Abstract
This review synthesizes the current understanding on the dynamic influence of light on the developmental morphology of woody plants. It explores the regulatory effects of photosynthesis and photomorphogenesis in response to varying light conditions including intensity, quality, and photoperiodicity, and their subsequent impact on plant growth and architecture. Additionally, this review elucidates the role of the circadian system in synchronizing internal rhythms with external light cycles, a process mediated by photoreceptors such as PHYTOCHROME A (PHYA) and PHYTOCHROME B (PHYB), which are pivotal for seasonal growth and dormancy in species like poplar. The molecular perspective is provided on the light-regulated transcription of genes, along with their influence on the plant’s growth cycles and seasonal adaptions. Furthermore, the interactive role of plant hormones, including auxin, ethylene, and abscisic acid (ABA), is explored in the context of light signal transduction and its subsequent effect on plant physiology. By providing a comprehensive view of the light-dependent mechanisms that govern woody plant growth, this review contributes to our understanding of plant adaptation strategies and informs approaches to enhance forestry production and biodiversity conservation in the face of climate change.
1. Introduction
Light, an essential element of the terrestrial ecosystem, engages in a sophisticated and elaborate interplay with plants [1,2]. This dynamic interaction serves not merely as a central focus for biological research but as a portal to a deeper understanding of life’s emergence and its evolutionary journey.
Woody plants are vital components of natural ecosystems and are critical resources for human existence [3,4]. However, the growth and morphological formation of woody plants are not merely spontaneous or unregulated processes, they result from delicate and complex physiological and molecular mechanisms modulated by light conditions [5,6,7]. Ranging from the capture and sensing of light to the gene expression regulatory network, from singular growth reactions to integrated morphological changes, light serves as a signal, continually sculping the life processes of woody plants by modulating the activation of plant photoreceptors and signal transduction pathways.
The modulation of woody plant morphogenesis by photic stimuli is a critical area of exploration within the disciplines of plant physiology and molecular biology. Photosynthesis, the foundational bioenergetic process, enables woody plants to transduce light energy into chemical energy through a series of sophisticated biochemical reactions, thereby facilitating their ontogenetic progression [8,9,10]. Beyond its bioenergetic role, photic exposure exerts a direct influence on morphological development, governing the leaf movements and density of branching, the morphology and anatomy of foliage, and the chronobiology of anthesis. Diverse photic parameters, including spectral composition, photon flux density, and photoperiodicity, intersect to form a delicate and precise regulatory matrix [11,12].
The orchestration of circadian rhythms is of paramount significance for woody plants [6,13,14]. This endogenous timekeeping mechanism, or biological clock, equips plants with the ability to anticipate and adapt to cyclical environmental fluctuations including diurnal and nocturnal transitions and the progression of the seasons [15,16,17,18]. Photoperiodic cues modulate these biological rhythms by interfacing with clock-associated genes, thereby exerting a profound influence on an array of phenological and physiological events including flowering, the induction of dormancy, and a myriad of metabolic functions [19,20,21]. In the context of seasonal growth regulation, the temporal variations in light intensity serve as the primary exogenous cue for plants. By monitoring the photoperiodic oscillations, plants fine-tune their phenological phases to synchronize with seasonal shifts [22,23]. For instance, in the temperate and polar latitudes, woody plants enter a state of quiescence during the protracted nights of autumn, a strategic adaptation to endure the ensuing frigid winter. Conversely, the lengthening days of spring signal the termination of dormancy, triggering the onset of a renewed growth cycle. These phenotypic modifications in woody plants are not confined to individual organisms but extend to population dynamics and broader ecosystem functions [24,25]. A comprehensive understanding of light-regulated growth and developmental mechanisms in woody plants not only augments our fundamental knowledge of plant biology but also has practical implications for agricultural and silvicultural practices. Furthermore, it contributes valuable insights into broader topics including global climate change adaptation and biodiversity preservation.
The scope of this review encompasses a detailed analysis of interactions between light and woody plant physiology; shedding light on the ways in which photic cues guide, we delve into the pivotal role of light in the orchestration of plant endogenous clocks and the modulation of their seasonal growth patterns.
2. Light Regulates Morphology and Development in Woody Plants
Light drives photosynthesis in plants, supplying the necessary energy for carbon assimilation and growth, and the regulation of plant growth, development, and morphology [26]. Plants respond to different light qualities, intensities, and durations by adjusting their morphology and growth, including the direction of growth, formation of structures, and differentiation of physiological functions (Figure 1). Within fluctuating lighting environments, plants exhibit different morphological traits, and the ability to acclimate to the changing light conditions plays a crucial role in determining the competitive capability of woody plants [27].
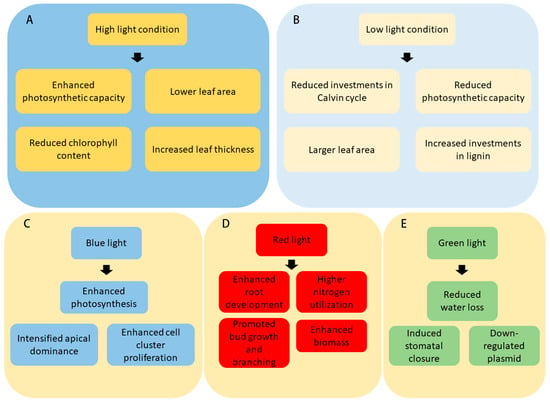
Figure 1.
Life of woody plant is regulated by varying light conditions. (A,B) Light intensity affects woody plant growth in various ways. (C–E) Lights of different spectral compositions regulate growth and physiological activities in woody plants.
2.1. Light Shapes Leaf Development
Since the seminal studies of 1998, empirical evidence has robustly demonstrated that robust and vertical branch growth in woody plants is augmented under conditions of high light intensity compared to low-light scenarios [28,29]. Investigations into four woody plant taxa—European white poplar (Populus tremula L.), common ash (Fraxinus excelsior L.), small-leaved lime (Tilia cordata Mill.) and hazel (Corylus avellana L.)—have elucidated that adaptability to varying photic environments is reflected through modifications in leaf anatomical structures and biochemical attributes. In high light conditions, light-averse pioneer species generally exhibit elevated photosynthetic capacity per area (), augmented leaf nitrogen allocation, as well as increased Maximum RuBisCO activity (Vcmax) and rates of photosynthetic electron transport (Jmax). Conversely, light-favoring species, despite their lower chlorophyll content, possess reduced leaf mass per area (MA), enabling them to expand their leaf area with minimal biomass investment for light interception [30]. Under low light conditions, shade-tolerant plants boost their light capture and utilization efficiency by optimizing their leaf chemical and structural composition compared to shade-intolerant species, including reduced , less investments of foliar nitrogen in Calvin cycle, photosynthetic electron transport proteins, and larger investment in lignin [31,32]. These evolutionary adaptations are pivotal for the enhancement of carbon sequestration in shade-tolerant species when light is scarce.
The spectrum of light significantly influences leaf development and growth, with specific wavelengths including red and blue light being integral for photosynthetic apparatus development and photomorphogenesis via phytochrome system transformations. The spectral composition of light, alters the chlorophyll content and nitrogen allocation within leaves, thereby influencing their morphophysiological traits and leading to variations in leaf chemical characteristics including pigment content and the ratio of photosystems, which are critical for optimizing light absorption and energy conversion efficiency under varying light conditions [27,33,34,35].
The blue light spectrum (400–500 nm) is particularly beneficial for the uptake and utilization of external nitrogen for photosynthesis and assimilation in leaves of Quercus variabilis, implying enhanced photosynthetic activity under blue light, while red light (600–700 nm) exposure results in a higher percentage of nitrogen (N) derived from internal sources compared to blue light [36]. Moreover, the green light spectrum contributes to physiological activities including stomatal closure and plastid downregulation, which can influence both photosynthesis and transpiration rates [36]. Interestingly, in Pinus sylvestris seedlings, it was found that red light (RL) notably enhances the biomass of both needles and root systems, outperforming the growth observed under white fluorescent light (WFL) control conditions. This increase in biomass under RL suggests a favorable effect on leaf development and overall plant growth. Moreover, the Pinus sylvestris seedlings exposed to RL and blue light (BL) experience lower rates of photosynthesis and respiration compared to those under blue–red light (BRL), with RL exhibiting the maximum difference between photosynthesis and respiration rates, which is crucial for optimal carbon balance [37].
Studies on various woody plants, including Asimina triloba, Aesculus glabra, Acer saccharum, Lindera benzoin, and Carpinus caroliniana indicated that high-intensity light within the spectrum of 400–700 nm can significantly enhance photosynthetic capacity in leaves. Canopy closure and light availability significantly influence leaf photosynthesis, during spring when the canopy is closing, light transmitted to the understory leaves decreases from 60% of above-canopy incident radiation to 35% after leaf drop in autumn. Photosynthetic capacity varied with leaf age and season. Young, expanding leaves at the top the crown exhibited the highest photosynthetic capacity and nitrogen content, whereas understory leaves showed a decline. Higher light levels in the spring support the development of photosynthetic apparatus in young leaves, leading to higher photosynthetic activity. Conversely, in autumn, declining light levels coincide with a decrease in photosynthetic capacity as leaves age and prepare for senescence [38].
Leaves exposed to more light exhibit increased thickness, more layers of palisade cells, and lower specific leaf area (SLA) compared to those grown in understory conditions, indicating structural adaptation to maximize light capture and minimize water loss, essential for photosynthesis and transpiration. Chlorophyll content, crucial for photosynthesis, shows a nonlinear negative relationship with leaf thickness, suggesting that thinner leaves may have higher chlorophyll concentration per unit weight, enhancing light absorption efficiency [39]. These adaptations reflect the plant’s response to optimize photosynthetic performance and water use efficiency under different light intensities.
