Involvement of the Transporter CgTrk1 in Potassium Uptake, Invasive Growth, and Full Virulence in Colletotrichum gloeosporioides
Abstract
1. Introduction
2. Material and Methods
2.1. Fungal Strains and Cultural Conditions
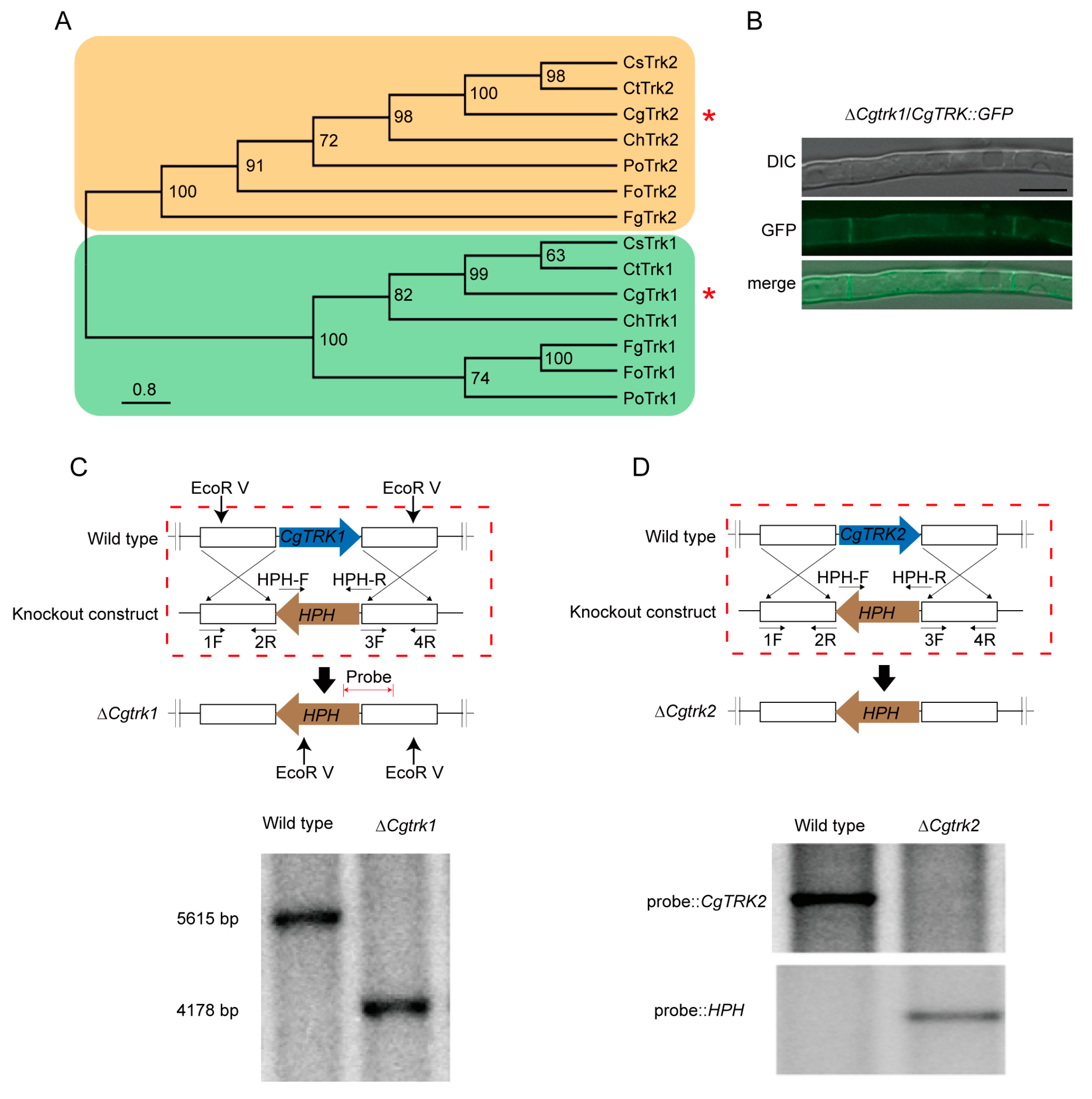
2.2. Generation of Gene-Knockout and Complementary Strains
2.3. Determination of Potassium Content in Fungal Mycelia
2.4. Vegetative Growth and Cell Wall Perturbs Resistance
2.5. Conidiation, Conidial Germination, and Appressorium Formation
2.6. Pathogenicity Assay
2.7. Statistical Analyses
3. Results
3.1. Identification and Localization Patterns of CgTrk1
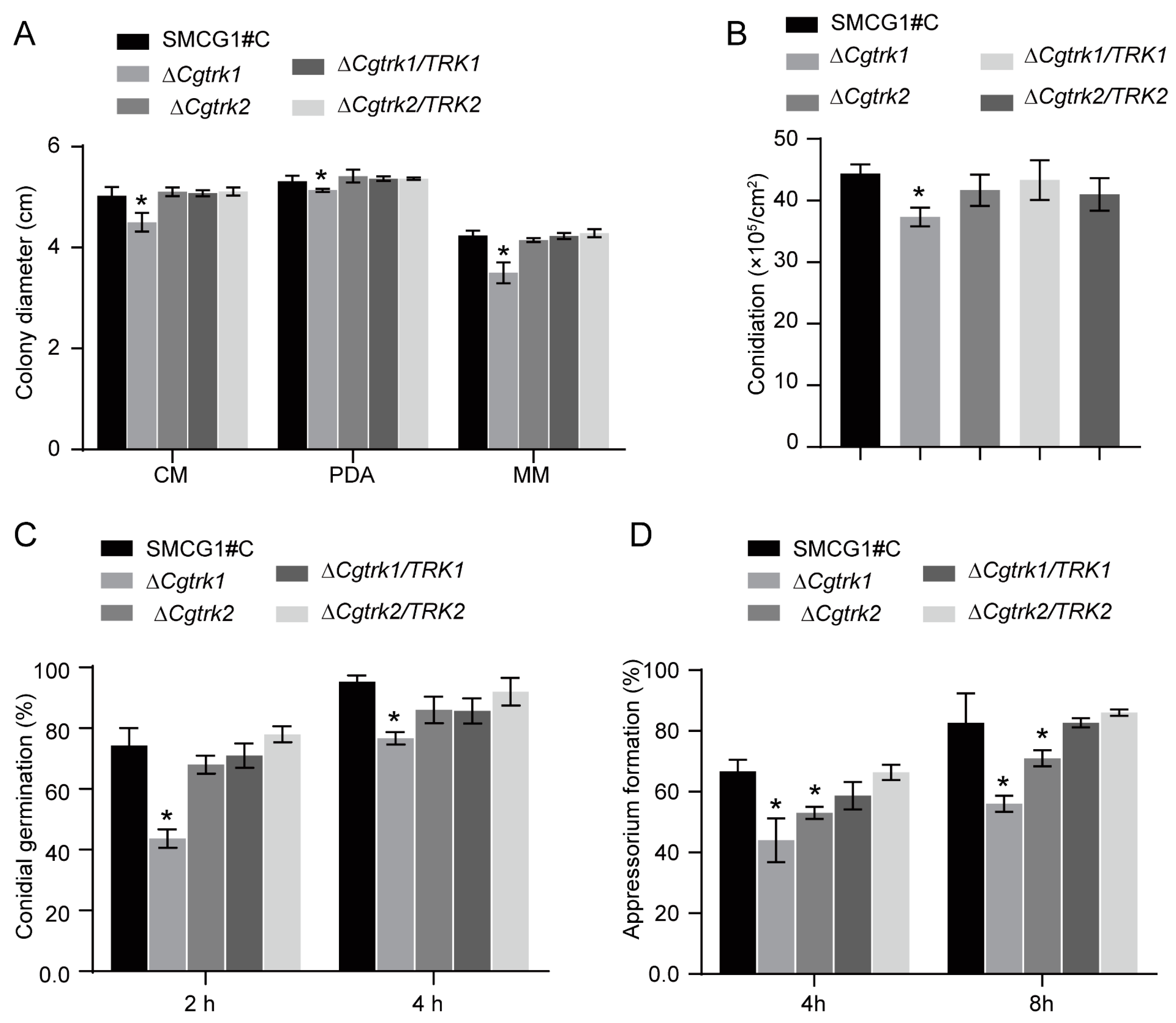
3.2. Involvement of CgTrk1 in Potassium Uptake and Cell Wall Integrity
3.3. CgTrk1 Involved in Conidiation and Appressorium Formation
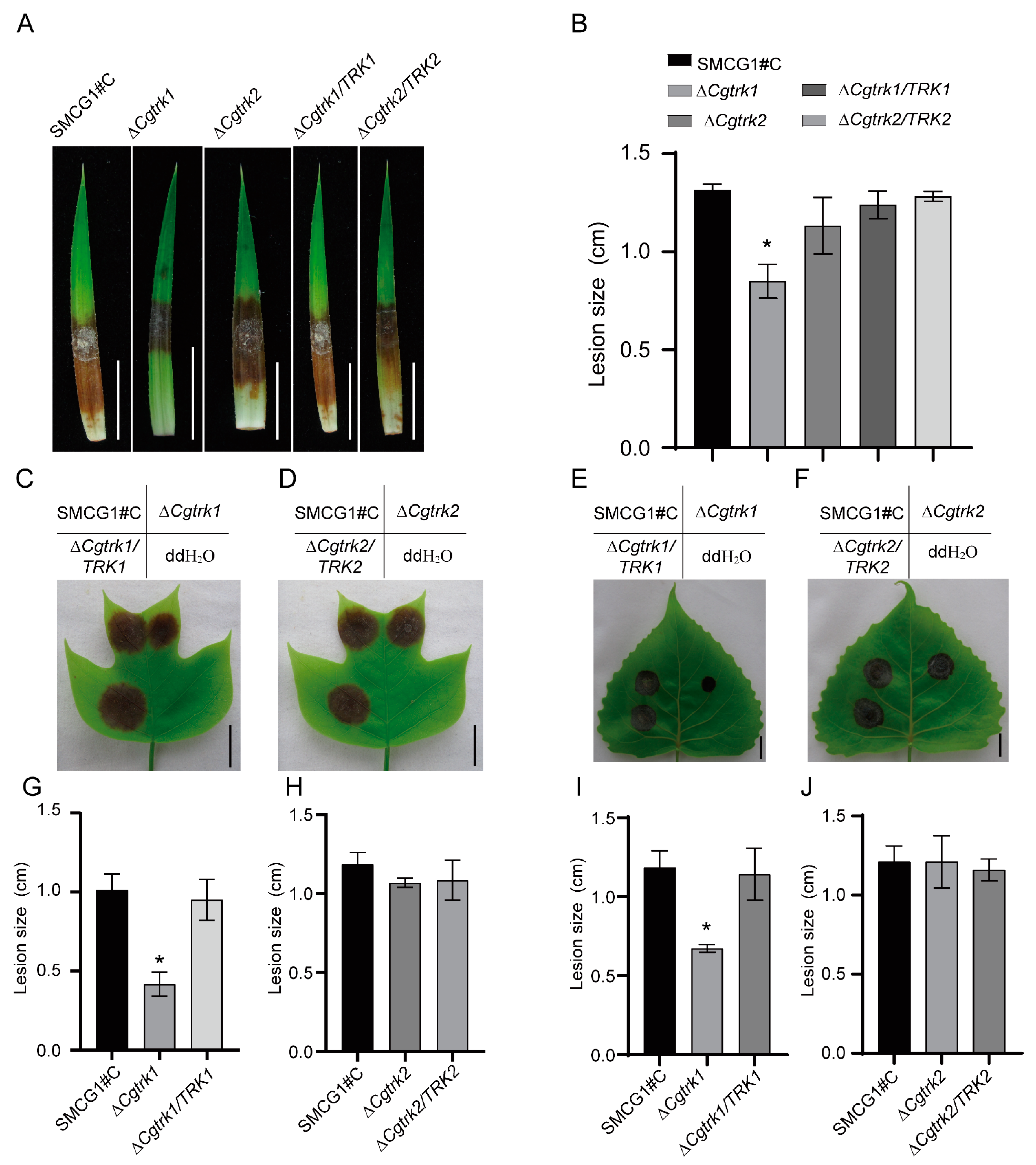
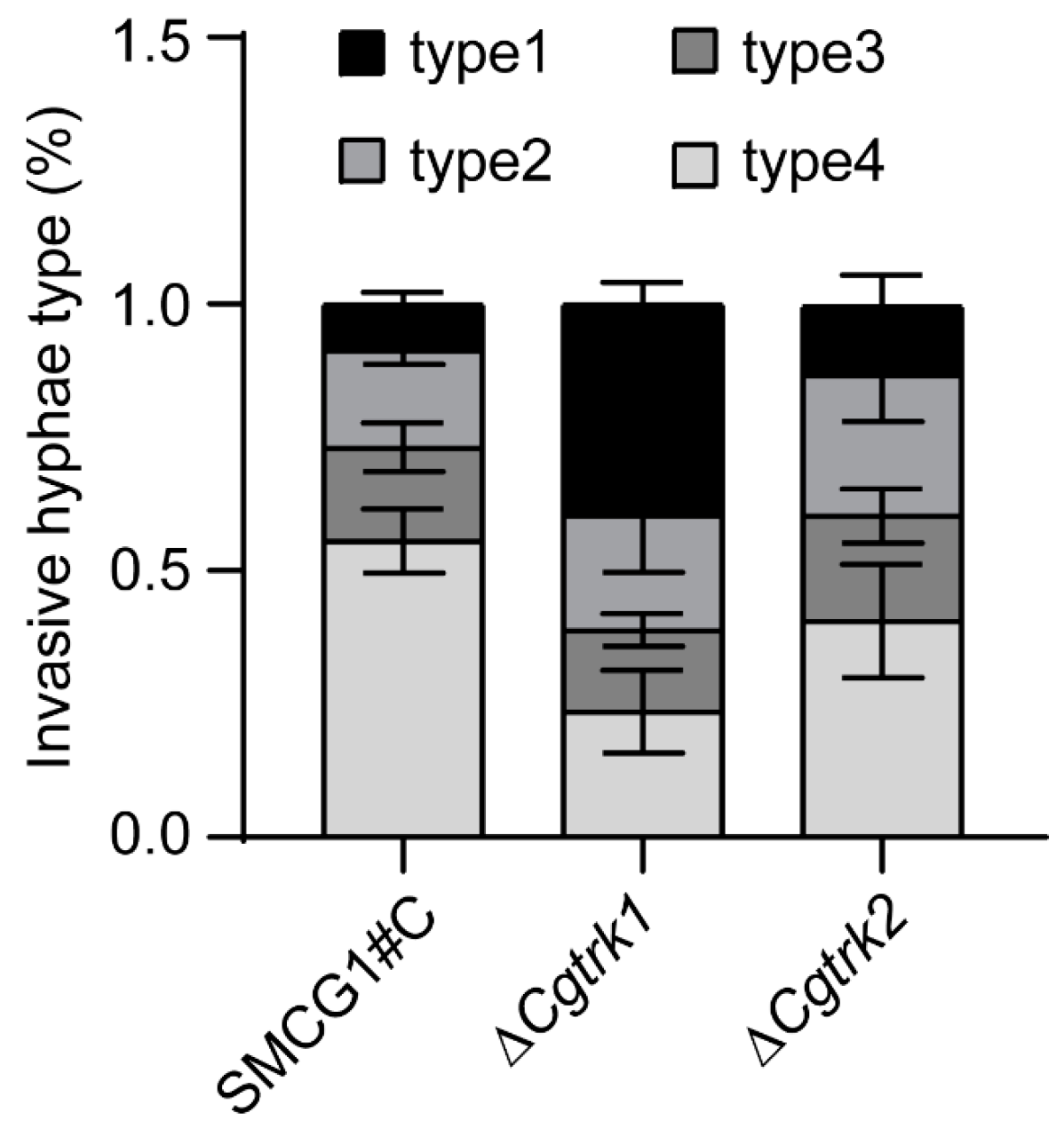
3.4. CgTrk1 Required for Full Virulence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ruiz-Castilla, F.J.; Ruiz Pérez, F.S.; Ramos-Moreno, L.; Ramos, J. Candida albicans Potassium Transporters. Int. J. Mol. Sci. 2022, 23, 4884. [Google Scholar] [CrossRef] [PubMed]
