Soil Carbon and Nitrogen Pools and Their Storage Characteristics under Different Vegetation Restoration Types on the Loess Plateau of Longzhong, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
2.3. Soil Sampling
2.4. Soil Analysis
2.4.1. Soil Environmental Factors
2.4.2. Soil Carbon and Nitrogen Pools
2.4.3. Soil Carbon and Nitrogen Storages
2.5. Statistical Analysis
3. Results
3.1. Changes in Soil Environmental Factors under Different Vegetation Restoration Types
3.2. Changes in Soil Carbon Pools under Different Vegetation Restoration Types
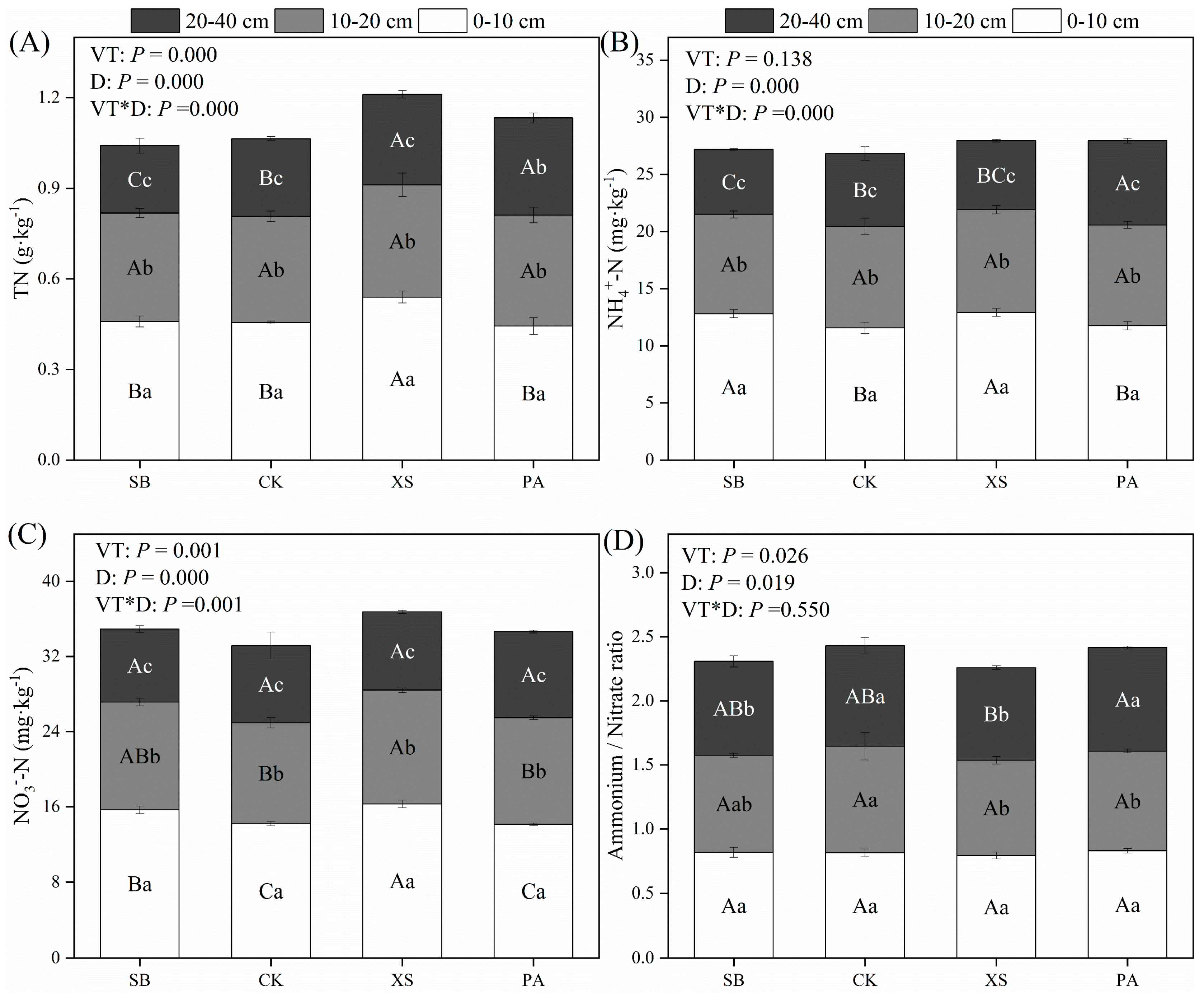
3.3. Changes in Soil N Pools under Different Vegetation Restoration Types
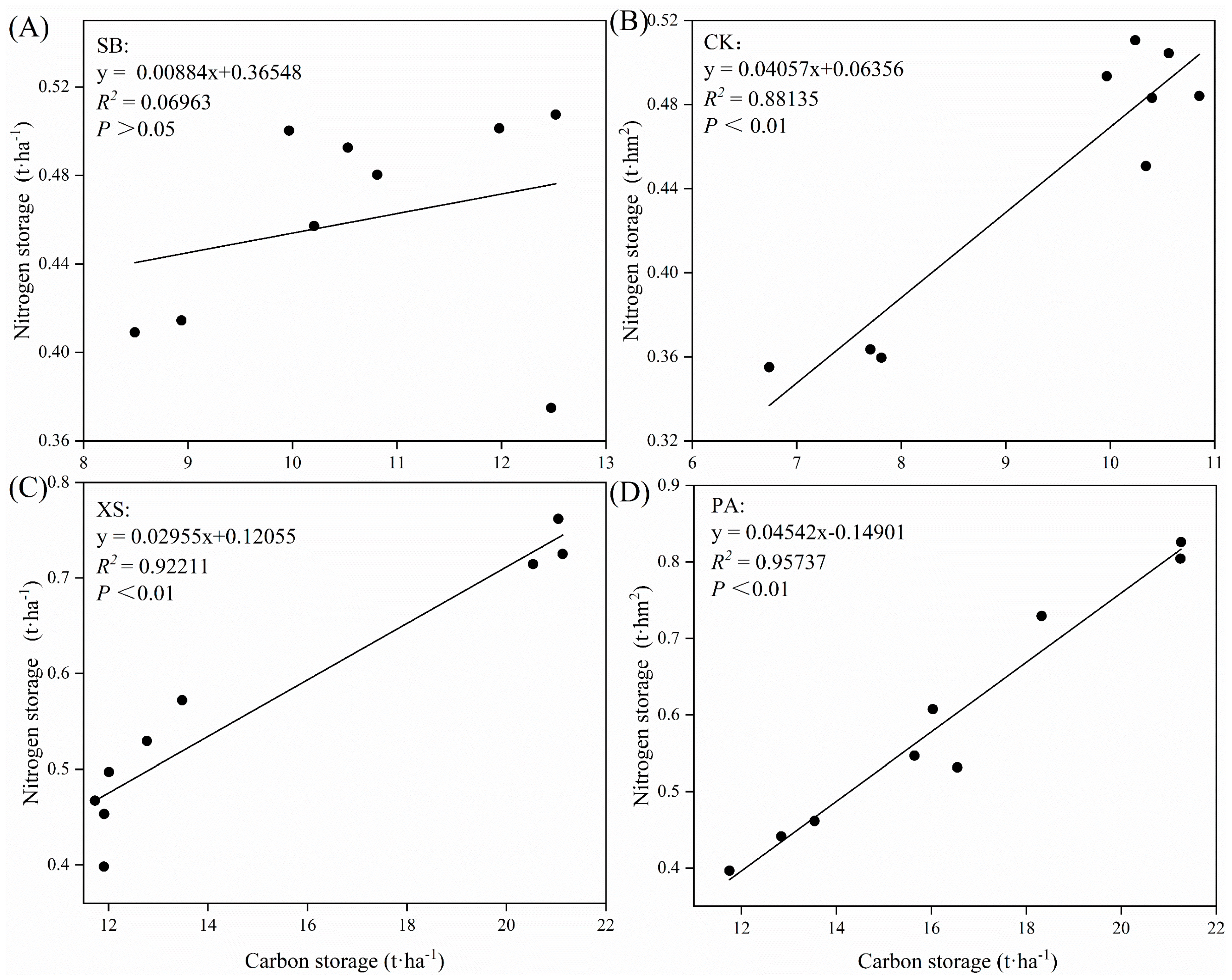
3.4. Changes in Soil Carbon and Nitrogen Storages and C/N under Different Vegetation Restoration Types
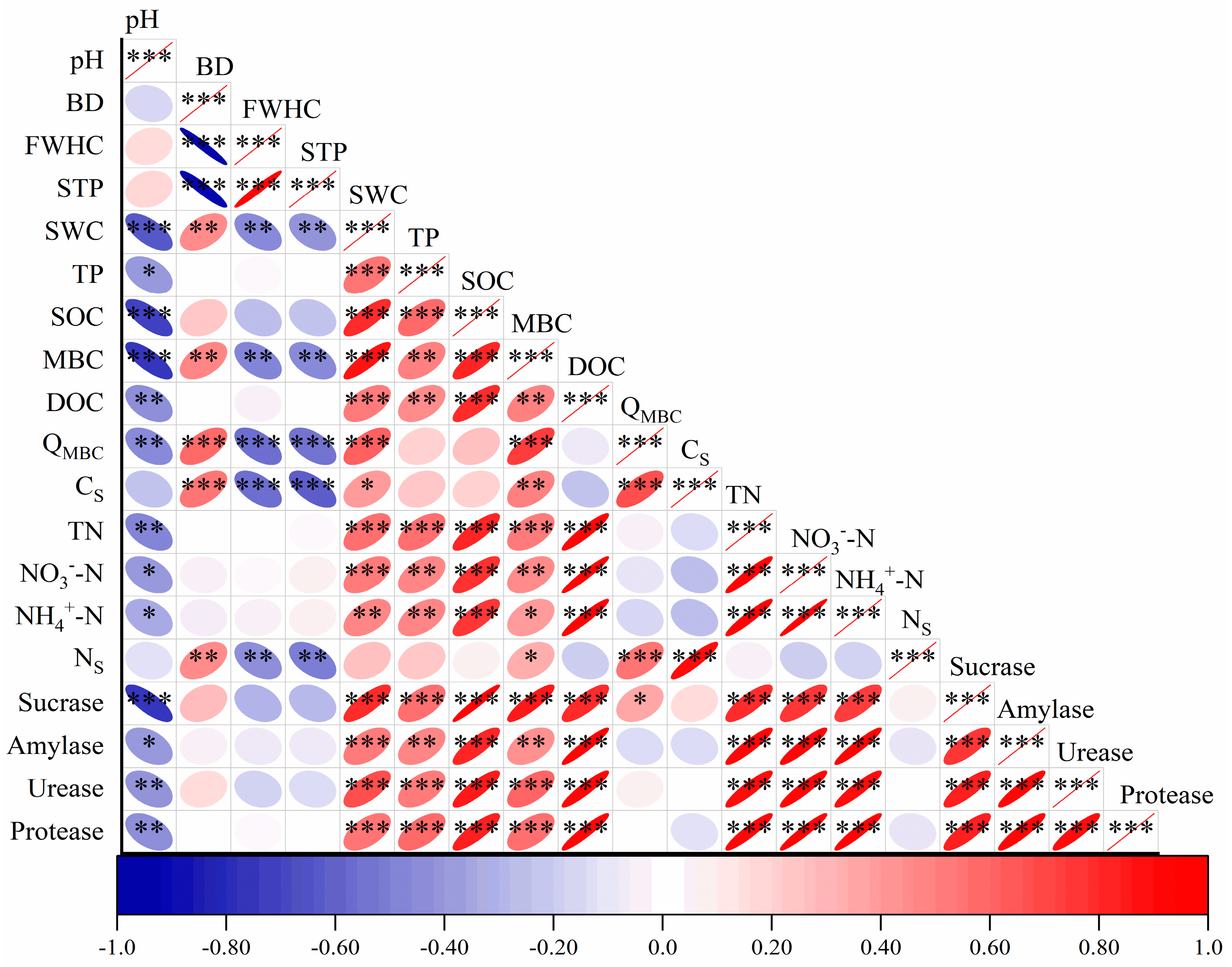
3.5. Relationships between Soil Carbon and Nitrogen Pools and Environmental Factors
4. Discussion
4.1. Effects of Different Vegetation Restoration Types on Soil Carbon and Nitrogen Storages
4.2. Effects of Different Vegetation Restoration Types on Soil Carbon Pools
4.3. Effects of Different Vegetation Restoration Types on Soil Nitrogen Pools
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, J.; Liang, Y.; Zhuo, M.; Sadiq, M.; Liu, L.; Wu, J.; Xu, G.; Liu, S.; Li, G.; Yan, L. Soil nitrogen and carbon storages and carbon pool management index under sustainable conservation tillage strategy. Front. Ecol. Evol. 2023, 10, 1082624. [Google Scholar] [CrossRef]
- De Marco, A.; Napoletano, P.; Panico, S.; Memoli, V.; Santorufo, L.; Ruggiero, A.; Colombo, C.; Barile, R.; Maisto, G. Combined effect of black locust invasion and fire on soils of Mediterranean shrublands and pine forests. Catena 2023, 220, 106656. [Google Scholar] [CrossRef]
- He, L.; Lu, S.; Wang, C.; Mu, J.; Zhang, Y.; Wang, X. Changes in soil organic carbon fractions and enzyme activities in response to tillage practices in the Loess Plateau of China. Soil Tillage Res. 2021, 209, 104940. [Google Scholar] [CrossRef]
- Omidvar, N.; Xu, Z.; Nguyen, T.; Salehin, B.; Ogbourne, S.; Ford, R.; Bai, S. A global meta-analysis shows soil nitrogen pool increases after revegetation of riparian zones. J. Soils Sediments 2021, 21, 665–677. [Google Scholar] [CrossRef]
- De Marco, A.; Panico, S.; Memoli, V.; Santorufo, L.; Zarrelli, A.; Barile, R.; Maisto, G. Differences in soil carbon and nitrogen pools between afforested pine forests and natural shrublands in a Mediterranean area. Appl. Soil Ecol. 2022, 170, 104262. [Google Scholar] [CrossRef]
- Bukomba, J.; Lusk, G. Spatial Variability in Inorganic Soil Nitrogen Production in a Mixed-Vegetation Urban Landscape. Nitrogen 2022, 3, 118–127. [Google Scholar] [CrossRef]
