The Changes in Soil Microbial Communities and Assembly Processes along Vegetation Succession in a Subtropical Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Soil Sampling
2.2. Determination of Soil Physicochemical Properties
2.3. DNA Extraction, Amplicon Library Preparation, and Sequencing
2.4. Sequence Data Processing
2.5. Statistical Analysis
3. Results
3.1. Soil Physicochemical Properties along the Succession Stages
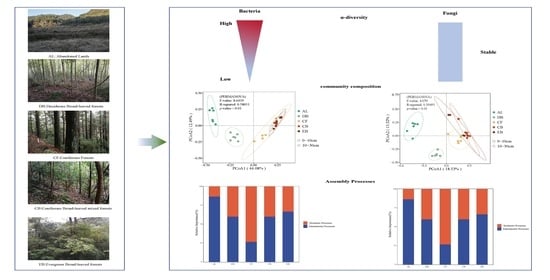
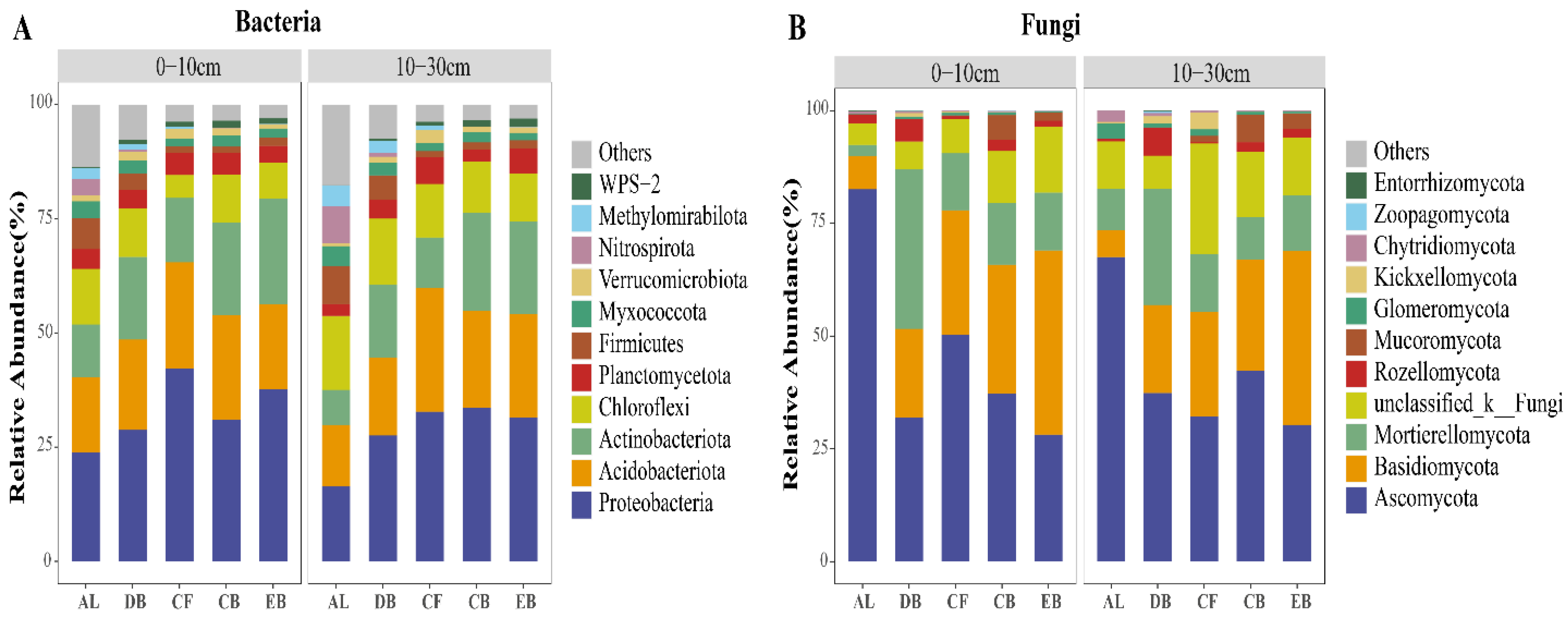
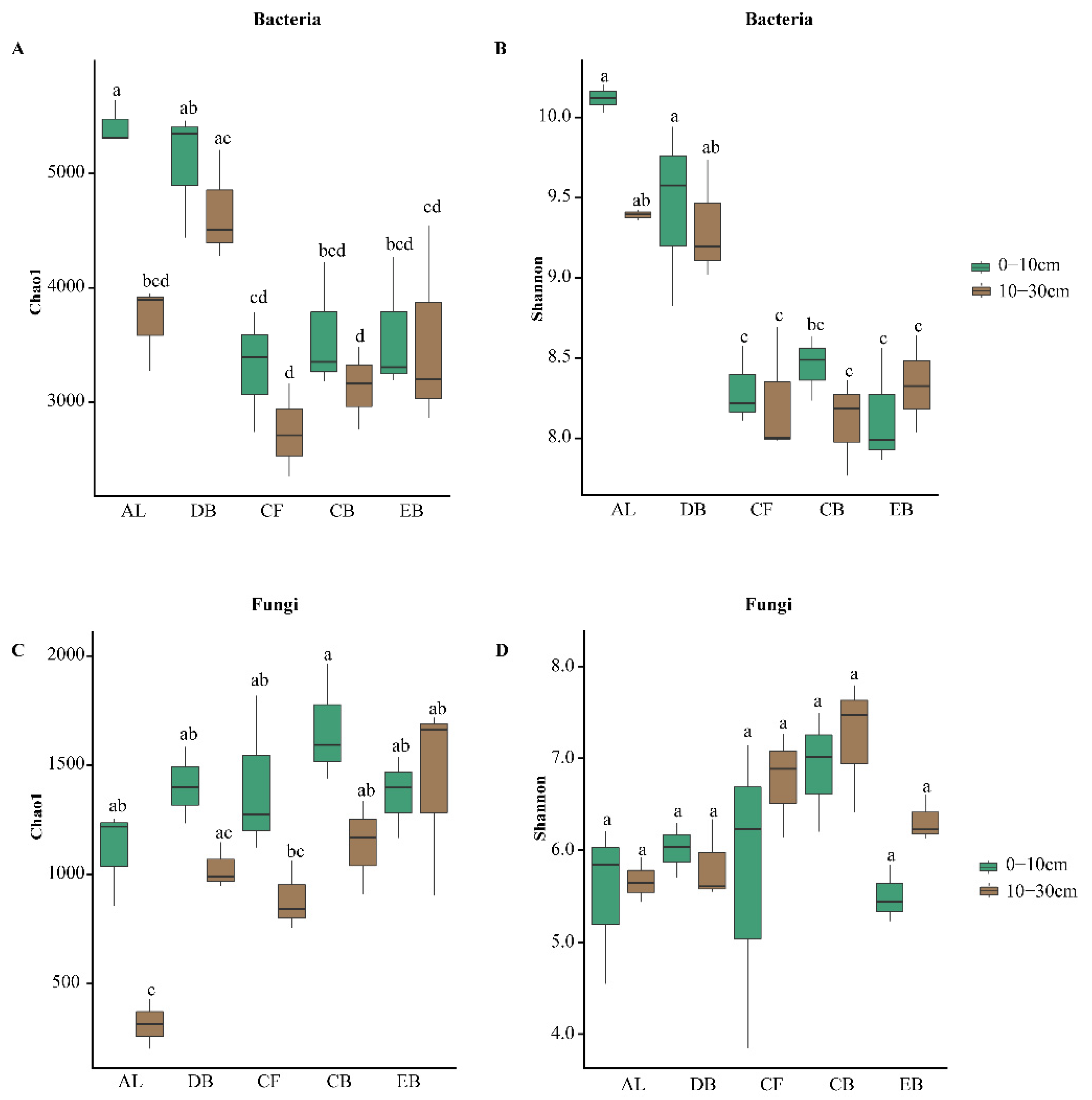
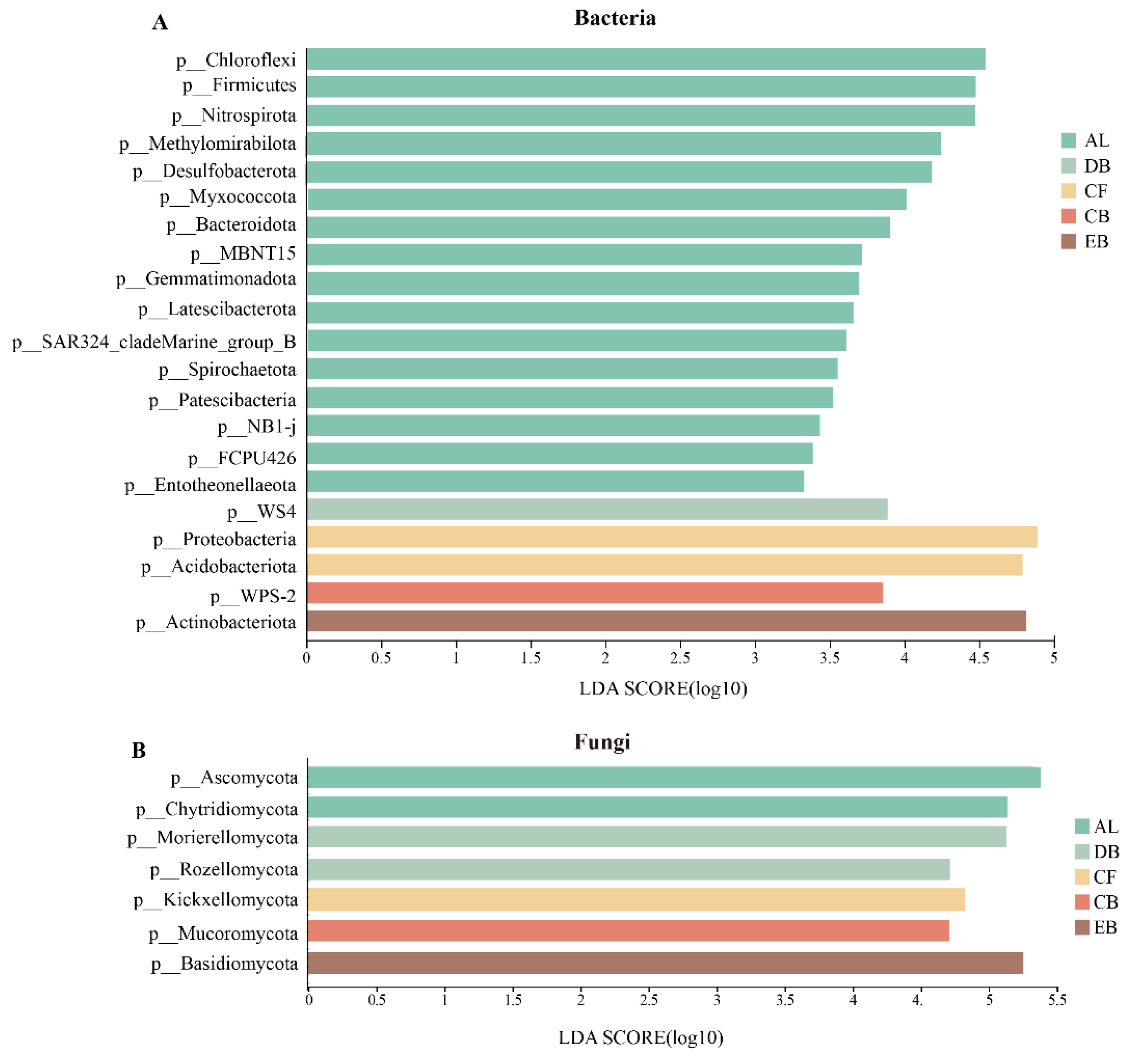
3.2. The Diversity and Composition of Soil Microbial Communities along the Succession Stages
3.3. The Beta Diversity of Soil Microbial Communities along the Succession Stages
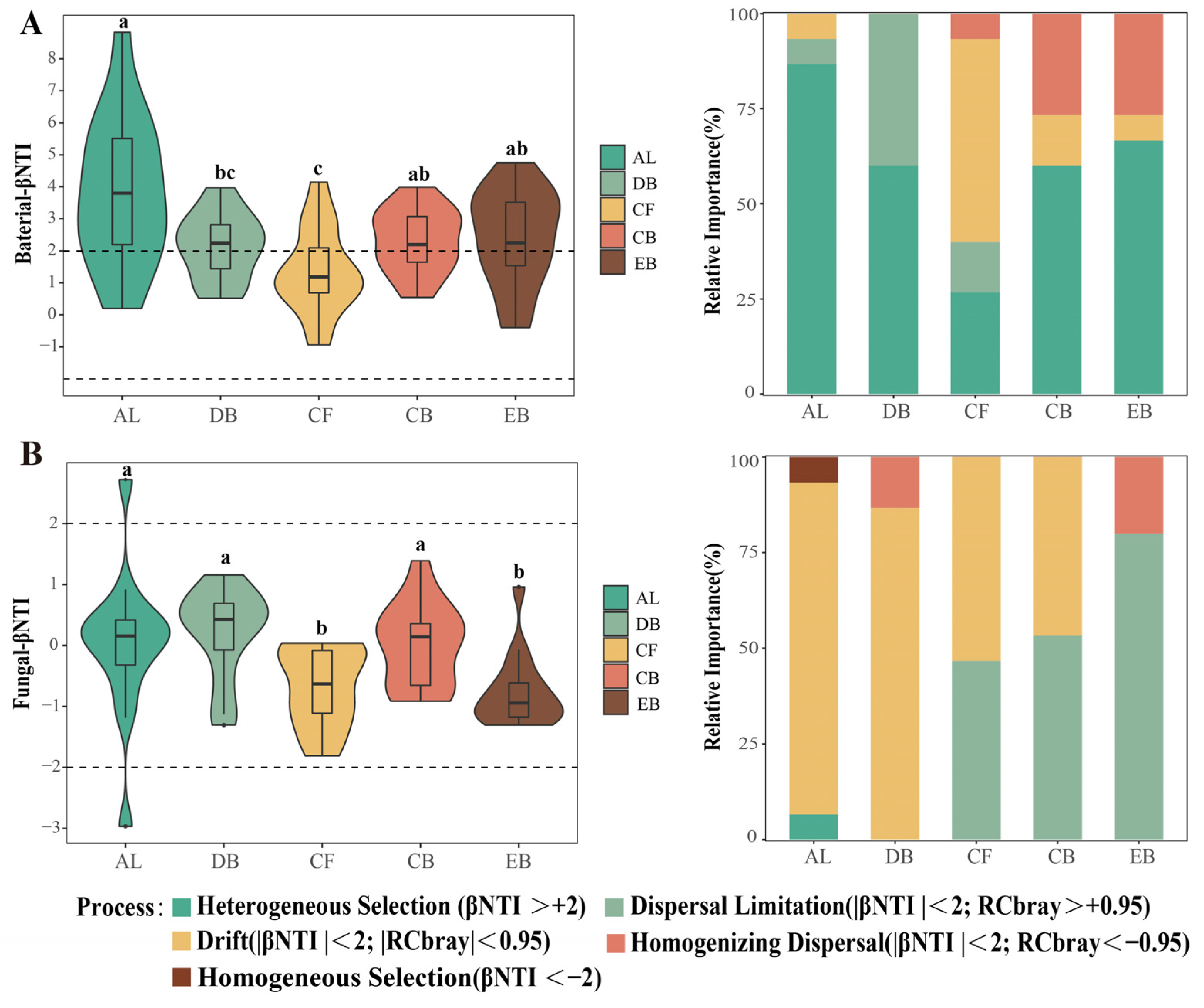
3.4. The Changes in Microbial Assembly Processes along the Succession Stages
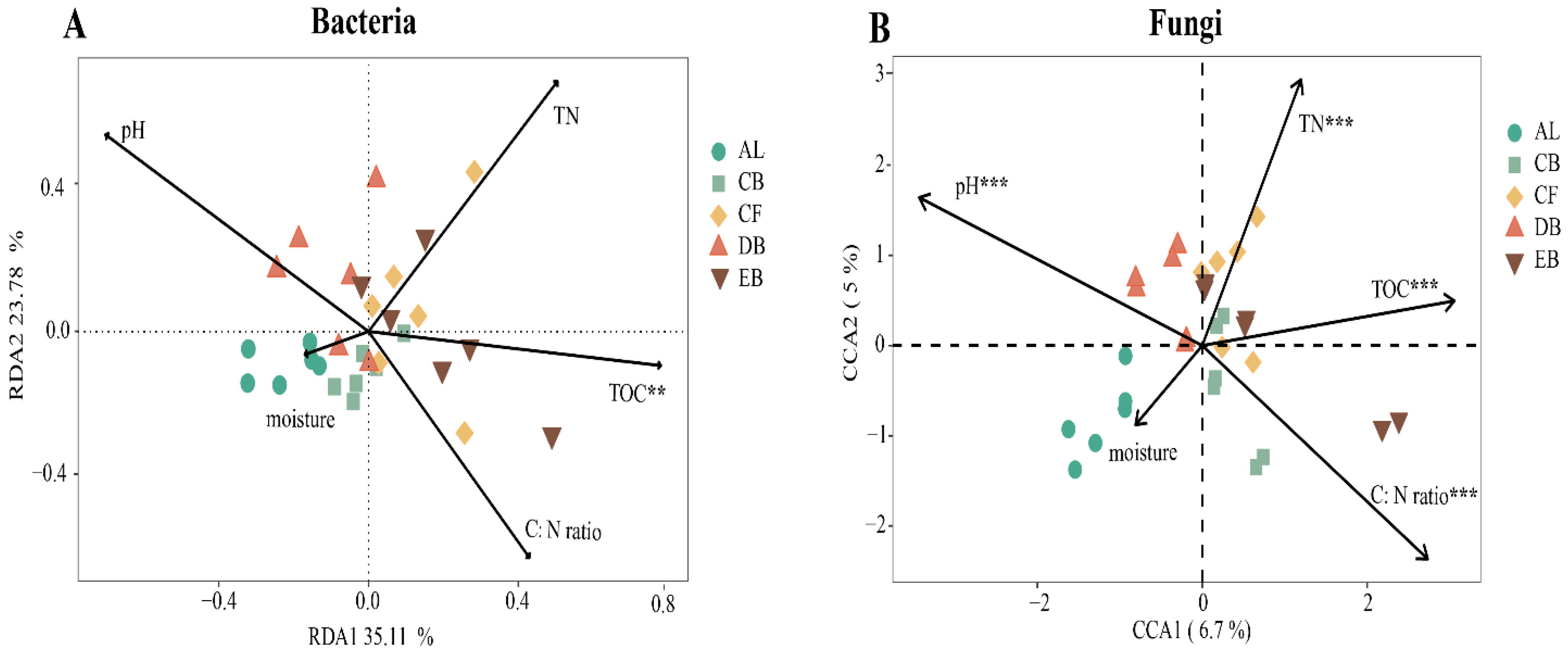
3.5. The Correlations between Soil Microbial Communities and Soil Properties
4. Discussion
4.1. The Structure and Diversity of Soil Microbial Communities during the Succession Stages
4.2. The Changes in Soil Microbial Community Assembly Processes during the Succession Stages
4.3. Soil Physicochemical Properties Drive Soil Microbial Community Changes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chazdon, R.L. Beyond Deforestation: Restoring Forests and Ecosystem Services on Degraded Lands. Science 2008, 320, 1458–1460. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.A.; Asner, G.P.; Costa, M.H.; Coe, M.T.; Defries, R.; Gibbs, H.K.; Howard, E.A.; Olson, S.; Patz, J.; Ramankutty, N.; et al. Forest Degradation and Loss of Ecosystem Goods and Services in the Amazon Basin. Front. Ecol. Environ. 2007, 5, 25–32. [Google Scholar] [CrossRef]
- Betts, M.G.; Wolf, C.; Ripple, W.J.; Phalan, B.; Millers, K.A.; Duarte, A.; Butchart, S.H.; Levi, T. Global Forest Loss Disproportionately Erodes Biodiversity in Intact Landscapes. Nature 2017, 547, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Strassburg, B.B.; Iribarrem, A.; Beyer, H.L.; Cordeiro, C.L.; Crouzeilles, R.; Jakovac, C.C.; Braga