Virulence and Pathological Characteristics of a New Metarhizium anisopliae Strain against Asian Long-Horn Beetle Anoplophora glabripennis Larvae
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Isolation and Identification
2.1.1. Fungal Isolation
2.1.2. Molecular Identification
2.1.3. Morphological Identification
2.2. Bioassays of the Metarhizium anisopliae Strain DES3 and Commercial Metarhizium anisopliae Strain against the ALB
2.2.1. Experimental Insects
2.2.2. Experimental Fungi
2.2.3. Bioassays
2.3. Histopathological Observation
2.4. Statistical Analysis
3. Results
3.1. Fungal Identification
3.2. Virulence of the Metarhizium anisopliae Strain DES3 against the ALB
3.3. Virulence of the Commercial Metarhizium anisopliae Strain against the ALB
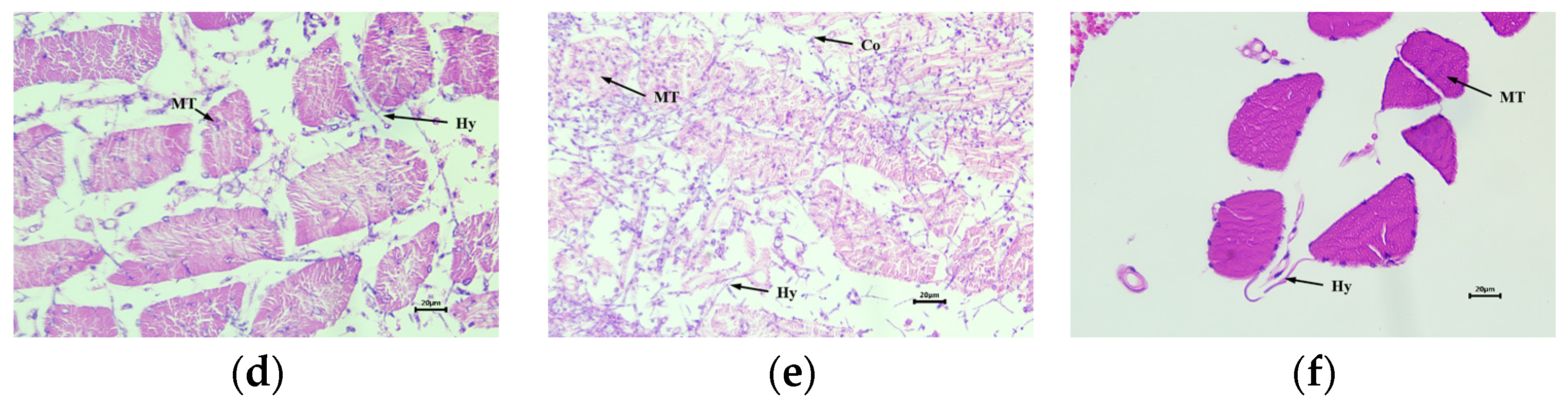
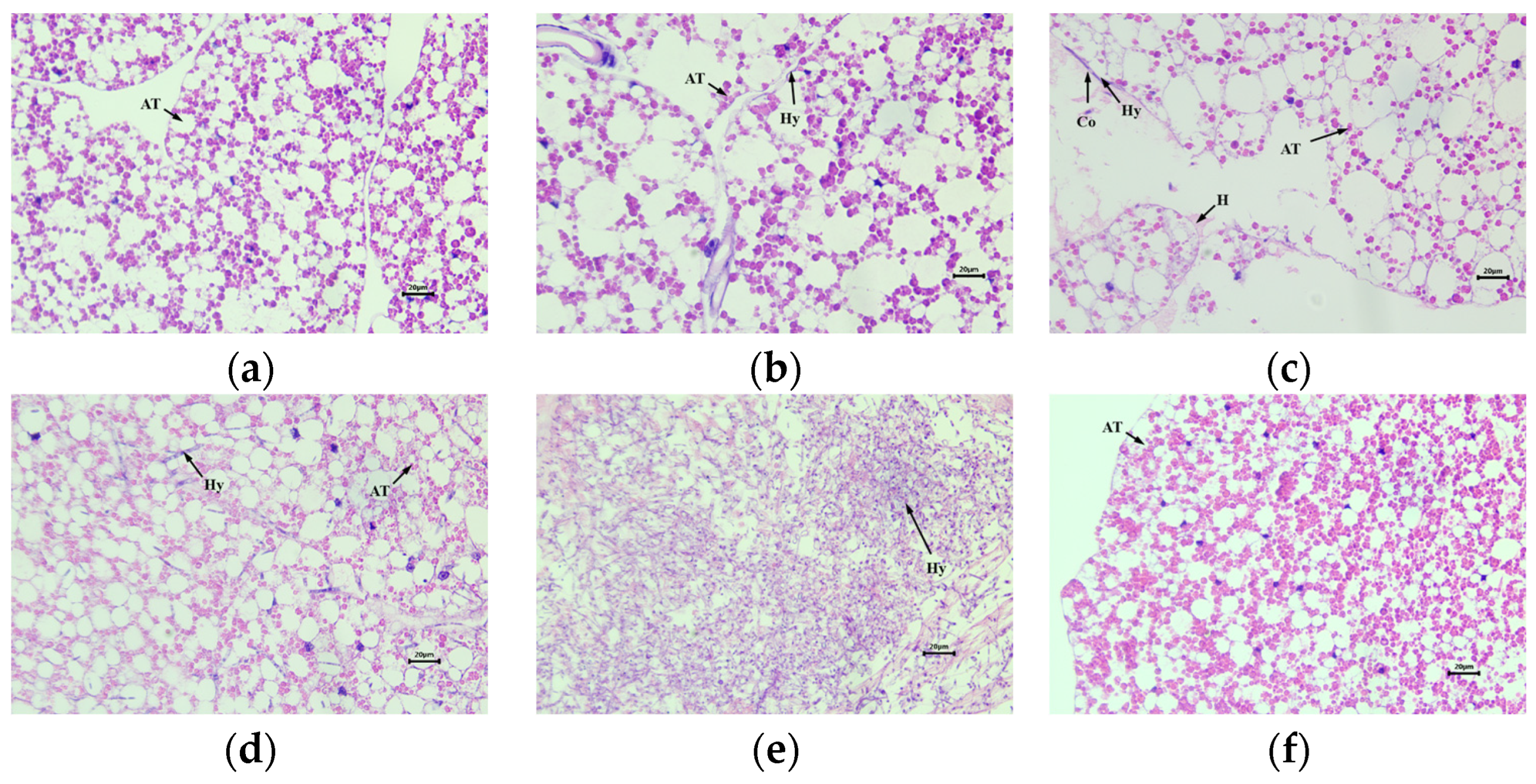
3.4. Histopathological Observation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.X.; Li, C.C.; Luo, Y.Q.; Wang, G.J.; Dou, Z.P.; Haq, I.U.; Shang, S.Q.; Cui, M.M. Current and future control of the wood-boring pest Anoplophora glabripennis. Insect Sci. 2023, 30, 1534–1551. [Google Scholar] [CrossRef] [PubMed]
- Haack, R.A.; Hérard, F.; Sun, J.H.; Turgeon, J.J. Managing invasive populations of Asian longhorned beetle and citrus longhorned beetle: A worldwide perspective. Annu. Rev. Entomol. 2010, 55, 521–546. [Google Scholar] [CrossRef] [PubMed]
- Cavagna, B.; Ciampitti, M.; Bianchi, A. Lombardy region experience to support the prediction and detection strategies. JJ. Entomol. Acarol. Res. 2013, 45, 1–6. [Google Scholar]
