Abstract
The enhancement of the physical and mechanical properties and the anti-mildew performance of wood–plastic composites are of great significance for broadening their application field. In this research, bamboo fibers underwent treatments with safe, environmentally friendly bio-enzymes. Subsequently, a bamboo–plastic composite (BPC) was developed using the modified bamboo fibers and polyethylene. The effects of biological enzymatic treatments on the surface free energy, the chemical composition of the bamboo fibers, water resistance, thermal stability, bending performance, impact performance, and anti-mildew performance of the BPC samples were analyzed. This study revealed that treating bamboo powder with bio-enzymes (xylanase, lipase, laccase, pectinase, hemicellulase, or amylase) decreased the surface free energy and the polar components of the bamboo fibers while improving the surface O/C atomic ratio of the bamboo fibers. These enzyme treatments enhanced the water resistance, bending performance, and anti-mildew performance of the BPC samples. However, on the whole, the thermal stability of the composites decreased. Particularly, after hemicellulase treatment, the composites had the lowest water absorption, reflecting a decrease of 68.25% compared to the control group. With xylanase modification, the 24 h water absorption thickness swelling rate of the composites was the lowest, reflecting a decrease of 71.27% compared to the control group. After pectinase modification, the static bending strength and elastic modulus of the prepared composites were the highest, with an increase of 15.45% and 13.31%, respectively, compared to the unmodified group. After xylanase modification, the composites exhibited the best anti-mildew effect, with an anti-mold effectiveness of 74.67%. In conclusion, bio-enzyme treatments can enhance the physical and mechanical properties and anti-mildew performance of BPCs. This research provides a theoretical foundation for the preparation of high-performance wood–plastic composites.
1. Introduction
A wood–plastic composite is a type of material that is primarily made from agricultural and forestry waste, along with thermoplastic and thermosetting resins, and it is well-known for its excellent strength, hardness, and recyclability, making it an environmentally friendly option. Nowadays, wood–plastic composite is widely used in fields such as automotive interior parts, decks, as well as interior and exterior decorations, etc. Bamboo plants represent a vital component of forest resources, exhibiting characteristics such as fast growth, a short production cycle, sustainable cultivation, excellent ecological performance, a wide range of uses, a high potential for carbon sequestration, and high comprehensive benefits [1]. Reports indicate that the current global annual bamboo production is around 20 million tons, with China, India, and Japan collectively contributing more than half of this production, making bamboo one of Asia’s most valuable forest resources [2,3,4]. However, the current level of bamboo utilization is low, with a utilization rate of only 30%~55% [5], meaning that the annual global processing residues of bamboo fragments, bamboo corner waste, and bamboo shavings can reach 9~14 million tons. Therefore, the preparation of high-performance bamboo–plastic composites (BPCs) using bamboo processing residues is of great significance for conserving resources and protecting the environment, in line with the requirements of a sustainable development strategy.
However, bamboo fibers contain substances such as hemicellulose, which have strong polarity and hydrophilicity, causing poor bonding between bamboo fibers and the hydrophobic plastic matrix. This negatively affects the water resistance, bending, and other physical and mechanical properties of BPCs, including their mold resistance. Traditional methods to counter this issue has typically involved either mechanical treatment (calendaring, stretching, electron beam irradiation, and plasma discharge) [6,7]; thermal treatment; or chemical treatment (alkali treatment and graft modification treatment) [8,9,10]. These processes, while somewhat effective in enhancing the physical and mechanical properties of the composite, are unfortunately complex and not particularly environmentally friendly.
The utilization of enzymatic modification treatment, compared to traditional physical and chemical methods, has proven to be more economical, gentler in its process, and more environmentally friendly. This method is broadly applied in various industries such as food processing, textile manufacturing, and papermaking, etc. In recent years, the exploration of enzymatic treatment has extended to the interfacial modification of wood–plastic composites. For instance, Agrawal et al. [11] enhanced jute fibers using a pectinase-modified treatment, which significantly improved the mechanical properties of the jute fiber/polypropylene composite. They discovered that the optimal mechanical properties of the composite were achieved when the pectinase addition was at 6 wt%. Furthermore, Chaari et al. [12] examined the effect of xylanase, pectinase, and a combination of both on the performance of a date palm fiber/poly (butylene succinate) composite. Their research indicated that enzymatic treatment greatly improved the tensile and impact performance of the composite. In general, the fundamental principle of enzymatic modification treatment involves the removal of some hemicellulose, lignin, and wax layer, among other components within plant fibers. This process aims to decrease the polarity of the plant’s fiber surface, to augment the surface area of plant fibers, and to enhance their surface roughness. Consequently, this promotes the interfacial bonding between plant fibers and the plastic matrix, thus improving the physical and mechanical properties and the biodegradability of wood–plastic composites [13,14]. Nevertheless, there is still a scarcity of reports on the preparation of wood–plastic composites using enzymatic methods to treat wood fibers. This field clearly has great potential for further study and development.
This study utilized xylanase-, lipase-, laccase-, pectinase-, hemicellulase-, and amylase-modified bamboo fibers as a reinforcement, with polyethylene (PE) serving as the matrix, and the enzymatically modified bamboo fibers/PE composites were prepared via hot-pressing technology. The changes in the polarity, chemical structure, and surface elements of the bamboo fiber surfaces before and after various enzymatic treatments were analyzed through contact angle measurements, Fourier transform infrared spectroscopy, and X-ray photoelectron spectroscopy. The effects of different enzymatic treatments on the water resistance, thermal stability, mechanical properties, and mold resistance of the bamboo fibers/PE composites were also explored, in order to provide some theoretical foundation and technical support for the foundation of high-performance wood–plastic composites.
2. Materials and Methods
2.1. Materials
The Moso bamboo (Phyllostachys heterocycla) processing residues, with a size range of 0.25~0.42 mm, was provided by Guizhou Xinjin Bamboo–Wood Products Co., Ltd., Chishui, Guizhou Province, China. The polyethylene powder (PE-600), with a particle size between 0.15–0.10 mm, was purchased from the Suyuan Plastic Material Mall, Zhangtoumu Town, Dongguan, Guangdong Province, China. The maleic anhydride grafted polyethylene (MAPE), with a grafting rate of 8%, was obtained from Shanghai Macklin Biochemical Co., Ltd., Shanghai, China. The bamboo fiber, PE, and MAPE were dried thoroughly before use, then sealed and stored for future use. The enzymes, citric acid (99.5%), sodium citrate (98%), and ethylene glycol (>99%) were purchased from ScienceLab Instrument Incorporated, Guiyang, Guizhou Province, China. The basic parameters of the different enzymes are listed in Table 1. Aspergillus niger was supplied by the Guangdong Microbial Culture Collection Center, Guangzhou, China.

Table 1.
Basic parameters of the different enzymes.
