Differences in Soil Solution Chemistry and Their Vertical Variation Between Moso Bamboo Forests and Japanese Cedar Plantations in Western Japan
Abstract
1. Introduction
2. Materials and Methods
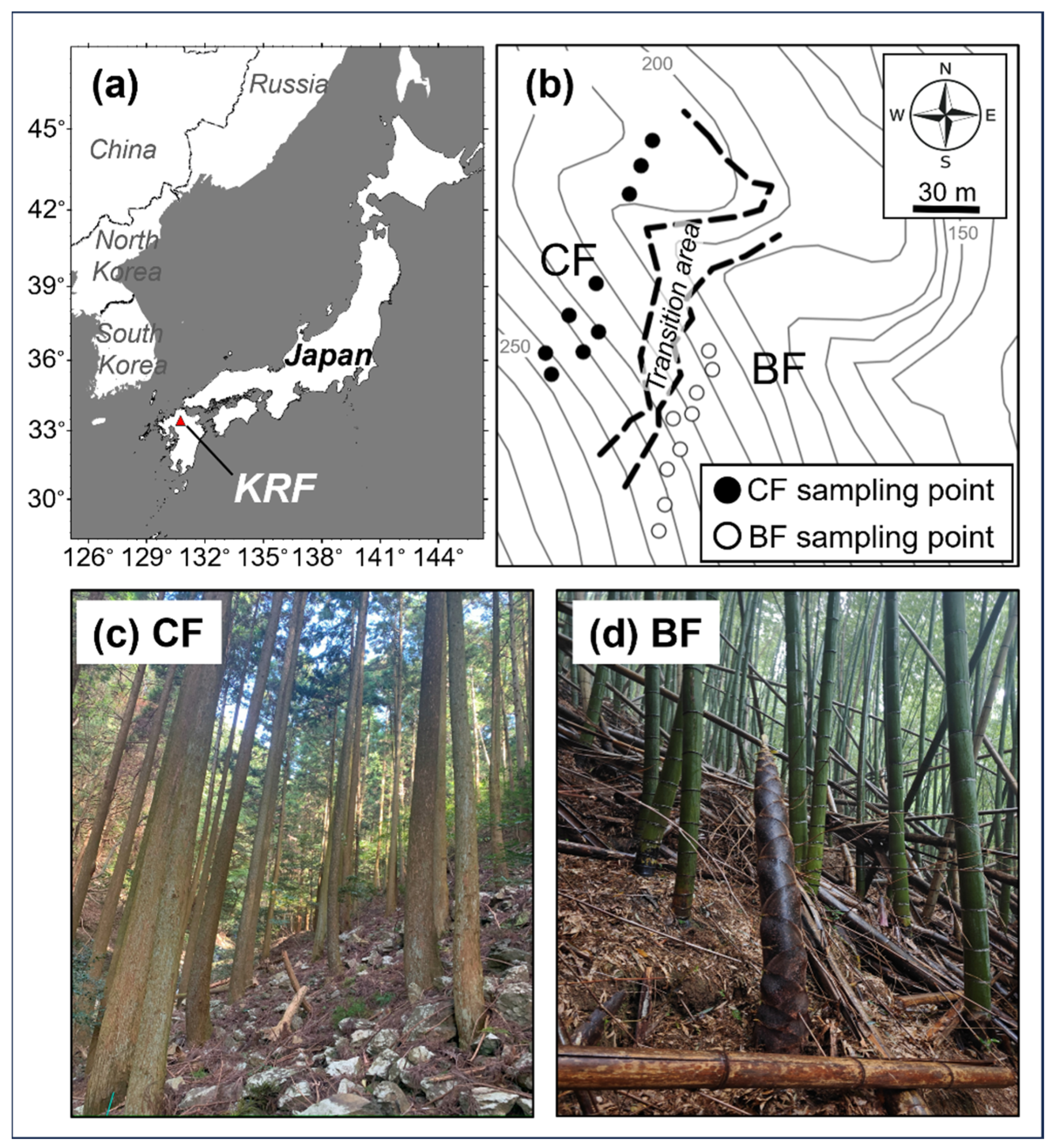
2.1. Site Description and Soil Solution Points
2.2. Soil Solution Collection and Chemical Analysis
2.3. Statistical Analysis
3. Results
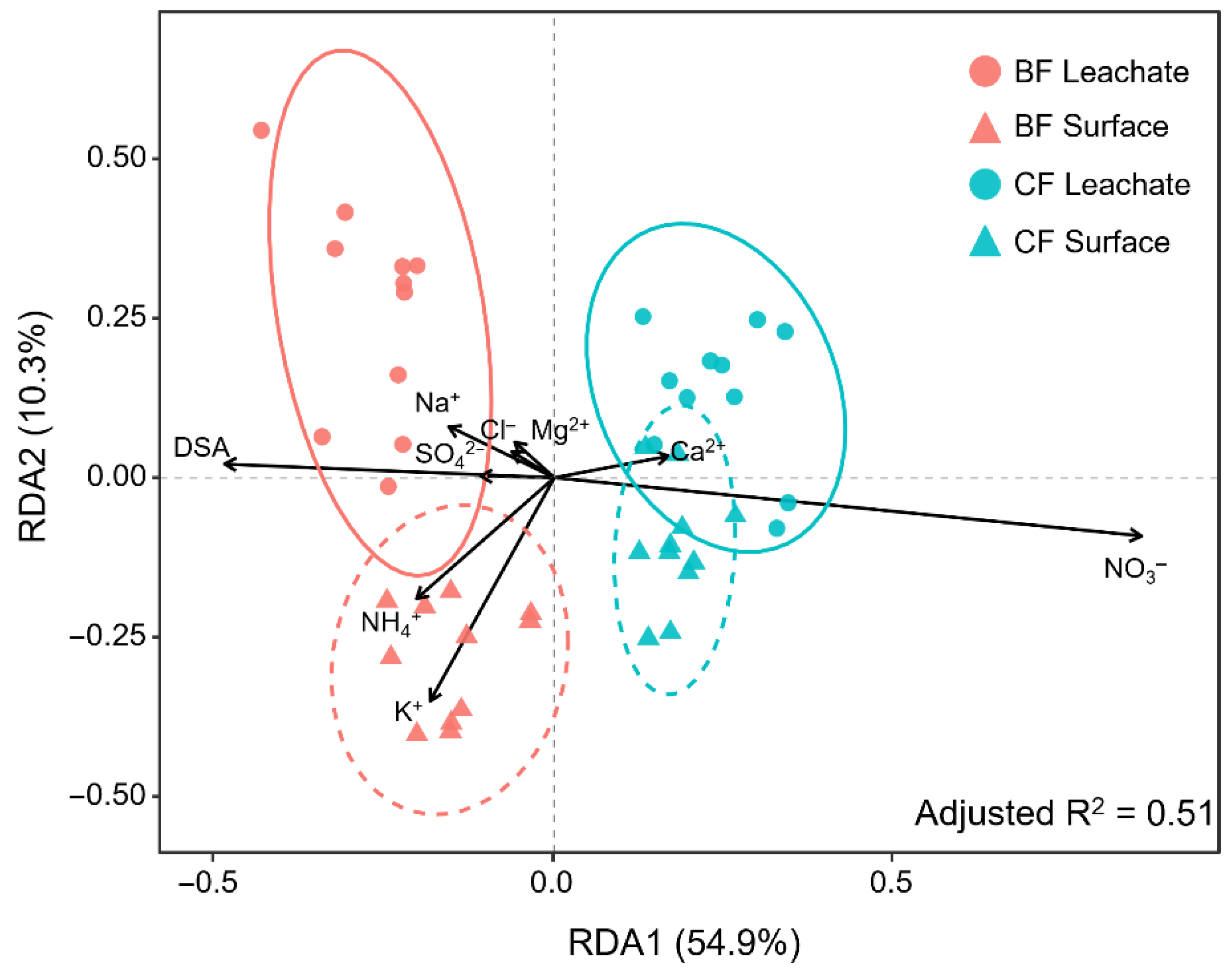
3.1. Multivariate Analysis of Soil Solution Chemistry
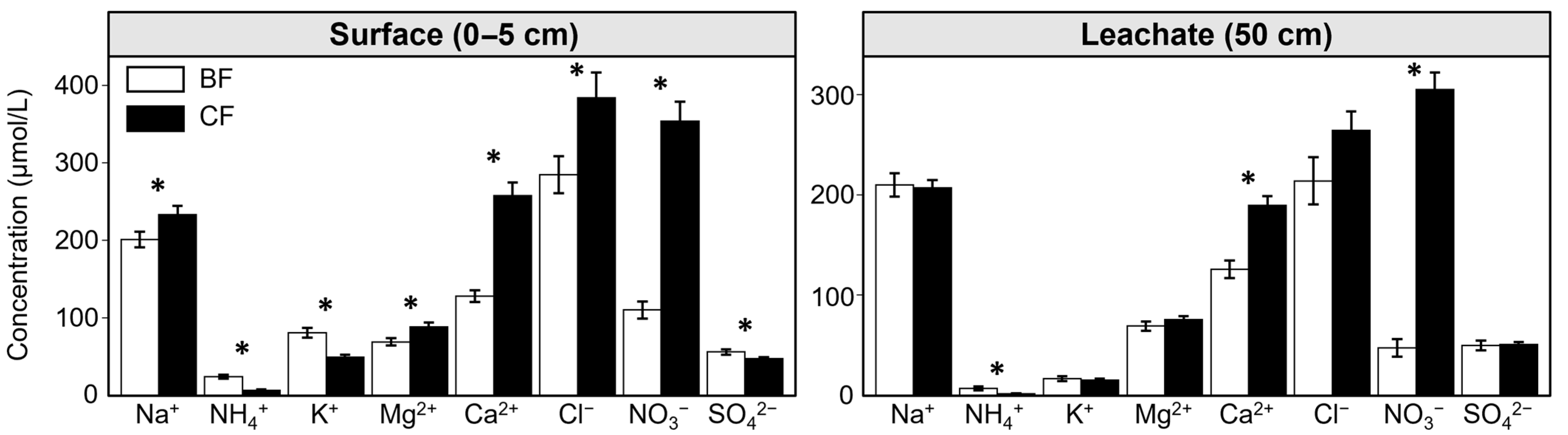
3.2. Soil Solution Chemistry in Different Forest Types
3.3. Vertical Distribution Patterns of Soil Solution Chemistry
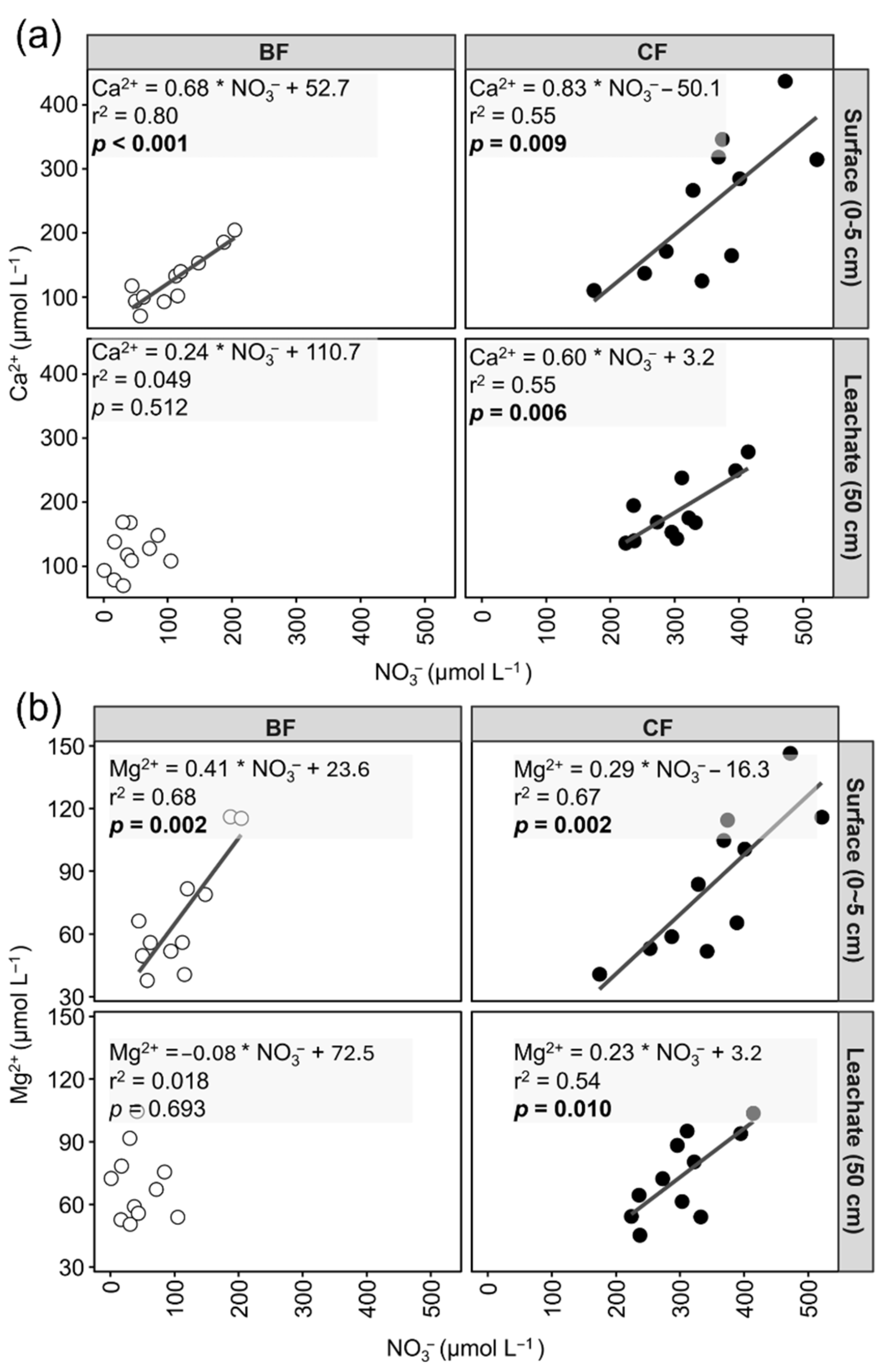
3.4. Correlations Between NO3− and Major Cations (Ca2+, Mg2+)
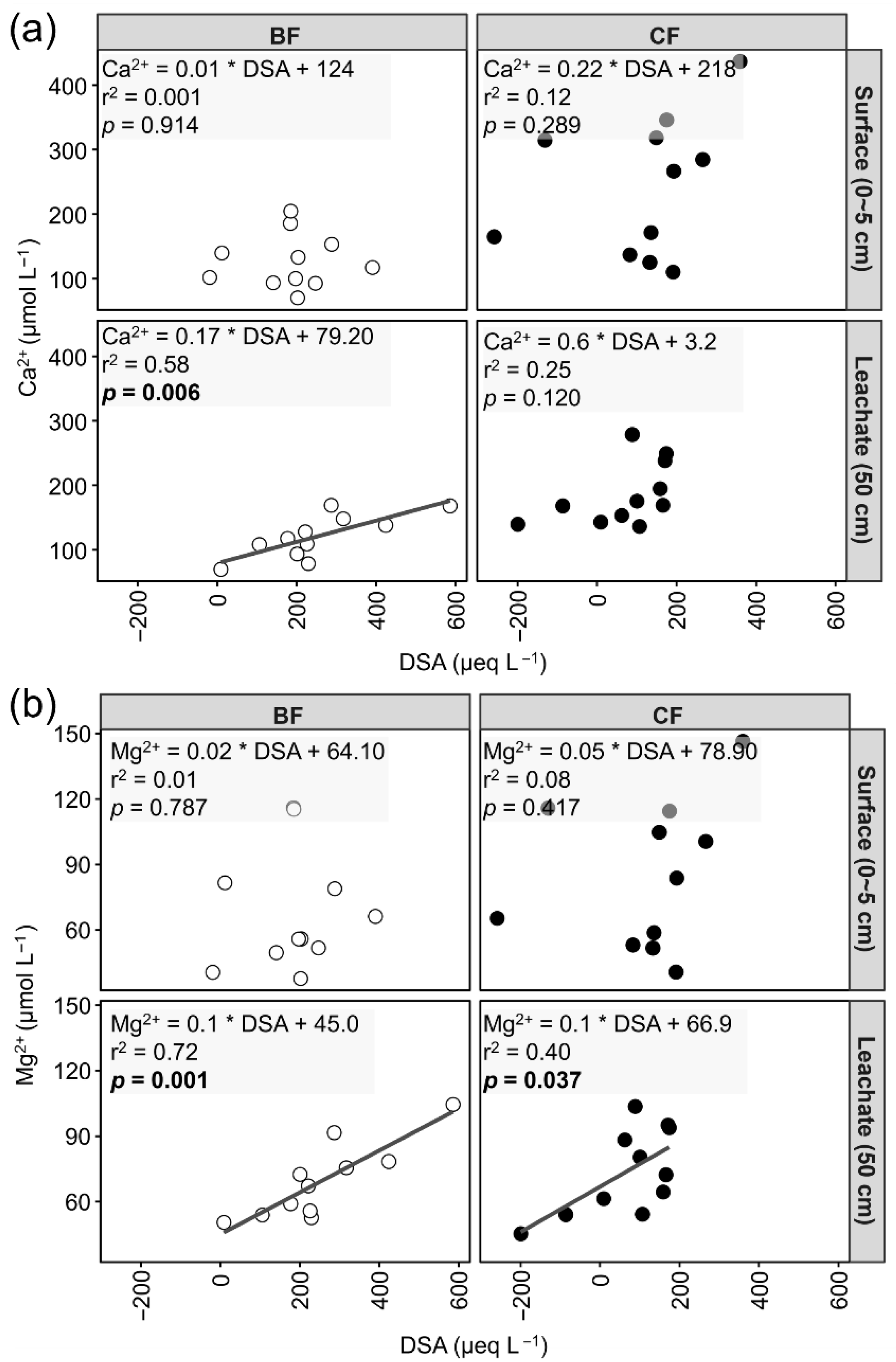
3.5. Deficit of Strong Anions (DSA) and Its Correlations with Ca2+ and Mg2+
4. Discussion
4.1. Mechanisms Underlying Differences in Soil Solution Chemistry Between BF and CF
4.2. Differences in Vertical Distribution Patterns for Major Ions Between BF and CF
4.3. Possible Mechanisms for Vertical Differences in Ionic Composition Maintaining Charge Balance (Ca2+, Mg2+, NO3−, Deficit of Strong Anions) Between BF and CF
4.4. Practical Implications, Limitations and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BF | Moso bamboo forest |
| CF | Japanese cedar plantation |
| N | Nitrogen |
| Ca | Calcium |
| Mg | Magnesium |
| K | Potassium |
| DSA | Deficit of strong anions |
References
- Gao, J. Breeding Status and Strategies of Moso Bamboo. In The Moso Bamboo Genome; Gao, J., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 193–208. ISBN 978-3-030-80836-5. [Google Scholar]
- Lee, S.H.; Md Tahir, P.; Osman Al-Edrus, S.S.; Uyup, M.K.A. Bamboo Resources, Trade, and Utilisation. In Multifaceted Bamboo: Engineered Products and Other Applications; Md Tahir, P., Lee, S.H., Osman Al-Edrus, S.S., Uyup, M.K.A., Eds.; Springer Nature: Singapore, 2023; pp. 1–14. ISBN 978-981-19-9327-5. [Google Scholar]