- Brabbs, T.; Collins, D.; Hérard, F.; Maspero, M.; Eyre, D. Prospects for the use of biological control agents against Anoplophora in Europe. Pest Manag. Sci. 2015, 71, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Cavey, J.F.; Hoebeke, E.R.; Passoa, S.; Lingafelter, S.W. A new exotic threat to North American hardwood forests: An Asian longhorned beetle, Anoplophora glabripennis (motschulsky) (Coleoptera: Cerambycidae): Larval description and diagnosis. Proc. Entomol. Soc. Wash. 1998, 100, 373–381. [Google Scholar]
- Wang, J.H.; Che, S.C.; Qiu, L.F.; Li, G.; Shao, J.L.; Zhong, L.; Zhang, G.F.; Xu, H. Efficacy of emamectin benzoate trunk injection against the Asian long-horned beetle [Anoplophora glabripennis (Coleoptera: Cerambycidae)]. J. Econ. Entomol. 2020, 113, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Barak, A.V.; Wang, Y.; Xu, L.; Rong, Z.; Hang, X.; Zhan, G. Methyl bromide as a quarantine treatment for Anoplophora glabripennis (Coleoptera: Cerambycidae) in regulated wood packing material. J. Econ. Entomol. 2005, 98, 1911–1916. [Google Scholar] [CrossRef] [PubMed]
- Barathi, S.; Sabapathi, N.; Kandasamy, S.; Lee, J.T. Present status of insecticide impacts and eco-friendly approaches for remediation—A review. Environ. Res. 2024, 240, 117432. [Google Scholar] [CrossRef] [PubMed]
- van Lenteren, J.C.; Bolckmans, K.; Köhl, J.; Ravensberg, W.J.; Urbaneja, A. Biological control using invertebrates and microorganisms: Plenty of new opportunities. Biocontrol 2018, 63, 39–59. [Google Scholar] [CrossRef]
- Perumal, V.; Kannan, S.; Alford, L.; Pittarate, S.; Mekchay, S.; Reddy, G.V.P.; Elangovan, D.; Marimuthu, R.; Krutmuang, P. Biocontrol effect of entomopathogenic fungi Metarhizium anisopliae ethyl acetate-derived chemical molecules: An eco-friendly anti-malarial drug and insecticide. Arch. Insect Biochem. Physiol. 2023, 114, e22037. [Google Scholar] [CrossRef]
