Abstract
The wood borer Cerambyx welensii Küster is a key contributor to Quercus open woodland (dehesa) decline. Among other factors, olfactory and visual cues could influence host colonisation by this species. In this study, we investigated whether the physiological performance and morphological features of Q. suber trees under summer stress are affected by C. welensii infestation. Additionally, we analysed the relation between morpho-physiological variables and the emission of monoterpenes that potentially mediate host selection by C. welensii. Thirty-six Q. suber trees with known monoterpene emission profiles were selected: 18 trees highly visited by C. welensii, and 18 neighbouring trees not visited or at least not visibly damaged by this wood borer. For each tree, we assessed photosynthesis, stomatal conductance, and transpiration during the early evening, and also the perimeter and crown projection. Trees visited by C. welensii maintained higher photosynthetic activity than non-visited trees (1.5–2.15 times) from 19:35 to 20:45 h. Visited trees had larger perimeters and smaller crown projection area-to-perimeter ratios than non-visited trees. Results suggest that, under stress conditions, the physiological performance of trees infested by C. welensii could have favoured foliar emission of certain monoterpenes influencing intraspecific host selection by this species.
Keywords:
Cerambycidae; dehesa; photosynthesis; stomatal conductance; transpiration; xylophagous; wood borer 1. Introduction
The sclerophyllous Mediterranean open woodland (dehesa) is an agrosilvopastoral system that, when properly put into practice, can serve as a sustainable approach to natural resource management, in harmony with the maintenance of high levels of biodiversity. From a socioeconomic perspective, for centuries, dehesas have potentially allowed sustainable social development and economic activity in certain traditionally disadvantaged areas. For these reasons, dehesas are considered a model of High Nature Value (HNV) farming [1]. HVN systems constitute a key part of the European Union’s biodiversity action plan and the Council of Europe’s strategy on biological and landscape diversity. Both European and national policy guidelines on rural development have considered research on dehesas a priority, particularly in relation to integrated pest management in these agroforestry systems.
Stands of cork oak (Quercus suber L.) are continuously exposed to anthropogenic and natural stressors [2]. Among natural stressors, attack by wood-boring insects, such as Cerambyx welensii Küster (Coleoptera, Cerambycidae), is believed to play a major role in Q. suber decline in southwest Spain [3,4]. Several types of deciduous trees, especially in the genus Quercus, are infested by C. welensii [5]. Adults of this large cerambycid (≥60 mm long) have a flight period that spans from mid-May to late July, with early night activity (mainly from 21:00 to 23:00 h) [3]. Only its larval stage causes damage; larvae bore into wood increasing the risk of branch and trunk breakage [3], as well as facilitating infection by wood-decaying fungi and plant pathogens [6]. Very high population densities of C. welensii in Q. suber stands are worrying since they have been shown to cause annual canopy cover loss of as much as 1.68% on average, potentially causing complete loss of the forest stand in a matter of decades (10–30 years) [7].
In addition to biotic stressors, trees in Mediterranean areas are exposed to severe summer stress, which causes physiological and biochemical changes, photosynthesis being one of the cellular functions most markedly affected by high temperatures and severe drought [8,9]. Notable decreases in both stomatal conductance (37%) and maximum carboxylation rate (43%) have been reported in Q. suber trees under severe drought [9]. Low photosynthetic and stomatal conductance rates, measured under extreme climatic conditions, have been considered an indicator of successful adaption to severe stress in Pinus halepensis Mill. [10]. Leaves of Q. suber showed higher photosystem II efficiency under light stress than those of Quercus ilex L. [11].
Terpenes and other volatile organic compounds (VOCs) have key functions in plant physiology and plant–environment interactions. They can protect plants against stressors and mediate plant communication [12,13]. Environmental factors such as temperature and photosynthetically active radiation are among the strongest determinants of short-term VOC emissions [14], as, for example, monoterpene precursors are derived from photosynthetic activity [15]. Under summer conditions, significant correlations were found between net photosynthetic rates and daily emission rates of terpenes (especially limonene, the least volatile monoterpene) by Q. ilex [16], though the correlations between monoterpene emissions and stomatal conductance were weaker [15]. Trees of the Quercus genus are considered among the highest VOC emitters [17], with Q. suber showing particularly strong monoterpene emission [11,18]. Further, Q. suber shows two marked foliar monoterpene emission patterns: a limonene chemotype (in which limonene is the most emitted compound) and a pinene chemotype (in which α– and β–pinene and sabinene dominate) [19]. Cerambycids seem to be attracted to VOCs such as monoterpenes, these compounds probably helping them identify weak and damaged host trees [20]. Higher rates of infestation by C. welensii have been observed in Q. suber with a limonene chemotype (limonene accounting for > 30% of total monoterpene emissions) [19,21].