2.2. Preparation of the Composites
2.2.1. Enzymatic Treatment of Bamboo Fibers
The method for processing the bamboo fiber (BFx) with xylanase involves the following steps. Firstly, a buffer solution with a pH of 5.0 was prepared with citric acid and sodium citrate. This solution was placed in a thermostatic water bath and heated to 50 °C. The chosen pH and temperature were optimal for a variety of biological enzymes, ensuring their activity was not inhibited (refer to Table 2 for details). Subsequently, 0.5 g of xylanase was slowly poured into the buffer solution and stirred for 8 min. After this, 100 g of the bamboo fiber was added into the mixture, and the combination was stirred for another 5 h with an electric agitator, keeping the temperature constant (50 °C) during this process. Upon completion of the stirring process, the modified bamboo fiber was separated from the buffer solution by a vacuum filter and rinsed several times with distilled water until the rinse water was neutral. The bamboo fiber was then placed in a blast air oven set at a temperature of 70 °C. Following a drying period of 13 h, the treated bamboo fiber was removed from the oven and sealed for later use.

Table 2.
Reaction conditions of bamboo fibers treated with different enzymes.
The method for treating the bamboo fiber with other enzymes is basically similar to the process detailed above for xylanase. Specific reaction conditions for the bamboo fiber when treated with different enzymes, based on their unique properties, are outlined in Table 2.
2.2.2. Preparation of Enzyme-Modified Bamboo Fibers/PE Composites
The bamboo fibers, modified with six types of enzymes, were thoroughly mixed with PE and MAPE in a mass ratio of 40:56:4. Subsequently, the mixture was poured into a homemade mold and evenly spread out. The various enzyme-treated bamboo fibers/polyethylene composites were manufactured by hot pressing using a flat vulcanizing machine. The hot-pressing operation was carried out at 180 °C for 8 min, with a hot-pressing pressure between 5 to 6 MPa and a targeted design density of 1.1 g/cm3.
2.3. Performance Testing and Characterization
2.3.1. Color Test
The bamboo fibers were pressed into sheets using a tablet machine. The CM-2003d colorimeter was used to measure the brightness value (L*), the red–green axis chromaticity index (a*), and the yellow–blue axis chromaticity index (b*) of each sample, based on the CIELAB color system. Five points on each sample were tested, and the average values were recorded. Moreover, the differences (∆L*, ∆a*, and ∆b*) were calculated by comparing the results with those of the control sample. The total color change (∆E*) was then determined using Formula (1).
2.3.2. Surface Free Energy Analysis
Distilled water and ethylene glycol were used as test fluids to measure the contact angle of the bamboo fiber before and after enzymatic treatment. A contact angle measuring instrument was used, and the surface free energy was calculated using the Owens–Wendt method with Formulas (2) and (3). The polar component and dispersion component of distilled water are 51.0 mJ/m2 and 21.8 mJ/m2 [15], respectively, and the polar component and dispersion component of ethylene glycol are 19.0 mJ/m2 and 29.3 mJ/m2 [16], respectively.
In the formulas, represents the surface energy of the test liquid, θ is the contact angle between the test liquid and the fiber, represents the polar surface energy components of the bamboo fiber, represents the polar surface energy components of the test liquid, represents the dispersive surface energy components of the bamboo fiber, represents the dispersive surface energy components of the test liquid, and represents the surface free energy of the bamboo fiber.
2.3.3. Infrared Spectroscopy Test
A Fourier Transform Infrared Spectrometer (FTIR) was used to qualitatively analyze the chemical composition of the bamboo fibers. The infrared spectrum of the bamboo fiber was tested using the potassium bromide pellet method, where the mass ratio between the bamboo fiber and potassium bromide was approximately 1:100. Both substances were thoroughly mixed and ground, subsequently being compressed into a transparent or semi-transparent sheet using a tableting machine. The test range was 4000~400 cm−1, the spectral resolution was set to 4 cm−1, and the total number of scans was 32.
2.3.4. X-ray Photoelectron Spectroscopy Test
The main surface elements of the bamboo fiber (C, O, N, Si, etc.) were analyzed using a K-Alpha XPS instrument (Thermo Fisher Scientific, Waltham, MA, USA). The full spectrum scan was conducted with a pass energy of 100 eV and a step size of 1 eV, while the narrow spectrum scan was performed with a pass energy of 50 eV and a step size of 0.05 eV. The C1s signal peak was fitted and deconvoluted using the Thermo Fisher Scientific Avantage software (version 5.979).
2.3.5. Water Absorption Test
This test referred to the method of GB/T 17657-2013 [17] to test and analyze the 24 h water absorption rate and the 24 h water absorption thickness expansion rate of the BPC samples. The dimensions of the samples were 20 mm in length, 20 mm in width, and 4 mm in thickness, and the water bath temperature was 20 °C. After a certain period of immersion, the sample was removed, the surface water was gently wiped off with a soft cloth, and the mass and dimensions of the sample were measured. Each sample group consisted of 6 specimens, and the average value was recorded.
The water absorption rate and the thickness swelling rate due to water absorption were computed using the following Formulas (4) and (5):
In these formulas, W0 and Wt represent the mass of the specimen before and after water immersion, respectively. T0 and Tt denote the thickness of the specimen before and after water immersion, respectively.
2.3.6. Thermal Performance
The thermal properties of the BPC samples were tested using a thermogravimetric analyzer (STA 2500, Netzsch, Selb, Germany). This experiment was analyzed according to the method of GB/T 27761-2011 [18], where 5 to 7 mg of the pulverized BPC powder was placed in a high-purity dynamic nitrogen environment. The pyrolysis temperature range was usually room temperature to 700 °C, maintaining a heating rate of 10 °C/min. Thermogravimetry can obtain thermogravimetric curves (TGs), and the mass loss rate curves (DTGs) can be obtained by the differential calculation of the TGs.
2.3.7. Mechanical Property
- Bending Performance
Three-point bending tests were carried out in a universal electronic testing machine to evaluate the bending performance of various samples. The experiments were conducted with a span of 64 mm and a loading speed of 1.9 mm/min. The dimensions of the specimens were 80 mm in length, 13 mm in width, and 4 mm in thickness. Six samples from each group were tested, and the average value was subsequently calculated using the method described in the literature [19].
- 2.
- Impact Performance
A pendulum impact test was conducted on the BPC samples using a simple supported beam, following the procedure outlined in GB/1043.1-2008 [20]. With a machine span of 60 mm and an impact speed of 2.9 m/s, the specimens with a length of 80 mm, a width of 10 mm, and a thickness of 4 mm were evaluated. Six specimens were tested in each group, and the average value was taken.
2.3.8. Anti-Mildew Performance Test
The anti-mold performance test of the BPCs was carried out according to the method in GB/T 18261-2013 [21]. The mold fungus used in the experiment was Aspergillus niger. The specimens with a length of 50 mm, a width of 20 mm, and a thickness of 4 mm were inoculated before being placed in a constant temperature and humidity test chamber for a period of 28 days. The test chamber was maintained at a consistent temperature of 28 °C, with a relative humidity of 85%. Damage values to the samples were evaluated based on the extent of visible mold growth on the sample surface (refer to Table 3). The anti-mold efficacy was subsequently computed using Formula (6).

Table 3.
Damage values of bamboo fibers/PE composites.