- Aw, K.M.S.; Hue, S.M. Mode of infection of Metarhizium spp. fungus and their potential as biological control agents. J. Fungi 2017, 3, 3020030. [Google Scholar] [CrossRef] [PubMed]
- Olombrada, M.; Medina, P.; Budia, F.; Gavilanes, J.G.; Martínez-del-Pozo, A.; García-Ortega, L. Characterization of a new toxin from the entomopathogenic fungus Metarhizium anisopliae: The ribotoxin anisoplin. Biol. Chem. 2017, 398, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.B.; Lu, H.L.; Sheng, H.Y.; St Leger, R.J. A Drosophila melanogaster model shows that fast growing Metarhizium species are the deadliest despite eliciting a strong immune response. Virulence 2023, 14, 2275493. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Jeong, S.G.; Choi, I.S.; Yang, J.E.; Lee, K.H.; Kim, J.; Kim, J.C.; Kim, J.S.; Park, H.W. Mechanisms of insecticidal action of Metarhizium anisopliae on adult Japanese pine sawyer beetles (Monochamus alternatus). ACS Omega 2020, 5, 25312–25318. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.Y.; Shi, H.L.; Wang, D. A new strain of Metarhizium robertsii isolated from Loess plateau and its virulence and pathological characteristics against Monochamus alternatus. Microorganisms 2024, 12, 514. [Google Scholar] [CrossRef] [PubMed]
- Jeon, I.; Kim, J.S. Soil treatment with Beauveria and Metarhizium to control fall armyworm, Spodoptera frugiperda, during the soil-dwelling stage. J. Asia-Pac. Entomol. 2024, 27, 102193. [Google Scholar] [CrossRef]
- Yang, X.M.; Zhang, Y.L.; Zhou, J.C.; Dong, H.; Bai, X.J.; Liu, W.; Gu, Z.M. Pathogenicity, infection process, physiological and biochemical effects of Metarhizium rileyi against Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) larvae. Egypt. J. Biol. Pest Control 2024, 34, 19. [Google Scholar] [CrossRef]
