Abstract
In cool temperate regions, soil respiration (Rs) data collected during the cold season is limited due to freezing and snow. This leads to a lack of understanding of Rs characteristics during the cold season and for ecosystems with long winters, it can significantly impact the annual carbon flux estimation. In this study, Rs data were collected from temperate deciduous forests to understand the characteristics of Rs values in the cold temperature season. To reflect spatial variation in Rs, five points were selected with different levels of litter layer development, ranging from Chamber 1 (almost no litter) to Chamber 5 (thick litter). Rs, air temperature (Ta) and rainfall, soil temperature (Ts) and soil moisture content (SMC) were collected every 30 min at each measurement point. As the litter layer developed, Ts tended to increase, but SMC tended to decrease, revealing that the degree of litter layer development had a clear effect on Ts and SMC. Rs showed a relatively high exponential correlation with Ts. However, the Rs−SMC functional relationship exhibited no correlation. Therefore, while the Ts-Rs functional equation can be used in the Rs calculator during the cold season, the SMC-Rs function would be suitable for use. Also, these deferent litter layers, TS, and SMC affected the Rs. The total Rs during the measurement period was various from 0.60 t C ha−1 for a thin litter layer to 1.88 t C ha−1 for a thick layer. This range of values may be appropriate for estimating Rs during the cold season in temperate regions. Also, the average across all plots was 6.05, ranging from 4.93 in no litter to 8.23 in thick litter layer.
1. Introduction
In the carbon cycle of terrestrial ecosystems, soil, as the largest carbon store [1] and a medium of circulation, is equally or more important than vegetation and the atmosphere. Therefore, understanding the carbon cycle of the soil layer is fundamental to comprehending the carbon cycle of terrestrial ecosystems, including their role and use in addressing climate change [2].
In the northern hemisphere, temperate-climate deciduous forests with four distinct seasons are a common vegetation type [3,4,5]. However, most studies have focused on collecting data during the plant growth season, and soil respiration (Rs) is mainly measured during the warm or growing season assuming that soil microbial activity is extremely low at cold temperature [6,7,8]. As a result, data on the soil carbon cycle during the winter season seemed insufficient. Therefore, many studies have shown functional correlations of soil respiration to various environmental factors, such as biotic phenology, soil temperature [9,10,11], precipitation [12,13], and soil moisture [14,15]. In addition, these factors were used to predict the soil carbon budget for the annual or long-term climate change environment. Most temperate and high-latitude boreal ecosystems have cold-temperature periods in which winter ice, snow cover, and cold temperatures limit access to measurement sites and influence behavioral and instrumental use during data collection. In addition, the strength and duration of these restrictions become greater at higher latitudes [8,16,17]. As a result, functional equations between Rs and environmental factors are often derived using Rs data, along with missing cold-temperature seasonal data. Moreover, this data is employed to calculate annual Rs values using a functional equation that does not reflect the cold-temperature seasonal data. Ultimately, because of these limitations, the suitability of the functional equation decreases and the error in the evaluated annual Rs value increases. The contribution of Rs during the winter season is relevant on an annual scale and should not be overlooked in the overall soil or ecosystem carbon balance [18]. Furthermore, many studies on soil carbon predict that global warming will accelerate the release of carbon stored in soil to the atmosphere through Rs [19,20,21]. Moreover, some studies suggest that in arctic, boreal, alpine, and temperate ecosystems, a significant portion of the fixed carbon during the photosynthetic season may be lost through soil respiration during the cold season [7,22,23,24]. Also, the observed wintertime CO2 fluxes were relatively low, but due to the long duration of the snow-covered period, they constitute an important part of the annual CO2 balance. The CO2 balance for the winter period of a subarctic fen in northern Europe was greater than the absolute value of the total annual balance, which shows the importance of the wintertime efflux [22]. The total respirations for the snow cover period of 4 months in a temperate deciduous temperate forest was 7.7% of the annual sum [7]. The contribution from the cold period to the annual CO2 flux in Moscow Region was substantial and averaged 21% and 14% for natural and agricultural ecosystems, respectively. The CO2 fluxes comprised approximately 7~10% in winter of the total annual carbon dioxide flux [23]. Future climate change in the Northern Hemisphere could affect the spatiotemporal distribution of snow cover and the frequency and duration of freeze–thaw cycles, and differences in the insulating effect of snow cover could affect soil respiration [24].
The Intergovernmental Panel on Climate Change 2007 report revealed that the observed global warming over the last 30 years was greatest at higher northern latitudes and during winter. This concentrated regional, temporal, and temporal warming is affecting a wide range of ecosystems in a variety of ways, with significant impacts on temperate and boreal ecosystems. In many cool temperate and boreal ecosystems, reduced snow cover during winter, leading to decreased soil insulation against freezing, is predicted to increase soil freeze–thaw frequency [25]. This pattern is expected to be exacerbated in the future. In addition, many studies have predicted that global warming will increase both the frequency and scale of precipitation and snowfall [26,27], which will affect various environmental factors involved in the carbon cycle, such as the soil carbon cycle during cold-temperature periods. Changes in temperature and moisture due to global warming significantly impact the soil environment not only in summer but also in winter, as well as the soil carbon balance by changing various Rs-related factors. Moreover, rising average temperatures and more frequent extreme temperature fluctuations are expected. In particular, increased winter temperature and reduced snowfall in temperate and subarctic ecosystems are anticipated to alter the soil conditions, including soil drying and changes in cold-temperature physical and chemical environments, ultimately impacting the soil carbon cycle and ecosystem [28,29,30].
In a related study, microbial processes in cold-temperature soils are particularly important in this context because the relative temperature sensitivity of organic matter decomposition is generally high at cold temperatures [31]. Removing snow cover created soils that were relatively dry, frequently frozen, and poor in carbon substrates, whereas adding snow cover created soils that were wetter, warmer, and relatively rich in carbon substrates [32]. In addition, there was improved respiratory sensitivity to moisture in the surrounding environment and reduced snowfall. Rs was only sensitive to temperature upon the addition of snow cover. More snowfall can induce higher soil respiration as a result of higher soil temperature under the deeper snow cover [33] or higher soil moisture content by snow melting [34]. Furthermore, it has been reported that changes in snowfall did not affect microbial biomass carbon, microbial biomass nitrogen, or soil available nitrogen content [35]. Ultimately, because soil carbon balance characteristics during cold-temperature periods are unclear, the collection of more data on winter Rs is required to gain a deeper understanding of the soil carbon cycle during cold-temperature periods. Therefore, understanding soil respiration and its characteristics during cold-temperature periods is crucial.
This study aims to evaluate the Rs values during the cold-temperature period in temperate deciduous forests and the Rs characteristics during the winter period. Thus, we attempted to identify the characteristics of winter Rs and its contribution to annual Rs values, as well as to interpret how environmental factors, such as soil temperature (Ts), soil moisture content (SMC), and litter layer are connected. In addition, the feasibility of using the functional formulas for the Ts and SMC factors in the winter season was examined. Through this, we aim to contribute to the accurate calculation of annual Rs values and improve the evaluation of the carbon cycle in temperate forest ecosystems.
2. Materials and Methods
2.1. Study Site Description
This study was conducted in a natural temperate deciduous forest located in the west-central part of the Korean Peninsula (36°34′17.4″ N, 127°00′34.9″ E; approximately 214 m above mean sea level, with a southern slope). The vegetation here is judged to belong to the Quercus serrata–Callicarpa japonica–Carpinus cordata community, in which Quercus serrata appears in the tree layer, based on the research results of the nearby Mt. Gyeryongsan National Park where vegetation was studied [36]. In the study site, Quercus species (Quercus serrata, Quercus mongolica, and Quercus variabilis) were dominant in the tree and sub-tree layers, whereas Styrax obassia, Prunus sargentii, and Lindera erythrocarpa were companion species in the shrub layer. The trees were approximately 60 to 70 years old, and Quercus serrata showed the highest coverage in the tree layer, with 19 individuals. Diameter breast height (DBH) ranged from 17.2 cm to 47.1 cm, with an average of 30.9 cm and an average tree height of 20.7 m. Next, Quercus aliena was present, with five individuals ranging from 13.7 cm to 27.4 cm, an average DBH of 20.5 cm, and an average tree height of 17.0 m. Pinus densiflora had one individual, with a height of 26 m and a DBH of 19.1 cm. In addition, 14 species, including Styrax japonicus, Prunus serrulata var. spontanea, and Lindera erythrocarpa, comprised the sub-tree layer, with an average height of 8.1 m and an average DBH of 8.0 cm. Also, the herb layer had very poor coverage. The tree biomass was calculated to be 1.2 tons for Quercus serrata and 2.1 tons for other trees.
