Abstract
This study aimed to evaluate the effect of inoculants of endophytic bacteria producing indoleacetic acid (IAA) on the physiological quality of seeds and the production of seedlings of Corymbia citriodora (Hook.) KD Hill & LAS Johnson. In the physiological quality test of the seeds, the treatments used were individual inoculation with Priestia megaterium, Exiguobacterium sibiricum, Pantoea vagans strain 45URP4-1, and Bacillus sp.; joint effect of the four strains (mix); inoculation only with the carrier (cassava starch and activated charcoal); carrier with 1.0 μg mL−1 of IAA; and non-inoculated control without IAA and without a carrier. In the production of seedlings in a greenhouse, the treatments were the same, except for the mix, which was replaced by P. vagans strain 7URP1-6 (Pvs7), as inoculation with the mix increased the number of abnormal seedlings. In the physiological quality test of seeds, seeds inoculated with the bacteria individually did not have the physiological quality impaired and the carrier created a microenvironment around the seeds, benefiting germination percentage, germination speed index, average germination time, and average germination speed. In the greenhouse, seedlings inoculated with Pvs7, P. megaterium and E. sibiricum were taller, with a larger stem diameter and dry mass of shoot, roots, and total. Seeds inoculated with E. sibiricum had higher averages for height, chlorophyll b content, and shoot and total dry mass, as well as a greater ability to colonize the rhizosphere and roots of C. citriodora, resulting in the production of higher-quality seedlings. Inoculation of seeds of C. citriodora with endophytic bacteria proved to be a promising alternative for plant development.
1. Introduction
Plant growth-promoting bacteria (PGPB) can be divided into two groups regarding their colonization: rhizospherical and endophytic [1]. The rhizosphere bacteria colonize the roots externally, while the endophytic ones colonize internally. Endophytes, by colonizing plant tissues internally, may be more likely to associate with plants, as they will be protected from the adverse effects of the environment, such as edaphoclimatic variations (rainfall, drought, and pH changes, among others) and competition with other microorganisms [2,3]. PGPB benefits plants through the synthesis of phytohormones, facilitating the absorption of nutrients in the soil by promoting root growth and the synthesis of enzymes such as nitrogenase, nitrate reductase, urease, and catalase; siderophores production; fixation of atmospheric nitrogen; antagonizing plant pathogens; and inducing resistance to biotic and abiotic factors [4,5,6]. In return, the plant provides protection and photoassimilates for bacterial growth and propagation.
Inoculating seeds with bacteria is a common practice in oilseed crops, such as soybeans, as it improves plant development [2]. According to Paravar et al. [6], coating seeds with microorganisms is a method that can improve the physiological and physical properties of seeds, as well as plant growth rates, and alleviate biotic and abiotic stresses. Despite the benefits, this technique has yet to be widespread in forest species, as is the case with species of the genus Corymbia. Corymbia species are difficult to propagate vegetatively due to recalcitrance to rooting. For this reason, many plantations end up being established via seminal means, as occurs with the species Corymbia citriodora [7,8]. C. citriodora is a species of Australian origin, adapted to different soil and climate conditions; wood is used in the production of charcoal, firewood, posts, and sawmills, among other uses [7,9]. The essential oil extracted from its leaves is used as a raw material for the perfumery, cleaning, and pharmaceutical industries [7].
Although bacteria inoculation of seeds benefits plant development, bacteria can produce compounds that, in large quantities, are harmful to the physiological quality of seeds, such as indoleacetic acid (IAA). IAA is a hormone belonging to the auxin group, which acts mainly in the formation of lateral roots and root hair. Excess IAA becomes harmful due to auxins, in general, having herbicidal action [10]. The production of 171.1 μg mL−1 of IAA by Rhizobium leguminosarum resulted in loss of seed vigor and the formation of abnormal lettuce seedlings [11]. The strains 7URP1-6 and 45URP4-1 of Pantoea vagans, 11URP4-2 of Priestia megaterium, 19RP3L2-7 of Exiguobacterium sibiricum, and 58CRP4-3 of Bacillus sp., used in the study, produce between 85 and 129 μg mL⁻¹ of IAA in vitro [12].
Therefore, physiological quality tests must be developed to verify whether the bacteria inhibit seed germination before inoculating them en masse. If bacteria do not inhibit the physiological quality of seeds, their potential in plant development can be explored. Thus, this study aimed to evaluate the effect of IAA-producing endophytic bacteria on the physiological quality of seeds and the production of C. citriodora seedlings.
2. Material and Methods
2.1. Physiological Quality of Seeds
2.1.1. Inoculant Production and Seed Inoculation
The experiment was implemented at the Soil Microbiology Laboratory, Department of Forest Engineering, Federal University of the Jequitinhonha and Mucuri Valleys (UFVJM), Diamantina, Minas Gerais State of Brazil, in April 2021. The seeds of C. citriodora were obtained from the company BENTEC-seeds®, inputs and technology, with a control record CITRINCO8NV and germination power of 80%. The seeds were disinfested in a laminar flow chamber (Filterflux, São Paulo, Brazil). For this, the seeds were washed six times with distilled and sterilized water and immersed in 70% alcohol solution for 30 s and 5% sodium hypochlorite for 15 min and then rinsed six times with distilled and sterilized water [13].
The bacteria were multiplied in 50 mL of Luria-Bertani liquid medium, composed of 10 g of tryptone, 5 g of yeast extract, and 5 g of sodium chloride per liter and incubated (Shaker Incubator Cienlab, São Paulo, Brazil) at 28 °C for 72 h under orbital agitation of 120 rpm. Then, the culture was centrifuged (Kasvi K14-400) at 4000 rpm for 10 min to remove the supernatant and preserve the bacterial mass. The bacterial cells were resuspended in 0.85% saline solution with the aid of the vortex (Kasvi K45-2810). Subsequently, the cell density was diluted in the saline solution until the cell suspension reached the optical density of 1.0 on a digital spectrophotometer (850MI) at a wavelength of 540 nm, which corresponds to 109 CFU mL−1, using the saline solution as a blank [14].
