A Novel System for the Characterization of Bark Macroscopic Morphology for Central European Woody Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Bark Guide Collection
2.2. Development of Identification Characters
2.3. Data Collection for Diagnostic Characters
2.4. Creating Identification Keys
3. Results
3.1. Bark Identification Bibliography
| Book | Identification Keys | Mature/Juvenile Bark Distinction | Scale Indication | Geographic Range | Note |
|---|---|---|---|---|---|
| Baraton A. Le monde des écorces. Editions du Rouergue: Parc Saint-Joseph, France, 2003 [41]. | × | × | × | Central Europe | Native and exotic woody species of horticultural importance |
| Godet, J.-D. Baumrinden. Eugen Ulmer KG: Stuttgart, Germany, 2011 [36]. | × | Somewhere | × | Europe | Infraspecific diversity of bark morphology |
| Mikolas, M. A Beginner’s Guide to Recognizing Trees of the Northeast. The Countryman Press: New York, USA, 2017 [40]. | × | × | × | Northeastern United States | |
| Spohn, M.; Spohn, R. Die Rinden unserer Bäume. Quelle & Meyer Verlag: Wiebelsheim, Germany, 2020 [32]. | ✓ | ✓ | ✓ | Central Europe | The most comprehensive book from Europe |
| Schwankl, A. Guide to Bark. Thames and Hudson: London, UK, 1956 [70]. | × | Somewhere | Indirect (DBH *) | Central Europe | Unique bark formations |
| Vaucher, H. Bäume. Prisma Verlag: Gütersloh, Germany, 1987 [71]. | × | × | × | Europe | |
| Vaucher, H. Baumrinden. Naturbuch-Verlag: Augsburg, Germany, 1997 [37]. | × | × | × | Europe and North America | |
| Vaucher, H. Tree Bark. Timber Press: Portland, Oregon, 2010 [72]. | × | somewhere | × | Worldwide | |
| Wojtech, M. Bark. Brandeis University Press: Waltham, Massachusetts, 2020 [73]. | ✓ | ✓ | ✓ | Northeastern United States | The most comprehensive book from North America |
3.2. Identification Characters
3.2.1. Bark Types
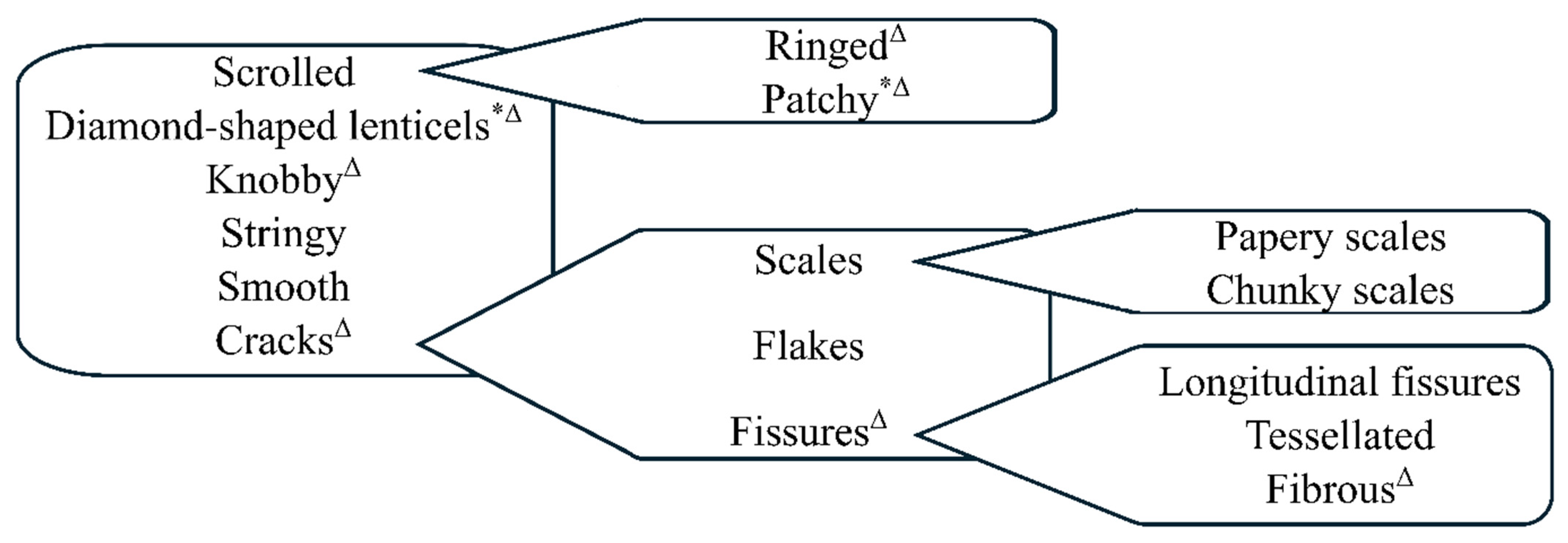
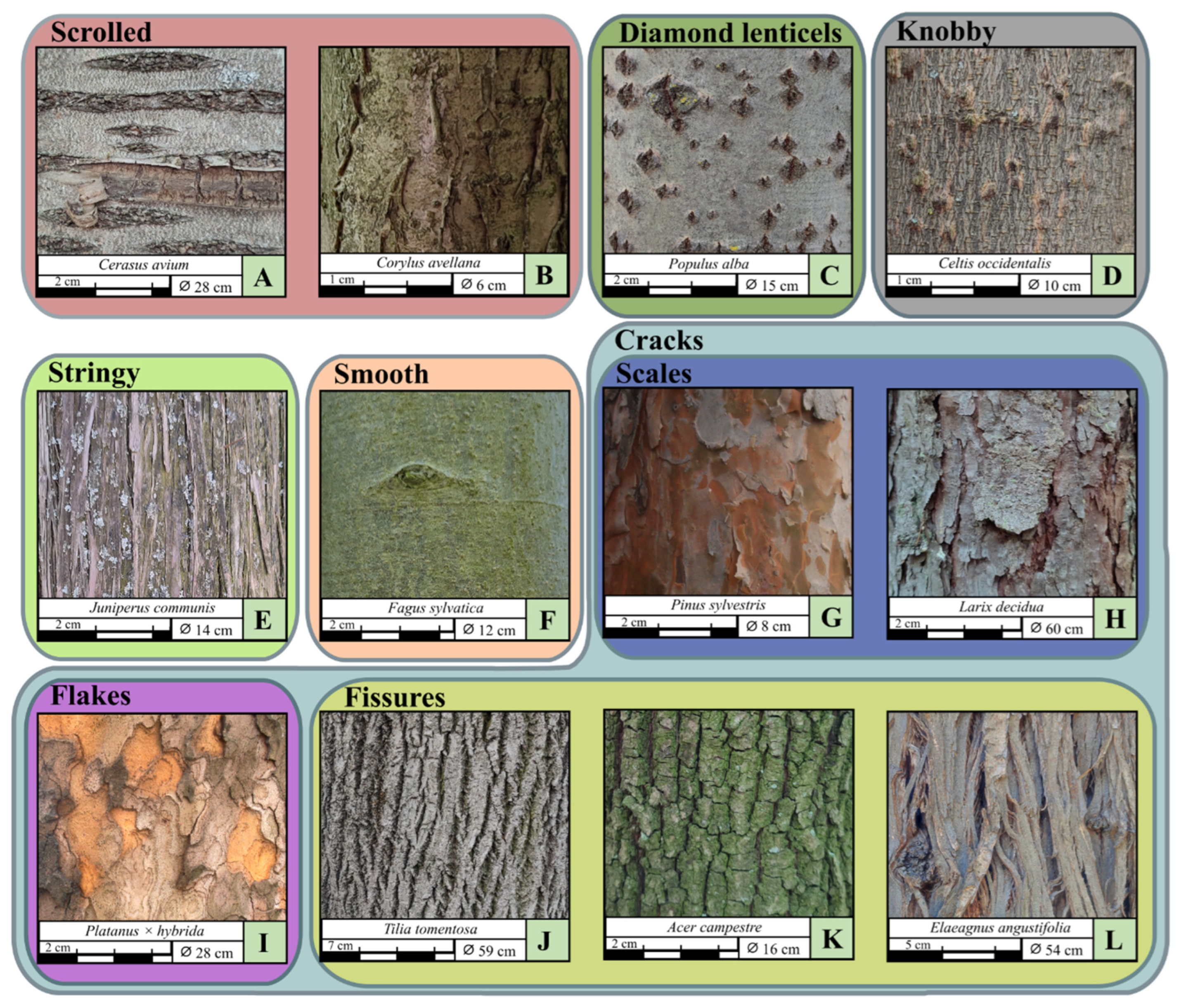
3.2.2. Bark Characters and Attributes
3.3. Characterization of Example Species
3.4. Construction of Identification Keys
4. Discussion
4.1. Bark Traits
4.2. Implementation
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Payette, S.; Delwaide, A.; Simard, M. Frost-ring chronologies as dendroclimatic proxies of boreal environments. Geophys. Res. Lett. 2010, 37, 1–6. [Google Scholar] [CrossRef]
- Rosner, S.; Morris, H. Breathing life into trees: The physiological and biomechanical functions of lenticels. IAWA J. 2022, 34, 234–262. [Google Scholar] [CrossRef]
- Roth, I. Structural Patterns of Tropical Barks; Borntraeger: Berlin, Germany, 1981. [Google Scholar]
- Nicolai, V.V. The ecological significances of trees’ bark during ecosystem dynamics. Spixiana 1995, 18, 187–199. [Google Scholar]
- Pausas, J.G. Bark thickness and fire regime. Funct. Ecol. 2015, 29, 315–327. [Google Scholar] [CrossRef]
- Rosell, J.A. Bark in Woody Plants: Understanding the Diversity of a Multifunctional Structure. Integr. Comp. Biol. 2019, 59, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, V.R.; Krokene, P.; Christiansen, E.; Krekling, T. Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol. 2005, 167, 353–376. [Google Scholar] [CrossRef] [PubMed]
- Morris, H.; Jansen, S. Bark: Its anatomy, function and diversity. In International Dendrology Society Yearbook; Anon, Ed.; International Dendrology Society: Kington, UK, 2016; pp. 51–61. [Google Scholar]
- Lev-Yadun, S. Why is the bark of common temperate Betula and Populus species white? Int. J. Plant Sci. 2019, 180, 632–642. [Google Scholar] [CrossRef]
- Paine, C.E.T.; Stahl, C.; Courtois, E.A.; Patiño, S.; Sarmiento, C.; Baraloto, C. Functional explanations for variation in bark thickness in tropical rain forest trees. Funct. Ecol. 2010, 24, 1202–1210. [Google Scholar] [CrossRef]
- Rosell, J.A.; Gleason, S.; Méndez-Alonzo, R.; Chang, Y.; Westoby, M. Bark functional ecology: Evidence for tradeoffs, functional coordination, and environment producing bark diversity. New Phytol. 2014, 201, 486–497. [Google Scholar] [CrossRef]
