Longleaf Pine Growth Divergence Increases over Time Across Its Geographic Range
Abstract
1. Introduction
2. Materials and Methods
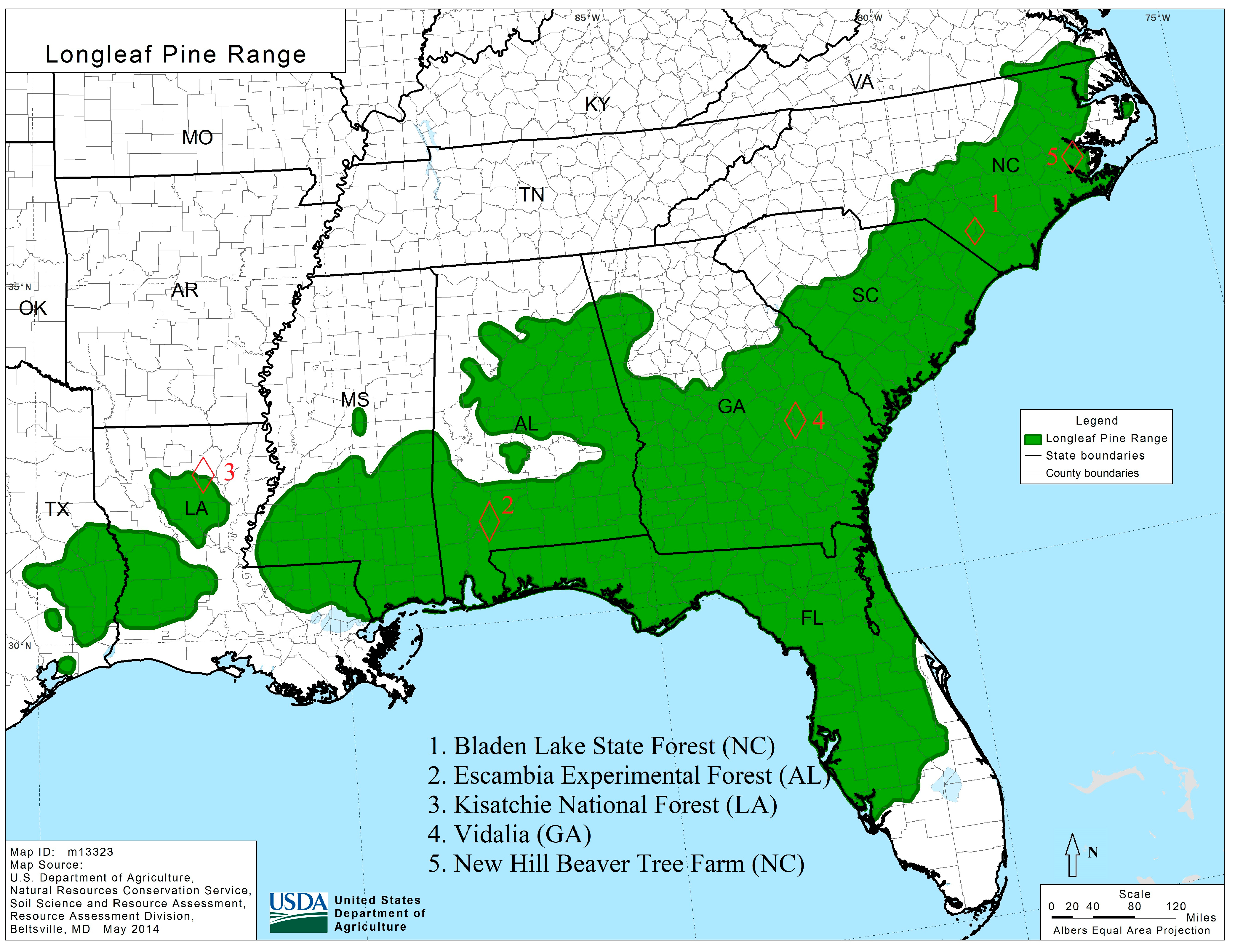
2.1. Study Sites
2.2. Tree Ring Analysis
2.3. Taylor’s Power Law
2.4. Divergence in Radial Growth
2.5. Standardized Precipitation-Evapotranspiration Index (SPEI)
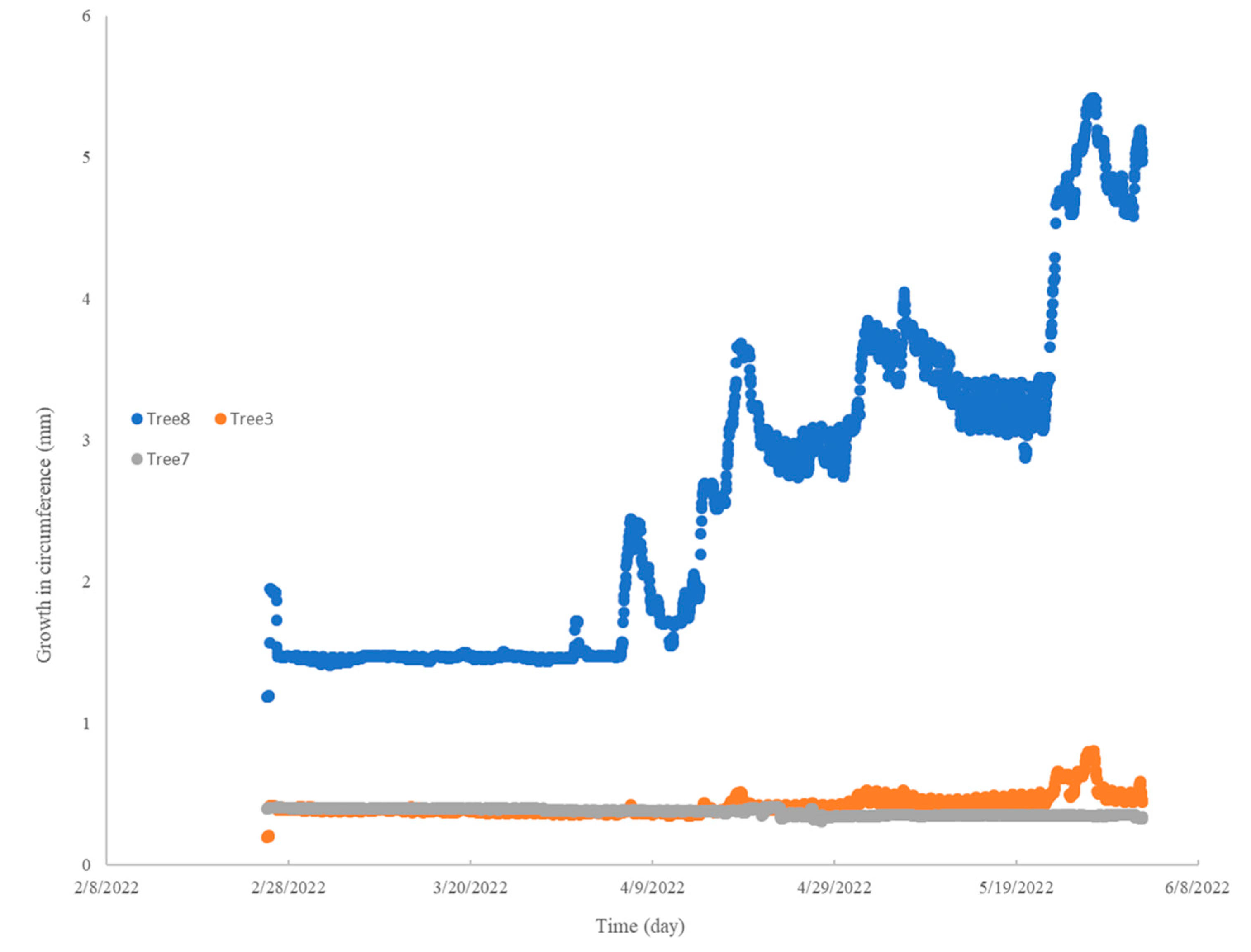
2.6. Dendrometer Measurements
2.7. Statistical Analysis
3. Results
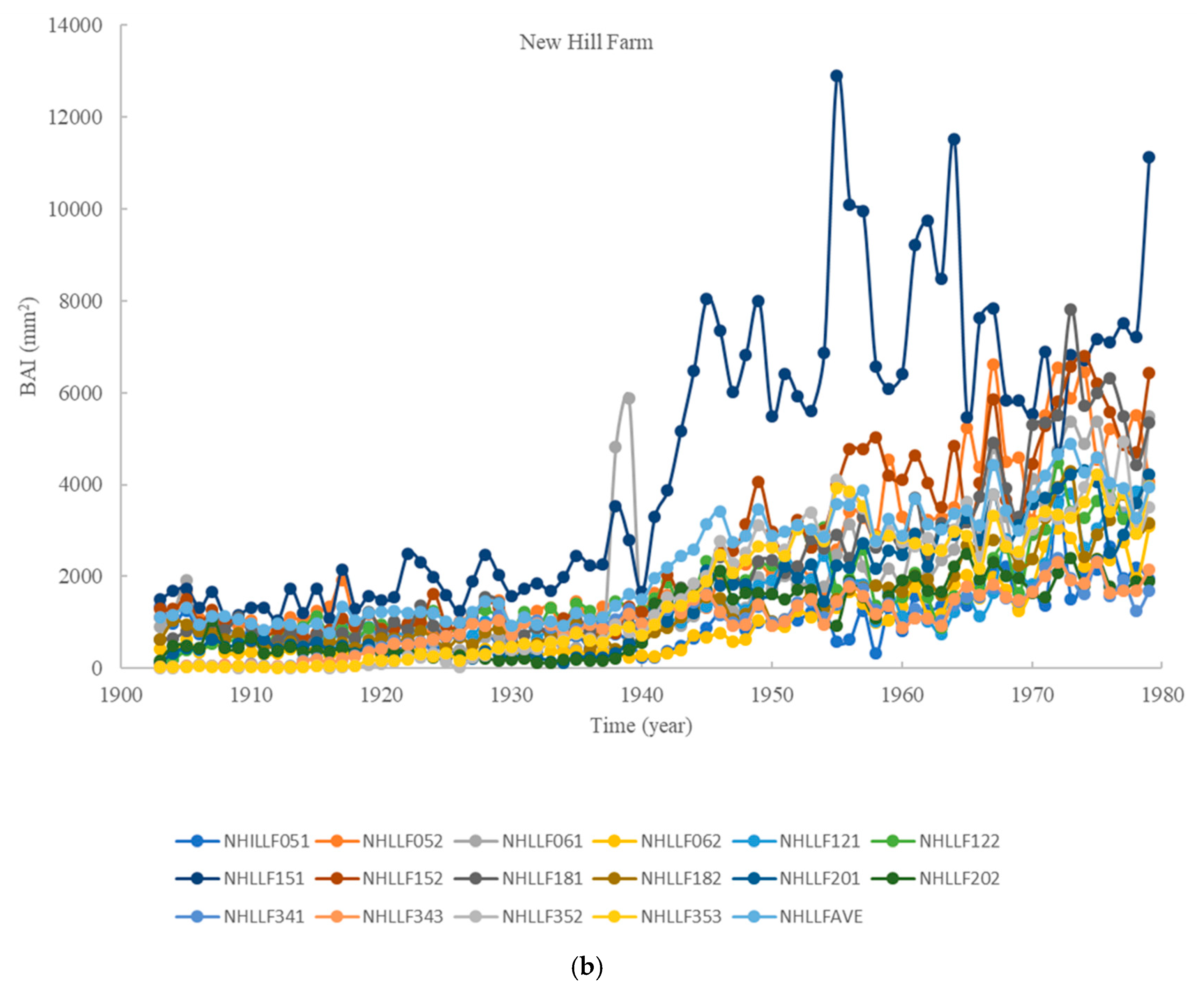
3.1. BAI Divergence
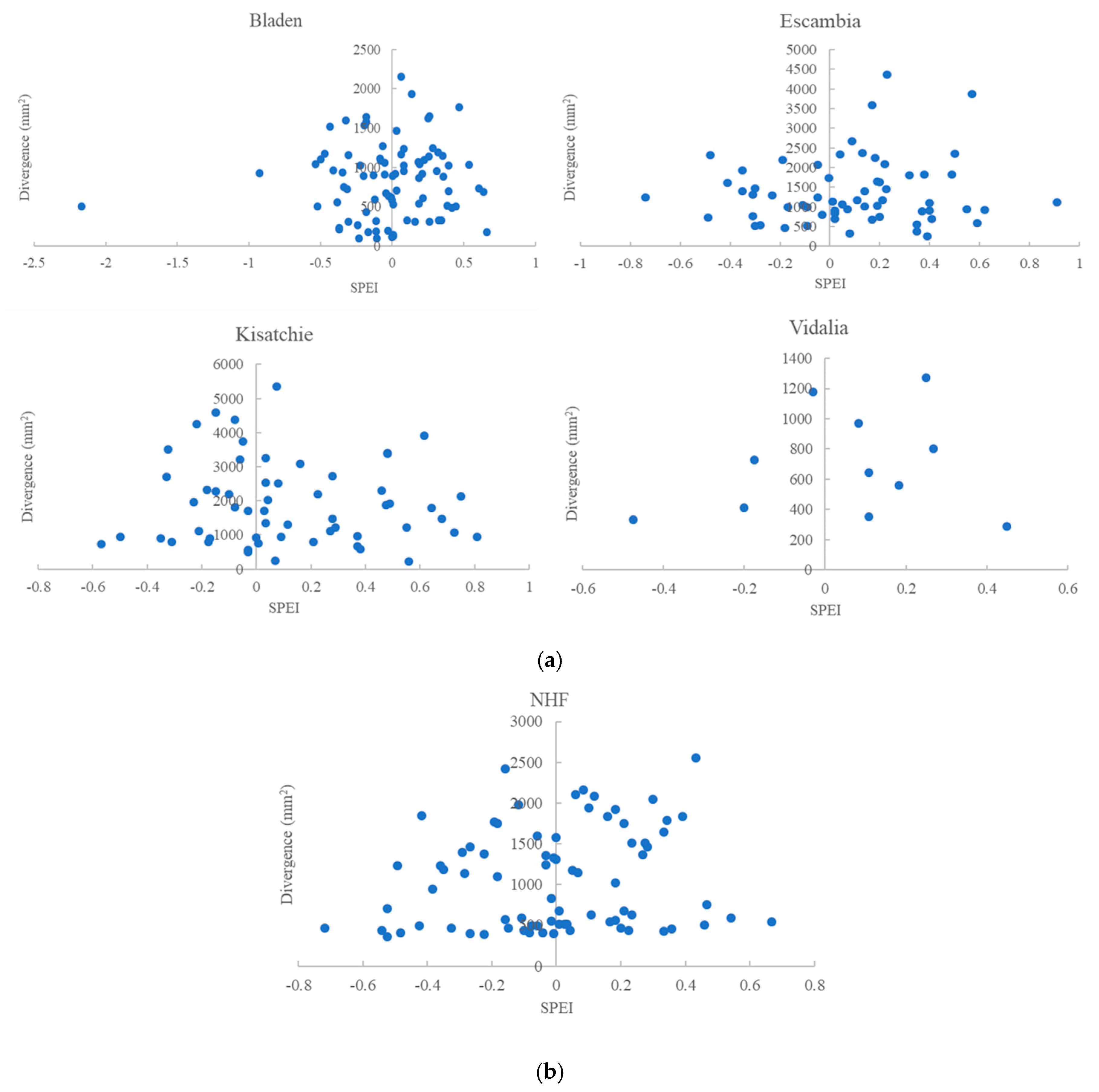
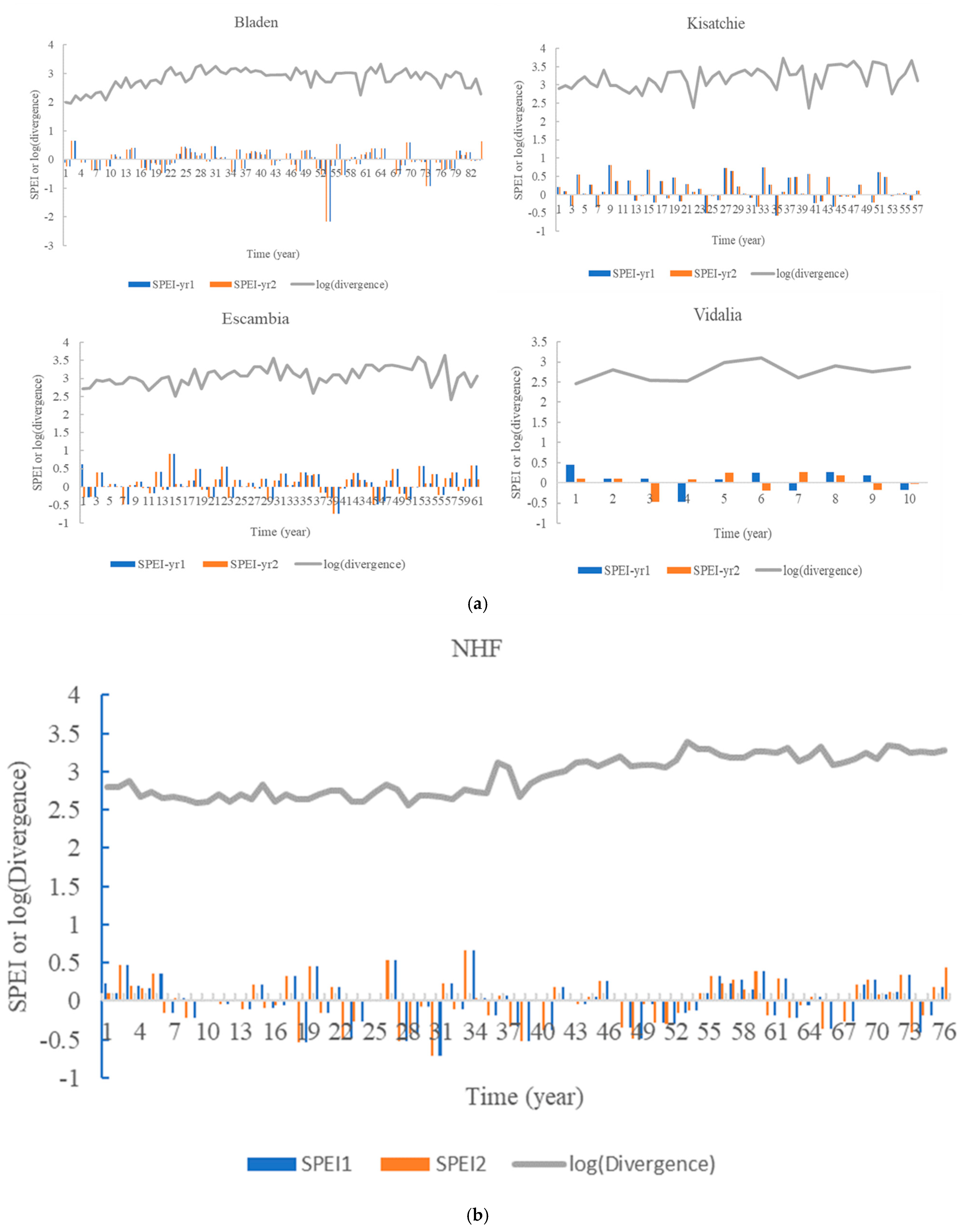
3.2. BAI Divergence and SPEI
3.3. Dendrometer Measurements at Escambia
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mann, M.E.; Bradley, R.; Hughes, M. Northern Hemisphere temperatures during the past millennium: Inferences, uncertainties and limitations. Geophys. Res. Lett. 1999, 26, 759–762. [Google Scholar] [CrossRef]
- D’Arrigo, R.; Wilson, R.; Jacoby, G. On the long-term context for late twentieth century warming. J. Geophys. Res. 2006, 111, D03103. [Google Scholar] [CrossRef]
- Jacoby, G.C.; D’Arrigo, R. Tree-ring width and density evidence of climatic and potential forest change in Alaska. Glob. Biogeochem. Cycles 1995, 9, 227–234. [Google Scholar] [CrossRef]
- Briffa, K.; Schweingruber, F.; Jones, P.; Osborn, T. Reduced sensitivity of recent tree growth to temperature at high northern latitudes. Nature 1998, 391, 678–682. [Google Scholar] [CrossRef]
- Wilmking, M.; D’Arrigo, R.; Jacoby, G.; Juday, G. Divergent growth responses in circumpolar boreal forests. Geophys. Res. Lett. 2005, 32, L15715. [Google Scholar] [CrossRef]
