Abstract
Compound fertilizer is generally applied to alleviate multi-nutrient deficiency problems in forest stands, but research on the effect of fertilizer application on soil CO2 efflux (Rs) processes has focused on the role of single-nitrogen (N) application. This study evaluates the effects of N addition in compound fertilizer on the rates in Pinus densiflora S. et Z. (Korean red pine) stands. Compound fertilizer with N (N3P4K1 = 113:150:37 kg ha−1 yr−1) and without N (P4K1 = 150:37 kg ha−1 yr−1) was applied on the forest floor for three years. Rs rates were measured for four years, from April 2011 to March 2015. The mean annual Rs rates during the study period were 3.10 µmol m−2 s−1 in the N3P4K1, 3.08 µmol m−2 s−1 in the P4K1, and 3.08 µmol m−2 s−1 in the control treatment. The rates in all treatments were significantly lower in 2013 (2.73 µmol m−2 s−1) than in other sampling years (3.03–3.58 µmol m−2 s−1) when the mean soil water content was the lowest (15.7%) during the four sampling years (other sampling years: 23.0–24.1%). The exponential relationships between Rs and the soil temperature were slightly more significant in the fertilized (N3P4K1: R2 = 0.72–0.80; P4K1: R2 = 0.70–0.81) treatments compared to the control (R2 = 0.62–0.74) treatment. The mean Q10 values for the four years were similar between the N3P4K1 treatment (4.19), the control (4.23) treatment, and the P4K1 (4.24) treatment. The results demonstrate that mean annual Rs rates in Korean red pine stands were not affected by the increased N availability in compound fertilizer, whereas decreases in mean annual Rs rates may be strongly attributed to the soil water content.
1. Introduction
Studies on the effect of fertilizer application on soil CO2 efflux (Rs) processes in forest stands have mainly focused on the role of N applications, because N can limit forest productivity [1,2,3] or because of elevated atmospheric N deposition [4]. Furthermore, N has been regarded as a significant factor that controls Rs in forest stands [1,2,4,5]. However, there are multi-nutrient deficiency problems in forest stands [6]. Forest management practices, such as multi-nutrient additions, have demonstrated the value of compound fertilizer to optimize the growth of tree species in nutrient-deficient forests [2,3,7].
The nutrient availability induced by compound fertilizer can influence the environmental conditions for the metabolic activity of tree roots and the decomposition of dead organic material [8,9]. Compound fertilizer did not affect Rs rates in a Japanese larch plantation [10] and a highly phosphorus-limited subtropical forest [9]. However, Rs rates increased when single-N fertilizer was added to forest soils [11]; an increase in forest production was reported in N-limited forest stands [6], as well as a decrease in coniferous forests with reduced heterotrophic respiration [1,6,9]. However, it is difficult to determine the effects of N in compound fertilizer because most studies were carried out to understand the responses of Rs rates following compound fertilizer [10] or single-N fertilizer applications [11].
Pinus densiflora S. et Z. (Korean red pine) occupies more than 23.5% (1.5 million ha) of Korean forests. Many studies have demonstrated the value of compound fertilizer for improving soil quality and tree growth in Korean forests [7,10]. However, there are few studies on the responses of Rs processes following compound fertilizer application with and without N addition [7]. Further, there is a need to understand the long-term effects of compound fertilization on Rs rates because most studies that report the effects of fertilizer additions on Rs were conducted over relatively short periods, e.g., two years [7,9]. The objectives of this study are to (1) evaluate the effects of N addition in compound fertilizer on annual Rs rates over four years and (2) determine the relationship between Rs rates and soil environmental factors following N addition in compound fertilizer applied to Korean red pine stands.
2. Materials and Methods
2.1. Experimental Design
This study was conducted on natural Korean red pine stands with low forest productivity (approximately 40 years old; site index: 8–10 at 20-year-old base age) in the Wola National Experimental Forest in Gyeongsangnam-do in South Korea. This area’s mean annual precipitation and temperature are 1490 mm yr−1 and 13.1 °C, respectively. The soil is a silt loam texture originating from sandstone and shale. The experimental design consisted of a complete randomized block design (block 1: 35°12′32″ N, 128°10′23″ E; 180 m; block 2: 35°12′ 26″ N, 128°10′25″ E, 195 m) and involved 18 plots [2 blocks × 3 treatments (N3P4K1, P4K1, control) × 3 replications] of 10 m × 10 m size, with a 5 m buffer zone between each plot (Figure 1). The understory tree species in the study sites were lespedeza (Lespedeza spp.), cork oak (Quercus variabilis Bl.), Serrata oak (Q. serrata Thunb.), wild smilax (Smilax china L.), and gray blue spicebush [Lindera glauca (Siebold & Zucc.) Blume]. More information on the study site is described elsewhere [7,12].