The inoculation carrier was prepared using 5 g of cassava starch dissolved in 100 mL of distilled and sterilized water (Autoclave Marte av 11), which was previously heated until the gum appearance was formed. Each 120 g of seeds was mixed with 5 mL of the meticulously prepared gum. The carrier was composed of activated charcoal (porosity 0.8–2.0 nm), which corresponded to 30%–50% of the volume of the bacterial mass resuspended in saline solution. The charcoal was added to 5 mL of the suspension of each bacterium, leaving for three minutes and, after this period, the mixture of charcoal and bacteria was added to the seeds pre-treated with the gum [14] and placed to grow in rolls of Germitest® papers.
2.1.2. Treatments and Experimental Design
The treatments used in this study were individual inoculation with Priestia megaterium strain 11URP4-2, Exiguobacterium sibiricum strain 19RP3L2-7, Pantoea vagans strain 45URP4-1, and Bacillus sp. strain 58CRP4-3; inoculation of seeds with four bacterial strains together (Mix); inoculation of seeds only with the carrier used in the composition of the inoculant; inoculation of seed with the carrier supplemented with 1.0 μg mL−1 of IAA previously dissolved in distilled and sterilized water [15]; and non-inoculated control, without IAA and without inoculation carrier. The inoculant mix was prepared using the volume of ¼ of each strain.
The bacteria were chosen from the collection of the Soil Microbiology Laboratory of the UFVJM for the greater production capacity of IAA in vitro by the cultivation method supplemented with 10% tryptophan and determined by the occurrence of oxidation by the Salkowski mixture [16]. The strains were isolated from eucalyptus roots previously superficially disinfested to obtain endophytic ones [12]; their characteristics are presented in Table 1.

Table 1.
Characteristics of the bacteria used in the experiment.
The experimental design was completely randomized, with five replicates of 50 seeds each per treatment. The seeds, which were inoculated with their respective treatments, were packed in rolls with two sheets of paper towels Germitest® type moistened with distilled and sterilized water in the proportion of 2.5 times the mass of the dry paper [17]. Then, the seeds were incubated in a BOD germination chamber (Solab Sb224) at 25 °C and a photoperiod of 12 h.
2.1.3. Evaluations
Daily and at the same time, the number of germinated seeds was counted and seeds with radicle length equal to or greater than 2 mm were considered germinated. The Germination Speed Index (GSI) was calculated by the equation GSI = ∑(ni/ti), where ni = the number of seeds that germinated at time “i”, ti = time after installation of the test; and i = 1 ⟶ 12 days [18]. The counts were made until the germination was stabilized.
After germination was stabilized, the following were evaluated:
- -
- Germination percentage (GP): Calculated by the formula GP = (NG/NP) × 100, where NG = number of seeds germinated at the end of the test and NP = number of seeds placed to germinate;
- -
- Average germination speed (AGS): Calculated by the equation AGS = 1/t where t = average germination time;
- -
- Average germination time (AGT): calculated by the equation AGT = (∑niti)/∑ni, where ni = number of germinated seeds per day; ti = incubation time; and i = 1 ⟶ 11 days;
- -
- Percentage of dead seeds (PDS): PDS = (ND/NG) × 100, where ND = number of dead seeds and NG = number of seeds placed to germinate;
- -
- Entropy: calculated according to the procedure adopted by Labouriau and Valadares [19]. where: E = informational entropy; fi = relative frequency of germination; and log2 = logarithm in base 2.
The number of normal and abnormal seedlings was also counted. The seedlings considered abnormal were those that did not have the potential to continue their development and give rise to normal plants, even growing in favorable conditions, and the following characteristics were analyzed: damage, deformations, and deterioration, both shoot and of the root system [17].
2.1.4. Statistical Analysis
The data were submitted to the Durbin–Watson test, to verify the independence of the errors, Shapiro–Wilk, to verify normality, and Bartlett, to verify the homogeneity of variances. Then, the analysis of variance was performed (p < 0.01 and p < 0.05) and, when significant, the means were grouped by the Scott-Knott test (p < 0.05). The statistical program R version 4.3.1 and the package ExpDes.pt were used [20].
2.2. Production of Seedlings in Greenhouse
2.2.1. Treatments, Substrate, Conduction, Design, and Evaluations
The experiment was set up at the Olericulture Sector of UFVJM in March 2022. The treatments were similar to those used in the previous experiment, with the difference that the mix was not used due to the increase in the number of abnormal seedlings in the germination test caused by it and the addition of the bacterium Pantoea vagans strain 7URP1-6 (GenBank code: OQ996385). This strain is gram-negative, N-fixative, and isolated from rooted cutting roots of the hybrid E. grandis × E. urophylla in a fertilized environment, with in vitro production of 85 μg mL−1 of IAA, synthesizing enzymes such as catalase, urease, and nitrate reductase, besides being able to solubilize inorganic and organic sources of phosphate, such as calcium phosphate and sodium phytate [12]. The inoculant production and inoculation were the same as in the previous experiment.
Tubes of 55 cm3 were filled with substrate composed of 70% coconut husk fiber and 30% carbonized rice husk and fertilized with 2 kg m−3 of osmocote (NPK: 10-06-10), 1 kg m−3 of Superphosphate Simple (NPK: 00-17-00) and 1 kg m−3 of Mono Ammonium Phosphate (NPK: 12-61-00) and then three seeds inoculated with their respective treatments were sown in the substrate of each tube. Twelve days after sowing, once the emergence was stabilized, thinning was carried out, leaving one plant per tube. Fifteen days after sowing, a reinoculation with 5 mL per plant with its respective treatment was performed. The reinoculation was performed with the aid of a 20 mL disposable syringe coupled to a breast probe. The inoculant was applied close to the roots. Until the 60th day, the plants were fertilized weekly with Clark’s nutrient solution with ½ of the original strength and from 60° to 120° the complete Clark solution was applied [21].
