MaxEnt-Based Habitat Suitability Assessment for Vaccinium mandarinorum: Exploring Industrial Cultivation Opportunities
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Data Collection and Screening
2.2. Acquisition and Processing of Bioclimatic Variable Data
2.3. Model Construction and Accuracy Analysis
2.4. Classification and Evaluation of Potential Suitable Habitats
2.5. Assessment of Environmental Variable Importance
3. Results
3.1. Model Accuracy Evaluation
3.2. Key Environmental Variables Influencing the Distribution of V. mandarinorum
3.3. Climate Factor Response Curve Analysis
3.4. Potential Distribution of V. mandarinorum Under Modern Climatic Conditions
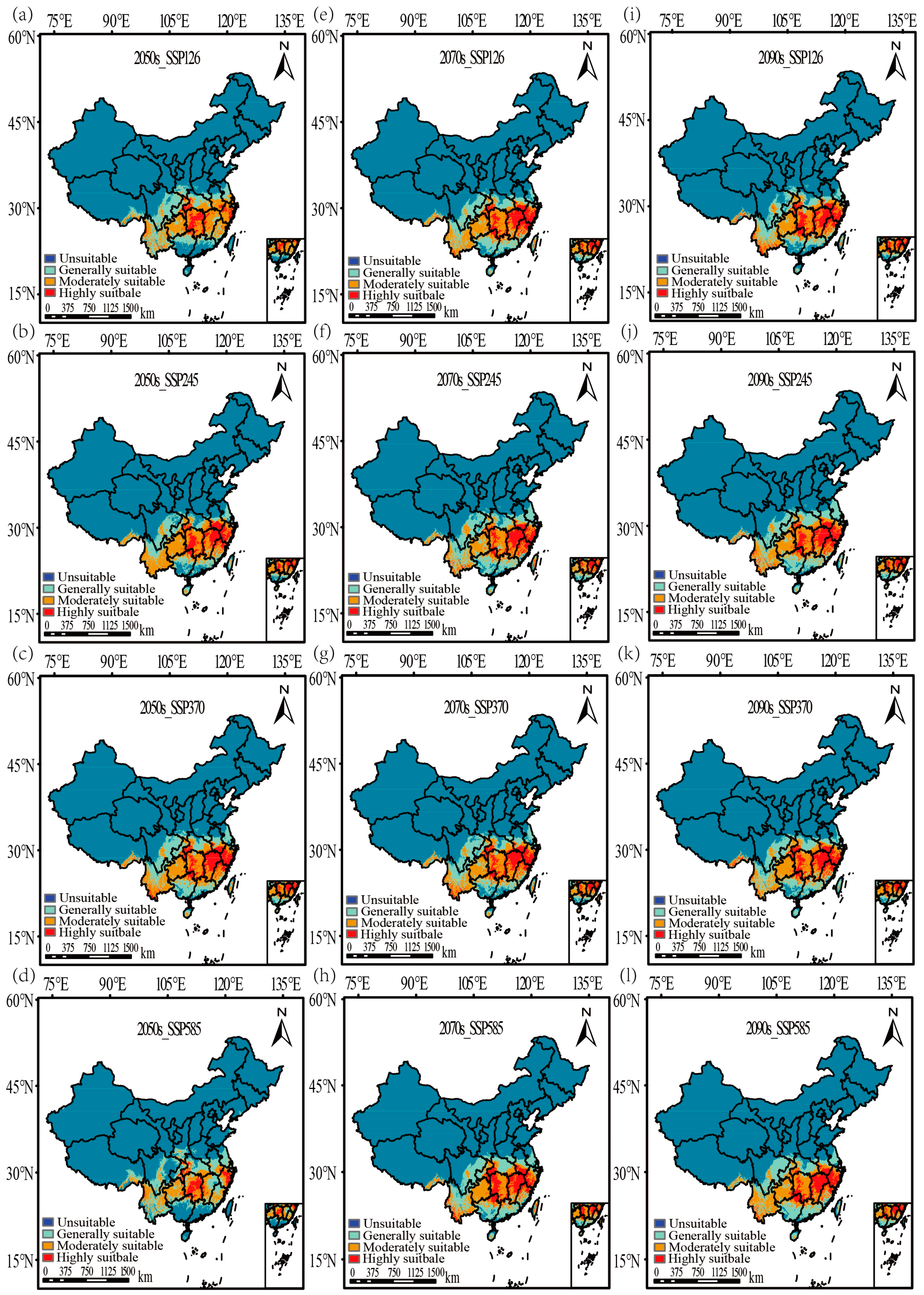
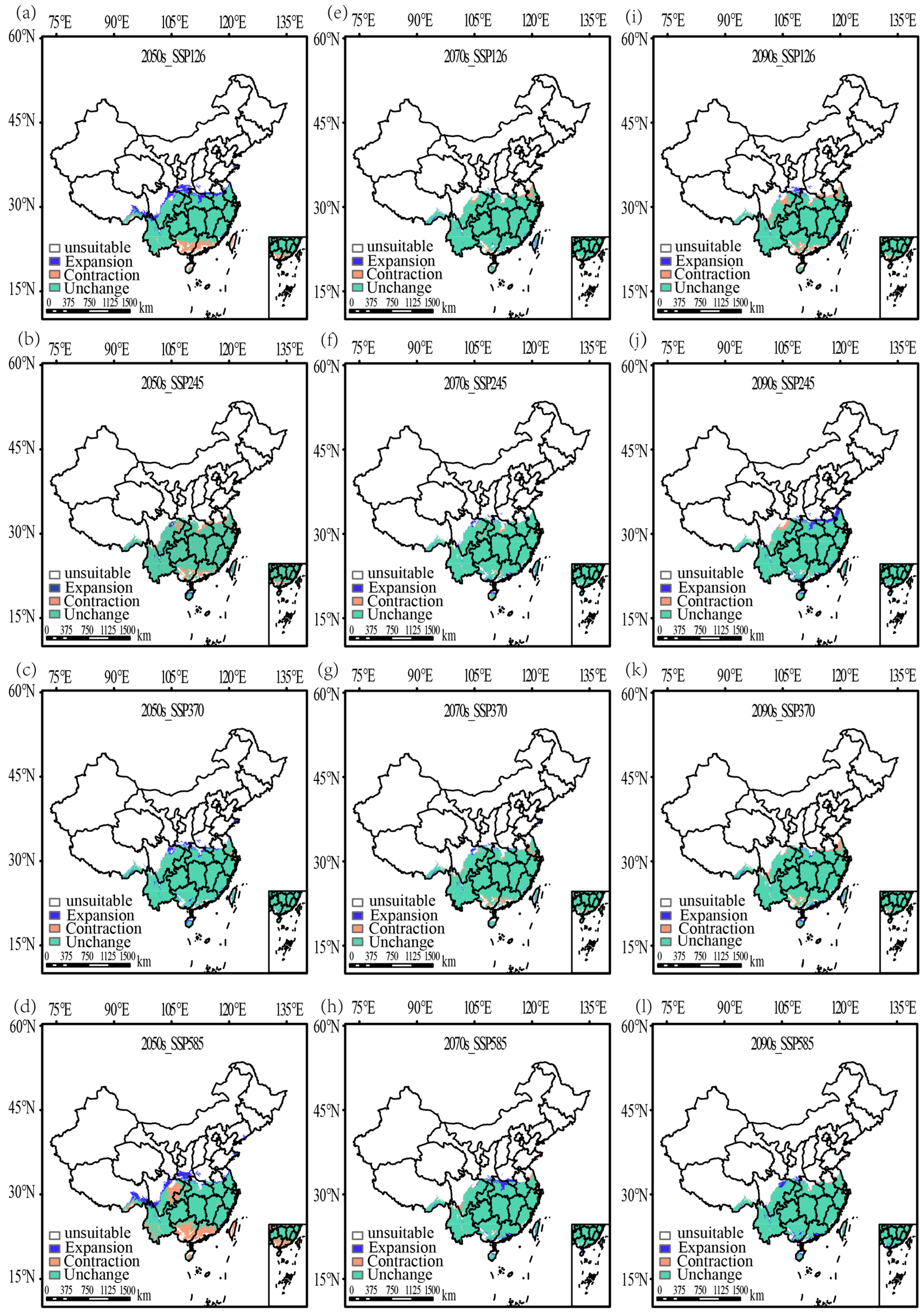
3.5. Changes in the Distribution of V. mandarinorum Under Future Climate Scenarios
4. Discussion
4.1. Constraints of Climate Factors on the Potential Geographic Distribution of V. mandarinorum
4.2. Analysis of Suitable Habitats for V. mandarinorum
4.3. Conservation and Introduction Strategies for V. mandarinorum
4.4. Limitations of This Study and Directions for Future Exploration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bonan, G.B.; Doney, S.C. Climate, ecosystems, and planetary futures: The challenge to predict life in earth system models. Science 2018, 359, eaam8328. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G.; Dawson, T.P. Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models useful? Global. Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef]
- Garcia, R.A.; Cabeza, M.; Rahbek, C.; Araújo, M.B. Multiple dimensions of climate change and their implications for biodiversity. Science 2014, 344, 1247579. [Google Scholar] [CrossRef] [PubMed]
- Giuseppe, Z.; Paulo, C.; Shepherd, T.G. Time-evolving sea-surface warming patterns modulate the climate change response of subtropical precipitation over land. Proc. Natl. Acad. Sci. USA 2020, 117, 4539–4545. [Google Scholar] [CrossRef]
- Jamieson, M.A.; Trowbridge, A.M.; Raffa, K.F.; Lindroth, R.L. Consequences of climate warming and altered precipitation patterns for plant-insect and multitrophic interactions. Plant Physiol. 2012, 160, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.T.; Ma, L.; Chen, C.B.; Zhou, H.K.; Yao, B.Q.; Ma, Z. Predicting the suitable geographical distribution of Sinadoxa corydalifolia under different climate change scenarios in the Three-River region using the MaxEnt model. Plants 2020, 9, 1015. [Google Scholar] [CrossRef] [PubMed]
- Thom, D.; Rammer, W.; Dirnböck, T.; Müller, J.; Kobler, J.; Katzensteiner, K.; Helm, N.; Seidl, R. The impacts of climate change and disturbance on spatio-temporal trajectories of biodiversity in a temperate forest landscape. J. Appl. Ecol. 2017, 54, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.R.; Finlayson, C.M.; Thwaites, R.; Shi, G.Q.; Cui, L.J. Using Government resettlement projects as a sustainable adaptation strategy for climate change. Sustainability 2017, 9, 1373. [Google Scholar] [CrossRef]
