Abstract
In tropical regions, the establishment of large-scale exotic plantations has addressed the demand for timber resources but has also disrupted the structural stability of native vegetation and altered soil nutrient cycling, thereby impairing ecosystem functions. Identifying effective restoration strategies for these plantations is crucial for sustainable forest management and ecological security. This study examined Acacia mangium Willd., Cunninghamia lanceolata (Lamb.) Hook., and Pinus caribaea Morelet. plantations in Hainan Tropical Rainforest National Park under three treatments: plantation control, girdling, and natural secondary forest. Vegetation surveys and soil analyses were conducted to explore the relationships between community structure, soil physicochemical properties, and enzyme activities. Diversity indices, Pearson correlations, and redundancy analysis were used to assess plant–soil relationships. The results showed that girdling significantly accelerated succession in C. lanceolata and P. caribaea plantations, increased species diversity, and enhanced the dominance of native species. Shrub-layer diversity indices (Hshrub, Dshrub, Eshrub) were the main drivers of soil properties and enzyme activities, while tree-layer effects were weaker. Girdling regulated soil nutrients and biological activity primarily via changes in community structure. These findings highlight the importance of optimizing shrub-layer structure and enhancing diversity for tropical plantation restoration. Combining forest type conversion with moderate interventions can promote coordinated plant–soil development over time.
1. Introduction
Tropical rainforests, among the most biodiverse and ecologically functional terrestrial ecosystems on Earth, play a critical role in global climate regulation, biodiversity conservation, and the carbon cycle [1,2]. Hainan Tropical Rainforest National Park, one of China’s first pilot sites for the national park system, preserves the most intact continental island-type tropical rainforest in the country and holds substantial ecological, scientific, and exemplary significance [3]. However, as a result of human activities in the past, extensive areas of natural forest within the park have been replaced by plantations of exotic fast-growing species, including Acacia mangium Willd., Cunninghamia lanceolata (Lamb.) Hook., and Pinus caribaea Morelet., leading to a simplified ecosystem structure and diminished ecological functions [4]. Current statistics indicate that plantations cover 842 km2 within the park, accounting for 19.3% of its total area, underscoring the urgent need for scientifically robust and effective approaches to facilitate the ecological transition of plantations toward zonal tropical rainforest vegetation [5].
In recent years, restoring the “authenticity” and “integrity” of ecosystems has become a core objective in the conservation and rehabilitation of tropical rainforests. Although previous studies have attempted to restore plantations through near-natural transformation measures such as clear-cutting, selective logging, enclosure, and enrichment planting [6,7], these approaches encounter substantial challenges under the complex environmental conditions and management constraints of Hainan’s tropical rainforests, including high operational difficulty, extended restoration timelines, and uncertainty in recovery outcomes. Within this context, tree girdling—a technique characterized by high controllability and minimal disturbance—has drawn increasing attention. This method severs the phloem transport system of the trunk, leading to the death of targeted trees within a certain period, thereby creating canopy gaps, freeing understory space, and promoting the natural regeneration of native species [8]. Girdling has been explored in some tropical rainforests overseas as a tool for habitat creation or as an alternative to logging [9,10]. Girdling has also been widely applied in studies of tropical plantations. In Brazil, girdling treatments in two Eucalyptus plantations were conducted to examine their effects on root respiration [11]; in Malaysia, selective girdling in Dryobalanops beccarii plantations was used to evaluate the growth performance of plantation trees [12]; and in Indonesia, girdling in Acacia mangium plantations was employed to investigate its effects on wood properties and drying characteristics [13]. These studies demonstrate that girdling can serve not only as a tool for habitat creation or production management, but also significantly influence tree physiological functions and wood traits, thereby providing valuable insights for the management of tropical plantation forests. However, its potential in structural reconstruction of plantation ecosystems and soil quality enhancement has yet to be systematically evaluated, and ecological restoration experiments in China that employ girdling specifically to induce the death of target trees remain particularly limited.
Previous studies have demonstrated that girdling can effectively accelerate the succession of plantation forests toward the community structure of natural forests, promoting the germination and growth of native broad-leaved tree species in the understory, thereby increasing the diversity and structural complexity of community species [14]. In addition, shifts in stand composition, the decomposition of deadwood, and changes in litter input patterns may have significant impacts on soil physicochemical properties, aggregate structure, and nutrient cycling [15,16]. In tropical regions, high temperatures and humidity accelerate material turnover, rendering soil structure and nutrient dynamics particularly sensitive to management interventions [1]. Therefore, examining the effects of girdling on community structure and soil quality in tropical plantations is of considerable importance for understanding restoration mechanisms and identifying ways to enhance ecological functions.
This study focuses on girdling experiments conducted in representative tropical plantations in Hainan, including A. mangium, C. lanceolata, and P. caribaea, and examines their effects on community composition, structural dynamics, and soil physicochemical properties. The objective is to identify scientifically robust and feasible ecological pathways and management strategies to accelerate the transition of tropical plantations toward zonal natural forests.
2. Materials and Methods
2.1. An Overview of the Study Area
The study area is located in the Jianfengling section of Hainan Tropical Rainforest National Park (108°44′32.74″–109°04′05.92″ E, 18°34′59.73″–18°52′46.77″ N), situated in the southwestern part of Hainan Island, spanning Ledong County and Dongfang City (Figure 1). It covers an area of 679 km2 with a forest cover reaching 98%. The region experiences a tropical marine monsoon climate, with an annual mean temperature of 24.5 °C. The rainy season occurs from May to October, with an average annual precipitation of 2265.8 mm, a mean relative humidity of 88%, and an average annual sunshine duration of 1625 h. Elevation ranges from 112 to 1412.5 m. Soils distributed along the elevation gradient primarily include lateritic soil, lateritic yellow soil, and mountain yellow soil, with lateritic red and yellow soils being the most prevalent. The zonal vegetation consists mainly of seasonal tropical rainforests, tropical lowland rainforests, and montane rainforests. Secondary forests, mainly resulting from commercial logging, constitute the remainder of the tropical natural forest cover.
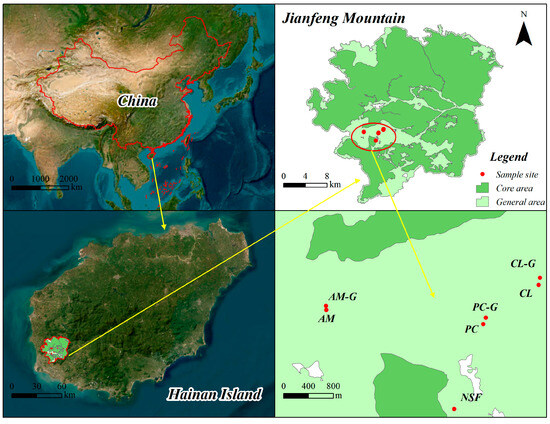
Figure 1.