- Buziquia, S.T.; Lopes, P.V.F.; Almeida, A.K.; De Almeida, I.K. Impacts of Bamboo Spreading: A Review. Biodivers. Conserv. 2019, 28, 3695–3711. [Google Scholar] [CrossRef]
- Xu, Q.-F.; Liang, C.-F.; Chen, J.-H.; Li, Y.-C.; Qin, H.; Fuhrmann, J.J. Rapid Bamboo Invasion (Expansion) and Its Effects on Biodiversity and Soil Processes +. Glob. Ecol. Conserv. 2020, 21, e00787. [Google Scholar] [CrossRef]
- Ouyang, M.; Eziz, A.; Xiao, S.; Fang, W.; Cai, Q.; Ma, S.; Zhu, J.; Yang, Q.; Hu, J.; Tang, Z.; et al. Effects of Bamboo Invasion on Forest Structures and Diameter–Height Allometries. For. Ecosyst. 2025, 12, 100256. [Google Scholar] [CrossRef]
- Isagi, Y.; Torii, A. Range Expansion and Its Mechanisms in a Naturalized Bamboo Species, Phyllostachys Pubescens, in Japan. J. Sustain. For. 1997, 6, 127–141. [Google Scholar] [CrossRef]
- Li, P.; Zhou, G.; Du, H.; Lu, D.; Mo, L.; Xu, X.; Shi, Y.; Zhou, Y. Current and Potential Carbon Stocks in Moso Bamboo Forests in China. J. Environ. Manag. 2015, 156, 89–96. [Google Scholar] [CrossRef]
- Kamada, M. Satoyama Landscape of Japan—Past, Present, and Future. In Landscape Ecology for Sustainable Society; Hong, S.-K., Nakagoshi, N., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 87–109. ISBN 978-3-319-74328-8. [Google Scholar]
- Suzuki, S. Chronological Location Analyses of Giant Bamboo (Phyllostachys Pubescens) Groves and Their Invasive Expansion in a Satoyama Landscape Area, Western Japan. Plant Species Biol. 2015, 30, 63–71. [Google Scholar] [CrossRef]
- Manabe, T.; Shibata, S.; Hasegawa, H.; Itoh, K. Trends and issues of landscape ecological studies on range expansion of bamboo forests in Japan—Perspective for sustainable use of bamboo forests. Jpn. J. Landsc. Ecol. 2020, 25, 119–135. [Google Scholar] [CrossRef]