- Shanley, R.P.; Hajek, A.E. Environmental contamination with Metarhizium anisopliae from fungal bands for control of the Asian longhorned beetle, Anoplophora glabripennis (Coleoptera: Cerambycidae). Biocontrol Sci. Technol. 2008, 18, 109–120. [Google Scholar] [CrossRef]
- Gomes, S.A.; Carolino, A.T.; Teodoro, T.B.P.; Silva, G.A.; Bitencourt, R.D.B.; Silva, C.P.; Alkhaibari, A.M.; Butt, T.M.; Samuels, R.I. The potential of Metarhizium anisopliae blastospores to control Aedes aegypti larvae in the field. J. Fungi 2023, 9, 759. [Google Scholar] [CrossRef]
- Jin, S.F.; Feng, M.G.; Chen, J.Q. Selection of global Metarhizium isolates for the control of the rice pest Nilaparvata lugens (Homoptera: Delphacidae). Pest Manag. Sci. 2008, 64, 1008–1014. [Google Scholar] [CrossRef]
- Adatia, A.; Johnson, D.; Entz, S. Pathogenicity of two new isolates of Metarhizium anisopliae from Canadian soil to Melanoplus bivittatus (Orthoptera: Acrididae) and Tenebrio molitor (Coleoptera: Tenebrionidae). Can. Entomol. 2010, 142, 128–134. [Google Scholar] [CrossRef]
- Mantzoukas, S.; Lagogiannis, I.; Ntoukas, A.; Eliopoulos, P.A.; Kouretas, D.; Karpouzas, D.G.; Poulas, K. Trapping entomopathogenic fungi from vine terroir soil samples with insect baits for controlling serious pests. Appl. Sci. 2020, 10, 3539. [Google Scholar] [CrossRef]
- Wendel, J.; Cisneros, J.; Jaronski, S.; Vitek, C.; Ciomperlik, M.; Flores, D. Screening commercial entomopathogenic fungi for the management of Diaphorina citri populations in the Lower Rio Grande Valley, Texas, USA. Biocontrol 2022, 67, 225–235. [Google Scholar] [CrossRef]
- Zimmermann, G. The galleria bait method for detection of entomopathogenic fungi in soil. J. Appl. Entomol. 1986, 102, 213–215. [Google Scholar] [CrossRef]