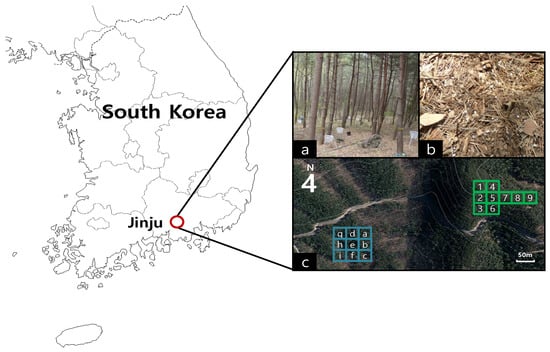
Figure 1.
Location of the study site (a), fertilizer application (b), and experimental design (c) [(Block 1: control (1, 4, 8), P4K1 (2, 5, 6), N3P4K1 (3, 7, 9); Block 2: control (a, e, g), P4K1 (b, h, i), N3P4K1 (c, d, f)].
Compound fertilizer with N (N3P4K1 = 113:150:37 kg ha−1 yr−1) is generally recommended for improvements in growth rates in Korean coniferous forests, whereas compound fertilizer (P4K1) without N is used where nutritional problems such as phosphorus (P) deficiency with low soil pH (below pH 5.0) exist in Korean forests [7,12]. Mixed urea, fused superphosphate, and potassium chloride fertilizers were employed as N, P, and K sources. Fertilizer was applied manually on the forest floor on 21 April 2011, 9 April 2012, and 15 March 2013 (total fertilizer amounts: N3P4K1 = 339:450:111 kg ha−1; P4K1 = 450:111 kg ha−1) for three years.
2.2. Rs Rates and Environmental Factors
Rs rates (μmol CO2 m−2 s−1) were measured once a month using an infrared gas analyzer (Model EGM-4, PP-Systems, Hitchin, Hertfordshire, UK) equipped with a flow-through closed chamber (Model SRC-2) between 10:00 a.m. and 13:00 p.m. at two randomly selected locations in each treatment plot during the study period (April 2011–March 2015). Han et al. [13] reported that the Rs measured around midday (09:00–15:00) provide the lowest bias to the daily mean Rs. Soil temperature was also measured at 8 cm depth adjacent to the chamber using a digital soil temperature probe (K-type, Summit SDT 200, Seoul, Republic of Korea). Rs rates in each treatment served to test exponential functions between Rs rates and soil temperature (Equation (1)):
where B0 and B1 are the model parameters and ST is the soil temperature. The Q10 values (Equation (2)) were calculated using the B1 coefficient:
Rs rate = B0eB1ST
Q10 = e10*B1
Soil samples were collected monthly at a 5 cm depth using a 100 cm3 core soil sampler. The soil core samples were transported to the laboratory and dried in an oven for 48 h at 105 °C to quantify the soil water content. The organic matter concentrations were determined by the loss-on-ignition method at 550 °C for 4 h. Organic C concentration (y) was calculated from the organic matter concentration (x) using a linear model (y = 1.3537x − 2.062, r2 = 0.56, p < 0.01) developed based on data from the study site. Soil pH (1:5 soil water suspension) was measured using an ion-selective glass electrode (Istec Model pH-220L, Seoul, Republic of Korea).
2.3. Stand and Soil Characteristics
All trees with >6 cm diameter at breast height (DBH) were measured annually to estimate the tree biomass C. The tree C stocks and increment in the tree biomass were calculated from the allometric regressions developed from this study site.
One pine tree with a DBH ranging from 13 to 15 cm was randomly selected in each treatment plot (a total of 18 trees: 6 trees from the control, 6 trees from the N3P4K1 treatment, and 6 trees from the P4K1 treatment). In April 2011, dendrometer bands (Series 5 Manual Band, Forestry Suppliers Inc., Jackson, MS, USA) were installed on each tree at DBH. The bands were used for repeated monthly measurements of DBH growth during the study period. To measure understory biomass, small trees < 1.3 m in height and herbs were cut into 1 m × 1 m areas in September 2014. The samples were oven-dried to measure the understory biomass.