Junqueira, A.; Lacerda, E.; Latawiec, A.E.; Balmford, A.; et al. Global Priority Areas for Ecosystem Restoration. Nature 2020, 586, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Uroz, S.; Buee, M.; Deveau, A.; Mieszkin, S.; Martin, F. Ecology of the Forest Microbiome: Highlights of Temperate and Boreal Ecosystems. Soil Biol. Biochem. 2016, 103, 471–488. [Google Scholar] [CrossRef]
- Lladó, S.; López-Mondéjar, R.; Baldrian, P. Forest Soil Bacteria: Diversity, Involvement in Ecosystem Processes, and Response to Global Change. Microbiol. Mol. Biol. Rev. 2017, 81, e00063-16. [Google Scholar] [CrossRef]
- Bahram, M.; Kohout, P.; Anslan, S.; Harend, H.; Abarenkov, K.; Tedersoo, L. Stochastic Distribution of Small Soil Eukaryotes Resulting from High Dispersal and Drift in a Local Environment. ISME J. 2016, 10, 885–896. [Google Scholar] [CrossRef]
- Meyer, K.M.; Memiaghe, H.; Korte, L.; Kenfack, D.; Alonso, A.; Bohannan, B.J. Why Do Microbes Exhibit Weak Biogeographic Patterns? ISME J. 2018, 12, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, G.B.; Xue, S.; Zhu, B. Changes in Soil Physico-Chemical and Microbiological Properties during Natural Succession on Abandoned Farmland in the Loess Plateau. Environ. Earth Sci. 2011, 62, 915–925. [Google Scholar] [CrossRef]
- Jiang, S.; Xing, Y.; Liu, G.; Hu, C.; Wang, X.; Yan, G.; Wang, Q. Changes in Soil Bacterial and Fungal Community Composition and Functional Groups during the Succession of Boreal Forests. Soil Biol. Biochem. 2021, 161, 108393. [Google Scholar] [CrossRef]
- Cline, L.C.; Zak, D.R. Soil Microbial Communities Are Shaped by Plant-driven Changes in Resource Availability during Secondary Succession. Ecology 2015, 96, 3374–3385. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.Z.; Bai, L.; Wang, J.Y.; Deng, J.; Ren, C.J.; Han, X.H.; Yang, G.H.; Wang, J. Change in Soil Bacterial Community during Secondary Succession Depend on Plant and Soil Characteristics. Catena 2019, 173, 246–252. [Google Scholar] [CrossRef]
- Prescott, C.E.; Grayston, S.J. Tree Species Influence on Microbial Communities in Litter and Soil: Current Knowledge and Research Needs. For. Ecol. Manag. 2013, 309, 19–27. [Google Scholar] [CrossRef]