- Pisaric, M.; Carey, S.; Kokelj, S.; Youngblut, D. Anomalous 20th century tree growth, Mackenzie Delta, Northwest Territories, Canada. Geophys. Res. Lett. 2007, 34, L05714. [Google Scholar] [CrossRef]
- Lloyd, A.; Fastie, C. Spatial and temporal variability in the growth and climate response of treeline trees in Alaska. Clim. Change 2002, 58, 481–509. [Google Scholar] [CrossRef]
- Büntgen, U.; Frank, D.; Schmidhalter, M.; Neuwirth, B.; Seifert, M.; Esper, J. Growth/climate response shift in a long subalpine spruce chronology. Trees 2006, 20, 99–110. [Google Scholar] [CrossRef]
- Vaganov, E.; Hughes, M.; Kirdyanov, A.; Schweingruber, F.; Silkin, P. Influence of snowfall and melt timing on tree growth in Subarctic Eurasia. Nature 1999, 400, 149–151. [Google Scholar] [CrossRef]
- D’Arrigo, R.; Kaufmann, R.; Davi, N.; Jacoby, G.; Laskowski, C.; Myneni, R.; Cherubini, P. Thresholds for warming-induced growth decline at elevational treeline in the Yukon Territory. Glob. Biogeochem. Cycles 2004, 18, GB3021. [Google Scholar] [CrossRef]
- Cook, E.; Johnson, A.; Blasing, T. Forest decline: Modeling the effect of climate in tree rings. Tree Physiol. 1987, 3, 27–40. [Google Scholar] [CrossRef]
- Yonenobu, H.; Eckstein, D. Reconstruction of early spring temperature for central Japan from the tree-ring widths of Hinoki cypress and its verification by other proxy records. Geophys. Res. Lett. 2006, 33, L10701. [Google Scholar] [CrossRef]