The goal of this study was to explore cues potentially affecting host selection by C. welensii, particularly its relation with both physiological performance and morphological features of Q. suber trees under summer stress, as well as the effect of physiological activity on the emission of VOCs potentially mediating intraspecific host selection by C. welensii. Hence, the specific objectives of this study were: (i) to analyse short-term changes in photosynthesis (as reflected by net CO2 assimilation, A), stomatal conductance (gs) and transpiration (E) in Q. suber trees classified according to sightings of and damage attributable to C. welensii; and (ii) to investigate the link between morpho-physiological features of Q. suber trees and their foliar monoterpene emission profiles.
2. Materials and Methods
2.1. Site and Trees Studied
The study area was a Q. suber open woodland (dehesa, with 75 trees ha−1) in southwest Spain (37°15′43.73″ N, 6°28′34.65″ O, 80 m a.s.l.) that is known to be majorly affected by C. welensii. The mean annual temperature and precipitation were 17.6 °C ± 0.2 and 588 mm, respectively. In 2008, we selected 36 trees classified into two groups (18 trees each) based on the history of sightings of C. welensii adults [3]. Specifically, the first group (here on called visited trees) included the trees visited most by different C. welensii adults (cumulative mean sightings of 54 ± 23 individuals per tree between 2002 and 2007, and evidence of substantial damage by large wood borers). The second group (here on called non-visited trees) comprised neighbouring Q. suber trees on which we had not observed either C. welensii adults or damage attributable to this wood borer (Figure 1). All the trees were estimated to be around 150 years old [7]. Cerambyx welensii identification was based on visual examination of all adults captured between 2002 and 2007, using a dichotomous key for adult longhorn beetles [22].
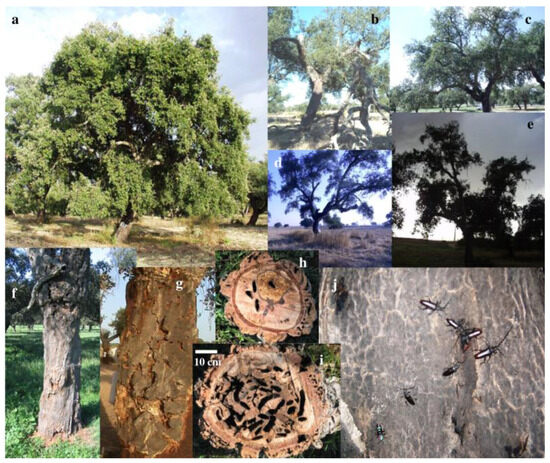
Figure 1.
(a) Quercus suber not infested by Cerambyx welensii; (b–e) Several degrees of crown architecture alteration in Q. suber, due to C. welensii damage; (f–i) damage caused by C. welensii; note the rotting process around a larval gallery in (h); (j) a cluster of C. welensii adults near a Q. suber bark exudate.
2.2. Physiological and Morphological Measurements
Measurements were made during the seasonal flight peak of C. welensii (last week of June; [3]), in the hours before the start of the nightly flight activity of C. welensii. Specifically, measurements were carried out in five rounds between 19:00 and 21:20, at 35 min intervals. Three or four trees in each group were randomly assigned to each round, and in each round, we collected one sprig from each of the selected trees (a total of 6–8 sprigs per round). We harvested 30 cm long sprigs from areas that were exposed to the sun and at ≈2 m from the ground. These sprigs had between 53 and 187 leaves, and after drying at 60 °C for ≥48 h, a mean dry weight of 14.48 ± 0.7 g. Immediately after the sprig harvest, we identified leaves that had grown the previous spring and measured photosynthesis (net CO2 assimilation, A), stomatal conductance (gs), and transpiration (E) in the field. Measurements of A, gs, and E were made using an infrared gas analyser with a wide leaf chamber (6.25 cm2; LCi system, ADC BioScientific, Hoddesdon, UK). Photosynthetic photon flux density, relative humidity, and temperature were measured during the aforementioned assessment of physiological parameters. Subsequently, we also assessed morphological variables that might be related to physiological performance, as well as host selection by C. welensii. Specifically, the perimeter of each tree was measured at breast height, and the crown projection area (CPA) was estimated from the vertical projection of the crown corresponding to each tree based on high-resolution satellite images [23] and using ArcGis 10.0.
The same evenings as the physiological measurements, foliar monoterpenes were sampled (see [21] for further details; data not included in this document). Briefly, monoterpene samples were obtained using an aeration system [24], comprising a measurement chamber (polyester oven bag; Albal, Cofresco, Madrid, Spain), connected to a Teflon sampling line leading to a pump (SP 200 EC–LC diaphragm pump; Schwarzer Precision, Essen, Germany). Charcoal-filtered air entering the chamber was drawn through a sorbent tube (403 Orbo Tenax TA glass tube, 150 mg, 60/80 mesh; Sigma-Aldrich, Madrid, Spain), for 5 min at a flow rate of 120 mL min−1. All Orbo tubes were sealed immediately after collection and stored at 4 °C in the field, and later, at −28 °C in the laboratory, until analysis which was always performed within 24 h. Blank controls (empty measurement chambers, not enclosing sprigs) were collected simultaneously using the same sampling system.
