Three Censuses of a Mapped Plot in Coastal California Mixed-Evergreen and Redwood Forest
Abstract
1. Introduction
2. Materials and Methods
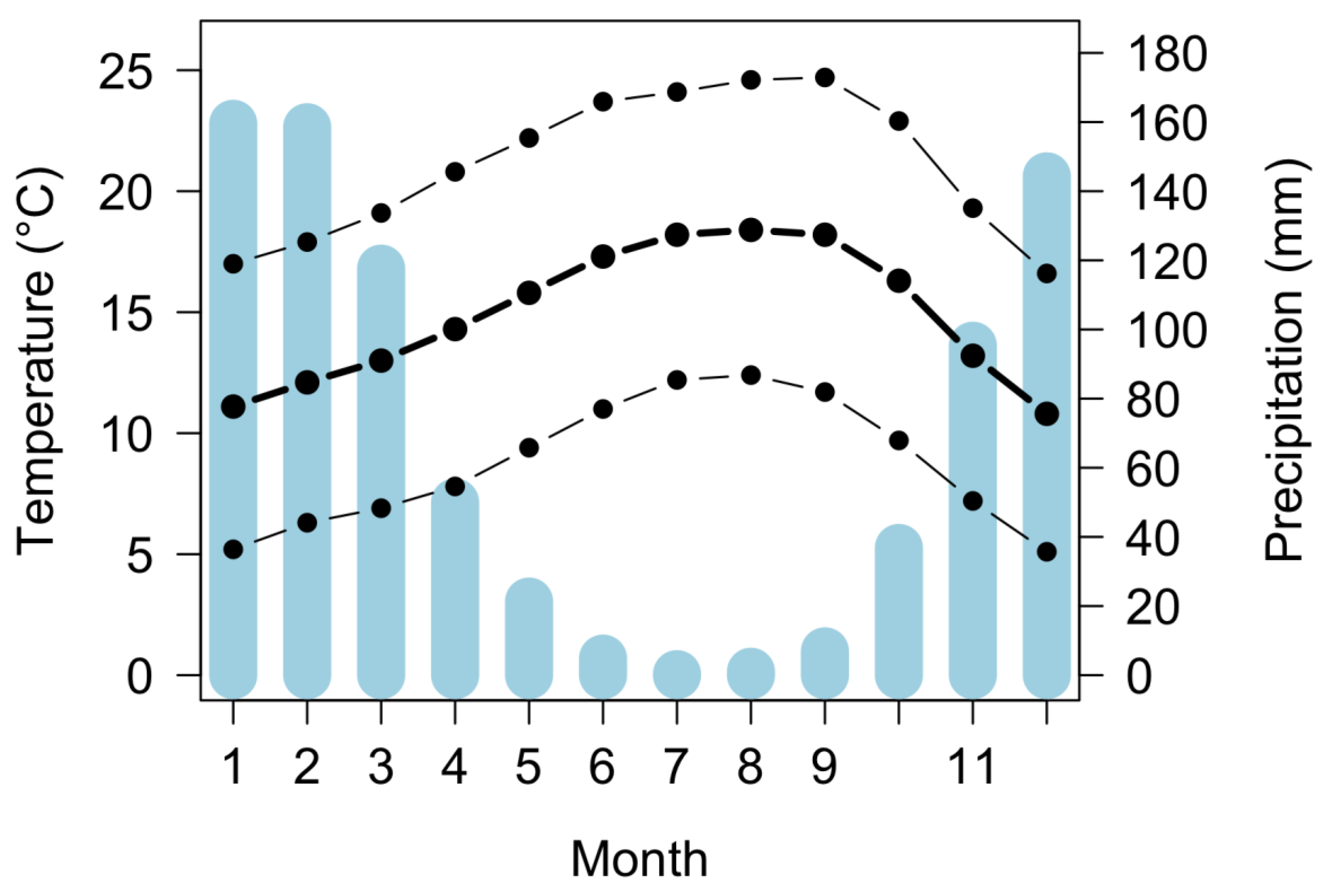
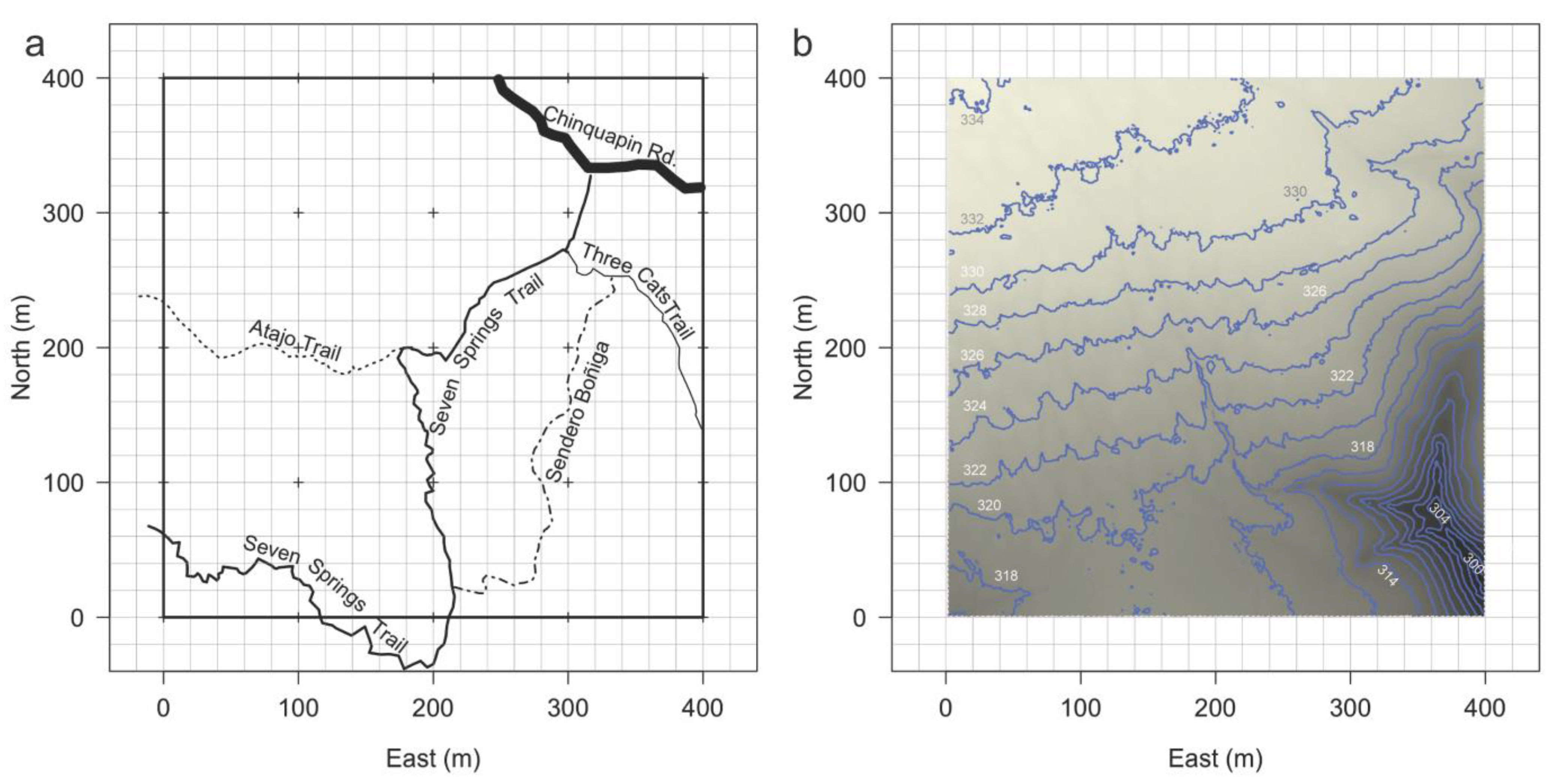
2.1. Site Description
2.2. Mapping, Measurement, and Data Protocols