Distribution map of sample plots. Note: AM: Acacia mangium Willd. plantation, AM-G: Acacia mangium plantation (Girdling), CL: Cunninghamia lanceolata (Lamb.) Hook. plantation, CL-G: Cunninghamia lanceolata plantation (Girdling), PC: Pinus caribaea Morelet.plantation, PC-G: Pinus caribaea plantation (Girdling), and NSF: Natural secondary forest.
2.2. Plot Establishment and Vegetation Survey
2.2.1. Plot Establishment
Between the 1960s and 2000, driven by the operational restructuring of forestry enterprises, the forest area of Jianfengling introduced several exotic plantation species into logged natural forest areas or degraded lands. These include A. mangium plantation (AM; planted in 2004), C. lanceolata plantation (CL; planted in 1991), and P. caribaea plantation (PC; planted in 1996). Under comparable elevation, aspect, and slope conditions, this study selected the plantations mentioned above, along with nearby undisturbed or minimally disturbed natural secondary forests, as controls to establish the study plots (Table 1). For each plantation species—A. mangium, C. lanceolata, and P. caribaea—two treatments were applied: (1) Plantation control with no intervention (Natural), and (2) Near-natural restoration via girdling treatment. Natural secondary forest (NSF) plots were established near the plantations as a reference. Three replicate plots of 20 m × 20 m were set up within each stand, spaced at least 20 m apart, where separate vegetation surveys of the tree and shrub layers were conducted. Plantation densities were recorded as 301 trees per hectare for A. mangium (Average height: 17.19 m, average DBH: 28.36 cm), 1108 trees per hectare for P. caribaea (Average height: 14.04 m, average DBH: 16.37 cm), and 2225 trees per hectare for C. lanceolata (Average height: 12.16 m, average DBH: 12.05 cm). The girdling treatment was implemented in July 2022, with all plantation tree trunks undergoing girdling. The procedure involved removing a continuous 35–40 cm wide strip of bark and cambium around the entire circumference of the trunk at a height of 40–100 cm, penetrating approximately 1 cm into the xylem to create a girdling wound (Figure 2). Precautions were taken to avoid damaging naturally regenerating seedlings within the stands during the treatment.

Table 1.
Basic information of sample plots in the study area.

Figure 2.
Treatment effects of different forest types. Note: (a): Acacia mangium plantation (Girdling), (b): Cunninghamia lanceolata plantation (Girdling), (c): Pinus caribaea plantation (Girdling), and (d): Natural secondary forest.
2.2.2. Vegetation Survey
Vegetation surveys were conducted in each plot in July 2022 (prior to girdling) and December 2024 (post-girdling). Within each 20 m × 20 m plot, all woody plants in the tree layer with a diameter at breast height (DBH) ≥ 3 cm (including both tree species and large-stemmed shrub species) were measured individually. Recorded parameters included species identity, number of individuals, DBH, tree height, and crown width. For the shrub layer, all woody plants with DBH < 3 cm (including saplings of tree species and shrub species) were surveyed, with species identity, number of individuals, basal diameter, and height recorded.
2.3. Analysis of Dominant Species
The importance values of species in each forest layer of various plantations and natural secondary forests were calculated. The top five species ranked by importance value within each layer were identified as the dominant species. The calculation formula [17] is as follows:
where IV denotes the importance value, RA the relative abundance, RF the relative frequency, and RDo the relative dominance.
For the shrub layer, relative dominance is substituted by relative height (RH), calculated as
2.4. Analysis of Species Diversity
Patrick’s richness index (R) [18], Shannon diversity index (H) [19], Simpson diversity index (D) [20], and Pielou’s evenness index (E) [21] were calculated to assess species diversity within different forest layers of various plantations and natural secondary forests. The relevant formulas [22] are as follows:
where S represents the total number of species within a sample plot and Pi represents the proportion of individuals belonging to the i-th species relative to the total number of individuals in the community.
2.5. Soil Sampling and Analysis
In December 2024, soil samples were collected from the 0–20 cm and 20–40 cm soil layers of each plot using a soil auger, following the five-point sampling method, which involved compositing soil samples from five points within each plot to form a single sample per depth. These samples were used to analyze soil physicochemical properties. Additionally, undisturbed soil samples were obtained for the measurement of soil physical properties. Soil physicochemical properties were determined according to the methods described by Bao et al. [23]. Soil pH was measured potentiometrically; soil organic carbon (SOC) was quantified using the potassium dichromate oxidation method with external heating; total nitrogen (TN) was determined by the Kjeldahl method following digestion with concentrated sulfuric acid; total phosphorus (TP) was analyzed using the molybdenum-antimony anti-spectrophotometric method after digestion with H2SO4-HClO4; total potassium (TK) was measured by flame photometry after digestion with HF-HClO4; alkali-hydrolyzable nitrogen (AN) was assessed via the alkaline hydrolysis method; available phosphorus (AP) was extracted using the Bray method and determined spectrophotometrically; and available potassium (AK) was extracted with NH4OAc and quantified by flame photometry. Soil texture was determined using the pipette method. Soil enzyme activities were also measured to evaluate biological activity [24]. Urease (URE) activity was assayed by the phenol-sodium hypochlorite colorimetric method, measuring the ammonium nitrogen released from urea hydrolysis; cellulase (Cx) activity was quantified using the DNS colorimetric method, determining the amount of reducing sugars produced; acid phosphatase (ACP) activity was measured using the pNPP colorimetric method, assessing the production of p-nitrophenol. All procedures strictly adhered to standard operating protocols to ensure data accuracy and reliability.
2.6. Data Analysis
This study used multiple statistical approaches for data analysis. One-way ANOVA combined with the post hoc LSD test was employed to compare significant differences in various indices among different forest types. Paired t-tests were used to analyze changes in diversity indices before and after girdling treatment, as well as differences in soil properties between soil layers. Pearson correlation analysis was performed to examine the relationships between diversity indices and physicochemical factors of soil. Additionally, redundancy analysis (RDA) was conducted to identify the primary environmental factors of variations in soil properties. The explanatory power and significance of each environmental factor were assessed via Monte Carlo permutation tests with 999 permutations. All statistical analyses were performed using R (version 4.2.0), utilizing packages such as psych, vegan, rdacca.hp, and ggplot2 for data visualization and analysis. The significance threshold was set at p < 0.05.
3. Results
3.1. Changes in Community Composition and Diversity Across Different Forest Types Before and After Girdling
3.1.1. Changes in Community Composition and Diversity of the Tree Layer
Between the pre-treatment period (2022) and post-treatment period (2024), significant changes occurred in the dominant species composition of the tree layer across different forest types (Table S1). In the AM plantation, A. mangium remained the absolute dominant species in the control plots; however, its importance value decreased from 28.60% to 24.90%. In addition, Alchornea rugosa and Microcos paniculata exchanged dominance rankings in 2024. In contrast, girdling-treated plots exhibited a pronounced decline in A. mangium, while native species such as M. paniculata and Ficus variegata showed significant increases in importance value. In CL and PC plantations, the dominant species composition in the tree layer remained relatively stable in the control plots, continuing to be dominated by the planted species C. lanceolata and P. caribaea. However, girdling treatment resulted in decreased importance values of these principal plantation species, which were partially supplanted by native broad-leaved species, including Psychotria asiatica and Engelhardia roxburghiana. The NSF exhibited no significant changes in the top five dominant tree species between 2022 and 2024, indicating a highly stable community structure.
