Distinct Roles of Forest Stand Types in Regulating Soil Organic Carbon Stability Across Depths
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
2.3. Soil Sample Collection and Processing
2.4. Soil Samples Determinations
2.5. Data Analysis
3. Results
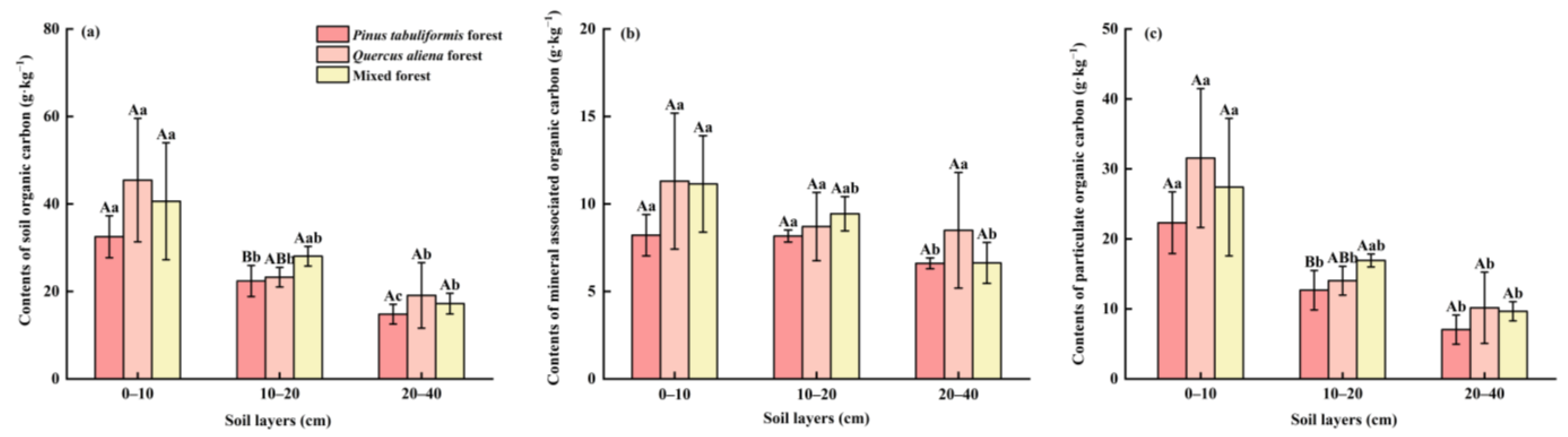
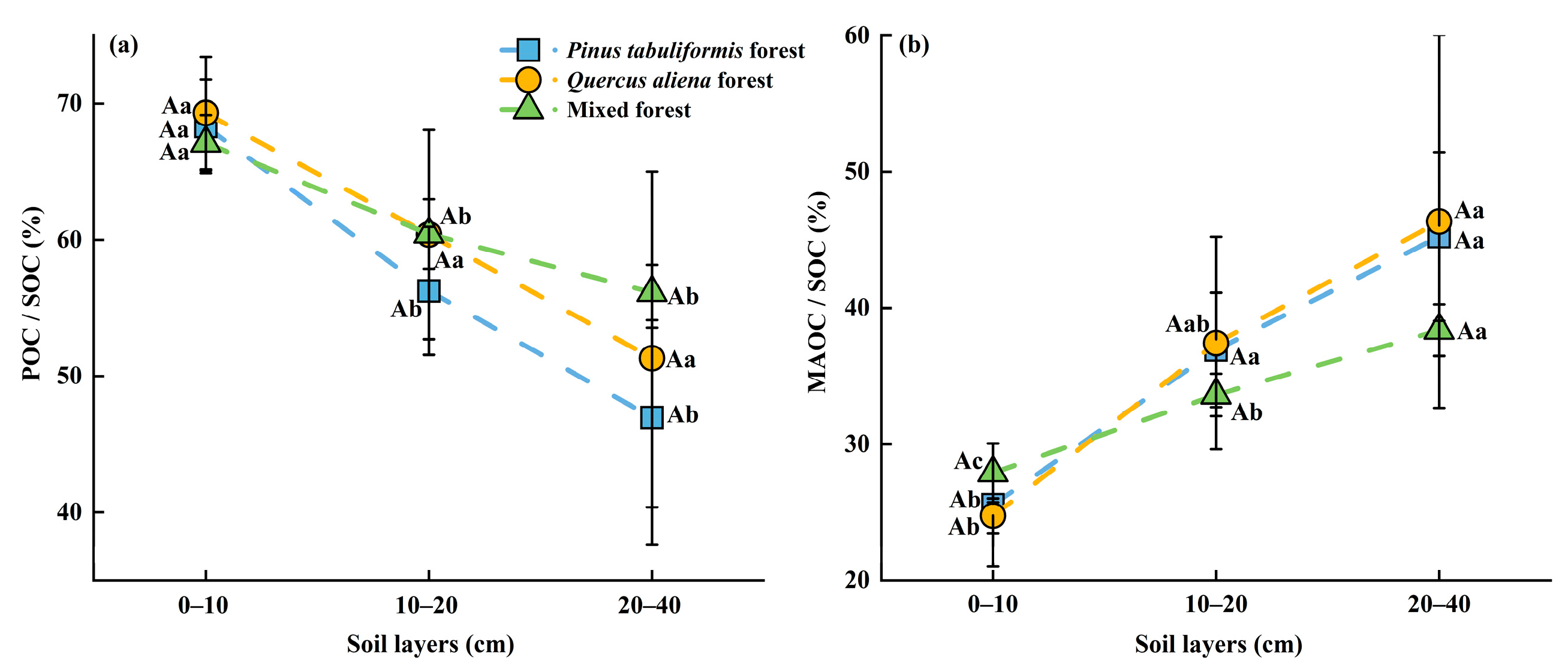
3.1. Distribution of SOC, POC, and MAOC Under Different Stand Types
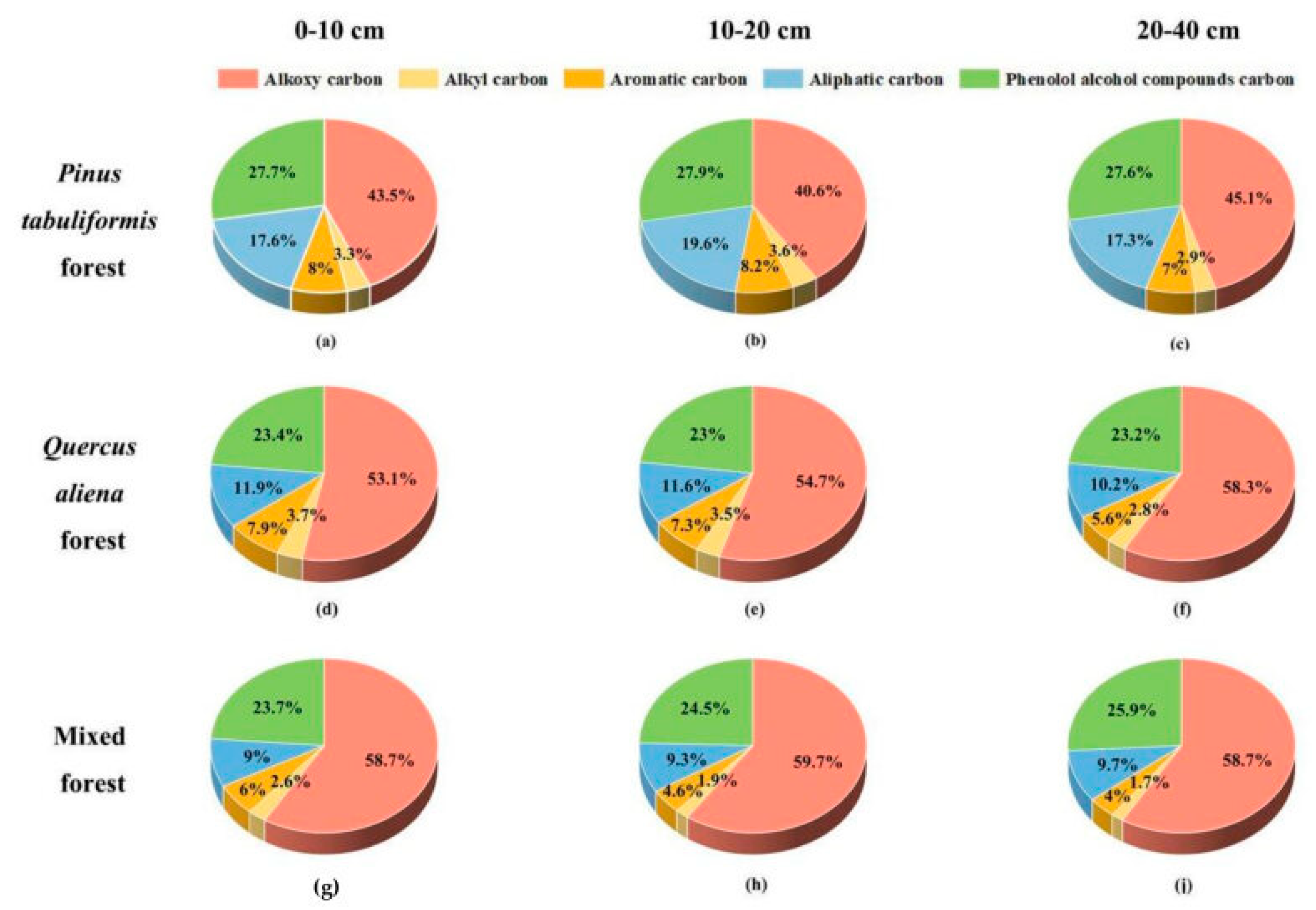
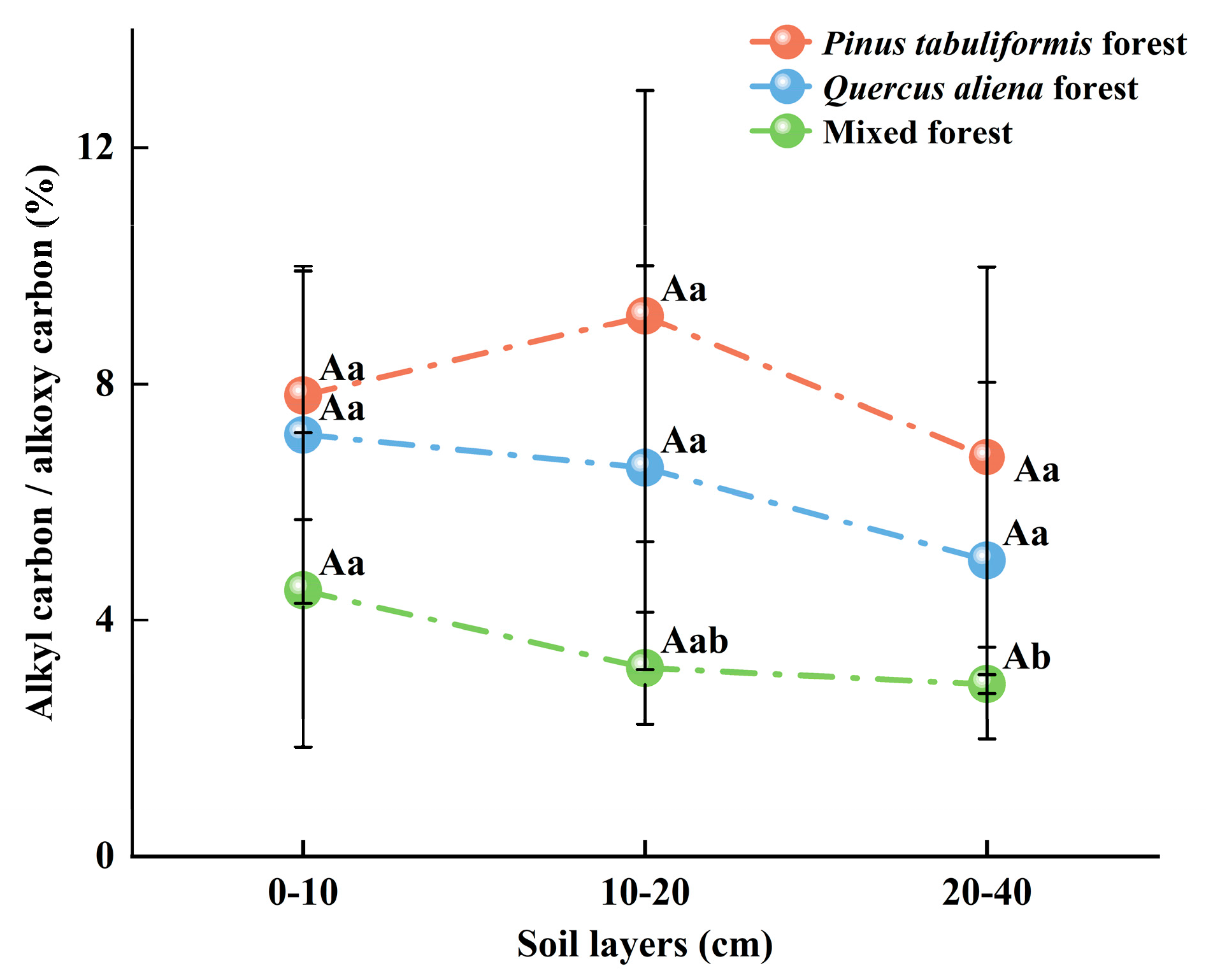
3.2. Characteristics of the Chemical Structure of MAOC Under Different Stand Types
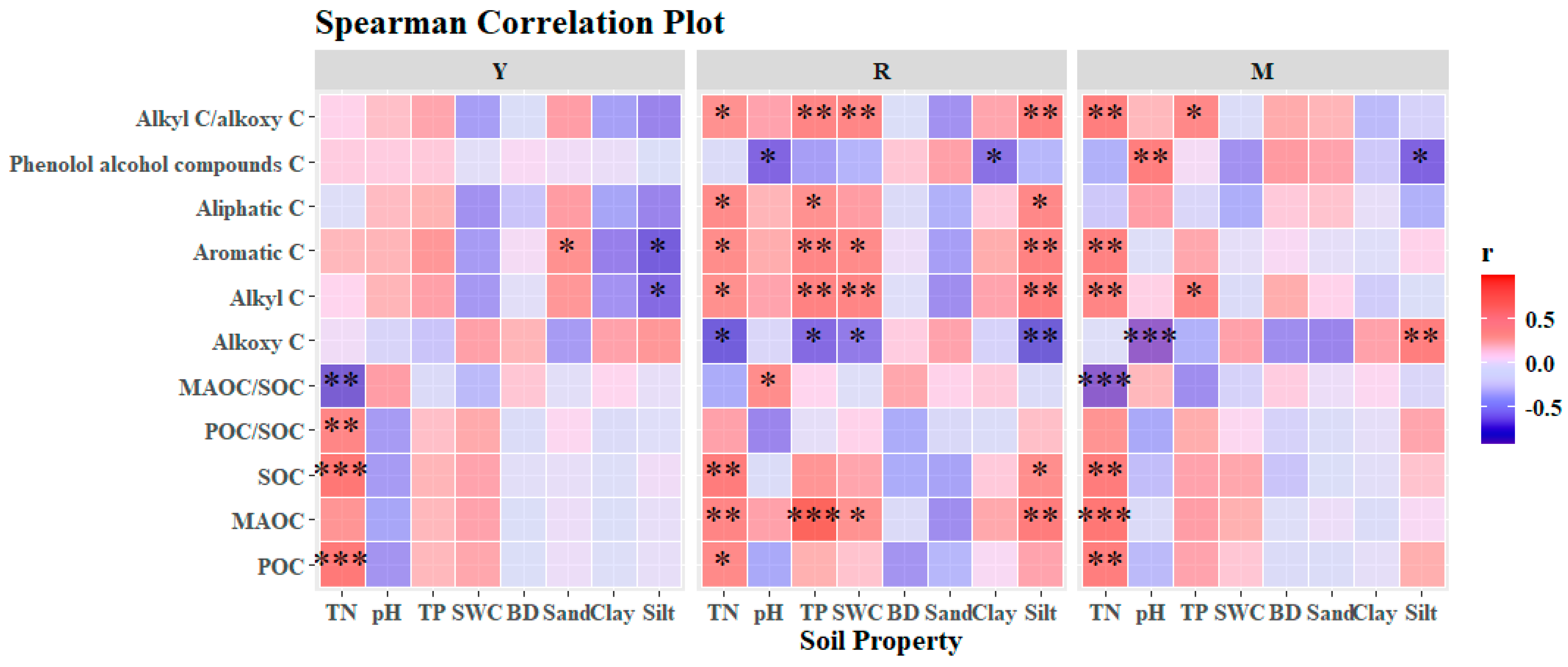
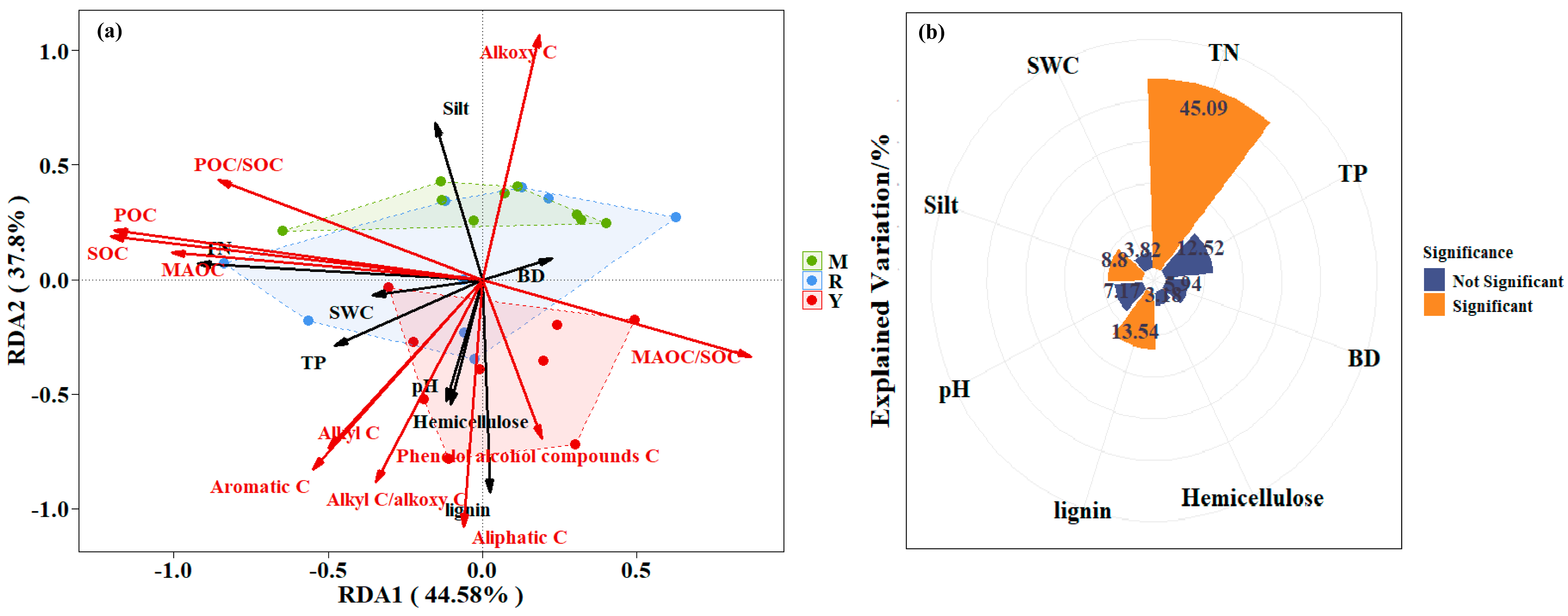
3.3. Correlation of SOC, POC, MAOC, and the Chemical Structure of MAOC with Properties of Soil and Litter
4. Discussion
4.1. Characteristics of SOC Stability Under Different Stand Types
4.1.1. Contents and Proportions of SOC Components
4.1.2. Chemical Structure of MAOC
4.2. Main Influencing Factors of SOC Stability Under Different Stand Types
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SOC | Soil organic carbon |
| MAOC | Mineral-associated organic carbon |
| POC | Particulate organic carbon |
| BD | Bulk density |
| FTIR | Fourier transform infrared |
| SWC | Soil water content |
| TP | Total phosphorus |
| TN | Total nitrogen |
References
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zheng, S.; Pendall, E.; Smith, P.; Liu, J.; Li, J.; Fang, C.; Li, B.; Nie, M. Unprotected carbon dominates decadal soil carbon increase. Nat. Commun. 2025, 16, 2008. [Google Scholar] [CrossRef]
- Sun, W.L.; Liu, X.H.; Tian, Z.X.; Shao, X.M. Analysis on Characteristics of Vegetation and Soil Bacterial Community under 20 Years’ Restoration of Different Tree Species: A Case Study of the Qinling Mountains. Forests 2021, 12, 562. [Google Scholar] [CrossRef]