In the given formula, E represents the anti-mildew effect, D0 denotes the damage value of the untreated composite sample, and D1 is the damage value of the composite sample prepared with enzyme-treated bamboo fibers.
2.3.9. Statistical Analysis
Significant procedures were carried out on the recorded data using the SPSS (Statistical Product and Service Solutions) 26.0 software. One-way analysis of variance (ANOVA) and least significant difference (LSD) multiple comparison tests were conducted. A significance level of α = 0.05 was used for all analyses.
3. Results and Discussion
3.1. Effect of Biological Enzymatic Treatment on the Surface Color of Bamboo Fibers
The image in Figure 1 illustrates the visual changes observed in the bamboo fibers before and after various enzymatic treatments. The specific results related to color parameters are detailed in Table 4. It can be seen that the red–green axis chroma index (a*) of most bamboo fibers did not change significantly compared with the untreated bamboo fibers. However, a notable decrease can be observed in the brightness value (L*) and the yellow–blue axis chroma index (b*). These findings indicate that enzymatic treatments can induce observable changes in the color attributes of bamboo fibers. The alterations in the color of enzymatically treated bamboo fibers are discernible to the naked eye, as they exceed the average capability of humans to distinguish color differences (∆E* greater than 5) [22].

Figure 1.
Appearance of bamboo fibers treated with biological enzymes.

Table 4.
Color parameters of bamboo fibers treated with biological enzymes.
3.2. Effect of Biological Enzymatic Treatment on the Surface Polarity of Bamboo Fibers
Table 5 presents the results of the contact angle and surface free energy of the untreated bamboo fibers compared with those treated with various bio-enzymes, using both distilled water and ethylene glycol as the test fluids. The data reveal that the enzymatic treatments led to a decrease in the surface free energy and the polar components while concurrently causing an increase in the dispersion components. Among the treated fibers, those treated with lipase demonstrated the lowest surface free energy (38.32 mJ/m2). The fibers treated with hemicellulase exhibited the smallest polar components, recorded at 4.72 mJ/m2. Meanwhile, the dispersion components were highest in fibers treated with pectinase, measuring at 36.76 mJ/m2. The decrease in the surface free energy and the polar surface energy components of the bamboo fibers may be primarily attributed to the degradation or removal of certain components, such as xylan, hemicellulose, and pectin, from the bamboo fibers. As a result, the content of hydrophilic groups on the bamboo fiber surface is diminished [23]. It has been reported that the extraction of hemicellulose can decrease the overall surface energy and hydrophilic character of wood [24].

Table 5.
Contact angle and surface free energy of bamboo fibers before and after biological enzymatic treatment a.
3.3. Effects of Biological Enzymatic Treatment on Main Functional Groups of Bamboo Fibers
The infrared spectra of the untreated and enzyme-treated bamboo fibers are shown in Figure 2. The absorption peak at 3429 cm−1 corresponds to the bending vibration of the hydroxyl group (O-H), and the absorption peak near 2918 cm−1 is attributed to the C-H bond. The band near 1730 cm−1 is assigned to the bending vibration of the non-conjugated carbonyl group (C=O) of hemicellulose and extractives, and the bands at 1632 cm−1 and 1514 cm−1 are characteristics of aromatic compounds associated with the C=C stretching of the aromatic ring skeleton of lignin [25,26,27]. As can be seen from Figure 2, after modifications with various biological enzymes, the intensity of the characteristic peaks of hydroxyl and carbonyl groups decreased to different extents. The weakest hydroxyl stretching vibration absorption peak was observed in bamboo fibers modified with xylanase and hemicellulase. Meanwhile, the C=O absorption peak at 1730 cm−1 in the bamboo fibers virtually vanishes following treatments with lipase, laccase, and amylase. This suggests that these enzyme treatments can decrease the hemicellulose or extractive content in bamboo fibers. Moreover, the characteristic peak of lignin weakens after laccase modification indicates a reduction in the lignin content of bamboo fibers.
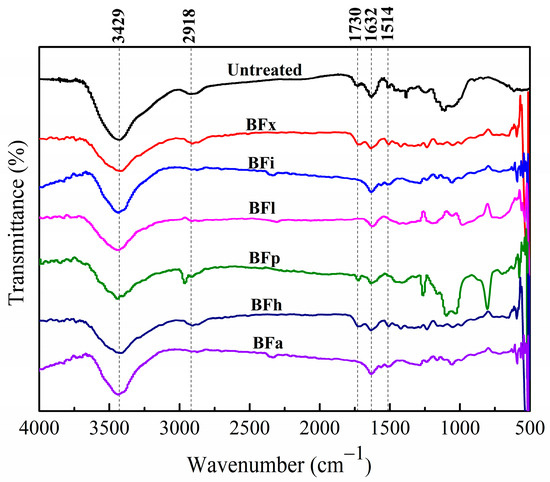
Figure 2.
Infrared spectra of bamboo fibers treated with biological enzymes.
Consequently, the components on the surface of the bamboo fibers undergo some degree of degradation or removal following modifications with various biological enzymes. Specifically, xylanase primarily degrades the xylan in bamboo fibers [28], lipase eliminates certain fat and lipids [29], laccase degrades some lignin and lipids [30], pectinase removes some pectin [31], hemicellulase removes some hemicellulose [32], and amylase eliminates some starch and other polysaccharide substances [33]. Thus, after treatment with biological enzymes, the surface structure of the bamboo fiber was damaged, making the surface of the bamboo fiber rough, which is conducive to its better combination with the plastic matrix.
3.4. Effect of Biological Enzymatic Treatment on Surface Element Composition of Bamboo Fibers
X-ray photoelectron spectroscopy (XPS) was used to analyze the surface element composition of the bamboo fibers. Figure 3 shows the XPS wide scanning map of the bamboo fibers treated with biological enzymes. It can be clearly observed from the figure that the peaks of C1s and O1s are located around 285 eV and 532 eV, respectively. This indicates the presence of a certain amount of carbon and oxygen elements in the bamboo fiber. Table 6 shows the relative content of the carbon and oxygen elements and the Oxygen/Carbon (O/C) ratio of the bamboo fibers before and after enzymatic modifications. The table reveals that the O/C ratio slightly decreased to 45.92% after hemicellulase treatment. The theoretical O/C ratios for cellulose, hemicellulose, and lignin are known to be 0.83, 0.81, and 0.33, respectively [34]. Therefore, this decrease is mainly due to the removal of low-molecular weight carbohydrates, such as hemicellulose, from the bamboo fiber. Moreover, except for the hemicellulase treatment, the O/C ratio of the bamboo fibers after enzymatic modifications was higher than that of the untreated bamboo fibers. This may be mainly caused by the following two reasons. Firstly, xylanase can selectively break the connection between lignin and polysaccharides, causing some lignin on the bamboo fiber surface to be shed. Secondly, although lipase, pectinase, and amylase do not affect lignin degradation, these enzymes can indirectly promote lignin removal by carrying away some tightly attached lignin while removing the wax layer, pectin, and starch. This leads to a decrease in the lignin content. Among the treated samples, the O/C ratio of the bamboo fibers treated with laccase was the highest, reaching 48.68%, which was 2.22% higher than the control sample (46.46%). This increase is attributed to laccase enhancing the surface activity of the bamboo fiber and promoting the accelerated degradation of lignin into decomposition products containing phenolic hydroxyl groups [35,36,37]. This process resulted in a decrease in the lignin content and an increase in the relative content of carbohydrates such as cellulose, thereby increasing the O/C ratio.