A 5 g subsample of fresh mineral soil to measure the change in inorganic soil N concentrations was extracted using a mechanical vacuum extractor (Model 24VE, SampleTek, Science Hill, KY, USA) with 50 mL of a 2 M KCl solution immediately after sampling. The soil extract solutions were immediately stored at 4 °C in a cooler. Inorganic N (NH4+, NO3−) concentrations in the soil extract solution were determined using an auto-analyzer (AQ2 Discrete Analyzer, Southampton, UK). The inorganic N concentration was measured during the fertilizer application (from April 2011 to April 2014).
Soil samples for chemical analysis before (March 2011) and after (October 2014) fertilizer application were collected from the top 15 cm from five randomly selected points in each treatment plot using a soil core sampler. The total N content was measured using an elemental analyzer (Vario Macro cube, Elementar Analysensysteme GmbH, Langenselbold, Germany). The available P concentration extracted by NH4F and HCl solutions [14] was measured using a UV spectrophotometer (Jenway 6505, Staffordshire, UK).
2.4. Data Analysis
Repeated-measures analysis of variance (ANOVA) and Tukey’s test (p < 0.05) were used to test for the significance in yearly means among the three treatments for Rs rates, tree growth (DBH, basal area, aboveground C stock, tree C stock), and soil environmental factors (soil temperature, soil water content, soil pH, organic C concentration) over the four-year sampling period between April 2011 and March 2015. One-way ANOVA was applied to each monthly variable to assess the differences among the treatments. All ANOVAs were executed using the general linear model procedure in SAS 9.1 [15]. Pearson’s correlation coefficients were calculated between Rs rates and the soil environmental factors.
3. Results
3.1. Soil and Stand Attributes
Soil fertility levels in the form of total N and available P measured in 2014 were significantly higher (p < 0.05) in the N3P4K1 treatment than in the P4K1 or control treatments (Table 1). However, N addition in compound fertilizer had an insignificant effect on the difference in monthly Rs rates, soil temperature, soil organic C concentration, and available soil N (Figure 2). Although the mean DBH and basal area during the study period were not affected by N addition in compound fertilizer (Table 2), the estimated aboveground C stocks in 2014 were highest for N3P4K1 (40.9 Mg C ha−1), followed by the control (37.4 Mg C ha−1) and P4K1 (35.4 Mg C ha−1) treatments. The C density expressed on a per tree basis using the stand density was also higher in the N3P4K1 (38.9 kg C tree−1) than in the P4K1 (32.7 kg C tree−1) and control (31.5 kg C tree−1) treatments (Table 2). There was a significant increase in the biomass of understory vegetation in the N4P3K1 treatment (Table 3).

Table 1.
Organic carbon, total nitrogen, and available phosphorus at the study site before (2010) and after (2014) fertilizer application.
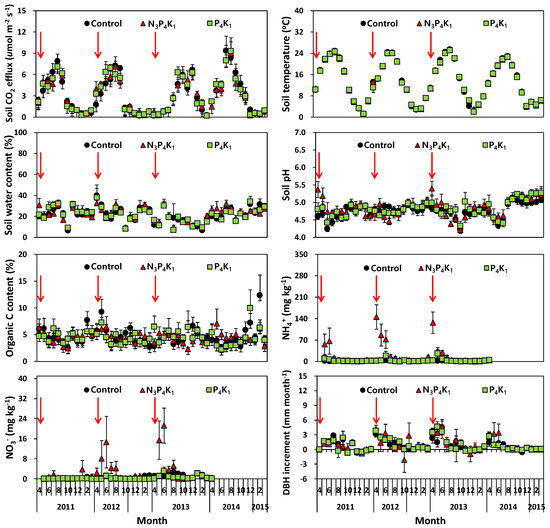
Figure 2.
Overview of monthly fluctuations in soil CO2 efflux, soil temperature, soil water content, soil pH, organic C content, soil NH4+ concentrations, soil NO3− concentrations, and DBH increment in fertilized and control treatments in Korean red pine stands. Error bars indicate standard errors (n = 6). Red arrows indicate fertilization events.

Table 2.
Annual stand density, DBH, basal area, aboveground C stocks, and tree C density in the fertilized and control treatments in Korean red pine stands.

Table 3.