2.3. Field Personnel and Data Management
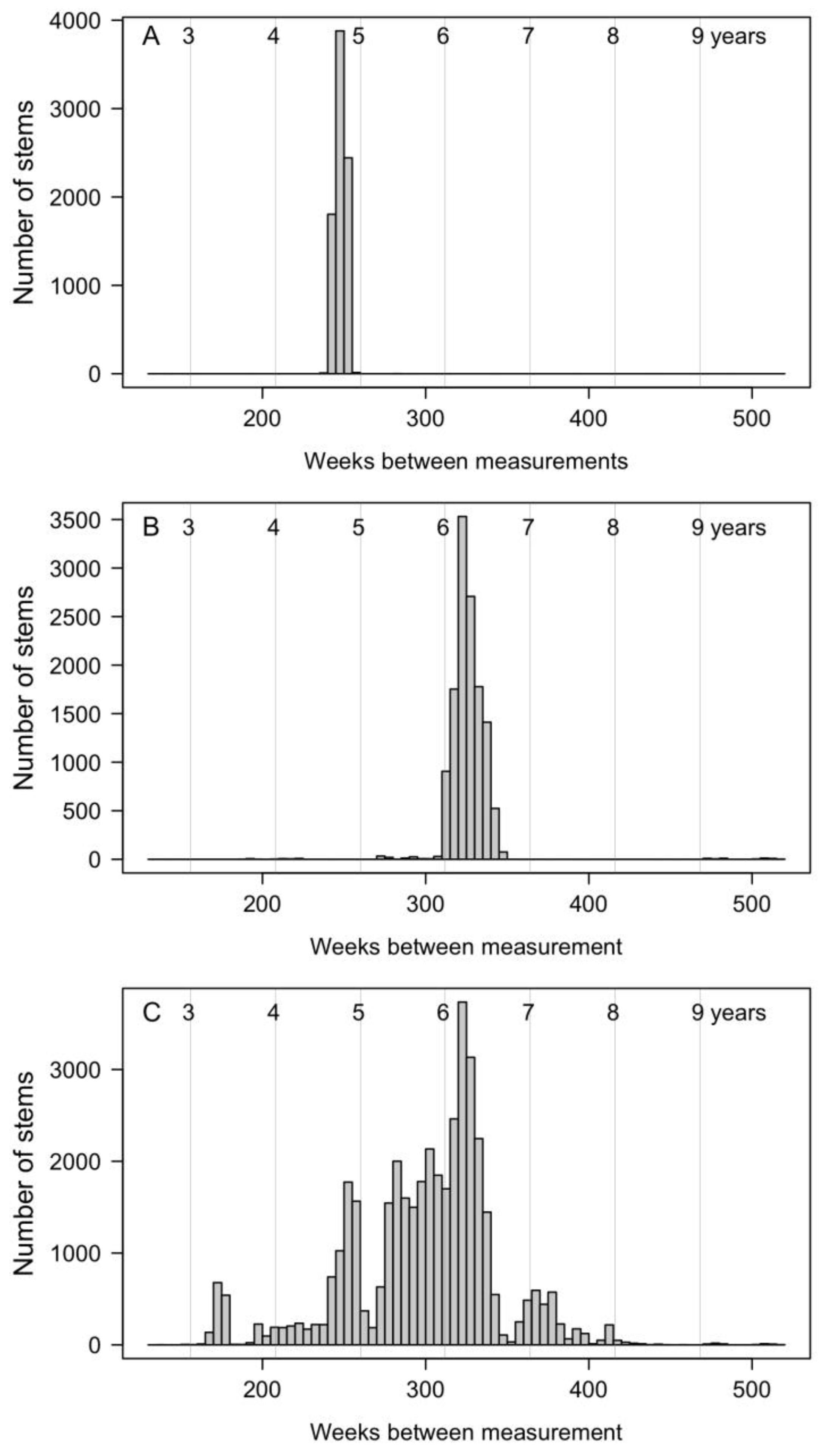
2.4. Census Periods
2.5. Mortality Rates
2.6. Growth Rates
2.7. Availability of Data
