a sample of 40 fully expanded leaves from the upper third of the individuals in each plot. From four to eight leaves per tree were collected from the outer crown until a total of 40 leaves was obtained for each treatment and for each of the four replicates. In the case of SG plots, the most susceptible and the most resistant trees from each of the five central rows of the plot were selected based on visual criteria of incidence and severity (as described in Section 2.4), resulting in the identification of the five most susceptible trees (SGs) and the five most resistant trees (SGr) to attack by G. platensis. In this case, eight leaves were removed from each tree to reach a total of 40 leaves.
The collected leaves were stored in paper bags and refrigerated in coolers before being transported to the Forest Health Laboratory at the Pontificia Universidad Católica de Chile in Santiago. The wet weight of each leaf sample was recorded using an analytical balance (Kern ABJ 220-4NM, Kern & Sohn GmbH, Balingen, Germany), and leaf area of each leaf was measured with a CI-202 leaf area meter (CID Bio-science, Camas, WA, USA) for all 40 samples. Each sample was subsequently dried to a constant wheight in an oven (Binder FD-260, Tuttlingen, Germany) at 65 °C for 10 days. To enhance the accuracy of the leaf area calculation, leaves collected from the second plot were photographed, and the leaf area was calculated using Fiji software (ImageJ2 version 2.14.0, open-source image processing software). After recording the dry weight of each leaf sample, the samples were pulverized using a commercial coffee grinder (Moulinex model D56) sent to the Soil, Water, Forest Research Analytical Laboratory at Universidad de Concepción (LISAB) for the determination of tissue nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), boron (B), magnesium (Mg) and carbon (C) concentrations.
2.6. Experimental Design and Data Analysis
The experimental design employed was a completely randomized block design with four replicates for each treatment (CH1, CH2, SG and SN). Nutritional variables and indicators of foliar damage, including incidence and severity, were assessed.
To analyze the obtained data, analyses of variance (ANOVA) followed by Tukey’s multiple comparison tests (p < 0.05) were performed to identify significant differences in nutritional composition and damage levels means among the various treatments and sampling instances (M1 and M2). To verify compliance with model assumptions, the normality of residuals was assessed using the Shapiro–Wilk test and residual plots, and homogeneity of variances was tested using Levene’s test. When the data did not meet the required statistical assumptions, nonparametric tests, such as the Kruskal–Wallis test and Dunnett’s test, were used.
To investigate the relationship between foliar nutritional status and field defoliation, a principal component analysis (PCA) was conducted. Based on the PCA results, linear regressions were performed to determine how each selected variable influenced the response variables. To satisfy the normality assumption required for regression analysis, a logarithmic transformation with a shift of 1 was applied to the incidence and severity data; specifically, the function log(x + 1) was utilized on the original values. For the PCA, a single average value labeled “SG” was calculated from the nutritional values of SGr and SGs. This unification was necessary because the distinction between SGr and SGs was made solely for nutritional analyses; in the defoliation assessment, no such separation existed, resulting in only a single “SG” variable being available.
All statistical analyses were conducted using a significance level of 0.05 in RStudio software (version 4.1.1).
3. Results
3.1. Defoliation Evaluation
In M1, the incidence and severity of attacks did not differ significantly between genotypes (Table 1 and Table 2). It is important to note that the severity levels for all treatments, when calculating the weighted average by category (refer to Section 2.4), fall within the initial range of 0 to 4%.

Table 1.
Incidence expressed as a percentage of Gonipterus platensis attacks on four Eucalyptus treatments during the M1 sampling (December 2022) and M2 sampling (May 2023).

Table 2.
Evaluation of the severity of Gonipterus platensis attacks on four Eucalyptus treatments during sampling M1 (December 2022) and M2 (May 2023).
In M2, the incidence was significantly higher in the SG genotype compared to the other genotypes evaluated (97.92%, Table 1). The severity exhibited a similar trend, with significantly higher values observed in the SG genotype, which recorded a value of 0.98, nearing severity category 1 (5 to 30% defoliation).
When comparing temporally, it was found that the incidence of the SN and CH2 genotypes remained constant from December 2022 to May 2023 (p = 0.315 and p = 1, respectively). In contrast, the incidence of the CH1 genotype decreased significantly over time (p = 0.0273), while the SG genotype showed a significant increase (p = 0.00109).
As mentioned earlier, in M2, a specific defoliation percentage was assigned within the damage category. In this instance, the severity was significantly higher in the SG treatment (6.79%, Table 3) compared to the other treatments, followed by CH2 (2.03%) and similar values in the SN and CH1 treatments (1.28% and 1.80%, respectively).

Table 3.
Severity expressed as a percentage of Gonipterus platensis attack during the M2 sampling (May 2023).
During the field visits, insect activity was documented. In December 2022, adult, larvae, and oothecae of the weevils were observed. However, during the visit in May 2023, only adult individuals were recorded.
3.2. Nutritional Analysis
No significant differences (p < 0.05) were observed in the concentrations of the foliar nutrients N, C, Mg, B and the carbon-to-nitrogen (C:N) ratio among the treatments. Table 4 and Table 5 present the foliar nutrient concentrations for each treatment based on analyses conducted in December 2022 (M1) and May 2023 (M2), respectively. Significant differences between treatments (p < 0.05) were detected for P concentrations in M1, with the SGs and SGr treatments exhibiting the highest concentrations, while CH1 had the lowest. For K, SGs and SGr had the highest concentrations in M1, whereas CH1 recorded the highest value in M2. The lowest K concentrations were found in the SN and CH1 treatments in M1, and in SN in M2. The Ca concentration was significantly higher in the CH1 treatment and lower in the SN treatment during both periods.

Table 4.
Comparison of foliar concentrations, expressed as percentages, of carbon (C), nitrogen (N), carbon-to-nitrogen ratio (C/N), and phosphorus (P) among Eucalyptus treatments for M1 (December 2022) and M2 (May 2023) sampling.

Table 5.
Comparison of foliar concentrations (in percentage) of potassium (K), calcium (Ca), magnesium (Mg), and boron (B, mg/kg) among Eucalyptus treatments for M1 (December 2022) and M2 (May 2023) samplings.
