1. Introduction
The majority of studies on the plant communities transformation have indicated progressive rates of species diversity reduction [
1,
2,
3]. A “toxic” increase in species richness in certain regions has been documented, led by the invasion of alien species and loss of native species [
4,
5]. Changes in species composition are typically the result of structural and functional disturbances of forest communities due to natural and/or anthropogenic causes [
6,
7]. The data increasingly indicate cascading effects of climate change on forest communities [
8]. Natural forest disturbances, such as wildfires, windfalls, and pest outbreaks, are becoming more frequent and severe due to climate change or land-use change [
9].
The functional properties of species assemblages in forest communities are an additional aspect of biodiversity [
9]. They provide a better understanding of the ecological consequences of disturbances or successional dynamics of communities [
10]. Functional properties are a set of ecological properties of species that determine the nature of plant growth and development, as well as its responses to environmental conditions [
11]. The grouping of species communities by functional traits is a long-standing idea recently applied to various ecological tasks [
12,
13].
Norway spruce (Picea abies (L.) H. Karst.) is one of the main forest-forming species in the European part of Russia, both in terms of its distribution and economic importance. In the hemiboreal zone (mixed forests), stands dominated by spruce are formed under the centuries-old human activity (logging, land plowing, fires, and silviculture in the modern era of forestry).
Repetitive vegetation monitoring in permanent relevés is a widely used method for assessing changes in plant communities [
6,
14,
15,
16]. The importance of long-term research in old-growth forests is consistently emphasized in relevant reviews [
17,
18,
19]. Most of these studies place great importance on the issues of successional dynamics in mature communities. However, identification of such forest areas is complicated even within specially protected territories.
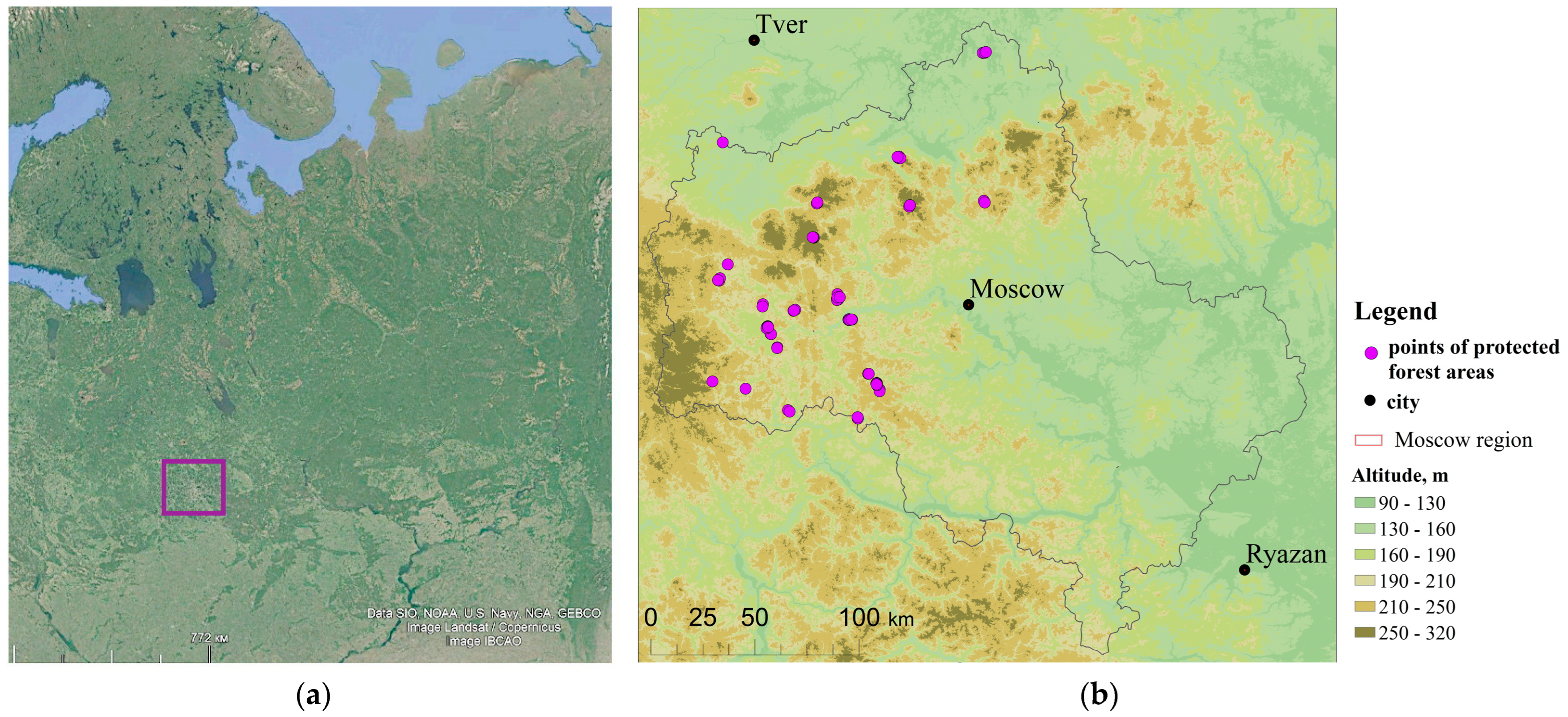
The forest cover of the Moscow region stands out against the background of neighboring territories with a greater representation of intact forests of natural origin due to the prohibition of large-scale industrial logging and the protective status of forests. This fact is a unique opportunity to study the peculiarities of autogenous successional dynamics of zonal forests. Therefore, the history and dynamics of rare old-growth spruce forests of the 19th and 20th centuries are of great interest to understand the potential of their preservation.
In the current study, we suggest a hypothesis of the critical state of old-growth spruce forests in the center of the East European Plain, the decline of which is escalating each year due to global climate change. The aim of our study is to identify the patterns of the structural and functional composition changes in mature spruce forests by repeating relevés after 40 years. The study area is the Moscow region, which is representative of the center of the East European Plain. The results allow us to identify dynamic trends of spruce forests and to forecast their further development in the territory of the East European Plain.