- Zhou, Z.; Ren, C.; Wang, C.; Delgado-Baquerizo, M.; Luo, Y.; Luo, Z.; Du, Z.; Zhu, B.; Yang, Y.; Jiao, S.; et al. Global turnover of soil mineral-associated and particulate organic carbon. Nat. Commun. 2024, 15, 5329. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Wang, H.; Zhou, T.; Wang, C.; Zhou, Z.; Liu, S. Storage Potential of Soil Functional Carbon Fractions in the World’s Largest Plantations. Adv. Sci. 2025, e04995. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Angst, G.; Mueller, K.E.; Eissenstat, D.M.; Trumbore, S.; Freeman, K.H.; Hobbie, S.E.; Chorover, J.; Oleksyn, J.; Reich, P.B.; Mueller, C.W. Soil organic carbon stability in forests: Distinct effects of tree species identity and traits. Glob. Change Biol. 2019, 25, 1529–1546. [Google Scholar] [CrossRef]
- Yang, E.; Wang, B.R.; Yao, H.J.; Huang, Y.M.; An, S.S. Dynamics of particulate and mineral-associated organic carbon during the development of biological soil crusts in the Loess Plateau. Res. Soil Water Conserv. 2023, 30, 25–33+40. [Google Scholar] [CrossRef]
- Deng, J.J.; Zhu, W.X.; Zhou, Y.B.; Yin, Y. Soil Organic Carbon Chemical Functional Groups under Different Revegetation Types Are Coupled with Changes in the Microbial Community Composition and the Functional Genes. Forests 2019, 10, 240. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, B.R.; Dou, Y.X.; Xue, Z.J.; Sun, H.; Wang, Y.Q.; Liang, C.; An, S.S. Advances in the research of transformation and stabilization of soil organic carbon from plant and microbe. J. Appl. Ecol. 2024, 35, 111–123. [Google Scholar] [CrossRef]
- Wang, H.; Ding, Y.; Zhang, Y.G.; Wang, J.X.; Freedman, Z.B.; Liu, P.C.; Cong, W.; Wang, J.; Zang, R.G.; Liu, S.R. Evenness of soil organic carbon chemical components changes with tree species richness, composition and functional diversity across forests in China. Glob. Change Biol. 2023, 29, 2852–2864. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Sheng, M.; You, M.Y.; han, X.Z. Advancement in research on application of 13 C NMR techniques to exploration of chemical structure of soil organic matter. Acta Pedol. Sin. 2019, 56, 796–812. [Google Scholar] [CrossRef]