Monoterpene samples were analysed by gas chromatography-mass spectrometry (GC type 6890N, MSD 5973; Agilent, Santa Clara, CA, USA), using an HP-5ms column (0.25 mm × 30 m × 0.25 μm) and helium as the carrier gas (1 mL min−1), with the following oven program: the starting temperature (46 °C) was increased to 70 °C (rate of 30 °C min−1), held steady (4 min), and then increased to 80 °C (rate of 5 °C min−1), to 90 °C (rate of 4.5 °C min−1), and to 300 °C (rate of 50 °C min−1). Peak identification was achieved by comparison with pure standards (Sigma-Aldrich; Madrid, Spain; purity ≥ 94%) and with mass spectra in the NIST 02 library (MSD Chemstation Build 75 software, G2070BA version). For quantitative analysis, calibration curves were obtained for α-pinene, β-pinene, sabinene, limonene, and myrcene.
Comparative analyses for each physiological variable were carried out using linear mixed models (LMM, lme4 package; [25]), with mean values of each variable as the dependent variables, sampling time and visits by C. welensii as fixed factors, and tree identity as a random factor (intercept). Likelihood ratios were used to assess the significance of the main factors. The Benjamini-Hochberg method was used to adjust p values for multiple comparisons [26] to control for the false discovery rate (expected proportion of tests that are classified as significant erroneously, Lsmeans package; [27]).
To investigate the relation of both physiological performance and morphological features with monoterpene emissions, we carried out partial canonical correspondence analysis (pCCA, Vegan package; [28]), taking the perimeter as a covariate. Previously, data were scaled to a mean of zero and unit variance within each sampling round. Multicollinearity was tested using variance inflation factors. Finally, the explanatory variables included photosynthesis (A), transpiration (E), and the crown projection area-to-perimeter (CPA/perimeter) ratio. The response variables were relative emissions of α-pinene, β-pinene, sabinene, limonene, and myrcene, and pinene-type VOCs, a new variable created by summing the relative emissions of α-pinene, β-pinene, and sabinene. These emissions were assessed for individual trees and expressed as percentages of the sum of observed emissions for the five main compounds emitted by Q. suber, namely, α-, and β-pinene, sabinene, limonene, and myrcene. The significance of the canonical axes was assessed with permutation tests using the Vegan package.
The aforementioned statistical analysis was carried out using R software, version 3.1.0. Thresholds for significance and marginal significance were set at α = 0.05 and α = 0.1, respectively.
3. Results
Over the early evening, the photosynthetic photon flux density decreased sharply (from 1097.5 to 75 µmol m–2 s−1). Nonetheless, the air temperature remained high (>28.1 °C), and relative humidity remained very low (<40% throughout the study period). Given the low light intensity at 21:20, we were not able to obtain accurate measurements for the three physiological variables (A, gs, and E) at this time, and hence, these data were not included in the analysis (Figure 2b–d).
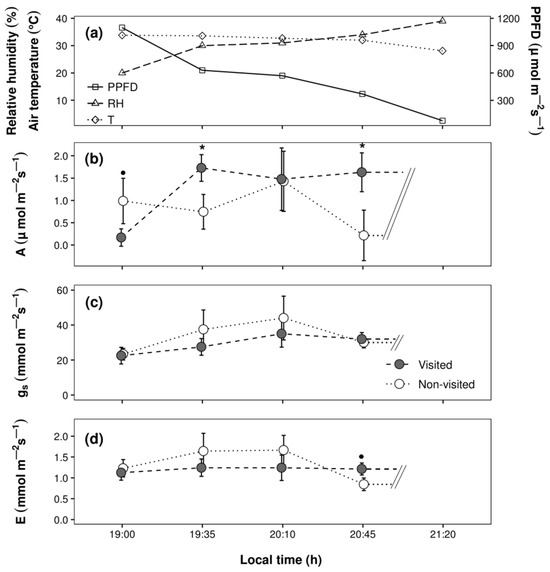
Figure 2.
(a) Environmental conditions over the early evening: relative humidity (RH), air temperature (T), and photosynthetic photon flux density (PPFD); (b) photosynthesis (A), (c) stomatal conductance (gs) and (d) transpiration (E) from Quercus suber trees classified as highly visited or not visited by Cerambyx welensii. Symbols over mean (±SE) values at some time points indicate significant differences by visit status (* p < 0.05; • p < 0.1 in LMM with Benjamini-Hochberg correction for multiple comparisons; N = 3–4/sampling round for each visit status). Data for A, gs, and E measured at 21:20 are not shown.