Biomass of understory vegetation in fertilized and control treatments in Korean red pine stands (September 2014).
3.2. Relationships Between Rs Rates and Soil or Stand Environmental Attributes
There was a significant main effect in the year concerning the mean annual Rs rates, soil temperature, soil water content, and soil pH, but there were no fertilization or interaction effects (Table 4). The mean soil NH4+ and NO3− concentrations were significantly higher in the N3P4K1 (NH4+: 20.20 mg kg−1; NO3−: 3.28 mg kg−1) than in the P4K1 (NH4+: 7.52 mg kg−1; NO3−: 0.48 mg kg−1) and control (NH4+: 2.11 mg kg−1; NO3−: 0.75 mg kg−1) treatments (Table 4). The mean annual Rs rates were not significantly affected by N addition in the compound fertilizer (N3P4K1: 3.10 µmol m−2 s−1; P4K1: 3.08 µmol m−2 s−1; control: 3.08 µmol m−2 s−1) during the four years (Table 4). However, the mean annual Rs rates were significantly lower in 2013 (2.73 µmol m−2 s−1) than in other years (3.02–3.58 µmol m−2 s−1). The exponential regression between Rs rates and the corresponding soil temperature (Figure 3) were significant in the fertilized (N3P4K1: R2 = 0.72–0.80; p < 0.05. P4K1: R2 = 0.72–0.81; p < 0.05) and control (R2 = 0.62–0.74, p < 0.05) treatments (Figure 3). Soil temperature explained from 62 to 81% of the variation in Rs rates. The mean Q10 values during the study period were 4.19 in the N3P4K1 treatment, 4.24 in the P4K1 treatment, and 4.23 in the control treatment. There was a positive correlation between Rs rates and the soil water content (r = 0.56, p < 0.01) and the soil NO3- concentration (r = 0.29, p < 0.05) in the N3P4K1 treatment. However, soil organic C availability was negatively correlated in the control (r = −0.30, p < 0.01) and the P4K1 treatment (r = −0.25, p = 0.03). Monthly fluctuations in Rs rates were negatively correlated (Figure 4) with the fluctuations in soil pH (N3P4K1: r = −0.56; p < 0.01. P4K1: r = −0.42; p < 0.01. Control: r = −0.43; p < 0.01), whereas monthly DBH increments were positively correlated (N3P4K1: r = 0.53; p < 0.01. P4K1: r = 0.57; p < 0.01. Control: r = 0.52; p < 0.01) with Rs rates.

Table 4.
Mean annual soil CO2 efflux rates and soil environmental attributes in fertilized and control treatments in Korean red pine stands.
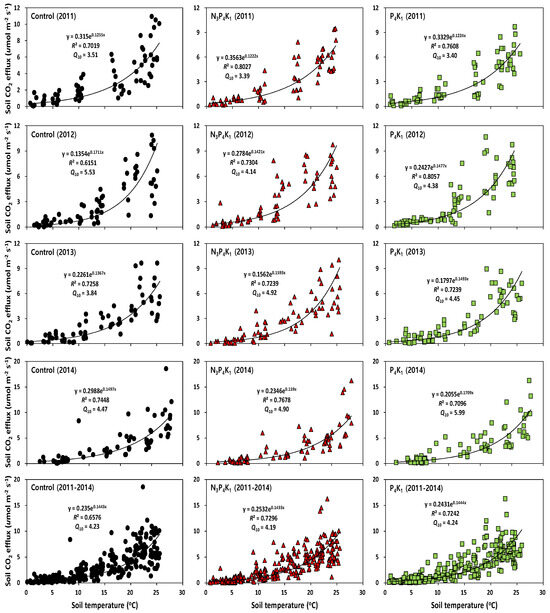
Figure 3.
Exponential regressions showing the relationship between soil temperature and soil CO2 efflux rates in fertilized and control treatments in Korean red pine stands.