- Chen, X.; Guo, Q.; Bowman, K.A. Climate variation within the range of longleaf pine forests during the past century. Atmosphere 2022, 13, 465. [Google Scholar] [CrossRef]
- Hodges, A.W. The naval stores industry. In The Longleaf Pine Ecosystem: Ecology, Silviculture, and Restoration; Jose, S., Jokela, E.J., Miller, D.L., Eds.; Springer: New York, NY, USA, 2006; pp. 43–48. [Google Scholar]
- Jose, S.; Jokela, E.J.; Miller, D.L. The Longleaf Pine Ecosystem: An overview. In The Longleaf Pine Ecosystem: Ecology, Silviculture, and Restoration; Jose, S., Jokela, E.J., Miller, D.L., Eds.; Springer: New York, NY, USA, 2006; pp. 3–8. [Google Scholar]
- Hausle, J.M.T.; Forrestor, J.A.; Moorman, C.E.; Martin, M.R. Tradeoffs between timber and wildlife habitat quality increase with density in longleaf pine (Pinus palustris) plantations. For. Ecol. Manag. 2023, 550, 121497. [Google Scholar] [CrossRef]
- Rother, M.T.; Huffman, J.M.; Guiterman, C.H.; Robertson, K.M.; Jones, N. A history of recurrent, low-severity fire without fire exclusion in southeastern pine savannas USA. For. Ecol. Manag. 2020, 475, 118406. [Google Scholar] [CrossRef]
- Platt, W.J.; Carr, S.M.; Reilly, M.; Fahr, J. Pine savanna overstory influences on ground-cover biodiversity. Appl. Veg. Sci. 2006, 9, 37–50. [Google Scholar] [CrossRef]
- Natural Resource Conservation Service (NRCS) Longleaf Pine Ecosystem Restoration. FY20-24 Implementation Strategy; USDA: Washington, DC, USA, 2020. [Google Scholar]
- Samuelson, L.J.; Stokes, T.A.; Butnor, J.R.; Johnsen, K.H.; Gonzalez-Benecke, C.A.; Martin, T.A.; Cropper, W.P., Jr.; Anderson, P.H.; Ramirez, M.R.; Lewis, J.C. Ecosystem carbon density and allocation across a chronosequence of longleaf pine forests. Ecol. Appl. 2017, 27, 244–259. [Google Scholar] [CrossRef]