- Kaufert, F. Factors Influencing the Formation of Periderm in Aspen. Am. J. Bot. 1937, 24, 24–30. [Google Scholar] [CrossRef]
- Langenfeld-Heyser, R.; Fiebelkorn, G.; Polle, A. Können Stichverletzungen oder Frühfrostereignisse bei Fagus sylvatica L. Rauborkigkeit und Holzstrahlproliferationen hervorrufen. In Strategien zur Sicherung von Buchenwäldern, 1st ed.; Petercord, R., Block, J., Eds.; Forschungsanstalt für Waldökologie und Forstwirtschaft Rheinland-Pfalz: Trippstadt, Germany, 2006; pp. 95–110. [Google Scholar]
- Ross, W.D.; Krahmer, R.L. Some sources of variation in structural characteristics of Douglas-fir bark. Wood Fiber 1971, 3, 35–46. [Google Scholar]
- Angyalossy, V.; Pace, M.R.; Evert, R.F.; Marcati, C.R.; Oskolski, A.A.; Terrazas, T.; Kotina, E.; Lens, F.; Mazzoni-Viveiros, S.C.; Angeles, G.; et al. IAWA List of Microscopic Bark Features. IAWA J. 2016, 37, 517–615. [Google Scholar] [CrossRef]
- Shtein, I.; Gričar, J.; Lev-Yadun, S.; Oskolski, A.; Pace, M.R.; Rosell, J.A.; Crivellaro, A. Priorities for Bark Anatomical Research: Study Venues and Open Questions. Plants 2023, 12, 1985. [Google Scholar] [CrossRef] [PubMed]
- Junikka, L. Survey of English macroscopic bark terminology. IAWA J. 1994, 15, 3–45. [Google Scholar] [CrossRef]
- Scott, A.W.; Hallam, C.J. Assessing Species Misidentification Rates through Quality Assurance of Vegetation Monitoring. Plant Ecol. 2002, 165, 101–115. [Google Scholar]
- Juhász, E.; Katona, K.; Molnár, Z.; Hahn, I.; Biró, M. A Reintroduced Ecosystem Engineer Species May Exacerbate Ongoing Biological Invasion: Selective Foraging of the Eurasian Beaver in Floodplains. Glob. Ecol. Conserv. 2020, 24, e01383. [Google Scholar] [CrossRef]
- Dexter, K.G.; Pennington, T.D.; Cunningham, C.W. Using DNA to Assess Errors in Tropical Tree Identifications: How Often Are Ecologists Wrong and When Does It Matter? Ecol. Monogr. 2010, 80, 267–286. [Google Scholar] [CrossRef]
- de Almeida, R.F.; Pellegrini, M.O.O.; de Morais, I.L.; Simão-Bianchini, R.; Rattanakrajang, P.; Cheek, M.; Simões, A.R.G. Barking up the Wrong Tree: The Dangers of Taxonomic Misidentification in Molecular Phylogenetic Studies. Plant Ecol. Evol. 2023, 156, 146–159. [Google Scholar] [CrossRef]
- Knott, J.; Wang, J.; Walker, D.; Domke, G.; Fei, S. Robustness of Ecological Indicators to Species Misidentification in the National Forest Inventory of the United States. Ecosphere 2025, 16, e70340. [Google Scholar] [CrossRef]
- Fitter, R.S.R.; Fitter, A.H.; Farrer, A. Collins Guide to the Grasses, Sedges, Rushes and Ferns of Britain and Northern Europe; HarperCollins: London, UK, 1984. [Google Scholar]
- Trockenbrodt, M. Survey and discussion of the terminology used in bark anatomy. IAWA Bull. 1990, 11, 141–166. [Google Scholar] [CrossRef]
- Bartha, D. Fa- és Cserjehatározó; Mezőgazda Kiadó: Budapest, Hungary, 1997. [Google Scholar]
- Bartha, D. Magyarország fa- és Cserjefajai; Mezőgazda Kiadó: Budapest, Hungary, 2018. [Google Scholar]
- Nagy, C. Erdészeti Növénytan; FVM Képzési és Szaktanácsadási Intézet: Budapest, Hungary, 2009. [Google Scholar]
- Schweingruber, F.H.; Steiger, P.; Börner, A. Bark Anatomy of Trees and Shrubs in the Temperate Northern Hemisphere; Springer: Berlin, Germany, 2019. [Google Scholar]
- Soó, R. A Magyar Flóra és Vegetáció Rendszertani-Növényföldrajzi Kézikönyve I; Akadémiai Kiadó: Budapest, Hungary, 1964. [Google Scholar]
- Soó, R. A Magyar Flóra és Vegetáció Rendszertani-Növényföldrajzi Kézikönyve II; Akadémiai Kiadó: Budapest, Hungary, 1966. [Google Scholar]
- Soó, R. A Magyar Flóra és Vegetáció Rendszertani-Növényföldrajzi Kézikönyve IV; Akadémiai Kiadó: Budapest, Hungary, 1970. [Google Scholar]
- Spohn, M.; Spohn, R. Die Rinden Unserer Bäume: Die 70 häufigsten Arten entdecken, Bestimmen und Verstehen; Quelle + Meyer Verlag: Wiebelsheim, Germany, 2020. [Google Scholar]
- Blakeslee, A.F.; Jarvis, C.D. Trees in Winter: Their Study, Planting, Care and Identification; The Macmillan Company: New York, NY, USA, 1913. [Google Scholar]