- Martins, K.G.; Marques, M.C.; dos Santos, E.; Marques, R. Effects of Soil Conditions on the Diversity of Tropical Forests across a Successional Gradient. For. Ecol. Manag. 2015, 349, 4–11. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Bardgett, R.D.; Vitousek, P.M.; Maestre, F.T.; Williams, M.A.; Eldridge, D.J.; Lambers, H.; Neuhauser, S.; Gallardo, A.; García-Velázquez, L.; et al. Changes in belowground biodiversity during ecosystem development. Proc. Natl. Acad. Sci. USA 2019, 116, 6891–6896. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ning, D. Stochastic Community Assembly: Does It Matter in Microbial Ecology? Microbiol. Mol. Biol. Rev. 2017, 81, e00002-17. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, K.; Krause, S.M.; Li, S.; Wang, X.; Zhang, Z.; Shen, M.; Yang, Q.; Lian, J.; Wang, X. Changes in Assembly Processes of Soil Microbial Communities during Secondary Succession in Two Subtropical Forests. Soil Biol. Biochem. 2021, 154, 108144. [Google Scholar] [CrossRef]
- Qiang, W.; He, L.; Zhang, Y.; Liu, B.; Liu, Y.; Liu, Q.; Pang, X. Aboveground Vegetation and Soil Physicochemical Properties Jointly Drive the Shift of Soil Microbial Community during Subalpine Secondary Succession in Southwest China. Catena 2021, 202, 105251. [Google Scholar] [CrossRef]
- Vandermeer, J.H. Niche Theory. Annu. Rev. Ecol. Syst. 1972, 3, 107–132. [Google Scholar] [CrossRef]
- Ning, D.; Yuan, M.; Wu, L.; Zhang, Y.; Guo, X.; Zhou, X.; Yang, Y.; Arkin, A.P.; Firestone, M.K.; Zhou, J. A Quantitative Framework Reveals Ecological Drivers of Grassland Microbial Community Assembly in Response to Warming. Nat. Commun. 2020, 11, 4717. [Google Scholar] [CrossRef] [PubMed]
- Vályi, K.; Mardhiah, U.; Rillig, M.C.; Hempel, S. Community Assembly and Coexistence in Communities of Arbuscular Mycorrhizal Fungi. ISME J. 2016, 10, 2341–2351. [Google Scholar] [CrossRef] [PubMed]
- Chesson, P. Mechanisms of Maintenance of Species Diversity. Annu. Rev. Ecol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef]