- Virk, L.; Liu, W.; Niu, J.; Xu, C.; Liu, Q.; Kan, Z.; Zhao, X.; Zhang, H. Effects of Diversified Cropping Sequences and Tillage Practices on Soil Organic Carbon, Nitrogen, and Associated Fractions in the North China Plain. J. Soil Sci. Plant Nutr. 2021, 21, 1201–1212. [Google Scholar] [CrossRef]
- Gao, G.; Tuo, D.; Han, X.; Jiao, L.; Li, J.; Fu, B. Effects of land-use patterns on soil carbon and nitrogen variations along revegetated hillslopes in the Chinese Loess Plateau. Sci. Total Environ. 2020, 746, 141156. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, H.; Xing, Z.; Zou, Y.; Cui, Z. Vegetation Degradation of Guanshan Grassland Suppresses the Microbial Biomass and Activity of Soil. Land 2021, 10, 203. [Google Scholar] [CrossRef]
- Xiao, R.; Man, X.; Duan, B. Carbon and Nitrogen Stocks in Three Types of Larix gmelinii Forests in Daxing’an Mountains, Northeast China. Forests 2020, 11, 305. [Google Scholar] [CrossRef]
- Li, D.; Niu, S.; Luo, Y. Global patterns of the dynamics of soil carbon and nitrogen stocks following afforestation: A meta-analysis. New Phytol. 2012, 195, 172–181. [Google Scholar] [CrossRef]
- Li, T.; Liu, G. Age-related changes of carbon accumulation and allocation in plants and soil of black locust forest on Loess Plateau in Ansai County, Shaanxi Province of China. Chin. Geogr. Sci. 2014, 24, 414–422. [Google Scholar] [CrossRef]
- Liu, M.; Li, P.; Liu, M.; Wang, J.; Chang, Q. The trend of soil organic carbon fractions related to the successions of different vegetation types on the tableland of the Loess Plateau of China. J. Soils Sediments 2020, 21, 203–214. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, D.; Cai, X.; Yang, X.; Zhang, H.; Wang, Y.; Diao, L.; Luo, Y.; Cheng, X. Natural revegetation over ~160 years alters carbon and nitrogen sequestration and stabilization in soil organic matter on the Loess Plateau of China. Catena 2023, 220, 106647. [Google Scholar] [CrossRef]
- Wang, H.; Wu, J.; Li, G.; Yan, L. Changes in soil carbon fractions and enzyme activities under different vegetation types of the northern Loess Plateau. Ecol. Evol. 2020, 10, 12211–12223. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Kang, D.; Wu, J.; Zhao, F.; Yang, G.; Han, X.; Feng, Y.; Re, G. Temporal variation in soil enzyme activities after afforestation in the Loess Plateau, China. Geoderma 2016, 282, 103–111. [Google Scholar] [CrossRef]
- Zhong, Z.; Wu, S.; Lu, X.; Ren, Z.; Wu, Q.; Xu, M.; Ren, C.; Yang, G.; Han, X. Organic carbon, nitrogen accumulation, and soil aggregate dynamics as affected by vegetation restoration patterns in the Loess Plateau of China. Catena 2021, 196, 104867. [Google Scholar] [CrossRef]
- Liu, S.; Wu, J.; Li, G.; Yang, C.; Yuan, J.; Xie, M. Seasonal freeze-thaw characteristics of soil carbon pools under different vegetation restoration types on the Longzhong Loess Plateau. Front. Ecol. Evol. 2022, 10, 1019627. [Google Scholar] [CrossRef]