A very significant (p < 0.01 overall) positive correlation was found between standardised values of E and gs in both non-visited (R = 0.61) and visited (R = 0.82) trees. Both gs and E increased until 20:10, after which the values dropped. Overall, these two variables had slightly higher values in non-visited than visited trees; however, there were neither significant effects of the main factors, time and visits, nor interaction effects on the rates of gs and E. In contrast, the main effect of time on A rates showed a trend towards significance (LMM: χ23 = 3.58, p = 0.06; Figure 2b) and was affected by visit status (significant time × visits interaction effect; LMM: χ23 = 9.83, p < 0.01). Specifically, the A rates of non-visited trees exceeded those of visited trees at 19:00 (t = 1.69, p = 0.08; Figure 2b), after which a sharp increase in A was observed in visited trees, reaching values significantly higher (2.15 times) than those measured in non-visited trees at 19:35 (t = −1.99, p = 0.04) and 20:45 (t = −2.35, p = 0.03).
The visited trees had a significantly larger mean perimeter than non-visited trees (168.4 and 131.1 cm, respectively; p = 0.01), and the two groups of trees had similar CPA values (48.2 and 46.4 m2 for visited and non-visited trees, respectively; p = 0.41). Accordingly, the CPA/perimeter ratio was slightly lower for visited than non-visited trees (31.5 and 36.3 m, respectively; t = −1.10, p = 0.2).
For non-visited trees, the pCCA model was not significant (Permutation test: p = 0.38). In contrast, for the two groups considered together, the two first pCCA axes explained a low amount of variance corresponding to the emissions of limonene, myrcene, and pinene-type VOCs (19%, Permutation test: F3 = 2.02, p = 0.07) (Figure 3a). The results of the pCCA model improved when it was applied to visited trees only, explaining 62% of the variance corresponding to limonene, myrcene, and pinene-type emissions (Permutation test: F3 = 6.01, p = 0.001) (Figure 3b).
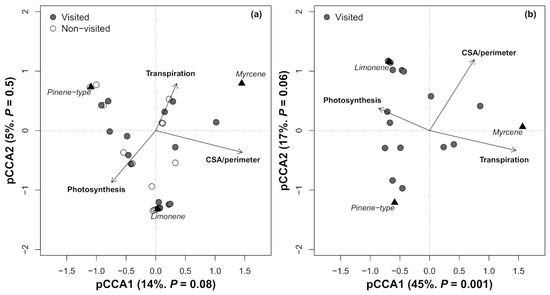
Figure 3.
Partial canonical correspondence analysis triplot (Scaling 1) for (a) All Quercus suber trees together, i.e., both those highly visited and those not visited by Cerambyx welensii, and (b) Q. suber trees classified as highly visited by C. welensii. The analysis illustrates the effect of physiological variables (photosynthesis and transpiration) as well as the crown projection area-to-perimeter ratio (CPA/perimeter) on emission composition, specifically on the emissions of limonene, myrcene and pinene-type VOCs (sum of α- and β-pinene and sabinene) expressed as a percentage of the total emissions of these five main monoterpenes.
Analysing both groups together, the first pCCA axis (pCCA1) was marginally significant (Figure 3a); in contrast, for visited trees only, pCCA1 was significant (p = 0.001), while the second pCCA axis (pCCA2) was marginally significant (p = 0.06) (Figure 3b). For all the trees, pCCA1 was related mainly to the CPA/perimeter ratio (biplot score: 0.17). For visited trees, pCCA1 was related primarily to A and secondarily to E (biplot scores: −0.46 and 0.42, respectively), and pCCA2 was mainly related to the CPA/perimeter ratio (biplot score: 0.41). In the model applied to visited trees, limonene emissions had moderate values (absolute value = 0.57) for both pCCA axes; in contrast, the pinene-type variable had a low value for pCCA1 (−0.16) but a moderate value for pCCA2 (0.63) (Figure 3b).
In brief, both pCCA models suggest that high limonene emissions were associated with low transpiration rates and high photosynthetic rates. High pinene-type emissions were found in trees with low CPA/perimeter ratios (smaller canopies). Conversely, high limonene emission was found in visited trees with high CPA/perimeter ratios (Figure 3). The least abundant monoterpene in Q. suber foliar emissions overall (myrcene) was strongly related to high transpiration rates; the association (positive) between the CPA/perimeter ratio and myrcene emissions was weaker in visited trees.
4. Discussion
In the Mediterranean area, environmental constraints, stress due to drought, high temperatures, and high insolation can cause oxidative stress, and reductions in photosynthesis linked to a decrease in stomatal conductance, even in species suited to the local environment, such as Q. suber and Q. ilex [29,30] and studies cited therein. Showing resiliency under such stressful conditions is an adaptive behaviour [31]. There is evidence that the impact of some abiotic stresses is mitigated by non-stored constitutively released VOCs, as these compounds can stabilise membranes and/or serve as antioxidants, at least in the absence of photorespiration [12,13,32,33]. On the other hand, less is known about the role isoprenoids and monoterpenes, especially limonene may play in such defence mechanisms [13].