The experiment was conducted in a randomized block design with five blocks, with each treatment, consisting of 15 plants. At 120 days after sowing, 10 plants were randomly harvested from each treatment and the following were evaluated: plant height, measured with a millimeter ruler; stem diameter, with a digital caliper (Digimess 100.179N); and chlorophyll a and b content, quantified directly with a ClorofiLOG brand chlorophyllometer (Falker CFL1030), according to the manufacturer’s instructions. Then, the shoot and roots were separated. These were dried in an oven (Solab SL100) at 65 °C until reaching constant weight and weighed on a precision analytical balance (Marte Ay220) to determine the shoot dry mass (SDM) and the root dry mass (RDM) and calculated the total dry mass (TDM), which was the sum of the SDM with the RDM.
2.2.2. Bacterial Quantification of Shoots, Roots, and Rhizosphere
Two plants from each treatment were harvested randomly and shoots and roots were separated and then weighed. The plant tissues were superficially disinfested by sequential immersion in 70% ethyl alcohol for one minute, in the mixture of 2% sodium hypochlorite +0.1 mL of polysorbate Tween® 80 for two minutes for shoots and three minutes for roots and, finally, in 70% ethyl alcohol for 30 s. Then, the plant tissues were immersed in distilled and sterilized water and shaken manually for one minute; finally, they were rinsed with distilled water and sterilized four more times, changing water with each new wash [22].
Before root disinfestation, the substrate was removed manually and the roots were placed in saline solution (9 mL of saline solution per 1 g of roots) and agitated orbitally for 30 min at 200 rpm to displace the bacteria from the rhizosphere. The saline solution was composed of one liter of distilled water plus 3.4 g of KH2PO4; 0.2 g of MgSO4; 0.1 g of NaCl; and 0.02 g of CaCl2; 2 mL of the micronutrient solution (0.04 g of CuSO4; 1.20 g of ZnSO4; 1.40 g of H3BO3; 1.00 g of Na2MoO4; and 1.175 g of MnSO4 per liter of distilled water); and 4 mL of ferric EDTA (1.64% solution) and 4.5 g of KOH, with the final pH adjusted at 7.0 [22]. Then, one gram of shoot and root tissue was macerated in grail containing 9 mL of the saline above the solution.
The suspensions resulting from maceration were transferred to 90 mL vials and shaken (Shaker Incubator Cienlab, São Paulo, Brazil) for 30 min at 200 rpm. Then, 1 mL of the suspension was transferred to a Falcon polypropylene tube containing 9 mL of saline, corresponding to the 10−1 dilution, with serial dilution up to 10−4 for shoots and 10−5 for roots and rhizosphere. From the tubes of the last three dilutions of each part (shoot, roots, and rhizosphere), 0.1 mL of the suspension was removed and transferred to Petri plates with TSA medium (Tryptone Soy Agar). For each dilution, four replications were used and the plates were incubated in a BOD (Solab Sb224, São Paulo, Brazil) chamber for three days at 28 °C. After this period, the number of colonies was counted in the dilutions that allowed growth between 25 and 300 colonies. The bacterial density was calculated considering the dilution and the aliquot inoculated in the plate and expressed in the number of CFU per gram of tissue, with the average of the plates of each treatment whose number of colonies was between 25 and 300.
2.2.3. Statistical Analysis
The data of height, stem diameter, chlorophyll a and b content, and dry mass of roots, shoot, and total were subjected to multivariate normality analysis, using the Doornik and Hansen [23] test (p < 0.05). Then, multivariate analysis of variance was conducted using Wilks Lambda, Pillai Trait, Hotelling–Lawley Trait, and Roy maximum root tests (p < 0.05). Grouping analysis of treatments was carried out using the Ward method (formation of homogeneous groups by the lowest minimum internal variance), being used as a reference to the Euclidean distance and the Pearson coefficient. To discriminate the treatment groups according to the agronomic variables of C. citriodora, canonical discriminant analysis was performed, which was represented in a biplot graph constructed for the first two canonical variables. Then, 95% confidence ellipses were constructed to detect statistical differences (p < 0.05) between treatment groups. All analyses were performed with R software version 4.2.2 [24]. The canonical discriminant analysis was performed with the aid of the candisc package [25].
The number of CFU in the rhizosphere, roots, and shoots was submitted to the Durbin–Watson, Shapiro-Wilk, Bartlett, and Tukey.1df tests to verify the independence of errors, normality, homogeneity of variances, and additivity, respectively, and then the analysis of variance. When there were significant differences, the means were grouped by the Scott-Knott test (p < 0.05) using the statistical program R [24].
Finally, Pearson correlation was performed between the variables (threshold set at 0.60). A correlation network was set up to graphically illustrate Pearson correlation analyses, in which the proximity between nodes is proportional to the absolute correlation values between the variables. These analyses were carried out using the Rbio version 190 software [26].
3. Results
3.1. Physiological Quality of Seeds
Inoculation influenced the germination percentage and dead seeds, germination speed index, average germination time, average germination speed, and number of normal and abnormal seedlings. Entropy was the only variable that was not influenced by the treatments (Table 2).

Table 2.
Analysis of variance for germination percentage (GP), percentage of dead seeds (PDS), germination speed index (GSI), average germination time (AGT), average germination speed (AGS), number of normal (NS) and abnormal (AS) seedlings, and entropy (ENT) of Corymbia citriodora seeds inoculated with Pantoea vagans strain 45URP4-1, Priestia megaterium, Exiguobacterium sibiricum, Bacillus sp., inoculation carrier, inoculation carrier with 1.0 μg mL−1 of IAA and control without inoculation.