- Ariño, J.; Ramos, J.; Sychrova, H. Monovalent Cation Transporters at the Plasma Membrane in Yeasts. Yeast 2018, 36, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Haro, R.; Rodríguez-Navarro, A. Functional Analysis of the M2D Helix of the TRK1 Potassium Transporter of Saccharomyces cerevisiae. Biochim. Biophys. Acta (BBA)-Biomembr. 2003, 1613, 1–6. [Google Scholar] [CrossRef]
- Do, E.A.; Gries, C.M. Beyond Homeostasis: Potassium and Pathogenesis During Bacterial Infections. Infect. Immun. 2021, 89, e0076620. [Google Scholar] [CrossRef] [PubMed]
- Kulik, N.; Kale, D.; Spurna, K.; Shamayeva, K.; Hauser, F.; Milic, S.; Janout, H.; Zayats, V.; Jacak, J.; Ludwig, J. Dimerisation of the Yeast K+ Translocation Protein Trk1 Depends on the K+ Concentration. Int. J. Mol. Sci. 2022, 24, 398. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.; Ariño, J.; Sychrová, H. Alkali-Metal-Cation Influx and Efflux Systems in Nonconventional Yeast Species. FEMS Microbiol. Lett. 2011, 317, 1–8. [Google Scholar] [CrossRef]
- Rosario, H.; Alonso, R.N. Molecular Analysis of the Mechanism of Potassium Uptake through the TRK1 Transporter of Saccharomyces cerevisiae. Biochim. Biophys. Acta (BBA)-Biomembr. 2002, 1564, 114–122. [Google Scholar]
- Jang, S.J.; Kim, S.S.; Kuk, Y.I. Effect of Plant Extracts and Emulsifiers on Control of Anthracnose (Colletotrichum coccodes) in Persimmon Trees. Biol. Agric. Hortic. 2018, 35, 123–131. [Google Scholar] [CrossRef]