- Bradter, U.; Kunin, W.E.; Altringham, J.D.; Thom, T.J.; Benton, T.G. Identifying appropriate spatial scales of predictors in species distribution models with the random forest algorithm. Methods Ecol. Evol. 2013, 4, 167–174. [Google Scholar] [CrossRef]
- Pouteau, R.; Meyer, J.; Taputuarai, R.; Stoll, B. Support vector machines to map rare and endangered native plants in Pacific islands forests. Ecol. Inform. 2012, 9, 37–46. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of maxent for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Belgiu, M.; Drăguţ, L. Random forest in remote sensing: A review of applications and future directions. ISPRS J. Photogramm. 2016, 114, 24–31. [Google Scholar] [CrossRef]
- Kumar, B.; Vyas, O.P.; Vyas, R. A comprehensive review on the variants of support vector machines. Mod. Phys. Lett. B. 2019, 33, 1950303. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Quan, Q.H.; Wu, Y.J. Integrating entropy weight and MaxEnt models for ecotourism suitability assessment in northeast China tiger and leopard national Park. Land 2024, 13, 1269. [Google Scholar] [CrossRef]
- Zurell, D.; Zimmermann, N.; Gross, H.; Baltensweiler, A.; Sattler, T.; Wuest, R.O. Testing species assemblage predictions from stacked and joint species distribution models. J. Biogeogr. 2020, 47, 101–113. [Google Scholar] [CrossRef]
- Kim, H.J.; Bae, M.; Jin, D. On a robust MaxEnt process regression model with sample-selection. Entropy 2018, 20, 262. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Bai, C.K.; Wu, K.Y.; Xue, Y.; Yang, J.J.; Gao, P.F.; Liang, H.; Li, G.S. Concentrated conservation and utilization: Four medicinal crops for diabetes treatment showed similar habitat distribution patterns in China. Ind. Crop Prod. 2020, 152, 12. [Google Scholar] [CrossRef]
- Manners, R.; Varela-Ortega, C.; van Etten, J. Protein-rich legume and pseudo- cereal crop suitability under present and future European climates. Eur. J. Agron. 2020, 113, 125974. [Google Scholar] [CrossRef]
- Rafatpey, P.; Lahout, M.; Rahnavard, A.; Biklarian, H.; Jafarzadeh, M. Ground squirrels (Spermophilus fulvus) habitat suitability using MaxEnt and ENFA modeling approaches. Sustain. Biodivers. Conserv. 2023, 2, 6–19. [Google Scholar] [CrossRef]
- Ahmadi, M.; Naderi, M.; Kaboli, M.; Nazarizadeh, M.; Karami, M.; Beitollahi, S.M. Evolutionary applications of phylogenetically-informed ecological niche modelling (ENM) to explore cryptic diversification over cryptic refugia. Mol. Phylogenet. Evol. 2018, 127, 712–722. [Google Scholar] [CrossRef]
- Melo-Merino, S.M.; Reyes-Bonilla, H.; Lira-Noriega, A. Ecological niche models and species distribution models in marine environments: A literature review and spatial analysis of evidence. Ecol. Model. 2020, 415, 108837. [Google Scholar] [CrossRef]
- Naqibzadeh, A.; Sarhangzadeh, J.; Sotoudeh, A.; Jafari, M.J. Habitat suitability modeling of Goiteredgazelle (Gazella subgutturosa): A Maximum Entropy approach from Samelghan plain, Iran. Sci. Rep. 2022, 3, 11–28. [Google Scholar] [CrossRef]