- Yang, C.; Li, G.; Yan, L.; Ma, W.; Wu, J.; Tan, Y.; Liu, S.; Zhang, S. Effects of Plant Community Type on Soil Methane Flux in Semiarid Loess Hilly Region, Central Gansu Province, China. Adv. Atmos. Sci. 2022, 39, 1360–1374. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Z.; Li, Y.; Sun, G.; Cen, Y.; Lou, Y.; Yao, Y.; Liu, W. Eco-Environment Quality Response to Climate Change and Human Activities on the Loess Plateau, China. Land 2023, 12, 1792. [Google Scholar] [CrossRef]
- Lv, P.; Sun, S.; Medina-Roladand, E.; Zhao, S.; Hu, Y.; Guo, A.; Zuo, X. Effects of habitat types on the dynamic changes in allocation in carbon and nitrogen storage of vegetation-soil system in sandy grasslands: How habitat types affect C and N allocation? Ecol. Evol. 2021, 11, 9079–9091. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Liu, S.; Liu, J.; Zhou, G. Effects of vegetation restoration and slope positions on soil aggregation and soil carbon accumulation on heavily eroded tropical land of Southern China. J. Soils Sediments 2009, 10, 505–513. [Google Scholar] [CrossRef]
- Yang, C.; Li, G.; Yan, L.; Wu, J.; Li, J.; Liu, S.; Lu, Y. Effects of different vegetation types on ecosystem respiration in semiarid Loess Hilly Region, Central Gansu Province, China. Ecol. Indic. 2022, 145, 109683. [Google Scholar] [CrossRef]
- Zhang, L.; Song, L.; Zhang, L.; Shao, H.; Chen, X.; Yan, K. Seasonal dynamics in nitrous oxide emissions under different types of vegetation in saline-alkaline soils of the Yellow River Delta, China and implications for eco-restoring coastal wetland. Ecol. Eng. 2013, 61, 82–89. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, Y.; Li, P.; Xu, G.; Shi, P.; Zhang, Y. Effects of freeze-thaw cycles on aggregate-associated organic carbon and glomalin-related soil protein in natural-succession grassland and Chinese pine forest on the Loess Plateau. Geoderma 2019, 334, 1–8. [Google Scholar] [CrossRef]
- Guan, Y.; Zhou, W.; Bai, Z.; Cao, Y.; Huang, Y.; Huang, H. Soil nutrient variations among different land use types after reclamation in the Pingshuo opencast coal mine on the Loess Plateau, China. Catena 2020, 188, 104427. [Google Scholar] [CrossRef]
- Xu, M.; Wang, J.; Zhu, Y.; Han, X.; Ren, C.; Yang, G. Plant Biomass and Soil Nutrients Mainly Explain the Variation of Soil Microbial Communities during Secondary Succession on the Loess Plateau. Microb. Ecol. 2022, 83, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Li, J.; Zhang, Y.; Bing, M.; Liu, Y.; Wu, J.; Hai, X.; Li, A.; Wang, K.; Wu, P.; et al. Effects of vegetation restoration types on soil nutrients and soil erodibility regulated by slope positions on the Loess Plateau. J. Environ. Manag. 2022, 302, 113985. [Google Scholar] [CrossRef]