- Gaber, R.F.; Styles, C.A.; Fink, G.R. TRK1 Encodes a Plasma Membrane Protein Required for High-Affinity Potassium Transport in Saccharomyces cerevisiae. Mol. Cell. Biol. 1998, 8, 2848–2859. [Google Scholar]
- Ariño, J.; Ramos, J.; Sychrová, H. Alkali Metal Cation Transport and Homeostasis in Yeasts. Microbiol. Mol. Biol. Rev. 2010, 74, 95–120. [Google Scholar] [CrossRef]
- Ko, C.H.; Gaber, R.F. TRK1 and TRK2 encode structurally related K+ transporters in Saccharomyces cerevisiae. Mol. Cell. Biol. 1991, 11, 4266–4273. [Google Scholar] [PubMed]
- Calero, F.; Gómez, N.; Ariño, J.; Ramos, J. Trk1 and Trk2 Define the Major K+ Transport System in Fission Yeast. J. Bacteriol. 2000, 182, 394–399. [Google Scholar] [CrossRef]
- Mulet, J.M.; Leube, M.P.; Kron, S.J.; Rios, G.; Fink, G.R.; Serrano, R. A Novel Mechanism of Ion Homeostasis and Salt Tolerance in Yeast: The Hal4 and Hal5 Protein Kinases Modulate the Trk1-Trk2 Potassium Transporter. Mol. Cell. Biol. 1999, 19, 3328–3337. [Google Scholar] [CrossRef]
- Pérez-Valle, J.; Jenkins, H.; Merchan, S.; Montiel, V.; Ramos, J.; Sharma, S.; Serrano, R.; Yenush, L. Key Role for Intracellular K+ and Protein Kinases Sat4/Hal4 and Hal5 in the Plasma Membrane Stabilization of Yeast Nutrient Transporters. Mol. Cell. Biol. 2007, 27, 5725–5736. [Google Scholar] [CrossRef] [PubMed]