Changes in diversity indices of the tree layer across different forest types are presented in Figure 3. The NSF maintained stable and relatively high levels of Rtree, Htree, Dtree, and Etree between the two sampling years. In the AM plantation and its girdled plots, diversity indices did not change significantly before and after girdling treatment (p > 0.05); however, Htree, Dtree, and Etree remained relatively high and continued to differ significantly from those in the natural forest (p < 0.05). In CL plots, girdling treatment significantly increased Htree (p < 0.05), with Dtree and Etree also exhibiting significant improvement. No significant differences were observed between the two years in the untreated CL plots, and overall diversity remained comparatively low. The PC plantation and its girdled plots exhibited an increasing trend in diversity indices in 2024. Notably, in the girdled PC plots, Htree and Etree approached or even surpassed values observed in the natural forest, although these changes had not yet reached statistical significance. Table S1. Dominant species (top 5) in the tree layer among different forest types pre-girdling (2022) and post-girdling (2024).
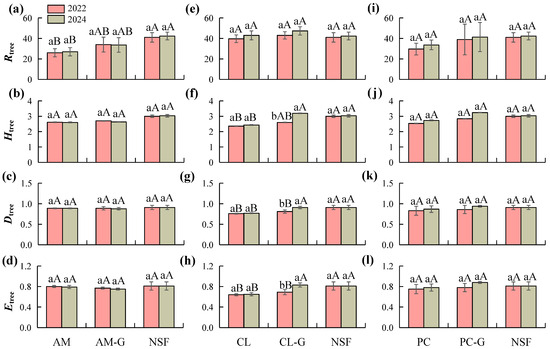
Figure 3.
Changes in tree layer plant diversity indices across different forest types between pre-girdling (2022) and post-girdling (2024). (a–d) Changes in tree layer diversity indices (Patrick index R tree, Shannon-Wiener index H tree, Simpson index D tree, Pielou index E tree,) for AM, AM-G, and NSF between 2022 and 2024. (e–h) Changes in tree layer diversity indices for AM, AM-G, and NSF between 2022 and 2024.for CL, CL-G, and NSF between 2022 and 2024. (i–l) Changes in tree layer diversity indices for PC, PC-G, and NSF between 2022 and 2024. Note: Different lowercase letters indicate significant differences (p < 0.05) between pre-girdling (2022) and post-girdling (2024) within the same forest type, while different uppercase letters represent significant differences (p < 0.05) among different forest types. AM: Acacia mangium plantation, AM-G: Acacia mangium plantation (Girdling), CL: Cunninghamia lanceolata plantation, CL-G: Cunninghamia lanceolata plantation (Girdling), PC: Pinus caribaea plantation, PC-G: Pinus caribaea plantation (Girdling), and NSF: Natural secondary forest.
3.1.2. Changes in Community Composition and Diversity of the Shrub Layer
As presented in Table S2, the community composition of the shrub layer in the natural control plots across different forest types remained relatively stable, dominated by shade-tolerant, broad-leaved species such as A. rugosa (AM) and P. asiatica (CL, PC, NSF), with minimal variation in dominant species. Following girdling treatment, more pronounced signs of community succession emerged within the plantations. In the AM plantation, the relative importance of Desmos chinensis and Mallotus anomalus increased significantly. In CL and PC plantations, native species, including Tabernaemontana bufalina, Lasianthus trichophlebus var. latifolius, and Polyspora hainanensis, were introduced. By contrast, natural forest communities exhibited high compositional stability with negligible changes in dominant species.
Comparisons of community diversity indices between 2022 (pre-girdling) and 2024 (post-girdling) further confirmed the recovery trend of community structure (Figure 4). Changes in Rshrub were not significant across forest types, and Hshrub, Dshrub, and Eshrub were relatively stable; however, significant differences among forest types persisted (p < 0.05). The NSF consistently maintained the highest species diversity and evenness (Hshrub = 3.51, Dshrub = 0.95, Eshrub = 0.87), significantly surpassing all plantation types. The PC and its girdled plots (PC-G) ranked second in diversity. Girdling treatment did not induce significant changes, but diversity indices approached those of the natural forest. The CL plantation exhibited the lowest diversity, with an Hshrub of 2.78 in 2024. Nonetheless, girdling treatment increased Hshrub to 3.13 and Eshrub from 0.68 to 0.73. Although these changes were not statistically significant, they indicated a positive trajectory toward ecological restoration. The AM plantation and its girdled plots exhibited minimal variation in community structure and diversity indices, maintaining an overall low level of diversity.
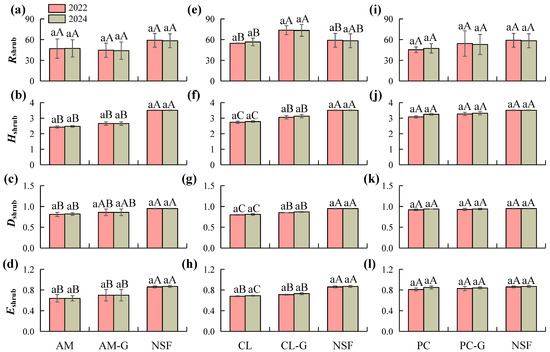
Figure 4.
Changes in shrub layer plant diversity indices across different forest types between pre-girdling (2022) and post-girdling (2024). (a–d) Changes in tree layer diversity indices (Patrick index R tree, Shannon-Wiener index H tree, Simpson index D tree, Pielou index E tree,) for AM, AM-G, and NSF between 2022 and 2024. (e–h) Changes in tree layer diversity indices for AM, AM-G, and NSF between 2022 and 2024.for CL, CL-G, and NSF between 2022 and 2024. (i–l) Changes in tree layer diversity indices for PC, PC-G, and NSF between 2022 and 2024. Note: Different lowercase letters indicate significant differences (p < 0.05) between pre-girdling (2022) and post-girdling (2024) within the same forest type, while different uppercase letters represent significant differences (p < 0.05) among different forest types. AM: Acacia mangium plantation, AM-G: Acacia mangium plantation (Girdling), CL: Cunninghamia lanceolata plantation, CL-G: Cunninghamia lanceolata plantation (Girdling), PC: Pinus caribaea plantation, PC-G: Pinus caribaea plantation (Girdling), and NSF: Natural secondary forest.