The highest percentage of germination, lowest percentage of dead seeds, and highest number of normal seedlings were observed in seeds treated only with the inoculation carrier and in those inoculated with the P. vagans strain 45URP4-1 (Table 3). The lowest germination speed index, highest average germination time, and lowest average germination speed were observed in the non-inoculated seeds, without carrier and without IAA, and the other treatments did not differ for these variables. The seeds inoculated with the mix generated a higher number of abnormal seedlings (Table 3).

Table 3.
Germination percentage (GP), percentage of dead seeds (PDS), germination speed index (GSI), average germination time (AGT), average germination speed (AGS), number of abnormal (AS), and normal (NS) seedlings of Corymbia citriodora seeds inoculated or not with endophytic bacteria.
3.2. Production of Seedlings in Greenhouse
There was a significant difference between the treatment by the multivariate Wilks Lambda test, Pillai Trait, Hotelling–Lawley Trait, and Roy maximum root for the variables plant height (PH), stem diameter (SD), and chlorophyll a (Ca) and b (Cb) content and dry mass of roots (RDM), shoot (SDM), and total (TDM) of C. citriodora plants (Table 4).

Table 4.
Multivariate analysis of variance for the vectors of treatment averages of Corymbia citriodora plants inoculated with Pantoea vagans strains 7URP1-6 and 45URP4-1, Priestia megaterium, Exiguobacterium sibiricum, Bacillus sp., inoculation carrier, and inoculation carrier with 1.0 μg mL−1 of IAA and control without inoculation.
The treatments were grouped into four groups according to the characteristics PH, SD, Ca, Cb, SDM, RDM, and TDM, being Group I. Control, control with carrier and carrier with 1.0 μg mL−1 of IAA. Group II: P. vagans strain 45URP4-1 and Bacillus sp. Group III: E. sibiricum. Group IV: P. vagans strain 7URP1-6 and P. megaterium (Figure 1).
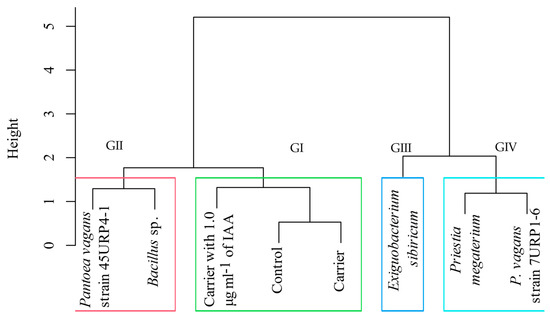
Figure 1.
Grouping of dendrogram treatments with Euclidean distance considering the variables plant height, stem diameter, chlorophyll a and b content, and dry mass of roots, shoot, and total plants of C. citriodora inoculated with endophytic bacteria and treated with cassava starch and activated charcoal (carrier), carrier with 1.0 μg mL−1 of IAA and control without inoculation (Control).
The plants inoculated with E. sibiricum (group III), P. megaterium, and P. vagans strain 7URP1-6 (group IV) had higher PH, SD, Cb content, RDM, SDM, and TDM, not differing significantly from each other but differing from the plants inoculated with the other treatments (Figure 2). The plants inoculated with E. sibiricum (group III) had PH = 16.97 cm, SD = 1.42 mm, Cb = 8.71, RDM = 1.04 g, SDM = 4.79 g, and TDM = 5.83 g; on average, the plants inoculated with P. megaterium and P. vagans strain 7URP1-6 (group IV) had PH = 16.04 cm, SD = 1.46 mm, Cb = 8.23, RDM = 1.05 g, SDM = 4.12 g, and TDM = 5.16 g; and while the plants that received the control treatments (group I) obtained the means of PH = 14.08 cm, SD = 1.20 mm, Cb = 8.43, RDM = 1.04 g, SDM = 3.78 g, and TDM = 4.82 g (Table 5). The highest Ca content was 29.4, obtained in the plants treated with the control treatments (Group I). The average Ca contents of plants inoculated with E. sibiricum (group III), P. megaterium, and P. vagans strain 7URP1-6 (group IV) were 28.75 and 28.09, respectively. Plants inoculated with P. vagans strain 45URP4-1 and Bacillus sp. (Group II) presented intermediate results, with means taller than Group I for PH (14.44 cm), SD (1.41 mm), SDM (4.31 g), and TDM (5.33 g) (Figure 2).
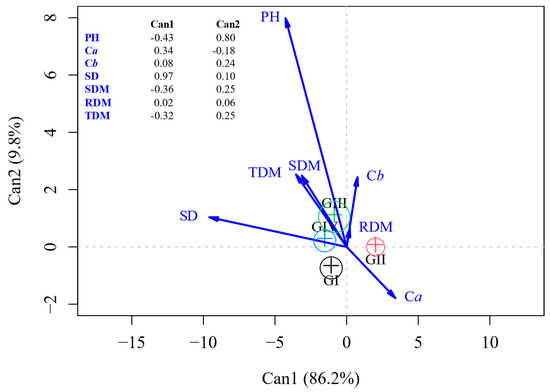
Figure 2.
Canonical discriminant analysis of the variables plant height (PH); stem diameter (SD); shoot dry mass (SDM), root dry mass (RDM); total dry mass (TDM); chlorophyll a (Ca) and b (Cb) content of Corymbia citriodora plants treated with cassava starch and activated charcoal; the carrier with 1.0 μg mL−1 of IAA and control without inoculation (GI) and inoculated with Pantoea vagans strain 45URP4-1 and Bacillus sp. (GII); Exiguobacterium sibiricum (GIII); and P. vagans strain 7URP1-6 and Priestia megaterium (GIV).

Table 5.
Means of plant height, stem diameter, chlorophyll a and b content, and dry mass of roots, shoot, and total of Corymbia citriodora plants treated with endophytic bacteria.