- Offley, S.R.; Schmidt, M.C. Protein Phosphatases of Saccharomyces cerevisiae. Curr. Genet. 2018, 65, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Yenush, L.; Mulet, J.M.; Ariño, J.; Serrano, R. The Ppz Protein Phosphatases are Key Regulators of K+ and pH Homeostasis: Implications for Salt Tolerance, Cell Wall Integrity and Cell Cycle Progression. EMBO J. 2002, 21, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Santolaria, C.; Velázquez, D.; Albacar, M.; Casamayor, A.; Ariño, J. Functional Mapping of the N-terminal Region of the Yeast Moonlighting Protein Sis2/Hal3 Reveals Crucial Residues for Ppz1 Regulation. FEBS J. 2022, 289, 7500–7518. [Google Scholar] [CrossRef] [PubMed]
- Yenush, L.; Merchan, S.; Holmes, J.; Serrano, R. pH-Responsive, Posttranslational Regulation of the Trk1 Potassium Transporter by the Type 1-Related Ppz1 Phosphatase. Mol. Cell. Biol. 2023, 25, 8683–8692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Z.; Li, B.; Pan, Y.-T.; Fang, Y.-L.; Li, D.-W.; Huang, L. Protein Phosphatase CgPpz1 Regulates Potassium Uptake, Stress Responses, and Plant Infection in Colletotrichum gloeosporioides. Phytopathology 2022, 112, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.-T.; Li, L.; Yang, J.-Y.; Li, B.; Zhang, Y.-Z.; Wang, P.; Huang, L. Involvement of Protein Kinase CgSat4 in Potassium Uptake, Cation Tolerance, and Full Virulence in Colletotrichum gloeosporioides. Front. Plant Sci. 2022, 13, 773898. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 Fungal Pathogens in Molecular Plant Pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Kim, K.T.; Yang, J.Y.; Song, H.; Choi, G.; Jeon, J.; Cheong, K.; Ko, J.; Xu, H.; Lee, Y.H. A High-Quality Draft Genome Sequence of Colletotrichum gloeosporioides sensu stricto SMCG1#C, a Causal Agent of Anthracnose on Cunninghamia lanceolata in China. Mol. Plant-Microbe Interact. 2019, 32, 139–141. [Google Scholar] [PubMed]
- Bonvicini, F.; Gentilomi, G.A.; Bressan, F.; Gobbi, S.; Rampa, A.; Bisi, A.; Belluti, F. Functionalization of the Chalcone Scaffold for the Discovery of Novel Lead Compounds Targeting Fungal Infections. Molecules 2019, 24, 372. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Fang, Y.L.; Wang, P.; Ye, J.R.; Huang, L. Pleiotropic Roles of ChSat4 in Asexual Development, Cell Wall Integrity Maintenance, and Pathogenicity in Colletotrichum higginsianum. Front. Microbiol. 2018, 9, 2311. [Google Scholar] [CrossRef] [PubMed]
- Binepal, G.; Gill, K.; Crowley, P.; Cordova, M.; Brady, L.J.; Senadheera, D.B.; Cvitkovitch, D.G. Trk2 Potassium Transport System in Streptococcus mutans and Its Role in Potassium Homeostasis, Biofilm Formation, and Stress Tolerance. J. Bacteriol. 2016, 198, 1087–1100. [Google Scholar] [CrossRef] [PubMed]