3.2. Soil Properties Across Different Forest Types
Soil properties of different forest types are detailed in Table 2 and Table S3. In the soil layer of 0–20 cm, the AM plantation and AM-G generally exhibited superior soil nutrient status compared to NSF, with AM-G showing significant increases in pH, AN, and AK. SOC, TN, and TP contents were higher in both AM and AM-G than in NSF. In the deeper soil layer of 20–40 cm, pH did not differ significantly among the three forest types; however, AM-G maintained higher levels of SOC and AN, while NSF exhibited significantly lower TP and TK contents. Regarding particle size distribution, NSF had the highest proportion of coarse sand in the surface layer (49.92%), whereas AM-G displayed higher clay content (22.74%). Differences in particle size distribution diminished with soil depth. With respect to enzyme activities, URE activity was highest in NSF, whereas β-glucosidase (Cx) activity peaked in AM-G, and ACP activity in AM-G also exceeded that in AM plots.

Table 2.
Changes in soil pH and nutrient contents among different forest types after girdling (2024).
In the surface soil layer (0–20 cm), the girdling-treated plots of C. lanceolata (CL-G) exhibited superior nutrient status, with slightly higher SOC and TN, significantly elevated TP and TK, and the highest AP and AK among the three forest types. AN remained at relatively high levels across all forest types. Regarding soil texture, both CL and CL-G exhibited higher content of coarse sand, whereas NSF contained greater proportions of clay and silt. Enzyme activities of URE and ACP did not differ significantly among forest types, while Cx activity was slightly higher in CL-G. In the soil layer of 20–40 cm, nutrient levels generally declined; however, CL-G maintained advantages in TP, TK, AP, and AK. Coarse particle content remained relatively high, and ACP activity was sustained at a comparatively high level.
In the surface soil layer, PC-G had the highest SOC and significantly higher AK compared to PC and NSF, with slight increases in AN and AP, reflecting optimal availability of soil nutrient. TK was significantly greater than in NSF, and pH was slightly higher than in PC but remained acidic. In terms of soil texture, PC-G exhibited the highest sand fraction, while NSF had a finer soil texture. Regarding enzyme activities, ACP activity peaked in PC-G, significantly exceeding that of other forest types, whereas URE and Cx activities were slightly lower than in NSF. In the soil layer of 20–40 cm, nutrient levels generally declined; however, PC-G maintained relatively high TP and AK. Deep soil ACP activity in PC-G was slightly higher than in PC but lower than in NSF.
3.3. Correlation Analysis Between Soil Factors and Plant Diversity
In the soil layer of 0–20 cm, diversity indices of the tree layer generally exhibited weak correlations with soil factors (Figure 5). Only Rtree showed significant correlations with nutrients, including TP, AP, and AK, as well as with coarse sand content and ACP activity. In contrast, diversity indices for the shrub layer—Hshrub, Dshrub, and Eshrub—demonstrated significant or highly significant correlations with multiple nutrients (TN, TP, AP, and AK) and enzyme activities (ACP, Cx, and URE).
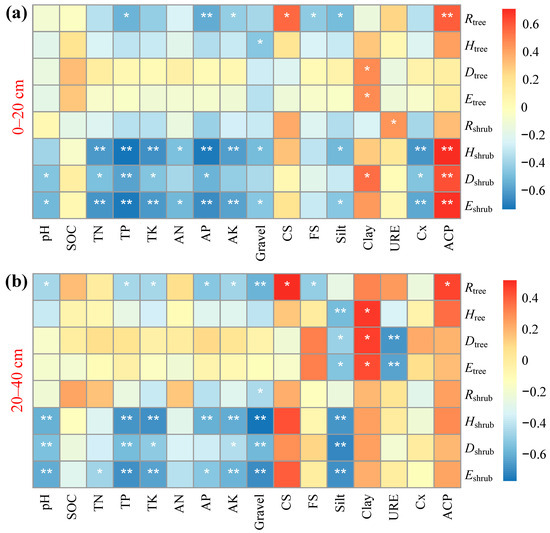
Figure 5.
Correlation analysis between soil factors and plant diversity. (a) in the 0–20 cm soil layer; (b) in the 20–40 cm soil layer. Note: p-values were used to test the level of significance for hypothesis testing. * and ** indicate p < 0.05 and p < 0.01, respectively. Rtree, Htree, Dtree, and Etree refer to the Patrick index, Shannon–Wiener index, Simpson index, and Pielou index of the tree layer, respectively. Rshrub, Hshrub, Dshrub, and Eshrub refer to the Patrick index, Shannon–Wiener index, Simpson index, and Pielou index of the shrub layer, respectively. SOC: Soil organic carbon, TN: Total nitrogen, TP: Total Phosphorus, TK: Total Potassium, AN: Alkaline hydrolyzable nitrogen, AP: Available Phosphorus, AK: Available Potassium, CS: Coarse sand, FS: Fine sand, URE: Urease, Cx: Cellulase, ACP: Acid phosphatase.
In the soil layer of 20–40 cm, the Rtree index of the tree layer continued to demonstrate the strongest responses to soil factors, exhibiting significant correlations with pH, TP, TK, AP, AK, and gravel content. Other indices showed limited correlations, mainly with soil texture (silt, coarse sand) and URE activity. Diversity indices for the shrub layer were significantly negatively correlated with phosphorus- and potassium-related nutrients, as well as gravel and silt content in the deeper soil layer.
3.4. Dominant Factors Driving Variation in Soil Properties
RDA results reveal that the explanatory effects of environmental factors on variations in soil properties differ significantly across soil layers, with forest type and the structural variables of the shrub layer exerting the most significant influence (Figure 6 and Table 3). In the surface soil layer of 0–20 cm, all explanatory variables collectively accounted for 89.56% of the variation in soil properties, with the first canonical axis explaining 73.05% and the second axis explaining 16.09%. Forest type emerged as the most important explanatory factor, independently contributing 44.00% of the explained variation, representing 49.11% of the total variance. Among factors for the structure of plant communities, Eshrub, Hshrub, and Dshrub also demonstrated significant explanatory power regarding the variation in soil properties. In contrast, the structural variable of the tree layer exhibited relatively weaker explanatory power. Only Rtree reached statistical significance, while other indices, such as Dtree, Htree, and Etree, did not show significant effects. The effect of treatment accounted for only 1.23% of the variation and was not statistically significant.
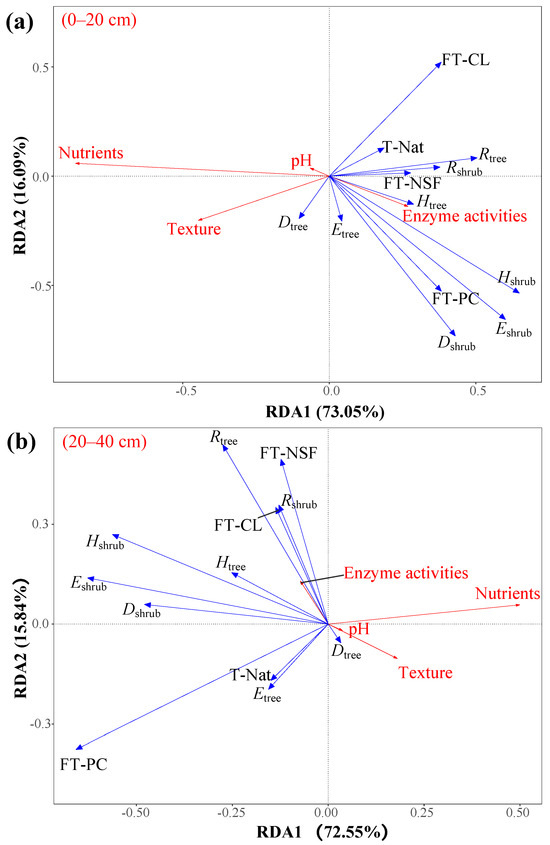
Figure 6.