Table 4 and Table 5 illustrate the differences in nutrient levels between the two periods (M1 and M2), with arrows (↑↓) indicating significant increases or decreases. A notable decrease in N and Mg concentrations was observed across all treatments from December 2022 to May 2023, possibly because as the leaves age they remobilize nutrients like N towards reserves [31]. P and B maintained relatively stable levels, except in SGs, where a significant decrease was observed. K and Ca showed significant increases in all treatments, except for SGs, which remained stable. Calcium increases because it does not move through phloem, so it is not remobilized and accumulates [32]. The C:N ratio increased significantly in all treatments, except for SN, where the increase was not statistically significant. Finally, C content tended to decrease in all treatments, with significant reductions noted for CH2, SGr, and SGs.
3.3. Relationship Between Foliage Nutritional Status and Weevil Preferences
Principal component analyses (PCA) were conducted to examine the relationship between the incidence and severity variables assessed in the field and the nutrient status of the foliage. Figure 1a illustrates the PCA based on the incidence of treatments in M1 and their corresponding nutrient values. The components account for 59.82% of the total variance, with incidence showing a positive correlation with Ca, Mg, and C. Correlation analyses revealed that only the relationship between incidence and Ca is statistically significant (rho = 0.618, p = 0.0107), while the correlations with Mg and C are not significant (p = 0.503 and p = 0.6714, respectively) (Table 6). Figure 1b depicts the grouping of treatments based on the principal component (PC). The overlap and size of the ellipses suggest that the data for the CH1 treatment are more dispersed and share characteristics with the other treatments, while the SG treatment exhibits lower dispersion. In contrast, the SN and CH2 treatments form less dispersed groups and differ from one another within the principal components. All ellipses are oriented along the diagonal, indicating that the groups are related to the two principal components.
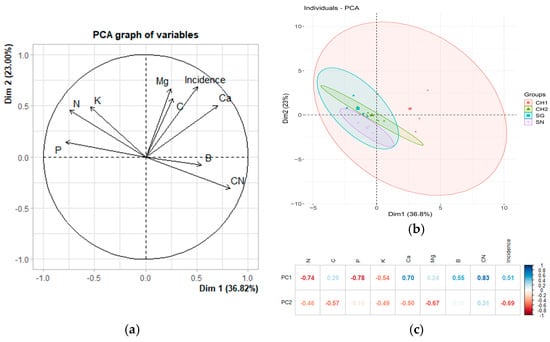
Figure 1.
The relationship between incidence and nutritional variables in M1 (December 2022). (a) The original variables are plotted in the principal component space (Dim 1 and Dim 2). The direction and length of the arrows indicate the contribution of each variable to the two principal components (PCs) displayed in the graph. In (b), each point represents an individual observation within the dataset. The ellipses denote the confidence regions for the groups (treatments) in the dataset, illustrating the dispersion and clustering of points associated with each treatment. (c) Heatmap displaying the loadings of the variables in the first two principal components of a principal component analysis (PCA). The loadings reflect the degree and direction of the correlation between the original variables and the principal components.

Table 6.
Correlations between defoliation incidence and nutrient concentration in M1.
In Figure 1c, it can be observed that the first principal component (PC) is strongly negatively correlated with N and P, while it is positively correlated with Ca and the C:N ratio. The second principal component is negatively associated with Mg and incidence.
The PCA conducted on defoliation severity in M1 and the nutritional analyses yielded results that are almost identical to information obtained from the PCA based on the incidence variable. This similarity arises because these two variables exhibit a perfect correlation (Spearman’s rho = 1, p < 2.2 × 10−16); most data fail in category 1, and mild defoliation and the nutrient values are identical (Figure A1 and Table A2).
The linear regression analysis conducted between incidence (normalized using the logarithmic function) and Ca content is illustrated in Figure 2. The linear regression indicates that an increase in incidence is observed as the Ca concentration rises, with a more pronounced linear trend for SG. The regression for severity gives essentially the same result and can be found in the annex to avoid data duplication (Figure A2). Furthermore, when performing individual regressions by treatment, SG emerges as the only significant factor for both incidence and severity (Table 7). In the case of the incidence of attack versus the Ca content in SG, the p-value of the regression is 0.02201, and the R2 value is 0.9565. Similarly, for the severity versus the Ca content in SG, the p-value of the regression is also 0.02201, with an R2 value of 0.9565.
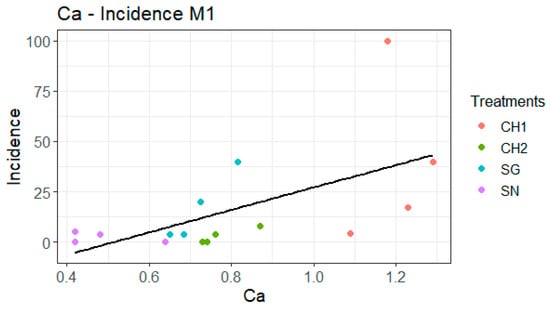
Figure 2.
The linear regression analysis between calcium concentration and incidence presents the original variables; however, the analysis utilizes the transformed variable (log(Incidence + 1)) for Model 1 (M1) (p = 0.008884, R2 = 0.3971).

Table 7.
Significance of the regression between incidence and severity in relation to the concentration (%) of calcium (Ca) for each Eucalyptus genotype in M1 (December 2022).
Figure 3a illustrates the principal component analysis (PCA) conducted on the incidence of treatments in M2 and their associated nutritional values. The components accounted for 53.18% of the total variance. The incidence of treatments is correlated with calcium (Ca), magnesium (Mg), and potassium (K) as depicted in Figure 3a. However, upon further analysis, these correlations were found to be statistically insignificant (Table 8). Figure 3b demonstrates the grouping of treatments based on the principal components. All treatments overlap, indicating that they share certain characteristics, and no treatment is entirely distinct from the others; there are notable similarities in their dispersion and the size of their ellipses. All ellipses are oriented along the diagonals, except for SN, which is distributed along Dimension 2. This distribution suggests a correlation with this component and the variables illustrated in Figure 3c, labeled as P.