Across our study period, we found low values for the physiological variables A, gs, and E, as reported before for similar times of day in Q. suber and Q. ilex [34,35,36]. High air temperatures (>25 °C) have been found to either reduce or inhibit photosynthesis in Q. suber and Q. ilex [37,38,39], and hence, the high temperatures during our samplings, presumably linked to low soil water availability, could also have negatively influenced A rates [14,36]. Nonetheless, even considering the moderate net CO2 assimilation rates found, the A values measured differed significantly between trees that were and were not visited by C. welensii.
There is a paucity of knowledge about cues used by xylophagous longhorn beetles to select deciduous host trees. It is known, however, that visual cues may influence host location by Cerambyx species [4]. In relation to this, considerable numbers of C. welensii individuals were found to be transient in our study area [3], supporting the hypothesis that host selection mechanisms mediate adult movement across adjacent areas. In the study reported herein, the visited trees had a significantly larger mean perimeter than non-visited trees, though a non-significantly smaller CPA/perimeter ratio. This pattern is consistent with an observed preference of C. welensii for old and/or damaged trees [3].
Secondly, VOCs released by damaged or stressed plants can serve as olfactory cues for cerambycids, for finding host plants as well as conspecific mates [20,40]. In particular, traps baited with sources of fermentation odours have been shown to attract C. welensii [41]. Further, this species is known to have olfactory sensitivity to a wide spectrum of tree volatiles, especially the main monoterpenes emitted by its hosts, namely, α- and β-pinene, sabinene, limonene, and myrcene [42]. In particular, colonisation by C. welensii was found to be more likely in Q. suber trees with a limonene chemotype; while the emission of pinene-type compounds was more common in trees with no signs of activity of this species [21]. It has been suggested that C. welensii may use certain ratios of the aforementioned five monoterpenes in host emissions to help it distinguish between leaf chemotypes, and in particular, females may be involved in the detection of the limonene chemotype [21]. Nonetheless, traps baited with monoterpenes alone only weakly attracted C. welensii, compared to the efficacy of traps baited with synthetic fermentation odours [43].
Both the significant pCCA model we obtained for trees visited by C. welensii and the differences we observed in net CO2 assimilation between trees that were and were not visited by this species, suggest, in accordance with [18], that the maintenance of photosynthetic activity during the early evening could favour the release of the less volatile monoterpene (limonene), precisely in a period when C. welensii starts its nightly flight activity. Further, infestation by C. welensii in old Q. suber trees increases the risk of modifying the crown architecture (e.g., changes in CPA/perimeter ratio) (Figure 1b–e), as a result of breakage of branches due to larval activity, and such crown alterations are expected to become increasingly common over the years in the study area [7]. We found the CPA/perimeter ratio was 14% lower in trees affected by C. welensii than in adjacent trees with no presence of this wood borer. This difference was not significant; however, this observation suggests an area for further research, because the pruning of large branches damaged by C. welensii is a common silvicultural practice in Q. suber stands, and it leads to a reduction in the CPA/perimeter ratio. These alterations in crown architecture could modify the tree silhouette, potentially influencing host selection through visual stimulation [4]. Such changes in crowns could also influence—due to changes in sunlight interception and/or hydraulic conductivity—trees’ physiological performance and VOC emission behaviour [44], as well as wood density—which affects larval growth in xylophagous insects [45].
5. Conclusions
Based on this study, photosynthetic rates seem to remain higher during the early evening in Q. suber trees highly visited by the wood borer C. welensii than neighbouring trees not visited or at least not visibly damaged by this species. The physiological performance we observed in trees infested by C. welensii could have favoured the foliar emission of certain VOCs, especially those less volatile and less influenced by environmental factors (in particular, light and temperature) such as limonene. These results are of interest as they help deepen our understanding of plant-insect interactions, in particular, those that may influence host selection by C. welensii, and in turn, may have applications in the integrated management of this pest.
Nonetheless, more studies are required to explore how the emission of given monoterpenes (especially, limonene and pinene-type compounds) affects the attraction of C. welensii in both laboratory and field conditions. Additionally, more research is needed to determine how changes in the physiological performance of Q. suber (associated with environmental stress and wood borer damage) influence, on the one hand, the foliar emission pattern of Q. suber, and on the other, the suitability of these trees for C. welensii larval growth and development.