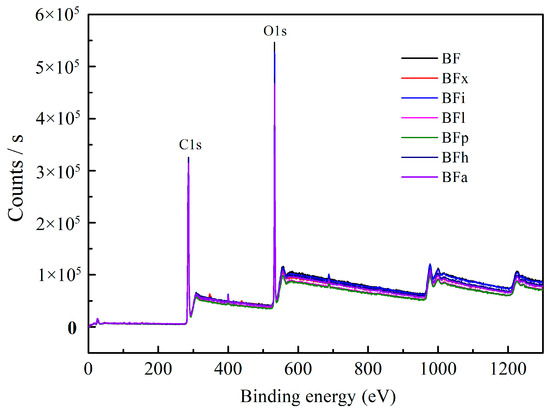
Figure 3.
XPS wide scan of bamboo fibers treated with biological enzymes.

Table 6.
The content of C and O elements and O/C value of bamboo fibers before and after biological enzymatic treatments.
In order to gain a deeper understanding of the surface elements of the bamboo fibers, Thermo Avantage software (5.979 version) was employed to fit the peaks of the C1s peak of the bamboo fibers before and after modifications, and the results are shown in Figure 4. The results, as shown in Figure 4, reveal four peaks, suggesting the existence of four different types of carbon on the bamboo fiber surface, namely, C1, C2, C3, and C4. The C1 component was referred to unoxidized carbon (i.e., C-C, C-H), mainly derived from lignin and extracts. The C2 component is due to a carbon atom bound to a single non-carbonyl oxygen atom (i.e., C-OH, C-O-C), and the C3 component is assigned to two carbon-atom-bonded oxygen atoms (i.e., C=O, O-C-O). The C2 and C3 component are mainly made from cellulose and hemicellulose. The C4 component is associated with carbon atoms that are bonded to one carbonyl oxygen atom and one non-carbonyl oxygen atom (i.e., O-C=O) and mainly come from hemicellulose and extracts [38,39]. The peak positions and relative content of the various chemical states of the carbon in the bamboo fibers both before and after enzymatic treatments are presented in Table 7. A comparison of the untreated bamboo fibers with the enzymatically modified fibers reveals that the relative contents of C1 and C4 in the bamboo fiber decreased after modification, while a significant increase is observed for C2 and C3. This can be attributed mainly to the varying degrees of degradation or elimination of non-cellulosic components in the bamboo fibers, such as hemicellulose, wax layers, lipids, pectin, lignin, and other extracts, following treatments with various biological enzymes, whereby such treatment results in the exposure of the fiber’s internal skeleton and cellulose structure [40]. Consequently, as the relative content of the cellulose increased, the components of C2 and C3 also increased. Notably, the bamboo fibers modified with lipase displayed the highest content of C2, measured at 51.20%, and the lowest content of C1 (34.16%).
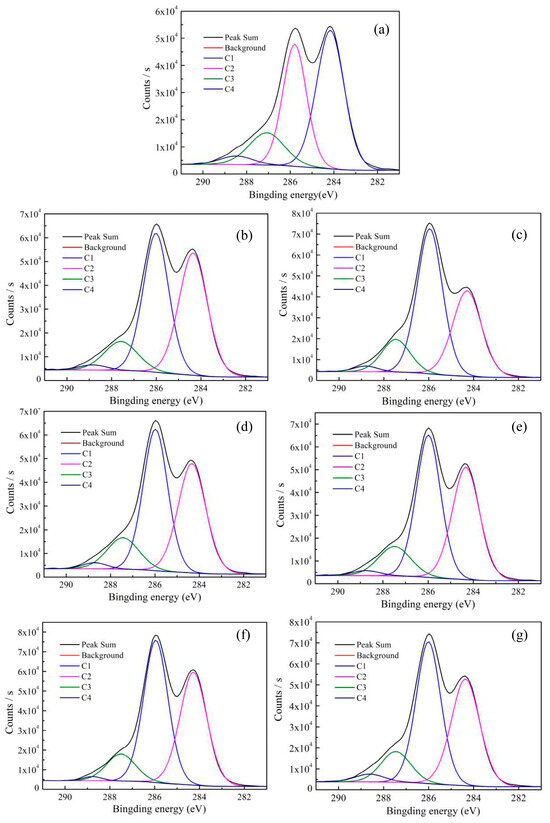
Figure 4.
C1s XPS spectra of untreated and bio-enzymatically treated bamboo fibers, where (a–g) are C1s XPS spectra on the surface of untreated fibers and the xylanase-, lipase-, laccase-, pectinase-, hemicellulase-, and amylase-treated fibers, respectively.

Table 7.
The percentage of different chemical states of carbon before and after enzymatic treatments of bamboo fibers.
3.5. Water Resistance of Bamboos/PE Composites Treated with Biological Enzymes
The moisture absorption of composites is regarded as an important determinant that affects the performance of these materials in almost all applications [41]. Figure 5 shows the 24 h water absorption and thickness expansion rates of the enzyme-modified bamboo fibers/PE composites. The statistical analysis indicated that treatment with various enzymes can significantly decrease the 24 h water absorption rate and thickness expansion rate of the composites, thereby enhancing their water resistance. Specifically, the composite that underwent hemicellulase modification exhibited the lowest 24 h water absorption rate (0.60%), which was 68.25% lower than that of the control group (1.89%). Meanwhile, the composite treated with xylanase had the lowest 24 h water absorption thickness expansion rate (0.52%), which was 71.27% lower than that of the control group (1.81%). The decrease in the water absorption of the composites was mainly attributed to the reduction in hemicellulose, lignin, pectin, and other substances in the bamboo fibers after enzymatic modifications. These modification processes roughen the bamboo fiber surface and decrease the hydrophilic group content. Consequently, the bamboo fibers are more uniformly distributed within the composites, the contact area between the bamboo fibers and the PE matrix is expanded, and the interfacial pores between the two are minimized. These changes hinder water infiltration into the BPC system, leading to lower 24 h water absorption and thickness expansion rates of the composites.
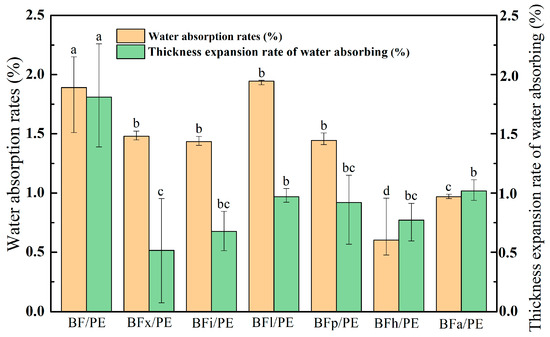
Figure 5.