The measurement site was preserved as a temple forest at Magoksa Temple, with little artificial disturbance, and environmental changes due to human activities were evaluated to be minimal. This site is a forest that is commonly found in the central temperate region of Korea and is considered the most suitable region for Rs characterization because it belongs to the forest ecosystem of the temperate monsoon climate region.
A 20 m × 20 m survey site was set up, and the survey points were determined based on the developmental gradient of the litter layer within the plot and the distance from the base of the trees. This was carefully monitored using a probe to ensure that thick roots of plants were not concentrated in a specific chamber and reflected in soil respiration. The area where the chamber was finally installed was approximately 8 m × 8 m. The leaf area index (LAI) of the canopy was measured to be 3.5 ± 0.12 on average (28 August 2021), with no significant difference within the measurement plots. The amount of fallen leaves supplied to the forest floor was approximately 457.7 ± 13.5 g m−2 showing little difference. At the study site, most leaves in the canopy layer fell in late October and new leaves expanded to close the canopy layer by early April. When the canopy opens around November, strong winds flow onto the forest floor, moving the fallen leaves. As a result, the fallen leaves accumulate unevenly at different points on the forest floor. This phenomenon is repeated every year, forming a thick litter layer at points where fallen leaves accumulate. Conversely, a thin or no litter layer is formed at points where the litter is swept away.
The data collection was conducted from 1 November 2021, to 31 March 2022, and the forest at this time was experiencing the winter season during which the leaves of the dominant deciduous trees fell off completely.
2.2. Soil Respiration Measurements
To reflect the spatial heterogeneity of soil respiration (Rs), five measurement points were established at specific distances from the base of the tree, each representing varying degrees of litter layer development. Five measurement points were established based on the gradient of litter layer development formed by accumulated litter: chamber 1 (Ch1; 457.8 g m−2, thin litter layer), chamber 2 (Ch2; 870.0 g m−2), chamber 3 (Ch3; 660.0 g m−2), chamber 4 (Ch4; 1102.2 g m−2), and chamber 5 (Ch5; 1583.3 g m−2, approximately 12 cm thick). Because the measurement points were selected based on litter layer development level, the five chambers represent a microsite gradient rather than independent replicates, and that the results should not be generalized beyond the studied stand/season without additional replication.
The setting point of the chambers was first determined by inserting a pointed probe into the soil layer to ensure that no excessively thick roots were contained in a particular chamber. This was to prevent excessively thick roots from affecting soil respiration. However, the possibility of a systematic shift in component shares (autotrophic vs. heterotrophic) is due to distance from the trunk/root density. After placing the chamber in its installed position, the litter hanging below was cut off with scissors. The chamber was installed so that it was embedded approximately 5 cm deep into the soil along the cracks in the litter layer. This was taken into consideration to ensure that the root respiration level was maintained homogeneously between chambers. However, we acknowledge that the absence of thick roots does not necessarily imply consistent root respiration.
Five chambers from Ch1 to Ch5 were sequentially measured at 5 min intervals for 25 min. Each chamber was automatically closed for 5 min, and the air within the closed-path system was circulated through an air pump system to an infrared gas analyzer (IRGA; LI-840, Li-Cor, Lincoln, NE, USA) to measure the CO2 concentration.
When the measurement starts, the chamber cap was closed, and the chamber, IRGA, and air pump system were connected to create a closed condition. This closed condition causes the concentration to gradually increase due to CO2 generated in the chamber. At this time, the CO2 concentration increase rate (a; ppm min−1) is the rate at which the CO2 concentration of the internal air increases per unit time as time passed after the chamber cap is closed. a is the value calculated by collecting five CO2 concentration values every 5 min at 1 min intervals and calculating the CO2 concentration increase rate per hour from the values at 2, 3, and 4 min. The measurement principles and process are described in the reference literature, so they are briefly described in this paper.
Rs was estimated from the increasing rate in CO2 concentration measured per unit of time Equation (1). It was possible to obtain continuous and high-resolution data on the Rs changes caused by Ts, SMC, rainfall, etc., using the automatic open/closed chamber (AOCC) system [8]. The Rs was defined as:
where a (∆CO2/∆t; ppm min−1) is the increasing rate of the CO2 concentration per unit time in the chamber, V is the total volume (m−3) when the chamber is closed phase, ρ is the density (mg m−3) of CO2 calculated from Ta and air pressure when measuring CO2 concentration, and A is the soil surface area (m2) inside the chamber where CO2 is released from the soil. Atmospheric pressure was collected using a CS106 (Campbell Scientific Inc., Logan, UT, USA).
Rs (mg CO2 m−2 h−1) = (a·V·ρ) A−1
IRGA was calibrated monthly using air-based zero and 915 ppm standard CO2 gas (span) according to the manual provided by the manufacturer. Additionally, the closure status of the chamber was checked at intervals of approximately two weeks.
Q10 value (the factor of respiration rate increased for an increase in temperature by 10 °C) is then calculated as:
where b is a parameter defining the temperature dependence of soil respiration, which can be derived from the exponential function.
Q10 = e10b
2.3. Environmental Factors
Air temperature (Ta) and precipitation were collected at the study site. Soil temperatures (Ts) from Ch1 to Ch5 were measured at a soil depth of 5 cm using thermocouples (T-CC, 0.32 mm, Ninomiya Electric Wire Co., Kanagawa, Japan) installed at each chamber location used for Rs measurement. Also, SMCs from Ch1 to Ch5 were also measured at a depth of 0~15 cm using CS616 (Campbell Scientific Inc., Logan, UT, USA). This depth corresponds to the layer with the highest organic matter content in the soil layer and where microbial respiration is active. Both Ts and SMC were measured simultaneously, and the averages were calculated every 30 s and recorded every 15 min using a data logger (CR1000, Campbell Scientific Inc., Logan, UT, USA). Precipitation, which directly affects SMC, was recorded by connecting a rain gauge (S-RGB-M002, Onset, MA, USA) on the forest floor.
To evaluate snowfall as rainfall, a transparent acrylic collar approximately 15 cm high was attached to the top of the rain gauge. Most of the snow accumulated in the cylinder and then melted and flowed into the rain gauge. Because snow melting takes time, the actual time of snowfall differs from the time rainfall was recorded. However, soil surface snow also melted and flowed into the soil in a similar manner, and since the timing of actual moisture inflow into the soil is crucial, the actual time of snowfall was not specifically considered. However, note that timestamps capture melt rather than snowfall; thus, our precipitation series represents precipitation-equivalent input with a variable melt lag that may blur event timing relative to SMC and Rs.
2.4. Data Treatments
To understand the characteristics of soil respiration changes over time, raw data collected every 30 min were hourly for time series analysis in each chamber and then organized into daily averages. This process identified periods exhibiting specific trends and summarized the Rs and environmental factors for each period. These daily average soil respiration (Rs) values were plotted against soil temperature (Ts), rainfall, and soil moisture content (SMC), and the change intervals were divided into three groups, as shown in Table 1. First, the cooling period, freezing period, and increasing temperature period refer to the period from Ts. For each grouped interval, the differences in Rs, Ts, and SMC were compared (p = 0.05). For Rs, an exponential regression function was used to derive missing values, from which full-period values were derived. In addition, the functional relationships between Rs and environmental factors such as Ts and SMC were analyzed, as well as between environments. A paired t-test was conducted to test significant differences between among-plot and within-plot variations. When groups were significantly different, ANOVAs were followed with Tukey’s HSD test (p = 0.05).