Author Contributions
Conceptualization, I.S.-O., G.L.-P., R.T. and L.D.; methodology, I.S.-O. and R.T.; formal analysis, I.S.-O. and E.P.-S.; investigation, I.S.-O., G.L.-P., R.T., E.P.-S. and L.D.; resources, I.S.-O. and R.T.; writing—original draft preparation, I.S.-O. and E.P.-S.; writing—review and editing, I.S.-O., G.L.-P., R.T. and L.D.; supervision, R.T. and L.D.; funding acquisition, I.S.-O. and G.L.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the research project: “Control de Cerambyx welensii en dehesa mediante capturas en trampa cebada con atrayentes de naturaleza cairomonal” (Specific Agreement for Research between the Andalusian Department of Fish and Agriculture and University of Córdoba, and Research Collaboration Agreement between University of Córdoba and University of Huelva, OTR-CONV-00246), Project: Net463168: “Contrato de consultoria y asistencia para el estudio de la distribucion geografica en Andalucia de Cerambyx cerdo y control de Cerambyx welensii” (Department of Agroforestry Science, University of Huelva and EGMASA, SA). The article processing charges were funded by two Andalusian Plan for Research, Development and Innovation (PAIDI) Research Groups at the University of Huelva: Gestión de Recursos Forestales (RNM301) and Análisis y Planificación del Medio Natural (RNM315).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
This paper is a translation of “Primera aproximación al estudio de la actividad fisiológica en Quercus suber con alta presencia de Cerambyx welensi Küster”, originally published in Spanish by the Spanish Society of Forestry Sciences (Cuadernos de la Sociedad Española de Ciencias Forestales, 2020, 46(1): 57–70). The English version was prepared by Israel Sánchez-Osorio, with support from the Andalusian Plan for Research, Development and Innovation (PAIDI) Research Groups: Gestión de Recursos Forestales (RNM301) and Análisis y Planificación del Medio Natural (RNM315). Permission to publish was granted by “Cuadernos de la Sociedad Española de Ciencias Forestales”, and the authors, Israel Sánchez-Osorio, Gloria López-Pantoja, Raúl Tapias, Evangelina Pareja-Sánchez, and Luis Domínguez. We also thank Agustín Rincón, María del Mar González, and Sebastiana Malia for assisting with the fieldwork and Manuel Fernández for his constructive feedback on an early version of this manuscript.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- World Wide Fund for Nature (WWF)/Adena. La Dehesa en los Programas de Desarrollo Rural 2007-13. Propuesta; World Wide Fund for Nature (WWF)/Adena: Madrid, Spain, 2006; p. 34. [Google Scholar]
- Aronson, J.; Pereira, J.S.; Pausas, J.G. (Eds.) Cork Oak Woodlands on the Edge. Ecology, Adaptive Management, and Restoration; Society for Ecological Restoration International, Island Press: Washington, DC, USA, 2009. [Google Scholar]
- López-Pantoja, G.; Domínguez, L.; Sánchez-Osorio, I. Mark-recapture estimates of the survival and recapture rates of Cerambyx welensii Küster (Coleoptera Cerambycidae) in a cork oak dehesa in Huelva (Spain). Cent. Eur. J. Biol. 2008, 3, 431–441. [Google Scholar] [CrossRef]
- Torres-Vila, L.M.; Mendiola-Díaz, F.J.; Sánchez-González, Á. Dispersal differences of a pest and a protected Cerambyx species (Coleoptera: Cerambycidae) in oak open woodlands: A mark-recapture comparative study. Ecol. Entomol. 2017, 42, 18–32. [Google Scholar] [CrossRef]
- Vives, E. Coleoptera, Cerambycidae. In Fauna Ibérica; Ramos, M.A., Ed.; Museo Nacional de Ciencias Naturales, CSIC: Madrid, Spain, 2000; Volume 12. [Google Scholar]
- Martín, J.; Cabezas, J.; Buyolo, T.; Patón, D. The relationship between Cerambyx spp. damage and subsequent Biscogniauxia mediterranum infection on Quercus suber forests. For. Ecol. Manag. 2005, 216, 166–174. [Google Scholar] [CrossRef]
- Domínguez, L.; López-Pantoja, G.; Cremades, D.; Paramio, A.; Hidalgo, P.J.; Sánchez-Osorio, I. Incidence of Large Wood Borers in the Conservation of dehesa Islands Forests in Southwestern Spain. Forests 2022, 13, 413. [Google Scholar] [CrossRef]
- Correia, B.; Rodriguez, J.L.; Valledor, L.; Almeida, T.; Santos, C.; Cañal, M.J.; Pinto, G. Analysis of the expression of putative heat-stress related genes in relation to thermotolerance of cork oak. J. Plant. Physiol. 2014, 171, 399–406. [Google Scholar] [CrossRef]