- Shi, X.Y.; Wang, C.; Zhao, J.C.; Wang, K.C.; Chen, F.; Chu, Q.Q. Increasing inconsistency between climate suitability and production of cotton (Gossypium hirsutum L.) in China. Ind. Crop Prod. 2021, 171, 113959. [Google Scholar] [CrossRef]
- Rosindell, J.; Harmon, L.J. OneZoom: A fractal explorer for the tree of life. PLoS Biol. 2012, 10, e1001406. [Google Scholar] [CrossRef]
- Kay, K.L.; Strauch, R.C.; Granillo, C.D.; Bame, M.W.; Xiong, J.; Mast, A.C.; Burton-Freeman, B.; Kay, C.D.; Lila, M.A. The berry health tool chest-an evidence map and interactive resource. Nutr. Rev. 2021, 80, 68–77. [Google Scholar] [CrossRef]
- Prevéy, J.S.; Parker, L.E.; Harrington, C.A.; Lamb, C.T.; Proctor, M.F. Climate change shifts in habitat suitability and phenology of huckleberry (Vaccinium membranaceum). Agr. Forest. Meteorol. 2020, 280, 107803. [Google Scholar] [CrossRef]
- Suárez-Seoane, S.; Jiménez-Alfaro, B.; Obeso, J.R. Habitat-partitioning improves regional distribution models in multi-habitat species: A case study with the European bilberry. Biodivers. Conserv. 2020, 29, 987–1008. [Google Scholar] [CrossRef]
- Hirabayashi, K.; Murch, S.J.; Erland, L.A.E. Predicted impacts of climate change on wild and commercial berry habitats will have food security, conservation and agricultural implications. Sci. Total. Environ. 2022, 845, 157341. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.J.; Zhang, C.; Suen, H.P.; Ai, S.Q.; Bai, Y.Q.; Bao, J.Z.; Chen, B.; Cheng, L.L.; Cui, X.Q.; Dai, H.C.; et al. The 2020 China report of the Lancet Countdown on health and climate change. Lancet Public Health 2021, 6, e64–e81. [Google Scholar] [CrossRef]
- Thompson, V.; Dunstone, N.; Scaife, A.A.; Smith, D.M.; Hardiman, S.C.; Ren, H.L.; Lu, B.; Belcher, S.E. Risk and dynamics of unprecedented hot months in South East China. Clim. Dyn. 2019, 52, 2585–2596. [Google Scholar] [CrossRef]
- Yang, Z.S.; He, J.W.; Tang, K.X.; He, Z.J.; Li, Y.; Yang, Y.L.; Wang, C.W. Resources of wild Vaccinium L. in the northwest of Yunnan province. Southwest. China J. Agric. Sci. 2008, 21, 1059–1062. [Google Scholar]
- Wei, Y.; Wei, L. Determination of Trace Elements in leaves of Vaccinium Mandarinorum. Stud. Trace Elem. Health 2003, 5, 28–29. [Google Scholar]
- Neto, C. Cranberry and blueberry: Evidence for protective effects against cancer and vascular diseases. Mol. Nutr. Food Res. 2007, 51, 652–664. [Google Scholar] [CrossRef]
- Zeng, B.; Yang, S.Z.; Yin, C.F.; Pan, M.S.; He, K.J. Wild blueberry resources (Vaccinium) in Hunan province: Preliminary investigation and utilization suggestions. J. Agric. 2018, 8, 73–78. [Google Scholar]
- Li, W.D.; Zhu, C.; Grass, I.; Vázquez, D.P.; Wang, D.R.; Zhao, Y.H.; Zeng, D.; Kang, Y.; Ding, P.; Si, X.F. Plant-frugivore network simplification under habitat fragmentation leaves a small core of interacting generalists. Commun. Biol. 2022, 5, 1214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Ni, J.; Tang, F.P.; Jiang, L.F.; Guo, T.R.; Pei, K.Q.; Sun, L.F.; Liang, Y. Diversity of root-associated fungi of Vaccinium mandarinorum along a human disturbance gradient in subtropical forests, China. J. Plant Ecol. 2017, 10, 56–66. [Google Scholar] [CrossRef]