- Fekete, L.; Mágocsy-Dietz, S. Erdészeti Növénytan II; Erdészeti Egyesület: Budapest, Hungary, 1896. [Google Scholar]
- Gencsi, L.; Vancsura, R. Dendrológia. Erdészeti Növénytan II; Mezőgazda Kiadó: Budapest, Hungary, 1992. [Google Scholar]
- Godet, J.-D. Baumrinden—Vergleichen und Bestimmen; Eugen Ulmer: Stuttgart, Germany, 2011. [Google Scholar]
- Vaucher, H. Baumrinden. Aussehen, Struktur, Funktion, Eigenschaften; Naturbuch Verlag: Augsburg, Germany, 2000. [Google Scholar]
- Idžojtić, M. Listopadno Drvece i Grmlje u Zimskom Razdoblju; Šumarski Fakultet: Zagreb, Croatia, 2005. [Google Scholar]
- Johnson, O. Európa Fái; HarperCollins Publishers Ltd.: London, UK, 2007. [Google Scholar]
- Mikolas, M. A Beginner’s Guide to Recognizing Trees of the Northeast; Countryman Press: Woodstock, VT, USA, 2017. [Google Scholar]
- Baraton, A. Le Monde des Écorces; Editions du Rouergue: Rodez, France, 2003. [Google Scholar]
- Bartha, D. Magyarország Ritka fa- és Cserjefajainak Atlasza; Kossuth Kiadó: Budapest, Hungary, 2012. [Google Scholar]
- Kiss, L. Fenyők. Fák és Cserjék 1.; Mezőgazda Kiadó: Budapest, Hungary, 1965. [Google Scholar]
- Krüssmann, G. Handbuch der Nadelgehölze; Paul Parey: Berlin, Germany, 1972. [Google Scholar]
- Majer, A. A Bakony Tiszafása; Akadémiai Kiadó: Budapest, Hungary, 1980. [Google Scholar]
- Mihály, B.; Botta-Dukát, Z. Biológiai Inváziók Magyarországon. Özönnövények I; TermészetBÚVÁR Alapítvány Kiadó: Budapest, Hungary, 2004. [Google Scholar]
- Price, D.; Bersweden, L. Winter Trees—A Photographic Guide to Common Trees and Shrubs; Field Studies Council Publications: Shrewsbury, UK, 2013. [Google Scholar]
- Schmidt, G. Rügyhatározó; Mezőgazda Kiadó: Budapest, Hungary, 2003. [Google Scholar]
- Vancsura, R. Lombos Fák és Cserjék. Fák és Cserjék 2; Mezőgazda Kiadó: Budapest, Hungary, 1960. [Google Scholar]
- Bokor, R. A Magyar Erdőkben (és Nyilvános Parkokban) Honos és Fontosabb Honosított Fás Növények Téli Állapotban való Határozója (Rügyhatározó); Röttig-Romwalter Nyomda Részvénytársaság: Budapest, Hungary, 1932. [Google Scholar]
- Caudullo, G.; De Rigo, D.; Mauri, A.; Houston Durrant, T.; San-Miguel-Ayanz, J. European Atlas of Forest Tree Species; European Commission: Luxembourg, 2011. [Google Scholar]
- Csapody, I.; Csapody, V.; Rott, F. Erdei Fák és Cserjék; Országos Erdészeti Főigazgatóság–Mezőgazdasági Könyv- és Folyóiratkiadó Vállalat: Budapest, Hungary, 1966. [Google Scholar]
- Csiszár, Á. Inváziós Növényfajok Magyarországon; Nyugat-Magyarországi Egyetem: Sopron, Hungary, 2012. [Google Scholar]
- Debreczy, I.; Rácz, Z. Conifers Around the World: Conifers of the Temperate Zones and Adjacent Regions I–II; DendroPress: Budapest, Hungary, 2012. [Google Scholar]
- Farjon, A.; Baas, P. A Handbook of the World’s Conifers; Brill Academic Publishers: Leiden, The Netherlands, 2010. [Google Scholar]
- Jagel, A.; Dörken, V.M. Weihnachtsgrün und Friedhofskoniferen—Bestimmung immergrüner Nadelbäume ohne Zapfen. Jahrb. Bochumer Bot. Vereins 2013, 4, 280–307. [Google Scholar]
- Keresztesi, B. A Tölgyek; Akadémiai Kiadó: Budapest, Hungary, 1967. [Google Scholar]
- Caccianiga, M.; Compostella, C.; Caccia, G.; Cattaneo, C. Contribution of plant anatomy to forensic investigation: Tree bark morphology. Forensic Sci. Int. 2021, 318, 110598. [Google Scholar] [CrossRef]
- Yunus, M.; Yunus, D.; Iqbal, M. Systematic bark morphology of some tropical trees. Bot. J. Linn. Soc. 1990, 103, 367–377. [Google Scholar] [CrossRef]
- Adams, D.C.; Jackson, J.F. Estimating the Allometry of Tree Bark. Am. Midl. Nat. 1995, 134, 99–106. [Google Scholar] [CrossRef]
- MacFarlane, D.W.; Luo, A. Quantifying tree and forest bark structure with a bark-fissure index. Can. J. For. Res. 2009, 39, 1859–1870. [Google Scholar] [CrossRef]
- Anna, I.; Jarosław, K. Hydrological properties of bark of selected forest tree species. Part I: The coefficient of development of the interception surface of bark. Trees Struct. Funct. 2014, 28, 831–839. [Google Scholar] [CrossRef]