- Boyer, W.D. Pinus palustris Mill. Longleaf pine. In Silvics of North America; Conifers. Agriculture Handbook; Burns, R.M., Honkala, B.H., Eds.; USDA Forest Service: Washington, DC, USA, 1990; pp. 405–412. [Google Scholar]
- Platt, W.J.; Evans, G.W.; Rathbun, S.L. The population dynamics of a long-lived conifer (Pinus palustris). Am. Nat. 1988, 131, 491–525. [Google Scholar] [CrossRef]
- Outcalt, K.W.; Sheffield, R.M. The Longleaf Pine Forest: Trends and Current Conditions; Resource Bulletin SRS-9; USDA Forest Service, Southern Research Station: Asheville, NC, USA, 1996. [Google Scholar]
- Chen, X.; Willis, J.L. Characterizing the growth of Pinus palustris and the relationship with cone production at the individual tree level. Dendrobiology 2025, 93, 98–107. [Google Scholar] [CrossRef]
- Taylor, L.R. Aggregation, variance and the mean. Nature 1961, 189, 732–735. [Google Scholar] [CrossRef]
- Cohen, J.E.; Xu, M.; Schuster, W.S.F. Allometric scaling of population variance with mean body sizes predicted from Taylor’s law and density-mass allometry. Proc. Natl. Acad. Sci. USA 2012, 109, 15829–15834. [Google Scholar] [CrossRef]
- Cohen, J.E.; Xu, M.; Schuster, W.S.F. Stochastic multiplicative population growth predicts and interprets Taylor’s power law of fluctuation scaling. Proc. R. Soc. London B 2013, 280, 20122955. [Google Scholar] [CrossRef]
- Keyantash, J.; Dracup, J. The quantification of drought: An evaluation of drought indices. Bull. Am. Meteorol. Soc. 2002, 83, 1167–1180. [Google Scholar] [CrossRef]