- Ge, F.; Xu, M.; Gong, C.; Zhang, Z.; Tan, Q.; Pan, X. Land cover changes the soil moisture response to rainfall on the Loess Plateau. Hydrol. Process. 2022, 36, e14714. [Google Scholar] [CrossRef]
- Jiang, X.; Shen, W.; Bai, X. Response of net primary productivity to vegetation restoration in Chinese Loess Plateau during 1986–2015. PLoS ONE 2019, 14, e0219270. [Google Scholar] [CrossRef]
- Deng, J.; Sun, P.; Zhao, F.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Soil C, N, P and Its Stratification Ratio Affected by Artificial Vegetation in Subsoil, Loess Plateau China. PLoS ONE 2016, 11, e0151446. [Google Scholar] [CrossRef] [PubMed]
- Meena, V.; Mondal, T.; Pandey, B.; Mukherjee, A.; Yadav, R.; Choudhary, M.; Singh, S.; Bisht, J.; Pattanayak, A. Land use changes: Strategies to improve soil carbon and nitrogen storage pattern in the mid-Himalaya ecosystem, India. Geoderma 2018, 321, 69–78. [Google Scholar] [CrossRef]
- Xiao, B.; Sun, F.; Yao, X.; Hu, K.; Kidron, G. Seasonal variations in infiltrability of moss-dominated biocrusts on aeolian sand and loess soil in the Chinese Loess Plateau. Hydrol. Process. 2019, 33, 2449–2463. [Google Scholar] [CrossRef]
- Zhao, J.; Luo, X.; Ma, Y.; Shao, T.; Yue, Y. Soil characteristics and new formation model of loess on the Chinese Loess Plateau. Geosci. J. 2017, 21, 607–616. [Google Scholar] [CrossRef]
- Ma, Y.; Bi, Q.; Li, G.; Liu, X.; Fu, G.; Zhao, Y.; Wang, L. Provenance variations in kernel oil content, fatty acid profile and biodiesel properties of Xanthoceras sorbifolium Bunge in northern China. Ind. Crops Prod. 2020, 151, 112487. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, W.; Xu, M.; Deng, J.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Response of forest growth to C:N:P stoichiometry in plants and soils during Robinia pseudoacacia afforestation on the Loess Plateau, China. Geoderma 2019, 337, 280–289. [Google Scholar] [CrossRef]
- Wu, J.; Wang, H.; Li, G.; Wu, J.; Gong, Y.; Wei, X. Responses of CH4 flux and microbial diversity to changes in rainfall amount and frequencies in a wet meadow in the Tibetan Plateau. Catena 2021, 202, 105253. [Google Scholar] [CrossRef]
- Lin, S.; Wang, S.; Si, Y.; Yang, W.; Zhu, S.; Ni, W. Variations in eco-enzymatic stoichiometric and microbial characteristics in paddy soil as affected by long-term integrated organic-inorganic fertilization. PLoS ONE 2017, 12, e0189908. [Google Scholar] [CrossRef]
- Zhu, X.; Fang, X.; Xiang, W.; Chen, L.; Shuai, O.; Li, P. Vegetation restoration drives dynamics of soil nitrogen content and availability in the subtropics. Catena 2023, 220, 106720. [Google Scholar] [CrossRef]
- Munoz-Rojas, M.; Ereckson, T.; Martini, D.; Dixon, K.; Merritt, D. Soil physicochemical and microbiological indicators of short, medium and long term post-fire recovery in semi-arid ecosystems. Ecol. Indic. 2016, 63, 14–22. [Google Scholar] [CrossRef]