- Jiménes Saa, J.H. La Identificación de los Arboles Tropicales por Medio de Caracteristicas del Tronco y la Corteza; Instituto Interamericano de Cooperación para la Agricultura (IICA): San José, Costa Rica, 1967. [Google Scholar]
- Rollet, B. Interet de l’etude des ecorces dans la determination des arbres tropicaux sur pied. Bois For. Trop. 1980, 194, 3–28. [Google Scholar]
- Rollet, B. Interet de l’etude des ecorces dans la determination des arbres tropicaux sur pied. Bois For. Trop. 1982, 195, 31–50. [Google Scholar] [CrossRef]
- Thorenaar, A. Naar Bruikbare Kenmerken Ter Identificatie Van Boomen Naar Hun Bast; Cambridge University: Cambridge, UK, 1926. [Google Scholar]
- Beard, F.S. Key for the identification of the more important trees of Tobago on characters of bark and blaze. Emp. For. J. 1944, 23, 34–36. [Google Scholar]
- De Vall, W.B. A bark character for the identification of certain Florida pines. Proc. Florida Acad. Sci. 1944, 7, 101–103. [Google Scholar]
- Kumari, A.K.R.; Kavitha, S.; Sheena, E.V. Bark morphology as a tool to identify the trees. Svādhyāya Int. J. Transdiscipl. Res. Dev. 2024, 4, 33–48. [Google Scholar]
- Schwankl, A. Guide to Bark; Thames and Hudson: London, UK, 1956. [Google Scholar]
- Vaucher, H. Bäume an den Rinden Erkennen und Bestimmen; Prisma Verlag: Güters-loh, Germany, 1987. [Google Scholar]
- Vaucher, H. Tree Bark: A Colour Guide; Timber Press: Portland, OR, USA, 2010. [Google Scholar]
- Wojtech, M. Bark: A Field Guide to Trees of the Northeast; Brandeis University Press: Waltham, MA, USA, 2020. [Google Scholar]
- Payne, W.W. A Glossary of Plant Hair Terminology. Brittonia 1978, 30, 239–255. [Google Scholar] [CrossRef]
- Wacowska, M. Ontogenesis and structure of periderm in Acer negundo L. and × Fatshedera lizei Guillaum. Acta Soc. Bot. Pol. 1985, 54, 17–27. [Google Scholar] [CrossRef]
- Ruffinatto, F.; Crivellaro, A.; Wiedenhoeft, A.C. Review of macroscopic features for hardwood and softwood identification and a proposal for a new character list. IAWA J. 2015, 36, 208–241. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; ter Steege, H.; Morgan, H.D.; van der Heijden, M.G.A.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Kattge, J.; Bönisch, G.; Díaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Tautenhahn, S.; Werner, G.D.A.; Aakala, T.; Abedi, M.; et al. TRY plant trait database—Enhanced coverage and open access. Glob. Change Biol. 2020, 26, 119–188. [Google Scholar] [CrossRef] [PubMed]
- Prentice, I.C.; Meng, T.; Wang, H.; Harrison, S.P.; Ni, J.; Wang, G. Evidence of a universal scaling relationship for leaf CO2 drawdown along an aridity gradient. New Phytol. 2011, 190, 169–180. [Google Scholar] [CrossRef]
- Wirth, C.; Lichstein, J.W. The Imprint of Species Turnover on Old-Growth Forest Carbon Balances—Insights from a Trait-Based Model of Forest Dynamics. In Old-Growth Forests: Function, Fate and Value; Wirth, C., Gleixner, G., Heimann, M., Eds.; Springer: New York, NY, USA, 2009; pp. 81–113. [Google Scholar]
- Milla, R.; Reich, P.B. Multi-trait interactions, not phylogeny, fine-tune leaf size reduction with increasing altitude. Ann. Bot. 2011, 107, 455–465. [Google Scholar] [CrossRef]
- Wang, H.; Harrison, S.P.; Prentice, I.C.; Yang, Y.; Bai, F.; Togashi, H.F.; Wang, M.; Zhou, S.; Ni, J. The China Plant Trait Database: Toward a comprehensive regional compilation of functional traits for land plants. Ecology 2018, 99, 500. [Google Scholar] [CrossRef]
- Rosell, J.A. Bark thickness across the angiosperms: More than just fire. New Phytol. 2016, 211, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Kucharzyk, S. Flora Wiosenna Lasów—Kluczyk Botaniczny do Oznaczania; Bieszczadzki Park Narodowy: Lutowiska, Poland, 2017. [Google Scholar]
- Kucharzyk, S. Flora Wiosenna Lasów—Kluczyk Botaniczny do Oznaczania Gatunków. Available online: https://archiwum.bdpn.pl/moje_php/kluczyk (accessed on 30 August 2025).