- Shimono, K.; Katayama, A.; Kume, T.; Enoki, T.; Chiwa, M.; Hishi, T. Differences in Net Primary Production Allocation and Nitrogen Use Efficiency between Moso Bamboo and Japanese Cedar Forests along a Slope. J. For. Res. 2022, 27, 28–35. [Google Scholar] [CrossRef]
- Song, Q.; Ouyang, M.; Yang, Q.; Lu, H.; Yang, G.; Chen, F.; Shi, J.-M. Degradation of Litter Quality and Decline of Soil Nitrogen Mineralization after Moso Bamboo (Phyllostachys pubescens) Expansion to Neighboring Broadleaved Forest in Subtropical China. Plant Soil 2016, 404, 113–124. [Google Scholar] [CrossRef]
- Yen, T.-M.; Lee, J.-S. Comparing Aboveground Carbon Sequestration between Moso Bamboo (Phyllostachys heterocycla) and China Fir (Cunninghamia lanceolata) Forests Based on the Allometric Model. For. Ecol. Manag. 2011, 261, 995–1002. [Google Scholar] [CrossRef]
- Zuo, K.; Fan, L.; Guo, Z.; Zhang, L.; Duan, Y.; Zhang, J.; Chen, S.; Lin, H.; Hu, R. High Nutrient Utilization and Resorption Efficiency Promote Bamboo Expansion and Invasion. J. Environ. Manag. 2024, 362, 121370. [Google Scholar] [CrossRef]
- Ni, H.; Su, W.; Fan, S.; Chu, H. Effects of Intensive Management Practices on Rhizosphere Soil Properties, Root Growth, and Nutrient Uptake in Moso Bamboo Plantations in Subtropical China. For. Ecol. Manag. 2021, 493, 119083. [Google Scholar] [CrossRef]
- Fu, D.; Chiwa, M. Contrasting Nitrate Leaching from an Abandoned Moso Bamboo Forest and a Japanese Cedar Plantation: Role of Vegetation in Mitigating Nitrate Leaching. Plant Soil 2023, 492, 229–240. [Google Scholar] [CrossRef]
- Song, Q.; Lu, H.; Liu, J.; Yang, J.; Yang, G.; Yang, Q. Accessing the Impacts of Bamboo Expansion on NPP and N Cycling in Evergreen Broadleaved Forest in Subtropical China. Sci. Rep. 2017, 7, 40383. [Google Scholar] [CrossRef]
- Luo, W.; Zhang, Q.; Wang, P.; Luo, J.; She, C.; Guo, X.; Yuan, J.; Sun, Y.; Guo, R.; Li, Z.; et al. Unveiling the Impacts Moso Bamboo Invasion on Litter and Soil Properties: A Meta-Analysis. Sci. Total Environ. 2024, 909, 168532. [Google Scholar] [CrossRef] [PubMed]