The highest numbers of CFU in the roots and rhizosphere were observed in the plants inoculated with E. sibiricum, followed by the roots of the plants inoculated with P. vagans strain 7URP1-6 and in the rhizosphere of the plants inoculated with P. vagans strain 45URP4-1 (Table 6). In the shoot, the highest number of CFU was observed in plants inoculated with P. vagans strain 7URP1-6. In the non-inoculated plants (control without inoculation, only carrier and carrier with IAA), lower numbers of CFU in the roots and rhizosphere were observed (Table 6). Overall, the plants had lower numbers of CFU in the shoot and the CFU numbers of the rhizosphere and roots were close.

Table 6.
Density of bacteria (CFU g of tissue−1) in the rhizosphere, roots, and shoot of Corymbia citriodora plants inoculated or not with endophytic bacteria.
The only significant positive correlations were between SDM and TDM (0.95) and between Ca and Cb content (0.65) (Figure 3).
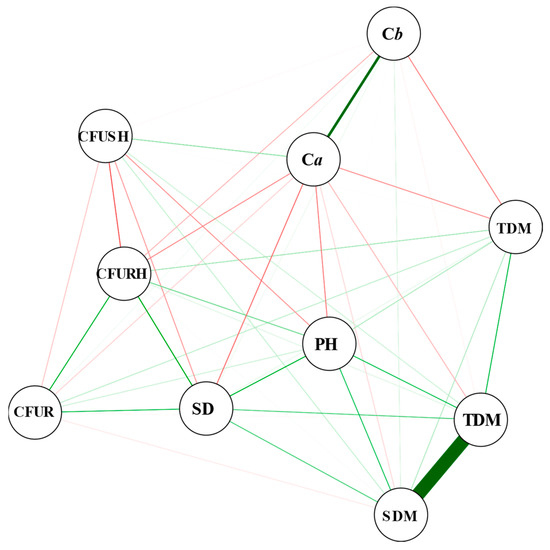
Figure 3.
Correlation network illustrating Pearson’s correlations between the variables chlorophyll a (Ca) and b (Cb); the number of colony-forming units of the shoot (CFUSH), roots (CFUR), and rhizosphere (CFURH); stem diameter (SD); plant height (PH); and dry mass of roots (RDM), shoot (SDM), and total (TDM) of Corymbia citriodora plants inoculated with Pantoea vagans strains 7URP1-6 and 45URP4-1, Priestia megaterium, Exiguobacterium sibiricum, Bacillus sp., inoculation carrier, and the carrier with 1.0 μg mL−1 of IAA and control without inoculation. The thicker greener lines represent the highest positive correlations. The red lines represent the negative correlations.
4. Discussion
The absence of s significant difference between the treatments for entropy (Table 2) shows that the bacteria and the carrier do not interfere in the organization and synchrony of seed germination. Entropy measures the organization of a system, so the lower the entropy, the greater the organization and synchrony of the system [27], in this case, represented by the population of seeds analyzed in the germination test. Therefore, the bacteria alone can be used in inoculant formulations for C. citriodora seeds as it does not interfere with entropy and other variables of the physiological quality of the seeds (Table 3).
The increase in the number of abnormal seedlings by mixed inoculation (Table 3) may have occurred due to competition between the strains for a niche [28], which may have resulted in the synthesis of compounds that impaired seedling development. Before establishing an association with plants, the bacterium–plant relationship can go through a parasitic phase, which is the period when bacteria require photosynthates from the plant to multiply and internally colonize the roots [29,30]. This may also have favored the increase in the number of abnormal seedlings due to the number of inoculated strains and the concentration of the inoculant. The success of co-inoculation is closely related to the genotype of the plant, with the proper selection of strains and the cellular concentration of each one [4]. In the present study, no previous tests were performed. Only the concentration of ¼ of each inoculant was used to formulate the mix. For further studies with co-inoculation, it is necessary first to select compatible strains and evaluate varying concentrations for each bacterium.
The benefits obtained with inoculations in the seed physiological quality test (Table 3) cannot be attributed to bacterial strains, as treatment with only the inoculation carrier also showed good results. Thus, it is inferred that the effects were not from the bacteria but from the carrier created a microenvironment around the seed, providing them with better germination conditions. One of the main characteristics of a good inoculant is its ability to retain moisture and avoid desiccation and, consequently, the death of microorganisms [31], which also stimulates the germination of the seeds because the humidity favors the germination process [32].
One of the main forms of application of inoculants is adhering the product to pre-inoculated seeds stored before sale or at sowing [31]. Bacterial survival in the seed is mainly diminished by three factors: desiccation, the toxic nature of the integument exudates, and high temperatures [33]. As the factors humidity and temperature were controlled in the physiological quality test of the seeds and the greenhouse experiment, the inoculation with P. vagans strain 7URP1-6, P. megaterium, and E. sibiricum benefited the growth of the plants (Figure 2); it is inferred that the benefits of the bacteria are not in the germination of the seeds but in the development of the seedlings.
In the greenhouse, the better development of plants after the seeds were inoculated with E. sibiricum (Figure 2) can be attributed to the colonization capacity of this species. Analyzing colonization, the numbers of CFU in the roots and rhizosphere were taller in the seeds inoculated with E. sibiricum (Table 6); one of the main points for the success of the inoculation is the ability of the bacteria to colonize the plants [3]. The E. sibiricum strain was the only one isolated from unfertilized environments and adult plants (Table 1). Exiguobacterium species can grow in extreme environments, with limited nutrients and temperatures ranging from −12 to 55 °C [34]. Thus, it is believed to have a greater ability to interact with C. citriodora plants; however, this effect may vary according to the genotype of the plant [35].
