Linking Morphological Traits of Fine Root to Soil CO2 Efflux in Middle-Aged Plantations of Four Tree Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Measurements of RS, TS, and SWC
2.3. Measurements of Soil Physicochemical Properties and Fine Root Traits
2.4. Statistical Analysis
3. Results
3.1. Soil Physicochemical Properties Among Different Plantations
3.2. Fine Root Biomass and Morphological Traits Among Different Plantations
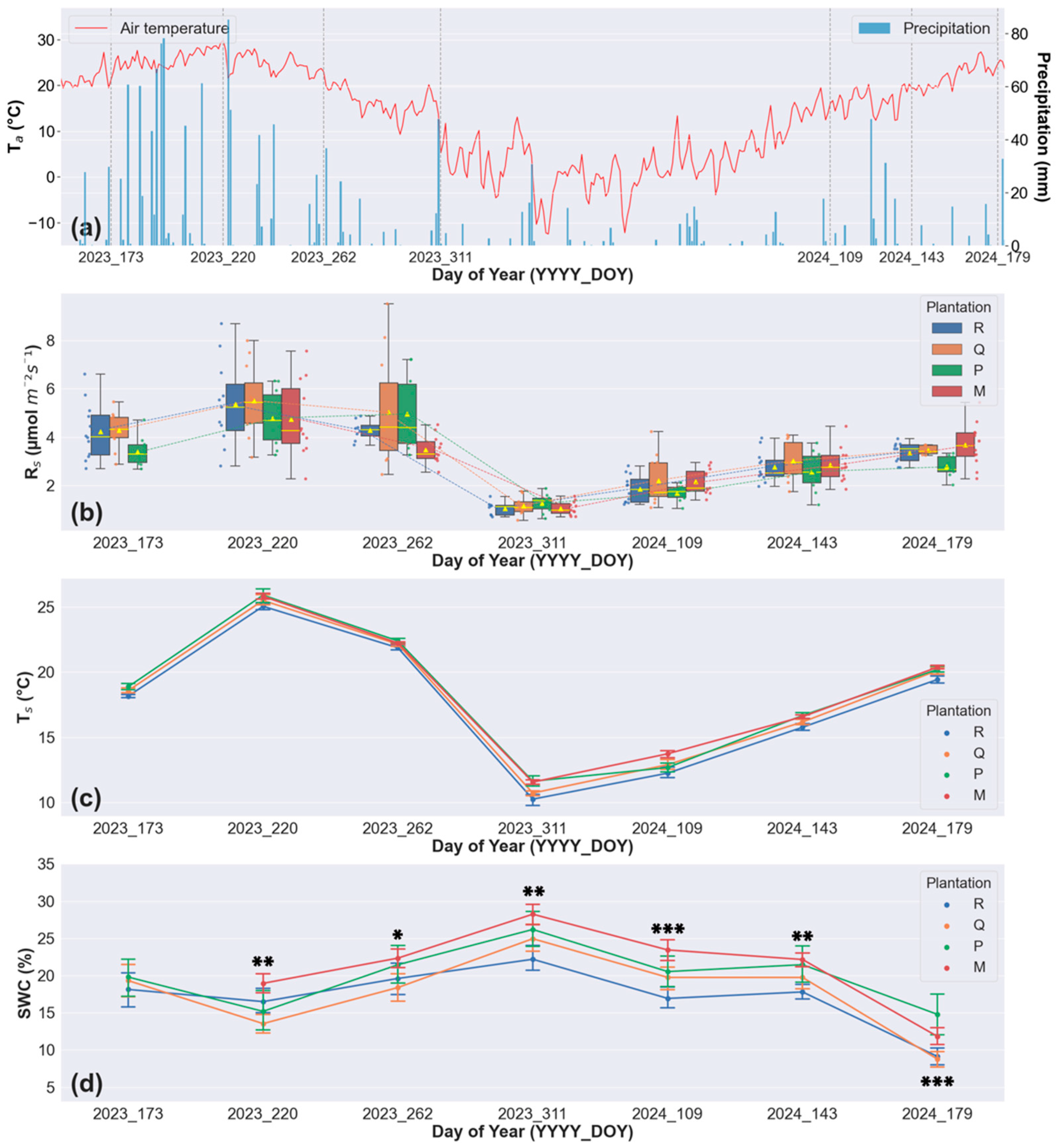
3.3. Soil CO2 Efflux
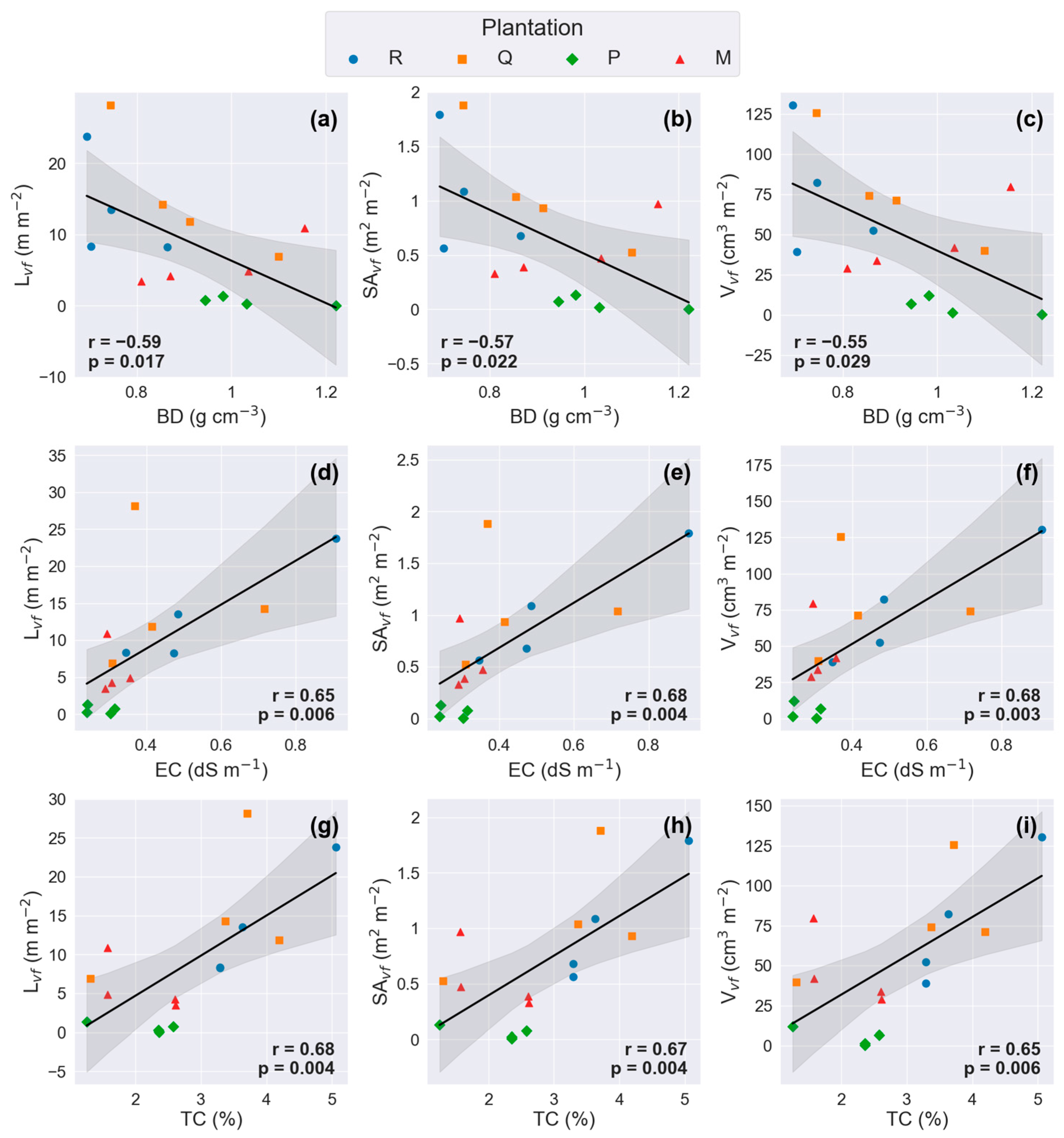
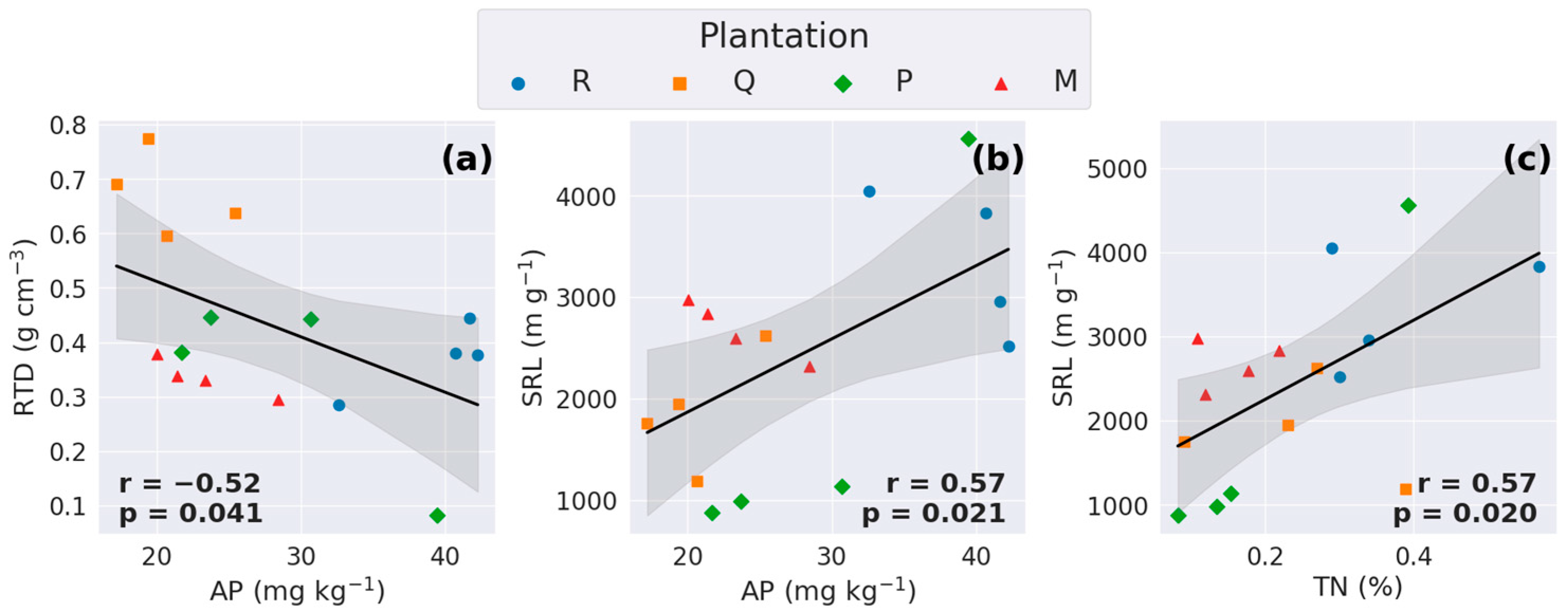
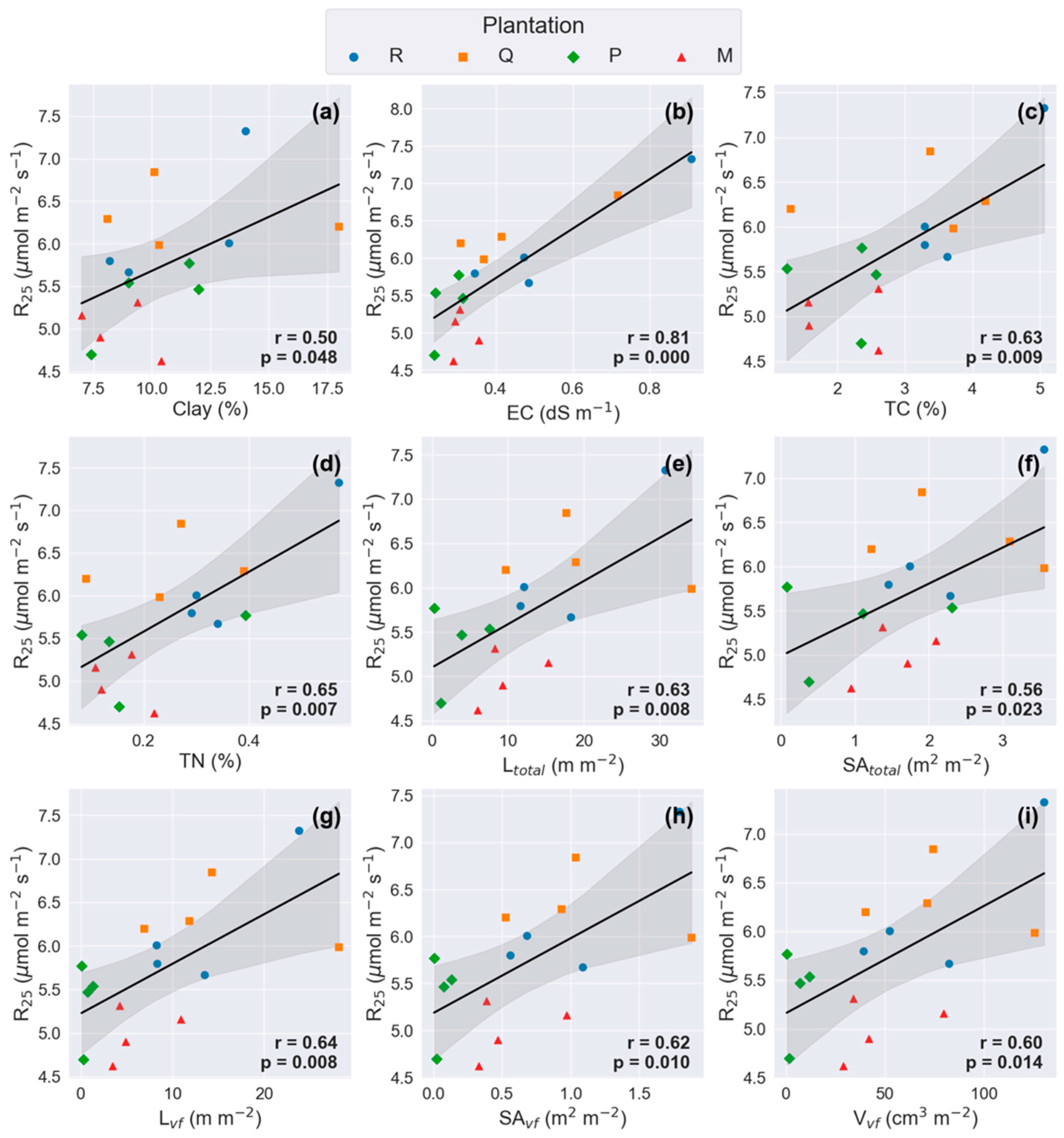
3.4. Relationships Between Soil Physicochemical Properties and Traits of Fine Roots