3. Results
3.1. Soil Respiration (Rs)
A clear seasonal pattern of soil respiration (Rs) was observed (Figure 1). Rs progressively declined early November 2021, dropping rapidly to about 20 mg CO2 m−2 h−1 in mid-January 2022. This low Rs persisted until early March, after which it began to rise gradually. Rs showed repeating small increases and decreases from early March until the end of March when measurements were halted. These seasonal changes in Rs were found to be highly correlated with changes in soil temperature (Ts) (Figure 2 and Figure 3). In other words, as Ts decreased, Rs also decreased, and as Ts increased, Rs also increased. Rs entered the freezing period as Ts decreased, and even when Ts increased to low levels, Rs barely increased, indicating reduced responsiveness to Ts.
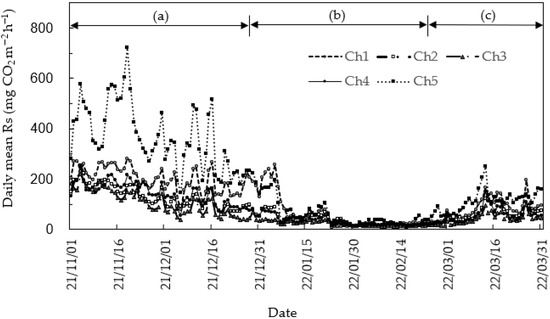
Figure 1.
Seasonal change in daily mean soil respiration (Rs) at points from chamber 1 (Ch1) without litter to chamber 5 (Ch5) with a developed litter layer of about 12 cm. The Rs of each chamber was calculated as a daily average with two data points per hour. In here, cooling period (a; 1 November–31 December 2021) refers to the period from the beginning of the low Ts period until the lowest Ts reaches below zero, freezing period (b; 1 January–26 February 2022) refers to the period during which the lowest Ts remains below zero, and increasing temperature period (c; 27 February–31 March 2022) refers to the period during which the lowest Ts rises above zero.
3.2. Air Temperature (Ta) and Soil Temperature (Ts)
The daily mean air temperature (Ta) was 2.7 °C for 151 days from November 2021 to March 2022 at the study site. The lowest mean daily Ta during the measurement period was −9.9 °C on 25 December 2021, and the maximum was 15.4 °C on 6 November 2021. The Ta decreased rapidly from November to December, and from January to mid-February, a freezing period dominated by almost subzero temperatures was maintained. The Ta tended to increase gradually, with recurring irregular increases and decreases from late February (Figure 2). In the central region of Korea, part of the cool temperate deciduous forest zone, subzero Ta during the cold season is common. In northern areas or at higher altitudes, the winter season starts earlier and ends later.
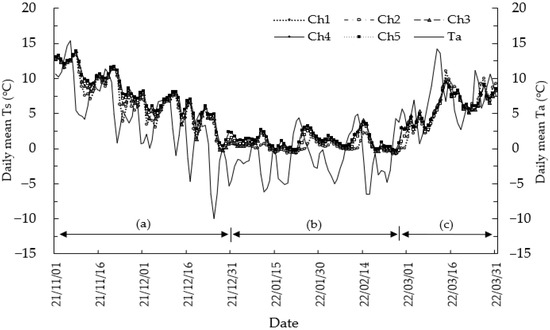
Figure 2.
Daily mean air temperature (Ta) and daily mean soil temperature (Ts) measured at a 5 cm depth from November 2021 to March 2022. Ts were collected at points from chamber 1 without litter to chamber 5 with a developed litter layer of approximately 12 cm, including chambers 2, 3, and 4, respectively. Cooling period (a; 1 November–31 December 2021) refers to the period from the beginning of the low Ts period until the lowest Ts reaches below zero, freezing period (b; 1 January–26 February 2022) refers to the period during which the lowest Ts remains below zero, and increasing temperature period (c; 27 February–31 March 2022) refers to the period during which the lowest Ts rises above zero.
Meanwhile, the soil temperature (Ts) measured at a depth of 5 cm during the study period ranged from 4.0 °C in chamber 1 (Ch1) to 4.9 °C in chamber 5 (Ch5), and the average Ts of all points was 4.6 °C. The lowest instantaneous minimum Ts was −2.0 °C measured in Ch1 on 21 February 2022. However, the daily mean Ts showed less fluctuation and a more stable trend than the daily mean Ta, and daily mean Ts never dropped −2 °C. When the variation in Ts is less pronounced than that of Ta, it is a general phenomenon and indicates that Ts remains relatively stable state compared to Ta (Figure 2).
Sites with a well-developed litter layer (Ch5) tended to maintain higher Ts values than those with an undeveloped litter (Ch1), and the range of change was also the lowest. In addition, the greatest difference in the mean Ts between points was 0.9 °C, with Ch5 being the highest at 4.9 °C and Ch1 being the lowest at 4.0 °C (Table 1). chamber 2 (Ch2) and chamber 3 (Ch3) were the same at 4.5 °C, and chamber 4 (Ch4) was the same as Ch5 at 4.9 °C.
Overall, the Ts tended to remain high as the litter layer developed (Figure 2). Moreover, the degree of formation of the litter layer at any point on the forest floor was closely related to the type or intensity of the diurnal and seasonal Ts changes. As a result, spatial differences in the effects of accumulated litter were found to be an important factor inducing the spatial nonuniformity of Ts, which can affect Rs.
Chamber 5 (Ch5), where the fallen leaf layer developed during the temperature decrease period, was most sensitive to changes in soil moisture content (SMC) compared to other measured plots. For example, the Rs in Ch5 on 10 November 2021, increased from 316.0 to 721.3 mg CO2 m−2 h−1 on 19 November 2021, sharply decreased to 269.1 mg CO2 m−2 h−1 on 26 November 2021, increased from 192.2 mg CO2 m−2 h−1 on 13 December to 517.7 mg CO2 m−2 h−1 on 16 December, and decreased again on 19 December to 184.5 mg CO2 m−2 h−1. Thereafter, this pattern of rapid increase and decrease was repeated, showing a tendency to gradually decrease, and by the end of January, it had decreased to the point where the difference between measurement sections was indistinguishable. In comparison, chamber 1 (Ch1) exhibited very low fluctuation of 264.9, 281.4, and 160.8 mg CO2 m−2 h−1 during the same period. Similar trends were observed at other points, and the range of change was very small. Even in the case of Ch1, where the litter layer was barely developed, it exhibited responsiveness to changes in Ts, although this value was relatively lower than that of Ch5, i.e., below 300 mg CO2 m−2 h−1. However, in the measurement area with intermediate development of the litter layer, the responsiveness was somewhat lower than that of Ch1 and Ch5. Moreover, within the same temperature range, the Rs values during the temperature decrease and increase phases exhibited different patterns. For example, on 18 November 2021, when the temperature was falling, the Ts and Rs was 9.9 °C and 604.0 mg CO2 m−2 h−1, respectively, but on 14 March 2022, when the temperature was rising, the Ts and Rs of Ch5 were 9.4 °C and 134.3 mg CO2 m−2 h−1, respectively. For the same Ts, the Rs values in the temperature decrease period tended to be higher than those during the temperature increase period.
In addition, overall Rs tended to change in response to changes in Ts, but the degree of fluctuation varied depending on the measurement point. Each measurement group showed a generally similar trend to Ch5. As a result, during the temperature decrease period, the developed litter layer exhibited relatively larger fluctuations than the no litter layer, and this fluctuation range was largest at Ch5, where the litter layer was the most developed. Although the highest Rs value was measured in Ch5, where the litter layer was most developed, no clear trend with the degree of litter layer development was observed in the other measurement groups. The average Rs for each measurement plot over the measurement period was highest at Ch5, where the litter layer was thick, at 190.6 mg CO2 m−2 h−1, and lowest at chamber 3 (Ch3), where the litter layer was poorly developed, at 60.7 mg CO2 m−2 h−1. In addition, chamber 1 (Ch1), where the litter layer was scarcely developed, had an average of 120.6 mg CO2 m−2 h−1, the second-highest value among the five measurement points. The average Rs of all five measurement points was 106.5 mg CO2 m−2 h−1, and the average of the total Rs during the all-measurement period was calculated as 1.1 t C ha−1.

Table 1.
Soil respiration (Rs), soil temperature (Ts), and soil moisture content (SMC) from chamber 1 (Ch1) to chamber 5 (Chr5). Data were collected at points from Ch1 without litter to Ch5 with a developed litter layer. The full-period Rs value was calculated as mean hour Rs 24 h·151 days. Within each row, the mean values sharing the same superscript letter indicate that there was no significant difference between the chambers.