3. Results
3.1. Species Composition
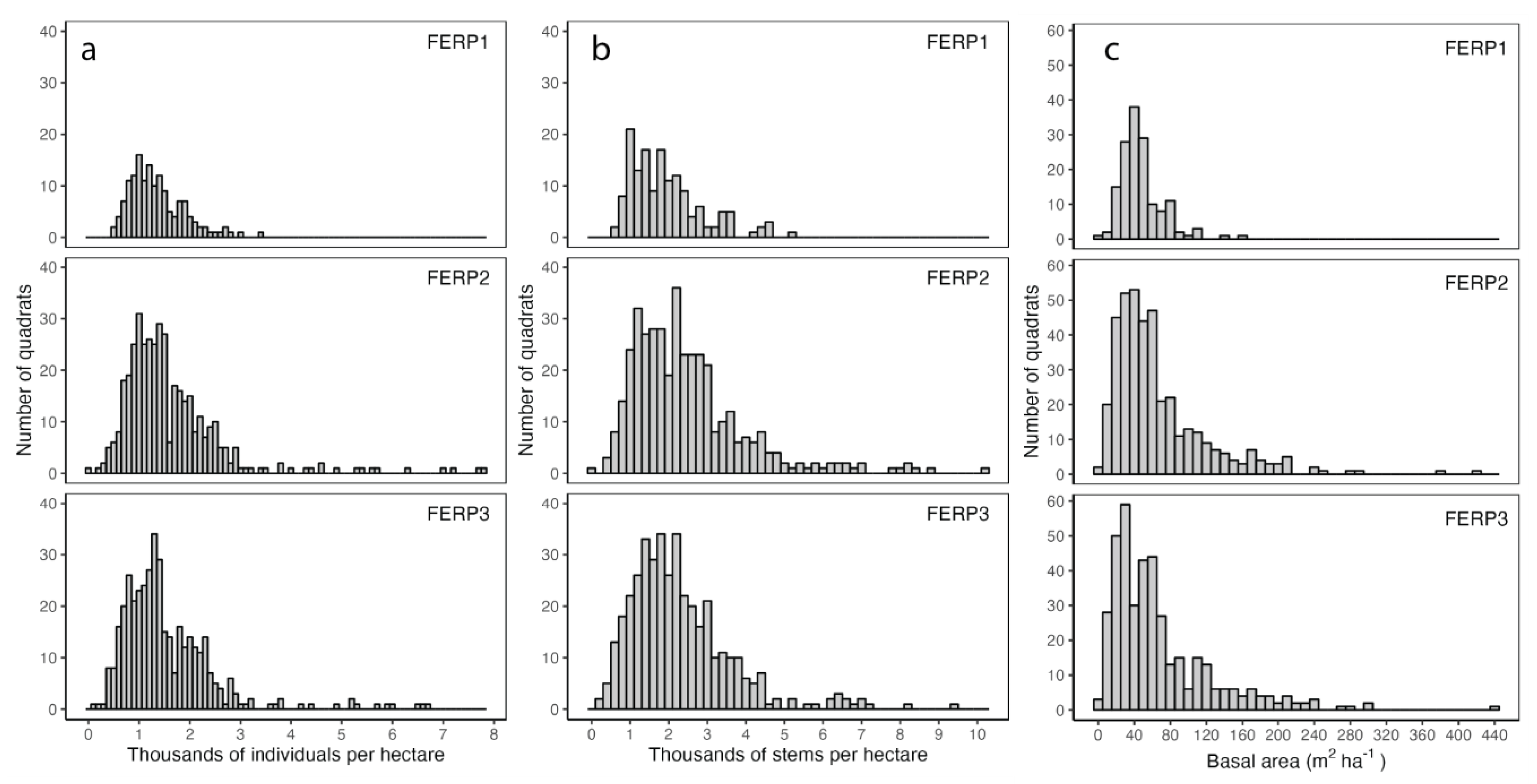
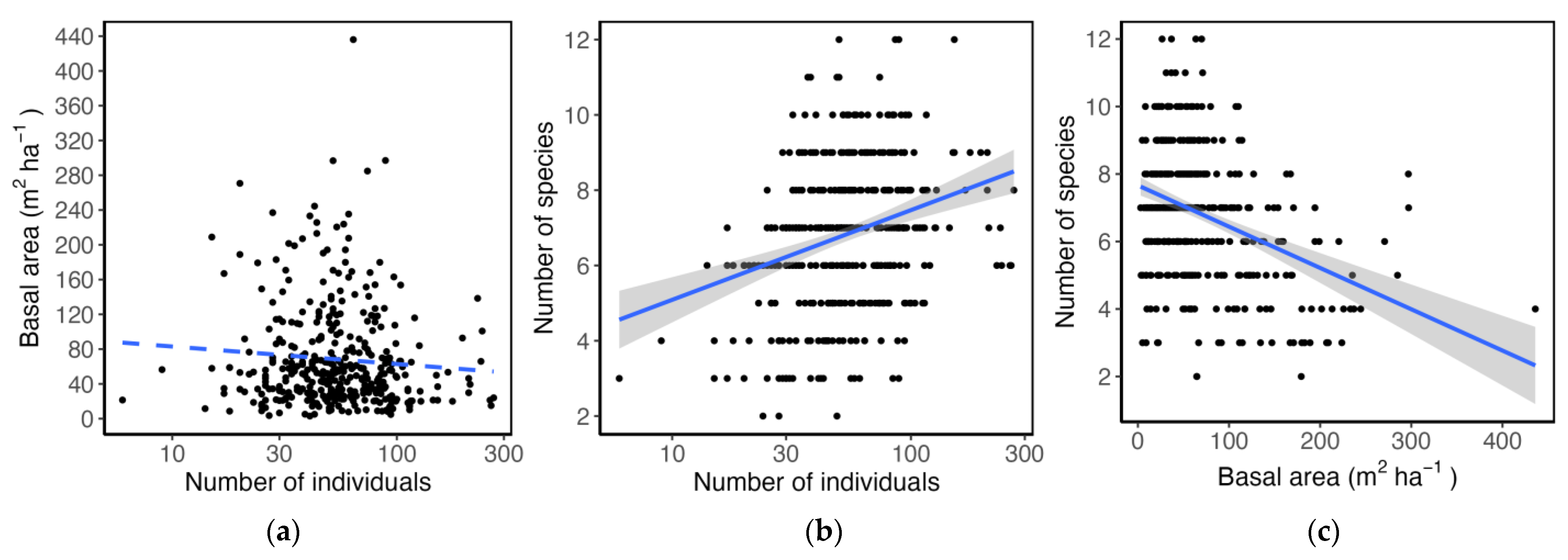
3.2. Abundance and Basal Area