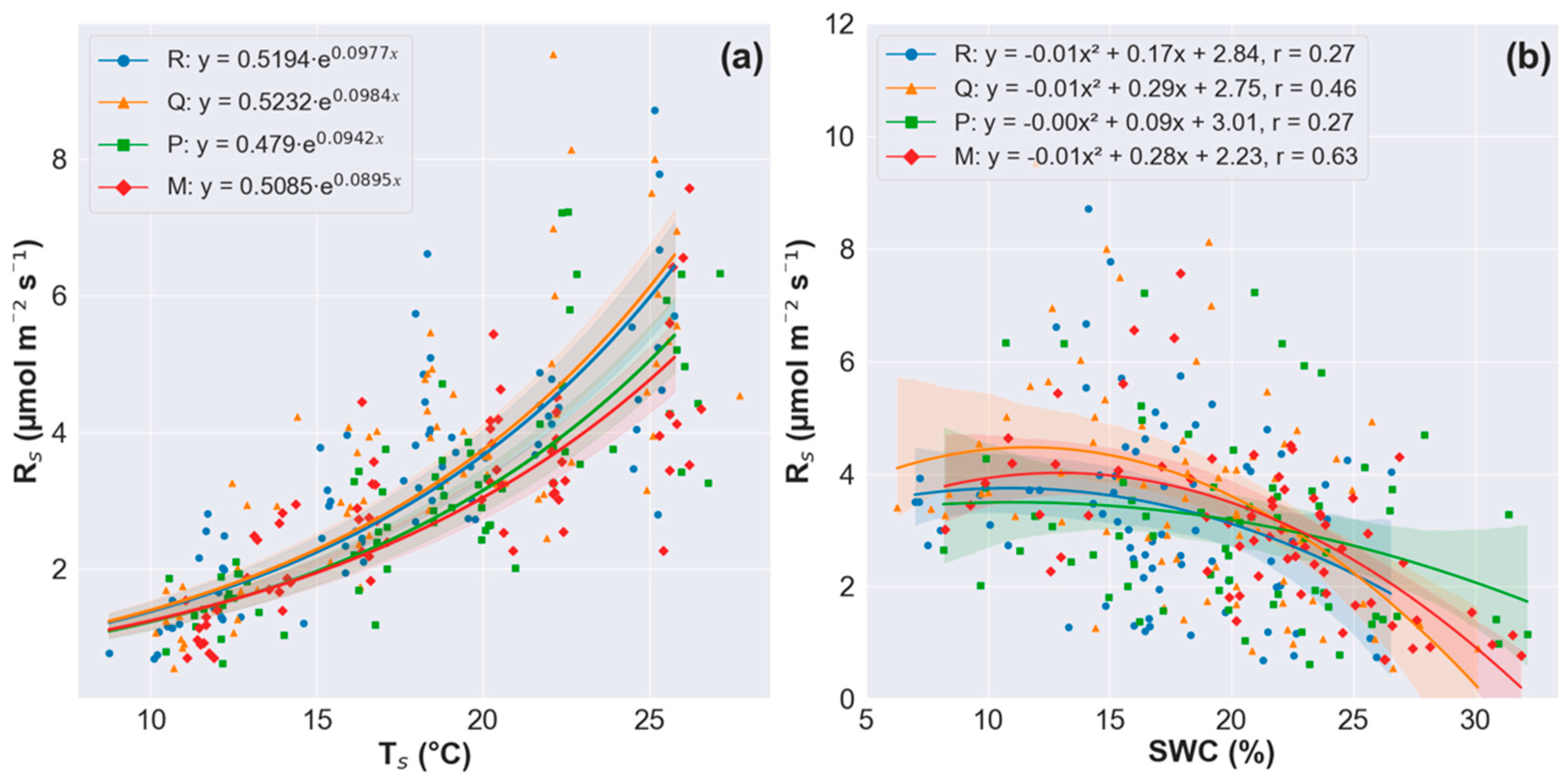
3.5. Relationships of RS with Soil Physicochemical Properties and Morphological Traits of Fine Roots
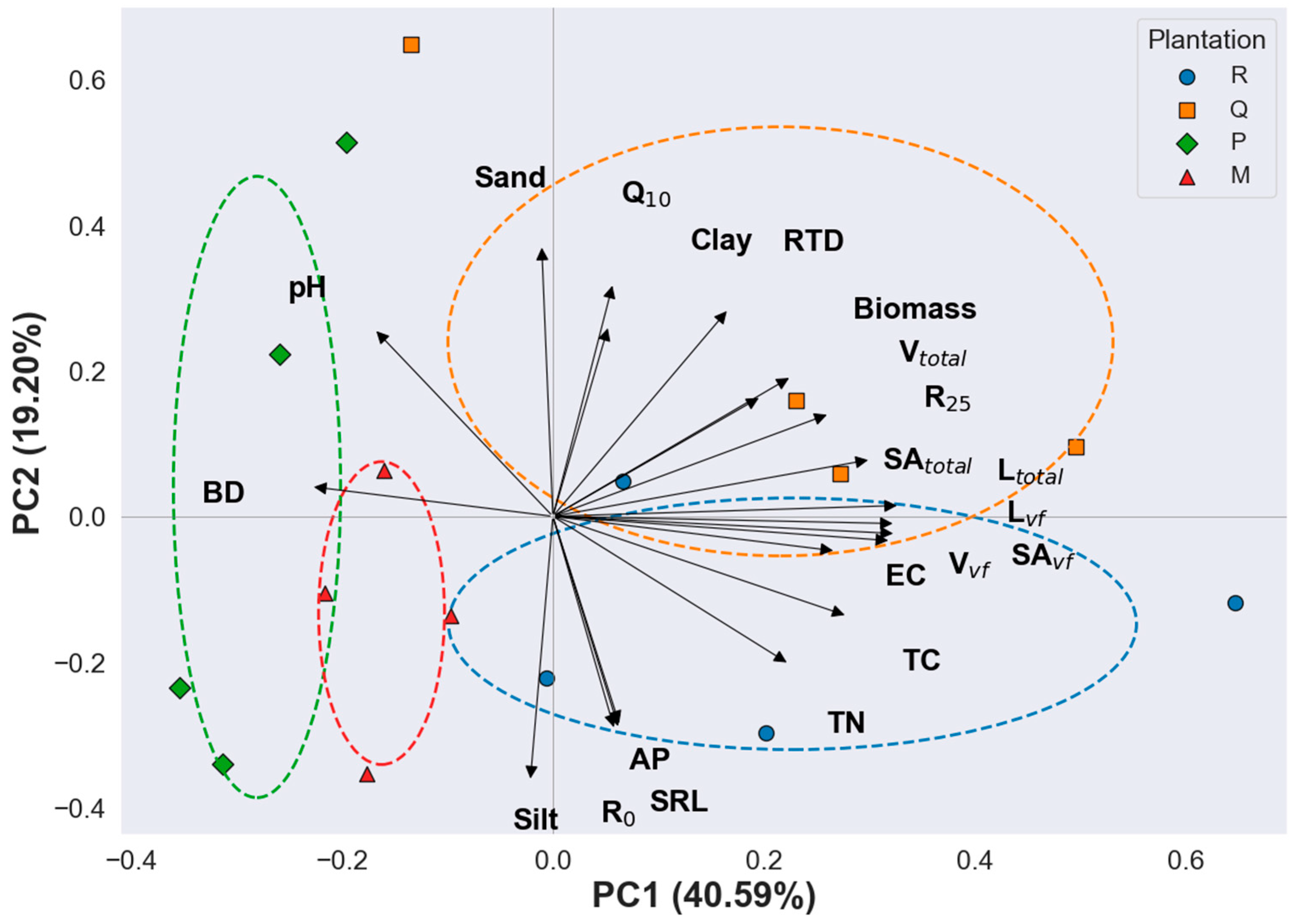
3.6. Multivariate Integration of Soil Properties, Fine Root Traits, and RS
4. Discussion
4.1. Species Effects on Soil Physicochemical Properties Across the Four Plantations
4.2. Species Effects on Morphological Traits of Fine Roots Across the Four Plantations
4.3. RS,TS, and SWC
4.4. The Correlation Between RS and Soil Physicochemical Properties
4.5. The Correlation Between RS and Morphological Traits of Very-Fine Roots
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; Available online: https://www.ipcc.ch/report/ar6/wg1/ (accessed on 15 June 2025).