Redundancy analysis of soil characteristics with plant diversity, forest type, and treatment. (a) in the 0–20 cm soil layer; (b) in the 20–40 cm soil layer. Note: Red lines indicate response variables, and blue lines indicate explanatory variables. Nutrients refer to the principal components with eigenvalues greater than 1 obtained from principal component analysis (PCA) based on soil nutrient indicators (Soil organic carbon [SOC], Total nitrogen [TN], Total Phosphorus [TP], Total Potassium [TK], Alkaline hydrolyzable nitrogen [AN], Available Phosphorus [AP], Available Potassium [AK]). Texture refers to the principal components with eigenvalues greater than 1 obtained from PCA based on soil particle composition (Gravel, Coarse sand [CS], Fine sand [FS], Silt, Clay). Enzyme activities refer to the principal components with eigenvalues greater than 1 obtained from PCA based on soil enzyme activity indicators (Urease [URE], Cellulase [Cx], and Acid phosphatase [ACP]). Rtree, Htree, Dtree, and Etree refer to the Patrick index, Shannon–Wiener index, Simpson index, and Pielou index of the tree layer, respectively. Rshrub, Hshrub, Dshrub, and Eshrub refer to the Patrick index, Shannon–Wiener index, Simpson index, and Pielou index of the shrub layer, respectively. FT-NSF: Forest type—Natural secondary forest, FT-CL: Forest type—Cunninghamia lanceolata, FT-PC: Forest type—Pinus caribaea, T-Nat: Treatment—Natural, CL: Cunninghamia lanceolata plantation, CL-G: Cunninghamia lanceolata plantation (Girdling), PC: Pinus caribaea plantation, PC-G: Pinus caribaea plantation (Girdling), and NSF: Natural secondary forest.

Table 3.
Assessment of the explanatory power of individual variables in redundancy analysis.
In the deep soil layer of 20–40 cm, the total explanatory power of the variables was 80.56%, with the first canonical axis accounting for 72.55% and the second axis explaining 15.84%. Forest type remained the dominant explanatory factor, explaining 53.59% of the total variance. The structural variables of the shrub layer continued to exhibit a significant influence, with Eshrub, Hshrub, and Dshrub all exhibiting strong explanatory power for variations in deep soil properties. The impact of the tree layer on deep soil properties remained relatively weak. Only Rtree was statistically significant, while other structural variables such as Dtree, Htree, and Etree did not show significant effects.
4. Discussion
4.1. Impact of Girdling Treatment on Community Structure in Different Forest Types
This study indicates that girdling treatment facilitates the replacement of dominant tree species in the tree layer and drives succession in the structure of the shrub layer across various plantation types, demonstrating its potential to accelerate ecological restoration in plantations. The decline of the planted species A. mangium in the AM plantation was particularly pronounced, accompanied by a significant increase in the importance values of native tree species. This suggests that girdling effectively reduces the competitive dominance of exotic species, thereby releasing understory resources and promoting the recovery of native species, which is consistent with previous findings on the positive impacts of thinning and girdling on biodiversity restoration [25].
The structural differences between exotic species and native plants are an important factor in these shifts. Exotic species like A. mangium, P. caribaea, and C. lanceolata typically form dense, closed canopies that limit light availability for understory plants. These species often exhibit simpler canopy structures compared to native plants, which typically have a more open canopy, supporting greater plant diversity in the understory. Dense canopies of exotic species reduce the light and space available for native species, suppressing their growth [26]. After girdling, the canopy gaps created by the mortality of the exotic trees allow more light to penetrate, promoting the growth of native plants. These changes in light availability are crucial for facilitating the regeneration of native species in the understory, as evidenced by the increase in native broad-leaved species in the CL and PC plantations following the girdling treatment [27]. These findings align with studies emphasizing the role of canopy closure regulation in enhancing biodiversity in both degraded and uneven-aged plantations [28,29].
The CL and PC plantations also exhibited noticeable shifts in community structure following girdling, primarily characterized by the invasion and colonization of several native broad-leaved species. This indicates that even within coniferous forests, manipulating canopy closure can facilitate plant community regeneration [30]. Conversely, the structure of both the tree layer and the shrub layer in NSF remained highly stable throughout the study period, thus further confirming its structural maturity and ecological stability [31].
Changes in diversity indices provide a more direct reflection of the responses of community structure. CL-G and PC-G exhibited increases in the Shannon diversity index and Pielou’s evenness index, indicating enhanced species evenness and more balanced competition. These findings suggest that girdling contributes to increased species diversity in plantations [32,33]. Although some of these changes have yet to reach statistical significance, the trends are evident. In contrast, the AM plantation exhibits minimal changes in community diversity, likely due to its distinctive nitrogen-fixation capacity and soil nutrient advantages, which continue to sustain the dominance of the original species [34].
4.2. Regulatory Effects of Forest Types and Treatments on Soil Properties
Different forest types and their respective treatments significantly influence soil physicochemical properties and enzyme activities. Overall, the AM plantation and AM-G exhibit superior soil nutrient levels compared to NSF, particularly in surface SOC, AN, and AK. This suggests that the exotic species and its litter decomposition products may contribute to long-term nutrient accumulation in the soil [35]. However, this advantage may also be associated with the simple structure of the understory community and the accumulation of organic layers on the forest floor [36,37].
In the girdling-treated CL and PC plantations, significant increases in TP, TK, AP, and AK are observed, indicating that reduced canopy closure likely enhances litter decomposition and nutrient cycling efficiency [38,39]. Notably, in the PC plantation, the simultaneous increase in AK and ACP activity further implies enhanced biological activity, potentially establishing a positive feedback mechanism that improves nutrient availability [40].
Enzyme activity results further corroborate the above inference. After girdling treatment in the AM plantation, activities of cellulase (Cx) and ACP increased, indicating intensified soil carbon and phosphorus cycling [41]. Conversely, NSF exhibited higher URE activity, reflecting its stronger nitrogen fixation capacity [42]. Overall, girdling treatment likely enhances functional activity of soil by indirectly stimulating the microbial community through improvements in soil permeability and microenvironmental conditions.