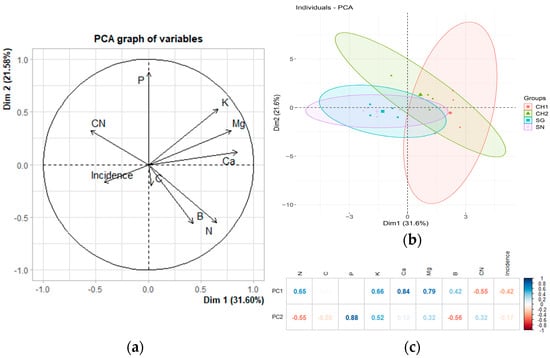
Figure 3.
The relationship between incidence and nutritional variables in M2 (May 2023). (a) The original variables are plotted in the principal component space (Dim 1 and Dim 2). The direction and length of the arrows indicate the contribution of each variable to the two principal components (PCs) displayed in the graph. In (b), each point represents an individual observation within the dataset. The ellipses denote the confidence regions for the groups (treatments) in the dataset, illustrating the dispersion and clustering of points associated with each treatment. (c) Heatmap displaying the loadings of the variables in the first two principal components of a principal component analysis (PCA). The loadings reflect the degree and direction of the correlation between the original variables and the principal components.

Table 8.
Correlations between defoliation parameters and nutrient concentration in M2 (May 2023).
Figure 4a illustrates the principal component analysis (PCA) conducted on the severity (%) of the treatments in M2 alongside their nutritional values. The components account for 53.9% of the total variance, revealing a negative correlation between defoliation severity and the levels of Ca, K, Mg, and possibly P. However, Spearman correlation analysis indicates that these relationships are not statistically significant (Table 8). In Figure 4b, it is evident that the treatments overlap, lacking complete differentiation in the principal component space. The overlap and size of the ellipses suggest that the data for the CH1 are more dispersed, while all treatments exhibit shared characteristics. Notably, all ellipses are oriented along the diagonal, indicating that the groups are related to the two principal components (PCs). Finally, Figure 4c demonstrates that the first principal component is strongly correlated with Ca, Mg, and K, whereas the second principal component is associated with P.
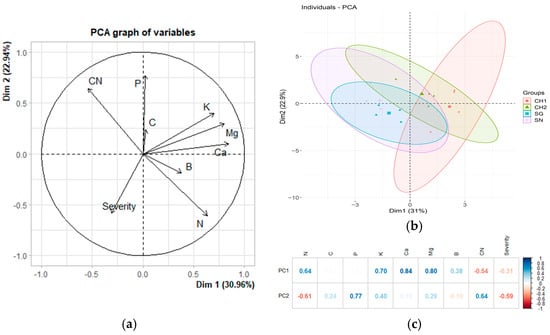
Figure 4.
The relationship between severity (%) and nutritional variables in M2 (May 2023). (a) The original variables are plotted in the principal component space (Dim 1 and Dim 2). The direction and length of the arrows indicate the contribution of each variable to the two principal components (PCs) displayed in the graph. In (b), each point represents an individual observation within the dataset. The ellipses denote the confidence regions for the groups (treatments) in the dataset, illustrating the dispersion and clustering of points associated with each treatment. (c) Heatmap displaying the loadings of the variables in the first two principal components of a principal component analysis (PCA). The loadings reflect the degree and direction of the correlation between the original variables and the principal components.
4. Discussion
4.1. Defoliation Evaluation
The overall defoliation levels observed in both evaluations, M1 and M2, were relatively low compared to findings from other studies. Goncalves et al. [4] reported defoliation percentages of approximately 45%, 70%, and 95% for E. globulus in the years 2012, 2013, and 2014, respectively, and 5%, 15%, and 75% for E. nitens during the same years in Portugal. In contrast, our study found average defoliation values of 6.79% for E. globulus and 1.28% for E. nitens in the second evaluation. Despite these differences in severity levels, it is noteworthy that we observed significantly higher defoliation levels for E. globulus compared to E. nitens, particularly in M2, as also reported by Goncalves et al. [4]. This trend has been documented in various studies [1,4,33,34]. The low levels of infestation by G. platensis may be attributed to the annual release of A. nitens in the study area, with the most recent application occurring in August 2022 [35], which likely reduced the insect’s presence on the trees.
Regarding the hybrids, it was observed that the severity values of CH1 and CH2 were intermediate between those of E. globulus and E. nitens, in M1 the differences were not significant. However, in M2, the severity was significantly higher for the SG treatment compared to the other treatments, with SN exhibiting the lowest severity. Campos et al. [36] reported defoliation levels that were comparatively higher than those observed in this study, although the patterns were similar. Since hybrids are expected to inherit their parent’s resistance to pests, as seen in G. platensis, it was found that hybrids between E. globulus and E. nitens exhibited lower percentages of defoliation than E. globulus, but not as low as those of E. nitens. Campos et al. [36] attributed these differences to the varying defensive metabolites present in both species.
When examining the incidence in M1, no statistically significant differences were found, possibly due to the high variability registered. Nevertheless, the trends observed in M2, where the SG treatment demonstrates a much higher defoliation than the other treatments, are consistent with the literature and our expectations regarding the resistance of hybrids.
In spring, G. plantensis reaches its peak population density, resulting in the most significant damage to foliage [37]. Given this and the observation that both adults and larvae in advanced developmental stages were present in December 2022, it is reasonable to conclude that M1 was evaluated during the height of the insect’s defoliation activity. Conversely, only adult insects were found in M2 (May 2023), indicating that defoliation remained relatively constant, with no significant differences observed. A decrease was noted for all treatments except for SG, where an increase was recorded.
Considering that adults from M1 to M2 continued to feed on foliage, the significant increase in incidence and the non-significant increase in severity in E. globulus could be attributed to the weevil’s known preferences for this species. For the remaining treatments, the decrease in incidence (significant only for CH1) and severity (not significant) can be attributed to the low levels of defoliation, which result from the insect’s reduced preference for this food source, as well as the tree’s ability to re-foliate.