- Beguería, S.; Vicente Serrano, S.M.; Reig-Gracia, F.; Latorre Garcés, B. SPEIbase v.2.10 [Dataset]; Digital CSIC: Madrid, Spain, 2024. [Google Scholar] [CrossRef]
- Zillio, T.; Condit, R. The impact of neutrality, niche differentiation and species input on diversity and abundance distributions. Oikos 2007, 116, 931–940. [Google Scholar] [CrossRef]
- Ballantyne, F. The upper limit for the exponent of Taylor’s power law is a consequence of deterministic population growth. Evolut. Ecol. Res. 2005, 7, 1213–1220. [Google Scholar]
- Cao, Y.; Zhao, Y.; Yue, X.; Xiong, F.; Sun, Y.; He, X.; Wang, L. Between disorder and order: A case study of power law. Phys. A 2016, 456, 244–255. [Google Scholar] [CrossRef]
- Suzuki, A.A.; Suzuki, M. Why do lower order branches show greater shoot growth than higher order branches? Considering space availability as a factor affecting shoot growth. Trees 2009, 23, 69–77. [Google Scholar] [CrossRef]
- Arruda-Neto, J.D.T.; Bittencourt-Oliveira, M.C.; Castro, A.C.; Rodrigues, T.E.; Harari, J.; Mesa, J.; Genofre, G.C. Global warming and the power-laws of ecology. Atmos. Climate Sci. 2012, 2, 8–13. [Google Scholar] [CrossRef][Green Version]
- Samuelson, L.J.; Stokes, T.A.; Ramirez, M.R.; Mendonca, C.C. Drought tolerance of a Pinus palustris plantation. For. Ecol. Manag. 2019, 451, 117557. [Google Scholar] [CrossRef]
- Shell, A.; Sharma, A.; Willis, J.L.; Tracy, J.; Polinko, A.; Ojha, S.; Vogel, J. Growth dynamics of longleaf pine during conversion to uneven-aged stands. For. Ecosys. 2025, 13, 100305. [Google Scholar] [CrossRef]