However, it is important to note that soil sampling was conducted only once, immediately after the girdling treatment in December 2024. This limitation prevents the assessment of temporal variability and the direct impact of girdling on soil physicochemical properties. The current study cannot capture any pre-treatment differences or longer-term trends. For future studies, we plan to include repeated soil sampling across multiple years to better capture temporal dynamics and assess the effects of girdling on soil physicochemical properties over time.
4.3. Response Relationships Between Soil Properties and Community Diversity
Analyzing the diversity of plant communities reveals that Hshrub, Dshrub, and Eshrub exhibit highly significant correlations with TN, TP, AP, AK, and enzyme activities such as ACP and Cx across both the 0–20 cm and 20–40 cm layers. This indicates that the community structure of the shrub layer strongly responds to variations in soil resources, likely due to shrub root systems being predominantly distributed in the surface soil layers and their heightened sensitivity to nutrient and moisture conditions [43,44]. However, changes in tree composition following girdling treatment also influence soil properties, likely through alterations in canopy structure, litter input, and root activity [45]. These indirect effects on soil properties may explain the observed relationships between the shrub layer and soil factors.
By contrast, the correlations between the diversity indices of the tree layer and soil factors are generally weaker, with only Rtree exhibiting significant associations with certain nutrient and structural factors across different soil depths. This difference suggests that, over short timescales, soil properties may exhibit a lagged response to changes in the structure of the tree layer, responding more rapidly to the dynamics of the shrub layer. This may also be influenced by the growth stability and inertia in resource utilization among mature trees [46,47].
Redundancy analysis further clarified the key drivers of soil variation. Forest type was the predominant explanatory factor in both soil layers. Structural variables of the shrub layer—Eshrub, Hshrub, and Dshrub—exhibited strong explanatory power and statistical significance at both depths, underscoring their critical ecological functions in modulating soil structure, rhizosphere processes, and microenvironmental regulation [48,49]. In contrast, structural variables of the tree layer generally had weaker influences, with the exception of Rtree. Treatment as an isolated factor contributed minimally, indicating that the short-term effects of treatment on soil properties are primarily mediated indirectly through alterations in vegetation structure rather than direct impacts. The short-term effects of the girdling treatment on soil properties are primarily mediated through indirect changes in vegetation structure rather than direct impacts.
4.4. Mechanisms of Girdling Effects and Management Implications
The results above reveal that girdling treatment not only impacts soil physicochemical properties and enzyme activities but also alters the relationships between the diversity of both shrub and tree layers and soil factors, highlighting a bidirectional feedback within the vegetation–soil system. These findings are consistent with previous studies on adjusting plantation structure and ecological restoration [50,51], which emphasize that interventions such as thinning and girdling can enhance community diversity, improve soil physicochemical properties, and stimulate microbial activity. However, unlike some studies focusing on tropical regions, enzyme activity levels in certain treated forest stands in this study even surpassed those found in natural forests, indicating a more complex dynamic between anthropogenic disturbance and ecological restoration [52]. Girdling modifies the composition of carbon input–litter input becomes more diverse while rhizosphere carbon declines. This alteration may promote the synthesis of microbial enzymes and the decomposition of organic matter via a “substrate regulation” mechanism. Additionally, the reconstruction of plant communities may indirectly influence soil microecological processes through root exudates and litter quality [53,54].
From a management perspective, girdling, as a plantation restoration technique characterized by minimal intervention, can effectively disrupt the monoculture structure of plantations in the short term, thereby promoting species replacement and the recovery of soil functions. This effect is especially pronounced in plantations of exotic species such as A. mangium and P. caribaea [25,55]. Future applications could integrate girdling with natural regeneration and mixed-species afforestation to achieve comprehensive improvements in ecosystem services. However, it should be noted that the observation period in this study was relatively short and did not cover the complete process of community succession. Additionally, the causal relationships between changes in the structure of microbial communities and enzyme activity require further in-depth investigation. Subsequent studies could leverage methods such as high-throughput sequencing and isotope tracing to better elucidate the microbial mechanisms driving carbon and nitrogen cycling.
5. Conclusions
Based on a girdling treatment experiment in tropical plantations of Hainan, China, this study systematically explores the response mechanisms of plant community composition, diversity, and soil properties across different forest types. The main conclusions are as follows:
(1) Girdling treatment significantly promotes successional dynamics and diversity restoration in plantation communities, particularly in CL and PC plantations. The increased establishment and dominance of native species demonstrate their potential for ecological restoration in plantations.
(2) Forest type and structural variables of the shrub layer serve as the primary factors of the variation in soil properties. The diversity indices of the shrub layer—Hshrub, Dshrub, and Eshrub—exhibit significant explanatory power regarding soil physicochemical properties and enzyme activities, underscoring their critical role in maintaining soil functions and stability.
(3) The tree layer showed a relatively weak response to soil variations, with tree diversity displaying limited correlation to soil indices. Among tree layer metrics, only Rtree showed a moderate relationship with soil properties, indicating that the influence of tree composition on soil physicochemical properties is weaker than that of the shrub layer.
(4) Girdling primarily influences soil nutrient availability and biological activity indirectly by altering community structure rather than directly causing changes in soil properties.
In summary, optimizing the shrub layer structure and enhancing community diversity are key strategies for the ecological restoration of plantations. Future research should employ multi-site, multi-year experimental designs, combining different interventions to further evaluate the most effective strategies for plantation restoration and to explore the long-term mechanisms of coordinated plant–soil development, providing more robust scientific guidance for the ecological restoration of degraded tropical plantations.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/f16101522/s1. Table S1: Dominant species (top 5) in the tree layer among different forest types pre-girdling (2022) and post-girdling (2024); Table S2: Dominant species (top 5) in the shrub layer among different forest types pre-girdling (2022) and post-girdling (2024); Table S3: Changes in soil texture and enzyme activity among different forest types after girdling (2024).
Author Contributions
X.W. and R.W.: conceptualization, writing—original draft, data curation writing; L.L. and B.Z.: data curation, visualization; J.Y., W.P., F.L. and X.L.: methodology, investigation; S.M., T.L. and J.L.: resources, supervision, project administration. All authors contributed to the article, approved the submitted version, revised the manuscript, and provided assistance with review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Hainan Province Department of Science and Technology (Technological Innovation Special Grant for Provincial Research Institutes, No. KYYSLK2023-012).