- Chase, J.M.; Myers, J.A. Disentangling the Importance of Ecological Niches from Stochastic Processes across Scales. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2351–2363. [Google Scholar] [CrossRef]
- Adler, P.B.; HilleRisLambers, J.; Levine, J.M. A Niche for Neutrality. Ecol. Lett. 2007, 10, 95–104. [Google Scholar] [CrossRef]
- Ren, C.; Liu, W.; Zhao, F.; Zhong, Z.; Deng, J.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Soil Bacterial and Fungal Diversity and Compositions Respond Differently to Forest Development. Catena 2019, 181, 104071. [Google Scholar] [CrossRef]
- Cutler, N.A.; Chaput, D.L.; van der Gast, C.J. Long-Term Changes in Soil Microbial Communities during Primary Succession. Soil Biol. Biochem. 2014, 69, 359–370. [Google Scholar] [CrossRef]
- Chen, H.-M.; Shi, F.-X.; Xu, J.-W.; Liu, X.-P.; Mao, R. Tree Mycorrhizal Type Controls over Soil Water-Extractable Organic Matter Quantity and Biodegradation in a Subtropical Forest of Southern China. For. Ecol. Manag. 2023, 535, 120900. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, F.; Li, Y. Key Issues of Land Use in China and Implications for Policy Making. Land Use Policy 2014, 40, 6–12. [Google Scholar] [CrossRef]
- Tong, X.; Brandt, M.; Yue, Y.; Ciais, P.; Rudbeck Jepsen, M.; Penuelas, J.; Wigneron, J.-P.; Xiao, X.; Song, X.-P.; Horion, S. Forest Management in Southern China Generates Short Term Extensive Carbon Sequestration. Nat. Commun. 2020, 11, 129. [Google Scholar] [CrossRef]
- Tang, C.Q.; He, L.-Y.; Su, W.-H.; Zhang, G.-F.; Wang, H.-C.; Peng, M.-C.; Wu, Z.-L.; Wang, C.-Y. Regeneration, Recovery and Succession of a Pinus Yunnanensis Community Five Years after a Mega-Fire in Central Yunnan, China. For. Ecol. Manag. 2013, 294, 188–196. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Jiang, L.; Luo, Y. Trends in Soil Microbial Communities during Secondary Succession. Soil Biol. Biochem. 2017, 115, 92–99. [Google Scholar] [CrossRef]
- Li, S.; Huang, X.; Shen, J.; Xu, F.; Su, J. Effects of Plant Diversity and Soil Properties on Soil Fungal Community Structure with Secondary Succession in the Pinus Yunnanensis Forest. Geoderma 2020, 379, 114646. [Google Scholar] [CrossRef]