Table 1.
Soil respiration (Rs), soil temperature (Ts), and soil moisture content (SMC) from chamber 1 (Ch1) to chamber 5 (Chr5). Data were collected at points from Ch1 without litter to Ch5 with a developed litter layer. The full-period Rs value was calculated as mean hour Rs 24 h·151 days. Within each row, the mean values sharing the same superscript letter indicate that there was no significant difference between the chambers.
| Ch1 | Ch2 | Ch3 | Ch4 | Ch5 | Average | ||
|---|---|---|---|---|---|---|---|
| Rs | Mean hour | 120.6 a | 80.1 b | 60.7 c | 80.5 b | 190.6 d | 106.5 |
| (mg CO2 m−2 h−1) | |||||||
| Full period | 1.19 | 0.79 | 0.60 | 0.80 | 1.88 | 1.05 | |
| (t C ha−1) | |||||||
| Mean Ts (°C) | a (n = 64 days) | 6.6 | 7.4 | 7.1 | 7.8 | 7.8 | 7.3 |
| b (n = 57 days) | 0.2 | 0.8 | 0.9 | 1.2 | 1.2 | 0.9 | |
| c (n = 22 days) | 5.9 | 5.6 | 6.0 | 6.0 | 6.0 | 5.9 | |
| Full period | 4.0 a | 4.5 a | 4.5 a | 4.9 a | 4.9 a | 4.6 | |
| Mean SMC (%) | a (n = 64 days) | 19.9 a | 19.7 a | 14.8 b | 9.6 c | 8.3 d | 14.5 |
| b (n = 57 days) | 15.8 a | 13.8 b | 11.9 c | 8.7 d | 8.0 e | 11.6 | |
| c (n = 22 days) | 19.0 a | 17.7 a | 14.6 b | 10.1 c | 9.2 c | 14.1 | |
| Full period | 18.1 a | 17.0 b | 13.7 c | 9.4 d | 8.4 e | 13.3 |
3.3. Soil Respiration (Rs) and Soil Temperature (Ts)
In the regression analysis of soil respiration (Rs) and soil temperature (Ts) for all measurement points, the R2 value varied from 0.39 to 0.72, but the average value was 0.60, showing a high R2 value. Chamber 3 (Ch3) exhibited the highest correlation with Rs, where the R2 was approximately 0.72. The R2 value for chamber 5 (Ch5), which had the most developed litter layer, was 0.52. In addition, chamber 1 (Ch1), which showed a thin litter layer, had the lowest R2 value of approximately 0.39.
Meanwhile, in the case of Ch5, for the same Ts, the Rs value tended to exhibit a relatively broad range compared to the other measurement points. For example, in Ch5 with a Ts range of approximately 7 °C, Rs had a wide range of 192.2 to 491.1 to 299.0 mg CO2 m−2 h−1 during the temperature drop period, but during the temperature rise period, it ranged from 105.8 to 161.7 mg CO2 m−2 h−1, with a relatively small difference of about 56.0 mg CO2 m−2 h−1.
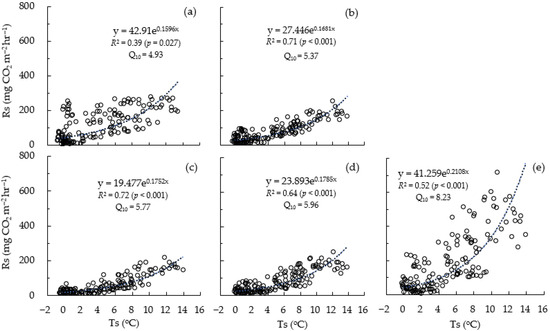
Figure 3.
Relationships between soil respiration (Rs) and soil temperature (Ts). (a–e) indicate chamber 1 (Ch1) to chamber 5 (Ch5), respectively.
By categorizing the cold season into cooling, freezing, and increasing temperature periods (Figure 2), it was divided that during the cooling and freezing periods, the Ts tended to remain higher in chamber with a well-developed litter layer compared to those without a developed litter layer (Table 1, Figure 2).
During the cooling period from November to December 2021 (a in Figure 2), chambers with a no or thin litter layer showed a decreasing trend with Ts remaining lower compared to chamber with a developed litter layer. During this period, it is likely that the newly supplied litter remained in the soil even in places where the litter layer was not developed, which prevented soil heating via solar radiation and maintained a low Ts.
In 2021, fresh new litter was supplied onto the forest floor from the forest canopy. By mid-February 2022, it began moving quickly, resulting in the nonuniform litter distribution typically observed by early March. In addition, this uneven litter layer distribution remained almost unchanged until mid-November when new litter was produced. Litter movement varies depending on the weather conditions of the year but generally occurs between early February and early March when strong winds blow.
During the temperature-increasing period from March to July, there was no significant difference in Ts, regardless of the litter layer development. This period corresponds to the time when litter movement caused by the wind was completed and differences in litter accumulation based on the location became visible.
Meanwhile, the Q10 values were 4.93 for Ch1, 5.37 for Ch2, 5.77 for Ch3, 5.96 for Ch4, and 8.23 for Ch5, showing a tendency to increase as the litter layer developed. The average across all plots was 6.05.
3.4. Rainfall and Soil Moisture Content (SMC)
During the measurement period, the maximum daily rainfall was 27.5 mm on 29 November 2021, and a total rainfall of 159.5 mm occurred during the entire study period (Figure 4). Meanwhile, during the almost freezing period from mid-December to mid-March, rainfall of approximately 2–3 mm occurred about five times, revealing a contrasting pattern between the early and late cold-temperature periods.
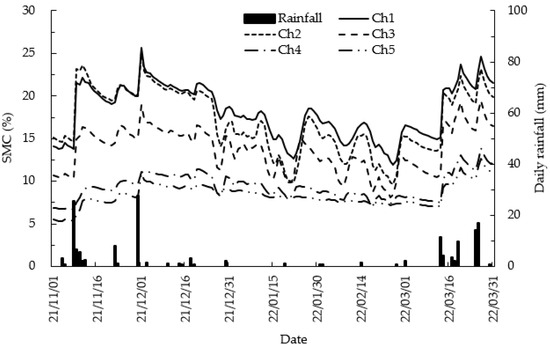
Figure 4.
Diurnal variations in soil moisture content (SMC) from chamber 1 (Ch1) to chamber 5 (Ch5) and daily rainfall. Ch1 (none) to Ch5 (most developed) represents the measurement points of different levels of litter layer development. SMC measured at a 0~15 cm depth from November 2021 to March 2022. When snow falls during the freezing period, it melts in the top collar of rain gauge and is measured as rainfall.
Most snowfall occurs during the freezing months of January and February; thus, the value may be inaccurate. Only a small amount of rainfall was recorded around 18 January 2022, but the soil moisture content (SMC) increased significantly from January 2022 (Figure 4). This is because the rain gauge could not convert the snowfall into rainfall. However, the increase in SMC can be used to estimate the amount of precipitation that fell as snow.
The average SMC of all measurement points was 13.3%. In addition, the minimum and maximum SMC for the study period was 18.1% at chamber 1 (Ch1) and 8.4% at Ch5, respectively. The average SMC at each point was 18.1% for Ch1, 17.0% for chamber 2 (Ch2), 13.8% for chamber 3 (Ch3), 9.4% for chamber 4 (Ch4), and 8.4% for chamber 5 (Ch5) (Table 1, Figure 4). In the case of Ch5, where the developed litter layer was the thickest, SMC exhibited the lowest value in full period, and the SMC of Ch1, which had almost no litter layer, had the highest value, which was a value approximately 2.2 times higher than that of Ch5. Furthermore, the range of changes in SMC remained smaller at points with a relatively thick litter layer (Ch4 and Ch5) compared to those with a thinner litter layer of Ch1 and Ch2 (Figure 4). In Ch4 and Ch5, where the litter layer was relatively developed, the maximum SMC did not exceed 12%, and the difference from the lowest value was 4.6% and 5.4%, respectively, whereas, in Ch2, it was 11.3%, revealing a very large difference. At these values, the SMC tended to be low at the point where the litter layer was thick and high at the point where the litter layer was thin.