- Paul, K.; Polglase, P.; Nyakuengama, J.; Khanna, P. Change in soil carbon following afforestation. For. Ecol. Manag. 2002, 168, 241–257. [Google Scholar] [CrossRef]
- Sartori, F.; Lal, R.; Ebinger, M.; Eaton, J. Changes in soil carbon and nutrient pools along a chronosequence of poplar plantations in the Columbia Plateau, Oregon, USA. Agric. Ecosyst. Environ. 2007, 122, 325–339. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Garcra-Palacios, P.; Milla, R.; Gallardo, A.; Maestre, F. Soil characteristics determine soil carbon and nitrogen availability during leaf litter decomposition regardless of litter quality. Soil Biol. Biochem. 2015, 81, 134–142. [Google Scholar] [CrossRef]
- Hussain, S.; Sharma, V.; Arya, V.; Sharma, K.; Rao, S. Total organic and inorganic carbon in soils under different land use/land cover systems in the foothill Himalayas. Catena 2019, 182, 104104. [Google Scholar] [CrossRef]
- Sun, X.; Wang, G.; Ye, Y.; Ma, Q.; Guan, Q.; Jones, D. Response of nitrogen fractions in the rhizosphere and bulk soil to organic mulching in an urban forest plantation. J. For. Res. 2021, 32, 2577–2588. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, Y.; Hu, X.; Wan, S.; Wang, H.; Liang, C.; Chen, F. Litter manipulation effects on microbial communities and enzymatic activities vary with soil depth in a subtropical Chinese fir plantation. For. Ecol. Manag. 2021, 480, 118641. [Google Scholar] [CrossRef]
- Qin, H.; Jiao, L.; Zhou, Y.; Wu, J.; Che, X. Elevation affects the ecological stoichiometry of Qinghai spruce in the Qilian Mountains of northwest China. Front. Plant Sci. 2022, 13, 917755. [Google Scholar] [CrossRef]
- Wang, D.; Yi, W.; Zhou, Y.; He, S.; Tang, L.; Yin, X.; Zhao, P.; Long, G. Intercropping and N application enhance soil dissolved organic carbon concentration with complicated chemical composition. Soil Tillage Res. 2021, 210, 104979. [Google Scholar] [CrossRef]
- Cai, Y.; Chang, S.; Ma, B.; Edwardw, B. Watering increased DOC concentration but decreased N2O emission from a mixed grassland soil under different defoliation regimes. Biol. Fertil. Soils 2016, 52, 987–996. [Google Scholar] [CrossRef]
- Xiao, K.; Li, D.; Wen, L.; Yang, L.; Luo, P.; Chen, H.; Wang, K. Dynamics of soil nitrogen availability during post-agricultural succession in a karst region, southwest China. Geoderma 2018, 314, 184–189. [Google Scholar] [CrossRef]
- Cong, W.; Ruijven, J.; Werf, V.; Dedeyn, G.; Mommer, L.; Berendse, F.; Hoffland, E. Plant species richness leaves a legacy of enhanced root litter-induced decomposition in soil. Soil Biol. Biochem. 2015, 80, 341–348. [Google Scholar] [CrossRef]