- Smith, I. Mycobacterium tuberculosis Pathogenesis and Molecular Determinants of Virulence. Clin. Microbiol. Rev. 2003, 16, 463–496. [Google Scholar] [CrossRef] [PubMed]
- MacGilvary, N.J.; Kevorkian, Y.L.; Tan, S. Potassium Response and Homeostasis in Mycobacterium tuberculosis Modulates Environmental Adaptation and is Important for Host Colonization. PLoS Pathog. 2019, 15, e1007591. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ho, K.K.; Su, J.; Gong, H.; Chang, A.C.; Lu, S. Potassium Transport of Salmonella is Important for Type III Secretion and Pathogenesis. Microbiology 2013, 159, 1705–1719. [Google Scholar] [CrossRef]
- Wang, P.; Li, B.; Pan, Y.-T.; Zhang, Y.-Z.; Li, D.-W.; Huang, L. Calcineurin-Responsive Transcription Factor CgCrzA Is Required for Cell Wall Integrity and Infection-Related Morphogenesis in Colletotrichum gloeosporioides. Plant Pathol. J. 2020, 36, 385–397. [Google Scholar] [CrossRef]
- Shi, X.; Long, Y.; He, F.; Zhang, C.; Wang, R.; Zhang, T.; Wu, W.; Hao, Z.; Wang, Y.; Wang, G.-L.; et al. The Fungal Pathogen Magnaporthe oryzae Suppresses Innate Immunity by Modulating a Host Potassium Channel. PLoS Pathog. 2018, 14, e1006878. [Google Scholar] [CrossRef]
- Muñoz-Planillo, R.; Kuffa, P.; Martínez-Colón, G.; Smith, B.L.; Rajendiran, T.M.; Núñez, G. K⁺ Efflux is the Common Trigger of NLRP3 Inflammasome Activation by Bacterial Toxins and Particulate Matter. Immunity 2013, 38, 1142–1153. [Google Scholar] [CrossRef] [PubMed]