Seasonal variations in SMC showed that it remained relatively high during periods of high rainfall and, conversely, low during periods of low rainfall. Furthermore, SMC increased rapidly immediately after rainfall occurred and then gradually decreased after the rainfall ended (Figure 4). This rainfall dependence of SMC showed an irregular pattern that repeated itself depending on rainfall amounts. As the litter layer developed, ΔSMC showed lower values for the same rainfall (Figure 5). For example, at 25.5 mm of rainfall, SMC in Ch1 increased by 11.3%, but in Ch5, with a thick litter layer, it increased only by 1.6%. While the overall R2 value was not high, the chambers with developed litter layers were calculated to be lower than those without.
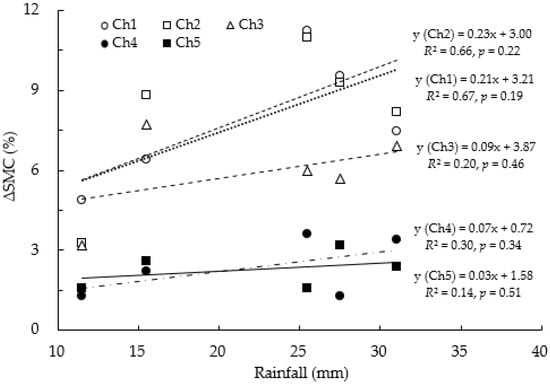
Figure 5.
The increasing rate (ΔSMC) in the soil moisture content to various precipitations from Ch1 without litter to Ch5 with a developed litter layer. ΔSMC was calculated as SMC before and after the precipitation event occurred.
3.5. Soil Respiration (Rs) and Soil Moisture Content (SMC)
Conversely, the analysis result of the correlation between soil respiration (Rs) and soil moisture content (SMC) (Figure 6) revealed that Rs showed only a weak association with SMC. The average R2 value from chamber 1 (Ch1) to chamber 5 (Ch5) was 0.11, which is a very low value, and chamber 4 (Ch4), which exhibited the lowest R2 value among the chambers, showed a value as low as 0.003 (Figure 6). As a result, Rs showed little correlation with SMC during the winter season, indicating that changes in SMC during winter had a minimal effect on Rs, regardless of the development of a litter layer.
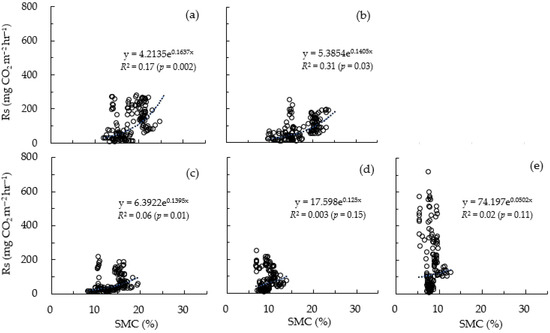
Figure 6.
Relationship between soil respiration (Rs) and soil moisture content (SMC). (a–e) indicate from Chamber 1 to Chamber 5, respectively.
4. Discussions
Rs displayed distinct seasonal patterns. During the cooling period, when temperatures were falling, it gradually decreased, repeatedly irregular decreases and increases. During the freezing period, it exhibited a relatively stable pattern with relatively low variability. Subsequently, during the increasing temperature period, it gradually increased, but the increase was more sensitive in layers with developed litter layers (Figure 1). Rs was significantly higher in the developed litter layer than undeveloped litter layer plot (Figure 1, Table 1). During the measurement period, the average Rs at Ch5, where the litter layer was the thickest, was 190.6 mg CO2 m−2 h−1, and Ch1, with the thinnest litter layer development, was 120.6 mg CO2 m−2 h−1. The Rs at Ch2, Ch3, and Ch4 were 80.1, 60.7, and 80.5 mg CO2 m−2 h−1, respectively, which was much lower than Ch5. However, even in Ch1 and Ch2, where the litter layer was not or thinly developed, the difference was approximately 66.6% (Table 1). These results show that when the litter layer is developed to a certain level, it significantly affects Rs, but when it is sufficiently developed, it has a complex effect due to factors beyond the litter layer. Rs is governed by various factors such as soil organic matter, soil moisture, amount of roots in the soil, Ts, and litter layer [17,37,38,39]. This is especially relevant for the amount of underground roots, which account for almost half of the Rs, thus exhibiting strong spatial heterogeneity [13,40]. In the case of Ch1, despite there being almost no litter layer, it exhibited the highest Rs value of all measurement points except Ch5, which suggests that factors other than the litter layer have a strong effect on Rs (Table 1, Figure 1). Before installing the chamber, a probe was used to exclude thick roots, but it was not possible to confirm whether this was actually performed or whether this was due to the large amount of fine roots. Therefore, for Ch5, it is believed that the thick developed litter layer contributed to Rs to an extent that exceeded the other respiratory control factors. Controlled experiments are needed to understand how much the litter layer contributes to Rs.
Meanwhile, Rs demonstrated an association with changes in Ts (Figure 1 and Figure 3). It is well-known that Rs is closely correlated with temperature changes. The seasonal change in Rs, in which Rs decreases as the Ts decreases, is a result that has been commonly observed in many studies on Rs and reveals that Rs is linked to Ts even during the coldest period of the year. In a study of Rs and root respiration in a Quercus acutissima forest in the vicinity of this study’s survey site, seasonal changes were investigated in high Rs during the high-temperature summer season and low Rs and root respiration during the winter season [37,41]. The results revealed seasonal changes in Rs, which have also been reported in several other studies [12,42]. Furthermore, this study revealed the tendency of Rs to gradually decrease until the freezing season, with Rs exhibiting repeated increases and decreases in response to the repeated changes in the Ts. Rs showed an increase in response to the increase in Ts during the temperature increase period after the freezing period. This reactivity to Ts was an exponential response of Rs to Ts, and the R2 value ranged from 0.386 at Ch1 to 0.721 at Ch3. However, there was no clear trend between high and low R2 values according to the litter layer development (Figure 3). Meanwhile, Q10 was highest at 8.23 in Ch5, a thick litter layer, and lowest at 4.93 in Ch1, a no-litter layer. The average of all measurement areas was 6.05. These results are thought to be due to the fact that the development of the litter layer broadened the range of Ts, thereby broadening the range of high Rs (Figure 3).
Considering these results, it was determined that the functional equation with Ts as a parameter is applicable even during the winter season. In contrast, the functional response of Rs to SMC showed an overall very low R2 value ranging from 0.003 at Ch4 to 0.057 at Ch3. Some studies have shown that Rs produces a parabolic relationship over a wide range [15,43,44], which suggests that under optimal SMC, the sensitivity of Rs increases to temperature changes. Therefore, it was suggested that moisture, including Ts, should be used as a predictor of Rs [45,46]. However, at least during the winter season, the use of functional equations with SMC as a variable was not considered appropriate.
Calculating the amount of carbon emitted through Rs during the cold-temperature period, the total Rs during the measurement period was the highest at 1.88 t C ha−1 for Ch5, followed by 1.19 t C ha−1 for Ch1, 0.79 t C ha−1 for Ch2, 0.80 t C ha−1 for Ch4, and 0.60 t C ha−1 for Ch3, respectively. The difference between Ch5, which had the highest Rs, and Ch3, which had the lowest Rs, was about 3.1 times. There was a large spatial difference within the small area where the Rs data were collected, which raises concerns about how to eliminate spatial variation when collecting Rs data. These results imply that careful selection of measurement points is necessary due to the spatial variation in soil respiration. Based on this finding, this study carefully selected measurement sites to ensure uniform root respiration.
Meanwhile, air temperature (Ta) and soil temperature (Ts), major factors affecting seasonal changes in soil respiration, clearly exhibited seasonal variations during the measurement period (Figure 2). Both decreased for a certain period (a in Figure 2), reached their lowest values (b in Figure 2), and then increasing again (c in Figure 2). Ts reflected the daily fluctuations of Ta but displayed different fluctuations in ranges and patterns, with Ts changing later than Ta (Figure 2). These seasonal changes reflect the typical seasonal changes that occur across a wide range of temperate regions [8,47]. In Korea’s central region, which falls within the cool temperate deciduous forest zone, freezing temperatures below zero are a normal occurrence during winter (Figure 2). This freezing season begins earlier and lasts longer in the north and at higher altitudes. Even in these seasonal changes, Ts exhibited different tendencies depending on the degree of the litter layer development. During the temperature decrease period, Ts dropped to a relatively lower level in chamber in the no litter layer, but during the temperature increasing period, it was shown to reach higher levels. It appears that during the temperature decrease period, the obstruction of solar radiation from reaching the ground because of freshly accumulated litter on the forest floor maintained relatively low Ts levels.