- Chen, H.; Huang, X.; Shi, W.; Herbert, K.; Hou, L.; Yang, H.; Song, Q.; Liu, J.; Shi, J.; Yang, Q.; et al. Coordination of nitrogen uptake and assimilation favours the growth and competitiveness of moso bamboo over native tree species in high-NH4+ environments. J. Plant Physiol. 2021, 266, 153508. [Google Scholar] [CrossRef]
- Wang, L.; Macko, S. Constrained preferences in nitrogen uptake across plant species and environments. Plant Cell Environ. 2011, 34, 525–534. [Google Scholar] [CrossRef]
- Nasholm, T.; Kielland, K.; Ganeteg, U. Uptake of organic nitrogen by plants. New Phytol. 2009, 182, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Bauhus, J.; Pare, D.; Te, L. Effects of tree species, stand age and soil type on soil microbial biomass and its activity in a southern boreal forest. Soil Biol. Biochem. 1998, 8, 1077–1089. [Google Scholar] [CrossRef]
- Jackson, L.; Burger, M.; Cavagnaro, T. Roots, nitrogen transformations, and ecosystem services. Annu. Rev. Plant Biol. 2008, 59, 341–363. [Google Scholar] [CrossRef]
- Wu, J.; Wang, H.; Li, G.; Ma, W.; Wu, J.; Gong, X.; Xu, G. Vegetation degradation impacts soil nutrients and enzyme activities in wet meadow on the Qinghai-Tibet Plateau. Sci. Rep. 2020, 10, 21271. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J. Nitrogen Mineralization: Challenges of a Changing Paradigm. Ecology 2004, 85, 591–602. [Google Scholar]
- Zhang, J.; Zhu, T.; Cai, Z.; Muller, C. Nitrogen cycling in forest soils across climate gradients in Eastern China. Plant Soil 2011, 342, 419–432. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Wang, H.; Wang, R.; Wang, X.; Li, J. Crop yield and soil properties of dryland winter wheat-spring maize rotation in response to 10-year fertilization and conservation tillage practices on the Loess Plateau. Field Crops Res. 2018, 225, 170–179. [Google Scholar] [CrossRef]

| Type of Vegetation Restoration | Geographical Coordinates | Altitude (m) | Coverage (%) | Main Species | Soil Disturbance History |
|---|---|---|---|---|---|
| Stipa bungeana Trin. (SB) | 104°39′1″ E, 35°34′48″ N | 1985 | >90 | Stipa bungeana Trin., Plantago asiatica L., Setaria viridis (L.) Beauv., Leymus secalinus (Georgi) Tzvel. | Abandoned at the end of 1990, naturally restored Stipa bungeana Trin. grassland. |
| Caragana korshinskii Kom. (CK) | 104°38′1″ E, 35°34′55″ N | 1980 | 30 | Caragana korshinskii Kom, Potentilla chinensis Ser., Picris hieracioides L. | Felled and planted until the 1990s; it was abandoned at the end of 2000 and naturally restored to a shrub community. |
| Xanthoceras sorbifolia Bunge. (XS) | 104°39′11″ E, 35°34′45″ N | 2010 | 60 | Xanthoceras sorbifolium Bunge., Bupleurum chinense DC., Gentiana macrophylla Pall. var. fetissowii (Regel et Winkl.) Ma et K.C.Hsia., Leontopodium leontopodioides (Willd.) Beauv. | Cleared by deforestation until the 1990s; the forest was restored by artificial planting in 2002. |