- Piayda, A.; Dubbert, M.; Rebmann, C.; Kolle, O.; Costa, F.; Correia, A.; Pereira, J.S.; Werner, C.; Cuntz, M. Drought impact on carbon and water cycling in a Mediterranean Quercus suber L. woodland during the extreme drought event in 2012. Biogeosciences 2014, 11, 7159–7178. [Google Scholar] [CrossRef]
- Llusià, J.; Roahtyn, S.; Yakir, D.; Rotenberg, E.; Seco, R.; Guenther, A.; Peñuelas, J. Photosynthesis, stomatal conductance and terpene emission response to water availability in dry and mesic Mediterranean forests. Trees 2015, 30, 749–759. [Google Scholar] [CrossRef]
- Lavoir, A.V.; Duffet, C.; Mouillot, F.; Rambal, S.; Ratte, J.P.; Schnitzler, J.P.; Staudt, M. Scaling-up leaf monoterpene emissions from a water limited Quercus ilex woodland. Atmos. Environ. 2011, 45, 2888–2897. [Google Scholar] [CrossRef]
- Grote, R.; Monson, R.; Niinemets, Ü. Leaf-level models of constitutive and stress-driven volatile organic compound emissions. In Biology, Controls and Models of Tree Volatile Organic Compound Emission; Niinemets, Ü., Monson, R.K., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 315–355. [Google Scholar]
- Loreto, F.; Pollastri, S.; Fineschi, S.; Velikovac, V. Volatile isoprenoids and their importance for protection against environmental constraints in the Mediterranean area. Environ. Exp. Bot. 2014, 103, 99–106. [Google Scholar] [CrossRef]
- Hakola, H.; Laurila, T.; Lindfors, V.; Hellén, H.; Gaman, A.; Rinne, J. Variation of the VOC emission rates of birch species during the growing season. Boreal. Environ. Res. 2001, 6, 237–249. [Google Scholar]
- Loreto, F.; Ciccioli, P.; Cecinato, A.; Bracaleoni, E.; Frattoni, M.; Tricoli, D. Influence of environmental α-pinene from factors and air composition on the emission of Quercus ilex leaves. Plant. Physiol. 1996, 110, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Llusia, J. Seasonal emission of monoterpenes by the Mediterranean tree Quercus ilex in field conditions: Relations with photosynthetic rates, temperature and volatility. Physiol. Plant. 1999, 105, 641–647. [Google Scholar] [CrossRef]
- Pearse, I.S.; Gee, W.S.; Beck, J.J. Headspace Volatiles from 52 oak species advertise induction, species identity, and evolution, but not Defense. J. Chem. Ecol. 2013, 39, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Pio, C.A.; Silva, P.A.; Cerqueira, M.A.; Nunes, T.V. Diurnal and seasonal emissions of volatile organic compounds from cork oak (Quercus suber) trees. Atmos. Environ. 2005, 39, 1817–1827. [Google Scholar] [CrossRef]
- Staudt, M.; Mir, C.; Joffre, R.; Rambal, S.; Bonin, A.; Landais, D.; Lumaret, R. Isoprenoid emission of Quercus spp. (Q. suber and Q. ilex) in mixed stands contrasting in interspecific genetic introgression. New Phytol. 2004, 163, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Allison, J.D.; Borden, J.H.; Seybold, J.H. A review of the chemical ecology of the Cerambycidae (Coleoptera). Chemoecology 2004, 14, 123–150. [Google Scholar] [CrossRef]
- Sánchez-Osorio, I.; López-Pantoja, L.; Tapias, R.; Pareja-Sánchez, E.; Domínguez, L. Monoterpene emission of Quercus suber L. highly infested by Cerambyx welensii Küster. Ann. For. Sci. 2019, 76, 98. [Google Scholar] [CrossRef]
- Bense, U. Longhorn Beetles: Illustrated Key to the Cerambycidae and Vesperidae of Europe; Margraf Verlag: Weikersheim, Germany, 1995. [Google Scholar]
- Google. Satellite Image of Dehesa San Enrique (37°15’43.73” N, 6°28’34.65” O). Almonte, Huelva, Spain. October 2015. Available online: http://earth.google.com (accessed on 3 December 2023).
- Zhang, Q.-H.; Birgersson, G.; Zhu, J.; Lofstedt, C.; Lofqvist, J.; Schlyter, F. Leaf volatiles from nonhost deciduous trees: Variation by tree species, season and temperature, and electrophysiological activity in Ips typographus. J. Chem. Ecol. 1999, 8, 1923–1943. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Lme4: Linear Mixed-Effects Models Using Eigen and S4. R Package, version 1.1-7; 2014; Available online: http://github.com/lme4/lme4/ (accessed on 1 December 2023).
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. 1995, B57, 289–300. [Google Scholar] [CrossRef]
- Lenth, R.V. Lsmeans: Least-Squares Means. R Package; Version 2.10; 2014; Available online: http://CRAN.R-project.org/package=lsmeans (accessed on 3 June 2016).
- Oksanen, F.; Simpson, G.L.; Blanchet, G.F.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package; Version 2.2-1; 2015; Available online: http://CRAN.R-project.org/package=vegan (accessed on 5 June 2016).