- Lucidcentral. Lucid Key: Identification and Diagnostic Tools for Biodiversity and Biosecurity. Available online: https://www.lucidcentral.org (accessed on 20 January 2025).
- Richardson, S.J.; Laughlin, D.C.; Lawes, M.J.; Holdaway, R.J.; Wilmshurst, J.M.; Wright, M.; Curran, T.J.; Bellingham, P.J.; McGlone, M.S. Functional and environmental determinants of bark thickness in fire-free temperate rain forest communities. Am. J. Bot. 2015, 102, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- Bartha, D. A közönséges bükk morfológiája. In A Bükk és a Bükkösök Magyarországon; Bartha, D., Csóka, G., Mátyás, C., Eds.; Soproni Egyetem Kiadó: Sopron, Hungary, 2024; pp. 28–45. [Google Scholar]
- Lima, M.T.; Guandique, M.E.G.; Tonello, K.C. Bark Morphology and Nutrient Flux in Urban Trees: Investigating Water Absorption and Ion Concentration Dynamics. Hydrology 2024, 11, 56. [Google Scholar] [CrossRef]
- Dowtin, A.L.; Cregg, B.C.; Nowak, D.J.; Levia, D.F. Towards optimized runoff reduction by urban tree cover: A review of key physical tree traits, site conditions, and management strategies. Landsc. Urban Plan. 2023, 239, 104849. [Google Scholar] [CrossRef]
- Kettlewell, H.B.D. Selection experiments on industrial melanism in the Lepidoptera. Heredity 1955, 9, 323–342. [Google Scholar] [CrossRef]
- Ontario Ministry of Natural Resources (OMNR). Ontario Tree Marking Guide, Version 1.1; OMNR: Toronto, ON, Canada, 2004. [Google Scholar]
- Godet, J.-D. Baumrinden; Arboris Verlag: Wohlen bei Bern, Switzerland, 2011. [Google Scholar]
- Godet, J.-D. Guide des Écorces des Arbres d’Europe; Delachaux et Niestlé: Paris, France, 2012. [Google Scholar]
- Godet, J.-D. Guide des Écorces des Arbres d’Europe: Reconnaître et Comparer les Espèces; Delachaux et Niestlé: Paris, France, 2022. [Google Scholar]
- Propat, P. Barks and Trunks: Rainforest Trees of South-Eastern Australia—Vol 1; Dragonwick: Goonellabah, Australia, 2009. [Google Scholar]
- Propat, P. Barks and Trunks: Rainforest Trees of South-Eastern Australia—Vol 2; Dragonwick: Goonellabah, Australia, 2013. [Google Scholar]
- Propat, P. Barks and Trunks: Rainforest Trees of South-Eastern Australia—Vol 1; Dragonwick: Goonellabah, Australia, 2020. [Google Scholar]
- Schwankl, A. Die Rinde, das Gesicht des Baumes. Ein Buch zum Bestimmen der Bäume und Hölzer nach ihren Rinden von 75 Arten des In- und Auslandes; Kosmos: Stuttgart, Germany, 1953. [Google Scholar]
- Vaucher, H. Les Arbres leurs Ecorces; Hatier: Paris, France, 1980. [Google Scholar]
- Vaucher, H. Bomen—Herkennen aan de Schors; Moussault: Bussum, The Netherlands, 1982. [Google Scholar]
- Vaucher, H. Les Arbres leurs Écorces; Hatier: Paris, France, 1985. [Google Scholar]
- Vaucher, H. Baumrinden; Verlag von Ferdinand Enke: Stuttgart, Germany, 1990. [Google Scholar]
- Vaucher, H. Guide des Écorces; Delachaux et Niestlé: Paris, France, 1993. [Google Scholar]
- Vaucher, H. Tree Bark: A Colour Guide; Timber Press: Portland, OR, USA, 2003. [Google Scholar]