A meta-analysis has shown that higher light intensity correlates with increased leaf thickness and enlarged mesophyll cell cross-sectional areas in woody plants [40]. Such morphological adaptations are instrumental in the amplification of photosynthetic efficiency across different light regimes. Distinct woody plant species implement disparate strategies for light adaptation. An exploration into the phenotypic responses of Japanese beech and alder revealed that beeches employ a strategy of synchronous multiple leaf outburst within a concentrated temporal frame, optimizing canopy architecture, including angled branches, to minimize self-shading and enhance light interception [41]. Alders, in contrast, adapt through the inclination and lateral extension of branches, particularly within the lower strata of the forest where light penetration is limited. Additionally, alders preferentially pursue horizontal branch growth to optimize sunlight reception under diminished light conditions.
2.2. Light Regulates Meristematic Processes of Woody Plants
The unveiling of the remarkable phenomenon of somatic embryogenesis, where carrot root cells in vitro could be coaxed into forming complete plants [42], catapulting plant tissue culture and micropropagation techniques into the research spotlight. The subsequent years have seen the successful application of these techniques to a variety of woody plant species, including poplars (Populus alba × grandidentata), eucalyptus (Eucalyptus globulus), larch (Larix olgensis), apples (Malus × domestica), and pears (Pyrus communis L.) [43,44,45,46]. These advancements have provided indispensable support for clonal propagation, genetic enhancement, and hybrid breeding programs for woody plants.
In the domain of plant tissue culture, the influence of light on the vegetative propagation and growth of woody plants is indispensable. Factors including light quality, intensity, and photoperiod profoundly impact the developmental trajectory of woody plants. Within the context of apple tissue cultures, the regulatory role of light quality on apical dominance and branching patterns in apple rootstocks has been underscored, the spectral quality of light has been demonstrated to affect axillary bud differentiation and shoot elongation rates [47,48]. It has been established that blue light intensifies apical dominance by temporarily arresting the development of lateral buds, thereby mitigating lateral growth. In contrast, red light can mitigate the influence of blue light, endorsing bud growth and branching, attributed to the lower red-to-far-red light ratio (R:FR ratio) inducing a shade avoidance response; higher R:FR ratios produce the converse effect. Further empirical evidence suggests that light quality regulates the development of individual and clustered branches in apple rootstocks, affecting bud formation and internodal length. Photoreceptor activity, particularly that of phytochromes (PHYs), is pivotal, where heightened PHY activity favors bud leaf formation while curtailing internode lengthening. The combined application of red and far-red light has been associated with increased branch multiplication, whereas blue and ultraviolet light enhanced cell cluster proliferation, and red, yellow, and green lights exhibited an inhibitory effect.
In apple, it has been discovered that the concurrent utilization of gibberellins under red light notably augmented the elongation of rootstocks, while under blue light, gibberellins favored shoot proliferation [49]. Under white light, however, gibberellins elicited a negligible impact on rootstock growth. This interplay between light and gibberellins hints at a possible synergistic mechanism in the in vitro propagation of woody plants.
An exploration into the tissue culture of the ‘Alshakr’ variety of date palm (Phoenix dactylifera L.) found that red and blue light-emitting diodes (CRB-LED) enhanced bud differentiation, phytochemical accumulation, and antioxidant enzyme activities [50]. Compared to conventional white fluorescent lighting, CRB-LED illumination resulted in an uptick of bud differentiation and growth, along with increased concentrations of carbohydrates, starch, amino acids, and peroxidase activity. Such findings affirm the potential of CRB-LED lighting in bolstering nutrient synthesis and antioxidant enzymatic functions in palm tissue cultures.
In studies concerning cherry tissue culture and ex vitro-grown seedlings, it has been found that root formation in vitro was contingent on the spectral quality illuminating the microcuttings [51]. Red light proved superior for root elongation relative to blue light, and under ex vitro conditions, red light-treated plants, as well as those exposed to a blend of red and blue light, exhibited the highest survival rates. Conversely, even though far-red light propelled internodal extension, it negatively affected root formation and length.
Moreover, it has been discovered that in Eucalyptus, donor plants exposed to far-red radiation yielded microcuttings with an elevated sugar profile, particularly soluble sugars and starch [52,53]. This enhanced carbohydrate content facilitated improved resource allocation during rooting, thereby ameliorating rooting efficacy.
2.3. Light Affects Xylem Development in Woody Plants
Studies have found that light has a significant impact on the development of xylem, especially secondary xylem. It has been reported that compared to the low light conditions, higher light density induces xylem differentiation in herbaceous species, potentially by promoting cell wall thickness and enhancing lignin deposition in xylem fibers [54,55]. Moreover, light quality was found to significantly affect the cell wall structure and composition in tomato seedlings, with distinct responses observed under different light conditions: blue light (BL) increased cell wall thickness to 256.8 nm, which is about three times thicker than under far-red light (FR), which had a thickness of only 80.5 nm, while white light (WL) also led to thick cell walls (223.9 nm) [56].
A comparative analysis on two Populus euramericana clones, ‘I-476’ and ‘Dorskamp’, examined the influence of light quality on regenerative capabilities, particularly focusing on the development of secondary xylem [57]. The study revealed differential responses to light spectra between the clones. ‘I-476’ demonstrated superior shoot regeneration under a 1:1 red-to-blue light ratio, with blue LED emission occurring at 440 nm and red at 650 nm (R50B50), indicating a more pronounced effect of balanced multispectral lighting on stem diameter growth and cellular differentiation. In contrast, ‘Dorskamp’ favored a red-shifted spectrum (R70B30) for maximal shoot induction, suggesting a genotype-specific requirement for red light in promoting growth. Monochromatic red light was shown to suppress bud initiation in ‘Dorskamp’, highlighting the necessity for a balanced spectral composition. The optimal regeneration of both clones was achieved under an equal red to blue light ratio. This finding underscores the importance of a judicious combination of red and blue wavelengths in enhancing the regenerative potential and facilitating the formation of secondary xylem in these clones.
Darkness can arrest the lignification process within the cell wall, thereby hindering secondary xylem development [58]. Additionally, a deficiency in light availability has been linked to a decrease in the formation of vascular tissues, compromising the transport efficiency of water and nutrients in the xylem. Grapevines exhibit a reduction in both the number and diameter of xylem vessels when exposed to low light conditions; this anatomical limitation translates to diminished hydraulic conductivity within the xylem, thereby directly impacting the plant’s water transport capabilities [59].
The knowledge on the regulatory factors of light influences on xylem development in woody plants remains superficial and limited; however, it has been unveiled recently that far-red light (730 nm) could play a crucial role in woody plant xylem development (Bao, unpublished).
3. Light Regulates Circadian Oscillation in Woody Plants
Light serves as a critical environmental cue in the orchestration of the plant circadian clock system, an intrinsic timing mechanism that empowers plants to anticipate and adapt to periodic fluctuations in their environment, including the diurnal cycle. The circadian clock regulates various physiological activities in woody plants. In the study conducted on Eucalyptus globulus trees, it was found that nocturnal stomatal conductance and sap flow were influenced by the circadian clock, especially during constant environmental conditions. Essentially, the circadian clock’s regulation of stomatal conductance at night plays a key role in determining how much water a tree uses [18]. The circadian clock functions in woody plants are influenced by environmental factors. Cold temperatures disrupt the normal function of the chestnut’s circadian clock, particularly affecting the primary oscillator feedback loop, which becomes non-functional at 4 °C or during winter [60].
In recent years, there has been an increase in research on the circadian system of woody plants, Figure 2 presents a model of the Populus circadian system, derived from Arabidopsis analogs [61,62,63,64,65,66,67,68,69] (Figure 2). Photoreceptors perceive morning far-red light and transduce the signal to LATE ELONGATED HYPOCOTYL 1 (LHY1) and LATE ELONGATED HYPOCOTYL 2 (LHY2). These proteins, together with TIMING OF CAB EXPRESSION 1 (TOC1), form a core oscillator, enhanced by factors that adjust timing and environmental responsiveness. The oscillator governs various outputs, including leaf movement, cold acclimation, water management, and seasonal adaptation. Auxin regulation is also clock-dependent. LHY1/LHY2 modulate cell proliferation through cytokinin and CYCLIN D3 (CYCD3), with auxin patterns suggesting evening clock regulation. Analogous to Arabidopsis’ EARLY BIRD (EBI), a nuclear factor affecting clock pace, Populus’ EBI1a/b modulates clock and growth, potentially by limiting the expression of LHY1 and LHY2 to morning, thus orchestrating growth timing [70,71].
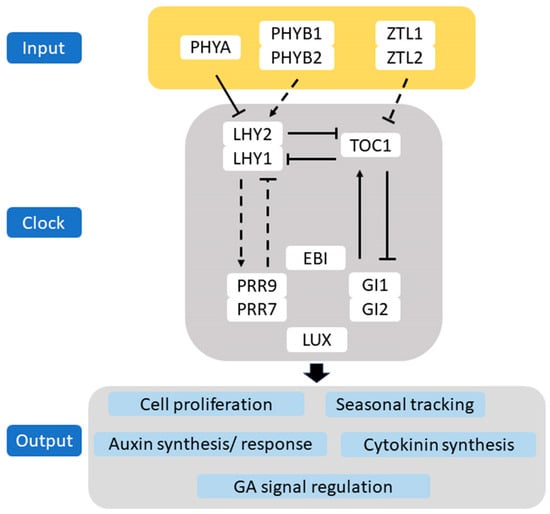
Figure 2.