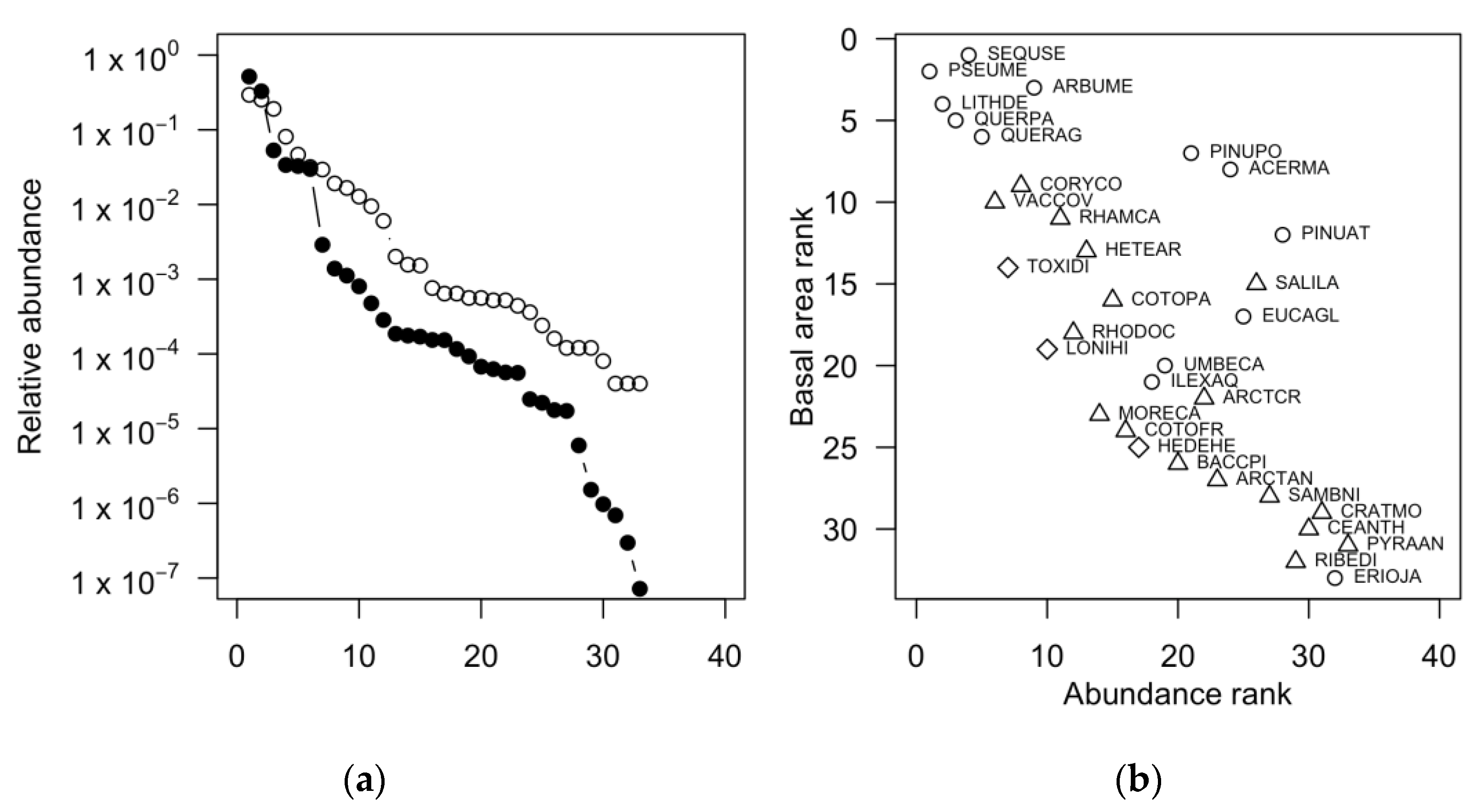
3.3. Community Structure and Species Relative Abundance
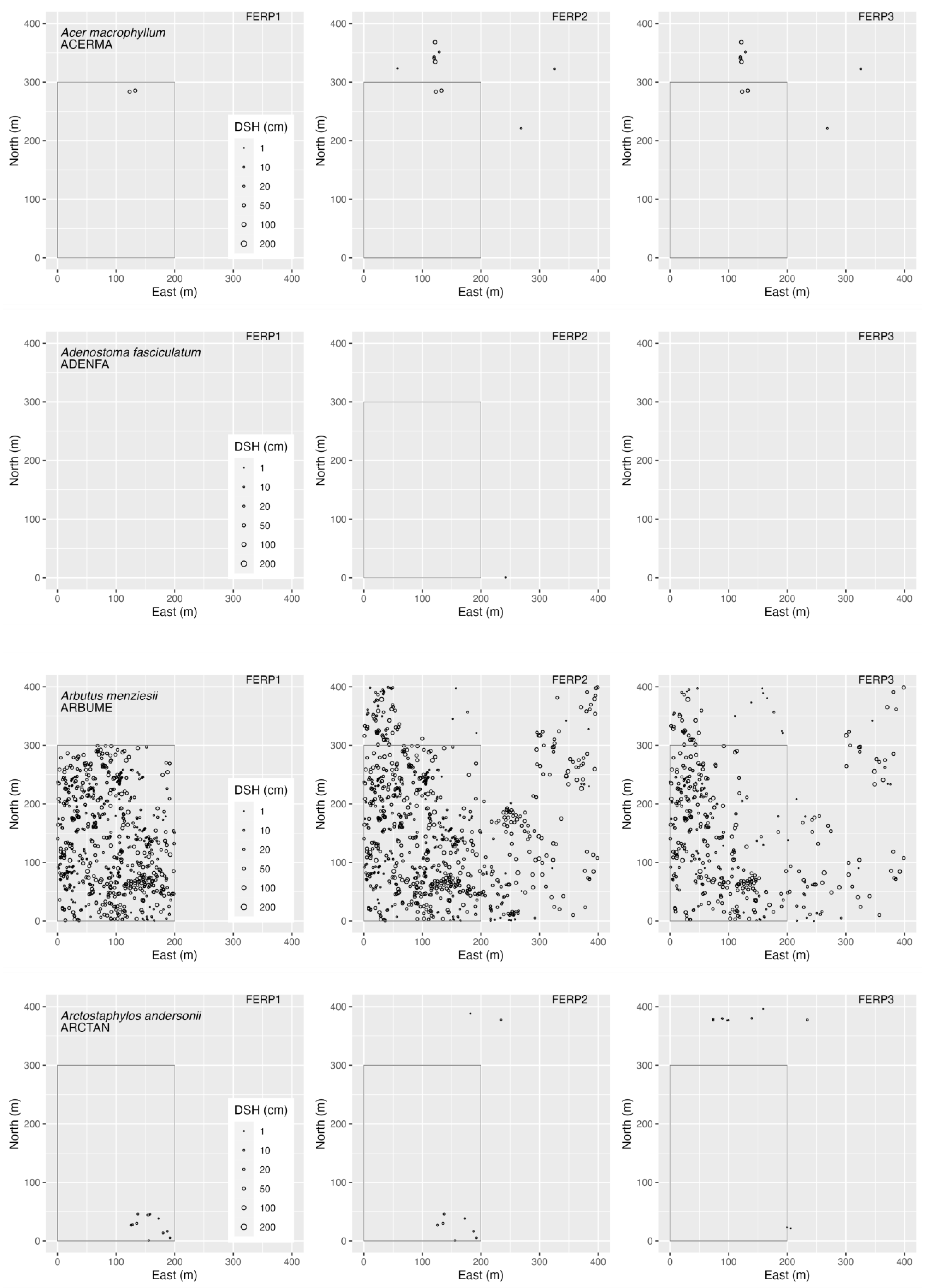
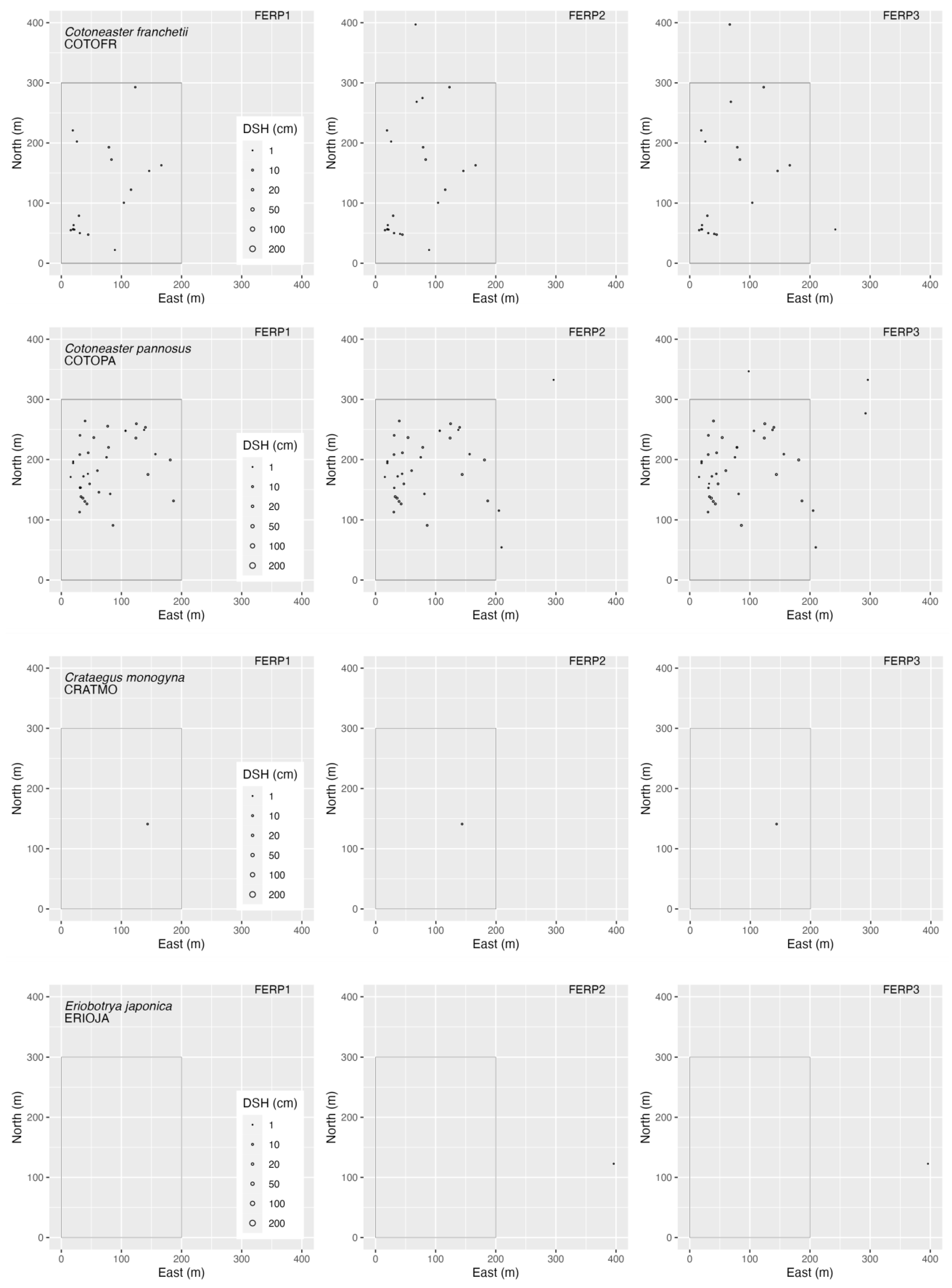