Data Availability Statement
Data is contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Artaxo, P.; Hansson, H.C.; Machado, L.A.T.; Rizzo, L.V. Tropical forests are crucial in regulating the climate on Earth. PLoS Clim. 2022, 1, e0000054. [Google Scholar] [CrossRef]
- Cavanaugh, K.C.; Gosnell, J.S.; Davis, S.L.; Ahumada, J.; Boundja, P.; Clark, D.B.; Mugerwa, B.; Jansen, P.A.; O’Brien, T.G.; Rovero, F.; et al. Carbon storage in tropical forests correlates with taxonomic diversity and functional dominance on a global scale. Glob. Ecol. Biogeogr. 2014, 23, 563–573. [Google Scholar] [CrossRef]
- Zong, L.P. The path to effective national park conservation and management: Hainan tropical rainforest National Park System Pilot Area. Int. J. Geoherit. Parks 2020, 8, 225–229. [Google Scholar] [CrossRef]
- Chen, H.H.; Huang, H.; Tian, L.Y.; Wei, J.X.; Yu, X.B.; Wang, X. Changes of vegetation community characteristics of Acacia mangium plantation under natural restoration. J. South Agric. 2024, 55, 2721–2733. [Google Scholar] [CrossRef]
- Liu, Z.J. Protection value and high-quality construction plans in National Park of Hainan Tropical Rainforest. Natl. Park 2023, 1, 250–254. [Google Scholar]
- Yamagawa, H.; Ito, S.; Nakao, T. Restoration of semi-natural forest after clearcutting of conifer plantations in Japan. Landsc. Ecol. Eng. 2010, 6, 109–117. [Google Scholar] [CrossRef]
- Piiroinen, T.; Nyeko, P.; Roininen, H. Natural establishment of indigenous trees under planted nuclei: A study from a clear-felled pine plantation in an afrotropical rain forest. Forest Ecol. Manag. 2015, 345, 21–28. [Google Scholar] [CrossRef]
- Wang, S.J.; Yan, M.H.; Huang, Q.L.; Song, L.; Peng, W.C. Research on girdling: A review. World For. Res. 2023, 36, 38–44. [Google Scholar] [CrossRef]
- Ohlson-Kiehn, C.; Pariona, W.; Fredericksen, T.S. Alternative tree girdling and herbicide treatments for liberation and timber stand improvement in Bolivian tropical forests. Forest Ecol. Manag. 2006, 225, 207–212. [Google Scholar] [CrossRef]
- Reque, J.A.; Bravo, F. Viability of thinning sessile oak stands by girdling. Forestry 2007, 80, 193–199. [Google Scholar] [CrossRef]
- Binkley, D.; Stape, J.L.; Takahashi, E.N.; Ryan, M.G. Tree-girdling to separate root and heterotrophic respiration in two Eucalyptus stands in Brazil. Oecologia 2006, 148, 447–454. [Google Scholar] [CrossRef]
- Wasli, M.E.; Ambun, D.B.; Kalu, M.; Sidi, M.; Nahrawi, H.; Elias, H. Assessment on the growth performance of planted Dryobalanops beccarii at reforestation sites after implementation of selective girdling. Biodiversitas 2020, 21, 1880–1889. [Google Scholar] [CrossRef]
- Basri, E.; Yuniarti, K.; Wahyudi, I.; Saefudin; Damayanti, R. Effects of girdling on wood properties and drying characteristics of Acacia mangium. J. Trop. For. Sci. 2015, 27, 498–505. [Google Scholar]
- Wang, S.J.; Yan, M.H.; Huang, Q.L.; Peng, W.C.; Liao, L.G.; Huang, S.Q.; Song, L. Experimental effect of trunk girdling on the semi-natural forest of Cunninghamia lanceolata in Hainan Tropical Rainforest National Park. Acta Ecol. Sin. 2025, 45, 2996–3005. [Google Scholar] [CrossRef]
- Krishna, M.P.; Mohan, M. Litter decomposition in forest ecosystems: A review. Energy Ecol. Environ. 2017, 2, 236–249. [Google Scholar] [CrossRef]
- Widyati, E.; Nuroniah, H.S.; Tata, H.L.; Mindawati, N.; Lisnawati, Y.; Darwo; Abdulah, L.; Lelana, N.E.; Mawazin; Octavia, D.; et al. Soil degradation due to conversion from natural to plantation forests in Indonesia. Forests 2022, 13, 1913. [Google Scholar] [CrossRef]
- Fang, J.Y. Exploring altitudinal patterns of plant diversity of China’s mountains. Biodivers. Sci. 2004, 1, 1–4. [Google Scholar] [CrossRef]
- Patrick, R. A proposed biological measure of stream conditions, based on a survey of the Conestoga Basin, Lancaster County, Pennsylvania. Proc. Acad. Nat. Sci. Phila. 1949, 101, 277–341. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Ma, K.P. Measurement of biotic community diversity I α diversity (Part 1). Biodivers. Sci. 1994, 2, 162–168. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agrochemical Analysis; China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Yang, W.Q.; Wang, K.Y. Advances in forest soil enzymology. Sci. Silvae Sin. 2004, 40, 152–159. [Google Scholar]
- Merceron, N.R.; Lamarque, L.J.; Delzon, S.; Porté, A.J. Killing it softly: Girdling as an efficient eco-friendly method to locally remove invasive Acer negundo. Ecol. Restor. 2016, 34, 297–305. [Google Scholar] [CrossRef]
- Cabin, R.J.; Weller, S.G.; Lorence, D.H.; Cordell, S.; Hadway, L.J.; Montgomery, R.; Goo, D.; Urakami, A. Effects of light, alien grass, and native species additions on Hawaiian dry forest restoration. Ecol. Appl. 2002, 12, 1595–1610. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Jagodziński, A.M. Similar impacts of alien and native tree species on understory light availability in a temperate forest. Forests 2019, 10, 951. [Google Scholar] [CrossRef]
- Rajeev, J.; Kumar, K.C.J.; Prasad, D.P.; Utpal, D. Exploring the impact of thinning operations on forest ecosystems in tropical and temperate regions worldwide: A comprehensive review. J. Resour. Ecol. 2023, 14, 1227–1242. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Fonseca, T.F. Influence management and disturbances on the regeneration of forest stands. Front. For. Glob. Change 2023, 6, 1123215. [Google Scholar] [CrossRef]
- Xu, X.L.; Wang, X.J.; Hu, Y.; Wang, P.; Saeed, S.; Sun, Y.J. Short-term effects of thinning on the development and communities of understory vegetation of Chinese fir plantations in Southeastern China. PeerJ 2020, 8, e8536. [Google Scholar] [CrossRef]
- Rosenfield, M.F.; Jakovac, C.C.; Vieira, D.L.M.; Poorter, L.; Brancalion, P.H.S.; Vieira, I.C.G.; de Almeida, D.R.A.; Massoca, P.; Schietti, J.; Albernaz, A.L.M.; et al. Ecological integrity of tropical secondary forests: Concepts and indicators. Biol. Rev. 2023, 98, 662–676. [Google Scholar] [CrossRef]
- Kerr, G. The use of silvicultural systems to enhance the biological diversity of plantation forests in Britain. Forestry 1999, 72, 191–205. [Google Scholar] [CrossRef]