- Ștefănescu, B.E.; Szabo, K.; Mocan, A.; Crişan, G. Phenolic compounds from five Ericaceae species leaves and their related bioavailability and health benefits. Molecules 2019, 24, 2046. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Tenuta, M.C.; Loizzo, M.R.; Bonesi, M.; Finetti, F.; Trabalzini, L.; Deguin, B. Vaccinium species (Ericaceae): From chemical composition to bio-functional activities. Appl. Sci. 2021, 11, 5655. [Google Scholar] [CrossRef]
- Xu, F.X.; Liu, S.Y.; Liu, Y.F.; Wang, S.H. Effect of mechanical vibration on postharvest quality and volatile compounds of blueberry fruit. Food Chem. 2021, 349, 129216. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.W.; Du, Q.H.; Zuo, S.Y.; Liu, G.T.; Wang, H.X.; Liu, G.L.; Zhao, L.; Xu, G.H. Assembly and comparative analysis of the complete mitochondrial genome of Vaccinium carlesii Dunn. Genomics 2024, 116, 110897. [Google Scholar] [CrossRef]
- Gillespie, E.; Kron, K. Molecular phylogenetic relationships and a revised classification of the subfamily Ericoideae (Ericaceae). Mol. Phylogenet. 2010, 56, 343–354. [Google Scholar] [CrossRef]
- Zhidkin, R.; Zhurbenko, P.; Bogomaz, O.; Gorodilova, E.; Katsapov, I.; Antropov, D.; Matveeva, T. Biodiversity of rolB/C-like natural transgene in the genus Vaccinium L. and its application for phylogenetic studies. Int. J. Mol. Sci. 2023, 24, 6932. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Liang, H.; Hu, W.; Yang, Y.; Tian, X.M.; Tsutsumi, C.; Fan, D.M.; Zhang, Z.Y. Molecular phylogeny of Enkianthus Lour. (Ericaceae) based on chloroplast and nuclear DNA sequences with an emphasis on the origin of polyploid species. Sci. Hortic. 2024, 328, 112960. [Google Scholar] [CrossRef]
- Khan, G.; Schepker, H.; Buhk, N.; Hahn, C.; Albach, D.C.; Zotz, G. Functional ecology and evolution of terrestrial and epiphytic species of Rhododendron section Schistanthe (Ericaceae). Prespect. Plant Ecol. 2024, 63, 125796. [Google Scholar] [CrossRef]
- Lou, H.; Cai, H.Y.; Fu, R.; Guo, C.; Fan, B.Z.; Hu, H.Q.; Zhang, J.; Sun, L. Effects of wildfire disturbance on forest soil microbes and colonization of ericoid mycorrhizal fungi in northern China. Environ. Res. 2023, 231, 116220. [Google Scholar] [CrossRef]
- Wei, X.Y.; Zhang, W.B.; Zulfiqar, F.; Zhang, C.Y.; Chen, J.J. Ericoid mycorrhizal fungi as biostimulants for improving propagation and production of ericaceous plants. Front. Plant Sci. 2022, 13, 1027390. [Google Scholar] [CrossRef] [PubMed]
- Amroun, D.; Hamoudi, M.; Khennouf, S.; Boutefnouchet, S.; Harzallah, D.; Amrane, M.; Dahamna, S. In-vivo anti-inflammatory activity and safety assessment of the aqueous extract of Algerian Erica arborea L. (Ericaceae) aerial parts. J. Ethnopharmacol. 2021, 271, 113881. [Google Scholar] [CrossRef]