A simplified model of the circadian system in Populus. The circadian clock aligns growth and physiological processes with the environmental cycle, day, and season. LHY1, LHY2, and TOC1 are fundamental to this oscillator, assisted by other elements. FLAVIN-BINDING, KELCH REPEAT, F BOX 1 (FKF1), and LOV KELCH PROTEIN 2 (LKP2) fulfill roles akin to ZEITLUPE(ZTL), maintaining oscillator tempo in its absence. ZTL influences both the circadian clock’s pace and phenological events like growth cessation and bud formation. PSEUDORESPONSE REGULATORS 7 (PRR7), PRR9, and TOC1 are expressed in waves between dawn and dusk. PRR7 and LUX ARRHYTHMO (LUX) are associated with bud set and leaf senescence. LHY1 and LHY2 orchestrate morning cell proliferation through cytokinin synthesis and CYCLIN D3 (CYCD3), while evening processes modulate auxin. EBI1a/b likely repress LHY1 and LHY2 at night. Arrows indicate stimulation; bars indicate inhibition. Established pathways are in black lines; hypothetical ones are in black dash lines.
As endogenous timekeeper, the circadian system is essential for aligning physiological processes with the external temporal landscape. Photoreceptors in plants, particularly phytochromes, are instrumental in transducing light signals into molecular messages that impact the expression of core circadian genes, thereby influencing a gamut of physiological and metabolic activities, including but not limited to flowering initiation, leaf expansion, stomatal regulation, and hormonal fluxes. These processes collectively fine-tune the efficiency of photosynthesis, water conservation, and energy utilization. The detection of photoperiod changes facilitates the plant’s transition between growth and dormancy states, highlighting the central role of light-mediated circadian clock modulation for plant fitness and reproductive success.
The pertinence of the circadian rhythm in governing perennial plant growth activities is exemplified in species including poplars. It has been demonstrated that the PHYTOCHROME A (PHYA) mediates the seasonal growth patterns in poplars by interfacing with the circadian clock’s temporal mechanism [65]. Attenuation of PHYA expression was associated with the cessation of growth and the premature setting of buds, an effect linked to the prolongation of the endogenous circadian period. PHYA is implicated in the transduction of light signals, modulating the transcriptional levels of circadian- and growth-related genes, including LATE ELONGATED HYPOCOTYL 1 (LHY1), FLAVIN-BINDING, KELCH REPEAT, F BOX 1 (FKF1), and FT, thus implicating a synergistic function of PHYA in circadian governance and photoperiodic growth response.
In poplars, the length of nocturnal intervals acts as a repressive signal that informs the photoperiodic integrator gene FT2 via the induction of the circadian regulator LHY2 [67]. The diurnal modulation of LHY2 expression, particularly its rapid downregulation under continuous light or upregulation in constant darkness, is indicative of a direct correlation with night length. This dynamic contributes to poplar’s photoperiod-responsive growth cessation by mediating the interplay between LHY2 expression and the nocturnal duration.
The circadian mechanism in poplars was discovered to meticulously regulate the plant growth cycle by modulating the expression of genes associated with cell division and auxin dynamics [63]. Suppression of key circadian genes, LHY1 and LHY2, truncated the circadian period, linking the expression of poplar CYCD3, a gene critical for cell division, to circadian regulation. Additionally, circadian control extends to phytohormone biosynthesis, with cytokinins accumulating during the daylight hours to regulate cellular division and expansion.
Some of the plant hormone gibberellic acids (GAs), namely GA1 and GA4, are integral to the photoperiodic and circadian regulation of poplar growth [72]. GA operates through two concurrent pathways: one where FT2 directly modulates photoperiodic response and another where GA bioactivity influences the photoperiodic/circadian rhythm axis. The harmonization of these pathways is crucial for the appropriate timing of growth cessation. Furthermore, GA concentrations modulate the responsiveness of poplars to short-day stimuli, affecting the timely induction of dormancy.
ALAN may influence phenology by changing plant perception of daylength and disturbing the circadian rhythms of plants [73]. It was elucidated that artificial light at night (ALAN), including street lights, vehicle lights, and building light-emitting diode (LED) light, exerts a pronounced effect on the phenological shifts of deciduous trees [74]. The investigation discerned that during the time from 2012 to 2016 in the conterminous United States, the presence of ALAN precipitated an advancement of budburst in deciduous trees by an average of 8.9 days in spring and delayed the autumnal leaf color transition by an average of 6.0 days. The magnitude of this effect is contingent upon the intensity of ALAN, with notable interactions with ambient temperature. At cooler temperatures (≤21 °C), ALAN was associated with a delay in leaf color change by approximately 15.4 days, whereas at elevated temperatures (≥22 °C), there was an advancement in this phenological event by about 5.7 days [74]. These findings suggest that ALAN modifies the perception of photoperiods and disrupts circadian rhythms, thereby altering the initiation and duration of phenological stages in plants.
4. Light Regulates Seasonal Growth in Woody Plants
In the diverse tapestry of environmental cues that guide plant growth, light signal is a paramount factor. Plants harness light as a sensory signal, orchestrating their growth and developmental trajectories, thereby deftly navigating the adaption to seasonal changes. The role of light in modulating plant seasonal growth is particularly pronounced in temperate and polar regions, where the ability to adapt to marked seasonal shifts is indispensable for survival [75,76,77,78,79]. Diminished light intensity and truncated photoperiods serve as harbingers of winter, eliciting a suite of adaptive responses in plants, notably the curtailment of growth, the formation of terminal buds, and the onset of dormancy—a survival strategy that conserves resources until favorable conditions return in spring.
PHYA plays a pivotal role in enabling woody plants to discern photoperiodic shifts, positing that PHYA modulates growth responses to shortening days by influencing gibberellin levels [79]. Alongside gibberellin, ethylene and ABA have been implicated in the induction of dormancy by short-day photoperiods in woody species [80]. ABA signal was demonstrated to modulate photoperiod-controlled seasonal growth by mediating changes in cellular communication within poplar tissues [81]. In response to photoperiodic shifts, alterations in ABA concentrations translate into modifications in cellular communicative dynamics, precipitating profound shifts in growth state.
In poplars with PHYA knocked down, a lengthened circadian rhythm, particularly under abbreviated photoperiods, precipitates an earlier termination of growth and bud dormancy onset [61]. Conversely, poplars overexpressing PHYA display a marked divergence from wild-type counterparts; they undergo accelerated internode elongation and maintain active apical buds under short-day conditions, with a propensity for active branching [82]. These findings further demonstrate that the dormancy and seasonal growth of poplar trees are closely related to the photoperiodism.
Norway spruce was discovered to respond to variable daylight duration and temperature across latitudes, and the growth cessation and bud set were primarily dictated by shortening daylight periods [83]. The cessation of growth layer expansion in the trunk and the initiation of needle primordia within the terminal buds coincided with an augmentation of cold resistance. Norway spruce modulates its growth–dormancy cycle by manipulating the bud–branch barrier in response to photoperiodic changes [84]. Long-day conditions were associated with the presence of highly methyl-esterified pectins in apical cell walls, promoting water conductivity, whereas short-day conditions induced pectin de-esterification, callose deposition, and xylem connection blockade, curtailing water transport into winter buds. The reversal of these processes under long-day conditions conducive to bud break, coupled with increased transcription of aquaporin proteins, underscores a sophisticated regulatory mechanism.
Carbon dynamics are tightly linked with light and play a role in the process of woody plant dormancy, with presented evidence that carbon scarcity, a consequence of inadequate daylight, leads to a stark reduction in cell division within buds, heralding dormancy [85]. This interplay between environmental light availability and carbon economics showcases a delicate balance upon which the dormancy and vitality of woody plants pivot.
5. Light Regulates Gene Expressions in Woody Plants
The transduction of light signals into meaningful biological responses is a complex and nuanced process in woody plants, involving an orchestration of transcriptional regulation that modulates the expression of a myriad of light-responsive genes. At the core of this process are photoreceptors, including phytochromes, that are exquisitely sensitive to specific wavelengths of light [86]. Upon absorption of light, these photoreceptors undergo conformational changes that trigger downstream signaling cascades, culminating in the modulation of transcription factor activity [87]. These transcription factors, once activated, seek out and bind to specific DNA sequences known as light-responsive elements. These elements are strategically positioned in the promoter regions of genes, which serve as molecular switchboards controlling when and where genes are expressed. The binding of transcription factors to these elements can either jump-start or suppress the transcription of adjacent genes, depending on the nature of the light signal received and the physiological needs of the plant.
By fine-tuning the transcription of genes related to critical physiological processes—including photosynthesis, the maintenance of circadian rhythms, the timing of flowering, and the modulation of hormone signaling pathways—plants can adapt their growth, development, and metabolism to the ever-changing light environment [88,89].
5.1. The Molecular Mechanism of Light-Induced Dormancy in Woody Plants
The perception of light signal is a sophisticated process in plants, mediated by phytochromes (PHYs), photoresponsive proteins that enable plants to attune their growth and developmental processes to the ambient light environment. In the plant model organism Arabidopsis thaliana, five PHY genes (PHYA-PHYE) serve the function of attuning growth and developmental processes [90,91]. In contrast, the genome of the model woody plant poplar encodes three phytochrome genes: PHYA, PHYB1, and PHYB2 [92]. Notably, poplar’s PHYB1 and PHYB2 share a high degree of amino acid similarity and act synergistically in the regulation of the shade avoidance response, yet they possess divergent roles. PHYB1 is the principal regulator of poplar’s shade avoidance, while PHYB2 predominantly governs seasonal and elongation growth, modulating the plant’s response under dynamic light conditions [93,94].