- Smethurst, P.J. Soil Solution and Other Soil Analyses as Indicators of Nutrient Supply: A Review. For. Ecol. Manag. 2000, 138, 397–411. [Google Scholar] [CrossRef]
- Yang, T.; Li, Y.; Ouyang, X.; Wang, B.; Ge, X.; Tang, L. Bamboo Plantation Establishment Changes Rainfall Partitioning and Chemistry. Ecosystems 2023, 26, 1326–1334. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Y.; Zhu, Q.; Lai, X.; Liao, K. Comparing the Variations and Controlling Factors of Soil N2O Emissions and NO3–-N Leaching on Tea and Bamboo Hillslopes. CATENA 2020, 188, 104463. [Google Scholar] [CrossRef]
- Ge, X.; Wang, C.; Wang, L.; Zhou, B.; Cao, Y.; Xiao, W.; Li, M.-H. Drought Changes Litter Quantity and Quality, and Soil Microbial Activities to Affect Soil Nutrients in Moso Bamboo Forest. Sci. Total Environ. 2022, 838, 156351. [Google Scholar] [CrossRef]
- Luan, J.; Li, S.; Dong, W.; Liu, Y.; Wang, Y.; Liu, S. Litter Decomposition Affected by Bamboo Expansion Is Modulated by Litter-Mixing and Microbial Composition. Funct. Ecol. 2021, 35, 2562–2574. [Google Scholar] [CrossRef]
- Kothawala, D.N.; Moore, T.R. Adsorption of Dissolved Nitrogen by Forest Mineral Soils. Can. J. For. Res. 2009, 39, 2381–2390. [Google Scholar] [CrossRef]
- Gundersen, P.; Schmidt, I.K.; Raulund-Rasmussen, K. Leaching of Nitrate from Temperate Forests—Effects of Air Pollution and Forest Management. Environ. Rev. 2006, 14, 1. [Google Scholar] [CrossRef]
- Ohta, T.; Shin, K.-C.; Saitoh, Y.; Nakano, T.; Hiura, T. The Effects of Differences in Vegetation on Calcium Dynamics in Headwater Streams. Ecosystems 2018, 21, 1390–1403. [Google Scholar] [CrossRef]
- Aber, J.; McDowell, W.; Nadelhoffer, K.; Magill, A.; Berntson, G.; Kamakea, M.; McNulty, S.; Currie, W.; Rustad, L.; Fernandez, I. Nitrogen Saturation in Temperate Forest Ecosystems: Hypotheses Revisited. BioScience 1998, 48, 921–934. [Google Scholar] [CrossRef]