- Aljanabi, S.M.; Martinez, I. Universal and rapid salt-extraction of high quality genomic DNA for PCR-based techniques. Nucleic Acids Res. 1997, 25, 4692–4693. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.J.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. 1990, 18, 315–322. [Google Scholar]
- Bischoff, J.F.; Chaverri, P.; White, J.F. Clarification of the host substrate of Ascopolyporus and description of Ascopolyporus philodendrus sp. nov. Mycologia 2005, 97, 710–717. [Google Scholar] [CrossRef]
- Zhou, Y.; Zou, X.; Zhi, J.; Xie, J.; Jiang, T. Fast recognition of Lecanicillium spp. and its virulence against Frankliniella occidentalis. Front. Microbiol. 2020, 11, 561381. [Google Scholar] [CrossRef]
- Zhang, D.F.; Gao, I.; Jakovlic, H.; Zou, J.; Zhang, W.; Li, X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Huang, J.F. Laboratory rearing of Anoplophora glabripennis. J. Beijing For. Univ. 1992, 14, 61–67. [Google Scholar]
- Dubois, T.; Lund, J.; Bauer, L.S.; Hajek, A.E. Virulence of entomopathogenic hypocrealean fungi infecting Anoplophora glabripennis. Biocontrol 2008, 53, 517–528. [Google Scholar] [CrossRef]
- Gebremariam, A.; Chekol, Y.; Assefa, F. Phenotypic, molecular, and virulence characterization of entomopathogenic fungi, Beauveria bassiana (Balsam) Vuillemin, and Metarhizium anisopliae (Metschn.) Sorokin from soil samples of Ethiopia for the development of mycoinsecticide. Heliyon 2021, 7, e07091. [Google Scholar] [CrossRef]
- Kim, J.C.; Lee, S.J.; Kim, S.; Lee, M.R.; Baek, S.; Park, S.E.; Kim, J.; Shin, T.Y.; Kim, J.S. Management of pine wilt disease vectoring Monochamus alternatus adults using spray and soil application of Metarhizium anisopliae JEF isolates. J. Asia-Pac. Entomol. 2020, 23, 224–233. [Google Scholar] [CrossRef]