The influence of PHYBs on growth is tightly coupled to photoperiodic conditions. Under long-day exposure, PHYBs suppress the activity of PIF4 and PIF8 transcription factors, thereby constraining growth. Conversely, as days shorten, the diminished activity of PHYBs facilitates increased PIF activity, promoting growth. In the context of autumnal growth cessation, PHYBs regulate PIF8 to suppress the function of FT2, thereby encouraging growth cessation and bud dormancy [76,94,95,96]. In the spring, the regulatory effects of PHYB on FT1 and CENTRORADIALIS(CENL1) genes contribute to dormancy release and bud break [97] (Figure 3A,B).
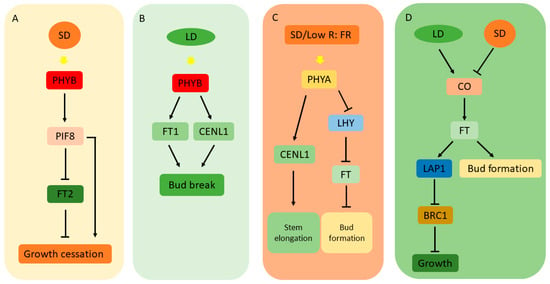
Figure 3.
(A) PHYB regulates growth cessation by modulating PIF8 protein function. (B) PHYB contributes to bud break by upregulating FT1 and CENL1 under long-day conditions. (C) PHYA, as a far-red light receptor, positively regulates stem elongation through CENL1 protein, while it negatively regulates bud formation through LHY/FT pathway. (D) CO/FT module coordinates growth and bud formation under different photoperiods. Yellow arrow indicates perceived light. Arrows indicate stimulation; bars indicate inhibition.
PHYA, a far-red light receptor, is integral to the regulation of the biological clock and seasonal growth in poplar. Under short-day conditions conducive to growth cessation, PHYA expression is modulated accordingly. The suppression of PHYA via RNAi in poplar prompts bud formation and growth cessation, through the regulation of LHY1 transcription and the induction of FKF1 and FT transcription [79,97]. Notably, overexpression of the oat PHYA gene in poplar enhances the expression of CENL1 in the rib meristem and adjacent tissues, fueling stem elongation and indicating the role of PHYA in modulating poplar’s photoperiod sensitivity and dormancy transition (Figure 3C).
The FT1 and FT2 genes are pivotal in the annual growth cycle of trees, each with disparate roles [98]. FT1, primarily expressed during the dormant winter season, is implicated in the initiation of reproductive growth, responsive to cold. In contrast, FT2, with its peak expression during the growing season, promotes vegetative growth and suppresses bud formation in response to warm temperatures and longer daylight hours [76,99]. The mobility of FT1 and FT2 also differs; FT1 remains localized, whereas FT2 is transmissible through grafting, highlighting their unique functional dynamics.
The CRISPR-Cas9 knockout mutants of FT2 exhibit severely dwarfed phenotypes, underscoring their essential roles in tree growth cycles [76]. In Norway spruce, the expression of FT4 gene is integral to the photoperiod and temperature-responsive growth rhythm. FT4 of Norway spruce is upregulated under short-day conditions, with its expression in leaves triggering a signaling cascade that influences apical tissues, analogous to angiosperm FT genes [83].
The flowering time in woody plants is coordinated by the CO/FT module, where the CONSTANS (CO) gene is central to the flowering response under long-day conditions. The diurnal pattern of CO expression in poplar parallels that in Arabidopsis, with CO and FT genes interacting under specific photoperiods to regulate flowering and seasonal growth [75,76]. In poplars, the biological clock member LHY2 modulates FT2 expression responsive to night length. Under long-day conditions, the low expression of LHY2 renders FT2 susceptible to activation by CO, while long nights induce LHY2, culminating in FT2 suppression, likely through LHY2 binding to the FT2’s regulatory region [67]. Furthermore, the BRANCHED1 (BRC1) protein regulates FT2 protein function in poplar, controlling branching and growth cessation under short-day conditions by interacting with FT2 and suppressing its function. BRC1 also represses the expression of LIKE-AP1 (LAP1) and AINTEGUMENTA LIKE 1 (AIL1), components downstream of the FT pathway essential for the short-day response [100,101,102,103] (Figure 3D).
The roles of the FD homologs FD-like 1 (FDL1) and FD-like 1 (FDL2) in hybrid poplar have been elucidated. The FDL1-FT protein complex predominantly governs photoperiod-driven seasonal growth, while FDL1 also contributes to adaptive responses and bud meristem maturation, potentially through interactions with the ABSCISIC ACID INSENSITIVE 3 (ABI3) transcription factor within the ABA signaling pathway [81]. Seasonal shifts, including decreased daylight, elevated ABA concentrations, which influence plant growth patterns, prompting growth retardation and dormancy in preparation for winter conditions [104].
5.2. Plant Hormone-Mediated Light Signal Transduction
In woody plants, the intricate ballet of growth and development is choreographed by a symphony of hormonal signals, with light serving as the conductor. The levels of auxin and the response to auxin signaling have been proven to be closely associated with the activity of cambial cells in woody plants [105,106] (Figure 4A). In poplar, the transition of the apical meristem from activity to dormancy under short-day conditions entails a meticulous regulation of auxin responsiveness. This transition is facilitated by the modulation of auxin response factors (ARFs), where the upregulation of repressive ARFs and downregulation of active ARFs occur during growth cessation. The dormancy phase is marked by the disruption of the AUX/indole-3-acetic acid (IAA) protein-associated SKP-Cullin-F-boxTIR (SCFTIR) complex, possibly increasing the stability of repressive auxin proteins [107]. Concurrently, microarray analyses have underscored the downregulation of auxin-responsive genes under short-day conditions—genes that pivotally influence processes including cold tolerance, starch degradation, and storage protein accumulation. Circadian rhythms also play a pivotal role in modulating these auxin-related genes, thus influencing the plant’s response to light and short-day-induced cessation of growth and dormancy [108].
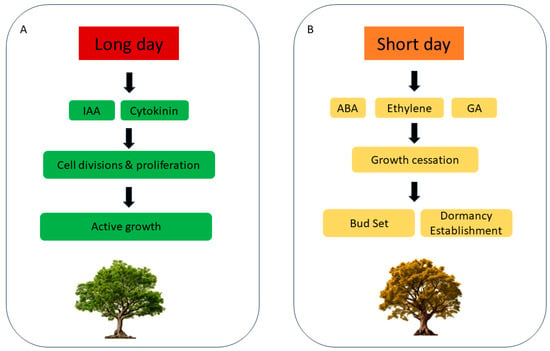
Figure 4.
(A) IAA and cytokinin prompt cell divisions and cell proliferation during long days. (B) Gibberellin, ethylene and ABA are involved in the induction of dormancy by short-day photoperiods in woody species.
In Eucalyptus, gene expression analyses of microcuttings have revealed a coordinated modulation of auxin signaling and sugar metabolism-related genes under varied light treatments [53]. Far-red light, in particular, enhances rooting ability, and this is coupled with the upregulation of auxin transport genes including Auxin Resistant 1 (AUX1), PIN-FORMED 1 (PIN1) and PIN-FORMED 2 (PIN2), ARFs including ARF6 and ARF8, and Argonaute1 (AGO1)—a gene implicated in rooting processes and possibly mitigating rooting inhibition through the negative regulation of ARF17. This is complemented by the increased expression of genes pivotal to carbohydrate transport and metabolism at the stem base following far-red light exposure [53].
Cytokinins, another class of hormones, along with cell division-related genes, are key players in integrating light signals, circadian rhythms, and growth in poplar (Figure 4A). The circadian clock, through LHY genes, modulates CYCLIN D3 genes, essential for cell division and proliferation. These genes, potentially through the mediation of B-BOX DOMAIN PROTEIN 19 (BBX19) and B-BOX DOMAIN PROTEIN 32 (BBX32) proteins, form part of the growth timing mechanism. Additionally, the CYCD3 promoter’s circadian elements and evening elements—acting in concert with transcription factors including CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) and LHY—regulate gene expression, ensuring that growth activities including cell division transpire at the optimal time, for instance, during the night, to minimize DNA damage from harmful solar radiation [63].
Gibberellin signaling plays a critical role in woody plant responses to short-day conditions. In poplar, the application of exogenous gibberellins can postpone growth cessation under short days (Figure 4B). Overexpression of genes involved in gibberellin biosynthesis and signaling results in elevated levels of bioactive gibberellins, which allows trees to sustain growth under short days and display insensitivity to FT2 expression levels [71].