- Huang, K.-X.; Xue, Z.-J.; Wu, J.-C.; Wang, H.; Zhou, H.-Q.; Xiao, Z.-B.; Zhou, W.; Cai, J.-F.; Hu, L.-W.; Ren, J.-S. Water Use Efficiency of Five Tree Species and Its Relationships with Leaf Nutrients in a Subtropical Broad-Leaf Evergreen Forest of Southern China. Forests 2023, 14, 2298. [Google Scholar] [CrossRef]
- Woese, C.R.; Kandler, O.; Wheelis, M.L. Towards a Natural System of Organisms: Proposal for the Domains Archaea, Bacteria, and Eucarya. Proc. Natl. Acad. Sci. USA 1990, 87, 4576–4579. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, R.H.; Larsson, K.-H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L. The UNITE Database for Molecular Identification of Fungi: Handling Dark Taxa and Parallel Taxonomic Classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing Taxonomic Classification of Marker-Gene Amplicon Sequences with QIIME 2’s Q2-Feature-Classifier Plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Paulson, J.N.; Stine, O.C.; Bravo, H.C.; Pop, M. Differential Abundance Analysis for Microbial Marker-Gene Surveys. Nat. Methods 2013, 10, 1200–1202. [Google Scholar] [CrossRef] [PubMed]
- Chase, J.M.; Kraft, N.J.; Smith, K.G.; Vellend, M.; Inouye, B.D. Using Null Models to Disentangle Variation in Community Dissimilarity from Variation in A-diversity. Ecosphere 2011, 2, 1–11. [Google Scholar] [CrossRef]
- Stegen, J.C.; Lin, X.; Fredrickson, J.K.; Chen, X.; Kennedy, D.W.; Murray, C.J.; Rockhold, M.L.; Konopka, A. Quantifying Community Assembly Processes and Identifying Features That Impose Them. ISME J. 2013, 7, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Stegen, J.C.; Lin, X.; Fredrickson, J.K.; Konopka, A.E. Estimating and Mapping Ecological Processes Influencing Microbial Community Assembly. Front. Microbiol. 2015, 6, 370. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Yan, W.; Wang, R.; Wang, W.; Shangguan, Z. Decreased Occurrence of Carbon Cycle Functions in Microbial Communities along with Long-Term Secondary Succession. Soil Biol. Biochem. 2018, 123, 207–217. [Google Scholar] [CrossRef]