4.2. Nutritional Analysis
According to Caetano-Madeira et al. [38] and Rodríguez & Álvarez [39], the identification of the nutritional status of plants should be conducted by establishing reference values for nutrient concentrations at critical levels or ranges. These values determine whether the foliar content of a nutrient is deficient or sufficient for a given insect, and indicate the threshold at which a reduction in growth rate occurs.
When comparing the nutritional standards presented in Rodríguez & Álvarez [39] for E. nitens, E. globulus and E. grandis with the values obtained in this study, it is evident that the N concentration is adequate in M1 but decreases in M2, becoming deficient (<1.3% N). The P levels are at the threshold of deficiency, with adequate ranges around 0.08% P; both nutrients exhibit values similar to those found in unfertilized plantations. In terms of K, Ca, Mg, and B, the values obtained fall within the typical ranges for Eucalyptus species, as reported by Gonzales-García et al. [40] and Viera et al. [41].
When comparing the nutrient concentrations of E. grandis with the nutritional standards found in the literature, it is observed that the K levels in M1 fall within the deficient range (<0.8%), while those in M2 are classified as adequate [39]. Calcium concentrations are found to be adequate in both samples, ranging from 0.3% to 1.1%. Magnesium levels are adequate in M1 (0.15% to 0.25%) but are close to deficient in M2 (<0.15%). Finally, the B content was adequate during both field visits, falling within the range of 15 to 27 mg/kg.
Regarding the nutritional differences between E. globulus and E. nitens, a study conducted in northeastern Spain that compared the foliar contents of nine eucalyptus species yielded results similar to those of the present work, revealing no significant differences in the concentrations of N, P, and Mg. In terms of K, the results in M1 were consistent with those of the study, showing differences between the species, with E. nitens exhibiting a lower concentration [26]. However, in M2, the concentrations of K were equal between both species. Español et al. [26] reported that E. globulus had a significantly higher foliar concentration of Ca than E. nitens, nevertheless we found no differences in this study.
On the other hand, there is limited information available regarding the nutritional differences that may exist between treatments of eucalyptus, particularly concerning how these differences can influence the preferences of the eucalyptus weevil. Caetano-Madeira et al. [38] investigated the tolerance to dieback in four hybrid genotypes of E. grandis × E. urophylla, discovering that certain nutritional variables exhibited significant differences among the genotypes. This suggests the possibility of genetic variations in the utilization of specific nutrients; however, further research is necessary to support this theory.
It is important to note that when comparing the concentrations of P and K in M1 among SGs, SGr, CH1, and CH2 treatments—excluding SN from the analysis—it was found that SGs and SGr exhibit higher concentrations of these nutrients (Table A1), with significant differences observed when compared to the CH1. Clonal hybrids are generally known to encounter challenges in root development, which can impair nutrient uptake, particularly for phosphorus, due to its low mobility in the soil [42]. This also suggests that the CH2 treatment could be more efficient in nutrient utilization than the CH1 treatment, as CH2 has nutrient concentrations similar to those of SGs and SGr.
4.3. Nutritional Differences Between M1 and M2
Differences in nutrient concentrations between the two sampling periods (December 2022 and May 2023) can be attributed to the seasonality of individual growth, re-translocation, and the mobility or immobility of nutrients within plants.
Regarding the seasonality of growth, despite the opportunistic characteristics of leaf development in eucalyptus, it has been observed that several regions exhibit a bimodal or seasonal growth rhythm throughout the year [43,44]. Generally, seasonal growth in wetter areas tends to occur in late spring and summer, continuing throughout this period as long as soil water is not a limiting factor. In more arid regions, shoot growth follows the late spring-summer pattern, but a secondary growth period can also be observed during autumn and even winter, provided that soil moisture is adequate and daily temperatures remain higher than normal for this time of year [43].
A study conducted in a nearby area [45] determined the optimal growth temperatures for E. globulus, E. nitens, and E. nitens × E. globulus to be 16.7 °C, 15.4 °C, and 15.7 °C, respectively. Statistical analysis in that study revealed no significant differences among these optimal temperatures, indicating a similar thermal requirement for maximum growth across these Eucalyptus species [45]. Based on this information, it can be inferred that the individuals in the study area were able to continue their growth until approximately March 2023. By the time of sampling in May 2023, they had entered a phase of declining growth, resulting in lower nutritional requirements.
Saur et al. [46] identified patterns in nutrient concentrations in southeastern Australia that are similar to the findings of this study. The concentrations of N, P, and K peaked at 1.9%, 0.12%, and 0.7%, respectively, during October and November, subsequently declining to minimum values of 0.9%, 0.05%, and 0.5% in May, with significant differences observed in N and P levels. The authors attribute these patterns to the re-translocation of N and P and their correlation with growth in basal area, as well as the close relationship between the movement of both nutrients. In contrast to the aforementioned study, we observed a significant increase in K from M1 to M2. It is important to note that K levels in M1 were low (<0.8%), which may have been influenced by several factors, including the susceptibility of this nutrient to leaching, as it primarily exists in cells as a free cation and is not integrated into cellular structures [47]. Additionally, K is a nutrient that is easily mobilized and can be readily replenished through the geochemical cycle. According to Rodríguez & Álvarez, adequate potassium nutrition can be achieved even with low levels of exchangeable K, which may be occurring in M2 [39].
Additionally, Saur et al. [46] found that the Ca and Mg concentrations increased progressively, from 0.8 to 1.9 and from 0.2 to 0.25, respectively. The increase in Ca was statistically significant, which aligns with our findings for Ca, although we did not observe a similar trend for Mg. The gradual rise in calcium concentration is expected, as Ca generally exhibits low physiological mobility. Consequently, Ca ions tend to accumulate in the leaves; once deposited in an organ, there is little to no redistribution. This phenomenon occurs primarily because calcium plays a crucial role in tissue formation [48,49,50].