Transcriptomic analyses have unveiled that during the progression from bud set to full dormancy induced by short days in poplar, the ethylene signaling pathway is activated by the photoperiod-related reduction in hexose levels, interfacing between light and ABA signals [80] (Figure 4B). Within a couple of weeks of short-day exposure, there is an induction of ethylene biosynthesis and response genes, including ACC SYNTHASE6 (ACS6), ETHYLENE TRIPLE RESPONSE2 (ETR2), ETHYLENE RESPONSE SENSOR1(ERS1), CONSTITUTIVE TRIPLE RESPONSE1 (CTR1), ETHYLENE INSENSITIVE3(EIN3), ETHYLENE RESPONSE FACTOR4 (ERF4), and ETHYLENE RESPONSE FACTOR5 (ERF5). Subsequently, the ABA signaling pathway peaks in activity, as evidenced by the pronounced upregulation of key ABA biosynthesis and signaling genes including NINE-CIS-EPOXYCAROTENOID DIOXYGENASE3 (NCED3), ABA DEFICIENT 2 (ABA2), MITOGEN-ACTIVATED PROTEIN KINASE3 (MPK3), FAR-RED-INSENSITIVE219 (FIN219), ABSCICIACID-INSENSITIVE1 (ABI1), and PROTEIN PHOSPHATASE2C (PP2C) [80,82,85,109]. Notably, alterations in the expression and functionality of the ethylene receptor ETHYLENE TRIPLE RESPONSE 1 (ETR1) impact bud morphogenesis and dormancy establishment [110]. A decrease in ETR1 expression or function, or an increase in ABI3 expression within the ABA signaling pathway, can prevent closed apical bud formation under short-day conditions, signifying that while dormancy is being established, bud formation is a partially independent process [80].
In summary, upon initiation of short-day light signals, a cascade of hormonal signals—ethylene followed by ABA—is triggered. Ethylene signaling paves the way for ABA signaling enhancement, which in turn promotes the entry of apical buds into dormancy by regulating various transcription factors. ABA concludes bud development by advancing dormancy and equipping the plant to withstand environmental stresses including low temperatures and dehydration. Together, ethylene and ABA orchestrate the formation of apical buds, environmental stress adaptation, and the culmination of dormancy, demonstrating the complexity and finesse of hormonal regulation in woody plants’ growth and development in response to light signals.
6. Discussion
Light is a pivotal environmental cue that profoundly shapes the morphology and development of woody plants, playing a multifaceted role that transcends simple energy provision for photosynthesis. The essence of plant growth, photosynthesis, harnesses light energy through the pigment chlorophyll in chloroplasts, transforming it into chemical energy that fuels developmental processes. The nuances of light exposure—quality, intensity, and duration—dictate photosynthetic efficiency and, consequently, growth patterns. In high-light environments, for instance, enhanced photosynthesis promotes rapid growth and increased branching, a testament to the adaptive capacity of plants to maximize light utilization. Through this process, plants make intricate adjustments in leaf architecture and biochemistry, including variations in leaf thickness and chlorophyll content, optimizing light capture and energy output. Light-induced morphological modifications extend beyond foliage, manifesting in buds, roots, and even the vascular architecture of xylem.
Integral to the plant’s adaptation to light is the circadian system, a temporal framework that synchronizes endogenous biological rhythms with the external cycle of day and night. Photoreceptors, particularly PHYA and PHYB, are key components in aligning the seasonal growth of plants with photoperiodic cues. In poplars, these photoreceptors regulate gene families such as FT and LHY, orchestrating the tree’s response to day length and the ensuing growth–dormancy cycles.
At the molecular helm of light signal regulation of gene transcription in woody plants lie light-responsive genes and transcription factors. The FT gene family, for instance, is a cornerstone in dictating the growth cycle, flowering timing, and seasonal responsiveness. Similarly, the CO gene is an integral determinant of flowering time, finely tuned to photoperiod fluctuations. These genes modulate the plant’s sensitivity to light cycles through interactions with transcription factors, ultimately sculpting plant growth patterns.
To decode complex light-dependent regulatory networks, understanding light perception mechanisms is essential. Woody plants are equipped with light receptors like phytochromes, which intercept light signals and transform them into biological messages that steer gene expression. These messages cascade through various physiological and metabolic processes, influencing the orchestration of photosynthesis, flowering, dormancy cycles, stomatal dynamics, and hormonal fluctuations.
Distinct from herbaceous models such as Arabidopsis thaliana, woody plants like poplars possess a reduced set of phytochromes, hinting at specialized functions and regulatory mechanisms tailored to support characteristics like seasonal growth. Investigating the unique roles of phytochromes and their associated transcription factors in woody plants promises to be a burgeoning area of research, offering fresh insights into light signal transduction.
7. Conclusions and Perspectives
Understanding the role of plant photoreceptors is a crucial part of elucidating the mechanism of plant light signal transduction. However, in woody plants, beyond phytochromes, there is very limited knowledge about other photoreceptors such as cryptochromes, phototropins, and the UV-B receptor. With the increasing amount of research on light signal transduction processes and pathways in woody plants in recent years, it is imperative that these other photoreceptors, beyond phytochromes, are devoted greater scrutiny in future research.
Secondary xylem, commonly referred to as wood, forms the predominant component of mature woody stems and roots. The mechanism of xylem development is a critical aspect in the study of woody plants, yet the molecular mechanisms by which light and light signaling regulate xylem development remain unclear. It is anticipated that future research will be able to shed light on the regulatory factors of light influence, including spectral composition, irradiance, and duration in regulating xylem development in woody plants.
The regulation of growth and development in woody plants by light involves various applied perspectives crucial for forestry, horticulture, and environmental conservation. As a pivotal environmental cue, light influences numerous aspects of woody plant physiology, morphology, and ecology, guiding their lifecycle from seed germination to maturity. A thorough comprehension of these mechanisms is essential for optimizing practices related to plant cultivation, breeding, and habitat restoration. The applied perspectives of light regulation in the growth and development of woody plants are fundamental to realizing targeted goals, such as augmenting productivity in agriculture and forestry, improving urban environments, or preserving natural ecosystems. Advances in research and technology continue to refine our understanding and manipulation of light’s role in woody plant biology, paving the way for more effective and sustainable practices.
Author Contributions
Conceptualization, Y.B. and X.L.; Visualization, C.-H.F. and M.-X.N.; Writing—review and editing, C.L. and H.-L.W. Manuscript and figures were critically revised by W.Y. and X.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Key R&D Program of China during the 14th Five-year Plan Period (2021YFD2200103) and the National Natural Science Foundation of China (32071734).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data availability statement is not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Darko, E.; Heydarizadeh, P.; Schoefs, B.; Sabzalian, M.R. Photosynthesis under artificial light: The shift in primary and secondary metabolism. Philos. Trans. R Soc. Lond. B Biol. Sci. 2014, 369, 20130243. [Google Scholar] [CrossRef] [PubMed]
- de Wit, M.; Galvao, V.C.; Fankhauser, C. Light-Mediated Hormonal Regulation of Plant Growth and Development. Annu. Rev. Plant Biol. 2016, 67, 513–537. [Google Scholar] [CrossRef]
- Luyssaert, S.; Schulze, E.D.; Borner, A.; Knohl, A.; Hessenmoller, D.; Law, B.E.; Ciais, P.; Grace, J. Old-growth forests as global carbon sinks. Nature 2008, 455, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.W.; Hanan, N.P.; Prihodko, L.; Anchang, J.; Ji, W.; Yu, Q. Woody-biomass projections and drivers of change in sub-Saharan Africa. Nat. Clim. Change 2021, 11, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, Y.; Shi, L.; Wang, L.; Li, W. Interaction of Phytohormones and External Environmental Factors in the Regulation of the Bud Dormancy in Woody Plants. Int. J. Mol. Sci. 2023, 24, 17200. [Google Scholar] [CrossRef]