- Remadevi, O.K.; Bai, N.S.; Sasidharan, T.O.; Balachander, M.; Dharmarajan, P. Attempts at controlling Teak Defoliator (Hyblaea puera Cramer, Lepidoptera, Hyblaeidae) with the entomopathogenic fungus, Metarhizium anisopliae (Metsch.): Laboratory, nursery and field trials. Int. J. Pest Manag. 2013, 59, 236–242. [Google Scholar] [CrossRef]
- Rudeen, M.L.; Jaronski, S.T.; Petzold-Maxwell, J.L.; Gassmann, A.J. Entomopathogenic fungi in cornfields and their potential to manage larval western corn rootworm Diabrotica virgifera virgifera. J. Invertebr. Pathol. 2013, 114, 329–332. [Google Scholar] [CrossRef]
- Wu, S.H.; Toews, M.D.; Oliveira-Hofman, C.; Behle, R.W.; Simmons, A.M.; Shapiro-Ilan, D.I. Environmental tolerance of entomopathogenic fungi: A new strain of Cordyceps javanica isolated from a whitefly epizootic versus commercial fungal strains. Insects 2020, 11, 711. [Google Scholar] [CrossRef]
- Meng, Y.; Don, P.I.D.W.H.; Wang, D. A new strain of Lecanicillium uredinophilum isolated from Tibetan plateau and its insecticidal activity. Microorganisms 2000, 10, 1832. [Google Scholar] [CrossRef]
- Tomer, H.; Blum, T.; Arye, I.; Faigenboim, A.; Gottlieb, Y.; Ment, D. Activity of native and commercial strains of Metarhizium spp. against the poultry red mite Dermanyssus gallinae under different environmental conditions. Vet. Parasitol. 2018, 262, 20–25. [Google Scholar] [CrossRef]
- Clifton, E.H.; Jaronski, S.T.; Hajek, A.E. Virulence of commercialized fungal entomopathogens against asian longhorned beetle (Coleoptera: Cerambycidae). J. Invertebr. Pathol. 2020, 20, 1. [Google Scholar] [CrossRef]
- Peng, G.X.; Jin, K.; Liu, Y.C.; Xia, Y.X. Enhancing the utilization of host trehalose by fungal trehalase improves the virulence of fungal insecticide. Appl. Microbiol. Biotechnol. 2015, 99, 8611–8618. [Google Scholar] [CrossRef]