Influence of enzyme-treated bamboo fibers on water absorption rate and water absorption thickness expansion rate of bamboo–plastic composites. Significant differences between enzymatic treatments are indicated by different letters (p < 0.05).
3.6. Thermal Performance Analysis of Bamboo Fibers/PE Composites Treated with Biological Enzymes
Figure 6 shows the TG (a) and DTG (b) curves of the untreated bamboo fibers/PE composites, the bamboo fibers/PE composites treated with different enzymes, and pure PE. It can be observed from the figure (a) that the thermogravimetric curve of the composites can be roughly divided into three stages: one is the evaporation of water in the composite, the second is the thermal decomposition of the bamboo fibers in the composite, and the third is the thermal decomposition of the PE. Table 8 presents the thermogravimetric characteristic data for the bamboo fibers/PE composites and the PE. It can be seen that during the second stage of the thermal decomposition of the bamboo fibers, the TP2 (representing the fastest rate of decomposition in the second stage) of the bamboo fibers/PE composites treated with various enzyme modifications was higher than that of the untreated bamboo fibers/PE composites. This suggests that different enzyme modifications can enhance the thermal performance of bamboo fibers. Among these modifications, the xylanase treatment resulted in the highest TP2, reaching 358.1 °C. However, the initial decomposition temperature T0, the temperature at TP3 (representing the fastest rate of decomposition in the third stage), and the residual carbon rate at 700 °C of the enzyme-modified bamboo fibers/PE composites were all lower than those of the untreated bamboo fibers/PE composites. Thus, while some aspects of thermal performance were improved, it appears that, overall, the various enzyme modification treatments may have reduced the thermal performance of the bamboo fibers/PE composites to varying degrees.
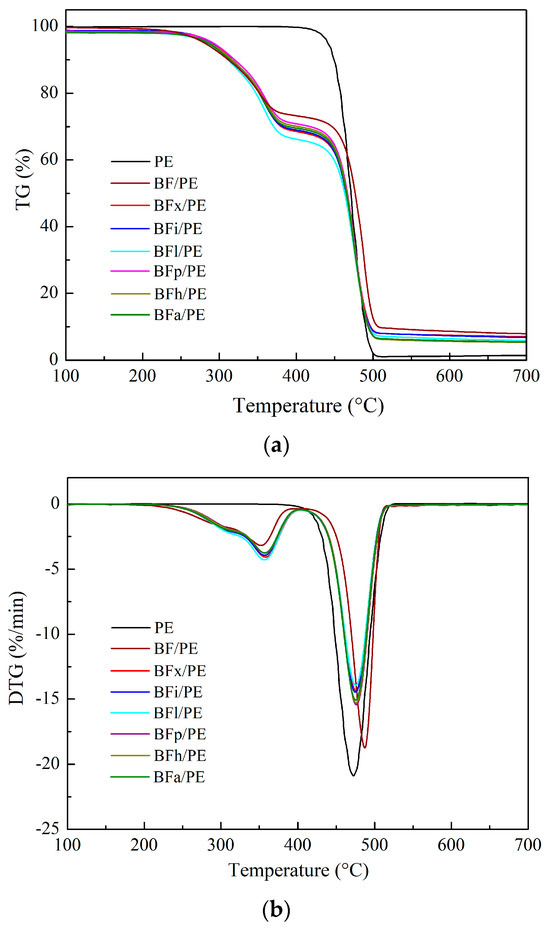
Figure 6.
(a) TG curves and (b) DTG curves of bamboo–plastic composites.

Table 8.
Thermogravimetric characteristics of untreated composites, bio-enzymatically treated composites, and PE.
3.7. Mechanical Performance of Bamboo Fibers/PE Composites Treated with Biological Enzymes
3.7.1. Bending Performance
Figure 7 illustrates the effects of various biological enzymes on the bending performance of the bamboo fiber/polyethylene (PE) composites. The results of the statistical analysis show that there were significant differences (at a significance level of 0.05) between the static bending strength and the elastic modulus of the enzyme-treated bamboo fibers/PE composites and the untreated bamboo fibers/PE composites. This suggests that such enzymatic modification treatments can improve the bending performance of composites. Among all treatments, the pectinase treatment resulted in the highest static bending strength and elastic modulus, measured at 48.71 MPa and 2.98 GPa, respectively. This represents an increase of 15.45% and 13.31% compared to the untreated composites (42.19 MPa and 2.63 GPa). The primary reason for this improvement is that the enzyme treatments effectively remove non-cellulosic components from the surface of the bamboo fiber, such as hemicellulose, lignin, pectin, and wax layers, etc. This process reduces the polarity of the fiber surface while increasing its roughness and specific surface area. As a result of this modification, the bamboo fiber exhibits improved mechanical interlocking with the plastic matrix. This enhances the interfacial bonding characteristics between the bamboo fiber and the PE matrix, which in turn elevates the bending performance of the composite [32,42,43].
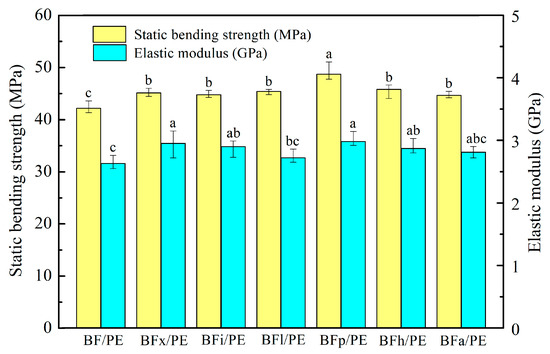
Figure 7.
Static bending strength and elastic modulus of bamboo fibers/PE composites treated with biological enzymes. Significant differences between enzymatic treatments are indicated by different letters (p < 0.05).
3.7.2. Impact Performance
The impact resistance of the unmodified and enzyme-modified bamboo fibers/PE composites was evaluated to determine the amount of absorbed energy during fractures, and the result is shown in Figure 8. From the statistical analysis, it can be seen that the impact strength of the bamboo–plastic composite was significantly diminished by amylase treatment. However, there was no significant difference in the impact strength of the composites reinforced with the bamboo fibers treated with other enzymes as compared to those that were untreated. Several factors such as inherent properties, the toughness of the reinforcing agent, the interface properties between the fiber and matrix, and the frictional work implicated in the fibers pulling out from resin impact the impact strength of fiber-reinforced polymer composites [41,44,45].
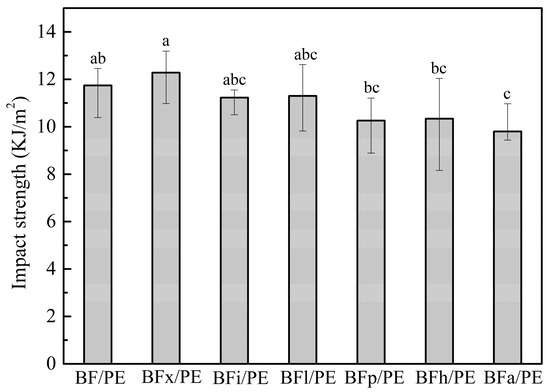
Figure 8.