3. Results
Historical cartographic materials indicated that 69% of the studied forest reserves had been forested for at least 300 years, on 28% of relevés the forests emerged about 250 years ago, and only 3% of relevés the forests were less than 200 years old. Remote sensing data from the 1960s and 1980s confirmed the absence of significant disturbance after 1945.
3.1. Changes in Structural and Functional Properties in Spruce Forests
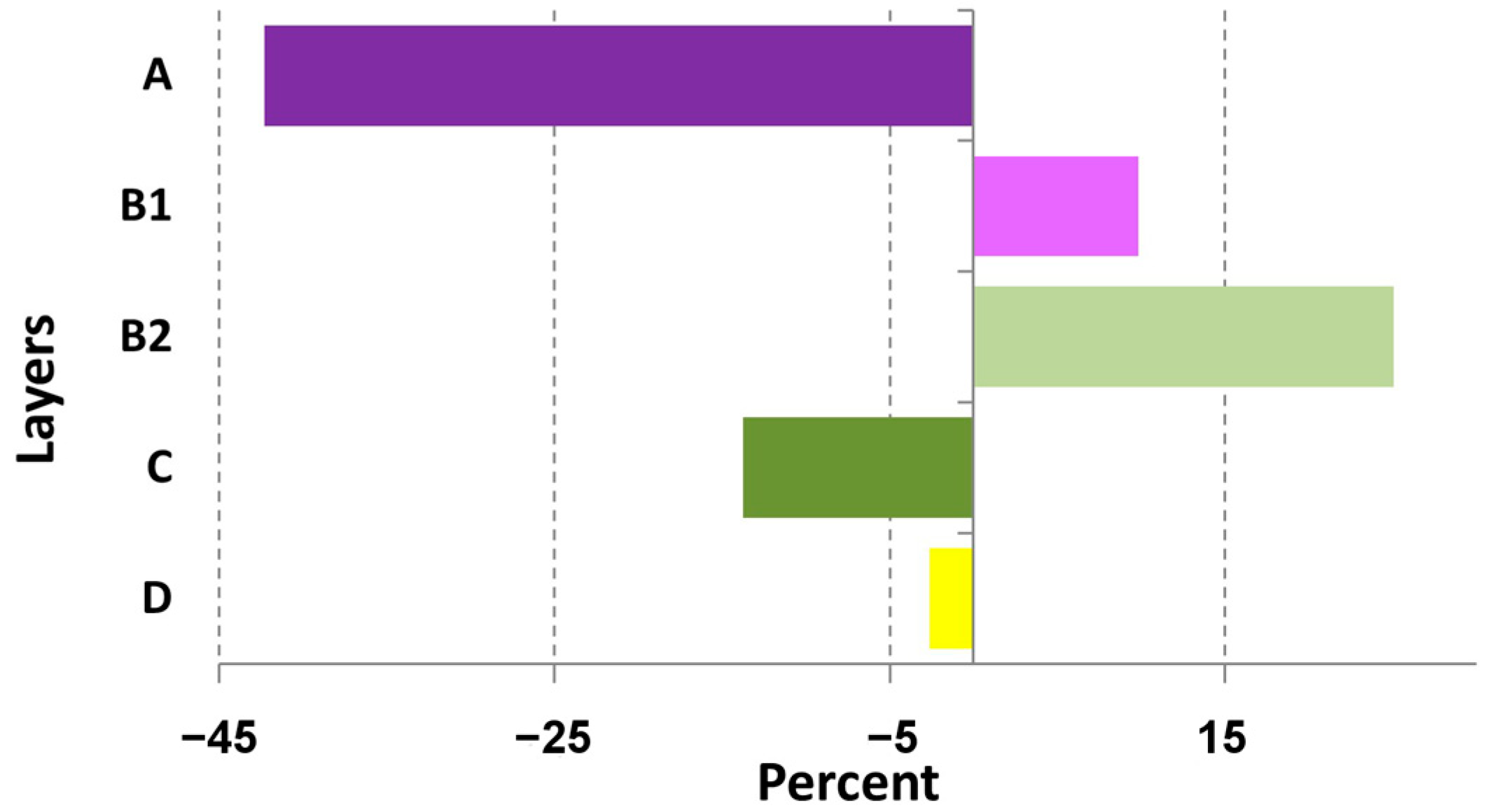
Changes in the general patterns of spruce communities’ structure over the 40-year period are the disruption of stand layer structure of the stand and changes in the species composition of forest-forming species of the tree and shrub layer (
Appendix B,
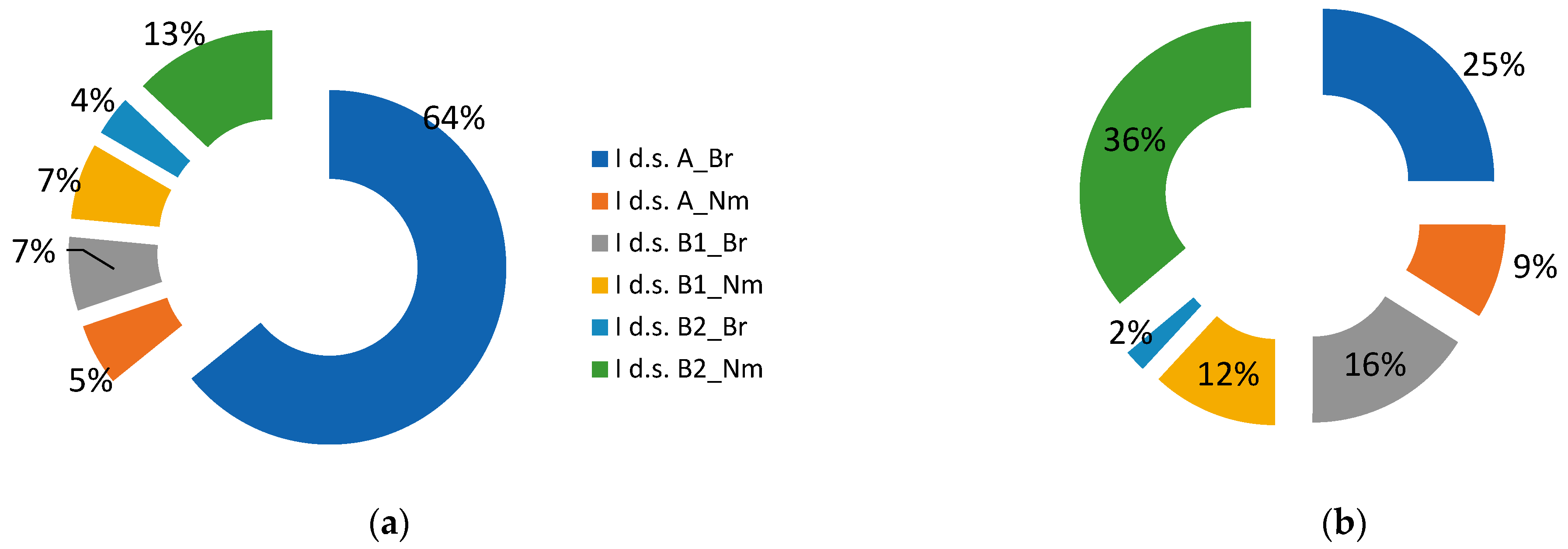
Figure A1a–f). Redistribution of the abundance of plants forming the main structural layers of spruce communities is evident (
Figure 2). It is noticeable that the tree layer (A) cover decreased by 2.5 times, and the understory (B1) and shrub (B2) layers cover increased twofold. Less significant change is lower layers (C and D) cover decrease. All differences were significant (at
p < 0.05) (
Table 1).