- Hagen-Thorn, A.; Callesen, I.; Armolaitis, K.; Nihlgård, B. The Impact of Six European Tree Species on the Chemistry of Mineral Topsoil in Forest Plantations on Former Agricultural Land. For. Ecol. Manag. 2004, 195, 373–384. [Google Scholar] [CrossRef]
- Strobel, B.W.; Hansen, H.C.B.; Borggaard, O.K.; Andersen, M.K.; Raulund-Rasmussen, K. Composition and Reactivity of DOC in Forest Floor Soil Solutions in Relation to Tree Species and Soil Type. Biogeochemistry 2001, 56, 1–26. [Google Scholar] [CrossRef]
- Legout, A.; van der Heijden, G.; Jaffrain, J.; Boudot, J.-P.; Ranger, J. Tree Species Effects on Solution Chemistry and Major Element Fluxes: A Case Study in the Morvan (Breuil, France). For. Ecol. Manag. 2016, 378, 244–258. [Google Scholar] [CrossRef]
- Ohta, T.; Niwa, S.; Hiura, T. Calcium Concentration in Leaf Litter Affects the Abundance and Survival of Crustaceans in Streams Draining Warm–Temperate Forests. Freshw. Biol. 2014, 59, 748–760. [Google Scholar] [CrossRef]
- Ohta, T.; Hiura, T. Root Exudation of Low-Molecular-Mass-Organic Acids by Six Tree Species Alters the Dynamics of Calcium and Magnesium in Soil. Can. J. Soil Sci. 2016, 96, 199–206. [Google Scholar] [CrossRef]
- D’Amore, D.V.; Hennon, P.E.; Schaberg, P.G.; Hawley, G.J. Adaptation to Exploit Nitrate in Surface Soils Predisposes Yellow-Cedar to Climate-Induced Decline While Enhancing the Survival of Western Redcedar: A New Hypothesis. For. Ecol. Manag. 2009, 258, 2261–2268. [Google Scholar] [CrossRef]
- Liu, Y.; Chiwa, M. Influence of Surface Soil Chemistry on Nutrient Leaching from Japanese Cedar Plantations and Natural Forests. Landsc. Ecol. Eng. 2024, 20, 187–194. [Google Scholar] [CrossRef]
- Pickett, S.T.A. Space-for-Time Substitution as an Alternative to Long-Term Studies. In Long-Term Studies in Ecology; Springer: New York, NY, USA, 1989; pp. 110–135. [Google Scholar] [CrossRef]
- Chiwa, M. Ten-Year Determination of Atmospheric Phosphorus Deposition at Three Forested Sites in Japan. Atmos. Environ. 2020, 223, 117247. [Google Scholar] [CrossRef]