Impact performance of bamboo fibers/PE composites treated with biological enzymes. Significant differences between enzymatic treatments are indicated by different letters (p < 0.05).
3.8. Anti-Mildew Performance of Bamboo Fibers/PE Composites Treated with Biological Enzymes
One of the main concerns regarding the use of wood–plastic composites in outdoor fields is their proneness to fungal attacks. This susceptibility is largely due to the wood fiber component of the composite, which is sensitive to fungi [46,47]. Figure 9 and Figure 10 show the changes in the anti-mold properties of the bamboo fibers/PE composites processed using various biological enzyme modification treatments. From the figures, it can be seen that these treatments significantly improved the anti-mold performance of the bamboo fibers/PE composites. The bamboo fibers/PE composites modified by xylanase demonstrated the highest mold resistance, with the lowest mold damage value of 0.95 and an anti-mold effectiveness of 74.67%. This result can be attributed to the enzymatic pretreatments of the bamboo fibers, which degrade and eliminate non-cellulosic components on their surface such as hemicellulose, xylan, the wax layer, lignin, extractives, pectin, starch, and others. This removal process achieves two main effects. Firstly, the reduction in substances like hemicellulose within the bamboo fiber can decrease the number of hydrophilic groups in the fiber, thereby weakening the wetness and hygroscopicity of the fiber surface. This enhances the water resistance of the composite, making it unable to satisfy the humidity conditions required for the growth of Aspergillus niger, thereby reducing the infection efficiency of the fungus and the speed of spread after infection. Secondly, after modification, the surface area of the bamboo fiber becomes larger and rougher, allowing it to bind more easily with the PE matrix, enhancing the interface performance of the composite. This makes it difficult for moisture and mold to penetrate the composite, effectively preventing the erosion of the composite by mold, thereby improving the mold resistance of the composite.
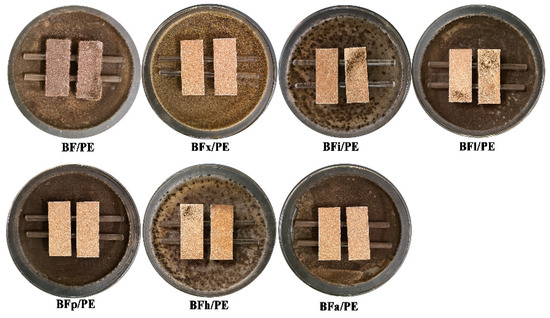
Figure 9.
Anti-mold effects of bamboo fibers/PE composites treated with different biological enzymes.
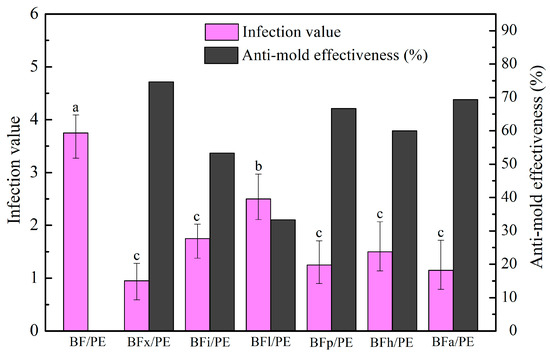
Figure 10.
Influence of biological enzyme treatments on the anti-mold performance of bamboo fibers/PE composites. Significant differences between enzymatic treatments are indicated by different letters (p < 0.05).
Furthermore, after the modification of the bamboo fibers with xylanase, some low molecular weight carbohydrates, xylan, and some lipids were degraded, and lignin-like substances were partially exposed. This increased the relative content of cellulose and lignin in the fibers. Lignin is chemically stable and not easily damaged by mold and even has a certain inhibitory effect on some fungi [48]. Therefore, in summary, the anti-mold performance of the composites was optimized after undergoing xylanase modification, with an anti-mold efficacy of 74.67%.
On the whole, this study found that enzymatic treatment has a certain positive effect on the surface characteristics of bamboo fibers used for bamboo–plastic composites, as well as on the physical, mechanical, and anti-mildew performance of bamboo–plastic composites. However, overall, it was observed that in several enzyme-modified bamboo fibers/PE composites, the composites prepared from bamboo fibers with the greatest reduction in surface free energy and polar components and the largest increase in the O/C atomic ratio did not exhibit the best water resistance, mechanical properties, and mold resistance. Therefore, further research is needed to establish a clear relationship between enzymatic modification treatments and the characteristics of lignocellulosic fibers, as well as the performance of wood–plastic composites. This will help in the development of the targeted biological modifications of wood fibers and the production of high-performance composites.
4. Conclusions
This study investigated the impact of six bio-enzymes, including xylanase, lipase, laccase, pectinase, hemicellulase, and amylase, on the surface characteristics of bamboo fibers as well as the physical and mechanical properties and anti-mildew performance of bamboo fibers/PE composites. The main results are as follows: Bio-enzyme treatments altered the color of the bamboo fibers to a certain degree and resulted in a decrease in the surface free energy and polar components of the fibers. Among them, the bamboo fibers treated with hemicellulase had the smallest polar component (4.72 mJ/m2). Overall, the O/C ratio of the bamboo fibers’ surface increased after the bio-enzyme treatments. In addition, there was a decrease in the water absorption rate and the thickness swelling rate of the BPCs upon treatment with the six bio-enzymes to varying degrees. Among all treatments, the hemicellulase treatment resulted in the smallest 24 h water absorption rate of the BPC (0.60), with a reduction of 68.71% compared to the control group. The 24 h water absorption thickness swelling rate of the composite treated with xylanase was the lowest (0.52%), which was 71.27% lower than the untreated group (1.81%). The bio-enzyme treatments decreased the thermal stability of the BPCs to some extent. The bio-enzyme modifications improved the bending performance of the BPCs. Specifically, the static bending strength and elastic modulus of the BPCs prepared with pectinase-treated bamboo fibers were the highest, at 48.71 MPa and 2.98 GPa, respectively, which were 15.45% and 13.31% higher than the untreated composites. The bio-enzyme treatments significantly improve the anti-mildew performance of the bamboo fibers/PE composites, and the anti-mildew effectiveness of the bamboo fibers/PE composites treated with xylanase reached 74.67%.
Overall, this study reveals the potential benefits of enzymatic treatments in enhancing the performance properties of bamboo fibers/PE composites. Specifically, treatments with xylanase, pectinase, and hemicellulose demonstrate a more considerable improvement on the properties of BPCs. Xylanase, in particular, significantly improved the water resistance, impact strength, and anti-mold performance of the composites. Consequently, xylanase emerged as a potentially valuable lignocellulosic fiber treatment enzyme, warranting its investigation in future studies.
Author Contributions
X.M., F.H., L.L., B.L., Y.C. and H.X. contributed to the preparation of the composites, data collection, and analysis. X.M., L.L. and L.Y. wrote the first draft of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (32160413 and 32060324), the Natural Science Foundation of Guizhou Province (No. Qiankehe ZK [2022] General 067), the International Joint Research Center for Biomass Materials, (Southwest Forestry University) (2023-GH06), and the 111 Project (D21027), Guizhou Provincial Department of Human Resources and Social Security high-level talent innovation and entrepreneurship project (2022-06).