- Si, Q.; Chen, K.L.; Wei, B.; Zhang, Y.W.; Sun, X.; Liang, J.Y. Dissolved carbon flow to particulate organic carbon enhances soil carbon sequestration. Soil 2024, 10, 441–450. [Google Scholar] [CrossRef]
- Guo, X.W.; Zhang, Y.X.; You, Y.M.; Sun, J.X. Research advance in the effects of litter input on forest soil organic carbon transformation and stability. Chin. J. Appl. Ecol. Yingyong Shengtai Xuebao 2024, 35, 2352–2361. [Google Scholar] [CrossRef]
- Lai, L.M.; Zhao, J.X.; Dou, Y.X. Fungal necromass carbon contributes more to POC and MAOC under different forest types of Qinling Mountains. Plant Soil 2025, 512, 603–618. [Google Scholar] [CrossRef]
- Pei, Z.F.; Hong, M.; Wu, Z.D.; Lu, J.Y.; Zhang, Y.X.; Shen, Q.G. Responses of Soil Organic Carbon Content and Its Chemical Structure of Different Components to Long-term Nitrogen Input in Meadow Steppe. Soils 2022, 54, 481–489. [Google Scholar] [CrossRef]
- Xu, S.; Yang, Y.; Sun, G.; Zhang, Q.; Wang, Y.; Zeng, H.; Simpson, M.J.; Wang, J. Aridity affects soil organic carbon concentration and chemical stability by different forest types and soil processes across Chinese natural forests. Sci. Total Environ. 2024, 944, 174002. [Google Scholar] [CrossRef]
- Guan, X.; Jiang, J.; Classen, A.T.; Ullah, S.; Wang, G. Disentangling the contribution of mycorrhizal fungi to soil organic carbon storage. Soil Biol. Biochem. 2025, 209, 109900. [Google Scholar] [CrossRef]
- Leifheit, E.F.; Verbruggen, E.; Rillig, M.C. Arbuscular mycorrhizal fungi reduce decomposition of woody plant litter while increasing soil aggregation. Soil Biol. Biochem. 2015, 81, 323–328. [Google Scholar] [CrossRef]