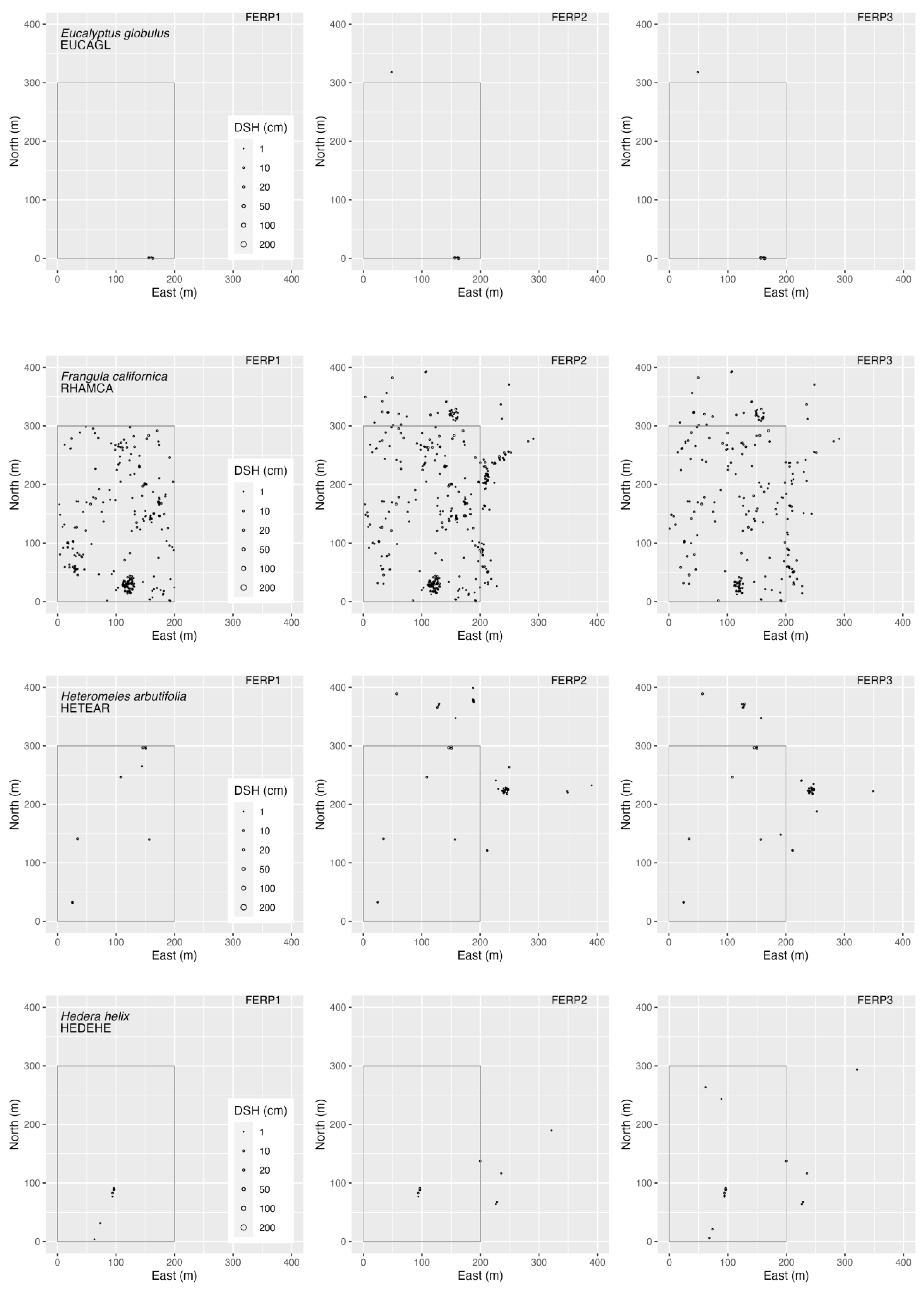
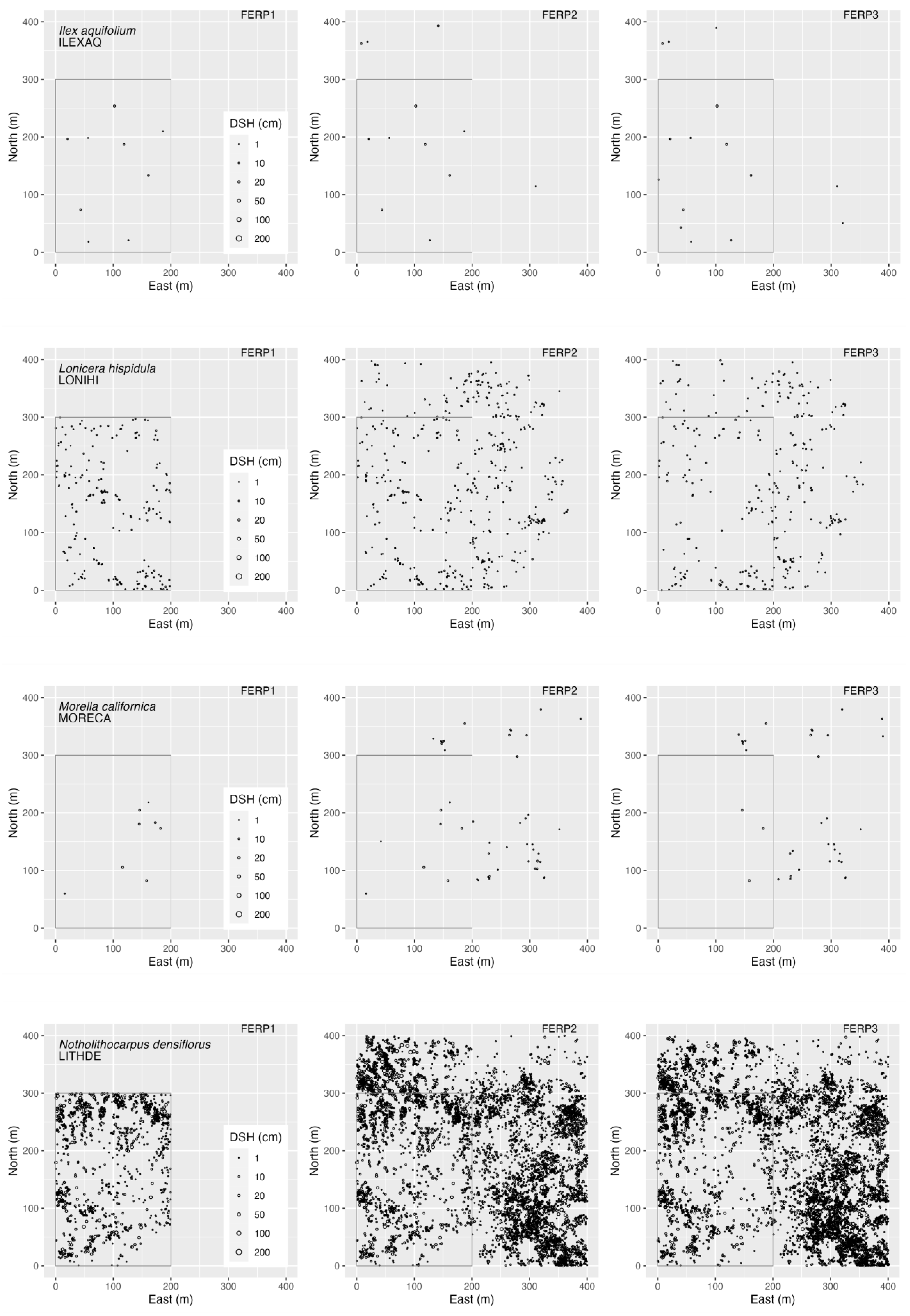
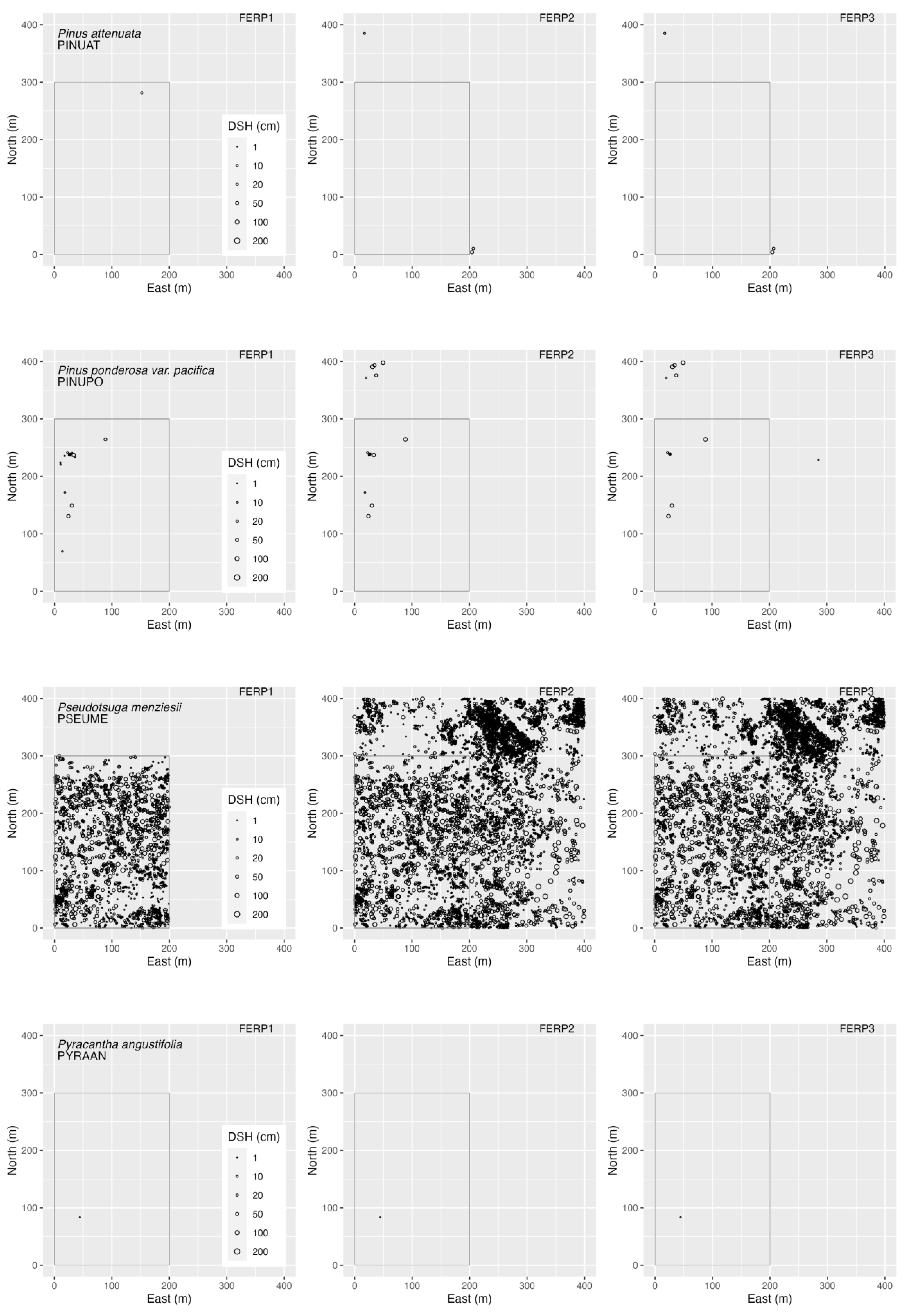