Regarding Mg, similar findings to those presented in this study on seasonal Mg concentrations have been reported by Bell and Ward [49] for E. saligna and E. wandoo in Western Australia. They observed a small peak in Mg levels during early summer, followed by a gradual decline over time. This pattern has also been documented in other genera, such as Olea europaea in eastern Greece, as noted by Stateras and Moustakas [51]. The observed fluctuations in Mg concentrations have been attributed to the ease of leaching and the increased mobility of Mg during rainy periods.
The results indicate a significant increase in the C:N ratio from late spring to autumn. This phenomenon can be attributed to the reduced incorporation and redistribution of nitrogen in the leaves that occurs at the end of their growth phase and the onset of aging. Concurrently, during this period, the joint production of RNA and proteins plays a crucial role in nitrogen regulation; as this activity ceases, nitrogen incorporation decreases and begins to be redistributed outside the leaf. In parallel, photosynthetic carbon fixation increases during leaf growth and subsequently declines. Nevertheless, the conversion of carbon into sugars begins early in leaf ontogeny and continues even when protein synthesis decreases during leaf senescence [52]. The results of the leaf analyses reflect this trend, showing a decrease in both macroelements (C and N) and an increase in the C:N ratio. Zhang et al. further develop this theory, positing that the nutritional and reproductive growth of a plant is governed by the ratio of carbohydrates to nitrogenous compounds, with a lower C:N ratio favoring nutritive growth [53].
4.4. Principal Component Analysis
Principal component analysis revealed a significant correlation between calcium concentration and defoliation parameters (incidence and severity) in M1. It has been suggested that this relationship may be mediated by the formation of calcium oxalate (CaOx) crystals in the leaves of Eucalyptus. Several studies have indicated that these crystals serve various functions in plants, including providing physical defense against herbivory by causing irritation and mechanical damage when ingested by insects [23,54,55]. Furthermore, Migacz et al. [22] reported the presence of CaOx crystals in six Eucalyptus species, including E. globulus, suggesting that these crystals may also be present in the analyzed genotypes.
It is important to note that, as previously mentioned, in M1, the herbivory activity of insects was higher, characterized by increased severity of attacks and the presence of all insect life stages. However, in May, when insect activity decreased, resulting in lower attack severity and only the presence of adult insects, no correlation was observed between calcium levels and defoliation parameters. This suggests that the nutritional status of the foliage may differentially affect the larvae and adults of G. platensis.
Consequently, the future identification and quantification of these crystals in the genotypes could confirm this hypothesis and provide a better understanding of the resistance mechanisms. Ultimately, this could assist in the selection of more resistant varieties or in proposing different fertilization strategies aimed at enhancing tree resistance to herbivory.
Although no significant correlations were found between defoliation parameters and nutritional concentrations, there is evidence that nutritional quality influences herbivorous insects. The literature indicates that, in general, an increase in the C:N ratio decreases plant palatability [56]. Our results support this trend, as shown in in sampling 1 (M1), where the C:N ratio was lower compared to M2, demonstrating a greater degree of overall defoliation.
5. Conclusions
This study provides the first field-based assessment in Chile examining the relationship between foliar nutrient status and herbivory caused by Gonipterus platensis across different Eucalyptus genotypes, thereby contributing novel insights into the nutritional and genetic factors underlying host susceptibility. Despite overall low defoliation levels under natural conditions, consistent differences among genotypes were detected, indicating that nutritional traits may partially explain the observed variation in herbivory intensity.
The inclusion of interspecific hybrids proved particularly relevant, as they exhibited intermediate susceptibility to G. platensis, confirming their hybrid behavior. These genotypes, which combine the superior pulping qualities of E. globulus with the frost tolerance of E. nitens, also demonstrated enhanced tolerance to the weevil. Such findings highlight their potential value in integrated pest management and breeding programs aimed at improving Eucalyptus productivity and resilience under Chilean conditions.
Overall, the results emphasize the importance of considering both nutritional and genetic factors when developing sustainable management strategies against Gonipterus in commercial Eucalyptus plantations, and they establish a baseline for future studies linking plant nutrition, genotype performance, and insect herbivory in South American forestry systems.
Author Contributions
Conceptualization, C.R., R.R., P.M.-S., C.B., M.P. and R.L.-F.; methodology, C.R., M.P., R.R. and P.M.-S.; formal analysis, R.L.-F. and P.M.-S.; investigation, C.R., R.R. and P.M.-S.; writing—original draft preparation, C.R., R.R., P.M.-S., C.B., M.P. and R.L.-F.; writing—review and editing, P.M.-S. and R.L.-F.; visualization, R.L.-F.; supervision, P.M.-S.; project administration, P.M.-S.; funding acquisition, P.M.-S. and R.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Chilean National Commission for Scientific and Technological Research with Project Grand ANID BASAL FB210015.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The financial support mentioned in the Funding part is gratefully acknowledged.
Conflicts of Interest
Author Matías Pincheira was employed by the company CMPC Forestal. The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A

Table A1.
Comparison of foliar concentration in percent of nitrogen (N), phosphorous (P) and potassium (K) on Eucalypts treatments SGr, SGs, CH1 and CH2 on M1 (December 2022).
Table A1.
Comparison of foliar concentration in percent of nitrogen (N), phosphorous (P) and potassium (K) on Eucalypts treatments SGr, SGs, CH1 and CH2 on M1 (December 2022).
| Treatment/Nutrient | N | P | K |
|---|---|---|---|
| SGr | 1.73 a | 0.0875 ab | 0.700 a |
| SGs | 1.74 a | 0.1000 a | 0.835 a |
| CH1 | 1.53 a | 0.0625 c | 0.440 b |
| CH2 | 1.63 a | 0.0825 b | 0.610 ab |
Different letters indicate significant differences at a 0.05 level of significance between genotypes using the Tukey test. Numbers indicate means and their standard deviations. CH1: Hybrid clone 1 (E. nitens × E. globulus); CH2: Hybrid clone 2 (E. nitens × E. globulus); SGs: E. globulus seed susceptible genotypes; SGr: E. globulus seed resistant genotypes, based on direct observation of the most affected and less affected trees, respectively.