Data Availability Statement
All the data are provided in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yu, L.S.; Wei, J.; Li, D.L.; Zhong, Y.D.; Zhang, Z.H. Explaining landscape levels and drivers of Chinese moso bamboo forests based on the plus model. Forests 2023, 14, 397. [Google Scholar] [CrossRef]
- Bahru, T.; Ding, Y.L. A review on bamboo resource in the african region: A call for special focus and action. Int. J. For. Res. 2021, 1, 8835673. [Google Scholar] [CrossRef]
- Gudainiyan, J.; Kishore, K. A review on cement concrete strength incorporated with agricultural waste. Mater. Today Proc. 2023, 78, 396–402. [Google Scholar] [CrossRef]
- Yang, F.; Jin, C.; Wang, S.; Wang, Y.J.; Wei, L.; Zheng, L.H.; Gu, H.P.; Lam, S.S.; Naushad, M.; Li, C.; et al. Bamboo-based magnetic activated carbon for efficient removal of sulfadiazine: Application and adsorption mechanism. Chemosphere 2023, 323, 138245. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.C.; Wang, Q.Y.; Sun, W.; Zhao, Y.H.; Wang, X.Z.; Liu, X.R.; Li, Y.J. Bamboo flattening technique: A literature and patent review. Eur. J. Wood Wood Prod. 2021, 79, 1035–1048. [Google Scholar] [CrossRef]
- Elsheikh, A.H.; Panchal, H.; Shanmugan, S.; Muthuramalingam, T.; El-Kassas, A.M.; Ramesh, B. Recent progresses in wood-plastic composites: Pre-processing treatments, manufacturing techniques, recyclability and eco-friendly assessment. Clean. Eng. Technol. 2022, 8, 100450. [Google Scholar] [CrossRef]
- Marais, S.; Gouanvé, F.; Bonnesoeur, A.; Grenet, J.; Poncin-Epaillard, F.; Morvan, C.; Métayer, M. Unsaturated polyester composites reinforced with flax fibers: Effect of cold plasma and autoclave treatments on mechanical and permeation properties. Compos. Part A Appl. Sci. Manuf. 2005, 36, 975–986. [Google Scholar] [CrossRef]
- Madhu, P.; Sanjay, M.R.; Jawaid, M.; Siengchin, S.; Khan, A.; Pruncu, C.I. A new study on effect of various chemical treatments on Agave Americana fiber for composite reinforcement: Physico-chemical, thermal, mechanical and morphological properties. Polym. Test. 2020, 85, 106437. [Google Scholar] [CrossRef]
- Rajeshkumar, G.; Hariharan, V.; Indran, S.; Sanjay, M.R.; Siengchin, S.; Maran, J.P.; Al-Dhabi, N.A.; Karuppiah, P. Influence of sodium hydroxide (NaOH) treatment on mechanical properties and morphological behaviour of Phoenix sp. fiber/epoxy composites. J. Polym. Environ. 2021, 29, 765–774. [Google Scholar] [CrossRef]
- Mohammed, M.; Rahman, R.; Mohammed, A.M.; Adam, T.; Betar, B.O.; Osman, A.F.; Dahham, O.S. Surface treatment to improve water repellence and compatibility of natural fiber with polymer matrix: Recent advancement. Polym. Test. 2022, 115, 107707. [Google Scholar] [CrossRef]
- Agrawal, M.; Naik, R.; Shetgar, S.; Purnima, D. Surface treatment of jute fibre using eco-friendly method and its use in PP composites. Mater. Today Proc. 2019, 18, 3268–3275. [Google Scholar] [CrossRef]
- Chaari, R.; Khlif, M.; Mallek, H.; Bradai, C.; Lacoste, C.; Belguith, H.; Tounsi, H.; Dony, P. Enzymatic treatments effect on the poly (butylene succinate)/date palm fibers properties for bio-composite applications. Ind. Crop. Prod. 2020, 148, 112270. [Google Scholar] [CrossRef]
- Mamun, A.A.; Bledzki, A.K. Micro fibre reinforced PLA and PP composites: Enzyme modification, mechanical and thermal properties. Compos. Sci. Technol. 2013, 78, 10–17. [Google Scholar] [CrossRef]
- Hasanin, M.S.; Abd El-Aziz, M.E.; El-Nagar, I.; Hassan, Y.R.; Youssef, A.M. Green enhancement of wood plastic composite based on agriculture wastes compatibility via fungal enzymes. Sci. Rep. 2022, 12, 19197. [Google Scholar] [CrossRef] [PubMed]
- Kusano, R.; Kusano, Y. Symmetric expressions of surface tension components. J. Adhes. 2023, 99, 2381–2401. [Google Scholar] [CrossRef]
- Yang, Z.H.; Cui, X. Effect of chain extenders with different functionalities on the properties of single-component waterborne polyurethane ink binders. RSC Adv. 2022, 12, 16696–16705. [Google Scholar] [CrossRef]
- GB/T 17657-2013; Test Methods of Evaluating the Properties of Wood-Based Panels and Surface Decorated Wood-Based Panels. China National Standardization Administration Committee: Beijing, China, 2013.
- GB/T 27761-2011; Standard Test Method of Mass Loss and Residue Measurement Validation of Thermogravimetric Analyzers. China National Standardization Administration Committee: Beijing, China, 2011.
- Guo, J.H.; Wang, C.; Li, C.; Liu, Y. Effect of acetylation on the physical and mechanical performances of mechanical densified spruce wood. Forests 2022, 13, 1620. [Google Scholar] [CrossRef]
- GB/1043.1-2008; Plastics—Determination of Charpy Impact Properties-Part 1: Non-Instrumented Impact Test. China National Standardization Administration Committee: Beijing, China, 2008.
- GB/T 18261-2013; Test method for anti-mildew agents in controlling wood mould and stain fungi. China National Standardization Administration Committee: Beijing, China, 2013.