- Chen, X.; Mou, Z.; Zhang, J.; Li, Y.; Wu, W.; Wang, T.; Zhu, X.; Wu, D.; Petticord, D.F.; Hui, D.; et al. Roots Dominate Over Extraradical Hyphae in Driving Soil Organic Carbon Accumulation During Tropical Forest Succession. Glob. Change Biol. 2025, 31, e70499. [Google Scholar] [CrossRef]
- Deng, F.B.; Liang, C. Revisiting the quantitative contribution of microbial necromass to soil carbon pool: Stoichiometric control by microbes and soil. Soil Biol. Biochem. 2022, 165, 108486. [Google Scholar] [CrossRef]
- Lu, M.Z.; He, G.; Fan, L.; Liu, G.H.; Wu, J.J.; Liu, W.Z.; Ma, L. Temperature sensitivity of aerobic and anaerobic organic carbon mineralization varies with climate and soil depth in riparian zones. Soil Biol. Biochem. 2024, 195, 109455. [Google Scholar] [CrossRef]
- Yu, Z.J.; Wang, K.B.; Li, J.W.; Shangguan, Z.P.; Deng, L. Mixed plantations have more soil carbon sequestration benefits than pure plantations in China. For. Ecol. Manag. 2023, 529, 120654. [Google Scholar] [CrossRef]
- Qin, Z.K.; Liu, R.H.; He, P.; Wang, C.; Nie, Y.X.; Shen, W.J. Effects of mixed broadleaved tree species with pure Pinus massoniana plantation on soil microbial necromass carbon and organic carbon fractions. Chin. J. Appl. Ecol. Yingyong Shengtai Xuebao 2024, 35, 141–152. [Google Scholar] [CrossRef]
- Yang, H.; Song, Y.H.; Pang, Y.; Kang, H.B.; Xue, Y.; Wang, D.X. Driving Factors of Chinese Pine Population Distribution in the Ridge Habitats of the Southern Slope of the Mid-Qinling Mountains, China. Forests 2023, 14, 2252. [Google Scholar] [CrossRef]
- Deng, W.B.; Wang, X.; Hu, H.B.; Zhu, M.D.; Chen, J.Y.; Zhang, S.; Cheng, C.; Zhu, Z.Y.; Wu, C.M.; Zhu, L. Variation Characteristics of Soil Organic Carbon Storage and Fractions with Stand Age in North Subtropical Quercus acutissima Carruth. Forest in China. Forests 2022, 13, 1649. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, B.; Wang, Q.; Cao, X.; Zhang, J. Differences in chemical composition of soil organic carbon resulting from long-term fertilization strategies. PLoS ONE 2015, 10, e0124359. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Yang, C.; Chen, Y.; Sun, W.; Zhang, Q.; Diao, M.; Sun, J. Extreme soil salinity reduces N and P metabolism and related microbial network complexity and community immigration rate. Environ. Res. 2025, 264, 120361. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Wu, F.Z.; Yang, W.Q.; Zhang, C.; Peng, Y.; Tan, B.; Xu, Z.F.; Huang, C.P. Cellulose Dynamics during Foliar Litter Decomposition in an Alpine Forest Meta-Ecosystem. Forests 2016, 7, 176. [Google Scholar] [CrossRef]
- Lai, J.; Zou, Y.; Zhang, J.; Peres-Neto, P. Generalizing hierarchical and variation partitioning in multiple regression and canonical analyses using the rdacca.hp R package. Methods Ecol. Evol. 2022, 13, 782–788. [Google Scholar] [CrossRef]
- Lu, S.; He, Y.H.; Chen, Y.Q.; Chen, L.J.; Wang, Z.Y.; Yuan, J.; Wu, L.C. Co-analysis of rhizosphere metabolomics and bacterial community structures to unfold soil ecosystem health in Camellia oleifera land under long-term cultivation. Appl. Soil Ecol. 2022, 171, 104336. [Google Scholar] [CrossRef]
- Hall, S.J.; Huang, W.; Timokhin, V.I.; Hammel, K.E. Lignin lags, leads, or limits the decomposition of litter and soil organic carbon. Ecology 2020, 101, e03113. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Change Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Gao, Y.; Peng, Y.; Chang, P.; Wu, Y.; Guo, L.; Luo, J.; Liu, L. Influences of plant functional traits on soil organic carbon stocks: The roles of carbon input quality and diversity. Ecology 2025, 106, e70148. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef]