- Yang, C.M.; Wu, L.F.; Wang, G.X.; Qu, Y.H.; Dan, Q.L.; Cao, H.; Ruan, J.W.; Li, J.Z.; Jiang, H.Y. Study on tissue culture and rapid propagation technique of Vaccinium mandarinorum Hance meristem. Southwest. China J. Agric. Sci. 2015, 28, 344–348. [Google Scholar]
- Li, Y.; Zhang, X.W.; Fang, Y.M. Predicting the impact of global warming on the geographical distribution pattern of Quercus variabilis in China. Chin. J. Appl. Ecol. 2014, 25, 3381–3389. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 12, 4302–4315. [Google Scholar] [CrossRef]
- Wu, T.W.; Lu, Y.X.; Fang, Y.J.; Xin, X.G.; Li, L.; Li, W.J.; Jie, W.H.; Zhang, J.; Liu, Y.M.; Zhang, L.; et al. The Beijing climate center climate system model (BCC—CSM): The main progress from CMIP5 to CMIP6. Geosci. Model. Dev. 2019, 12, 1573–1600. [Google Scholar] [CrossRef]
- Jiang, T.; Lv, Y.R.; Huang, J.L.; Wang, Y.J.; Su, B.D.; Tao, H. New scenarios of CMIP6 model (SSP-RCP) and its application in the Huaihe river basin. Adv. Meteorol. Sci. Technol. 2020, 10, 102–109. [Google Scholar] [CrossRef]
- Sıkdokur, E.; Naderi, M.; Çeltik, E.; Aytekin, M.Ç.K.; Kusak, J.; Sağlam, S.K.; Şekercioğlu, Ç.H. Human-brown bear conflicts in Türkiye are driven by increased human presence around protected areas. Ecol. Inform. 2024, 81, 102643. [Google Scholar] [CrossRef]
- Hijmans, R.J.; VanEtten, J. raster: Geographic Data Analysis and Modeling, R Package Version 2.2-31; 2012. Available online: https://CRAN.R-project.org/package=raster (accessed on 15 July 2024).
- Puchałka, R.; Dyderski, M.K.; Vítková, M.; Sádlo, J.; Klisz, M.; Netsvetov, M.; Prokopuk, Y.; Matisons, R.; Mionskowski, M.; Wojda, T.; et al. Black locust (Robinia pseudoacacia L.) range contraction and expansion in Europe underchanging climate. Glob. Chang. Biol. 2021, 27, 1587–1600. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open–source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Bradie, J.; Leung, B. A quantitative synthesis of the importance of variables used in MaxEnt species distribution models. J. Biogeogr. 2017, 44, 1344–1361. [Google Scholar] [CrossRef]
- Hessburg, P.F.; Miller, C.; Parks, S.A.; Povak, N.; Taylor, A.H.; Higuera, P.E.; Prichard, S.; North, M.P.; Collins, B.M.; Hurteau, M.D.; et al. Climate, environment, and disturbance history govern resilience of western north American forests. Front. Ecol. Evol. 2019, 7, 239. [Google Scholar] [CrossRef]
- Bombi, P.; D’Andrea, E.; Rezaie, N.; Cammarano, M.; Matteucci, G. Which climate change path are we following? Bad news from Scots pine. PLoS ONE 2017, 12, e0189468. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Paź, S.; Frelich, L.E.; Jagodziński, A.M. How much does climate change threaten European forest tree species distributions? Glob. Chang. Biol. 2018, 24, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Ali, Q.; Ali, M.; Khan, F.Z.A.; Noureldeen, A.; Alghamdi, A.; Darwesh, H.; Fatima, A.; Jalali, A.I.; Prendergast, K.; Saeed, S. Water Deprivation and Sowing Times Alter Plant–Pollination Interactions and Seed Yield in Sunflower, Helianthus annuus L. (Asteraceae). Plants 2024, 13, 3194. [Google Scholar] [CrossRef] [PubMed]