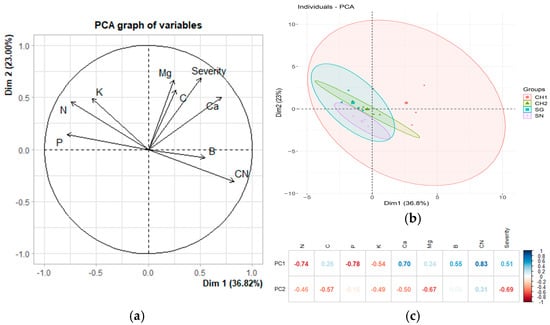
Figure A1.
The relationship between severity and nutritional variables in M1 (December 2022). (a) The original variables are plotted in the principal component space (Dim 1 and Dim 2). The direction and length of the arrows indicate the contribution of each variable to the two principal components displayed in the graph. In (b), each point represents an individual observation within the dataset. The ellipses denote the confidence regions for the groups (treatments) in the dataset, illustrating the dispersion and clustering of points associated with each treatment. (c) A heatmap displaying the loadings of the variables in the first two principal components of a Principal Component Analysis (PCA). The loadings reflect the degree and direction of the correlation between the original variables and the principal components.

Table A2.
Correlations between defoliation severity and nutrient concentration in M1.
Table A2.
Correlations between defoliation severity and nutrient concentration in M1.
| Variable | Nutrient | rho | p-Value |
|---|---|---|---|
| Severity | Ca | 0.618 | 0.0107 |
| Mg | 0.181 | 0.503 | |
| C | 0.115 | 0.6714 |
The Spearman correlation, which is applicable for nonparametric data, between severity and nutritional values in M1 are presented. The correlation coefficient (rho) and the significance level of the correlation (alpha = 0.05), with the null hypothesis stating that rho equals 0, are also included.
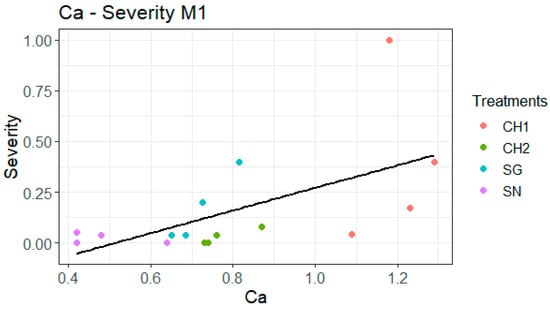
Figure A2.
Linear regression analysis between calcium concentration and severity showing the original variables in M1. The analysis is based on the transformed variable (log(Severity + 1)) (p = 0.01126, R2 = 0.378).
References
- Campos, J.V.; Riquelme, S.; Pecio, Ł.; Guedes, L.; Mardones, C.; Alzamora, R.; Arteaga-Pérez, L.E.; Rubilar, R.; Fiehn, O.; Pérez, A.J. Constitutive and inducible defense in Eucalyptus determines the feeding host of Gonipterus platensis, denoting specific plant-insect coevolution and a strategy for resistance improvement. Ind. Crops Prod. 2022, 189, 115811. [Google Scholar] [CrossRef]
- Poblete Hernández, P.; Gysling Caselli, J.; Alvarez González, V.; Bañados, M.; Carlos, J.; Kahler González, C.; Aguirre, D.S.; Baeza Rocha, D. Anuario Forestal 2023; Instituto Forestal: Santiago, Chile, 2023. [Google Scholar]
- Volker, P.W.; Potts, B.M.; Borralho, N.M. Genetic parameters of intra-and inter-specific hybrids of Eucalyptus globulus and E. nitens. Tree Genet. Genomes 2008, 4, 445–460. [Google Scholar] [CrossRef]
- Gonçalves, C.I.; Vilas-Boas, L.; Branco, M.; Rezende, G.D.; Valente, C. Host susceptibility to Gonipterus platensis (Coleoptera: Curculionidae) of Eucalyptus species. Ann. For. Sci. 2019, 76, 63. [Google Scholar] [CrossRef]
- Ayuga-Téllez, E.; García-Iruela, A.; Rielo, J.C.; González-García, C. Actions for Monitoring the Gonipterus Pest in Eucalyptus on the Cantabrian Coast. Agronomy 2022, 12, 1692. [Google Scholar] [CrossRef]
- Mapondera, T.S.; Burgess, T.; Matsuki, M.; Oberprieler, R.G. Identification and molecular phylogenetics of the cryptic species of the Gonipterus scutellatus complex (Coleoptera: Curculionidae: Gonipterini). Aust. J. Entomol. 2012, 51, 175–188. [Google Scholar] [CrossRef]
- Branco, S.; Mateus, E.P.; da Silva, M.D.G.; Mendes, D.; Rocha, S.; Mendel, Z.; Schütz, S.; Paiva, M.R. Electrophysiological and behavioral responses of the Eucalyptus weevil, Gonipterus platensis, to host plant volatiles. J. Pest Sci. 2019, 92, 221–235. [Google Scholar] [CrossRef]
- Echeverri-Molina, D.; Santolamazza-Carbone, S. Toxicity of synthetic and biological insecticides against adults of the Eucalyptus snout-beetle Gonipterus scutellatus Gyllenhal (Coleoptera: Curculionidae). J. Pest Sci. 2010, 83, 297–305. [Google Scholar] [CrossRef]
- Reis, A.R.; Ferreira, L.; Tomé, M.; Araujo, C.; Branco, M. Efficiency of biological control of Gonipterus platensis (Coleoptera: Curculionidae) by Anaphes nitens (Hymenoptera: Mymaridae) in cold areas of the Iberian Peninsula: Implications for defoliation and wood production in Eucalyptus globulus. For. Ecol. Manag. 2012, 270, 216–222. [Google Scholar] [CrossRef]
- Alvarado, A.; Sartori, A.; CONAF. Gonipterus scutellatus Gyllenhal (Coleoptera: Curculionidae), «Gorgojo del Eucalipto». Nota técnica año 24, n° 47. 2006. Available online: https://bibliotecadigital.ciren.cl/handle/20.500.13082/147597 (accessed on 10 October 2022).