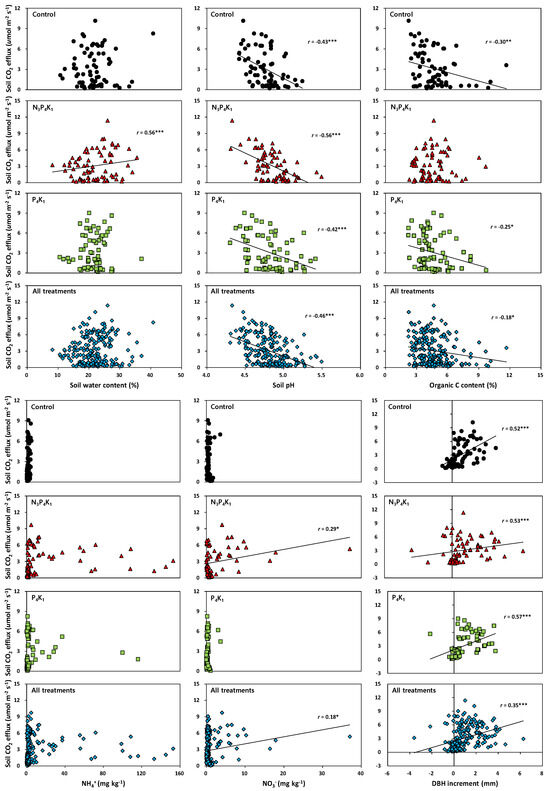
Figure 4.
Correlation between soil CO2 efflux and soil or stand attributes in fertilized and control treatments in Korean red pine stands (*: <0.05; **: <0.01; ***: <0.001).
4. Discussion
4.1. Stand and Soil Attributes
Because N or P availability is generally considered the major soil factor that limits the growth of many temperate forest tree species [3,16,17], N and P fertilizer application would have been expected to have a more significant growth response compared to the control treatment. However, fertilizer application had little influence on the DBH and basal area increment throughout the experiment. The lack of a significant growth response to fertilizer application could be due to an insufficient annual rate to meet the nutritional requirement for a growth response [7,16], high N immobilization due to the high N fluxes induced by N4P3K1 treatment, or the limiting possibility of other soil nutrients [12]. In addition, no P4K1 treatment effects on tree growth suggest that P may not be a limiting soil nutrient for tree growth in this mature pine stand. Soil N fertility levels, i.e., NH4+ and NO3− concentrations in mineral soil, were significantly improved more in the N4P3K1 than in the P4K1 or control treatment. Thus, the highest aboveground C increment and density were observed in the N4P3K1 treatment between 2010 and 2014, although there was no significance among the treatments.
4.2. Factors Controlling the Monthly Fluctuation of Rs Rates
Nitrogen addition in compound fertilizer had little influence on the difference in monthly Rs rates. This result agrees with previous compound fertilizer studies [7,18], which showed insignificant effects on monthly Rs rates because microbial decay and root growth activities are temperature-dependent [19]. However, it is important to note that the monthly soil water content (r = 0.56, p < 0.01) and soil NO3− concentration (r = 0.29, p < 0.05) in the N3P4K1 treatment were primary drivers of the monthly variation in Rs rates. The positive correlation between the soil water content and Rs rates indicates that the soil water content changed following N addition in compound fertilizer. A similar result was reported, i.e., a significant positive linear relationship between Rs rates and soil water content following N application in subtropical forest ecosystems in China [20,21]. The rapid disappearance of available N within several months in the N3P4K1 treatment (Figure 2) could be due to microbial immobilization [12,22] or plant uptake during the growing season. Tietema et al. [23] reported that net N immobilization rates in coniferous forest soils were positively correlated with Rs. This result suggests that the soil water content and soil NO3− concentration appear to be primary predicting factors that affect the monthly fluctuation of Rs rates following N addition in compound fertilizer.
Monthly fluctuations in Rs were positively correlated with monthly DBH increments and negatively correlated with soil pH, which depends on soil temperature. For example, soil pH is generally lower in the growing season than in the dormant season in temperate coniferous forests [10]. However, monthly fluctuations in Rs were not affected by soil environmental factors, such as organic C and NH4+ concentrations, which were independent of fluctuations in soil temperature. Similar results were observed in other Korean coniferous stands, with no correlation between the monthly Rs rates and these soil environmental factors [8,10].