- Austin, M.W.; Cole, P.O.; Olsen, K.M.; Smith, A.B. Climate change is associated with increased allocation to potential outcrossing in a common mixed mating species. Am. J. Bot. 2022, 109, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Kupec, P.; Deutscher, J.; Futter, M. Longer growing seasons cause hydrological regime shifts in central European forests. Forests 2021, 12, 1656. [Google Scholar] [CrossRef]
- Selås, V. Seed production of a masting dwarf shrub, Vaccinium myrtillus, in relation to previous reproduction and weather. Can. J. Bot. 2000, 78, 423–429. [Google Scholar] [CrossRef]
- Krebs, C.J.; Boonstra, R.; Cowcill, K.; Kenney, A.J. Climatic determinants of berry crops in the boreal forest of the southwestern Yukon. Botany 2009, 87, 401–408. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Liang, P.; Sun, Y. Chapter 1-basic features of the Asian summer monsoon system. In The Asian Summer Monsoon; Elsevier: Amsterdam, The Netherlands, 2019; pp. 3–22. [Google Scholar]
- Pan, J.W.; Fan, X.; Luo, S.Q.; Zhang, Y.Q.; Yao, S.; Guo, Q.Q.; Qian, Z.Q. Predicting the potential distribution of two varieties of Litsea coreana (Leopard-Skin Camphor) in China under climate change. Forests 2020, 11, 1159. [Google Scholar] [CrossRef]
- Jian, S.Q.; Zhu, T.S.; Wang, J.Y.; Yan, D.H. The current and future potential geographical distribution and evolution process of Catalpa bungei in China. Forests 2022, 13, 96. [Google Scholar] [CrossRef]
- Hickler, T.; Vohland, K.; Feehan, J.; Miller, P.A.; Smith, B.; Costa, L.; Giesecke, T.; Fronzek, S.; Carter, T.R.; Cramer, W.; et al. Projecting the future distribution of European potential natural vegetation zones with a generalized, tree species-based dynamic vegetation model. Glob. Ecol. Biogeogr. 2012, 21, 50–63. [Google Scholar] [CrossRef]
- Tichý, L.; Axmanová, I.; Dengler, J.; Guarino, R.; Jansen, F.; Midolo, G.; Nobis, M.P.; van Meerbeek, K.; Aćić, S.; Attorre, F.; et al. Ellenberg-type indicator values for European vascular plant species. J. Veg. Sci. 2023, 34, e13168. [Google Scholar] [CrossRef]
- Vasseur, D.; DeLong, J.; Gilbert, B.; Greig, H.; Harley, C.; McCann, K.; O’Connor, M. Increased temperature variation poses a greater risk to species than climate warming. P. Roy. Soc. B Biol. Sci. 2014, 281, 20132612. [Google Scholar] [CrossRef] [PubMed]
- Ellison, D.; Morris, C.E.; Locatelli, B.; Sheil, D.; Cohen, J.; Murdiyarso, D.; Gutierrez, V.; van Noordwijk, M.V.; Creed, I.F.; Pokorny, J.; et al. Trees, forests and water: Cool insights for a hot world. Glob. Environ. Chang. 2017, 43, 51–61. [Google Scholar] [CrossRef]
- Kotowski, W.; Beauchard, O.; Opdekamp, W.; Meire, P.; Van Diggelen, R. Waterlogging and canopy interact to control species recruitment in floodplains. Funct. Ecol. 2010, 24, 918–926. [Google Scholar] [CrossRef]
- Hillman, A.; Nielsen, S.E. Climate refugia along Lake Superior’s shores: Disjunct arctic-alpine plants rely on cool shoreline temperatures but are restricted to highly exposed habitat under climate warming. J. Plant Ecol. 2024, 17, rtae050. [Google Scholar] [CrossRef]