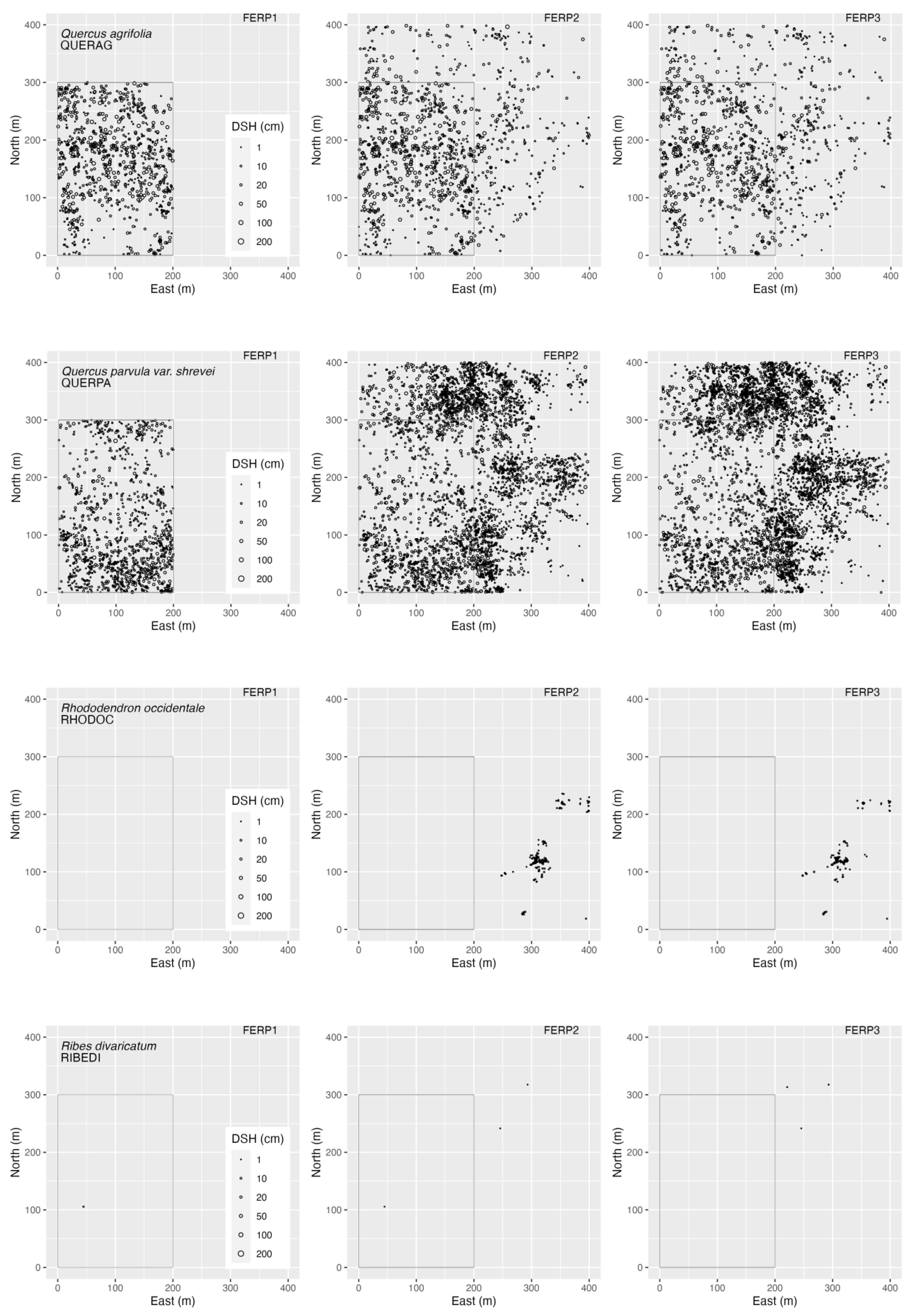
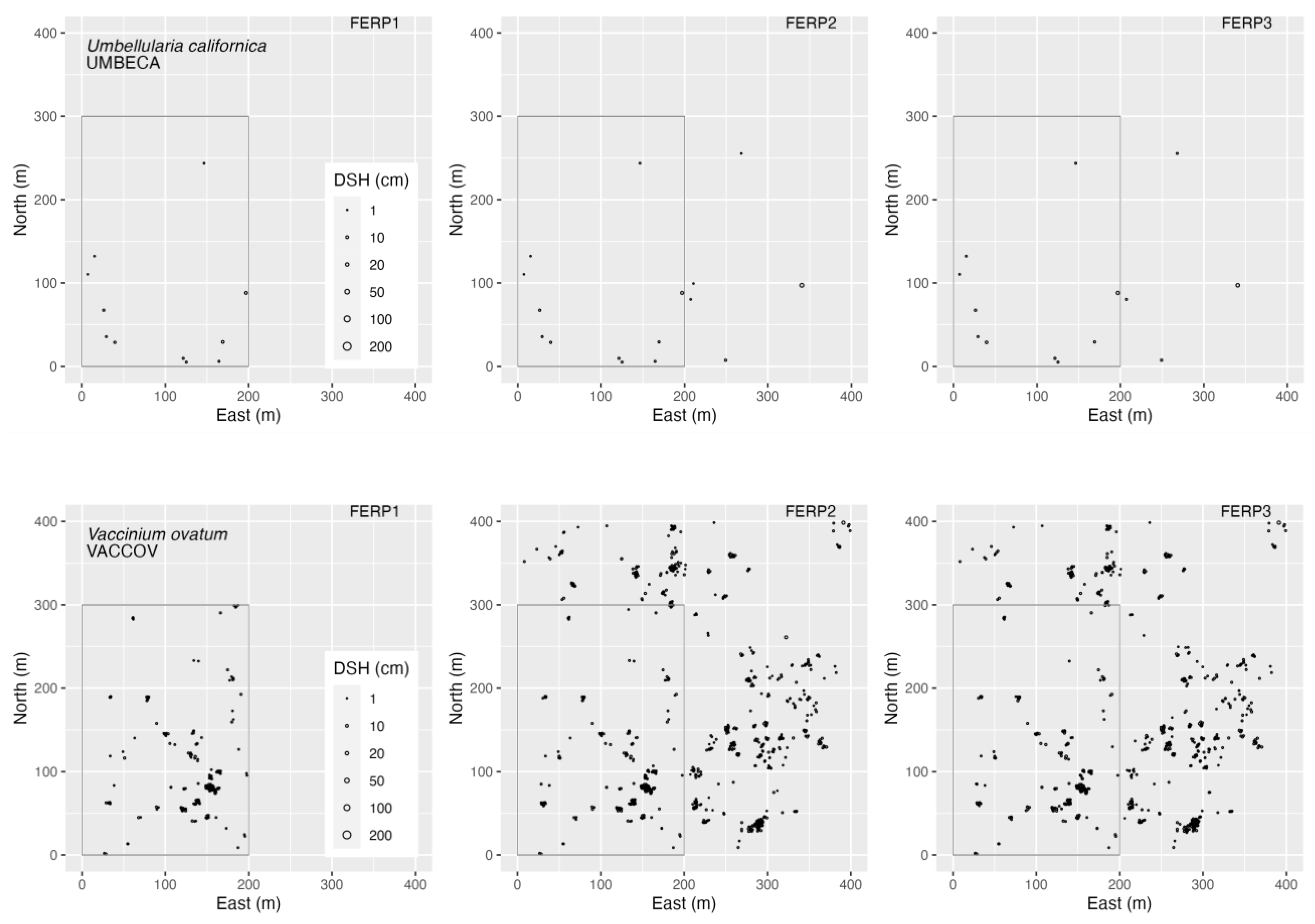
3.4. Spatial Patterns
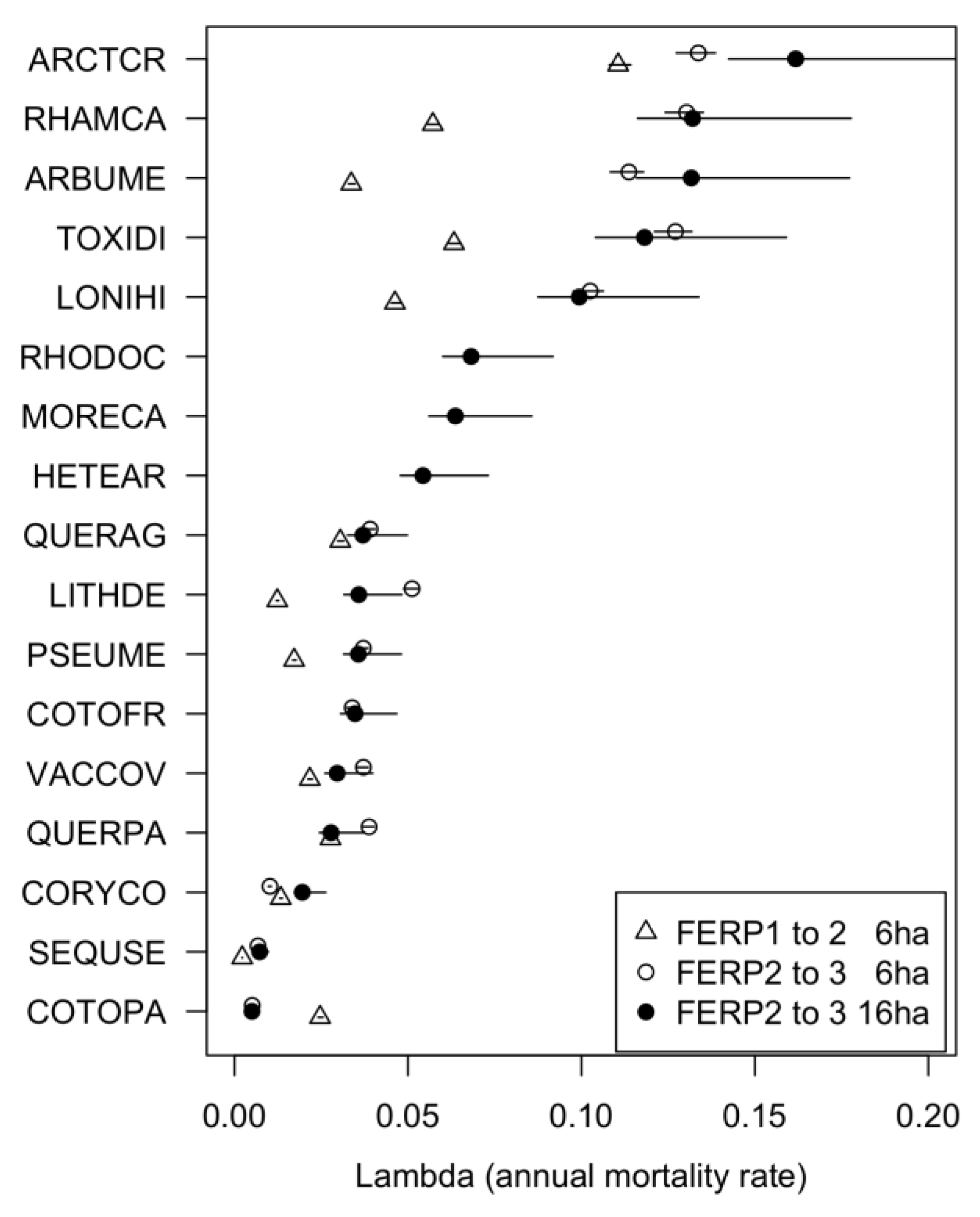
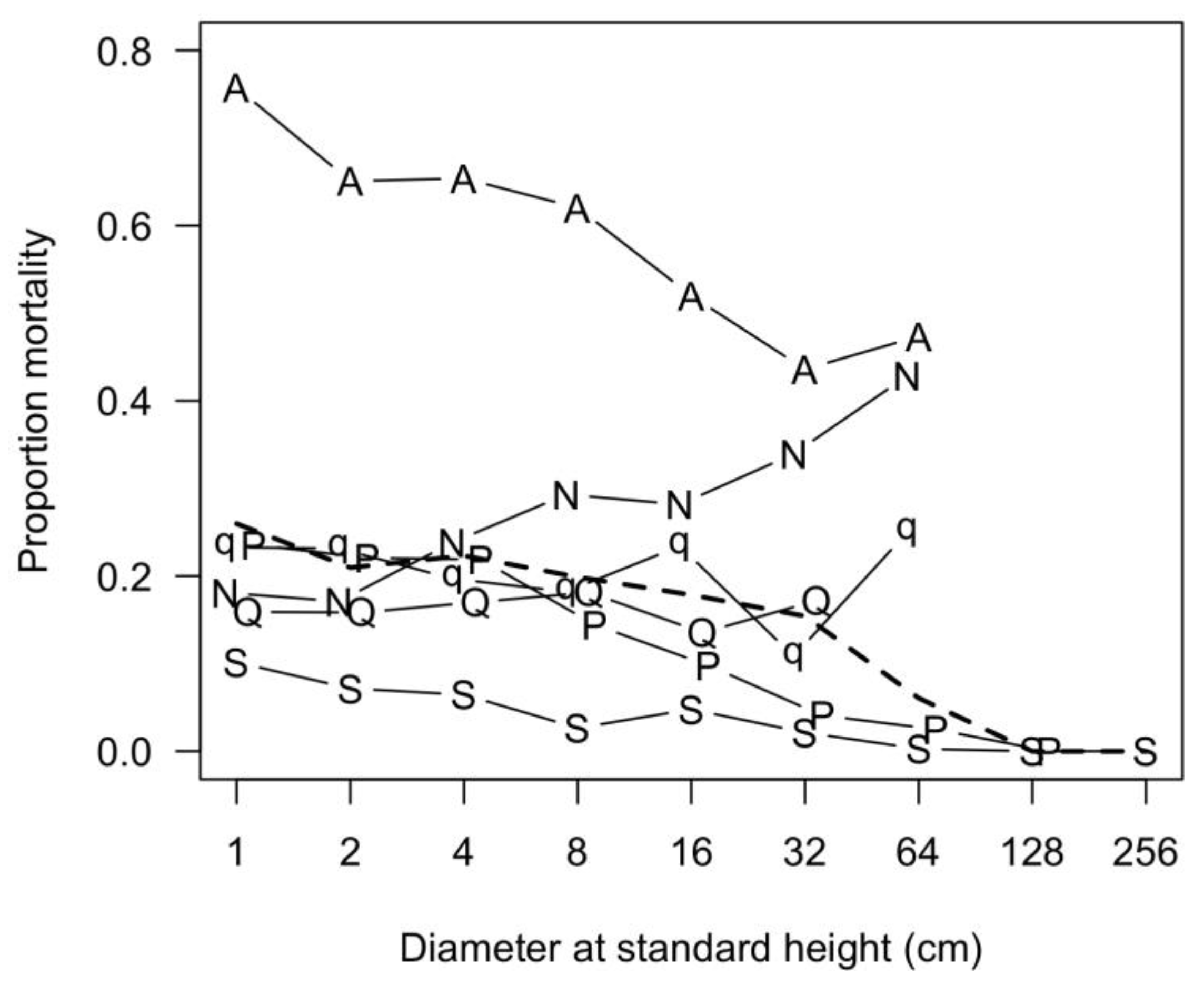