| Picea asperata Mast. (PA) | 104°38′51″ E, 35°34′51″ N | 1990 | 60 | Picea asperata Mast., Stipa bungeana Trin., Leymus secalinus (Georgi) Tzvel. | Picea asperata Mast. with a tree age of 10 years was transplanted in 2005 and naturally restored to the Picea asperata Mast. forest. |
| Type of Vegetation Restoration | pH | BD (g·cm−3) | FWHC (g·kg−1) | STP (%) | SOC (g·kg−1) | TN (g·kg−1) | TP (mg·kg−1) |
|---|---|---|---|---|---|---|---|
| SB | 7.88 ± 0.01 A | 1.10 ± 0.04 BC | 576.26 ± 23.83 AB | 67.62 ± 1.09 AB | 8.51 ± 0.90 A | 0.43 ± 0.02 AB | 65.67 ± 2.66 C |
| CK | 7.84 ± 0.01 A | 1.02 ± 0.03 C | 650.30 ± 34.98 A | 70.03 ± 1.41 A | 8.17 ± 0.86 A | 0.39 ± 0.02 B | 95.00 ± 10.22 BC |
| XS | 7.38 ± 0.09 C | 1.13 ± 0.04 AB | 535.23 ± 36.69 BC | 64.32 ± 1.82 BC | 10.68 ± 1.18 A | 0.52 ± 0.06 A | 113.33 ± 6.79 AB |
| PA | 7.57 ± 0.02 B | 1.22 ± 0.03 A | 467.20 ± 28.86 C | 61.18 ± 1.98 C | 9.83 ± 0.36 A | 0.52 ± 0.03 A | 144.89 ± 21.13 A |
| Soil Layer (cm) | Vegetation Restoration Types | SWC (%) | TP (mg·kg−1) | Sucrase (mg·g−1) | Amylase (mg·g−1) | Urease (mg·g−1) | Protease (μg·g−1) |
|---|---|---|---|---|---|---|---|
| 0–10 | SB | 17.10 ± 0.67 BCa | 0.16 ± 0.01 ABa | 401.68 ± 5.91 Ca | 45.09 ± 0.18 Ba | 67.26 ± 0.69 Aa | 208.58 ± 5.87 Ba |
| CK | 15.63 ± 0.70 Ca | 0.14 ± 0.12 Ba | 279.59 ± 7.95 Da | 47.53 ± 0.36 ABa | 56.89 ± 0.13 Ca | 152.38 ± 2.52 Ca | |
| XS | 19.40 ± 0.06 Aa | 0.21 ± 0.02 Aa | 545.83 ± 2.65 Aa | 48.83 ± 1.08 ABa | 61.88 ± 2.66 Ba | 236.71 ± 0.92 Aa | |
| PA | 18.43 ± 0.38 ABa | 0.16 ± 0.01 ABa | 519.81 ± 2.45 Ba | 50.66 ± 2.62 Aa | 60.89 ± 0.73 BCa | 213.74 ± 7.38 Ba | |
| 10–20 | SB | 15.43 ± 0.66 Bab | 0.12 ± 0.00 Ba | 277.99 ± 7.45 Cb | 30.68 ± 0.81 Bb | 37.53 ± 2.19 Bb | 115.61 ± 4.39 Ab |
| CK | 12.77 ± 0.73 Cb | 0.14 ± 0.00 Aa | 225.01 ± 6.67 Db | 34.97 ± 0.24 Ab | 35.53 ± 2.21 Bb | 103.46 ± 16.18 Ab | |
| XS | 18.77 ± 0.12 Ab | 0.12 ± 0.01 Bb | 425.26 ± 2.11 Ab | 36.14 ± 0.12 Ab | 48.66 ± 1.50 Ab | 113.46 ± 4.59 Ab | |
| PA | 17.57 ± 0.26 Aab | 0.15 ± 0.00 Aa | 401.83 ± 9.69 Bb | 35.79 ± 0.74 Ab | 44.03 ± 00.61 Ab | 129.06 ± 1.21 Ab | |
| 20–40 | SB | 14.40 ± 0.64 Bb | 0.14 ± 0.02 Aa | 124.77 ± 5.82 Bc | 22.31 ± 0.18 Bc | 21.20 ± 0.66 Cc | 54.96 ± 1.64 Cc |
| CK | 9.07 ± 0.61 Cc | 0.09 ± 0.00 Ba | 136.00 ± 4.77 Bc | 24.63 ± 0.94 ABc | 18.94 ± 0.60 Dc | 56.16 ± 1.11 Cc | |
| XS | 17.37 ± 0.12 Ac | 0.14 ± 0.01 Ab | 280.23 ± 1.01 Ac | 28.34 ± 1.21 Ac | 37.54 ± 0.52 Ac | 61.85 ± 0.98 Bc | |
| PA | 16.90 ± 0.35 Ab | 0.16 ± 0.01 Aa | 292.34 ± 4.91 Ac | 27.31 ± 2.01 Ac | 35.31 ± 0.71 Bc | 87.58 ± 0.79 Ac |
| Soil Layer/cm | SB | CK | XS | PA | |
|---|---|---|---|---|---|
| CS/t·ha−1 | 0–10 | 10.52 ± 0.17 Cb | 10.40 ± 0.09 Ca | 12.76 ± 0.42 Bb | 16.08 ± 0.26 Ab |