- Valente, C.; Gonçalves, C.I.; Reis, A.; Branco, M. Pre-selection and biological potential of the egg parasitoid Anaphes inexpectatus for the control of the Eucalyptus snout beetle, Gonipterus platensis. J. Pest Sci. 2017, 90, 911–923. [Google Scholar] [CrossRef]
- Wallis, I.R.; Keszei, A.; Henery, M.L.; Moran, G.F.; Forrester, R.; Maintz, J.; Marsh, K.J.; Andrew, R.L.; Foley, W.J. A Chemical perspective on the evolution of variation in Eucalyptus globulus. Perspect. Plant Ecol. Evol. Syst. 2011, 13, 305–318. [Google Scholar] [CrossRef]
- Henery, M.; Henson, M.; Wallis, I.; Stone, C.; Foley, W. Predicting crown damage to Eucalyptus grandis by Paropsis atomaria with direct and indirect measures of leaf composition. For. Ecol. Manag. 2008, 255, 3642–3651. [Google Scholar] [CrossRef]
- Centella, C.; Jerez, V.; Gonzalez, U.; Bittner, M. Especialización en el uso de hospederos de Dictyneis asperatus (Blanchard 1851) en un fragmento de vegetación esclerófila-higrófila en la Península de Hualpén, Chile. Rev. Chil. De Hist. Nat. 2003, 76, 391–400. [Google Scholar] [CrossRef]
- Awmack, C.S.; Leather, S.R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef]
- Vergara, O.; Jerez, V. Insectos e infestaciones asociadas al follaje de Nothofagus antarctica (Forst) Oerst (Nothofagaceae) en la cuenca del río Baker, Región de Aysén, Chile. Gayana 2010, 74, 83–93. [Google Scholar] [CrossRef]
- Riquelme Virgala, M.; Di Silvestro, G.; Martínez, C.; Santadino, M.; Poretti, T.; Ansa, A.; Coviella, C. Consumo larval y preferencia de oviposición de Gonipterus pulverulentus (Coleoptera: Curculionidae) asociados a distintas especies de Eucalyptus (Myrtaceae). Bosque 2018, 39, 291–297. [Google Scholar] [CrossRef]
- Ohmart, C.P.; Edwards, P.B. Insect herbivory on Eucalyptus. Annu. Rev. Èntomol. 1991, 36, 637–657. [Google Scholar] [CrossRef]
- Fox, L.R.; Macauley, B.J. Insect grazing on Eucalyptus in response to variation in leaf tannins and nitrogen. Oecologia 1977, 29, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Ohmart, C.P.; Stewart, L.G.; Thomas, J.R. Effects of food quality, particularly nitrogen concentrations, of Eucalyptus blakelyi foliage on the growth of Paropsis atomaria larvae (Coleoptera: Chrysomelidae). Oecologia 1985, 65, 543–549. [Google Scholar] [CrossRef]
- Schoonhoven, L.M.; Van Loon, J.J.; Dicke, M. Insect-Plant Biology; Oxford University Press on Demand: Oxford, UK, 2005. [Google Scholar]
- Migacz, I.P.; Raeski, P.A.; de Almeida, V.P.; Raman, V.; Nisgoski, S.; de Muniz, G.I.; Farago, P.V.; Khan, I.A.; Budel, J.M. Comparative leaf morpho-anatomy of six species of Eucalyptus cultivated in Brazil. Rev. Bras. Farmacogn. 2018, 28, 273–281. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Nakata, P.A. Calcium oxalate in plants: Formation and function. Annu. Rev. Plant Biol. 2005, 56, 41–71. [Google Scholar] [CrossRef] [PubMed]
- Molano-Flores, B. Herbivory and calcium concentrations affect calcium oxalate crystal formation in leaves of Sida (Malvaceae). Ann. Bot. 2001, 88, 387–391. [Google Scholar] [CrossRef]
- Jáuregui-Zuñiga, D.A.; Moreno, A. La biomineralización del oxalato de calcio en plantas: Retos y potencial. REB 2004, 23, 18–23. [Google Scholar]
- Español, E.; Zas Arregui, R.; Vega, G. Contenidos Foliares en Macro y Micronutrientes en Nueve Especies de Eucalyptus en el Noroeste español; Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (INIA): Madrid, Spain, 2000. [Google Scholar]
- Judd, T.S. Nutrient Concentrations in Eucalyptus: A Synthesis in Relation to Differences Between Taxa, Sites and Components; Nutrition of Eucalyptus; CSIRO Publishing: Clayton, Australia, 1996. [Google Scholar]
- SitRural. (s.f.). SitRural. Obtenido de CIREN. Available online: https://www.sitrural.cl/ (accessed on 20 November 2022).
- Stolpe, N.B. Descripciones de Los Principales Suelos de la VIII Región de Chile; Universidad de Concepción: Concepción, Chile, 2006. [Google Scholar]
- Cooperativa de Productividad Forestal. (s.f.). Protocolo de muestreo de follaje para estudios PFC (Eucaliptus sp.). Available online: https://www.forestproductivity.org/ (accessed on 3 November 2022).
- Bonomelli, C.; Suarez, D. Eucalyptus fertilization. N, P and K accumulation. Ciencia Inv. Agr. 1999, 26, 11–20. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cordero Rivera, A.; Santolamazza Carbone, S. The effect of three species of Eucalyptus on growth and fecundity of the Eucalyptus snout beetle (Gonipterus scutellatus). Forestry 2000, 73, 21–29. [Google Scholar] [CrossRef]
- Lanfranco, D.; Dungey, H.S. Insect damage in Eucalyptus: A review of plantations in Chile. Austral Ecol. 2001, 26, 477–481. [Google Scholar] [CrossRef]
- Pincheira, M. (CMPC, Santiago, Chile). Personal communication, 2024.