- Flores-Rentería, D.; Sánchez-Gallén, I.; Morales-Rojas, D.; Larsen, J.; Alvarez-Sanchez, J. Changes in the Abundance and Composition of a Microbial Community Associated with Land Use Change in a Mexican Tropical Rain Forest. J. Soil Sci. Plant Nutr. 2020, 20, 1144–1155. [Google Scholar] [CrossRef]
- Eilers, K.G.; Lauber, C.L.; Knight, R.; Fierer, N. Shifts in Bacterial Community Structure Associated with Inputs of Low Molecular Weight Carbon Compounds to Soil. Soil Biol. Biochem. 2010, 42, 896–903. [Google Scholar] [CrossRef]
- Ho, A.; Di Lonardo, D.P.; Bodelier, P.L. Revisiting Life Strategy Concepts in Environmental Microbial Ecology. FEMS Microbiol. Ecol. 2017, 93, fix006. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an Ecological Classification of Soil Bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Filippidou, S.; Wunderlin, T.; Junier, T.; Jeanneret, N.; Dorador, C.; Molina, V.; Johnson, D.R.; Junier, P. A Combination of Extreme Environmental Conditions Favor the Prevalence of Endospore-Forming Firmicutes. Front. Microbiol. 2016, 7, 1707. [Google Scholar] [CrossRef]
- Chai, Y.; Cao, Y.; Yue, M.; Tian, T.; Yin, Q.; Dang, H.; Quan, J.; Zhang, R.; Wang, M. Soil Abiotic Properties and Plant Functional Traits Mediate Associations between Soil Microbial and Plant Communities during a Secondary Forest Succession on the Loess Plateau. Front. Microbiol. 2019, 10, 895. [Google Scholar] [CrossRef]
- Dong, K.; Tripathi, B.; Moroenyane, I.; Kim, W.; Li, N.; Chu, H.; Adams, J. Soil Fungal Community Development in a High Arctic Glacier Foreland Follows a Directional Replacement Model, with a Mid-Successional Diversity Maximum. Sci. Rep. 2016, 6, 26360. [Google Scholar] [CrossRef]
- Urbanová, M.; Šnajdr, J.; Baldrian, P. Composition of Fungal and Bacterial Communities in Forest Litter and Soil Is Largely Determined by Dominant Trees. Soil Biol. Biochem. 2015, 84, 53–64. [Google Scholar] [CrossRef]
- Yan, B.; Sun, L.; Li, J.; Liang, C.; Wei, F.; Xue, S.; Wang, G. Change in Composition and Potential Functional Genes of Soil Bacterial and Fungal Communities with Secondary Succession in Quercus Liaotungensis Forests of the Loess Plateau, Western China. Geoderma 2020, 364, 114199. [Google Scholar] [CrossRef]