| 10–20 | 9.13 ± 0.44 Bc | 7.42 ± 0.34 Cb | 11.85 ± 0.06 Ab | 12.71 ± 0.52 Ac | |
| 20–40 | 12.32 ± 0.17 Ba | 10.39 ± 0.26 Ca | 20.90 ± 0.19 Aa | 20.28 ± 0.98 Aa | |
| 0–40 | 31.97 ± 0.34 C | 28.21 ± 0.48 D | 45.51 ± 0.47 B | 49.06 ± 1.48 A | |
| NS/t·ha−1 | 0–10 | 0.48 ± 0.01 Ba | 0.50 ± 0.01 Ba | 0.53 ± 0.02 ABb | 0.56 ± 0.02 Ab |
| 10–20 | 0.44 ± 0.03 Aa | 0.36 ± 0.00 Bb | 0.44 ± 0.02 Ac | 0.43 ± 0.02 Ac | |
| 20–40 | 0.46 ± 0.04 Ba | 0.48 ± 0.01 Ba | 0.73 ± 0.01 Aa | 0.79 ± 0.03 Aa | |
| 0–40 | 1.38 ± 0.02 B | 1.34 ± 0.02 B | 1.71 ± 0.05 A | 1.78 ± 0.03 A | |
| C/N | 0–10 | 22.07 ± 0.35 BCab | 20.84 ± 0.43 Ca | 23.95 ± 0.19 Bb | 28.72 ± 1.37 Aa |
| 10–20 | 20.75 ± 0.47 Bb | 20.63 ± 0.84 Ba | 27.10 ± 1.45 Aa | 29.36 ± 0.16 Aa | |
| 20–40 | 27.28 ± 3.01 Aa | 21.85 ± 0.84 Ba | 28.50 ± 0.45 Aa | 25.76 ± 0.37 ABb | |
| 0–40 | 23.37 ± 1.33 B | 21.11 ± 0.41 B | 26.52 ± 0.80 A | 27.94 ± 0.69 A |
| C/N | RDA Test Results | Factors | Explains (%) | Contribution (%) | F | p |
|---|---|---|---|---|---|---|
| C | Environmental factors | Sucrase | 73.6 | 76.6 | 94.9 | 0.002 |
| Amylase | 17.0 | 17.7 | 59.7 | 0.002 | ||
| SWC | 2.9 | 3.0 | 14.2 | 0.002 | ||
| STP | 1.0 | 1.0 | 5.6 | 0.012 | ||
| FWHC | 0.6 | 0.7 | 3.9 | 0.022 | ||
| RDA twin axis | Axis 1 | 77.48 | - | - | - | |
| Axis 2 | 18.49 | - | - | - | ||
| N | Environmental factors | Protease | 89.7 | 92.3 | 297 | 0.002 |
| Amylase | 2.5 | 2.5 | 10.4 | 0.002 | ||
| STP | 2.0 | 2.1 | 11.3 | 0.004 | ||
| Urease | 1.5 | 1.5 | 10.7 | 0.006 | ||
| BD | 0.7 | 0.7 | 5.6 | 0.016 | ||
| RDA twin axis | Axis 1 | 96.74 | - | - | - | |
| Axis 2 | 0.39 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, M.; Yuan, J.; Liu, S.; Xu, G.; Lu, Y.; Yan, L.; Li, G. Soil Carbon and Nitrogen Pools and Their Storage Characteristics under Different Vegetation Restoration Types on the Loess Plateau of Longzhong, China. Forests 2024, 15, 173. https://doi.org/10.3390/f15010173
Xie M, Yuan J, Liu S, Xu G, Lu Y, Yan L, Li G. Soil Carbon and Nitrogen Pools and Their Storage Characteristics under Different Vegetation Restoration Types on the Loess Plateau of Longzhong, China. Forests. 2024; 15(1):173. https://doi.org/10.3390/f15010173
Chicago/Turabian StyleXie, Mingjun, Jianyu Yuan, Shuainan Liu, Guorong Xu, Yanhua Lu, Lijuan Yan, and Guang Li. 2024. "Soil Carbon and Nitrogen Pools and Their Storage Characteristics under Different Vegetation Restoration Types on the Loess Plateau of Longzhong, China" Forests 15, no. 1: 173. https://doi.org/10.3390/f15010173
APA StyleXie, M., Yuan, J., Liu, S., Xu, G., Lu, Y., Yan, L., & Li, G. (2024). Soil Carbon and Nitrogen Pools and Their Storage Characteristics under Different Vegetation Restoration Types on the Loess Plateau of Longzhong, China. Forests, 15(1), 173. https://doi.org/10.3390/f15010173