- Cao, Y.Q.; Li, M.; Xia, Y.X. Mapmi gene contributes to stress tolerance and virulence of the entomopathogenic fungus, Metarhizium acridum. J. Invertebr. Pathol. 2011, 108, 7–12. [Google Scholar] [CrossRef]
- Bawin, T.; Seye, F.; Boukraa, S.; Zimmer, J.Y.; Raharimalala, F.N.; Ndiaye, M.; Compere, P.; Delvigne, F.; Francis, F. Histopathological effects of Aspergillus clavatus (Ascomycota: Trichocomaceae) on larvae of the southern house mosquito, Culex quinquefasciatus (Diptera: Culicidae). Fungal Biol. 2016, 120, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tong, X.G.; Awasthi, M.K.; Wu, F.Y.; Ha, S.; Ma, J.Y.; Sun, X.H.; He, C. Dynamics of soil microbial biomass and enzyme activities along a chronosequence of desertified land revegetation. Ecol. Eng. 2018, 111, 22–30. [Google Scholar] [CrossRef]
- Hu, J.F.; Angeli, S.; Schuetz, S.; Luo, Y.Q.; Hajek, A.E. Ecology and management of exotic and endemic Asian longhorned beetle Anoplophora glabripennis. Agric. For. Entomol. 2009, 11, 359–375. [Google Scholar] [CrossRef]
- Qayyum, M.A.; Saeed, S.; Wakil, W.; Nawaz, A.; Iqbal, N.; Yasin, M.; Chaurdhry, M.A.; Bashir, M.A.; Ahmed, N.; Riaz, H.; et al. Diversity and correlation of entomopathogenic and associated fungi with soil factors. J. King Saud Univ. Sci. 2021, 33, 101520. [Google Scholar] [CrossRef]
- Goble, T.A.; Dames, J.F.; Hill, M.P.; Moore, S.D. Investigation of native isolates of entomopathogenic fungi for the biological control of three citrus pests. Biocontrol Sci. Technol. 2011, 21, 1193–1211. [Google Scholar] [CrossRef]
- Tang, H.Y.; Zheng, J.Y.; Wang, D. An effective method for biological control of Anoplophora glabripennis (Coleoptera: Cerambycidae) by using a vector mite, Pyemotes moseri (Acarina: Pyemotidae), carrying insecticidal fungi. Sci. Silvae Sin. 2022, 58, 157–164. [Google Scholar]