- Tonin, R.; Wilhelmi, S.; Gultas, M.; Gerdol, R.; Paun, O.; Trucchi, E.; Schmitt, A.O.; Wellstein, C. Ice holes microrefugia harbor genetically and functionally distinct populations of Vaccinium vitis-idaea (Ericaceae). Sci. Rep. 2023, 13, 13055. [Google Scholar] [CrossRef]
- Yang, H.; Wu, Y.Q.; Zhang, C.H.; Wu, W.L.; Lyu, L.F.; Li, W.L. Growth and physiological characteristics of four blueberry cultivars under different high soil pH treatments. Environ. Exp. Bot. 2022, 197, 104842. [Google Scholar] [CrossRef]
- Payá-Milans, M.; Nunez, G.H.; Olmstead, J.W.; Rinehart, T.A.; Staton, M. Regulation of gene expression in roots of the pH-sensitive Vaccinium corymbosum and the pH-tolerant Vaccinium arboreum in response to near neutral pH stress using RNA-Seq. BMC Genom. 2017, 18, 580. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.L.; Wang, S.Q.; Dai, N.F.; Shen, S.K. Interspecific variance of molecular response to external pH conditions in two Rhododendron species. Plant Soil. 2024, 504, 111–127. [Google Scholar] [CrossRef]
- Li, J.G.; Mavrodi, O.V.; Hou, J.F.; Blackmon, C.; Babiker, E.M.; Mavrodi, D.V. Comparative analysis of rhizosphere microbiomes of southern highbush blueberry (Vaccinium corymbosum L.), Darrow’s Blueberry (V. darrowii Camp), and Rabbit eye Blueberry (V. virgatum Aiton). Front. Microbiol. 2020, 11, 370. [Google Scholar] [CrossRef]
- Rains, K.C.; Bledsoe, C.; Kraus, T.E.C.; Wurzburger, N. Evidence that ericoid mycorrhizal shrubs can outcompete ectomycorrhizal trees for nitrogen in tannin-rich litter. Ecosphere 2023, 15, e4818. [Google Scholar] [CrossRef]
- Gorzelak, M.A.; Hambleton, S.; Massicotte, H.B. Community structure of ericoid mycorrhizas and root-associated fungi of Vaccinium membranaceum across an elevation gradient in the Canadian Rocky Mountains. Fungal Ecol. 2012, 5, 36e45. [Google Scholar] [CrossRef]
- Zheng, Y.X.; Yuan, C.; Matsushita, N.; Lian, C.L.; Geng, Q.F. Analysis of the distribution pattern of the ectomycorrhizal fungus Cenococcum geophilum under climate change using the optimized MaxEnt model. Ecol. Evol. 2023, 13, 10565. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.E.; Classen, A.T. Climate change influences mycorrhizal fungal-plant interactions, but conclusions are limited by geographical study bias. Ecol. 2020, 101, e02978. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Guo, B.; Zhang, L.; Li, J.; Li, S.; Zhao, W.; Yang, G.; Zhou, S.; Zhou, C.; Song, P.; et al. Interactive climate-soil forces shape the spatial distribution of foliar N:P stoichiometry in Vaccinium uliginosum planted in agroforests of Northeast China. Front. Ecol. Evol. 2022, 10, 1065680. [Google Scholar] [CrossRef]
- Hill, N.M.; Vander Kloet, S.P. Longevity of experimentally buried seed in Vaccinium relationship to climate, reproductive factors and natural seed banks. J. Ecol. 2005, 93, 1167–1176. [Google Scholar] [CrossRef]

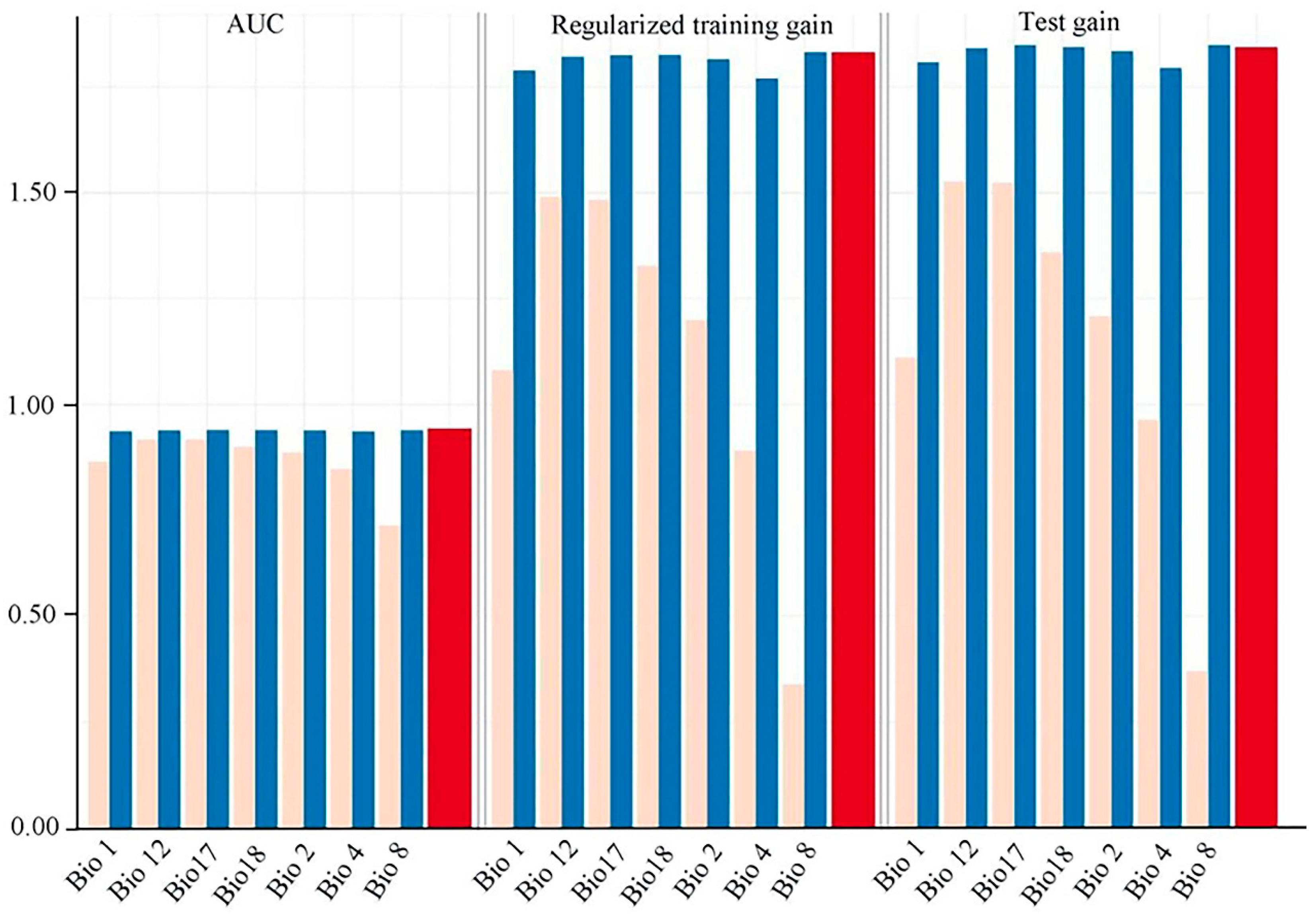