- Matsushita, M.; Nishikawa, H.; Tamura, A. Effects of girdling intensity, pruning season and thinning on tree growth, crown vigor and wound recovery in Japanese larch. Forests 2022, 13, 449. [Google Scholar] [CrossRef]
- Mendham, D.S.; White, D.A. A review of nutrient, water and organic matter dynamics of tropical acacias on mineral soils for improved management in Southeast Asia. Aust. For. 2019, 82 (Suppl. 1), 45–56. [Google Scholar] [CrossRef]
- Ouyang, S.N.; Tie, L.H.; Rao, X.Q.; Cai, X.A.; Liu, S.P.; Vitali, V.; Wei, L.Y.; Yu, Q.S.; Sun, D.; Lin, Y.B.; et al. Mixed-species Acacia plantation decreases soil organic carbon and total nitrogen concentrations but favors species regeneration and tree growth over monoculture: A thirty-three-year field experiment in Southern China. Forests 2023, 14, 968. [Google Scholar] [CrossRef]
- Vogt, K.A.; Vogt, D.J.; Brown, S.; Tilley, J.P.; Edmonds, R.L.; Silver, W.L.; Siccama, T.G. Dynamics of Forest Floor and Soil Organic Matter Accumulation in Boreal, Temperate, and Tropical Forests; CRC Press: Boca Raton, FL, USA, 1995; pp. 159–178. [Google Scholar] [CrossRef]
- Deng, J.J.; Fang, S.; Fang, X.M.; Jin, Y.Q.; Kuang, Y.W.; Lin, F.M.; Liu, J.Q.; Ma, J.R.; Nie, Y.X.; Ouyang, S.N.; et al. Forest understory vegetation study: Current status and future trends. For. Res. 2023, 3, 6. [Google Scholar] [CrossRef]
- Gliksman, D.; Haenel, S.; Osem, Y.; Yakir, D.; Zangy, E.; Preisler, Y.; Grünzweig, J.M. Litter decomposition in Mediterranean pine forests is enhanced by reduced canopy cover. Plant Soil 2018, 422, 317–329. [Google Scholar] [CrossRef]
- Prescott, C.E. The influence of the forest canopy on nutrient cycling. Tree Physiol. 2002, 22, 1193–1200. [Google Scholar] [CrossRef]
- Ndabankulu, K.; Egbewale, S.O.; Tsvuura, Z.; Magadlela, A. Soil microbes and associated extracellular enzymes largely impact nutrient bioavailability in acidic and nutrient poor grassland ecosystem soils. Sci. Rep. 2022, 12, 12601. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.H.; Long, X.Y.; Liao, Y.Q.; Lin, Y.H.; He, Z.H.; Kong, Q.; Kong, X.S.; He, X.B. Influence of arbuscular mycorrhizal fungi on nitrogen dynamics during Cinnamomum camphora litter decomposition. Microorganisms 2025, 13, 151. [Google Scholar] [CrossRef]
- Lavres, J.; Castro Franco, G.; de Sousa Câmara, G.M. Soybean seed treatment with nickel improves biological nitrogen fixation and urease activity. Front. Environ. Sci. 2016, 4, 37. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Plant-soil interactions in Mediterranean forest and shrublands: Impacts of climatic change. Plant Soil 2013, 365, 1–33. [Google Scholar] [CrossRef]
- Bayala, J.; Prieto, I. Water acquisition, sharing and redistribution by roots: Applications to agroforestry systems. Plant Soil 2020, 453, 17–28. [Google Scholar] [CrossRef]
- Chen, D.M.; Zhou, L.X.; Wu, J.P.; Hsu, J.; Lin, Y.B.; Fu, S.L. Tree girdling affects the soil microbial community by modifying resource availability in two subtropical plantations. Appl. Soil Ecol. 2012, 53, 108–115. [Google Scholar] [CrossRef]
- Millard, P.; Sommerkorn, M.; Grelet, G.A. Environmental change and carbon limitation in trees: A biochemical, ecophysiological and ecosystem appraisal. New Phytol. 2007, 175, 11–28. [Google Scholar] [CrossRef] [PubMed]
- De Pauw, K.; Sanczuk, P.; Meeussen, C.; Depauw, L.; De Lombaerde, E.; Govaert, S.; Vanneste, T.; Brunet, J.; Cousins, S.A.O.; Gasperini, C.; et al. Forest understorey communities respond strongly to light in interaction with forest structure, but not to microclimate warming. New Phytol. 2022, 233, 219–235. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Hai, L.; Qin, F.C.; Liu, L.; Hong, G.Y.; Li, Z.H.; Li, L.; Yue, Y.J.; Dong, X.Y.; He, R.; et al. Effects of micro-topography on soil nutrients and plant diversity of artificial shrub forest in the Mu Us Sandy Land. Plants 2025, 14, 2163. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.Y.; Fan, F.; Lin, Q.M.; Guo, S.Z.; Li, S.M.; Zhang, Y.P.; Feng, Z.Y.; Wang, X.X.; Rensing, C.; Cao, G.Q.; et al. Effects of different stand densities on the composition and diversity of soil microbiota in a Cunninghamia lanceolata plantation. Plants 2025, 14, 98. [Google Scholar] [CrossRef]
- Xing, G.T.; Wang, X.F.; Jiang, Y.M.; Yang, H.; Mai, S.W.; Xu, W.X.; Hou, E.Q.; Huang, X.Z.; Yang, Q.; Liu, W.J.; et al. Variations and influencing factors of soil organic carbon during the tropical forest succession from plantation to secondary and old–growth forest. Front. Ecol. Evol. 2023, 10, 1104369. [Google Scholar] [CrossRef]
- Iddrisu, A.Q.; Hao, Y.Q.; Issifu, H.; Getnet, A.; Sakib, N.; Yang, X.B.; Abdallah, M.M.; Zhang, P. Effects of stand density on tree growth, diversity of understory vegetation, and soil properties in a Pinus koraiensis plantation. Forests 2024, 15, 1149. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Fu, D.J.; Lu, C.X.; Xu, X.M.; Tang, Q.H. Positive effects of ecological restoration policies on the vegetation dynamics in a typical ecologically vulnerable area of China. Ecol. Eng. 2021, 159, 106087. [Google Scholar] [CrossRef]
- Machado, D.L.; Engel, V.L.; Podadera, D.S.; Sato, L.M.; de Goede, R.G.M.; de Moraes, L.F.D.; Parrotta, J.A. Site and plant community parameters drive the effect of vegetation on litterfall and nutrient inputs in restored tropical forests. Plant Soil 2021, 464, 405–421. [Google Scholar] [CrossRef]
- Wang, C.J.; Lin, W.S.; Jia, S.X.; Chen, S.D.; Xiong, D.C.; Xu, C.; Yang, Z.J.; Liu, X.F.; Yang, Y.S. Effects of litter and root inputs on soil microbial community structure in subtropical natural and plantation forests. Plant Soil 2025, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.P.; Liu, Z.F.; Wang, X.L.; Sun, Y.X.; Zhou, L.X.; Lin, Y.B.; Fu, S.L. Effects of understory removal and tree girdling on soil microbial community composition and litter decomposition in two Eucalyptus plantations in South China. Funct. Ecol. 2011, 25, 921–931. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).