The in vitro IAA production of P. vagans strain 7URP1-6, P. megaterium, and E. sibiricum is close, ranging from 85 to 90 μg mL−1 (Table 1). The best-known effect of auxins is the stimulation of rooting, increasing the area of soil exploitation by the roots and also the absorption of water and nutrients [36,37], consequently favoring plant development, as observed in the study, namely with respect to taller plants, with larger stem diameter and dry mass of the roots, shoot, and total (Figure 2). Sousa et al. [38] inoculated E. urophylla seeds with Azospirillum amazonense and Stenotrophomonas maltophilia and also observed better development of height, stem diameter, root length, and dry matter.
In addition to the synthesis of IAA, these strains synthesize catalase (Table 1), an enzyme responsible for remediating the toxic effects of hydrogen peroxide on plant metabolism. The E. sibiricum strain also synthesizes the enzyme nitrate reductase, mainly responsible for the assimilation of nitrogen by plants, and P. megaterium to urease, an enzyme responsible for the hydrolysis of urea into carbon dioxide and ammonia. The 7URP1-6 strain of P. vagans is N-fixing and synthesizes enzymes such as urease and nitrate reductase, which, in the long term, can improve plant development, as well as being able to solubilize inorganic and organic phosphate sources, such as calcium phosphate and sodium phytate. Inoculation of rice seeds with P. vagans strain LYY2b, also producing IAA, resulted in abundant root hair production, increased root and shoot length, and root hair formation [39].
Although the strains 7URP1-6 and 45URP4-1 of P. vagans are of the same species, their effects were different. 7URP1-6 improved seedling quality, while 45URP4-1 provided intermediate seedling quality (Figure 2). This can be explained by the genetic variability that exists within species, causing them to provide different results [35].
The lower number of CFU in the shoot of plants and the numbers of CFU in the rhizosphere and nearby roots (Table 6) are explained by the colonization process since endophytes usually colonize plants through the root system from sites with epidermal damage, which arise naturally, due to the development of lateral roots, or by means of root hairs. In the rhizosphere, exudates are also released in large quantities by the roots and both endophytic and rhizosphere bacteria grow. For this reason, it is believed that the highest bacterial densities are in the rhizosphere and progressively decrease from the roots through the stem and reach the leaves, where the density is lower [40].
The levels of chlorophyll a and b were positively and significantly correlated (Figure 3). Chlorophyll a and b are pigments are associated with photosynthesis. They are usually found in nature in a ratio of 3:1 [41], which may have balanced the effect of chlorophylls on plant development because the non-inoculated plants (group I) had the highest chlorophyll a content, while those inoculated with E. sibiricum (group III) had a taller chlorophyll b content. It acts in the production of organic substances, being used in the photochemical stage of photosynthesis since chlorophyll b is an accessory pigment and acts by increasing the range of light absorption that can be used in the photosynthetic process [41].
Given the importance of inoculation and not yet having an inoculant for eucalyptus seeds, the best development of plants after inoculation with P. vagans strain 7URP1-6, P. megaterium, and E. sibiricum is a promising result, given the importance of C. citriodora for the production of coal, cellulose, furniture, firewood, poles, sawmill, rails, manufacture of floors, and extraction of essential oils from its leaves, among other applications [42,43]. The production of seedlings with taller quality in the greenhouse increases the chances of survival in the field as it has greater chances of withstanding the adverse conditions of the environment. However, it is still necessary to expand the studies to field conditions and other tree species.
5. Conclusions
The inoculation of C. citriodora seeds with P. megaterium, E. sibiricum, P. vagans strain 45URP4-1, and Bacillus sp. does not interfere with the physiological quality of the seeds. The seeds of C. citriodora inoculated with P. vagans strain 7URP1-6, P. megaterium, and E. sibiricum present similar results in plant development. However, those inoculated with E. sibiricum had taller averages for height, chlorophyll b content, and shoot and total dry mass and greater ability to colonize the roots and rhizosphere of C. citriodora, resulting in the production of better-quality seedlings. Given the benefits of inoculating C. citriodora seeds with PGPB on plant development, future studies can evaluate the effect of PGPB on other forest species that are also propagated via seed, as inoculation of seeds of C. citriodora with endophytic bacteria proved to be a promising alternative in the plant development.