- Munné-Bosch, S.; Peñuelas, J.; Asensio, D.; Llusià, J. Airborne ethylene may alter antioxidant protection and reduce tolerance of holm oak to heat and drought stress. Plant Physiol. 2004, 136, 2937–2947. [Google Scholar] [CrossRef]
- Vaz, M.; Pereira, J.S.; Gazarini, L.C.; David, T.S.; David, J.S.; Rodrigues, A.; Maroco, J.; Chaves, M.M. Drought-induced photosynthetic inhibition and autumn recovery in two Mediterranean oak species (Quercus ilex and Quercus suber). Tree Physiol. 2010, 30, 946–956. [Google Scholar] [CrossRef]
- Vaz, M.; Maroco, J.; Ribeiro, N.; Gazarini, L.C.; Pereira, J.S.; Chaves, M.M. Leaf-level responses to light in two co-occurring Quercus (Quercus ilex and Quercus suber): Leaf structure, chemical composition and photosynthesis. Agroforest. Syst. 2011, 82, 173–181. [Google Scholar] [CrossRef]
- Peñuelas, J.; Llusià, J. Linking photorespiration, monoterpenes and thermotolerance in Quercus. New Phytol. 2002, 155, 227–237. [Google Scholar] [CrossRef]
- Llusià., J.; Peñuelas, J.; Asensio, D.; Munné-Bosch, S. Airborne limonene confers limited thermotolerance to Quercus ilex. Physiol. Plant. 2005, 123, 40–48. [Google Scholar] [CrossRef]
- Tenhunen, J.D.; Reynolds, J.F.; Lange, O.L.; Dougherty, R.L.; Harley, P.C.; Kummerow, J.; Rambal, S. Quinta. A physiologically-based growth simulator for drought adapted woody plant species. In Biomass Production by Fast-Growing Trees; Pereira, J.S., Landsberg, J.J., Eds.; NATO ASI Series; Springer: Dordrecht, The Netherlands, 1989; Volume 166, pp. 135–168. [Google Scholar]
- Oliveira, G.; Werner, C.; Mertens, C.; Correia, O. Influencia de la Posición de la Copa Sobre la Fenología y las Relaciones Hídricas en Alcornoque (Quercus suber); Actas del I. Congreso Forestal Español: Lourizán, Spain, 1993; pp. 277–282. [Google Scholar]
- Niinemets, U.; Seufert, G.; Steinbrecher, R.; Tenhunen, J.D. A model coupling foliar monoterpene emissions to leaf photosynthetic characteristics in Mediterranean evergreen Quercus species. New Phytol. 2002, 153, 257–275. [Google Scholar] [CrossRef]
- Delfine, S.; Csiky, O.; Seufert, G.; Loreto, F. Fumigation with exogenous monoterpenes of a non-isoprenoid-emitting oak (Quercus suber): Monoterpene acquisition, translocation, and effect on the photosynthetic properties at high temperatures. New Phytol. 2000, 146, 27–36. [Google Scholar] [CrossRef]
- Peñuelas, J.; Llusià, J.; Asensio, D.; Munné-Bosch, S. Linking isoprene with plant thermotolerance, antioxidants and monoterpene emissions. Plant. Cell Environ. 2005, 28, 278–286. [Google Scholar] [CrossRef]
- Núñez, L.; Plaza, J.; Pérez-Pastor, R.; Pujadas, M.; Gimeno, B.; Bermejo, V.; García-Alonso, S. High water vapour pressure deficit influence on Quercus ilex and Pinus pinea field monoterpene emission in the central Iberian Peninsula (Spain). Atmos. Environ. 2002, 36, 4441–4452. [Google Scholar] [CrossRef]
- Millar, J.G.; Hanks, L.M. Chemical ecology of cerambycid beetles. In Cerambycidae of the World: Biology and Management; Wang, Q., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2017. [Google Scholar]
- Torres-Vila, L.M.; Sanchez-González, A.; Ponce-Escudero, F.; Martín-Vertedor, D.; Ferrero-García, J.J. Assessing mass trapping efficiency and population density of Cerambyx welensii Küster by mark-recapture in dehesa open woodlands. Eur. J. Forest. Res. 2012, 131, 1103–1116. [Google Scholar] [CrossRef]
- Sánchez-Osorio, I.; Tapias, R.; Domínguez, L.; López-Pantoja, G.; González, M.d.M. Electroantennographic Responses of Cerambyx welensii Küster to Host-Related Volatiles. Forests 2021, 12, 1168. [Google Scholar] [CrossRef]
- Sánchez-Osorio, I.; López-Pantoja, L.; Paramio, A.M.; Lencina, J.L.; Gallego, D.; Domínguez, L. Field attraction of Cerambyx welensii to fermentation odors and host monoterpenes. J. Pest. Sci. 2016, 89, 59–68. [Google Scholar] [CrossRef]
- Grote, R. Sensitivity of volatile monoterpene emission to changes in canopy structure: A model-based exercise with a process-based emission model. New Phytol. 2007, 173, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Hoeber, S.; Leuschner, C.; Köhler, L.; Arias-Aguilar, D.; Schuldt, B. The importance of hydraulic conductivity and wood density to growth performance in eight tree species from a tropical semi-dry climate. For. Ecol. Manag. 2014, 330, 126–136. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).