- Abbass, K.; Qasim, M.Z.; Song, H.; Murshed, M.; Mahmood, H.; Younis, I. A review of the global climate change impacts, adaptation, and sustainable mitigation measures. Environ. Sci. Pollut. Res. 2022, 29, 42539–42559. [Google Scholar] [CrossRef] [PubMed]
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.M.; Hauck, J.; Landschützer, P.; Le Quéré, C.; Li, H.; Luijkx, I.T.; Olsen, A.; et al. Global Carbon Budget 2024. Earth Syst. Sci. Data Discuss. 2024, 17, 965–1039. [Google Scholar] [CrossRef]
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef]
- Baccini, A.; Walker, W.S.; Carvalho, L.; Farina, M.; Sulla-Menashe, D.; Houghton, R.A. Tropical forests are a net carbon source based on aboveground measurements of gain and loss. Science 2017, 358, 230–234. [Google Scholar] [CrossRef]
- Jobbágy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Thomson, A. Temperature-associated increases in the global soil respiration record. Nature 2010, 464, 579–582. [Google Scholar] [CrossRef]
- Phillips, R.P.; Finzi, A.C.; Bernhardt, E.S. Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long-term CO2 fumigation. Ecol. Lett. 2011, 14, 187–194. [Google Scholar] [CrossRef]
- Watts, J.D.; Natali, S.; Minions, C. Soil Respiration Maps for the Above Domain, 2016–2017; ORNL DAAC Data Set; Oak Ridge National Laboratory Distributed Active Archive Center: Oak Ridge, TN, USA, 2022. [CrossRef]
- Lee, M.S.; Lee, J.S.; Koizumi, H. Temporal variation in CO2 efflux from soil and snow surfaces in a Japanese cedar (Cryptomeria japonica) forest. Ecol. Res. 2007, 22, 215–222. [Google Scholar]
- Noh, N.J.; Son, Y.; Lee, S.K.; Yoon, T.K.; Seo, K.W.; Kim, C.; Lee, W.K.; Bae, S.W.; Hwang, J. Influence of stand density on soil CO2 efflux for a Pinus densiflora forest in Korea. J. Plant Res. 2010, 123, 411–419. [Google Scholar] [CrossRef]
- Fekete, I.; Varga, C.; Biró, B.; Tóth, J.A.; Várbíró, G.; Szabó, G.; Kotroczó, Z. The effects of litter production and litter depth on soil microclimate in a Central European deciduous forest. Plant Soil 2016, 398, 291–300. [Google Scholar] [CrossRef]
- Staněk, L.; Neruda, J.; Ulrich, R. Changes in the concentration of CO2 in forest soils resulting from the traffic of logging machines. J. For. Sci. 2025, 71, 250–267. [Google Scholar] [CrossRef]
- Lal, R. Managing soils for negative feedback to climate change and positive impact on food and nutritional security. Soil Sci. Plant Nutr. 2020, 66, 1–9. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, M.W.; Chang, H.; Je, S.M.; Kim, G.J.; Noh, N.J. Effects of soil physical ameliorants on the growth and root morphology of Prunus yedoensis and Ginkgo biloba seedlings in compacted soils. J. For. Res. 2025, 30, 1–10. [Google Scholar] [CrossRef]
- Hobbie, S.E.; Oleksyn, J.; Eissenstat, D.M.; Reich, P.B. Plant species effects on nutrient cycling: Revisiting litter feedbacks. Trends Ecol. Evol. 2015, 30, 357–363. [Google Scholar] [CrossRef]
- Freschet, G.T.; Valverde-Barrantes, O.J.; Tucker, C.M.; Craine, J.M.; McCormack, M.L.; Violle, C.; Fort, F.; Blackwood, C.B.; Urban-Mead, K.R.; Iversen, C.M.; et al. Climate, soil and plant functional types as drivers of global fine-root trait variation. J. Ecol. 2017, 105, 1182–1196. [Google Scholar] [CrossRef]
- Makita, N.; Kosugi, Y.; Dannoura, M.; Takanashi, S.; Niiyama, K.; Kassim, A.R.; Nik, A.R. Patterns of root respiration rates and morphological traits in 13 tree species in a tropical forest. Tree Physiol. 2012, 32, 303–312. [Google Scholar] [CrossRef]
- Miyatani, K.; Tanikawa, T.; Makita, N.; Hirano, Y. Relationships between specific root length and respiration rate of fine roots across stands and seasons in Chamaecyparis obtusa. Plant Soil 2018, 423, 215–227. [Google Scholar] [CrossRef]
- Makita, N.; Kawamura, A.; Osawa, A. Size-dependent morphological and chemical properties of fine-root litter decomposition. Plant Soil 2015, 393, 283–295. [Google Scholar] [CrossRef]