- Olsen, J.E. Light and temperature sensing and signaling in induction of bud dormancy in woody plants. Plant Mol. Biol. 2010, 73, 37–47. [Google Scholar] [CrossRef]
- Osakabe, Y.; Kawaoka, A.; Nishikubo, N.; Osakabe, K. Responses to environmental stresses in woody plants: Key to survive and longevity. J. Plant Res. 2012, 125, 1–10. [Google Scholar] [CrossRef]
- De Baerdemaeker, N.J.F.; Salomon, R.L.; De Roo, L.; Steppe, K. Sugars from woody tissue photosynthesis reduce xylem vulnerability to cavitation. New Phytol. 2017, 216, 720–727. [Google Scholar] [CrossRef]
- De Roo, L.; Salomon, R.L.; Oleksyn, J.; Steppe, K. Woody tissue photosynthesis delays drought stress in Populus tremula trees and maintains starch reserves in branch xylem tissues. New Phytol. 2020, 228, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Liu, X.; Lu, W.; Chen, P.; Quan, M.; Si, J.; Du, Q.; Zhang, D. Genetic dissection of the gene coexpression network underlying photosynthesis in Populus. Plant Biotechnol. J. 2020, 18, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Cinnamon, Y.; Genin, O.; Yitzhak, Y.; Riov, J.; David, I.; Shaya, F.; Izhaki, A. High-resolution episcopic microscopy enables three-dimensional visualization of plant morphology and development. Plant Direct 2019, 3, e00161. [Google Scholar] [CrossRef] [PubMed]
- Iwabe, R.; Koyama, K.; Komamura, R. Shade Avoidance and Light Foraging of a Clonal Woody Species, Pachysandra terminalis. Plants 2021, 10, 809. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Bhalerao, R.P.; Eriksson, M.E. Growing in time: Exploring the molecular mechanisms of tree growth. Tree Physiol. 2021, 41, 657–678. [Google Scholar] [CrossRef]
- Song, Y.H.; Ito, S.; Imaizumi, T. Similarities in the circadian clock and photoperiodism in plants. Curr. Opin. Plant Biol. 2010, 13, 594–603. [Google Scholar] [CrossRef]
- Heide, O.M.; Prestrud, A.K. Low temperature, but not photoperiod, controls growth cessation and dormancy induction and release in apple and pear. Tree Physiol. 2005, 25, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, A.K.; Buonaiuto, D.M.; Chamberlain, C.J.; Morales-Castilla, I.; Wolkovich, E.M. Spatial and temporal shifts in photoperiod with climate change. New Phytol. 2021, 230, 462–474. [Google Scholar] [CrossRef]
- Ibanez, C.; Ramos, A.; Acebo, P.; Contreras, A.; Casado, R.; Allona, I.; Aragoncillo, C. Overall alteration of circadian clock gene expression in the chestnut cold response. PLoS ONE 2008, 3, e3567. [Google Scholar] [CrossRef]
- Resco de Dios, V.; Diaz-Sierra, R.; Goulden, M.L.; Barton, C.V.M.; Boer, M.M.; Gessler, A.; Ferrio, J.P.; Pfautsch, S.; Tissue, D.T. Woody clockworks: Circadian regulation of night-time water use in Eucalyptus globulus. New Phytol. 2013, 200, 743–752. [Google Scholar] [CrossRef]
- Dong, X.; Jiang, X.; Kuang, G.; Wang, Q.; Zhong, M.; Jin, D.; Hu, J. Genetic control of flowering time in woody plants: Roses as an emerging model. Plant Divers. 2017, 39, 104–110. [Google Scholar] [CrossRef]
- Gendron, J.M.; Leung, C.C.; Liu, W. Energy as a seasonal signal for growth and reproduction. Curr. Opin. Plant Biol. 2021, 63, 102092. [Google Scholar] [CrossRef]
- Shim, D.; Ko, J.H.; Kim, W.C.; Wang, Q.; Keathley, D.E.; Han, K.H. A molecular framework for seasonal growth-dormancy regulation in perennial plants. Hortic. Res. 2014, 1, 14059. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Bae, E.K.; Tran, T.N.A.; Lee, H.; Ko, J.H. Exploring the Seasonal Dynamics and Molecular Mechanism of Wood Formation in Gymnosperm Trees. Int. J. Mol. Sci. 2023, 24, 8624. [Google Scholar] [CrossRef]
- Petterle, A.; Karlberg, A.; Bhalerao, R.P. Daylength mediated control of seasonal growth patterns in perennial trees. Curr. Opin. Plant Biol. 2013, 16, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Flynn, D.F.B.; Wolkovich, E.M. Temperature and photoperiod drive spring phenology across all species in a temperate forest community. New Phytol. 2018, 219, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.G.; Ma, Q.; Rossi, S.; Biondi, F.; Deslauriers, A.; Fonti, P.; Liang, E.; Makinen, H.; Oberhuber, W.; Rathgeber, C.B.K.; et al. Photoperiod and temperature as dominant environmental drivers triggering secondary growth resumption in Northern Hemisphere conifers. Proc. Natl. Acad. Sci. USA 2020, 117, 20645–20652. [Google Scholar] [CrossRef] [PubMed]
- Kami, C.; Lorrain, S.; Hornitschek, P.; Fankhauser, C. Light-regulated plant growth and development. Curr. Top. Dev. Biol. 2010, 91, 29–66. [Google Scholar] [CrossRef] [PubMed]
- Hallik, L.; Niinemets, U.; Kull, O. Photosynthetic acclimation to light in woody and herbaceous species: A comparison of leaf structure, pigment content and chlorophyll fluorescence characteristics measured in the field. Plant Biol. 2012, 14, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Stoll, P.; Schmid, B. Plant foraging and dynamic competition between branches of Pinus sylvestris in contrasting light environments. J. Ecol. 2002, 86, 934–945. [Google Scholar] [CrossRef]
- King, D.A. Relationship between Crown Architecture and Branch Orientation in Rain Forest Trees. Ann. Bot. 1998, 82, 1–7. [Google Scholar] [CrossRef][Green Version]
- Niinemets, U.; Kull, O.; Tenhunen, J.D. An analysis of light effects on foliar morphology, physiology, and light interception in temperate deciduous woody species of contrasting shade tolerance. Tree Physiol. 1998, 18, 681–696. [Google Scholar] [CrossRef]
- Seemann, J.R.; Sharkey, T.D.; Wang, J.; Osmond, C.B. Environmental effects on photosynthesis, nitrogen-use efficiency, and metabolite pools in leaves of sun and shade plants. Plant Physiol. 1987, 84, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.R. Photosynthetic acclimation and nitrogen partitioning within a lucerne canopy. I. Canopy characteristics. Funct. Plant Biol. 1993, 20, 55–67. [Google Scholar] [CrossRef]
- Arena, C.; Tsonev, T.; Doneva, D.; De Micco, V.; Michelozzi, M.; Brunetti, C.; Centritto, M.; Fineschi, S.; Velikova, V.; Loreto, F. The effect of light quality on growth, photosynthesis, leaf anatomy and volatile isoprenoids of a monoterpene-emitting herbaceous species (Solanum lycopersicum L.) and an isoprene-emitting tree (Platanus orientalis L.). Environ. Exp. Bot. 2016, 130, 122–132. [Google Scholar] [CrossRef]
- Tripathi, S.; Bhadouria, R.; Srivastava, P.; Devi, R.S.; Chaturvedi, R.; Raghubanshi, A.S. Effects of light availability on leaf attributes and seedling growth of four tree species in tropical dry forest. Ecol. Process. 2020, 9, 2. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Li, J.; Wu, P.; Hu, D.; Zhong, Q.; Cheng, D. Respiration in light of evergreen and deciduous woody species and its links to the leaf economic spectrum. Tree Physiol. 2024, 44, tpad129. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, J.; He, C.; Wang, Q. Effects of light spectra and 15N pulses on growth, leaf morphology, physiology, and internal nitrogen cycling in Quercus variabilis Blume seedlings. PLoS ONE 2021, 16, e0243954. [Google Scholar] [CrossRef]
- Pashkovskiy, P.; Kreslavski, V.D.; Ivanov, Y.; Ivanova, A.; Kartashov, A.; Shmarev, A.; Strokina, V.; Kuznetsov, V.V.; Allakhverdiev, S.I. Influence of Light of Different Spectral Compositions on the Growth, Photosynthesis, and Expression of Light-Dependent Genes of Scots Pine Seedlings. Cells 2021, 10, 3284. [Google Scholar] [CrossRef]
- Augspurger, C.K.; Cheeseman, J.M.; Salk, C.F. Light gains and physiological capacity of understorey woody plants during phenological avoidance of canopy shade. Funct. Ecol. 2005, 19, 537–546. [Google Scholar] [CrossRef]
- Cao, K.F. Leaf anatomy and chlorophyll content of 12 woody species in contrasting light conditions in a Bornean heath forest. Can. J. Bot. 2000, 78, 1245–1253. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Ntagkas, N.; Siebenkäs, A.; Mäenpää, M.; Matsubara, S.; Pons, T. A meta-analysis of plant responses to light intensity for 70 traits ranging from molecules to whole plant performance. New Phytol. 2019, 223, 1073–1105. [Google Scholar] [CrossRef] [PubMed]
- Kikuzawa, K. Phenological and morphological adaptations to the light environment in two woody and two herbaceous plant species. Funct. Ecol. 2003, 17, 29–38. [Google Scholar] [CrossRef]