- Japan Meteorological Agency. Available online: https://www.jma.go.jp/jma/indexe.html (accessed on 8 September 2025).
- Shinohara, Y.; Misumi, Y.; Kubota, T.; Nanko, K. Characteristics of Soil Erosion in a Moso-Bamboo Forest of Western Japan: Comparison with a Broadleaved Forest and a Coniferous Forest. CATENA 2019, 172, 451–460. [Google Scholar] [CrossRef]
- Inagaki, Y.; Sakai, H.; Shinomiya, Y.; Yoshinaga, S.; Torii, A.; Yamada, T.; Noguchi, K.; Morishita, T.; Fujii, K. Effects of Climate and Acidic Deposition on Interannual Variations of Stream Water Chemistry in Forested Watersheds in the Shimanto River Basin, Southern Japan. Ecol. Res. 2025, 40, 249–263. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package Version 2.6-4. 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 23 September 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://Www.R-Project.Org/ (accessed on 23 September 2025).
- Asiedu, D.K.; Suzuki, S.; Shibata, T. Provenance of Sandstones from the Lower Cretaceous Sasayama Group, Inner Zone of Southwest Japan. Sediment. Geol. 2000, 131, 9–24. [Google Scholar] [CrossRef]
- Nakanishi, A.; Shibata, H.; Inokura, Y.; Nakao, T.; Toda, H.; Satoh, F.; Sasa, K. Chemical Characteristics in Stemflow of Japanese Cedar in Japan. Water Air Soil Pollut. 2001, 130, 709–714. [Google Scholar] [CrossRef]
- Ding, W.; Tsunogai, U.; Nakagawa, F.; Sambuichi, T.; Chiwa, M.; Kasahara, T.; Shinozuka, K. Stable Isotopic Evidence for the Excess Leaching of Unprocessed Atmospheric Nitrate from Forested Catchments under High Nitrogen Saturation. Biogeosciences 2023, 20, 753–766. [Google Scholar] [CrossRef]
- Jobbágy, E.G.; Jackson, R.B. The Distribution of Soil Nutrients with Depth: Global Patterns and the Imprint of Plants. Biogeochemistry 2001, 53, 51–77. [Google Scholar] [CrossRef]