4.3. Factors Affecting Mean Annual Rs Rates
The mean annual rates of Rs did not respond to N addition in the compound fertilizer. Similarly, Liu et al. [9] reported that N + P additions in subtropical forest ecosystems did not affect Rs rates. They found an antagonistic interaction in which increased N stimulated root growth, but inhibited soil microbial activities. Many studies have reported adverse effects on Rs rates after N fertilizer application [1,4,5]. Reduced Rs rates following N fertilizer application were attributed to the change in the conditions for decomposition of organic matter, such as reduced microbial biomass [1,4,24]. For example, microbial biomass was reduced by 15% under N addition globally [25]. Similarly, microbial respiration rates decreased by 27% in the N3P4K1 treatment compared to the control treatment at the same study site [7]. However, the reduced microbial respiration at this study site could be compensated for by stimulating the growth activity of understory vegetation following N addition in compound fertilizer (Table 3). For example, increased Rs by understory vegetation in mixed forest plantations in China was attributed to root respiration as a result of changes in soil environments [21]. In addition, understory biomass positively regulated the spatial variation in Rs rates in a temperate–subtropical transitional oak forest [26]. Furthermore, understory vegetation was important in enhancing the soil microbial biomass and extracellular enzymes in subtropical Chinese fir (Cunninghamia lanceolata) forests [27]. In contrast to the N4P3K1 treatment, there was a lack of Rs response in the P4K1 treatment. Similarly, P addition did not change Rs rates in temperate forests at a global scale if P was not limited for tree growth [16]. In addition, the change in Rs rates induced by P addition may mask the effects of N addition on Rs rates when N and P additions occur concurrently [10]. Rs rates in tropical, temperate, and boreal forests decreased by 1.44% under N addition based on a meta-analysis of Rs rates among biomes [28].
The mean annual Rs rates of all treatments rapidly declined by 17–27% in 2013 compared to other sampling years (2011, 2012, 2014). This could be associated with reduced microbial activity or root respiration under limited soil water conditions, which were significantly lower in 2013 (15.2–16.3%) than in other sampling years (21.4–25.3%). Similarly, Rs rates under N addition were significantly reduced when the soil water content was less than 15% in young subtropical forest ecosystems in China [21]. Reduced Rs rates under a low soil water content were due to limited respiratory enzymatic activity and the diffusion of soluble substrates [29]. Similarly, Wood et al. [30] observed a significant parabolic relationship between the soil water content and Rs rates. This result suggests that the annual change in the soil water content was a key factor that influenced the annual Rs rates at the study site, as opposed to nutrient availability induced by fertilizer application.
The temperature sensitivity of Rs rates is commonly expressed by the Q10 value [31]. No obvious thermal acclimation of Rs rates under N addition in compound fertilizer existed. These results are consistent with a study conducted in Japanese larch stands, in which compound fertilizer application had little effect on the temperature sensitivity of Rs rates [11]. In contrast, N addition in forests may affect Q10 values by changing the metabolism of root growth and soil microbes [32]. However, N addition in compound fertilizer did not change the fundamental exponential relationships between the soil temperature and Rs rates, which is consistent with other fertilizer studies [8,11]. The Q10 values in this study are comparable to or higher than those observed in other coniferous forests in Korea [7,10], which have been reported as 3.45–3.77 at 12 cm soil depth [7,10], and the global mean value ranges from 1.3 to 3.3 [33]. The variability in Q10 values between previous and current studies may be due to the difference in soil depth at which temperature was measured [34,35] or the difference in soil water conditions [31], rather than fertilization effects.
5. Conclusions
Mean annual Rs rates in Korean red pine stands reveal the independent effects of increased N availability resulting from N addition in compound fertilizer. In addition, nitrogen addition did not change soil temperature and water content, which are the main factors involved in controlling Rs. However, significant decreases in mean annual Rs rates were observed in 2013 when the mean soil water content was the lowest among other sampling years. The decrease in mean annual Rs rates may have contributed to the limited soil water content for root growth or microbial activities. A negative and positive correlation between Rs and soil pH or DBH increments in fertilized and control treatments confirms that abiotic and biotic factors significantly drive the monthly fluctuation of Rs with soil temperature. These results indicate that Rs rates in Korean red pine stands might be independent of N because soil temperature and soil water content are more likely to control Rs activity at a given site. Consequently, future studies of the responses of Rs rates in fertilizers with and without N addition should evaluate soil microbial metabolism and root activity rather than soil environmental factors.
Author Contributions
Conceptualization, C.K. and G.B.; methodology, C.K.; software, G.B.; validation, C.K.; formal analysis, G.B.; investigation, G.B.; resources, C.K.; data curation, G.B.; writing—original draft preparation, G.B.; writing—review and editing, G.B. and C.K.; visualization, G.B.; supervision, G.B.; project administration, G.B.; funding acquisition, G.B. and C.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2010-0022193; RS-2023-00271791).