- Ames, G.M.; Vineyard, D.L.; Anderson, S.M.; Wright, J.P. Annual growth in longleaf (Pinus palustris) and pond pine (P. serotina) in the Sandhills of North Carolina is driven by interactions between fire and climate. For. Ecol. Manag. 2015, 340, 1–8. [Google Scholar] [CrossRef]
- Zampieri, N.E.; Pau, S.; Rother, M.T. Variation in the strength and stationarity of southern longleaf pine seasonwood climate-growth relationships. Dendrochronology 2024, 85, 126209. [Google Scholar] [CrossRef]
- Ford, C.R.; Minor, E.S.; Fox, G.A. Long-term effects of fire and fire-return interval on population structure and growth of longleaf pine (Pinus palustris). Can. J. For. Res. 2010, 40, 1410–1420. [Google Scholar] [CrossRef]
- Willis, J.L.; Sharma, A.; Kush, J.S. Seasonality of biennial burning has no adverse effects on mature longleaf pine survival or productivity. Front. For. Glob. Chang. 2021, 4, 684087. [Google Scholar] [CrossRef]
- Peltier, D.M.P.; Anderegg, W.R.L.; Guo, J.S.; Ogle, K. Contemporary tree growth shows altered climate memory. Ecol. Lett. 2022, 25, 2663–2674. [Google Scholar] [CrossRef] [PubMed]
- Barber, V.; Juday, G.; Finney, B. Reduced growth of Alaska white spruce in the twentieth century from temperature-induced drought stress. Nature 2000, 405, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, W.; Wiles, G.; D’Arrigo, R.; Wilmking, M. Divergent tree growth response to recent climatic warming, Lake Clark National Park and Preserve, Alaska. Geophys. Res. Lett. 2005, 32, L20703. [Google Scholar] [CrossRef]