- Campos Rivera, J.V. Incidencia del Estrés Hídrico Sobre la Biosíntesis de Metabolitos Secundarios y la Predisposición a la Defoliación: Un Análisis Metabolómico en Especies Forestales de Interés Comercial Para Chile. Ph.D. Thesis, Universidad de Concepción, Concepción, Chile, 2023. [Google Scholar]
- Baldini, A. GONIPTERUS PLATENSIS: Biología, Daños, Control, Efecto Sobre Eucalyptus globulus, y su Impacto Económico Sobre la Industria Forestal. 2020. Available online: https://www.researchgate.net/publication/343599142_GONIPTERUS_PLATENSIS_Biologia_danos_control_efecto_sobre_Eucalyptus_globulus_y_su_impacto_economico_sobre_la_Industria_Forestal/ (accessed on 2 December 2022).
- Caetano-Madeira, D.D.; Omena-Garcia, R.P.; Elerati, T.L.; da Silva Lopes, C.B.; Corrêa, T.R.; de Souza, G.A.; Oliveira, L.A.; Cruz, C.D.; Bhering, L.L.; Nunes-Nesi, A.; et al. Metabolic, Nutritional and Morphophysiological Behavior of Eucalypt Genotypes Differing in Dieback Resistance in Field When Submitted to PEG-Induced Water Deficit. Agronomy 2023, 13, 1261. [Google Scholar] [CrossRef]
- Rodríguez, J.; Álvarez, J. Nutrición y Fertilización de las Plantaciones Forestales; Forestal Mininco SA: Santiago, Chile, 2010. [Google Scholar]
- Gonzalez-Garcia, M.; Hevia, A.; Majada, J.; Rubiera, F.; Barrio-Anta, M. Nutritional, carbon and energy evaluation of Eucalyptus nitens short rotation bioenergy plantations in northwestern Spain. Iforest-Biogeosciences For. 2015, 9, 303. [Google Scholar] [CrossRef]
- Márcio, V.; Fernandez, F.R.; Rodríguez-Soalleiro, R. Nutritional prescriptions for Eucalyptus plantations: Lessons learned from Spain. Forests 2016, 7, 84. [Google Scholar] [CrossRef]
- de Assis, T.F.; Rodríguez, F. La propagación vegetativa de eucaliptos en Chile. In Mejoramiento genético de eucaliptos en Chile; Instituto Forestal: Santiago, Chile, 2014; pp. 137–148. Available online: https://bibliotecadigital.infor.cl/bitstream/20.500.12220/20506/1/31039.pdf/ (accessed on 2 February 2023).
- Specht, R.L.; Brouwer, Y.M. Seasonal shoot growth of Eucalyptus spp. in the Brisbane area of Queensland (with notes on shoot growth and litter fall in other areas of Australia). Aust. J. Bot. 1975, 23, 459–474. [Google Scholar] [CrossRef]
- Millner, J.P.; Kemp, P.D. Seasonal growth of Eucalyptus species in New Zealand hill country. New For. 2012, 43, 31–44. [Google Scholar]
- Watt, M.S.; Rubilar, R.; Kimberley, M.O.; Kriticos, D.J.; Emhart, V.; Mardones, O.; Acevedo, M.; Pincheira, M.; Stape, J.; Fox, T. Using seasonal measurements to inform ecophysiology: Extracting cardinal growth temperatures for process-based growth models of five Eucalyptus species/crosses from simple field trials. New Zealand J. For. Sci. 2014, 44, 9. [Google Scholar] [CrossRef]
- Saur, E.; Nambiar, E.K.S.; Fife, D.N. Foliar nutrient retranslocation in Eucalyptus globulus. Tree Physiol. 2000, 20, 1105–1112. [Google Scholar] [CrossRef]
- Paredes Grieve, J. Lixiviación artificial de potasio en plantas de cacao y su relación con antracnosis foliar (Colletotrichum gloeosporoides penz). Master’s Thesis, Instituto Interamericano de Ciencias Agrícolas de la OEA, Turrialba, Costa Rica, 1967. [Google Scholar]
- Hanger, B.C. The movement of calcium in plants. Commun. Soil Sci. Plant Anal. 1979, 10, 171–193. [Google Scholar] [CrossRef]
- Bell, D.T.; Ward, S.C. Seasonal changes in foliar macronutrients (N, P, K, Ca and Mg) in Eucalyptus saligna Sm. and E. wandoo Blakely growing in rehabilitated bauxite mine soils of the Darling Range, Western Australia. Plant Soil 1984, 81, 377–388. [Google Scholar] [CrossRef]
- Gómez, V.B. El calcio y su asimilación por parte de las plantas. Cannabis Mag. La Rev. Los Prof. Y Amantes Del Cáñamo 2014, 125, 58–63. [Google Scholar]
- Stateras, D.C.; Moustakas, N.K. Seasonal changes of macro-and micro-nutrients concentration in olive leaves. J. Plant Nutr. 2018, 41, 186–196. [Google Scholar] [CrossRef]
- Altamira, S.P.; Trippi, V.S. Las relaciones C/N y PS/N durante el crecimiento y la senescencia de hojas de Phaseolus vulgaris L. AgriScientia 1991, 8, 3–7. [Google Scholar] [CrossRef]
- Zhang, J.; He, N.; Liu, C.; Xu, L.; Chen, Z.; Li, Y.; Wang, R.; Yu, G.; Sun, W.; Xiao, C.; et al. Variation and evolution of C: N ratio among different organs enable plants to adapt to N-limited environments. Glob. Change Biol. 2020, 26, 2534–2543. [Google Scholar] [CrossRef]
- Lawrie, N.S.; Cuetos, N.M.; Sini, F.; Salam, G.A.; Ding, H.; Vancolen, A.; Nelson, J.M.; Erkens, R.H.J.; Perversi, G. Systematic review on raphide morphotype calcium oxalate crystals in angiosperms. AoB Plants 2023, 15, plad031. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Pandith, S.A.; Shah, M.A.; Reshi, Z.A. Calcium Oxalate Crystals, the Plant ‘Gemstones’: Insights into Their Synthesis and Physiological Implications in Plants. Plant Cell Physiol. 2023, 64, 1124–1138. [Google Scholar] [CrossRef] [PubMed]
- Schädler, M.; Jung, G.; Auge, H.; Brandl, R. Palatability, decomposition and insect herbivory: Patterns in a successional old-field plant community. Oikos 2003, 103, 121–132. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).