| Environmental Variable | Unit | Relative Contribution (%) | Permutation Importance (%) |
|---|---|---|---|
| Precipitation of the Driest Quarter (Bio17) | mm | 61.3 | 16.9 |
| Annual Precipitation (Bio12) | mm | 23.4 | 28.9 |
| Mean Temperature of the Wettest Quarter (Bio8) | °C | 6.4 | 0.02 |
| Annual Mean Temperature (Bio1) | °C | 3.6 | 16.0 |
| Temperature Seasonality (Bio4) | °C | 2.7 | 16.6 |
| Precipitation of the Warmest Quarter (Bio18) | mm | 1.7 | 9.9 |
| Mean Diurnal Range (Bio2) | °C | 0.8 | 11.6 |
| Scenarios | Generally Suitable (104 km2) | Moderately Suitable (104 km2) | Highly Suitable (104 km2) | Total Suitable (104 km2) |
|---|---|---|---|---|
| Current | 86.66 | 68.57 | 42.57 | 197.8 |
| 2050s-SSP126 | 91.08 | 85.06 | 26.10 | 202.24 |
| 2050s-SSP245 | 67.25 | 84.58 | 37.55 | 189.38 |
| 2050s-SSP370 | 84.24 | 79.63 | 43.81 | 207.68 |
| 2050s-SSP585 | 95.63 | 66.51 | 20.93 | 183.07 |
| 2070s-SSP126 | 83.25 | 72.12 | 41.61 | 196.98 |
| 2070s-SSP245 | 92.26 | 66.68 | 40.92 | 199.86 |
| 2070s-SSP370 | 78.01 | 72.21 | 43.46 | 193.68 |
| 2070s-SSP585 | 87.87 | 69.18 | 45.18 | 202.23 |
| 2090s-SSP126 | 74.67 | 69.37 | 44.40 | 188.44 |
| 2090s-SSP245 | 89.89 | 71.69 | 43.69 | 205.27 |
| 2090s-SSP370 | 78.54 | 70.58 | 42.54 | 191.66 |
| 2090s-SSP585 | 85.21 | 76.59 | 40.61 | 202.41 |
| Climate Scenario | Period | Generally Suitable (104 km2) | Moderately Suitable (104 km2) | Highly Suitable (104 km2) | Total Suitable (104 km2) |
|---|---|---|---|---|---|
| 2050s | 4.42 | 16.49 | −16.47 | 4.44 | |
| SSP126 | 2070s | −3.41 | 3.55 | −0.96 | −0.82 |
| 2090s | −11.99 | 0.8 | 1.83 | −9.36 | |
| 2050s | −19.41 | 16.01 | −5.02 | −8.42 | |
| SSP245 | 2070s | 5.6 | −1.89 | −1.65 | 2.06 |
| 2090s | 3.23 | 3.12 | 1.12 | 7.47 | |
| 2050s | −2.42 | 11.06 | 1.24 | 9.88 | |
| SSP370 | 2070s | −8.65 | 3.64 | 0.89 | −4.12 |
| 2090s | −8.12 | 2.01 | −0.03 | −6.14 | |
| 2050s | 8.97 | −2.06 | −21.64 | −14.73 | |
| SSP585 | 2070s | 1.21 | 0.61 | 2.61 | 4.43 |
| 2090s | −1.45 | 8.02 | −1.96 | 4.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, X.; Zhou, P.; Zhang, M.; Fang, Y.; Zhang, Q. MaxEnt-Based Habitat Suitability Assessment for Vaccinium mandarinorum: Exploring Industrial Cultivation Opportunities. Forests 2024, 15, 2254. https://doi.org/10.3390/f15122254
Bao X, Zhou P, Zhang M, Fang Y, Zhang Q. MaxEnt-Based Habitat Suitability Assessment for Vaccinium mandarinorum: Exploring Industrial Cultivation Opportunities. Forests. 2024; 15(12):2254. https://doi.org/10.3390/f15122254
Chicago/Turabian StyleBao, Xuxu, Peng Zhou, Min Zhang, Yanming Fang, and Qiang Zhang. 2024. "MaxEnt-Based Habitat Suitability Assessment for Vaccinium mandarinorum: Exploring Industrial Cultivation Opportunities" Forests 15, no. 12: 2254. https://doi.org/10.3390/f15122254
APA StyleBao, X., Zhou, P., Zhang, M., Fang, Y., & Zhang, Q. (2024). MaxEnt-Based Habitat Suitability Assessment for Vaccinium mandarinorum: Exploring Industrial Cultivation Opportunities. Forests, 15(12), 2254. https://doi.org/10.3390/f15122254