- Farooq, T.H.; Xincheng, X.; Shakoor, A.; Rashid, M.H.U.; Bashir, M.F.; Nawaz, M.F.; Kumar, U.; Shahzad, S.M.; Yan, W. Spatial Distribution of Carbon Dynamics and Nutrient Enrichment Capacity in Different Layers and Tree Tissues of Castanopsis Eyeri Natural Forest Ecosystem. Environ. Sci. Pollut. Res. 2022, 29, 10250–10262. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Zu, Y. Temporal Changes in SOM, N, P, K, and Their Stoichiometric Ratios during Reforestation in China and Interactions with Soil Depths: Importance of Deep-Layer Soil and Management Implications. For. Ecol. Manag. 2014, 325, 8–17. [Google Scholar] [CrossRef]
- Guan, F.; Xia, M.; Tang, X.; Fan, S. Spatial Variability of Soil Nitrogen, Phosphorus and Potassium Contents in Moso Bamboo Forests in Yong’an City, China. CATENA 2017, 150, 161–172. [Google Scholar] [CrossRef]
- Fang, Y.; Koba, K.; Makabe, A.; Takahashi, C.; Zhu, W.; Hayashi, T.; Hokari, A.A.; Urakawa, R.; Bai, E.; Houlton, B.Z.; et al. Microbial Denitrification Dominates Nitrate Losses from Forest Ecosystems. Proc. Natl. Acad. Sci. USA 2015, 112, 1470–1474. [Google Scholar] [CrossRef]
- Urakawa, R.; Toda, H.; Cao, Y. Long-term Changes in Stream Water Chemistry in Small Forested Watersheds in the Northern Kanto Region. Ecol. Res. 2025, 40, 264–276. [Google Scholar] [CrossRef]
- Nye, P.H. Changes of pH across the Rhizosphere Induced by Roots. Plant Soil 1981, 61, 7–26. [Google Scholar] [CrossRef]
- Bassirirad, H. Kinetics of Nutrient Uptake by Roots: Responses to Global Change. New Phytol. 2000, 147, 155–169. [Google Scholar] [CrossRef]
- Li, Q.; Song, X.; Chang, S.X.; Peng, C.; Xiao, W.; Zhang, J.; Xiang, W.; Li, Y.; Wang, W. Nitrogen Depositions Increase Soil Respiration and Decrease Temperature Sensitivity in a Moso Bamboo Forest. Agric. For. Meteorol. 2019, 268, 48–54. [Google Scholar] [CrossRef]
- Huang, K.; Li, Y.; Hu, J.; Tang, C.; Zhang, S.; Fu, S.; Jiang, P.; Ge, T.; Luo, Y.; Song, X.; et al. Rates of Soil Respiration Components in Response to Inorganic and Organic Fertilizers in an Intensively-Managed Moso Bamboo Forest. Geoderma 2021, 403, 115212. [Google Scholar] [CrossRef]