- Tranquillini, W. Physiological Ecology of the Alpine Timberline; Springer: New York, NY, USA, 1979. [Google Scholar]
- Kozlowski, T.; Karmer, P.; Pallardy, S. The Physiological Ecology of Woody Plants; Academic Press: San Diego, CA, USA, 1991. [Google Scholar]
- Maxwell, J.T.; Au, T.F.; Kannenberg, S.A.; Harley, G.L.; Dannenberg, M.P.; Ficklin, D.L.; Pederson, N. Asymmetric effects of hydroclimate extremes on eastern US tree growth: Implications on current demographic shifts and climate variability. Glob. Change Biol. 2024, 30, e17474. [Google Scholar] [CrossRef]
- Campoa, J.; Puhlick, J. Influence of climate fluctuations on Pinus palustris growth and drought resilience. For. Ecosys. 2023, 10, 100151. [Google Scholar] [CrossRef]
- Henderson, J.P.; Grissino-Mayer, H.D. Climate-tree growth relationships of longleaf pine (Pinus palustris Mill.) in the Southeastern Coastal Plain, USA. Dendrochronologia 2009, 27, 31–43. [Google Scholar] [CrossRef]
- Chen, X.; Willis, J.L. Individuals’ behaviors of cone production in longleaf pine trees. Forests 2023, 14, 494. [Google Scholar] [CrossRef]
- Boyer, W.D. Volume growth loss: A hidden cost of periodic prescribed burning in longleaf pine? South. J. Appl. For. 1987, 11, 154–157. [Google Scholar] [CrossRef]
- Munn, J.E. Investigating Among-Individual Growth Heterogeneity in Longleaf Pine: Advancing Dendrochronological Approaches. Ph.D. Thesis, University of South Florida, Tampa, FL, USA, 2019. Available online: https://digitalcommons.usf.edu/etd/8061 (accessed on 22 September 2025).