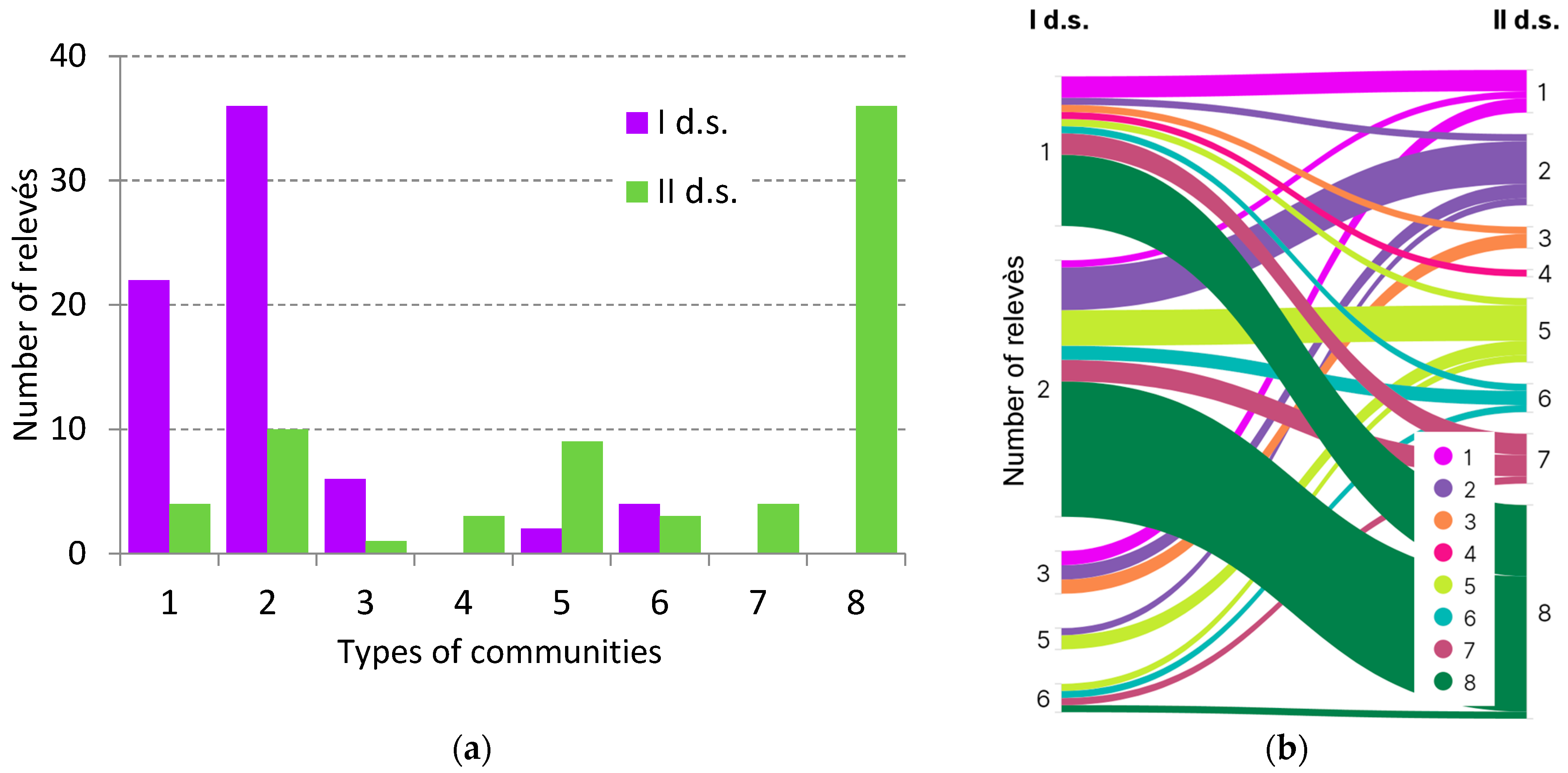
The typological composition of spruce forests has also changed (
Figure 3a). The disintegration of the tree layer in spruce forests caused a change in the set of community types. Five community types were identified in I d.s., and eight in II d.s. There was an increase in typological diversity through the appearance mainly of two new types—with disintegrated tree layers (7 and 8) in place of boreal and nemoral spruce forests (1, 2). In the first case, active regeneration of spruce was observed mainly in point of relevés of boreal (type 1) and nemoral spruce forests (type 2) (
Figure 3b). The composition of communities in destroyed spruce forests (type 8) with regeneration of hazel (
Corylus avellana) and rowan (
Sorbus aucuparia) under the canopy of linden (
Tilia cordata) and maple (
Acer platanoides) was formed mainly from nemoral (type 2) and, to a lesser extent, boreal spruce forests (type 1). Several relevés of nemoral pine–spruce forests (4) were recorded at the point of the spruce boreal type (1).