- Wojtech, M. Bark: A Field Guide to Trees of the Northeast; Brandeis University Press: Waltham, MA, USA, 2011. [Google Scholar]
- Core, E.L.; Ammons, N.P. Woody Plants in Winter; The Boxwood Press: Pacific Grove, CA, USA, 1958. [Google Scholar]
- Johnson, O.; More, D. Collins Tree Guide; HarperCollins Publishers Ltd.: London, UK, 2006. [Google Scholar]
- Pikula, J. Stromové a Keřové Dřeviny lesů a Volné Krajiny České Republiky; Akademické nakladatelství CERM: Brno, Czech Republic, 2013. [Google Scholar]
- Sterry, P. Collins Complete Guide to British Trees: A Photographic Guide to Every Common Species; HarperCollins Publishers Ltd.: London, UK, 2008. [Google Scholar]
- Trelease, W. Winter Botany: A Companion Volume to the Author’s Plant Materials of Decorative Gardening; Trelease, W., Ed.; Wentworth Press: Wilmington, DE, USA, 1918. [Google Scholar]
- Watts, M.T.; Watts, T. Winter Tree Finder: A Manual for Identifying Deciduous Trees in Winter; Nature Study Guild: Berkeley, CA, USA, 1970. [Google Scholar]
- Forel, O. Synchromies; Temps: Paris, France, 1961. [Google Scholar]
- Forel, O. Synchromies; Le Manoir: Suisse, Switzerland, 1967. [Google Scholar]
- Forel, O. Secrets des Ecorces. Synchromies; Edita: Edita, Finland, 1972. [Google Scholar]
- Forel, O. Geheimnisvolle Rinden Synchromien; Edita: Edita, Finland, 1972. [Google Scholar]
- Forel, O. Hidden Art in Nature. Synchromies; Harper & Row: New York, NY, USA, 1972. [Google Scholar]
- Forel, O. Secrets des Ecorces. Synchromies; Éditions Denoël: Paris, France, 1980. [Google Scholar]
- Johnson, D. Bark; Dojopico Books: Hudson, NY, USA, 2022. [Google Scholar]
- Pollet, C. Écorces: Voyage dans l’intimité des Arbres du Monde; Eugen Ulmer KG: Stuttgart, Germany, 2008. [Google Scholar]
- Pollet, C. Bark: An Intimate Look at the World’s Trees; Frances Lincoln Publishers: London, UK, 2010. [Google Scholar]
- Pollet, C. Rinde: Die Wunderwelt der Bäume Entdecken; Eugen Ulmer KG: Stuttgart, Germany, 2012. [Google Scholar]
- Pollet, C. Rinde: Die Wunderwelt der Bäume Entdecken; Eugen Ulmer KG: Stuttgart, Germany, 2016. [Google Scholar]
- Pollet, C. Cortecce: Viaggio Nell’intimità Degli Alberi del Mondo; L’Ippocampo: Milano, Italy, 2017. [Google Scholar]
- Pollet, C. Écorces: Voyage dans L’intimité des Arbres du Monde; Eugen Ulmer KG: Stuttgart, Germany, 2022. [Google Scholar]
- Pollet, C. Écorces: Galerie d’art à ciel Ouvert; Eugen Ulmer KG: Stuttgart, Germany, 2011. [Google Scholar]
- Pollet, C. Beautiful Trees: Close-Ups of Amazing Tree Bark from Around the World; Frances Lincoln Publishers: London, UK, 2012. [Google Scholar]
- Sandved, K.B.; Prance, A.E.; Prance, G.T. Bark: The Formation, Characteristics, and Uses of Bark Around the World; Timber Press: Portland, OR, USA, 1993. [Google Scholar]
- Evert, R.F. Periderm. In Esau’s Plant Anatomy, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; pp. 427–445. [Google Scholar]
- Smithson, E. Development of winged cork in Acer campestre L. Proc. Leeds Philos. Lit. Soc. Sci. Sect. 1952, 6, 97–103. [Google Scholar]