| BF | CF | |
|---|---|---|
| Stem density (No. ha−1) | 6900 | 1020 |
| Stem diameter (cm) | 8.6 | 34.1 |
| Plant height (m) | 10.9 | 23.6 |
| Soil solution pH (5 cm depth) | 6.08 | 6.32 ns |
| Soil solution pH (50 cm depth) | 7.05 | 6.82 * |
| Surface | Leachate | p Value | |
|---|---|---|---|
| BF | |||
| Na+ | 200.9 | 210.1 | 0.336 |
| NH4+ | 24.5 | 7.0 | <0.001 |
| K+ | 80.6 | 16.7 | <0.001 |
| Ca2+ | 128.1 | 126.0 | 0.773 |
| Mg2+ | 69.1 | 69.2 | 0.706 |
| Cl− | 284.7 | 214.1 | 0.186 |
| NO3− | 110.3 | 47.5 | 0.030 |
| SO42− | 56.2 | 50.1 | 0.259 |
| CF | |||
| Na+ | 233.2 | 207.4 | 0.044 |
| NH4+ | 6.8 | 1.7 | 0.008 |
| K+ | 50.0 | 15.4 | <0.001 |
| Ca2+ | 257.6 | 189.8 | <0.001 |
| Mg2+ | 88.7 | 75.7 | 0.045 |
| Cl− | 383.9 | 264.4 | <0.001 |
| NO3− | 354.1 | 305.6 | 0.036 |
| SO42− | 47.5 | 50.9 | 0.394 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, D.; Chiwa, M. Differences in Soil Solution Chemistry and Their Vertical Variation Between Moso Bamboo Forests and Japanese Cedar Plantations in Western Japan. Forests 2025, 16, 1519. https://doi.org/10.3390/f16101519
Fu D, Chiwa M. Differences in Soil Solution Chemistry and Their Vertical Variation Between Moso Bamboo Forests and Japanese Cedar Plantations in Western Japan. Forests. 2025; 16(10):1519. https://doi.org/10.3390/f16101519
Chicago/Turabian StyleFu, Dongchuan, and Masaaki Chiwa. 2025. "Differences in Soil Solution Chemistry and Their Vertical Variation Between Moso Bamboo Forests and Japanese Cedar Plantations in Western Japan" Forests 16, no. 10: 1519. https://doi.org/10.3390/f16101519
APA StyleFu, D., & Chiwa, M. (2025). Differences in Soil Solution Chemistry and Their Vertical Variation Between Moso Bamboo Forests and Japanese Cedar Plantations in Western Japan. Forests, 16(10), 1519. https://doi.org/10.3390/f16101519