Detailed structures of tree layers and diagnostic species of herb-dwarf shrubs and moss layers for the identified forest types are given below (
Table 2). The results of LDA showed different accuracy for the selected types. The overall accuracy of the field relevés typology was 90.0% (
Table A3).
3.2. Changes in Common Patterns of Tree and Shrub Layers
Let us consider in more detail the main changes in the tree and shrub layers that determine the redistribution of habitat-forming conditions (light, moisture, and nutrients) for plants of lower levels. Drastic changes are noticeable between the composition and structure of vegetation of the upper layers. The main changes are the decrease in the tree layer cover, dominated by
Picea abies, and the increase in the shrub layer cover, dominated by
Corylus avellana and
Sorbus aucuparia (
Figure 2).
The decrease in the total cover of the tree layer was not only due to the loss of
Picea abies (2.5-fold), but also due to the loss of
Betula sp. (5-fold),
Populus tremula,
Pinus sylvestris, and disappearance of
Alnus incana. The loss of these species is a natural process associated with overmaturity (100 years and more). In contrast, the participation of
Quercus robur has slightly increased (
Table 3).
The cover of understory tends to increase (
Table 4). During the study period,
Picea abies understory increased more than 3-fold, indicating a recovery process as a form of secondary succession and potentially successful regeneration in place of decayed stands.
Acer platanoides and
Quercus robur increased yet more, indicating potential for spruce-broadleaved stands in the future.
The shrub layer was poorly represented in old-growth spruce forests (
Table 5). The predominant species (in descending order) were
Corylus avellana,
Sambucus racemosa,
Sorbus aucuparia, and
Frangula alnus; the participation of other species was almost negligible. The most significant structural change is the fivefold coverage increase in
C.avellana (from 5.7 to 26.36% to 80%–90% in some communities). In many cases hazel shrub communities with woody undergrowth were formed in place of old-growth spruce forests. There is also a tendency of cover increase in rowan (
Sorbus aucuparia) and elderberry (
Sambucus racemosa) to a lesser extent. Other species under the overgrown hazel canopy have decreased their participation.
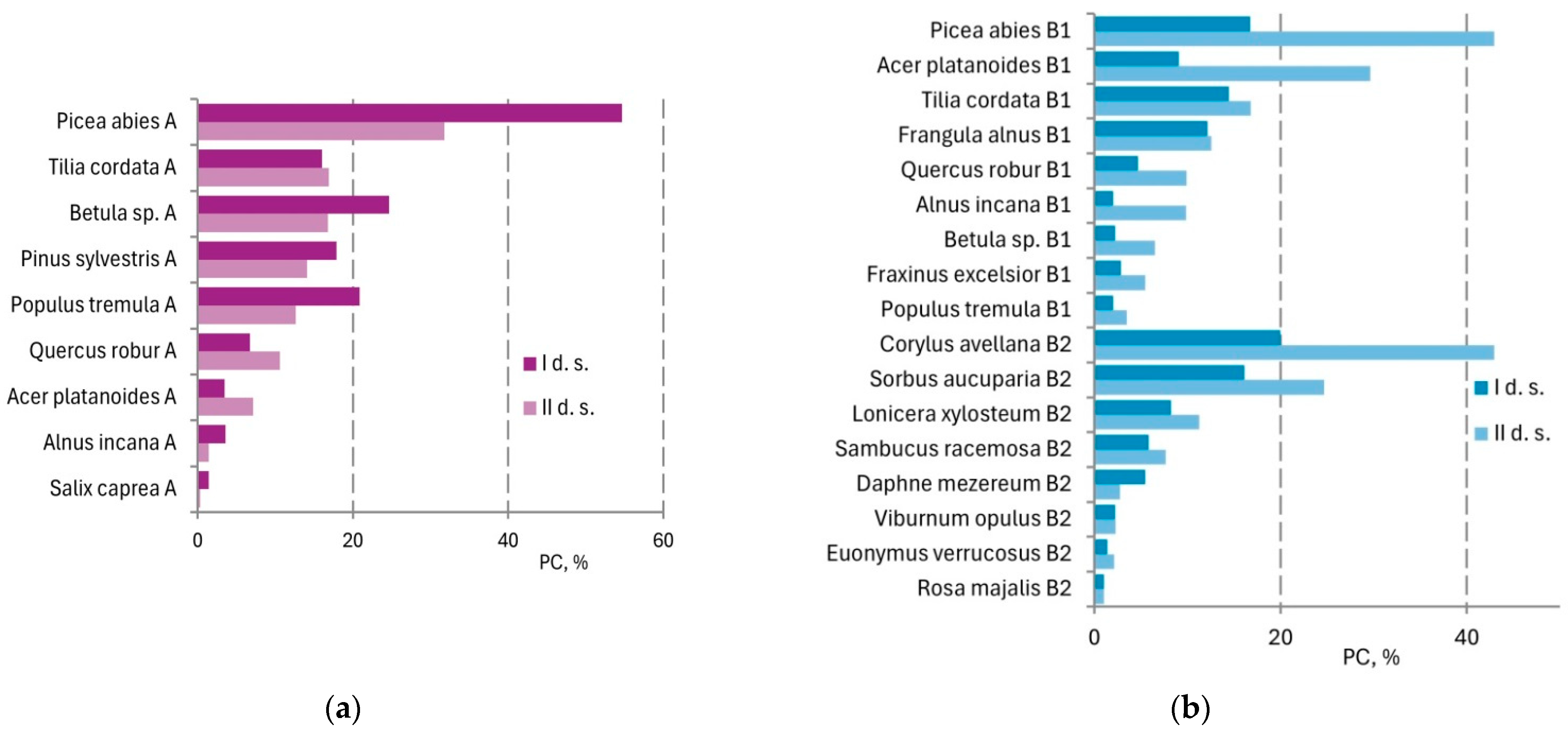
Structural reorganization of the tree and understory layers was reflected in the species activity index (
A) shift during the study period.