- Smithson, E. Development of winged cork in Ulmus × hollandica Mill. Proc. Leeds Philos. Lit. Soc. Sci. Sect. 1952, 6, 211–220. [Google Scholar]
- Majer, A. Magyarország Erdőtársulásai (az Erdőműveléstan Alapjai); Akadémiai Kiadó: Budapest, Hungary, 1968. [Google Scholar]
- Serra, O.; Mähönen, A.P.; Hetherington, A.J.; Ragni, L. The Making of Plant Armor: The Periderm. Annu. Rev. Plant Biol. 2022, 73, 405–432. [Google Scholar] [CrossRef]
- Gill, A.M. Stems and fires. In Plant Stems; Academic Press Inc.: San Diego, CA, USA, 1995; pp. 323–342. [Google Scholar]

| Prerequisite | Character | Character States |
|---|---|---|
| Color main category | Dark, light, green-red, white (partially) | |
| Color | Free description | |
| Phyllotaxis | Opposite, alternate, spiral, whorled | |
| Branching | Monopodial, sympodial, twisted | |
| Cross-section | Round, oval, ribbed, angular | |
| Shiny bark | Yes/no | |
| Winged cork | Yes/no | |
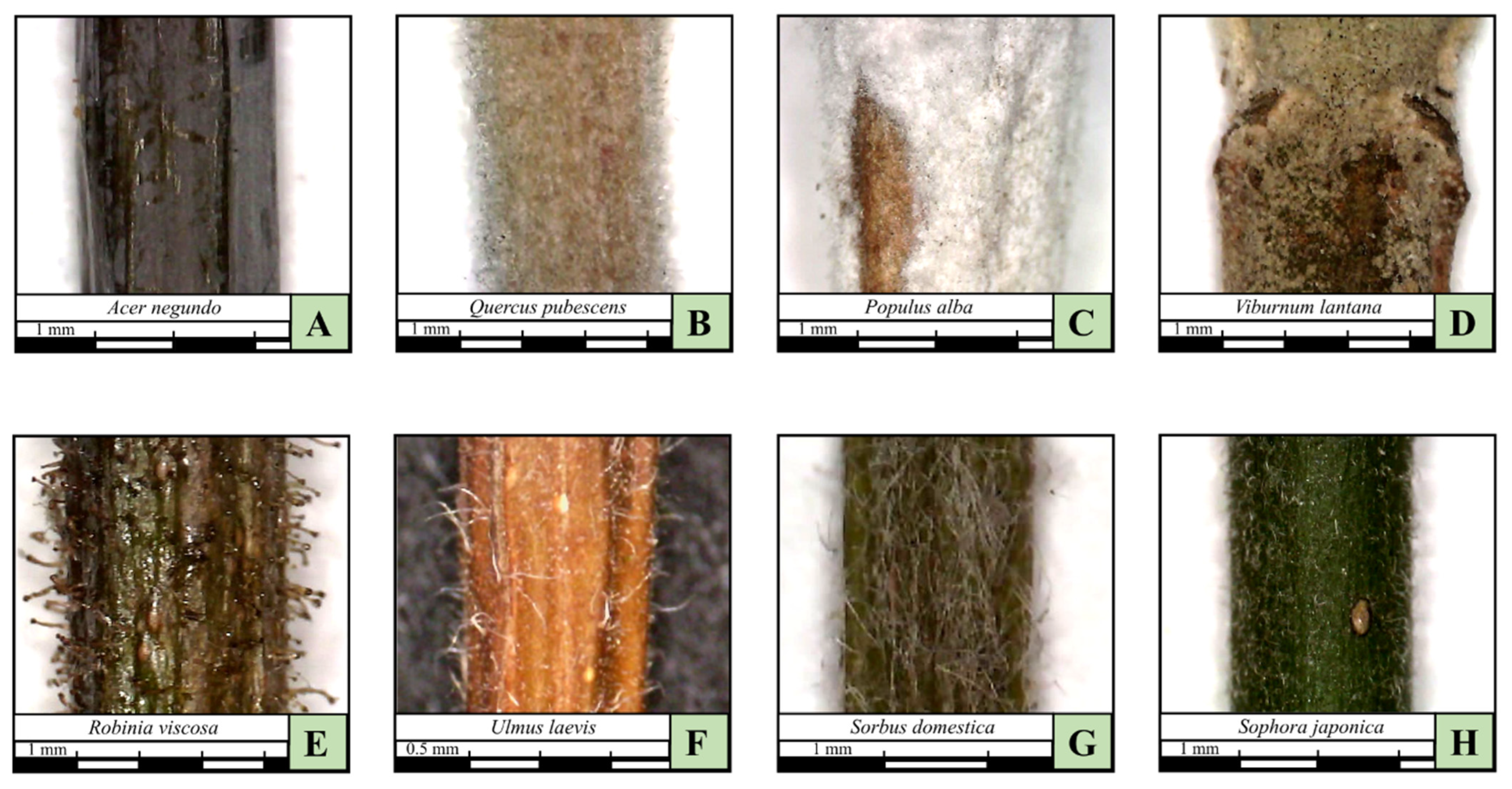
| Trichomes | Glabrous, sparse pilose, dense pilose, tomentose, woolly, pulverulent, glandular, felted | |
| Trichomes | Trichome color | Free description |
| Visible lenticels | Yes/no | |
| Visible lenticels | Lenticel shape | Rounded, transversely elongated, longitudinally elongated |
| Lenticel color | Free description | |
| Visible leaf scar | Yes/No | |
| Leaf scar size | Small, large | |
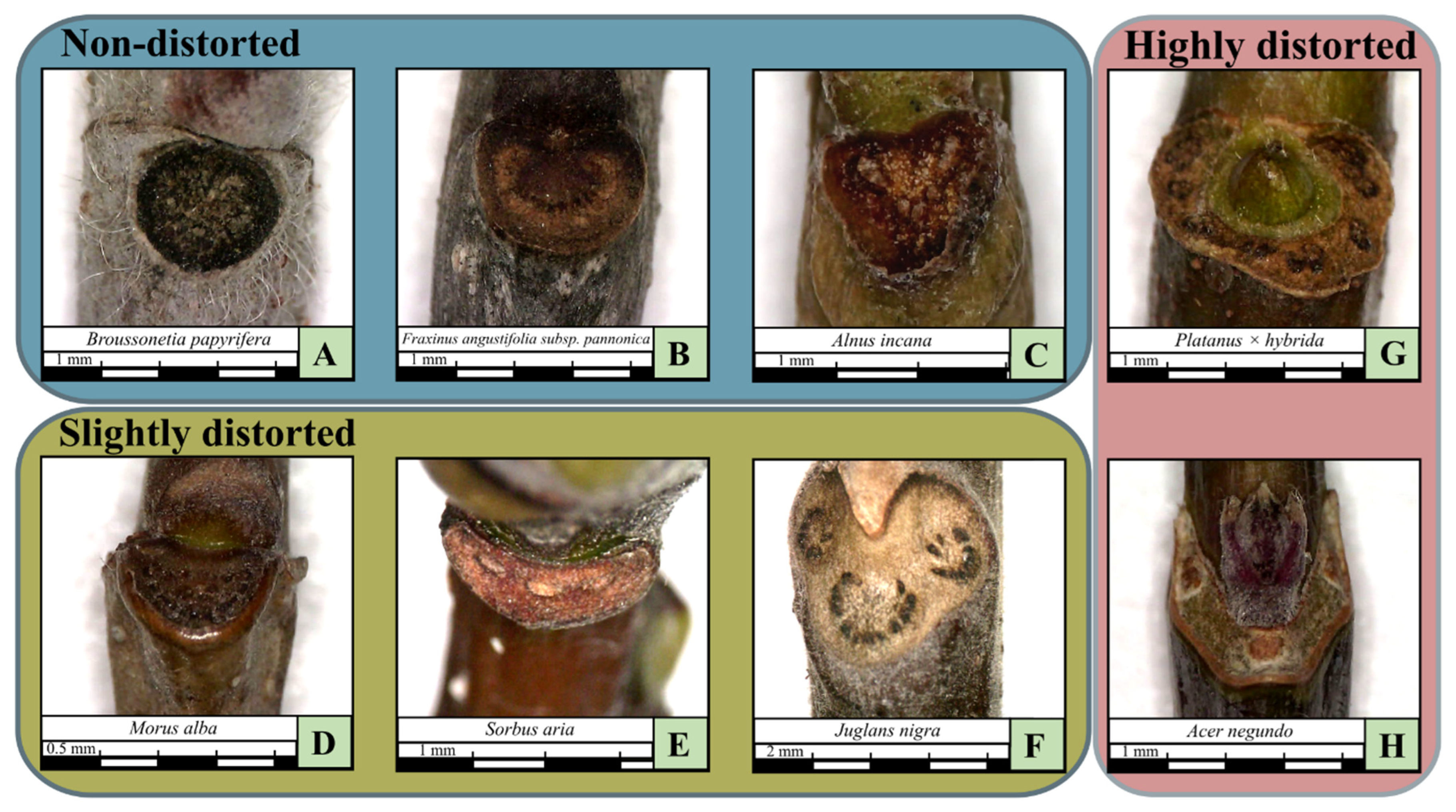
| Leaf scar main category | Non-distorted, slightly distorted, highly distorted | |
| Leaf scar shape | Circle, semicircle, oval, crescent (“C”), horseshoe (“U”), triangular, heart, annular | |
| Raised leaf cushion | Yes/no | |
| Bundle scars | Free description, but common forms include 1, 3, 1–3, 3–5, irregular, many along the margin | |
| Opposite phyllotaxis | Touching leaf scars | Yes/no |
| Other characters | Free description |
| Prerequisite | Character | Character States | |
|---|---|---|---|
| Color main category | Dark, light, green-red, white (partially) | ||
| Color | Free description | ||
| Bark development direction | Bark main and subtypes (indicating transitions) | ||
| Non-persistent smooth bark | Transitional bark | Yes/no | |
| Bark development time (years) | Free description (accuracy within 5 years)–only literature data | ||
| Bark development time (diameter) | Free description | ||
| Winged cork | Yes/no | ||
| Shiny bark | Yes/no | ||
| Persistent epidermis | Yes/no | ||
| Slow periderm formation | Yes/no | ||
| Knobby short shoots | Yes/no | ||
| Visible lenticels | Yes/no /No | ||
| Visible lenticels | Lenticel shape | Rounded, transversely elongated, longitudinally elongated, diamond | |
| Lenticel color | Free description | ||
| Thorns/spines | Yes/no | ||
| Thorns/spines presence | Thorn/spine type | Prickles, branched thorns, simple thorns, stipular spines, multiple types | |
| Scaly/needle-leaved species | Leaf fall time | Free description (years) | |
| Transitional bark | Color main category | Dark, light, green-red, white (partially) | |
| Color | Free description | ||
| Further transition stages | Yes/no | ||