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The authors have reviewed and edited the output, and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bowden, R.D.; Davidson, E.; Savage, K.; Arabia, C.; Steudler, P. Chronic nitrogen additions reduce total soil respiration and microbial respiration in temperate forest soils at the Harvard Forest. For. Ecol. Manag. 2004, 196, 43–56. [Google Scholar] [CrossRef]
- Jandl, R.; Lindner, M.; Vesterdal, L.; Bauwens, B.; Baritz, R.; Hagedorn, F.; Johnson, D.W.; Minkkinen, K.; Byrne, K.A. How strongly can forest management influence soil carbon sequestration? Geoderma 2007, 137, 253–268. [Google Scholar] [CrossRef]
- Binkley, D.; Fisher, R.F. Ecology and Management of Forest Soils, 5th ed.; John Wiley and Sons Ltd.: Chichester, UK, 2020; p. 440. [Google Scholar]
- Janssens, I.A.; Dieleman, W.; Luyssaert, S.; Subke, J.-A.; Reichstein, M.; Ceulemans, R.; Ciais, P.; Dolman, A.J.; Grace, J.; Matteucci, G.; et al. Reduction of forest soil respiration in response to nitrogen deposition. Nat. Geosci. 2010, 3, 315–322. [Google Scholar] [CrossRef]
- Zhong, Y.; Yan, W.; Shangguan, Z. The effects of nitrogen enrichment on soil CO2 fluxes depending on temperature and soil properties. Glob. Ecol. Biogeogr. 2016, 25, 475–488. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, W.; Sun, T.; Chen, L.; Pang, X.; Wang, Y.; Xiao, F. N and P fertilization reduced soil autotrophic and heterotrophic respiration in a young Cunninghamia lanceolata forest. Agric. For. Meteorol. 2017, 232, 66–73. [Google Scholar] [CrossRef]
- Jeong, J.; Bolan, N.; Kim, C. Heterotrophic soil respiration affected by compound fertilizer types in red pine (Pinus densiflora S. et Z.) stands of Korea. Forests 2016, 7, 309. [Google Scholar] [CrossRef]
- He, T.; Wang, Q.; Wang, S.; Zhang, F. Nitrogen addition altered the effect of belowground C allocation on soil respiration in a subtropical forest. PLoS ONE 2016, 11, e0155881. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, G.; Bai, S.H.; Song, J.; Shang, Y.; He, M.; Wang, X.; Zheng, Z. Differential response of soil respiration to nitrogen and phosphorus addition in a highly phosphorus-limited subtropical forest, China. For. Ecol. Manag. 2019, 448, 499–508. [Google Scholar] [CrossRef]
- Kim, C. Soil carbon storage, litterfall and CO2 efflux in fertilized and unfertilized larch (Larix leptolepis) plantations. Ecol. Res. 2008, 23, 757–763. [Google Scholar] [CrossRef]
- Hasselquist, N.J.; Metcalfe, D.B.; Högberg, P. Contrasting effects of low and high nitrogen additions on soil CO2 flux components and ectomycorrhizal fungal sporocarp production in a boreal forest. Glob. Change Biol. 2012, 18, 3596–3605. [Google Scholar] [CrossRef]
- Baek, G.; Lim, H.; Noh, N.J.; Kim, C. No impact of nitrogen fertilization on carbon sequestration in a temperate Pinus densiflora forest. Sci. Rep. 2023, 13, 1743. [Google Scholar] [CrossRef]
- Han, Y.; Wang, G.; Zhou, S.; Li, W.; Xiong, L. Day-night discrepancy in soil respiration varies with seasons in a temperate. Func. Ecol. 2023, 37, 2002–2013. [Google Scholar] [CrossRef]
- Kalra, Y.P.; Maynard, D.G. Methods Manual for Forest Soil and Plant Analysis. Forestry Canada Northwest Region Information Report NOR-X-319; Forestry: Edmonton, AB, Canada, 1991. [Google Scholar]
- SAS Institute Inc. SAS/STAT Statistical Software, Version 9.1; SAS Publishing: Cary, NC, USA, 2003.