A of
Picea abies in the tree layer was reduced by almost half;
Betula sp.,
Pinus sylvestris, and
Populus tremula decreased to a lesser extent. At the same time,
A of
Tilia cordata and
Acer platanoides has increased (
Figure 4a). Significant changes were observed in the understory—
Picea abies increased more than 2 times, and
Acer platanoides 3 times. In the shrub layer,
A of
Corylus avellana increased more than 2 times, and
Sorbus aucuparia and
Lonicera xylosteum increased to a lesser extent (
Figure 4b).
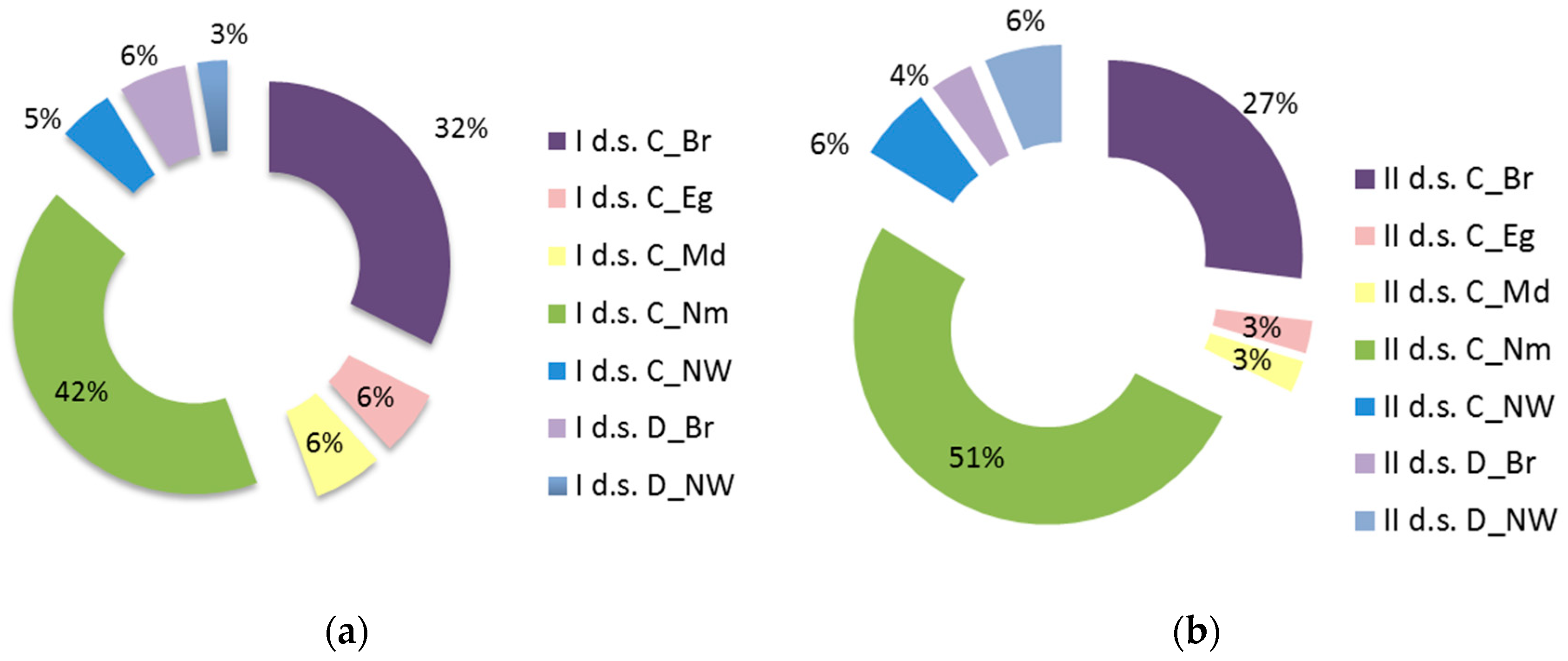
ECGs grouping allows us to assess functional shifts in tree (A), undergrowth (B1), and shrub (B2) layers. The main changes here were observed in the redistribution of species in the boreal and nemoral groups. The coverage of boreal species in the tree layer (A) decreased by 2.5 times, and coverage of the nemoral species increased by 1.5 times. The coverage of boreal species in the undergrowth layer (B1) increased by more than 2 times; coverage of nemoral species changed slightly. Significant reduction in boreal species coverage in the shrub layer (B2), nemoral species coverage increases more than 3 times (
Figure 5a,b). All mentioned changes are significant at
p < 0.05.
There are different trends of phytocenotic links, calculated based on gamma-correlation of abundance of the main forest-forming species in communities for two time periods (
Table A2). The highest positive relationship is for
Tilia cordata and
Pinus sylvestris (r = 0.85, r = 0.81), and the negative correlation is for
Picea abies (r = −0.44) between the study period I d.s. and II d.s. in the tree layer.
The PC of Picea abies in the main layer in II d.s. has a negative correlation with I d.s. values, indicating a reliable change in the distribution of the main forest-forming species at the relevés, while the PC of associated species remained positively correlated over the study period. This indicates that the PC of Corylus avellana and especially Acer platanoides in II d.s. were determined by the values for the previous measurement period. On the contrary, the results of comparison of Picea abies in the main layer cover between the indicators of I d.s. and II d.s. showed their relative independence, and the strong disturbance of the spruce mother layer confirms again cardinal transformations in the structure of communities.