- Gawron, J.; Marchwicka, M. Color changes of ash wood (Fraxinus excelsior L.) caused by thermal modification in air and steam. For. Wood Technol. 2021, 116, 21–27. [Google Scholar] [CrossRef]
- Yingkamhaeng, N.; Nimchua, T.; Pinmanee, P.; Suwanprateep, J.; Rungmekarat, S.; Sukyai, P. Synergistic effect of xylanase and laccase on structural features of energy cane. Ind. Crop. Prod. 2022, 176, 114410. [Google Scholar] [CrossRef]
- Hosseinaei, O.; Wang, S.Q.; Enayati, A.A.; Rials, T.G. Effects of hemicellulose extraction on properties of wood flour and wood-plastic composites. Compos. Part A Appl. Sci. Manuf. 2012, 43, 686–694. [Google Scholar] [CrossRef]
- Li, H.Y.; Wang, S.; Zhang, X.; Wu, H.; Wang, Y.J.; Zhou, N.; Zhao, Z.J.; Wang, C.; Zhang, X.F.; Wang, X.; et al. Synthesis and characterization of an environmentally friendly phenol-formaldehyde resin modified with waste plant protein. Polymers 2023, 15, 2975. [Google Scholar] [CrossRef] [PubMed]
- Popescu, C.M.; Jones, D.; Krzisnik, D.; Humar, M. Determination of the effectiveness of a combined thermal/chemical wood modification by the use of FT-IR spectroscopy and chemometric methods. J. Mol. Struct. 2019, 1200, 127133. [Google Scholar] [CrossRef]
- Li, H.Y.; Zou, Y.M.; Liang, J.Y.; Zhao, Z.J.; Zhou, N.; Gao, Y.; Yan, R.H.; Zhou, Q.Q.; Li, C. The potential of Platanus orientalis L. Bark for high-grade resource utilization. Forests 2023, 14, 2002. [Google Scholar] [CrossRef]
- Lei, X.C.; Lin, L.; Li, K.C. Effect of xylanase pretreatment of wood chips on fiber separation in the CTMP refining process. BioRes. 2008, 3, 801–815. [Google Scholar] [CrossRef]
- Jia, Q.Q.; Chen, J.C.; Yang, G.H.; Liu, K.F.; Wang, Y.Y.; Zhang, K. Effects of Lipase and Xylanase Pretreatment on the Structure and Pulping Properties of Wheat Straw. Polymers 2022, 14, 5129. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, A.; del Río, J.C.; Martínez, A.T. Microbial and enzymatic control of pitch in the pulp and paper industry. Appl. Microbiol. Biotechnol. 2009, 82, 1005–1018. [Google Scholar] [CrossRef]
- Li, X.P.; Xiao, R.; Morrell, J.J.; Wu, Z.K.; Du, G.B.; Wang, S.G.; Zou, C.G.; Cappellazzi, J. Improving the performance of bamboo and eucalyptus wood fiber/polypropylene composites using pectinase pre-treatments. J. Wood Chem. Technol. 2017, 38, 44–50. [Google Scholar] [CrossRef]
- Zhao, B.; Al Rasheed, H.; Ali, I.; Hu, S.L. Efficient enzymatic saccharification of alkaline and ionic liquid-pretreated bamboo by highly active extremozymes produced by the co-culture of two halophilic fungi. Bioresour. Technol. 2021, 319, 124115. [Google Scholar] [CrossRef]
- Akhtar, H.M.S.; Abdin, M.; Hamed, Y.S.; Wang, W.; Chen, G.J.; Chen, D.; Chen, C.X.; Li, W.; Mukhtar, S.; Zeng, X.X. Physicochemical, functional, structural, thermal characterization and α-amylase inhibition of polysaccharides from chickpea (Cicer arietinum L.) hulls. LWT Food Sci. Technol. 2019, 113, 108265. [Google Scholar] [CrossRef]
- Laine, J.; Stenius, P.; Carlsson, G.; Ström, G. Surface characterization of unbleached kraft pulps by means of ESCA. Cellulose 1994, 1, 145–160. [Google Scholar] [CrossRef]
- Kalia, S.; Thakur, K.; Kumar, A.; Celli, A. Laccase-assisted surface functionalization of lignocellulosics. J. Mol. Catal. B Enzym. 2014, 102, 48–58. [Google Scholar] [CrossRef]
- Shuddhodana, G.M.N.; Bisaria, V.S. Effectiveness of cross-linked enzyme aggregates of cellulolytic enzymes in hydrolyzing wheat straw. J. Biosci. Bioeng. 2018, 126, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Várnai, A.; Siika-Aho, M.; Viikari, L. Restriction of the enzymatic hydrolysis of steam-pretreated spruce by lignin and hemicellulose. Enzyme Microb. Technol. 2010, 46, 185–193. [Google Scholar] [CrossRef]
- Sun, H.; Yang, Y.; Han, Y.; Tian, M.; Li, B.; Han, L.; Wang, A.; Wang, W.; Zhao, R.; He, Y. X-ray photoelectron spectroscopy analysis of wood degradation in old architecture. BioRes 2020, 15, 6332–6343. [Google Scholar] [CrossRef]
- Bouafif, H.; Koubaa, A.; Perré, P.; Cloutier, A.; Riedl, B. Analysis of among-species variability in wood fiber surface using DRIFTS and XPS: Effects on esterification efficiency. J. Wood Chem. Technol. 2008, 28, 296–315. [Google Scholar] [CrossRef]
- Kalia, S.; Thakur, K.; Celli, A.; Kiechel, M.A.; Schauer, C.L. Surface modification of plant fibers using environment friendly methods for their application in polymer composites, textile industry and antimicrobial activities: A review. J. Environ. Chem. Eng. 2013, 1, 97–112. [Google Scholar] [CrossRef]
- Werchefani, M.; Lacoste, C.; Elloumi, A.; Belghith, H.; Gargouri, A.; Bradai, C. Enzyme-treated Tunisian Alfa fibers reinforced polylactic acid composites: An investigation in morphological, thermal, mechanical, and water resistance properties. Polym. Compos. 2020, 41, 1721–1735. [Google Scholar] [CrossRef]
- Saleem, Z.; Rennebaum, H.; Pudel, F.; Grimm, E. Treating bast fibers with pectinase improves mechanical characteristics of reinforced thermoplastic composites. Compos. Sci. Technol. 2008, 68, 471–476. [Google Scholar] [CrossRef]
- Li, X.; Xiao, R.; Morrell, J.J.; Zhou, X.; Du, G. Improving the performance of hemp hurd/polypropylene composites using pectinase pre-treatments. Ind. Crops Prod. 2017, 97, 465–468. [Google Scholar] [CrossRef]
- Fu, S.Y.; Feng, X.Q.; Lauke, B.; Mai, Y.W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate–polymer composites. Compos. Part B Eng. 2008, 39, 933–961. [Google Scholar] [CrossRef]
- Liu, L.; Yu, J.; Cheng, L.; Qu, W. Mechanical properties of poly(butylene succinate) (PBS) biocomposites reinforced with surface modified jute fibre. Compos. Part A Appl. Sci. Manuf. 2009, 40, 669–674. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, X.; Peng, Y.; Cao, J.Z. Water absorption and mold susceptibility of wood flour/polypropylene composites modified with silane-wax emulsions. Polym. Compos. 2019, 40, 141–148. [Google Scholar] [CrossRef]
- Dong, Y.M.; Tan, Y.; Wang, K.L.; Cai, Y.H.; Li, J.Z.; Sonne, C.; Li, C. Reviewing wood-based solar-driven interfacial evaporators for desalination. Water Res. 2022, 223, 119011. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, H.; Umezawa, T.; Yoshikawa, T.; Koyama, Y.; Fumoto, E.; Sato, S.; Nakasaka, Y.; Masuda, T. Antifungal activity of simply fractionated organosolv lignin against Trametes versicolor. J. Biotechnol. 2023, 364, 23–30. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).