Conversely, in the case of the temperature increase period, litter from the previous year was almost completely removed by the wind in places where the litter layer was not developed, and as a result, solar radiation passing through the forest canopy reached the soil surface, contributing to an increase in ground temperature. However, in places where the litter layer was developed, relatively low Ts was observed because the litter layer blocks the solar radiation. In general, the litter layer acts as a protective barrier between the soil and atmosphere. This layer has high porosity and low thermal conductivity, which insulates the underlying soil layers [48]. Therefore, the development of the litter layer contributes to suppressing changes in Ts caused by solar radiation or Ta. Ultimately, the litter layer development could reduce soil freezing through the insulating effect of the litter layer by suppressing heat release during temperature decreases and, conversely, blocking heat gain during temperature or solar radiation increases [49]. The change in Ts at each measurement point confirmed that the litter layer influenced the Ts, as it tended to change at a higher temperature as the litter layer developed (Figure 2). Generally, in the winter season, both Ta and precipitation interact to influence Ts [25]. Therefore, areas where the litter layer is not developed can be more affected by rainfall, snow, and temperature changes than areas where the litter layer is developed.
Differences in litter layer development range from a narrow range in front of and behind large trees to the entire slope of a mountain. This suggests that spatial differences in the complex litter layer development ultimately contribute to creating a fine soil environment, allowing for the complex control of the soil microenvironment, such as the Ts. Meanwhile, areas with an undeveloped litter layer may have stronger and more frequent thaw–freeze cycles than developed areas, which suggests that this causes differences in ecosystem carbon and nutrient losses [50,51]. This is because during the winter season, the cold temperatures change the freezing dynamics of the soil, contributing to changes in the ecosystem’s carbon and nutrient cycles. The litter layer has the potential to modify ecosystem carbon and nutrient cycling by altering the soil’s freezing dynamics. In this study, the degree of litter layer development had the most evident influence on the soil moisture environment. During the study period, areas with a developed litter layer did not maintain a relatively high soil moisture content (SMC) compared to areas where the litter layer was developed (Figure 4, Table 1). In addition, the average SMC during the study period tended to decrease as the litter layer developed (Table 1). The SMC of chamber 5 (Ch5), which had the most developed litter layer, was 9.7% lower than that of chamber 1 (Ch1), which had almost no litter layer.
In relation to this, when a certain amount of rainfall or snowfall occurs, the degree of SMC increases according to the degree of litter layer development, with the increase in SMC being significantly higher in areas where the litter layer is not developed compared to areas with a developed litter layer (Figure 5). These results indicate that the litter layer is an influential factor in SMC changes. Therefore, although the degree of litter layer development may vary, it appears that the litter layer intercepts a large amount of water supplied by rain or snow, impeding the inflow of moisture into the soil and thus maintaining the SMC in a relatively low state [52]. In this regard, the amount of rainfall intercepted by the litter layer varies depending on the season, litter condition, vegetation, and rainfall amount, but it has been reported to account for approximately 2–70% of the total rainfall [53,54,55,56]. These SMCs can affect Rs, and the degree of influence may be greater in summer when Rs is active.
SMC varies temporally as a result of soil water input (e.g., precipitation and snow) and output (e.g., root uptake, evaporation, and gravity drainage) differences, which are driven by seasonal and annual weather events. SMC also varies spatially because of soil texture, vegetation canopy, litter formation, root distribution, and other topographic heterogeneities [57,58,59,60]. In addition to these various SMC control factors, differences in rainfall inflow depending on the degree of litter layer development are factors that affect the spatial heterogeneity of the soil moisture environment.
Most snowfall was concentrated during the cooling and increasing temperature periods. There is some uncertainty about rainfall during the winter period because there has been no evaluation of whether the collar installed on top of the rain gauge accurately converts snowfall into rainfall. Furthermore, because the soil surface freezes in cold period, the supplied moisture may be stored as frozen water, which may melt and temporarily infiltrate the soil as ground temperature rises. This could result in a delay in snowfall and SMC increases. The phenomenon of snowfall being transferred to soil moisture occurs due to various factors [61].
In addition, due to the decrease in snow cover and the increase in rainfall freezing season due to global warming [25], the frequency of freezing and thawing in soils in temperate regions may increase. However, in stands with a developed litter layer, the intensity and frequency of these events may be alleviated. In areas where the litter layer is developed, this increase in frequency can also be mitigated by a low SMC and relatively high Ts. However, there is little understanding of how these changes are connected to the soil carbon cycle and to what extent they will affect it.
Overall, the uneven accumulation of litter on the forest floor significantly influenced Ts and SMC during the cold season, which, in turn, influenced Rs. This study did not separately measure respiration rates for the soil and litter layers to determine how much the litter layer influenced soil respiration. Therefore, it remains unclear to what extent the litter layer influences TS and SMC, respectively, on individual respiration in the litter layer and soil. Further detailed research is needed to understand these limitations.
5. Conclusions
In many mid-latitude ecosystems, it is challenging to collect Rs data because of cold temperature, snow cover, etc., in winter; therefore, winter Rs data are often not obtained. In this study, Rs data were collected from different litter layer development levels in the field during the cold-season, and we examined the applicability of the Ts–Rs functional equation for general use in the cold-temperature season. Ts showed atypical seasonal patterns, ranging from a minimum of −0.7 °C to a maximum of 13.9 °C and remained more stable than Ta, never dropping below −2 °C. Plots with developed litter layers maintained higher Ts than those no or thin litter layers. The daily mean Ts difference between the measurement points was very small, at around 0.9 °C. In this regard, soil respiration (Rs) exhibited seasonal variation with changes in Ts, decreasing during winter and increasing again in spring. Consequently, Rs showed a high exponential relationship with Ts even during the cold-temperature periods. In response to Rs to Ts, the R2 value ranged from 0.386 to 0.721, and the average value was 0.60, revealing a relatively high value. There was no clear trend between high and low values of R2 according to the litter layer development. As a result, the results demonstrated that the functional equation with Ts as a parameter could be applied even during the cold season.
Litter layer development significantly influenced the soil moisture content (SMC), with less developed areas maintaining higher moisture levels. In the area with almost no litter layer, the SMC was 2.2 times greater than that of the thickest litter layer. Moreover, the SMC range of change was smaller in areas with thicker litter layers. Therefore, as the litter layer developed, Ts tended to increase, but SMC tended to decrease. In the most developed litter layer, the average SMC during the study period remained 9.7% lower than that of the almost no litter layer. The response of Rs to SMC demonstrated very low R2 values overall and as a result, SMC showed little influence on Rs regulation. These results indicate that at least in the case of the broad-leaved forest in monsoon Asia, the effects of Ts exert a greater influence on Rs than SMC in winter. During the cold period from November to March, Rs showed high spatial variation depending on the degree of litter layer development. Overall, it was a high amount, accounting for about 12.3% of annual soil respiration on average.
In this study, estimation of the amount of carbon emitted through Rs during the cold-season from November to March revealed that the significant spatial variation, with values differing greatly depending on the measurement point. One of the main causes of such variation was the degree of litter layer development. Therefore, the degree of litter layer development at the measurement site should be considered when measuring Rs. As a result, it was concluded that the functional equation of Rs–Ts can be used even during the cold season when measurement data cannot be obtained because of winter snowfall and freezing. However, the Rs−SMC functional equation was determined as having very low or no correlation, and it is considered difficult to use the Rs−SMC correlation equation. It is thought that the accuracy thereof will be higher in an area where a normal more uniform litter layer level is formed.
When evaluating annual Rs, the Rs value of the winter season should be reflected as it is not small enough to be disregarded. Moreover, it is judged that the Ts−Rs functional equation can be used in cases where direct data collection is challenging. However, the five chambers above represent a microsite gradient rather than independent replicates, and causal attribution/generalization is limited to the studied stand and season.