- Liang, Y.; Ning, D.; Lu, Z.; Zhang, N.; Hale, L.; Wu, L.; Clark, I.M.; McGrath, S.P.; Storkey, J.; Hirsch, P.R. Century Long Fertilization Reduces Stochasticity Controlling Grassland Microbial Community Succession. Soil Biol. Biochem. 2020, 151, 108023. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.; Hu, A.; Wang, F.; Chen, Y.; Gu, Z.; Anslan, S.; Hou, J. Different Community Assembly Mechanisms Underlie Similar Biogeography of Bacteria and Microeukaryotes in Tibetan Lakes. FEMS Microbiol. Ecol. 2020, 96, fiaa071. [Google Scholar] [CrossRef]
- Zhang, Q.; Goberna, M.; Liu, Y.; Cui, M.; Yang, H.; Sun, Q.; Insam, H.; Zhou, J. Competition and Habitat Filtering Jointly Explain Phylogenetic Structure of Soil Bacterial Communities across Elevational Gradients. Environ. Microbiol. 2018, 20, 2386–2396. [Google Scholar] [CrossRef]
- Phillips, R.P.; Brzostek, E.; Midgley, M.G. The Mycorrhizal-associated Nutrient Economy: A New Framework for Predicting Carbon–Nutrient Couplings in Temperate Forests. New Phytol. 2013, 199, 41–51. [Google Scholar] [CrossRef]
- Smith, A.P.; Marín-Spiotta, E.; Balser, T. Successional and Seasonal Variations in Soil and Litter Microbial Community Structure and Function during Tropical Postagricultural Forest Regeneration: A Multiyear Study. Glob. Change Biol. 2015, 21, 3532–3547. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Zhao, Y.; Zhang, W.; Hu, G.; Xie, H.; Yan, J.; Han, S.; He, H.; Zhang, X. Linkage of Microbial Residue Dynamics with Soil Organic Carbon Accumulation during Subtropical Forest Succession. Soil Biol. Biochem. 2017, 114, 114–120. [Google Scholar] [CrossRef]
- Landesman, W.J.; Nelson, D.M.; Fitzpatrick, M.C. Soil Properties and Tree Species Drive SS-Diversity of Soil Bacterial Communities. Soil Biol. Biochem. 2014, 76, 201–209. [Google Scholar] [CrossRef]
- Yan, G.; Luo, X.; Huang, B.; Wang, H.; Sun, X.; Gao, H.; Zhou, M.; Xing, Y.; Wang, Q. Assembly Processes, Driving Factors, and Shifts in Soil Microbial Communities across Secondary Forest Succession. Land Degrad. Dev. 2023, 34, 3130–3143. [Google Scholar] [CrossRef]