- Thiessen, S.; Gleixner, G.; Wutzler, T.; Reichstein, M. Both priming and temperature sensitivity of soil organic matter decomposition depend on microbial biomass—An incubation study. Soil Biol. Biochem. 2013, 57, 739–748. [Google Scholar] [CrossRef]
- Templer, P.H.; Findlay, S.; Lovett, G. Soil microbial biomass and nitrogen transformations among five tree species of the Catskill Mountains, New York, USA. Soil Biol. Biochem. 2003, 35, 607–613. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Tang, Z.X.; You, Y.M.; Guo, X.W.; Wu, C.J.; Liu, S.R.; Sun, O.J. Differential effects of forest-floor litter and roots on soil organic carbon formation in a temperate oak forest. Soil Biol. Biochem. 2023, 180, 109017. [Google Scholar] [CrossRef]
- Mathers, N.J.; Xu, Z.H. Solid-state 13C NMR spectroscopy: Characterization of soil organic matter under two contrasting residue management regimes in a 2-year-old pine plantation of subtropical Australia. Geoderma 2003, 114, 19–31. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chen, F.S.; Zheng, Z.Y.; Peng, Y.T.; Liu, Q.; Wang, S.N.; Wang, F.C. Effects of litter management on chemical structure and thermal stability of soil organic carbon in a Chinese fir forest. Shengtaixue Zazhi 2024, 43, 2641–2649. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.R.; Song, Z.C.; Yang, Y.J.; Wang, J.X.; You, Y.M.; Zhang, X.; Shi, Z.M.; Nong, Y.; Ming, A.G.; et al. Introducing nitrogen-fixing tree species and mixing with Pinus massoniana alters and evenly distributes various chemical compositions of soil organic carbon in a planted forest in southern China. For. Ecol. Manag. 2019, 449, 117477. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, Y.; Hu, X.F.; Wan, S.Z.; Wang, H.M.; Liang, C.; Chen, F.S. Litter manipulation effects on microbial communities and enzymatic activities vary with soil depth in a subtropical Chinese fir plantation. For. Ecol. Manag. 2021, 480, 118641. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Ji, X.D.; Zhang, Z.Q.; Zhang, H.S.; Zha, T.G.; Jiang, L. Elevation and total nitrogen are the critical factors that control the spatial distribution of soil organic carbon content in the shrubland on the Bashang Plateau, China. Catena 2021, 204, 105415. [Google Scholar] [CrossRef]
- Xu, J.H.; Sun, Y.; Gao, L.; Cui, X.Y. A review of the factors influencing soil organic carbon stability. Chin. J. Eco-Agric. 2018, 26, 222–230. [Google Scholar] [CrossRef]
- Chang, Y.; Sokol, N.W.; Groenigen, K.J.; Bradford, M.A.; Ji, D.; Crowther, T.W.; Liang, C.; Luo, Y.; Kuzyakov, Y.; Wang, J.; et al. A stoichiometric approach to estimate sources of mineral-associated soil organic matter. Glob. Change Biol. 2024, 30, e17092. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, E.M.; Kane, J.; Tripathi, B.M.; Rion, M.S.I.; Hungate, B.A.; Franklin, R.; Walter, C.; Sulman, B.; Brzostek, E. Carbon acquisition ecological strategies to connect soil microbial biodiversity and carbon cycling. Soil Biol. Biochem. 2023, 177, 108893. [Google Scholar] [CrossRef]
- Malik, A.A.; Puissant, J.; Buckeridge, K.M.; Goodall, T.; Jehmlich, N.; Chowdhury, S.; Gweon, H.S.; Peyton, J.M.; Mason, K.E.; van Agtmaal, M.; et al. Land use driven change in soil pH affects microbial carbon cycling processes. Nat. Commun. 2018, 9, 3591. [Google Scholar] [CrossRef]
- Mao, H.R.; Cotrufo, M.F.; Hart, S.C.; Sullivan, B.W.; Zhu, X.F.; Zhang, J.C.; Liang, C.; Zhu, M.Q. Dual role of silt and clay in the formation and accrual of stabilized soil organic carbon. Soil Biol. Biochem. 2024, 192, 109390. [Google Scholar] [CrossRef]
- Lazarevic, J.; Menkis, A. Exploring Fungal Communities in the Needles of Marginal Conifer Tree Populations. Forests 2025, 16, 968. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Luo, Z.; Sun, O.J. Long-term litter type treatments alter soil carbon composition but not microbial carbon utilization in a mixed pine-oak forest. Biogeochemistry 2021, 152, 1–17. [Google Scholar] [CrossRef]