- Steward, F.C.; Mapes, M.O.; Mears, K. Growth and Organized Development of Cultured Cells. II. Organization in Cultures Grown from Freely Suspended Cells. Am. J. Bot. 1958, 45, 705–708. [Google Scholar] [CrossRef]
- Wilson, P.J. Multiplication Rates In Vitro and by Stem Cuttings Propagation, and Clonal Development from Eucalyptus globulus Seedlings. For. Sci. 1996, 42, 415–418. [Google Scholar] [CrossRef]
- Bommineni, V.R.; Mathews, H.; Samuel, S.B.; Kramer, M.; Wagner, D.R. A New Method for Rapid In Vitro Propagation of Apple and Pear. HortScience 2001, 36, 1102–1106. [Google Scholar] [CrossRef]
- Fillatti, J.J.; Sellmer, J.; McCown, B.; Haissig, B.; Comai, L. Agrobacterium mediated transformation and regeneration of Populus. Mol. Gen. Genet. 1987, 206, 192–199. [Google Scholar] [CrossRef]
- Song, Y.; Bai, X.; Dong, S.; Yang, Y.; Dong, H.; Wang, N.; Zhang, H.; Li, S. Stable and Efficient Agrobacterium-Mediated Genetic Transformation of Larch Using Embryogenic Callus. Front. Plant Sci. 2020, 11, 584492. [Google Scholar] [CrossRef]
- Muleo, R.; Morini, S. Light quality regulates shoot cluster growth and development of MM106 apple genotype in in vitro culture. Sci. Hortic. 2006, 108, 364–370. [Google Scholar] [CrossRef]
- Muleo, R.; Morini, S. Physiological dissection of blue and red light regulation of apical dominance and branching in M9 apple rootstock growing in vitro. J. Plant Physiol. 2008, 165, 1838–1846. [Google Scholar] [CrossRef] [PubMed]
- Geng, F.; Moran, R.; Day, M.; Halteman, W.; Zhang, D. In Vitro Shoot Proliferation of Apple Rootstocks ‘B.9’, ‘G.30’, and ‘G.41’ Grown under Red and Blue Light. HortScience 2015, 50, 430–433. [Google Scholar] [CrossRef]
- Al-Mayahi, A.M. Effect of red and blue light emitting diodes “CRB-LED” on in vitro organogenesis of date palm (Phoenix dactylifera L.) cv. Alshakr. World J. Microbiol. Biotechnol. 2016, 32, 160. [Google Scholar] [CrossRef] [PubMed]
- Iacona, C.; Muleo, R. Light quality affects in vitro adventitious rooting and ex vitro performance of cherry rootstock Colt. Sci. Hortic. 2010, 125, 630–636. [Google Scholar] [CrossRef]
- Ruedell, C.M.; de Almeida, M.R.; Fett-Neto, A.G. Concerted transcription of auxin and carbohydrate homeostasis-related genes underlies improved adventitious rooting of microcuttings derived from far-red treated Eucalyptus globulus Labill mother plants. Plant Physiol. Biochem. 2015, 97, 11–19. [Google Scholar] [CrossRef]
- Ruedell, C.M.; de Almeida, M.R.; Schwambach, J.; Posenato, C.F.; Fett-Neto, A.G. Pre and post-severance effects of light quality on carbohydrate dynamics and microcutting adventitious rooting of two Eucalyptus species of contrasting recalcitrance. Plant Growth Regul. 2012, 69, 235–245. [Google Scholar] [CrossRef]
- Falcioni, R.; Moriwaki, T.; de Oliveira, D.M.; Andreotti, G.C.; de Souza, L.A.; Dos Santos, W.D.; Bonato, C.M.; Antunes, W.C. Increased Gibberellins and Light Levels Promotes Cell Wall Thickness and Enhance Lignin Deposition in Xylem Fibers. Front. Plant Sci. 2018, 9, 1391. [Google Scholar] [CrossRef]
- Ghosh, S.; Nelson, J.F.; Cobb, G.M.C.; Etchells, J.P.; de Lucas, M. Light regulates xylem cell differentiation via PIF in Arabidopsis. Cell Rep. 2022, 40, 111075. [Google Scholar] [CrossRef] [PubMed]
- Falcioni, R.; Moriwaki, T.; Perez-Llorca, M.; Munne-Bosch, S.; Gibin, M.S.; Sato, F.; Pelozo, A.; Pattaro, M.C.; Giacomelli, M.E.; Ruggeberg, M.; et al. Cell wall structure and composition is affected by light quality in tomato seedlings. J. Photochem. Photobiol. B 2020, 203, 111745. [Google Scholar] [CrossRef] [PubMed]
- Kwon, A.R.; Cui, H.-Y.; Lee, H.; Shin, H.; Kang, K.-S.; Park, S.-Y. Light quality affects shoot regeneration, cell division, and wood formation in elite clones of Populus euramericana. Acta Physiol. Plant. 2015, 37, 65. [Google Scholar] [CrossRef]
- Nečesaný, V. The effect of light deficiency on the formation of secondary xylem. Wood Sci. Technol. 1969, 3, 100–108. [Google Scholar] [CrossRef]
- Schultz, H.R.; Matthews, M.A. Xylem development and hydraulic conductance in sun and shade shoots of grapevine (Vitis vinifera L.): Evidence that low light uncouples water transport capacity from leaf area. Planta 1993, 190, 393–406. [Google Scholar] [CrossRef]
- Ibanez, C.; Kozarewa, I.; Johansson, M.; Ogren, E.; Rohde, A.; Eriksson, M.E. Circadian clock components regulate entry and affect exit of seasonal dormancy as well as winter hardiness in Populus trees. Plant Physiol. 2010, 153, 1823–1833. [Google Scholar] [CrossRef]
- Baudry, A.; Ito, S.; Song, Y.H.; Strait, A.A.; Kiba, T.; Lu, S.; Henriques, R.; Pruneda-Paz, J.L.; Chua, N.H.; Tobin, E.M.; et al. F-box proteins FKF1 and LKP2 act in concert with ZEITLUPE to control Arabidopsis clock progression. Plant Cell 2010, 22, 606–622. [Google Scholar] [CrossRef]
- Ding, J.; Bohlenius, H.; Ruhl, M.G.; Chen, P.; Sane, S.; Zambrano, J.A.; Zheng, B.; Eriksson, M.E.; Nilsson, O. GIGANTEA-like genes control seasonal growth cessation in Populus. New Phytol. 2018, 218, 1491–1503. [Google Scholar] [CrossRef]
- Edwards, K.D.; Takata, N.; Johansson, M.; Jurca, M.; Novak, O.; Henykova, E.; Liverani, S.; Kozarewa, I.; Strnad, M.; Millar, A.J.; et al. Circadian clock components control daily growth activities by modulating cytokinin levels and cell division-associated gene expression in Populus trees. Plant Cell Environ. 2018, 41, 1468–1482. [Google Scholar] [CrossRef]
- Jurca, M.; Sjolander, J.; Ibanez, C.; Matrosova, A.; Johansson, M.; Kozarewa, I.; Takata, N.; Bako, L.; Webb, A.A.R.; Israelsson-Nordstrom, M.; et al. ZEITLUPE Promotes ABA-Induced Stomatal Closure in Arabidopsis and Populus. Front. Plant Sci. 2022, 13, 829121. [Google Scholar] [CrossRef]
- Kozarewa, I.; Ibanez, C.; Johansson, M.; Ogren, E.; Mozley, D.; Nylander, E.; Chono, M.; Moritz, T.; Eriksson, M.E. Alteration of PHYA expression change circadian rhythms and timing of bud set in Populus. Plant Mol. Biol. 2010, 73, 143–156. [Google Scholar] [CrossRef]
- McKown, A.D.; Klapste, J.; Guy, R.D.; Geraldes, A.; Porth, I.; Hannemann, J.; Friedmann, M.; Muchero, W.; Tuskan, G.A.; Ehlting, J.; et al. Genome-wide association implicates numerous genes underlying ecological trait variation in natural populations of Populus trichocarpa. New Phytol. 2014, 203, 535–553. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Sanchez, J.M.; Triozzi, P.M.; Alique, D.; Geng, F.; Gao, M.; Jaeger, K.E.; Wigge, P.A.; Allona, I.; Perales, M. LHY2 Integrates Night-Length Information to Determine Timing of Poplar Photoperiodic Growth. Curr. Biol. 2019, 29, 2402–2406. [Google Scholar] [CrossRef] [PubMed]
- Takata, N.; Saito, S.; Saito, C.T.; Uemura, M. Phylogenetic footprint of the plant clock system in angiosperms: Evolutionary processes of pseudo-response regulators. BMC Evol. Biol. 2010, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Takata, N.; Saito, S.; Tanaka Saito, C.; Nanjo, T.; Shinohara, K.; Uemura, M. Molecular phylogeny and expression of poplar circadian clock genes, LHY1 and LHY2. New Phytol. 2009, 181, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.; McWatters, H.G.; Bako, L.; Takata, N.; Gyula, P.; Hall, A.; Somers, D.E.; Millar, A.J.; Eriksson, M.E. Partners in time: EARLY BIRD associates with ZEITLUPE and regulates the speed of the Arabidopsis clock. Plant Physiol. 2011, 155, 2108–2122. [Google Scholar] [CrossRef]
- Ashelford, K.; Eriksson, M.E.; Allen, C.M.; D’Amore, R.; Johansson, M.; Gould, P.; Kay, S.; Millar, A.J.; Hall, N.; Hall, A. Full genome re-sequencing reveals a novel circadian clock mutation in Arabidopsis. Genome Biol. 2011, 12, R28. [Google Scholar] [CrossRef]
- Eriksson, M.E.; Hoffman, D.; Kaduk, M.; Mauriat, M.; Moritz, T. Transgenic hybrid aspen trees with increased gibberellin (GA) concentrations suggest that GA acts in parallel with FLOWERING LOCUS T2 to control shoot elongation. New Phytol. 2015, 205, 1288–1295. [Google Scholar] [CrossRef]
- Briggs, W.R. Physiology of plant responses to artificial lighting. In Ecological Consequences of Artificial Night Lighting; Island Press: Washington, DC, USA, 2006; pp. 389–411. [Google Scholar]
- Meng, L.; Zhou, Y.; Roman, M.O.; Stokes, E.C.; Wang, Z.; Asrar, G.R.; Mao, J.; Richardson, A.D.; Gu, L.; Wang, Y. Artificial light at night: An underappreciated effect on phenology of deciduous woody plants. PNAS Nexus 2022, 1, pgac046. [Google Scholar] [CrossRef]
- Böhlenius, H.; Huang, T.; Charbonnel-Campaa, L.; Brunner, A.M.; Jansson, S.; Strauss, S.H.; Nilsson, O. CO/FT regulatory module controls timing of flowering and seasonal growth cessation in trees. Science 2006, 312, 1040–1043. [Google Scholar] [CrossRef]