- Hayward, J.A.; Mathur, A.; Ngo, C.; Man, S.M. Cytosolic Recognition of Microbes and Pathogens: Inflammasomes in Action. Microbiol. Mol. Biol. Rev. 2018, 82, e00015-18. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Yang, J.; Sun, M.; Pan, Y.; Huang, L. Involvement of the Transporter CgTrk1 in Potassium Uptake, Invasive Growth, and Full Virulence in Colletotrichum gloeosporioides. Forests 2024, 15, 1044. https://doi.org/10.3390/f15061044
Wang Z, Yang J, Sun M, Pan Y, Huang L. Involvement of the Transporter CgTrk1 in Potassium Uptake, Invasive Growth, and Full Virulence in Colletotrichum gloeosporioides. Forests. 2024; 15(6):1044. https://doi.org/10.3390/f15061044
Chicago/Turabian StyleWang, Zhi, Jiyun Yang, Meiling Sun, Yuting Pan, and Lin Huang. 2024. "Involvement of the Transporter CgTrk1 in Potassium Uptake, Invasive Growth, and Full Virulence in Colletotrichum gloeosporioides" Forests 15, no. 6: 1044. https://doi.org/10.3390/f15061044
APA StyleWang, Z., Yang, J., Sun, M., Pan, Y., & Huang, L. (2024). Involvement of the Transporter CgTrk1 in Potassium Uptake, Invasive Growth, and Full Virulence in Colletotrichum gloeosporioides. Forests, 15(6), 1044. https://doi.org/10.3390/f15061044