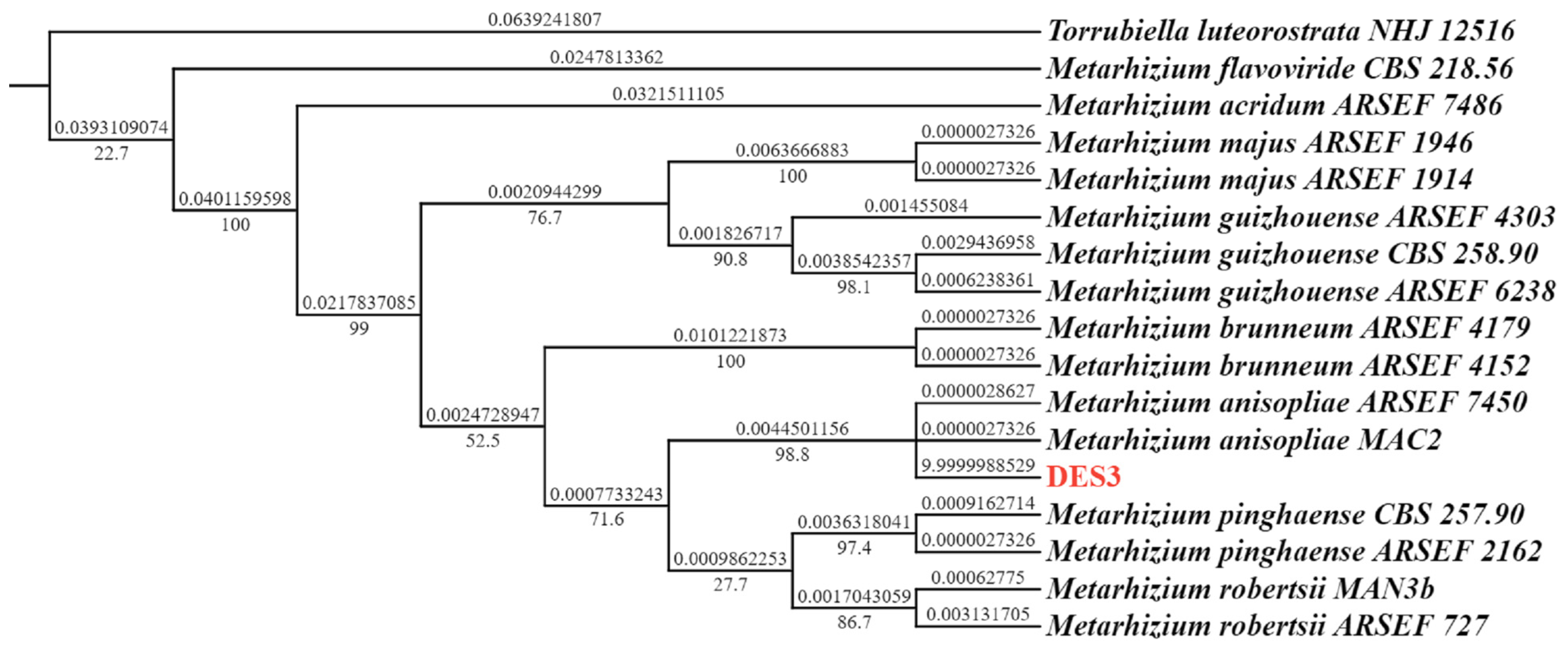
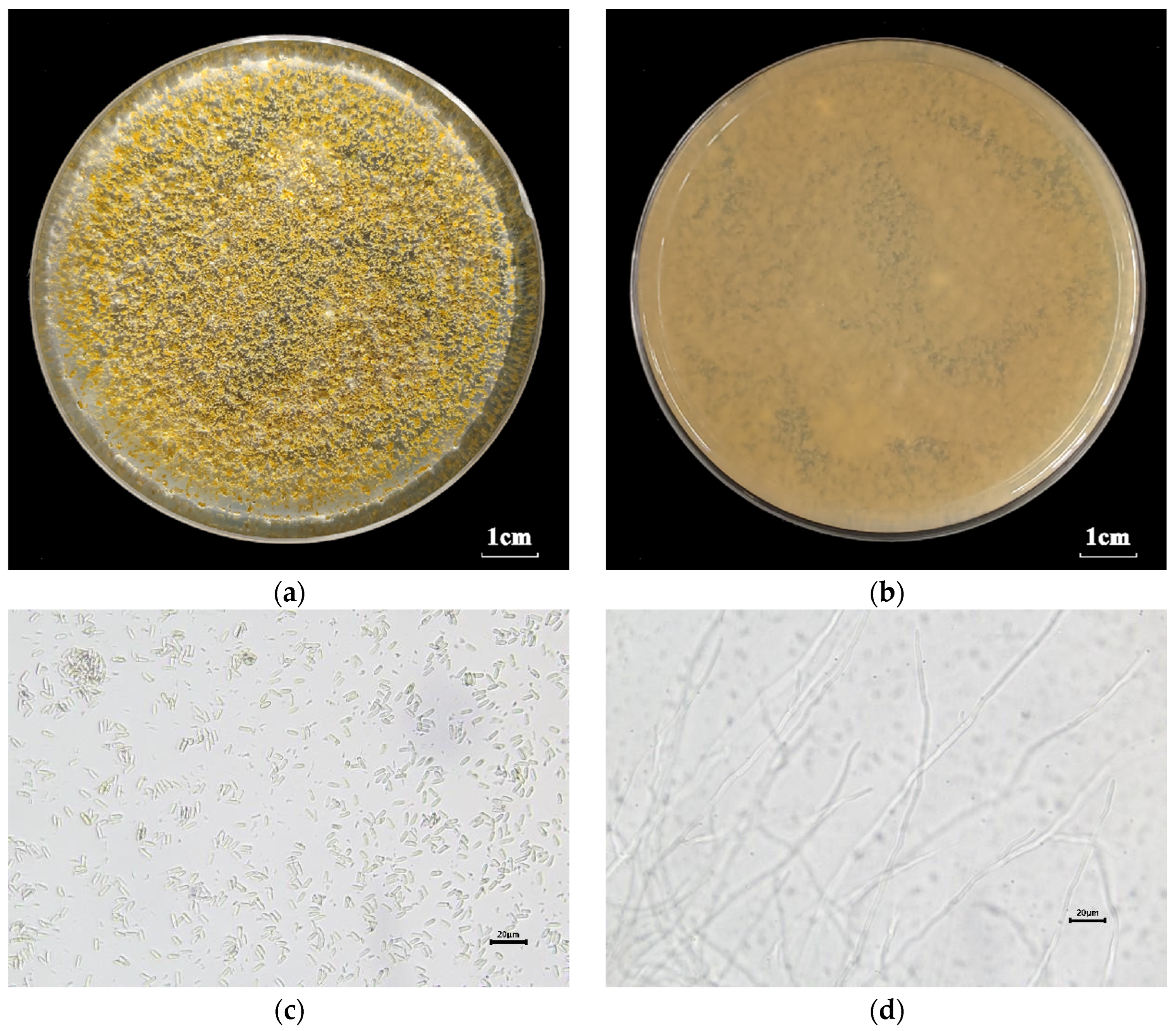
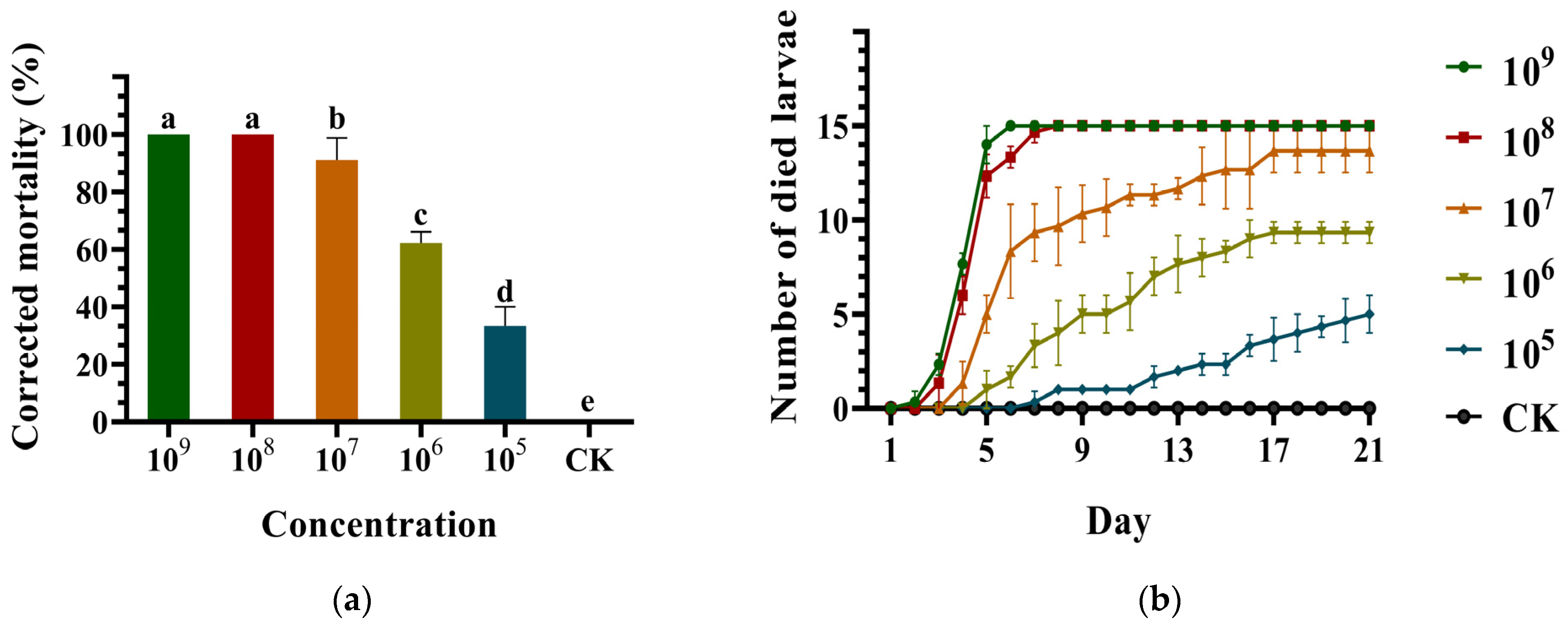
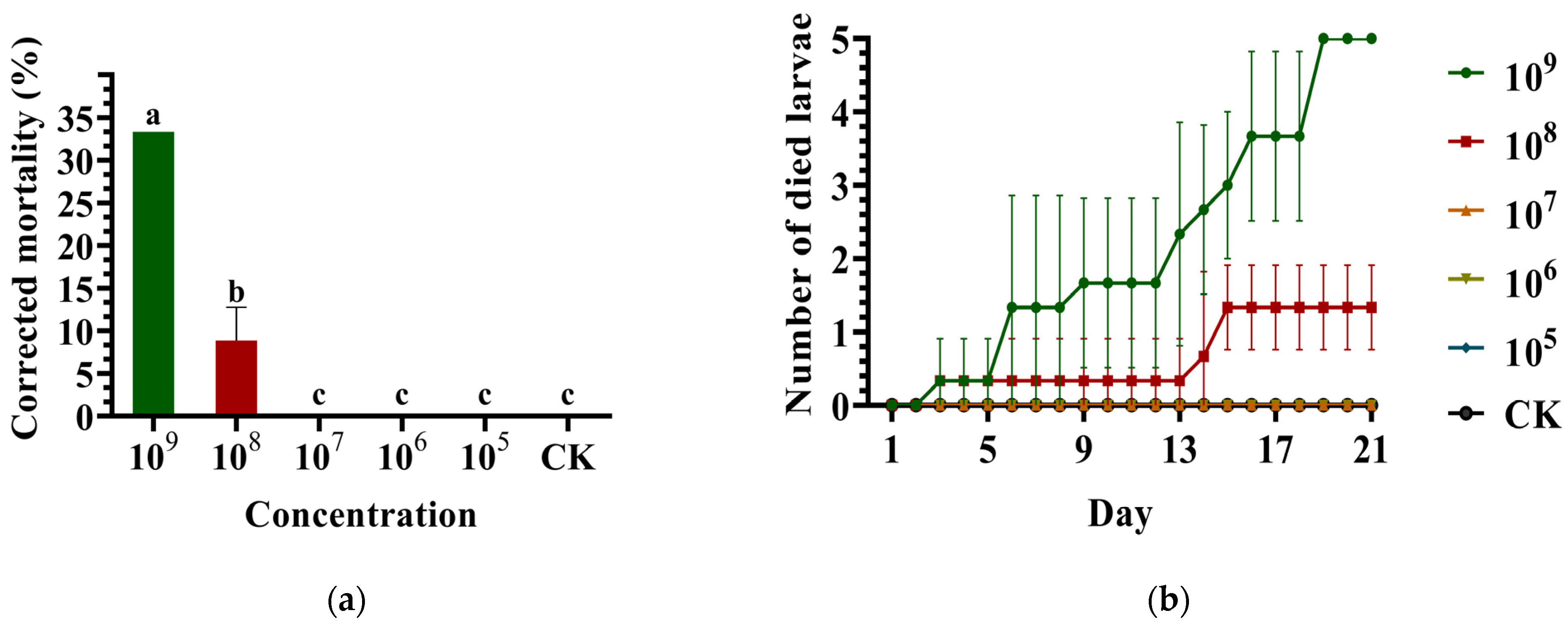



| Fungal Number | LC50 (Spore/mL) | 95% Confidence Interval | LC90 (Spore/mL) | 95% Confidence Interval |
|---|---|---|---|---|
| DES3 | 1.39 × 106 | 1.02 × 106–1.94 × 106 | 1.31 × 107 | 4.04 × 106–4.55 × 107 |
| Fungal Number | Conidial Concentration | LT50 (Days) | 95% Confidence Interval | LT90 (Days) | 95% Confidence Interval |
|---|---|---|---|---|---|
| DES3 | 109 | 3.90 | 3.78–4.01 | 5.43 | 4.95–6.18 |
| 108 | 4.19 | 4.07–4.30 | 5.56 | 5.09–6.20 | |
| 107 | 6.10 | 5.72–6.48 | 11.39 | 9.15–15.41 | |
| 106 | 9.51 | 8.70–10.70 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.-Y.; Jian, C.-C.; Wang, D. Virulence and Pathological Characteristics of a New Metarhizium anisopliae Strain against Asian Long-Horn Beetle Anoplophora glabripennis Larvae. Forests 2024, 15, 1045. https://doi.org/10.3390/f15061045
Zheng J-Y, Jian C-C, Wang D. Virulence and Pathological Characteristics of a New Metarhizium anisopliae Strain against Asian Long-Horn Beetle Anoplophora glabripennis Larvae. Forests. 2024; 15(6):1045. https://doi.org/10.3390/f15061045
Chicago/Turabian StyleZheng, Ji-Yang, Chun-Cheng Jian, and Dun Wang. 2024. "Virulence and Pathological Characteristics of a New Metarhizium anisopliae Strain against Asian Long-Horn Beetle Anoplophora glabripennis Larvae" Forests 15, no. 6: 1045. https://doi.org/10.3390/f15061045
APA StyleZheng, J.-Y., Jian, C.-C., & Wang, D. (2024). Virulence and Pathological Characteristics of a New Metarhizium anisopliae Strain against Asian Long-Horn Beetle Anoplophora glabripennis Larvae. Forests, 15(6), 1045. https://doi.org/10.3390/f15061045