Funding
This research was supported by the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2020R1I1A2073000).
Data Availability Statement
The raw data included in the datasets presented in this article can be processed in various ways to create new articles. This research is currently underway, so their disclosure is restricted for the time being. Therefore, requests to access the datasets should be directed to contact the author directly via email for consultation.
Acknowledgments
I am deeply grateful to Magok-sa and the monk Dosang of Muwiam for their cooperation in providing the research site and installing the equipment.
Conflicts of Interest
The author declares no conflicts of interest.
Abbreviations
| Rs | Soil respiration |
| Ts | Soil temperature |
| Ta | Air temperature |
| SMC | Soil moisture content |
| AOCC | Automatic open/closed chamber |
References
- Striegl, R.; Wickland, K. Effects of a clear-cut harvest on soil respiration in a jack pine—Lichen woodland. Can. J. For. Res. 1998, 28, 534–539. [Google Scholar] [CrossRef]
- Hirano, T.; Kim, H.; Tanaka, Y. Long-term half-hourly measurement of soil CO2 concentration and soil respiration in a temperate deciduous forest. J. Geophys. Res. Atmos. 2003, 108, D20. [Google Scholar] [CrossRef]
- Hanson, P.J.; Edwards, N.T.; Garten, C.T.; Andrews, J.A. Separating root and soil microbial contributions to soil respiration: A review of methods and observations. Biogeochemistry 2000, 48, 115–146. [Google Scholar] [CrossRef]
- Wen, J. Evolution of eastern Asian and eastern North American disjunct distributions in flowering plants. Annu. Rev. Ecol. Syst. 1999, 30, 421–455. [Google Scholar] [CrossRef]
- Keddy, P.A. Ecological properties for the evaluation, management, and restoration of temperate deciduous forest ecosystems. Ecol. Appl. 1996, 6, 748–762. [Google Scholar] [CrossRef]
- Fahnestock, J.T.; Jones, M.H.; Welker, J.M. Wintertime CO2 efflux from Arctic soils: Implications for annual carbon budgets. Glob. Biogeochem. Cycles 1999, 13, 775–779. [Google Scholar] [CrossRef]
- Hirano, T. Seasonal and diurnal variations in topsoil and subsoil respiration under snowpack in a temperate deciduous forest. Glob. Biogeochem. Cycles 2005, 19, GB2011. [Google Scholar] [CrossRef]
- Lee, J. Effect of micro-environment in ridge and southern slope on soil respiration in Quercus mongolica forest. J. Ecol. Environ. 2018, 42, 210–218. [Google Scholar] [CrossRef]
- Peng, S.; Piao, S.; Wang, T.; Sun, J.; Shen, Z. Temperature sensitivity of soil respiration in different ecosystems in China. Soil Bol. Biochem. 2009, 41, 1008–1014. [Google Scholar] [CrossRef]
- Joo, S.; Park, S.; Park, M.; Lee, C. Estimation of soil respiration using automated chamber systems in an oak (Quercus mongolica) forest at the Nam-San site in Seoul. Kor. Sci. Total Environ. 2012, 416, 400–409. [Google Scholar] [CrossRef]
- Lee, Y.; Moon, Y. A study on the soil respiration in a Quercus acutissima forest. Kor. J. Environ. Ecol. 2001, 24, 141–147. [Google Scholar]
- Jeong, S.; Eom, J.; Lee, J.; Lee, J. Effect of rainfall events on soil carbon flux in mountain pastures. J. Environ. Ecol. 2017, 41, 203–309. [Google Scholar] [CrossRef]
- Lee, M.; Nakane, K.; Nakatsubo, T.; Mo, W.; Koizumi, H. Effects of rainfall events on soil CO2 flux in a cool temperate deciduous broad-leaved forest. Ecol. Res. 2002, 17, 401–409. [Google Scholar] [CrossRef]
- Orchard, V.A.; Cook, F.J. Relationship between soil respiration and soil moisture. Soil Biol. Biochem. 1983, 15, 447–453. [Google Scholar] [CrossRef]
- Suh, S.; Lee, E.; Lee, J. Temperature and moisture sensitivities of CO2 efflux from lowland and alpine meadow soils. J. Plant Ecol. 2009, 2, 225–231. [Google Scholar] [CrossRef][Green Version]
- Bond-Lamberty, B.; Bunn, A.G.; Thomson, A.M. Multi-year lags between forest browning and soil respiration at high northern latitudes. PLoS ONE 2012, 7, e50441. [Google Scholar] [CrossRef]
- Lee, M.-S.; Nakane, K.; Nakatsubo, T.; Koizumi, H. Seasonal changes in the contribution of root respiration to total soil respiration in a cool-temperate deciduous forest. Plant Soil 2003, 255, 311–318. [Google Scholar] [CrossRef]
- Azizi-Rad, M.; Guggenberger, G.; Ma, Y.; Sierra, C.A. Sensitivity of soil respiration rate with respect to temperature, moisture and oxygen under freezing and thawing. Soil Biol. Biochem. 2022, 165, 108488. [Google Scholar] [CrossRef]
- Raich, J.; Schlesinger, W. The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus B 1992, 44, 81–99. [Google Scholar] [CrossRef]
- Rustad, L.E.; Huntington, T.G.; Boone, R.D. Controls on soil respiration: Implication for climatic change. Biogeochemistry 2000, 48, 1–6. [Google Scholar] [CrossRef]
- Woodwell, G.; Mackenzie, F.; Houghton, R.; Apps, M.; Gorham, E.; Davidson, E. Biotic feedbacks in the warming of the earth. Clim. Change 1998, 40, 495–518. [Google Scholar] [CrossRef]
- Aurela, M.; Laurila, T.; Tuovinen, J.P. Annual CO2 balance of a subarctic fen in northern Europe: Imortance of the wintertime efflux. J. Geophys. Res. 2002, 107, 4607. [Google Scholar] [CrossRef]
- Kurganova, I.; Gerenyu, V.L.D.; Rozanova, L.; Sapronov, D.; Myakshina, T.; Kudeyarov, V. Annual and seasonal CO2 fluxes from Russian southern taiga soils. Tellus B 2003, 54, 338–344. [Google Scholar] [CrossRef]
- Du, E.; Zhou, Z.; Li, P.; Jiang, L.; Hu, X.; Fang, J. Winter soil respiration during soil-freezing process in a boreal forest in Northeast China. J. Plant Ecol. 2013, 6, 349–357. [Google Scholar] [CrossRef]
- Henry, H.A.L. Climate change and soil freezing dynamics: Historical trends and projected changes. Clim. Change 2008, 87, 421–434. [Google Scholar] [CrossRef]
- Donat, M.G.; Lowry, A.L.; Alexander, L.V.; O’Gorman, P.A.; Maher, N. More extreme precipitation in the world’s dry and wet regions. Nat. Clim. Change 2016, 6, 508–513. [Google Scholar] [CrossRef]
- Quante, L.; Willner, S.N.; Middelanis, R.; Levermann, A. Regions of intensification of extreme snowfall under future warming. Sci. Rep. 2021, 11, 16621. [Google Scholar] [CrossRef]
- Goulden, M.L.; Wofsy, S.C.; Harden, J.W.; Trumbore, S.E.; Crill, P.M.; Gower, S.T.; Fries, T.; Daube, B.C.; Fan, S.-M.; Sutton, D.J.; et al. Sensitivity of boreal forest carbon balance to soil thaw. Science 1998, 279, 214–217. [Google Scholar] [CrossRef]
- Haei, M.; Öquist, M.G.; Kreyling, J.; Ilstedt, U.; Laudon, H. Winter climate controls soil carbon dynamics during summer in boreal forests. Environ. Res. Lett. 2013, 8, 024017. [Google Scholar] [CrossRef]
- Smith, P.; Fang, C.; Dawson, J.J.C.; Moncrieff, J.B. Impact of global warming on soil organic carbon. Advan. Agron. 2008, 97, 1–43. [Google Scholar]
- Fang, C.; Moncrieff, J. The dependence of soil CO2 efflux on temperature. Soil Biol. Biochem. 2001, 33, 155–165. [Google Scholar] [CrossRef]