- Andre, D.; Marcon, A.; Lee, K.C.; Goretti, D.; Zhang, B.; Delhomme, N.; Schmid, M.; Nilsson, O. FLOWERING LOCUS T paralogs control the annual growth cycle in Populus trees. Curr. Biol. 2022, 32, 2988–2996. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Adams, J.P.; Kim, H.; No, K.; Ma, C.; Strauss, S.H.; Drnevich, J.; Vandervelde, L.; Ellis, J.D.; Rice, B.M.; et al. FLOWERING LOCUS T duplication coordinates reproductive and vegetative growth in perennial poplar. Proc. Natl. Acad. Sci. USA 2011, 108, 10756–10761. [Google Scholar] [CrossRef] [PubMed]
- Triozzi, P.M.; Ramos-Sanchez, J.M.; Hernandez-Verdeja, T.; Moreno-Cortes, A.; Allona, I.; Perales, M. Photoperiodic Regulation of Shoot Apical Growth in Poplar. Front. Plant Sci. 2018, 9, 1030. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.E.; Junttila, O.; Nilsen, J.; Eriksson, M.E.; Martinussen, I.; Olsson, O.; Sandberg, G.; Moritz, T. Ectopic expression of oat phytochrome A in hybrid aspen changes critical daylength for growth and prevents cold acclimatization. Plant J. 2002, 12, 1339–1350. [Google Scholar] [CrossRef]
- Ruttink, T.; Arend, M.; Morreel, K.; Storme, V.; Rombauts, S.; Fromm, J.; Bhalerao, R.P.; Boerjan, W.; Rohde, A. A molecular timetable for apical bud formation and dormancy induction in poplar. Plant Cell 2007, 19, 2370–2390. [Google Scholar] [CrossRef] [PubMed]
- Tylewicz, S.; Tsuji, H.; Miskolczi, P.; Petterle, A.; Azeez, A.; Jonsson, K.; Shimamoto, K.; Bhalerao, R.P. Dual role of tree florigen activation complex component FD in photoperiodic growth control and adaptive response pathways. Proc. Natl. Acad. Sci. USA 2015, 112, 3140–3145. [Google Scholar] [CrossRef] [PubMed]
- Ruonala, R.; Rinne, P.L.; Kangasjarvi, J.; van der Schoot, C. CENL1 expression in the rib meristem affects stem elongation and the transition to dormancy in Populus. Plant Cell 2008, 20, 59–74. [Google Scholar] [CrossRef]
- Gyllenstrand, N.; Clapham, D.; Kallman, T.; Lagercrantz, U. A Norway spruce FLOWERING LOCUS T homolog is implicated in control of growth rhythm in conifers. Plant Physiol. 2007, 144, 248–257. [Google Scholar] [CrossRef]
- Lee, Y.; Karunakaran, C.; Lahlali, R.; Liu, X.; Tanino, K.K.; Olsen, J.E. Photoperiodic Regulation of Growth-Dormancy Cycling through Induction of Multiple Bud-Shoot Barriers Preventing Water Transport into the Winter Buds of Norway Spruce. Front. Plant Sci. 2017, 8, 2109. [Google Scholar] [CrossRef]
- Tarancon, C.; Gonzalez-Grandio, E.; Oliveros, J.C.; Nicolas, M.; Cubas, P. A Conserved Carbon Starvation Response Underlies Bud Dormancy in Woody and Herbaceous Species. Front. Plant Sci. 2017, 8, 788. [Google Scholar] [CrossRef]
- Smith, H. Phytochromes and light signal perception by plants--an emerging synthesis. Nature 2000, 407, 585–591. [Google Scholar] [CrossRef]
- Legris, M.; Ince, Y.Ç.; Fankhauser, C. Molecular mechanisms underlying phytochrome-controlled morphogenesis in plants. Nat. Commun. 2019, 10, 5219. [Google Scholar] [CrossRef]
- Venkat, A.; Muneer, S. Role of Circadian Rhythms in Major Plant Metabolic and Signaling Pathways. Front. Plant Sci. 2022, 13, 836244. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Wang, Y.; Yu, Y.; He, Y.; Wang, L. Coordinative regulation of plants growth and development by light and circadian clock. Abiotech 2021, 2, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Sharrock, R.A.; Quail, P.H. Novel phytochrome sequences in Arabidopsis thaliana: Structure, evolution, and differential expression of a plant regulatory photoreceptor family. Genes Dev. 1989, 3, 1745–1757. [Google Scholar] [CrossRef] [PubMed]
- Mathews, S. Evolutionary studies illuminate the structural-functional model of plant phytochromes. Plant Cell 2010, 22, 4–16. [Google Scholar] [CrossRef]
- Howe, G.T.; Bucciaglia, P.A.; Hackett, W.P.; Furnier, G.R.; Cordonnier-Pratt, M.M.; Gardner, G. Evidence that the phytochrome gene family in black cottonwood has one PHYA locus and two PHYB loci but lacks members of the PHYC/F and PHYE subfamilies. Mol. Biol. Evol. 1998, 15, 160–175. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, B.; Li, Y.; Andre, D.; Nilsson, O. Phytochrome B and PHYTOCHROME INTERACTING FACTOR8 modulate seasonal growth in trees. New Phytol. 2021, 232, 2339–2352. [Google Scholar] [CrossRef]
- Karve, A.A.; Jawdy, S.S.; Gunter, L.E.; Allen, S.M.; Yang, X.; Tuskan, G.A.; Wullschleger, S.D.; Weston, D.J. Initial characterization of shade avoidance response suggests functional diversity between Populus phytochrome B genes. New Phytol. 2012, 196, 726–737. [Google Scholar] [CrossRef]
- Wei, H.; Luo, M.; Deng, J.; Xiao, Y.; Yan, H.; Liu, H.; Li, Y.; Song, Q.; Xiao, X.; Shen, J.; et al. SPL16 and SPL23 mediate photoperiodic control of seasonal growth in Populus trees. New Phytol. 2024, 241, 1646–1661. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Su, Y.; Klintenäs, M.; Li, Y.; Sane, S.; Wu, Z.; Chen, Q.; Zhang, B.; Nilsson, O.; Ding, J. Age-dependent seasonal growth cessation in Populus. Proc. Natl. Acad. Sci. USA 2023, 120, e2311226120. [Google Scholar] [CrossRef]
- Mohamed, R.; Wang, C.T.; Ma, C.; Shevchenko, O.; Dye, S.J.; Puzey, J.R.; Etherington, E.; Sheng, X.; Meilan, R.; Strauss, S.H.; et al. Populus CEN/TFL1 regulates first onset of flowering, axillary meristem identity and dormancy release in Populus. Plant J. 2010, 62, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Leseberg, C.H.; Li, A.; Kang, H.; Duvall, M.; Mao, L. Genome-wide analysis of the MADS-box gene family in Populus trichocarpa. Gene 2006, 378, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Liu, Y.; Luthe, D.S.; Yuceer, C. Poplar FT2 shortens the juvenile phase and promotes seasonal flowering. Plant Cell 2006, 18, 1846–1861. [Google Scholar] [CrossRef]
- Azeez, A.; Miskolczi, P.; Tylewicz, S.; Bhalerao, R.P. A tree ortholog of APETALA1 mediates photoperiodic control of seasonal growth. Curr. Biol. 2014, 24, 717–724. [Google Scholar] [CrossRef]
- Karlberg, A.; Bako, L.; Bhalerao, R.P. Short day-mediated cessation of growth requires the downregulation of AINTEGUMENTALIKE1 transcription factor in hybrid aspen. PLoS Genet. 2011, 7, e1002361. [Google Scholar] [CrossRef]
- Maurya, J.P.; Singh, R.K.; Miskolczi, P.C.; Prasad, A.N.; Jonsson, K.; Wu, F.; Bhalerao, R.P. Branching Regulator BRC1 Mediates Photoperiodic Control of Seasonal Growth in Hybrid Aspen. Curr. Biol. 2020, 30, 122–126. [Google Scholar] [CrossRef]
- Miskolczi, P.; Singh, R.K.; Tylewicz, S.; Azeez, A.; Maurya, J.P.; Tarkowska, D.; Novak, O.; Jonsson, K.; Bhalerao, R.P. Long-range mobile signals mediate seasonal control of shoot growth. Proc. Natl. Acad. Sci. USA 2019, 116, 10852–10857. [Google Scholar] [CrossRef]
- Tylewicz, S.; Petterle, A.; Marttila, S.; Miskolczi, P.; Azeez, A.; Singh, R.K.; Immanen, J.; Mähler, N.; Hvidsten, T.R.; Eklund, D.M.; et al. Photoperiodic control of seasonal growth is mediated by ABA acting on cell-cell communication. Science 2018, 360, 212–215. [Google Scholar] [CrossRef]
- Tuominen, H.; Puech, L.; Fink, S.; Sundberg, B. A Radial Concentration Gradient of Indole-3-Acetic Acid Is Related to Secondary Xylem Development in Hybrid Aspen. Plant Physiol. 1997, 115, 577–585. [Google Scholar] [CrossRef]
- Nilsson, J.; Karlberg, A.; Antti, H.; Lopez-Vernaza, M.; Mellerowicz, E.; Perrot-Rechenmann, C.; Sandberg, G.; Bhalerao, R.P. Dissecting the molecular basis of the regulation of wood formation by auxin in hybrid aspen. Plant Cell 2008, 20, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Baba, K.; Karlberg, A.; Schmidt, J.; Schrader, J.; Hvidsten, T.R.; Bako, L.; Bhalerao, R.P. Activity-dormancy transition in the cambial meristem involves stage-specific modulation of auxin response in hybrid aspen. Proc. Natl. Acad. Sci. USA 2011, 108, 3418–3423. [Google Scholar] [CrossRef]
- Resman, L.; Howe, G.; Jonsen, D.; Englund, M.; Druart, N.; Schrader, J.; Antti, H.; Skinner, J.; Sjodin, A.; Chen, T.; et al. Components acting downstream of short day perception regulate differential cessation of cambial activity and associated responses in early and late clones of hybrid poplar. Plant Physiol. 2010, 154, 1294–1303. [Google Scholar] [CrossRef]
- Rohde, A.; Prinsen, E.; De Rycke, R.; Engler, G.; Van Montagu, M.; Boerjan, W. PtABI3 impinges on the growth and differentiation of embryonic leaves during bud set in poplar. Plant Cell 2002, 14, 1885–1901. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Gao, Y.; Wu, X.; Moriguchi, T.; Bai, S.; Teng, Y. Bud endodormancy in deciduous fruit trees: Advances and prospects. Hortic. Res. 2021, 8, 139. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).