- Karst, J.; Gaster, J.; Wiley, E.; Landhäusser, S.M. Relationships between root respiration rate and root morphology, chemistry, and anatomy in Larix gmelinii and Fraxinus mandshurica. Tree Physiol. 2013, 33, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Noh, N.J.; Crous, K.Y.; Li, J.; Choury, Z.; Barton, C.V.M.; Arndt, S.K.; Reich, P.B.; Tjoelker, M.G.; Pendall, E. Does root respiration in Australian rainforest tree seedlings acclimate to experimental warming? Tree Physiol. 2020, 40, 1192–1204. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Zhu, B. Linking Root Respiration to Chemistry and Morphology across Species. Glob. Change Biol. 2021, 27, 1367–1381. [Google Scholar] [CrossRef] [PubMed]
- McCormack, M.L.; Guo, D.; Iversen, C.M.; Chen, W.; Eissenstat, D.M.; Fernandez, C.W.; Li, L.; Ma, C.; Ma, Z.; Poorter, H.; et al. Building a better foundation: Improving root-trait measurements to understand and model plant and ecosystem processes. New Phytol. 2017, 215, 27–37. [Google Scholar] [CrossRef]
- Freschet, G.T.; Pagès, L.; Iversen, C.M.; Comas, L.H.; Rewald, B.; Roumet, C.; Klimešová, J.; Zadworny, M.; Poorter, H.; Postma, J.A.; et al. A starting guide to root ecology: Strengthening ecological concepts and standardising root classification, sampling, processing and trait measurements. New Phytol. 2021, 232, 973–1122. [Google Scholar] [CrossRef]
- Valverde-Barrantes, O.J.; Smemo, K.A.; Blackwood, C.B. Fine Root Morphology Is Phylogenetically Structured, but Nitrogen Is Related to the Plant Economics Spectrum in Temperate Trees. Funct. Ecol. 2015, 29, 796–807. [Google Scholar] [CrossRef]
- Norris, M.D.; Blair, J.M.; Johnson, L.C. Effects of fire frequency on litter decomposition and nutrient release in a tallgrass prairie. Oecologia 2012, 170, 587–598. [Google Scholar]
- Aponte, C.; García, L.V.; Marañón, T.; Gardes, M. Tree species effects on nutrient cycling and soil biota: A feedback mechanism favouring species coexistence. For. Ecol. Manag. 2013, 309, 36–46. [Google Scholar] [CrossRef]
- Russell, A.E.; Raich, J.W.; Valverde-Barrantes, O.J.; Fisher, R.F. Tree species effects on soil properties in experimental plantations in tropical moist forest. Soil Sci. Soc. Am. J. 2007, 71, 1389–1397. [Google Scholar] [CrossRef]
- Pérez-Bejarano, A.; Mataix-Solera, J.; Zornoza, R.; Guerrero Maestre, C.; Arcenegui, V.; Mataix-Beneyto, J.J.; Cano-Amat, S. Influence of plant species on physical, chemical and biological soil properties in a Mediterranean forest soil. Eur. J. For. Res. 2010, 129, 15–24. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, W.; Xu, M.; Deng, J.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Response of forest growth to C:N:P stoichiometry in plants and soils during Robinia pseudoacacia afforestation on the Loess Plateau, China. Geoderma 2019, 337, 280–289. [Google Scholar] [CrossRef]
- Lambers, H.; Plaxton, W.C. Phosphorus: Back to the Roots. In Annual Plant Reviews, Volume 48: Phosphorus Metabolism in Plants; Plaxton, W.C., Lambers, H., Eds.; John Wiley & Sons: Chichester, UK, 2015; pp. 3–22. [Google Scholar] [CrossRef]
- McCormack, M.L.; Adams, T.S.; Smithwick, E.A.H.; Eissenstat, D.M. Variability in root production, phenology, and turnover rate among 12 temperate tree species. Ecology 2014, 95, 2224–2235. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Q.; Liu, L.; Liu, S.; Zhang, C. Fine root phenology differs among subtropical evergreen broadleaved forests with increasing tree diversities. For. Ecol. Manag. 2017, 404, 326–334. [Google Scholar] [CrossRef]
- Comas, L.H.; Eissenstat, D.M. Patterns in root trait variation among 25 co-existing North American forest species. New Phytol. 2009, 182, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B. The world-wide ‘fast–slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Kramer-Walter, K.R.; Bellingham, P.J.; Millar, T.R.; Smissen, R.D.; Richardson, S.J.; Laughlin, D.C. Root traits are multidimensional: Specific root length is independent from root tissue density and the plant economic spectrum. J. Ecol. 2016, 104, 1299–1310. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Sources of CO2 efflux from soil and review of partitioning methods. Soil Biol. Biochem. 2006, 38, 425–448. [Google Scholar] [CrossRef]