- Aanderud, Z.T.; Jones, S.E.; Schoolmaster, D.R., Jr.; Fierer, N.; Lennon, J.T. Sensitivity of soil respiration and microbial communities to altered snowfall. Soil Biol. Biochem. 2013, 57, 217–227. [Google Scholar] [CrossRef]
- Björkman, M.P.; Morgner, E.; Cooper, E.J.; Elberling, B.; Klemedtsson, L.; Björk, R.G. Winter carbon dioxide effluxes from Arctic ecosystems: An overview and comparison of methodologies. Glob. Biogeochem. Cycles 2010, 24, GB3010. [Google Scholar] [CrossRef]
- Wu, X.; Yao, Z.; Brüggemann, N.; Shen, Z.; Wolf, B.; Dannenmann, M.; Zheng, X.; Butterbach-Bahl, K. Effects of soil moisture and temperature on CO2 and CH4 soil atmosphere exchange of various land use/cover types in a semi-arid grassland in Inner Mongolia, China. Soil Biol. Biochem. 2010, 42, 773–787. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, Y.; Zhou, X.; Tang, J.; Kuzyakov, Y.; Yu, H.; Fan, J.; Ding, W. Extreme rainfall and snowfall alter responses of soil respiration to nitrogen fertilization: A 3-year field experiment. Glob. Change Biol. 2017, 23, 3403–3417. [Google Scholar] [CrossRef]
- Song, J.; Kwon, S.; Kim, H.; Lee, J.; Yun, I.; Yun, C. Temple forest vegetation structure of cultural heritage site in Mt. Gyeryongsan national park—Focused on Donghaksa, Gapsa and Sinwonsa. Kor. J. Environ. Ecol. 2019, 33, 722–733. [Google Scholar] [CrossRef]
- Davidson, E.A.; Belk, E.; Boone, R.D. Soil water content and temperature as independent or confounded factors controlling soil respiration in a temperate mixed hardwood forest. Glob. Change Biol. 1998, 4, 217–227. [Google Scholar] [CrossRef]
- Knohl, A.; Werner, R.A.; Brand, W.A.; Buchmann, N. Short-term variations in δ13C of ecosystem respiration reveals link between assimilation and respiration in a deciduous forest. Oecologia 2005, 142, 70–82. [Google Scholar] [CrossRef]
- Misson, L.; Gershenson, A.; Tang, J.; McKay, M.; Cheng, W.; Goldstein, A. Influences of canopy photosynthesis and summer rain pulses on root dynamics and soil respiration in a young ponderosa pine forest. Tree Physiol. 2006, 26, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Son, Y.; Jin, H.; Park, I.; Kim, D.; Kim, Y.; Shin, D. Belowground carbon allocation of natural Quercus mongolica forests estimated from litterfall and soil respiration measurements. Kor. J. Agric. For. Meteorol. 2005, 7, 227–234. [Google Scholar]
- Lee, K.; Won, H.; Moon, H. Contribution of root respiration to soil respiration for Quercus acutissima Forest. J. Environ. Ecol. 2012, 26, 780–786. [Google Scholar]
- Liang, N.; Nakadai, T.; Hirano, T.; Qu, L.; Koike, T.; Fujinuma, Y.; Inoue, G. In situ comparison of four approaches to estimating soil CO2 efflux in a northern larch (Larix kaempferi Sarg.) forest. Agric. For. Meteorol. 2004, 123, 97–117. [Google Scholar] [CrossRef]
- Peng, Y.; Thomas, S.C.; Tian, D. Forest management and soil respiration: Implications for carbon sequestration. Environ. Rev. 2008, 16, 93–111. [Google Scholar] [CrossRef]
- Webster, K.L.; Creed, I.F.; Bourbonnière, R.A.; Beall, F.D. Controls on the heterogeneity of soil respiration in a tolerant hardwood forest. J. Geophys. Res. 2008, 113, G03018. [Google Scholar] [CrossRef]
- Mariko, S.; Nishimura, N.; Mo, W.; Matsuri, Y.; Kibe, T.; Koizumi, H. Winter CO2 flux from soil and snow surfaces in a cool-temperate deciduous forest, Japan. Ecol. Res. 2000, 15, 363–372. [Google Scholar] [CrossRef]
- Mielnick, P.C.; Dugas, W.A. Soil CO2 flux in a tallgrass prairie. Soil Biol. Chem. 2000, 32, 221–228. [Google Scholar] [CrossRef]
- Mo, W.; Lee, M.S.; Uchida, M.; Inatomi, M.; Saigusae, N.; Mariko, S.; Koizumi, H. Seasonal and annual variations in soil respiration in a cool-temperate deciduous broad-leaved forest in Japan. Agric. For. Meteorol. 2005, 134, 81–94. [Google Scholar] [CrossRef]
- Zweigel, R.B.; Dashtseren, A.; Temuujin, K.; Aalstad, K.; Webster, C.; Stuenzi, S.M.; Aas, K.S.; Lee, H.; Westermann, S. Simulating the thermal regime and surface energy balance of a permafrost-underlain forest in Mongolia. JGR Earth Surf. 2024, 129, e2023JF007609. [Google Scholar] [CrossRef]
- Johnson, L.C.; Shaver, G.R.; Cades, D.H.; Rastetter, E.; Nadelhoffer, K.; Giblin, A.; Laundre, J.; Stanley, A. Plant carbon–nutrient interactions control CO2 exchange in Alaskan wet sedge tundra ecosystems. Ecology 2000, 81, 453–469. [Google Scholar]
- Hobbie, S.; Chapin, F.S. Winter regulation of tundra litter carbon and nitrogen dynamics. Biogeochemistry 1996, 35, 327–338. [Google Scholar] [CrossRef]
- Schimel, J.P.; Kielland, K.; Chapin, F.S. Nutrient availability and uptake by tundra plants. In Landscape Function: Implications for Ecosystem Response to Disturbance. A Case Study of Arctic Tundra; Reynolds, F., Tenhunen, J.D., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 203–221. ISBN 978-3-662-01147-8. [Google Scholar]
- Chang, M. Change Forest Hydrology: An Introduction to Water and Forests, 2nd ed.; Chang, M., Ed.; CRC: Boca Raton, FL, USA, 2012; p. 569. ISBN 978-0-8493-5332-1. [Google Scholar]
- Dabral, B.G.; Premnath, R. Some preliminary investigation on the rainfall interception by leaf litter. Indian For. 1963, 89, 112–116. [Google Scholar]
- Helvey, J.D.; Patric, J.H. Canopy and litter interception of rainfall by hardwood of eastern United States. Water Resour. Res. 1965, 1, 193–206. [Google Scholar] [CrossRef]
- Sun, W.; Shao, Q.; Liu, J.; Zhai, J. Assessing the effects of land use and topography on soil erosion on the Loess Plateau in China. CATENA 2014, 121, 151–163. [Google Scholar] [CrossRef]
- Pradhan, I.P. Preliminary study on rainfall interception through leaflitter. Indian For. 1973, 99, 440–445. [Google Scholar]
- Wilson, K.B.; Hanson, P.J.; Baldocchi, D.D. Factors controlling evaporation and energy partitioning beneath a deciduous forest over an annual cycle. Agric. For. Meteorol. 2000, 10, 83–103. [Google Scholar] [CrossRef]
- Griffiths, R.P.; Madritch, M.D.; Swanson, A.K. The effects of topography on forest soil characteristics in the Oregon Cascade Mountains (USA): Implications for the effects of climate change on soil properties. For. Ecol. Manag. 2009, 257, 1–7. [Google Scholar] [CrossRef]
- Xu, S.; Liu, L.L.; Sayer, E.J. Variability of above-ground litter inputs alters soil physicochemical and biological processes: A meta-analysis of litterfall-manipulation experiments. Biogeosciences 2013, 10, 7423–7433. [Google Scholar] [CrossRef]
- He, L.; Ivanov, V.Y.; Bohrer, G.; Maurer, K.D.; Vogel, C.S.; Moghaddam, M. Effects of fine-scale soil moisture and canopy heterogeneity on energy and water fluxes in a northern temperate mixed forest. Agric. For. Meteorol. 2014, 184, 243–256. [Google Scholar] [CrossRef]
- Shinoda, M. Climate memory of snow mass as soil moisture over central Eurasia. J. Geophys. Res. Atmos. 2001, 106, 33393–33403. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).