Author Contributions
Conceptualization, A.M.d.O., M.R.d.C. and P.H.G.; methodology, A.M.d.O., M.R.d.C. and C.M.d.A.; software, A.M.d.O.; validation, J.F.S.M., N.R.A., G.F.P.d.A., J.V.G. and P.H.G.; formal analysis, A.M.d.O., G.F.P.d.A., J.V.G., N.R.A. and C.M.d.A.; investigation, M.R.d.C., P.H.G., N.R.A. and C.M.d.A.; data curation, A.M.d.O., G.F.P.d.A. and J.V.G.; funding acquisition, J.B.d.S., G.M.B. and C.M.d.A.; writing—original draft preparation, J.V.G., G.F.P.d.A. and A.M.d.O.; writing—review and editing, A.M.d.O., C.M.d.A., M.R.d.C., G.M.B., J.B.d.S. and P.H.G.; visualization, G.M.B., J.F.S.M., N.R.A., J.B.d.S., P.H.G. and M.R.d.C.; supervision, M.R.d.C. and P.H.G.; project administration, A.M.d.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Acknowledgments
With acknowledgements to the Foundation for Research Support of the State of Minas Gerais (FAPEMIG), the National Council for Scientific and Technological Development (CNPq), and the Coordination for the Improvement of Higher Education Personnel (CAPES)—Financial Code 001.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hardoim, P.R.; Overbeek, L.S.V.; Berg, G.; Pirttila, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef]
- Oliveira, A.M.; Costa, M.R.; Grazziotti, P.H.; Abreu, C.M.; Bispo, N.S.; Roa, J.P.B.; Silva, D.M.; Miranda, J.M. Brazilian scenario of inoculant production: A look at patents. Rev. Bras. Cienc. Solo 2022, 46, e0210081. [Google Scholar] [CrossRef]
- Souza, R.D.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.S.; Nogueira, M.A.; Hungria, M. Microbial inoculants: Reviewing the past, discussing the present and previewing an outstanding future for the use of beneficial bacteria in agriculture. AMB Express 2019, 9, 1–22. [Google Scholar] [CrossRef]
- Eid, A.M.; Fouda, A.; Abdel-Rahman, M.A.; Salem, S.S.; Elsaied, A.; Oelmüller, R.; Hijri, M.; Bhowmik, A.; Elkelish, A.; Hassan, S.E.D. Harnessing bacterial endophytes for promotion of plant growth and biotechnological applications: An overview. Plants 2021, 10, 935. [Google Scholar] [CrossRef] [PubMed]
- Paravar, A.; Piri, R.; Balouchi, H.; Ma, Y. Microbial seed coating: An attractive tool for sustainable agriculture. Biotechnol. Rep. 2023, 37, e00781. [Google Scholar] [CrossRef]
- Reis, C.A.F.; Assis, T.F.; Santos, A.M.; Paludzyszyn Filho, E. Corymbia Citriodora: Estado da Arte de Pesquisas no Brasil; Embrapa Florestas: Colombo, Brazil, 2013; 59p. [Google Scholar]
- Lima, M.S.; Araujo, M.M.; Berghetti, Á.L.P.; Aimi, S.C.; Costella, C.; Griebeler, A.M.; Somavilla, L.M.; Santos, O.P.; Valente, B.M.R.T. Mini-cutting technique application in Corymbia and Eucalyptus: Effects of mini-tunnel use across seasons of the year. New For. 2022, 53, 161–179. [Google Scholar] [CrossRef]
- Bonora, F.S.; Nahrung, H.F.; Hayes, R.A.; Scharaschkin, T.; Pegg, G.; Lee, D.J. Changes in leaf chemistry and anatomy of Corymbia citriodora subsp. variegata (Myrtaceae) in response to native and exotic pathogens. Australas. Plant Pathol. 2020, 49, 641–653. [Google Scholar] [CrossRef]
- Todd, O.E.; Figueiredo, M.R.; Morran, S.; Soni, N.; Preston, C.; Kubeš, M.F.; Napier, R.; Gaines, T.A. Synthetic auxin herbicides: Finding the lock and key to weed resistance. Plant Sci. 2020, 300, 110631. [Google Scholar] [CrossRef] [PubMed]
- Schlindwein, G.; Vargas, L.K.; Lisboa, B.B.; Azambuja, A.C.; Granada, C.E.; Gabiatti, N.C.; Prates, P.; Stumpf, R. Influência da inoculação de rizóbios sobre a germinação e o vigor de plântulas de alface. Cienc. Rural 2008, 38, 658–664. [Google Scholar] [CrossRef]
- Ramires, R.V. Microrganismos Endofíticos em Eucalyptus. Ph.D. Thesis, Universidade Federal dos Vales do Jequitinhonha e Mucuri, Diamantina, Brazil, 2021. 110 f. [Google Scholar]
- Titon, M.; Xavier, A.; Otoni, W.C.; Motoike, S.Y. Efeito dos reguladores de crescimento dicamba e picloram na embriogênese somática em Eucalyptus grandis. Rev. Árvore 2007, 31, 417–426. [Google Scholar] [CrossRef]
- Oliveira, C.A.; Marriel, I.E.; Gomes, E.A.; Mattos, B.B.; Santos, F.C.; Oliveira, M.C.; Alves, V.M.C. Metodologia de Aplicação de Microrgabnismos Solubilizadores de Fósforo em Sementes Visando Melhor Aproveitamento deste Nutriente Pelas Plantas; Embrapa: Sete Lagoas, Brazil, 2013; 29p, Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/95211/1/bol-88.pdf (accessed on 5 April 2021).
- Maravilha, L.F.; Titon, M.; Canguçu, V.S.; Rocha, F.M.; Oliveira, M.R. Enraizamento in vitro e aclimatação de plântulas de Corymbia citriodora. Pesq. Flor. Bras. 2023, 43, e202102232. [Google Scholar] [CrossRef]
- Patten, C.L.; Glick, B.R. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl. Environ. Microbiol. 2002, 68, 3795–3801. [Google Scholar] [CrossRef]
- Mapa. Ministério da Agricultura, Pecuária e Abastecimento. Regras Para Análise de Sementes. 1a ed. Brasília, 2009. 398p. Available online: https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/arquivos-publicacoes-insumos/2946_regras_analise__sementes.pdf (accessed on 2 January 2022).
- Maguire, J.D. Speed of germination-aid selection and evaluation for seedling emergence and vigor. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Labouriau, L.G.; Valadares, M.B. On the germination of seed of Calotropis procera. An. Acad. Bras. Ciênc. 1976, 48, 263–284. [Google Scholar]
- Ferreira, E.B.; Cavalcanti, P.P.; Nogueira, D.A. ExpDes: An R package for ANOVA and experimental designs. Appl. Math. 2014, 5, 2952. [Google Scholar] [CrossRef]
- Clark, R.B. Characterization of phosphatase of intact maize roots. J. Agric. Food Chem. 1975, 23, 458460. [Google Scholar] [CrossRef]
- Döbereiner, J.; Baldani, V.L.D.; Baldani, J.I. Como Isolar e Identificar Bactérias Diazotróficas de Plantas Não Leguminosas; Embrapa-SPI: Itaguaí, Brazil, 1995; 60p. [Google Scholar]
- Doornik, J.A.; Hansen, H. An omnibus test for univariate and multivariate normality. Oxf. B Econ. Stat. 2008, 70, 927–939. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 10 November 2022).