- Lee, J.S. Monitoring soil respiration using an automatic operating chamber in a Gwangneung temperate deciduous forest. J. Ecol. Environ. 2011, 34, 411–423. [Google Scholar] [CrossRef]
- Klimek, B.; Chodak, M.; Niklińska, M. Soil respiration in seven types of temperate forests exhibits similar temperature sensitivity. J. Soils Sediments 2021, 21, 338–345. [Google Scholar] [CrossRef]
- Possinger, A.R.; Driscoll, C.T.; Green, M.B.; Fahey, T.J.; Johnson, C.E.; Koppers, M.M.K.; Martel, L.D.; Morse, J.L.; Templer, P.H.; Uribe, A.M.; et al. Increasing soil respiration in a northern hardwood forest indicates symptoms of a changing carbon cycle. Commun. Earth Environ. 2025, 6, 418. [Google Scholar] [CrossRef]
- Curiel-Yuste, J.; Janssens, I.A.; Carrara, A.; Ceulemans, R. Annual Q10 of soil respiration reflects plant phenological patterns as well as temperature sensitivity. Glob. Change Biol. 2004, 10, 161–169. [Google Scholar] [CrossRef]
- Phillips, C.L.; Nickerson, N.; Risk, D.; Bond, B.J. Interpreting diel hysteresis between soil respiration and temperature. Glob. Change Biol. 2011, 17, 515–527. [Google Scholar] [CrossRef]
- Seyfried, M.S.; Flerchinger, G.N.; Bryden, S.; Link, T.; Marks, D.G.; McNamara, J.P. Slope and aspect controls on soil climate: Field documentation and implications for large-scale simulation of critical zone processes. Vadose Zone J. 2021, 20, e20158. [Google Scholar] [CrossRef]
- Lenk, A.; Richter, R.; Kretz, L.; Wirth, C. Effects of canopy gaps on microclimate, soil biological activity and their relationship in a European mixed floodplain forest. Sci. Total Environ. 2024, 941, 173572. [Google Scholar] [CrossRef] [PubMed]
- Robakowski, P.; Wyka, T.P.; Kowalkowski, W.; Barzdajn, W.; Pers-Kamczyc, E.; Jankowski, A.; Politycka, B. Practical Implications of Different Phenotypic and Molecular Responses of Evergreen Conifer and Broadleaf Deciduous Forest Tree Species to Regulated Water Deficit in a Container Nursery. Forests 2020, 11, 1011. [Google Scholar] [CrossRef]
- Yuan, B.C.; Li, Z.Z.; Liu, H.; Gao, M.; Zhang, Y.Y. Microbial biomass and activity in salt-affected soils under arid conditions. Appl. Soil Ecol. 2007, 35, 319–328. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Sarikhani, M.R.; Sinegani, A.A.; Ahmadi, A.; Keesstra, S. Estimating soil respiration under different land uses using artificial neural network and linear regression models. Catena 2019, 174, 371–382. [Google Scholar] [CrossRef]
- Cui, H.; Bai, J.; Du, S.; Wang, J.; Keculah, G.N.; Wang, W.; Zhang, G.; Jia, J. Interactive effects of groundwater level and salinity on soil respiration in coastal wetlands of a Chinese delta. Environ. Pollut. 2021, 286, 117400. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Miao, F.; Li, Z.; Tang, W.; Sun, J.; Wang, Q.; Liu, J. Assessing the effect of soil salinization on soil microbial respiration and diversities under incubation conditions. Appl. Soil Ecol. 2020, 155, 103671. [Google Scholar] [CrossRef]
- Drake, P.; McCormick, C.A.; Smith, M.J.A. Controls of soil respiration in a salinity-affected ephemeral wetland. Geoderma 2014, 221–222, 96–102. [Google Scholar] [CrossRef]
- Ahmad, M.N.; Anuar, M.I.; Abd Aziz, N.; Murdi, A.A. Function and application of soil electrical conductivity (EC) sensor in agriculture: A review. Adv. Agric. Food Res. J. 2025, 6, a0000552. [Google Scholar]
- Kim, H.N.; Park, J.H. Monitoring of soil EC for the prediction of soil nutrient regime under different soil water and organic matter contents. Appl. Biol. Chem. 2024, 67, 1. [Google Scholar] [CrossRef]
- Makita, N.; Fujii, S. Tree species effects on microbial respiration from decomposing leaf and fine root litter. Soil Biol. Biochem. 2015, 88, 39–47. [Google Scholar] [CrossRef]
- Saiz, G.; Byrne, K.A.; Butterbach-Bahl, K.; Kiese, R.; Blujdea, V.; Farrell, E.P. Stand age-related effects on soil respiration in a first rotation Sitka spruce chronosequence in central Ireland. Glob. Change Biol. 2006, 12, 1007–1020. [Google Scholar] [CrossRef]
- Jagodziński, A.M.; Ziółkowski, J.; Warnkowska, A.; Prais, H. Tree Age Effects on fine root biomass and morphology over chronosequences of Fagus sylvatica, Quercus robur and Alnus glutinosa stands. PLoS ONE 2016, 11, e0148668. [Google Scholar] [CrossRef] [PubMed]