- Feng, J.; Zhu, B. A global meta-analysis of soil respiration and its components in response to phosphorus addition. Soil Biol. Biochem. 2019, 135, 38–47. [Google Scholar] [CrossRef]
- Goswami, S.; Fisk, M.C.; Vadeboncoeur, M.A.; Garrison-Johnston, M.; Yanai, R.D.; Fahey, T.J. Phosphorus limitation of aboveground production in northern hardwood forests. Ecology 2018, 99, 438–449. [Google Scholar] [CrossRef]
- Håkansson, C.; Hedwall, P.; Bader, M.; Strömgren, M.; Axelsson, M.; Bergh, J. Forest fertilization transiently increases soil CO2 efflux in young Norway spruce stands in Sweden. Agric. For. Meteorol. 2025, 360, 110287. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Zhou, G.; Liu, J.; Liu, S.; Duan, H.; Zhang, D. Responses of soil respiration to elevated carbon dioxide and nitrogen addition in young subtropical forest ecosystems in China. Biogeosciences 2010, 7, 315–328. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, J.; Wu, J.; Chen, H.; Lin, Y.; Zhou, L.; Fu, S. Impacts of understory species removal and/or addition on soil respiration in mixed forest plantation with native species in southern China. For. Ecol. Manag. 2011, 261, 1053–1060. [Google Scholar] [CrossRef]
- Micks, P.; Aber, J.D.; Boone, R.D.; Davidson, E.A. Short-term soil respiration and nitrogen immobilization response to nitrogen applications in control and nitrogen-enriched temperate forests. For. Ecol. Manag. 2004, 196, 57–70. [Google Scholar] [CrossRef]
- Tietema, A.; Emmett, B.A.; Gundersen, P.; Kjønaas, O.J.; Koopmans, C.J. The fate of 15N-labelled nitrogen deposition in coniferous forest ecosystems. For. Ecol. Manag. 1998, 101, 19–27. [Google Scholar] [CrossRef]
- Zang, H.; Wang, J.; Kuzyakov, Y. N fertilization decreases soil organic matter decomposition in the rhizosphere. Appl. Soil Ecol. 2016, 108, 47–53. [Google Scholar] [CrossRef]
- Treseder, K.K. Nitrogen additions and microbial biomass: A meta-analysis of ecosystem studies. Ecol. Lett. 2008, 11, 1111–1120. [Google Scholar] [CrossRef]
- Liu, Y.; Shang, Q.; Wang, L.; Liu, S. Effects of understory shrub biomass on variation of soil respiration in a temperate-subtropical transitional oak forest. Forests 2019, 10, 88. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Zhang, C.; Wang, H.; Fu, X.; Chen, F.; Wan, S.; Sun, X.; Wen, X.; Wang, J. Understory vegetation plays the key role in sustaining soil microbial biomass and extracellular enzyme activities. Biogeosciences 2018, 15, 4481–4494. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, X.; Zhang, B.; Lu, M.; Luo, Y.; Liu, L.; Li, B. Different responses of soil respiration and its components to nitrogen addition among biomes: A meta-analysis. Glob. Change Biol. 2014, 20, 2332–2343. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A.; Luo, Y. On the variability of respiration in terrestrial ecosystems: Moving beyond Q10. Glob. Change Biol. 2006, 12, 154–164. [Google Scholar] [CrossRef]
- Wood, T.E.; Detto, M.; Silver, W.L. Sensitivity of soil respiration to variability in soil moisture and temperature in a humid tropical forest. PLoS ONE 2013, 8, e80965. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.; Welp, G.; Amelung, W. The temperature sensitivity (Q10) of soil respiration: Controlling factors and spatial prediction at regional scale based on environmental soil classes. Glob. Biogeochem. Cycles 2018, 32, 306–323. [Google Scholar] [CrossRef]
- Zhang, C.; Niu, D.; Hall, S.J.; Wen, H.; Li, X.; Fu, H.; Wan, C.; Elser, J.J. Effects of simulated nitrogen deposition on soil respiration components and their temperature sensitivities in a semiarid grassland. Soil Biol. Biochem. 2014, 75, 113–123. [Google Scholar] [CrossRef]
- Tjoelker, M.G.; Oleksyn, J.; Reich, P.B. Modelling respiration of vegetation: Evidence for a general temperature-dependent Q10. Glob. Change Biol. 2001, 7, 223–230. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Thomson, A. A global database of soil respiration data. Biogeosciences 2010, 7, 1915–1926. [Google Scholar] [CrossRef]
- Song, X.; Peng, C.; Zhao, Z.; Zhang, Z.; Guo, B.; Wang, W.; Jiang, H.; Zhu, Q. Quantification of soil respiration in forest ecosystems across China. Atmos. Environ. 2014, 94, 546–551. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).