| Sites | Trees | Linear Fitting Equation for log (V) = bˑ log (M) + log (a) | R2 | p |
|---|---|---|---|---|
| Bladen | Tree #1 | y = 1.8818x − 0.0992 | 0.976 | <0.01 |
| Tree #2 | y = 2.0217x − 0.3243 | 0.9711 | <0.01 | |
| Tree #3 | y = 2.1948x − 0.7831 | 0.9681 | <0.01 | |
| Escambia | Tree #1 | y = 2.7985x − 2.5056 | 0.9901 | <0.01 |
| Tree #2 | y = 2.0397x − 0.3923 | 0.9727 | <0.01 | |
| Tree #3 | y = 1.8797x − 0.0843 | 0.9781 | <0.01 | |
| Kisatchie | Tree #1 | y = 1.7691x + 0.4529 | 0.9898 | <0.01 |
| Tree #2 | y = 1.6532x + 0.7477 | 0.9406 | <0.01 | |
| Tree #3 | y = 1.7181x + 0.538 | 0.9746 | <0.01 | |
| Vidalia | Tree #1 | y = 2.3498x − 0.7555 | 0.9401 | <0.01 |
| Tree #2 | y = 2.7717x − 1.7201 | 0.8689 | <0.01 | |
| Tree #3 | y = 2.6982x − 1.218 | 0.8847 | <0.01 | |
| Tree #4 | y = 1.3007x − 0.1279 | 0.6018 | <0.05 | |
| Tree #5 | y = 0.7544x + 34.901 | 0.1261 | >0.05 | |
| NHF | NHILLF051 | y = 1.1097x + 1.7618 | 0.1123 | >0.05 |
| NHILLF052 | y = 5.5497x − 12.061 | 0.7445 | <0.05 | |
| NHILLF061 | y = 3.4442x − 4.728 | 0.5956 | <0.05 | |
| NHILLF062 | y = 5.259x − 9.548 | 0.942 | <0.01 | |
| NHILLF121 | y = 2.0701x − 0.9439 | 0.678 | <0.05 | |
| NHILLF122 | y = 1.9109x − 0.3969 | 0.869 | <0.01 | |
| NHILLF151 | y = 4.7994x − 10.237 | 0.9154 | <0.01 | |
| NHILLF152 | y = 5.0763x − 10.569 | 0.57 | <0.05 | |
| NHILLF181 | y = 5.2092x − 10.602 | 0.8642 | <0.01 | |
| NHILLF182 | y = 4.9798x − 9.557 | 0.9022 | <0.01 | |
| NHILLF201 | y = 3.469x − 4.5928 | 0.8812 | <0.01 | |
| NHILLF202 | y = 3.089x − 3.4024 | 0.8582 | <0.01 | |
| NHILLF341 | y = 2.2158x − 0.8736 | 0.9471 | <0.01 | |
| NHILLF343 | y = 2.2438x − 0.9342 | 0.939 | <0.01 | |
| NHILLF352 | y = 2.2435x − 0.6069 | 0.985 | <0.01 | |
| NHILLF353 | y = 2.5019x − 1.3833 | 0.9688 | <0.01 | |
| NHILLFAVE | y = 6.4933x − 15.275 | 0.8696 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Willis, J.L.; Clabo, D.C. Longleaf Pine Growth Divergence Increases over Time Across Its Geographic Range. Forests 2025, 16, 1512. https://doi.org/10.3390/f16101512
Chen X, Willis JL, Clabo DC. Longleaf Pine Growth Divergence Increases over Time Across Its Geographic Range. Forests. 2025; 16(10):1512. https://doi.org/10.3390/f16101512
Chicago/Turabian StyleChen, Xiongwen, John L. Willis, and David C. Clabo. 2025. "Longleaf Pine Growth Divergence Increases over Time Across Its Geographic Range" Forests 16, no. 10: 1512. https://doi.org/10.3390/f16101512
APA StyleChen, X., Willis, J. L., & Clabo, D. C. (2025). Longleaf Pine Growth Divergence Increases over Time Across Its Geographic Range. Forests, 16(10), 1512. https://doi.org/10.3390/f16101512