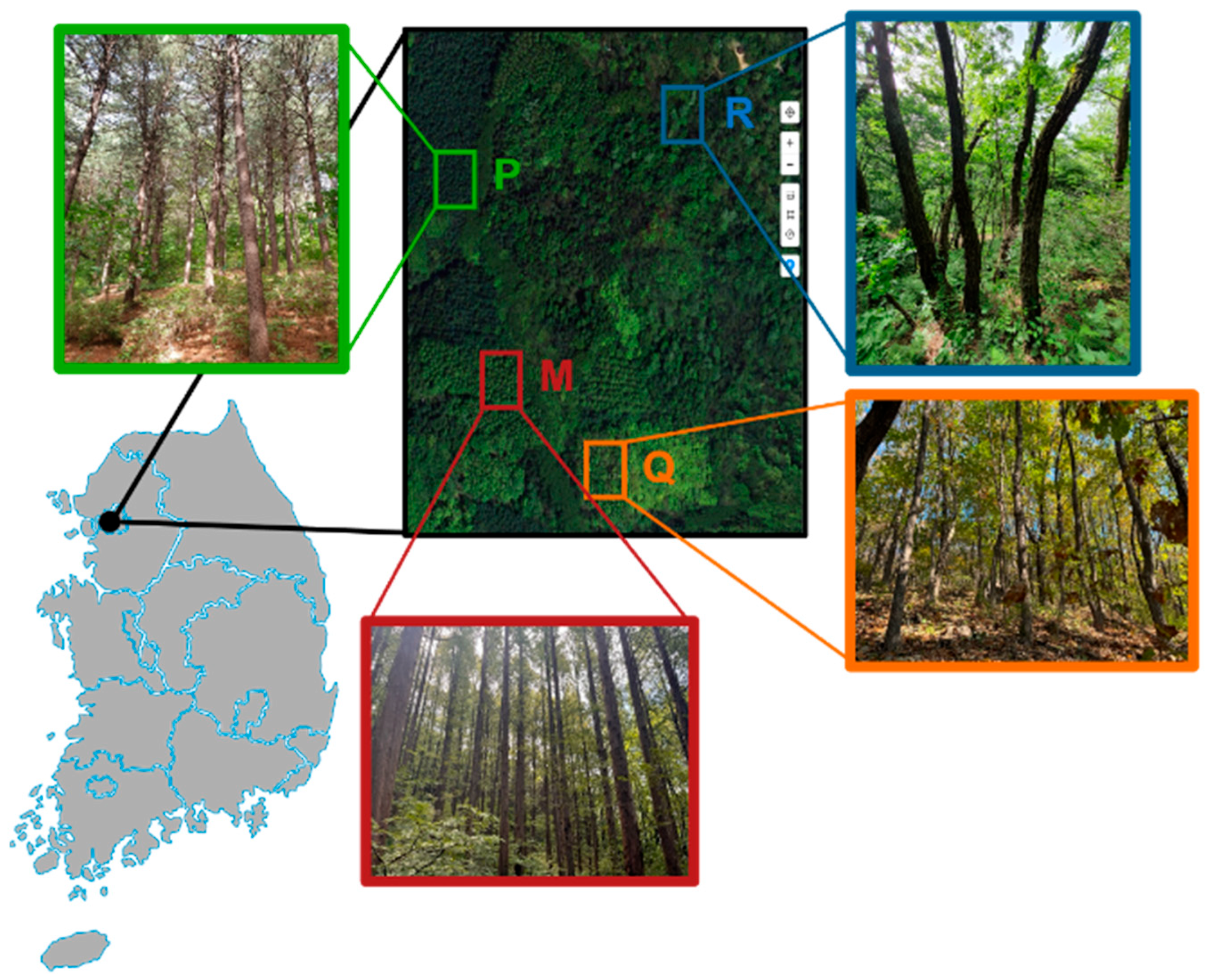
| Characteristics | Plantation | |||
|---|---|---|---|---|
| R | Q | P | M | |
| Stand age class | IV | IV | IV | IV |
| Altitude (m a. s. l.) | 195–200 | 190–200 | 135–140 | 145–155 |
| Slope (°) | 31–33 | 26–30 | 20–22 | 22–24 |
| Aspect (°) | NW19 | NW3 | NW40 | NW10 |
| Stand density (tree ha−1) | 550 ± 87 | 767 ± 68 | 650 ± 76 | 959 ± 31 |
| Mean D.B.H. (cm) | 23.85 ± 0.56 | 20.98 ± 1.47 | 22.50 ± 1.16 | 23.87 ± 1.48 |
| Basal area (m2 ha−1) | 26.2 ± 2.97 | 25.7 ± 3.92 | 28.0 ± 0.89 | 46.7 ± 5.5 |
| Properties | Plantation | ||||
|---|---|---|---|---|---|
| R | Q | P | M | p-Value | |
| Sand (%) | 45.1 ± 1.7 | 47.6 ± 1.8 | 46.4 ± 1.8 | 43.3 ± 0.9 | >0.1 |
| Silt (%) | 43.8 ± 2.8 | 40.8 ± 3.7 | 43.6 ± 2.0 | 48.1 ± 1.6 | >0.1 |
| Clay (%) | 11.1 ± 1.5 | 11.6 ± 2.2 | 10.0 ± 1.1 | 8.7 ± 0.8 | >0.1 |
| BD (g cm−3) | 0.75 ± 0.04 | 0.90 ± 0.08 ab | 1.05 ± 0.06 a | 0.97 ± 0.08 ab | 0.045 * |
| pH | 4.34 ± 0.03 | 4.51 ± 0.09 | 4.90 ± 0.23 | 4.69 ± 0.08 | 0.056 |
| EC (dS m−1) | 0.55 ± 0.12 | 0.45 ± 0.09 | 0.28 ± 0.02 | 0.31 ± 0.02 | 0.088 |
| TC (%) | 3.82 ± 0.42 a | 3.15 ± 0.63 a | 2.14 ± 0.30 a | 2.09 ± 0.30 a | 0.043 |
| TN (%) | 0.38 ± 0.07 | 0.25 ± 0.06 | 0.19 ± 0.07 | 0.16 ± 0.0 3 | 0.090 |
| AP (mg kg−1) | 39.3 ± 2.3 a | 20.7 ± 1.7 b | 28.9 ± 4.0 ab | 23.3 ± 1.8 b | 0.002 ** |
| Traits | Diameter (mm) | Plantation | ||||
|---|---|---|---|---|---|---|
| R | Q | P | M | p-Value | ||
| Biomass (kg m−2) | Total | 1.39 ± 0.28 | 2.89 ± 0.78 | 1.19 ± 0.59 | 0.95 ± 0.16 | 0.080 |
| L (m m−2) | Total | 18.15 ± 4.45 a | 20.09 ± 5.13 a | 3.19 ± 1.63 b | 9.70 ± 1.99 ab | 0.022 * |
| Very-fine | 13.46 ± 3.66 ab | 15.28 ± 4.56 a | 0.60 ± 0.28 b | 5.86 ± 1.70 ab | 0.018 * | |
| 0.5–2.0 | 4.69 ± 0.80 | 5.68 ± 1.03 | 2.58 ± 1.35 | 3.84 ± 0.45 | >0.1 | |
| SA (m2 m−2) | Total | 2.26 ± 0.47 | 2.44 ± 0.54 | 0.96 ± 0.50 | 1.53 ± 0.24 | >0.1 |
| Very-fine | 1.03 ± 0.28 a | 1.09 ± 0.28 a | 0.06 ± 0.03 b | 0.54 ± 0.15 ab | 0.016 * | |
| 0.5–2.0 | 1.23 ± 0.19 | 1.57 ± 0.35 | 0.91 ± 0.47 | 0.99 ± 0.14 | >0.1 | |
| V (cm3 m−2) | Total | 368.3 ± 61.5 | 429.6 ± 116.1 | 297.3 ± 152.0 | 281.7 ± 46.1 | >0.1 |
| Very-fine | 76.0 ± 20.3 a | 77.7 ± 17.7 a | 5.1 ± 2.7 b | 46.1 ± 11.5 ab | 0.014 * | |
| 0.5–2.0 | 292.5 ± 42.0 | 406.5 ± 106.7 | 292.2 ± 149.3 | 235.6 ± 39.2 | >0.1 | |
| RTD (g cm−3) | Total | 0.37 ± 0.03 b | 0.67 ± 0.04 a | 0.34 ± 0.09 b | 0.34 ± 0.02 b | 0.001 ** |
| SRL (m g−1) | Total | 3339 ± 360 | 1879 ± 295 | 1890 ± 892 | 2682 ± 145 | >0.1 |
| Parameter | Plantation | ||||
|---|---|---|---|---|---|
| R | Q | P | M | p-Value | |
| Q10 | 2.80 ± 0.11 | 2.76 ± 0.15 | 2.62 ± 0.15 | 2.54 ± 0.17 | 0.583 |
| R0 (µmol m−2 s−1) | 0.48 ± 0.04 | 0.52 ± 0.06 | 0.50 ± 0.06 | 0.52 ± 0.08 | 0.970 |
| R25 (µmol m−2 s−1) | 6.20 ± 0.38 a | 6.33 ± 0.18 a | 5.37 ± 0.23 ab | 5.00 ± 0.45 b | 0.007 ** |
| r2 | 0.80 ± 0.05 | 0.79 ± 0.03 | 0.80 ± 0.05 | 0.82 ± 0.06 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, S.W.; Song, K.H.; Jang, J.W.; Lee, S.H.; Koo, N.; Kim, S.; Noh, N.J. Linking Morphological Traits of Fine Root to Soil CO2 Efflux in Middle-Aged Plantations of Four Tree Species. Forests 2025, 16, 1513. https://doi.org/10.3390/f16101513
Lim SW, Song KH, Jang JW, Lee SH, Koo N, Kim S, Noh NJ. Linking Morphological Traits of Fine Root to Soil CO2 Efflux in Middle-Aged Plantations of Four Tree Species. Forests. 2025; 16(10):1513. https://doi.org/10.3390/f16101513
Chicago/Turabian StyleLim, Seung Won, Kyu Hong Song, Ji Won Jang, Se Hee Lee, Namin Koo, Sukwoo Kim, and Nam Jin Noh. 2025. "Linking Morphological Traits of Fine Root to Soil CO2 Efflux in Middle-Aged Plantations of Four Tree Species" Forests 16, no. 10: 1513. https://doi.org/10.3390/f16101513
APA StyleLim, S. W., Song, K. H., Jang, J. W., Lee, S. H., Koo, N., Kim, S., & Noh, N. J. (2025). Linking Morphological Traits of Fine Root to Soil CO2 Efflux in Middle-Aged Plantations of Four Tree Species. Forests, 16(10), 1513. https://doi.org/10.3390/f16101513