- Goldstein, A.; Turner, W.R.; Spawn, S.A.; Anderson-Teixeira, K.J.; Cook-Patton, S.; Fargione, J.; Gibbs, H.K.; Griscom, B.; Hewson, J.H.; Howard, J.F.; et al. Protecting irrecoverable carbon in Earth’s ecosystems. Nat. Clim. Change 2020, 10, 287–295. [Google Scholar] [CrossRef]

| Litter Types | Chemical Properties of the Litter | ||
|---|---|---|---|
| Cellulose/% | Hemicellulose/% | Lignin/% | |
| Quercus aliena forests litter | 8.07 ± 0.41A | 5.87 ± 1.93A | 40.12 ± 4.15A |
| Pinus tabuliformis forests litter | 10.74 ± 2.25A | 5.65 ± 0.43A | 46.11 ± 7.31A |
| Mixed forests litter | 8.55 ± 0.74A | 4.92 ± 0.29A | 36.89 ± 3.06A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Lai, L.; Mei, Y.; Zhao, Y.; Li, Z.; Dou, Y.; Hou, L.; Geng, Q.; Zhang, S. Distinct Roles of Forest Stand Types in Regulating Soil Organic Carbon Stability Across Depths. Forests 2025, 16, 1585. https://doi.org/10.3390/f16101585
Zhao J, Lai L, Mei Y, Zhao Y, Li Z, Dou Y, Hou L, Geng Q, Zhang S. Distinct Roles of Forest Stand Types in Regulating Soil Organic Carbon Stability Across Depths. Forests. 2025; 16(10):1585. https://doi.org/10.3390/f16101585
Chicago/Turabian StyleZhao, Jiaxi, Liming Lai, Ye Mei, Yanming Zhao, Zimo Li, Yanxing Dou, Lin Hou, Qinghong Geng, and Shuoxin Zhang. 2025. "Distinct Roles of Forest Stand Types in Regulating Soil Organic Carbon Stability Across Depths" Forests 16, no. 10: 1585. https://doi.org/10.3390/f16101585
APA StyleZhao, J., Lai, L., Mei, Y., Zhao, Y., Li, Z., Dou, Y., Hou, L., Geng, Q., & Zhang, S. (2025). Distinct Roles of Forest Stand Types in Regulating Soil Organic Carbon Stability Across Depths. Forests, 16(10), 1585. https://doi.org/10.3390/f16101585