| Bark development direction | Bark main and subtypes (indicating transitions) | ||
| Bark development time (diameter) | Free description | ||
| Shiny bark | Yes/no | ||
| Visible lenticels | Yes/no /No | ||
| Visible lenticels | Lenticel shape | Rounded, transversely elongated, longitudinally elongated, diamond | |
| Lenticel color | Free description | ||
| Thorns/spines | Yes/no | ||
| Thorns/spines presence | Thorn/spine type | Prickles, branched thorns, simple thorns, stipular spines, multiple types | |
| Further transitional bark | Bark development direction | Bark main and subtypes (indicating transitions) | |
| Bark development time (diameter) | Free description | ||
| Other characters | Free description | ||
| Prerequisite | Character | Character States |
|---|---|---|
| Color main category | Dark, light, green-red, white (partially) | |
| Color | Free description | |
| Eye-marks | Yes/no | |
| Texture | Soft/Hard | |
| Lenticels | Yes/no | |
| Shiny bark (ridge) | Yes/no | |
| Combined bark | Yes/no | |
| Bark main type | Knobby, cracks, diamond-shape lenticels, smooth, stringy, scrolled | |
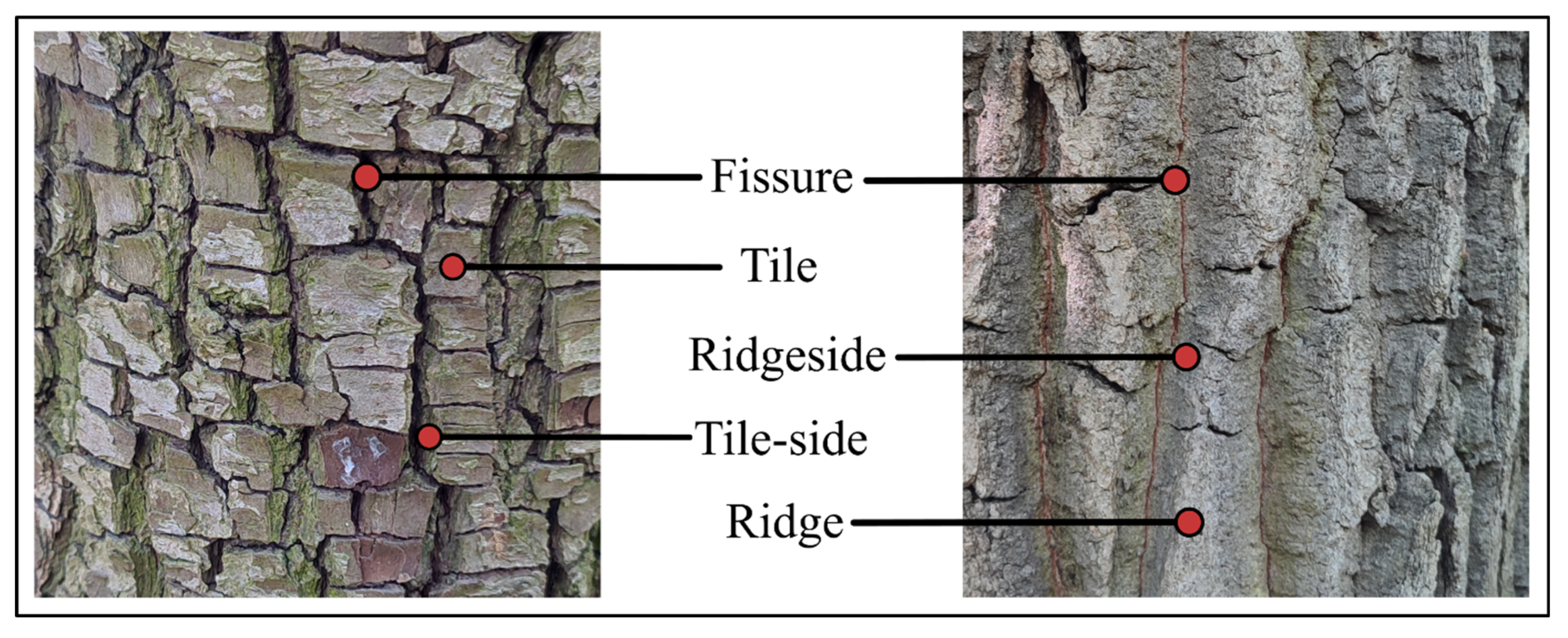
| Cracked bark | Cracked subtypes | Scales, flakes, fissures |
| Fissured bark | Fissured subtypes | Longitudinal fissures, tessellated, fibrous |
| Fissure depth and density | Shallow and sparse; shallow and dense; deep and sparse; deep and dense | |
| Fissure network | Longitudinal ridges dominate, reticulate ridges | |
| Fissure color | No difference, free description | |
| Ridgeside color | No difference, free description | |
| Tessellated bark | Tile shape | Square, rectangular |
| Removable tiles | Yes/no | |
| Scales, tessellated bark | Separated edges | Yes/no |
| Scales, flakes, tessellated, stringy, scrolled bark | Lower layer color | Free description |
| Scaly bark | Scaly subtype | Papery, chunky |
| Scale shape | Rectangular, rounded, irregular | |
| Scrolled bark | Scrolled subtype | Patchy, ringed |
| Smooth bark | Pattern | None, reticulate, patchy, mixed |
| Veteran bark | Yes/no | |
| Veteran bark | Fissured veteran bark | Yes/no |
| Veteran bark at trunk base | Yes/no | |
| Other characters | Free description |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zoltán, L.; Korda, M. A Novel System for the Characterization of Bark Macroscopic Morphology for Central European Woody Species. Forests 2025, 16, 1586. https://doi.org/10.3390/f16101586
Zoltán L, Korda M. A Novel System for the Characterization of Bark Macroscopic Morphology for Central European Woody Species. Forests. 2025; 16(10):1586. https://doi.org/10.3390/f16101586
Chicago/Turabian StyleZoltán, László, and Márton Korda. 2025. "A Novel System for the Characterization of Bark Macroscopic Morphology for Central European Woody Species" Forests 16, no. 10: 1586. https://doi.org/10.3390/f16101586
APA StyleZoltán, L., & Korda, M. (2025). A Novel System for the Characterization of Bark Macroscopic Morphology for Central European Woody Species. Forests, 16(10), 1586. https://doi.org/10.3390/f16101586