- Friendly, M.; Fox, J. Candisc: Visualizing Generalized Canonical Discriminant and Canonical Correlation Analysis, R package version 0.8-0.2017; R Foundation for Statistical Computing: Vienna, Austria, 2015. Available online: https://CRAN.R-project.org/package=candisc (accessed on 9 November 2022).
- Bhering, L.L. Rbio: A tool for biometric and statistical analysis using the R platform. Crop Breed. Appl. Biotechnol. 2017, 17, 187–190. [Google Scholar] [CrossRef]
- Nassif, S.M.L.; Perez, S.C.J.G. Efeito da temperatura na germinação de sementes de amendoim-do-campo (Pterogyne nitens Tul.). Rev. Bras. Sementes 2000, 22, 1–6. [Google Scholar] [CrossRef]
- Leppyanen, I.; Shtark, O.; Pavlova, O.; Bovin, A.; Ivanova, K.; Serova, T.; Dolgikh, E. Analysis of the effects of joint inoculation by arbuscular mycorrhizal fungi and rhizobia on the growth and development of pea plants Pisum sativum L. Agric. Biol. 2021, 6, 475–486. [Google Scholar] [CrossRef]
- Toft, C.; Andersson, S. Evolutionary microbial genomics: Insights into bacterial host adaptation. Nat. Rev. Genet. 2010, 11, 465–475. [Google Scholar] [CrossRef]
- Lyu, D.; Msimbira, L.A.; Nazari, M.; Antar, M.; Pagé, A.; Shah, A.; Monjezi, N.; Zajonc, J.; Tanney, C.A.S.; Backer, R.; et al. The coevolution of plants and microbes underpins sustainable agriculture. Microorganisms 2021, 9, 1036. [Google Scholar] [CrossRef]
- Albareda, M.; Rodríguez-Navarro, D.N.; Camacho, M.; Temprano, F.J. Alternatives to peat as a carrier for rhizobia inoculants: Solid and liquid formulations. Soil. Biol. Biochem. 2008, 40, 2771–2779. [Google Scholar] [CrossRef]
- Mbi, T.K.; Ntsefong, N.G.; Lenzemo, T.E. Seed Dormancy: Induction, maintenance and seed technology approaches to break dormancy. In Seed Biology Updates; Jimenez-Lopez, J.C., Ed.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Deaker, R.; Roughley, R.J.; Kennedy, I.R. Legume seed inoculation technology—A review. Soil. Biol. Biochem. 2004, 36, 1275–1288. [Google Scholar] [CrossRef]
- Vishnivetskaya, T.A.; Kathariou, S.; Tiedje, J.M. The Exiguobacterium genus: Biodiversity and biogeography. Extremophiles 2009, 13, 541–555. [Google Scholar] [CrossRef]
- Razgour, O.; Forester, B.; Taggart, J.B.; Bekaert, M.; Juste, J.; Ibáñez, C.; Puechmaille, S.J.; Novella-Fernandez, R.; Alberdi, A.; Manel, S. Considering adaptive genetic variation in climate change vulnerability assessment reduces species range loss projections. Proc. Natl. Acad. Sci. USA 2019, 116, 10418–10423. [Google Scholar] [CrossRef]
- Pascale, S.; Rouphael, Y.; Colla, G. Plant biostimulants: Innovative tool for enhancing plant nutrition in organic farming. Eur. J. Hortic. Sci. 2017, 82, 277–285. [Google Scholar] [CrossRef]
- Nakhforoosh, A.; Nagel, K.A.; Fiorani, F.; Bodner, G. Deep soil exploration vs. topsoil exploitation: Distinctive rooting strategies between wheat landraces and wild relatives. Plant Soil. 2021, 459, 397–421. [Google Scholar] [CrossRef]
- Sousa, F.G.; Mielke, K.C.; Caldeira, D.R.M.; Baldani, V.L.D.; Baldani, J.I.; Silva, R.F.; Balbinot, E.; Klein, V.A.C. Genetic diversity and inoculation of plant-growth promoting diazotrophic bacteria for production of Eucalyptus urophylla seedlings. Aust. J. Crop Sci. 2022, 16, 35–44. [Google Scholar] [CrossRef]
- Verma, S.K.; Kingsley, K.; Bergen, M.; English, C.; Elmore, M.; Kharwar, R.N.; White, J.F. Bacterial endophytes from rice cut grass (Leersia oryzoides L.) increase growth, promote root gravitropic response, stimulate root hair formation, and protect rice seedlings from disease. Plant Soil 2018, 422, 223–238. [Google Scholar] [CrossRef]
- Souza, C.R.S.; Barbosa, A.C.O.; Ferreira, C.F.; Souza, F.V.D.; Rocha, L.S.; Souza, E.H.; Oliveira, S.A.S. Diversity of microorganisms associated to Ananas spp. from natural environment, cultivated and ex situ conservation areas. Sci. Hortic. 2019, 243, 544–551. [Google Scholar] [CrossRef]
- Streit, N.M.; Canterle, L.P.; Canto, M.W.D.; Hecktheuer, L.H.H. The chlorophylls. Cienc. Rural 2005, 35, 748–755. [Google Scholar] [CrossRef]
- Lin, L.; Chen, W.; Li, C.; Cui, H. Enhancing stability of Eucalyptus citriodora essential oil by solid nanoliposomes encapsulation. Ind. Crops Prod. 2019, 140, 111615. [Google Scholar] [CrossRef]
- Souza, B.M.; Freitas, M.L.M.; Sebbenn, A.M.; Gezan, S.A.; Zanatto, B.; Zulian, D.F.; Lopes, M.T.G.; Longui, E.L.; Guerrini, I.A. Genotype-by-environment interaction in Corymbia citriodora (Hook.) KD Hill, & LAS Johnson progeny test in Luiz Antonio, Brazil. For. Ecol. Manag. 2020, 460, 117855. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).