3.3. Changes in Common Patterns in Herb-Dwarf Shrub and Moss Layers
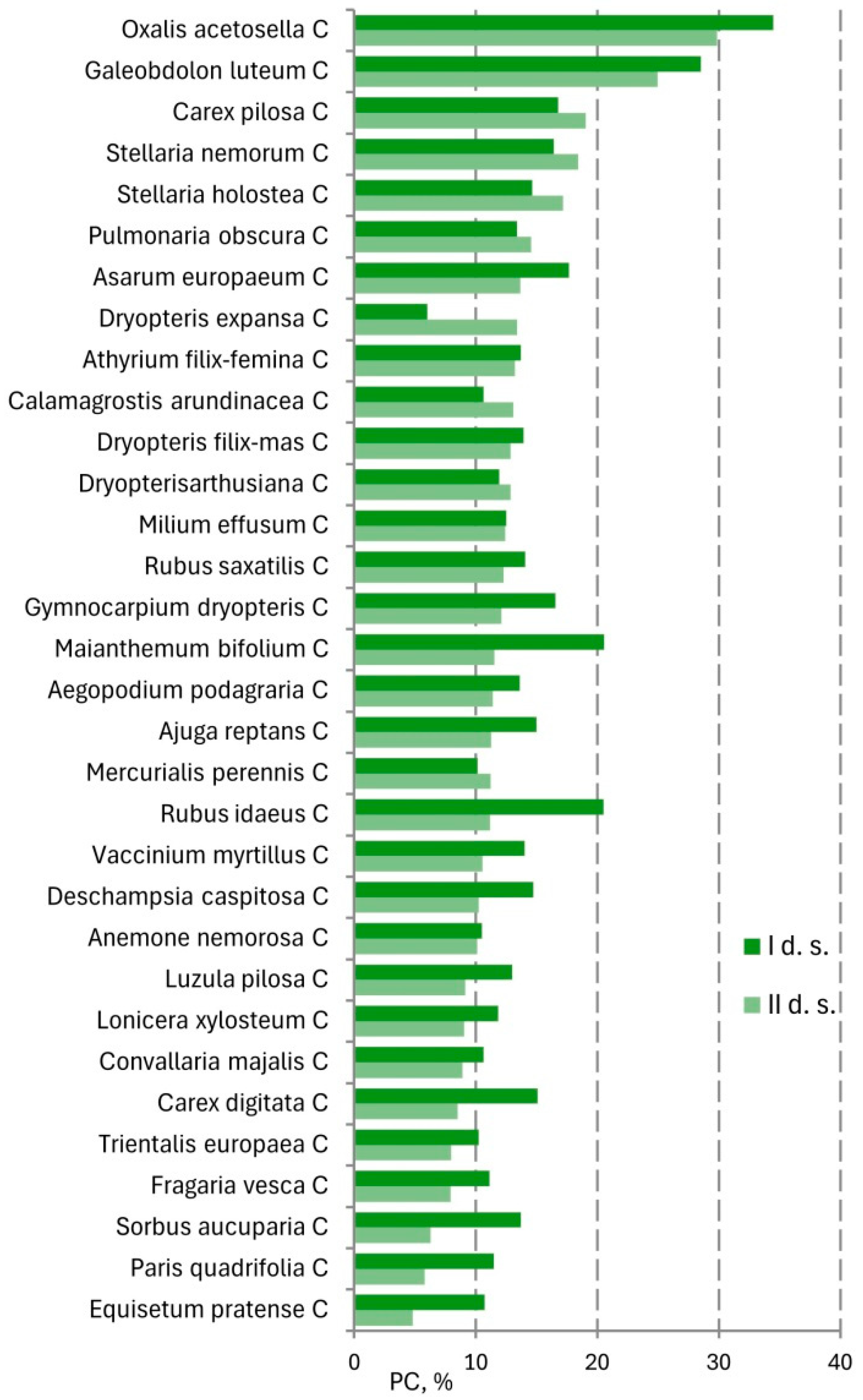
There are no drastic changes in the species composition of the herb-dwarf shrub (C) layers according to the species activity index (
A). The changes were mostly related to the redistribution of species abundance while maintaining the general composition of species (
Figure 6).
There was also a restructuring pattern in the ecological-phytocoenotic structure of herb-dwarf shrub (C) and moss (D) layers (
Figure 7). A minor significant (
p < 0.05) decrease in the proportion of boreal vascular species and a minor increase in nemoral species. A decrease in edge-herb (Eg) and meadow (Md) species, significant changes in boreal (Br) and edge-herb (Eg) species of the moss layer (D).
3.4. Relationship with Environmental Factors
The relationship of spruce community type’s organization with external environmental factors, in particular, climate and terrain conditions, allows us to reveal specific patterns.
The mean value of maximum temperatures of I d.s. is 17.9 ± 0.7 °C, and II d.s.—19.5 ± 0.7 °C. Precipitation accumulation of I d.s. is 69.9 ± 4.7 mm, II d.s.—65.4 ± 4.2 mm. Climate water deficit of I d.s. is 12.1 ± 2.5, II d.s.—20.6 ± 3.3. According to Mann–Whitney U tests, the differences are significant for maximum temperatures and climate water deficit, which are higher for the II d.s.
Spearman rank correlations between the indicators of forest-forming species abundance and terrain elevation, slope, and curvature were calculated. There was a significant correlation between elevation and undergrowth (B1) PC, positive: Corylus avellana (r = 0.49 in I d.s., r = 0.35 in II d.s.), Acer platanoides (r = 0.31 in I d.s., r = 0.35 in II d.s.), negative: Pinus sylvestris (r = −0.33 in I d.s., r = −0.29 in II d.s.), and Picea abies (r = −0.32 in I d.s., r = −0.36 in II d.s.). No significant relationships with slope and curvature.