- Goldmann, K.; Schöning, I.; Buscot, F.; Wubet, T. Forest Management Type Influences Diversity and Community Composition of Soil Fungi across Temperate Forest Ecosystems. Front. Microbiol. 2015, 6, 1300. [Google Scholar] [CrossRef] [PubMed]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil Bacterial and Fungal Communities across a pH Gradient in an Arable Soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef] [PubMed]

| Successional Stages | Dominant Tree Species | Soil Types | Altitude (m) |
|---|---|---|---|
| AL | Euphorbia latiris and Echinochloa crus galli | yellow-red soil | 250–318 |
| DB | Liquidambar formosana | yellow-red soil | 273–488 |
| CF | Cunninghamia lanceolata and Pinus massoniana | yellow-red soil | 452–501 |
| CB | Cunninghamia lanceolata, Alniphyllum fortunei, Sassafras tzumu, Syzygium buxifolium | yellow-red soil | 297–453 |
| EB | Castanopsis eyrei, Castanopsis nigrescens, Cyclobalanopsis glauca and Syzygium buxifolium | yellow-red soil | 287–454 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, J.; Huang, K.; Xu, F.; Zhang, Y.; Yuan, B.; Chen, H.; Shi, F. The Changes in Soil Microbial Communities and Assembly Processes along Vegetation Succession in a Subtropical Forest. Forests 2024, 15, 242. https://doi.org/10.3390/f15020242
Ren J, Huang K, Xu F, Zhang Y, Yuan B, Chen H, Shi F. The Changes in Soil Microbial Communities and Assembly Processes along Vegetation Succession in a Subtropical Forest. Forests. 2024; 15(2):242. https://doi.org/10.3390/f15020242
Chicago/Turabian StyleRen, Jiusheng, Kangxiang Huang, Fangfang Xu, Yuan Zhang, Bosen Yuan, Huimin Chen, and Fuxi Shi. 2024. "The Changes in Soil Microbial Communities and Assembly Processes along Vegetation Succession in a Subtropical Forest" Forests 15, no. 2: 242. https://doi.org/10.3390/f15020242
APA StyleRen, J., Huang, K., Xu, F., Zhang, Y., Yuan, B., Chen, H., & Shi, F. (2024). The Changes in Soil Microbial Communities and Assembly Processes along Vegetation Succession in a Subtropical Forest. Forests, 15(2), 242. https://doi.org/10.3390/f15020242