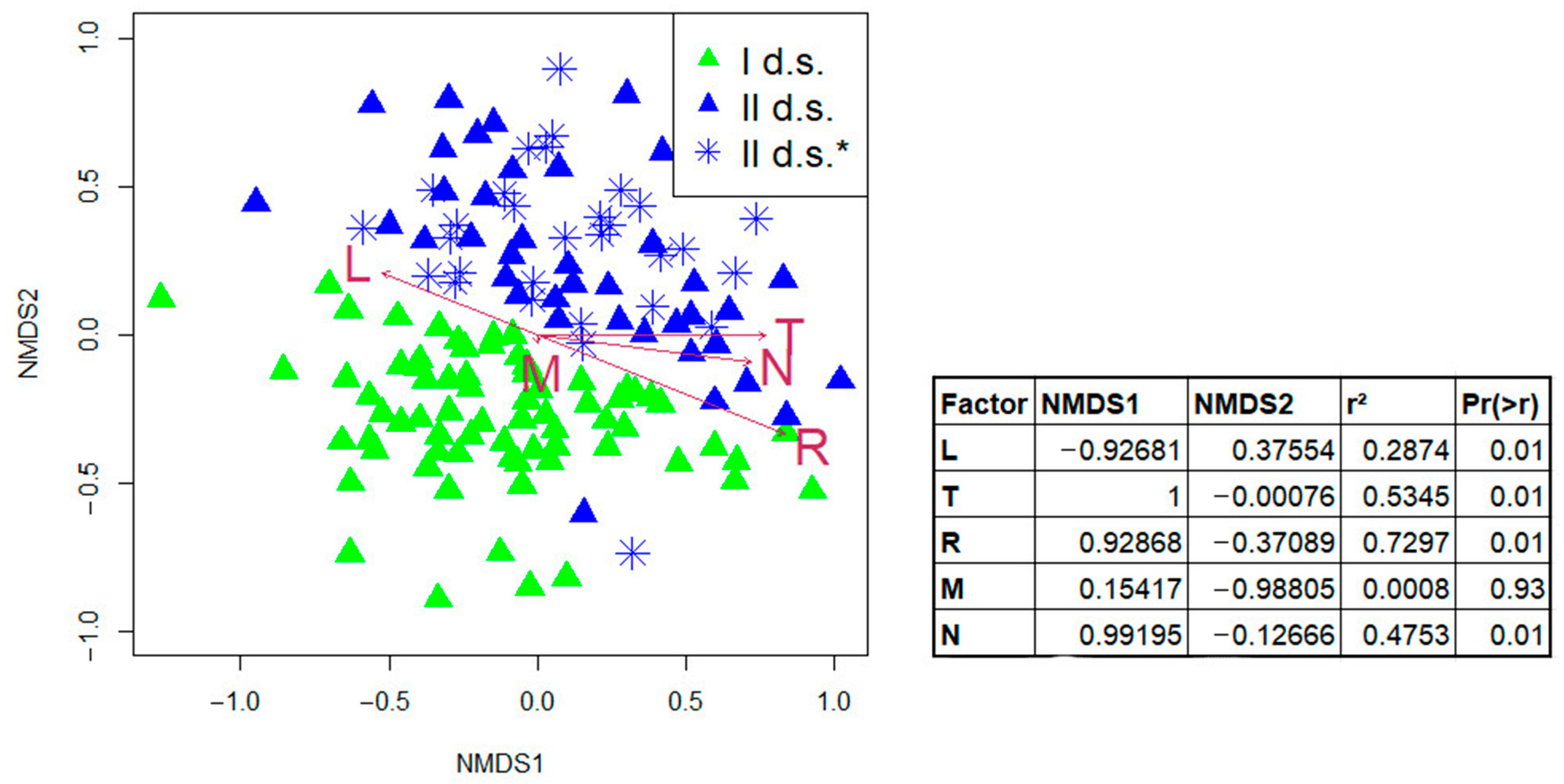
Changes in ecological conditions of habitats were assessed by linking community types to biotopic factors expressed through Ellenberg ecological scales using non-metric multidimensional scaling (NMDS ordination). Changes in light (L), temperature (T), soil reaction (R), and soil nitrogen (N) factors were associated with the first axis of variation (NMDS1). Changes in moisture content (M) and, to a lesser extent, soil reaction (R)—associated with the second axis (NMDS2). The correlations between environmental factors and ordination axes and regression coefficient (r2) are high enough, which indicates significant change in environmental factors for the studied time interval. The stress value was 0.24 when reducing the multidimensional space to a two-dimensional one.
In general, the gradient of displacement of the set of relevés in II d.s. indicated a tendency of maximum increase in the values of temperature and decrease in soil reaction, contributing to the formation of a nemoral spectrum of species. Soil reaction and moisture content were relatively “weak” factors (
Figure 8).
4. Discussion
There are many cases of transformations of community composition and structure in the course of community dynamics in the literature, in which typical mosaic-cyclic processes have been described [
42,
43,
44]. Strong winds tend to act as the main disturbance type in European temperate forests. This leads to small-scale episodic disturbances [
45,
46], where disturbed areas are usually reforested by highly competitive, shade-tolerant climax tree species. Outbreaks of the bark beetle
Ips typographus often occurred after large windthrow events in Western Europe [
47]. Mass spruce dieback in the forests of Central Russia was preceded by unfavorable climatic conditions (droughts) during the vegetation period [
48,
49], which weakened trees and reduced their ability to resist pests and diseases [
50,
51]. Observations of spontaneous dynamics of spruce forest communities in the Central Forest Reserve confirmed a similar tendency of dieback within 1–2 years as a result of recurrent meteorological anomalies, further weakening of trees, and insect pest outbreaks [
52].
Our study recorded drastic changes in spruce communities within the center of the East European Plain that occurred over a period of 40 years and confirmed our assumptions. The restructuring in mature communities of natural origin is associated with the peculiarities of the formation of cenopopulations of tree species. The tree layer in mature communities is characterized by a gradual decrease in PC from the upper to the lower layers and mutual oppression of trees of younger generations. Phytocenotic significance of interaction as a differentiation factor and high competition for environmental factors (light, moisture, soil nutrients) was investigated in other studies [
53,
54,
55]. In trees of older generations, the significance of phytocenotic factors is inferior to environmental factors, in particular, hurricane winds or sharp fluctuations in the water table. Therefore, the impact of extreme factors turns out to be the most dangerous for the largest trees of older generations [
56]. In our case, such dramatic changes reflect the fact of complete or partial mortality of the mother spruce stand, including as a result of mass outbreaks of bark beetle in 1999–2003 and 2010–2013.
Two directions of secondary succession were recorded after the collapse of the mother spruce stand: (1) with restoration of
Picea abies undergrowth and (2) with active development of shrubs (
Corylus avellana and
Sorbus aucuparia) and broadleaved undergrowth. Analysis of the sensitivity of forest-forming species to environmental conditions was used in the study of successional direction. In our study we observed a significant relationship of
Picea abies,
Acer platanoides, and
Corylus avellana undergrowth with morphometric indicators of terrain. This is consistent with other data—boreal spruce forests are typical for flat terrain. While nemoral (complex) spruce forests with participation of broadleaved tree species are typical for convex moraine watershed plateaus [
57].
It has been established that the mean annual temperature has increased over 40 years by 2 °C [
58]. For spruce forests at their southern limit of distribution, such warming corresponds to a shift in the vegetation period isotherms by about 150 km to the south [
59]. This leads to a transformation of the formational composition of forests. Indeed, in our study, typological diversity of the studied communities increased after 40 years not only due to the loss of the tree layer and the formation of new “non-forest” community types, but also due to the transition of a part of mixed spruce–broadleaf communities to broadleaf communities and of pine–spruce communities of the boreal type to the nemoral type. Analysis of the complete species composition of spruce forests and the ratio of species of different ECGs confirmed the general nemoralization of the composition—while the species composition as a whole remained the same, the abundance of boreal-type species in all structural layers decreased, while the abundance of nemoral species increased. The gradient of the shift in the set of modern relevés in the ecological space indicated a tendency of increasing values of temperature and decreasing soil acidity, which contributed to the formation of a nemoral spectrum of species. The increase in the proportion of nemoral species in the forests of the Moscow region has been noted already since the 1990s in other studies [
60].
In this case, it is difficult to separate the interrelated effects of natural succession and climate warming, as soil enrichment and increased availability of nutrients favoring the development of the nemoral plant spectrum occur in both cases. There is evidence that complex patterns of change in species biodiversity are also observed when combining factors of abandonment of traditional management in Czech plant communities and in association with increased litter accumulation [
61,
62]. Both processes support stronger competitors and likely contribute to the co-evolution of vegetation [
63]. In addition, climate warming may extend the growing season, contribute to biomass increases, and alter successional processes [
64,
65]. The results of the study of oak stands in Poland revealed different effects of an increase in air temperature of 1 °C on the two oak species (slowing down the growth of
Quercus robur but improving the growth of
Q. petraea) [
66]. This indicates different responses of species to changing environmental conditions.
It should also be considered that the mechanisms of intra- and interspecific competition are important driving forces in the feedback loop of the structure and functioning of the stand [
67,
68]. Analysis of interspecific relationships in the composition of tree species in the upper and subordinate layers (undergrowth and shrubs) demonstrated significant relationships that predetermine the likely course of development of spruce communities. Thus, on the one hand, a significant positive relationship was observed between different generations of tree (
Acer platanoides,
Quercus robur, and
Pinus sylvestris) and shrub (
Corylus avellana) species at different study times; on the other hand, a negative correlation was noted between the abundance of
Picea abies in the upper layer in I d.s. and II d.s. At the same time, the active development of young trees of
Acer platanoides in the undercanopy space of the main layer is currently negatively associated with the formation of
Picea abies undergrowth but positively with
Populus tremula in the upper layer. Ecological features of the species, primarily the illumination factor, explain this picture. As a whole, the dynamics of correlation links indicate changes in the structure and interactions between species of the ecosystem between two study periods. Strengthening of positive links may indicate closer cooperation or joint response of species to environmental conditions in the process of successional dynamics. Whereas the emergence of negative links indicates competition or division of resources.
Considering the life cycles of vegetation (10–20 years for the shrub layer with understory and 40 years for the tree layer) [
69], we can assume that if the protected status is maintained, over the next 40–60 years the composition of spruce forests will approach coniferous–broadleaved forests, and the latter will transition to broadleaved forests. This rate of transition of successional stages is close to the pattern of forest succession in a number of spruce and mixed (with undergrowth of
Quercus robur,
Tilia cordata, and
Picea abies) communities in the south of the Moscow region [
70]. An assessment of coniferous forest dynamics in the Moscow region based on historical Landsat images in the period from 1990 to 2020 showed that the area of spruce forests of natural and artificial origin decreased from 19.6 to 7% [
23]. Thus, the total area and composition of spruce forests in the region will decrease in the future due to direct loss of spruce in mature stands of both artificial and natural origin and subsequent transition to mixed forests.
5. Conclusions
The presence of mature forests of natural origin in the Smolensk–Moscow Upland is a unique opportunity to study the peculiarities of autogenous successional dynamics of zonal forests. In this study we considered disturbances in the structural and functional organization of old-growth spruce forests over the last four decades, caused by the dieback of upper canopy trees. The initiation of this type of disturbance was a general increase in temperatures and especially extreme droughts provoking weakening of spruce stands and invasions of insect pests. Restoration of mixed and broadleaved communities through active development of Corylus avellana and Acer platanoides undergrowth in higher elevation positions with better drainage was more common. The development of a young generation of Picea abies in canopy windows with subsequent restoration of boreal-type spruce forests was observed in flat terrain forms. In general, there was an undoubted trend of nemoralization of plant species composition, confirmed by quantitative assessment of the shift in biotopic characteristics towards an increase in average temperature, decrease in soil acidity, and increase in soil fertility.
In the next 40–60 years, under the current forest management regime in the Moscow region, as well as in the center of the East European Plain, Picea abies is expected to be present as a companion species in mixed stands with a complete loss of “pure” spruce forests.
The main avenues for future research are (1) a more detailed assessment of the dynamics of species diversity of spruce communities and their derivatives; (2) identifying differences in the dynamics of natural forests in reserves and plantation forests; and (3) comparison of dynamic processes not only in spruce, but also in pine forests.