3.5. Patterns of Mortality
3.6. Patterns of Recruitment
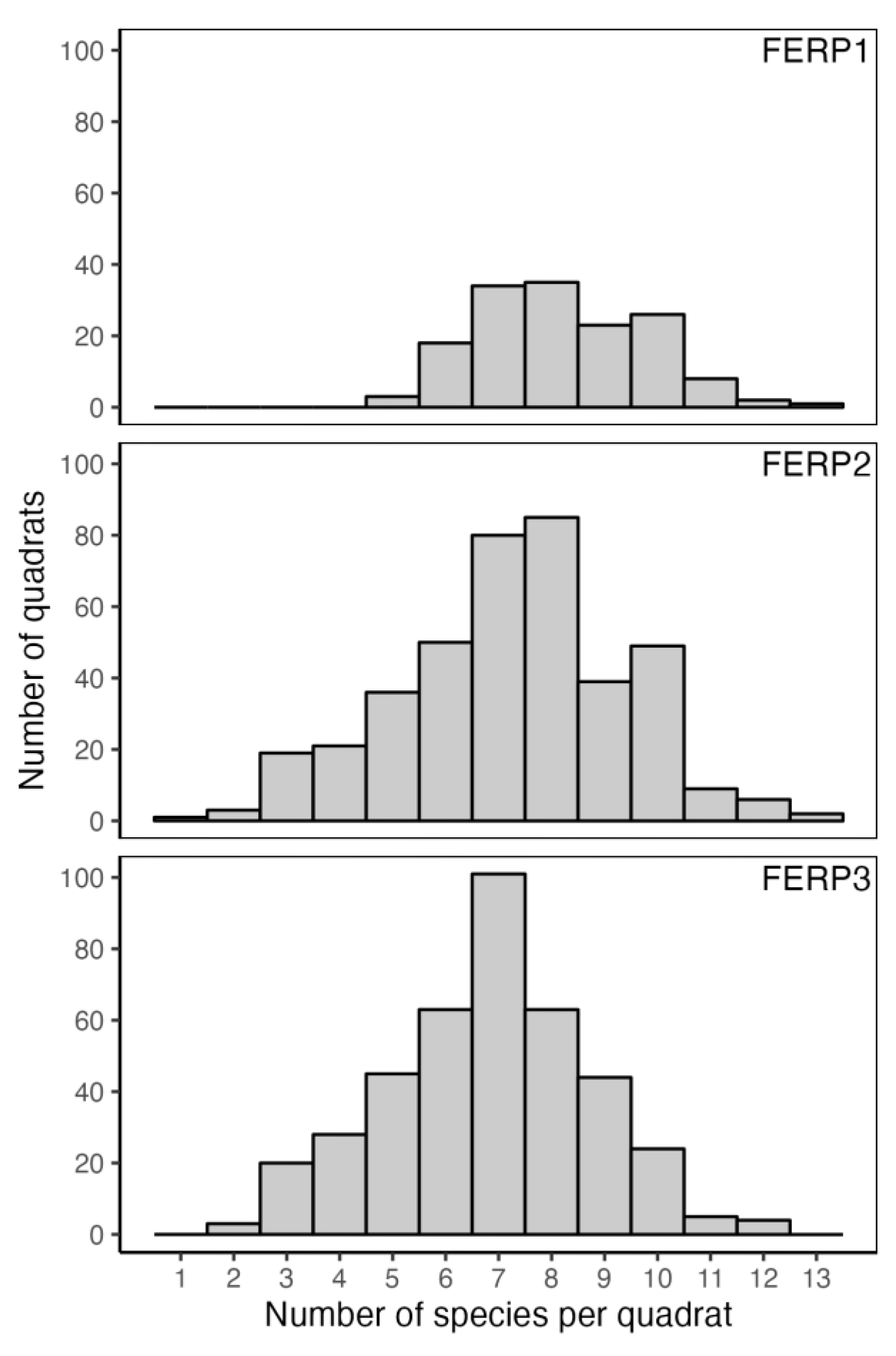
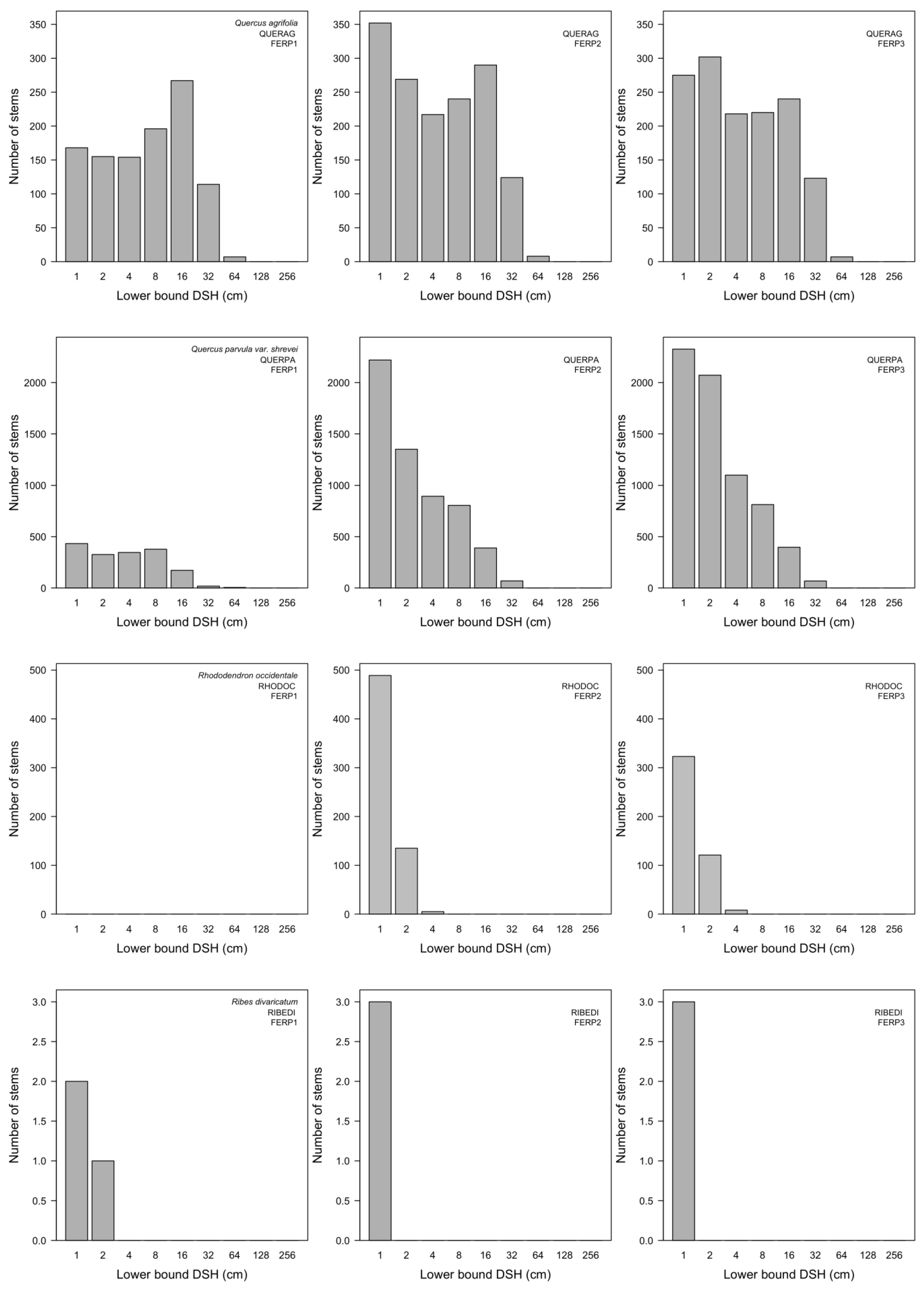
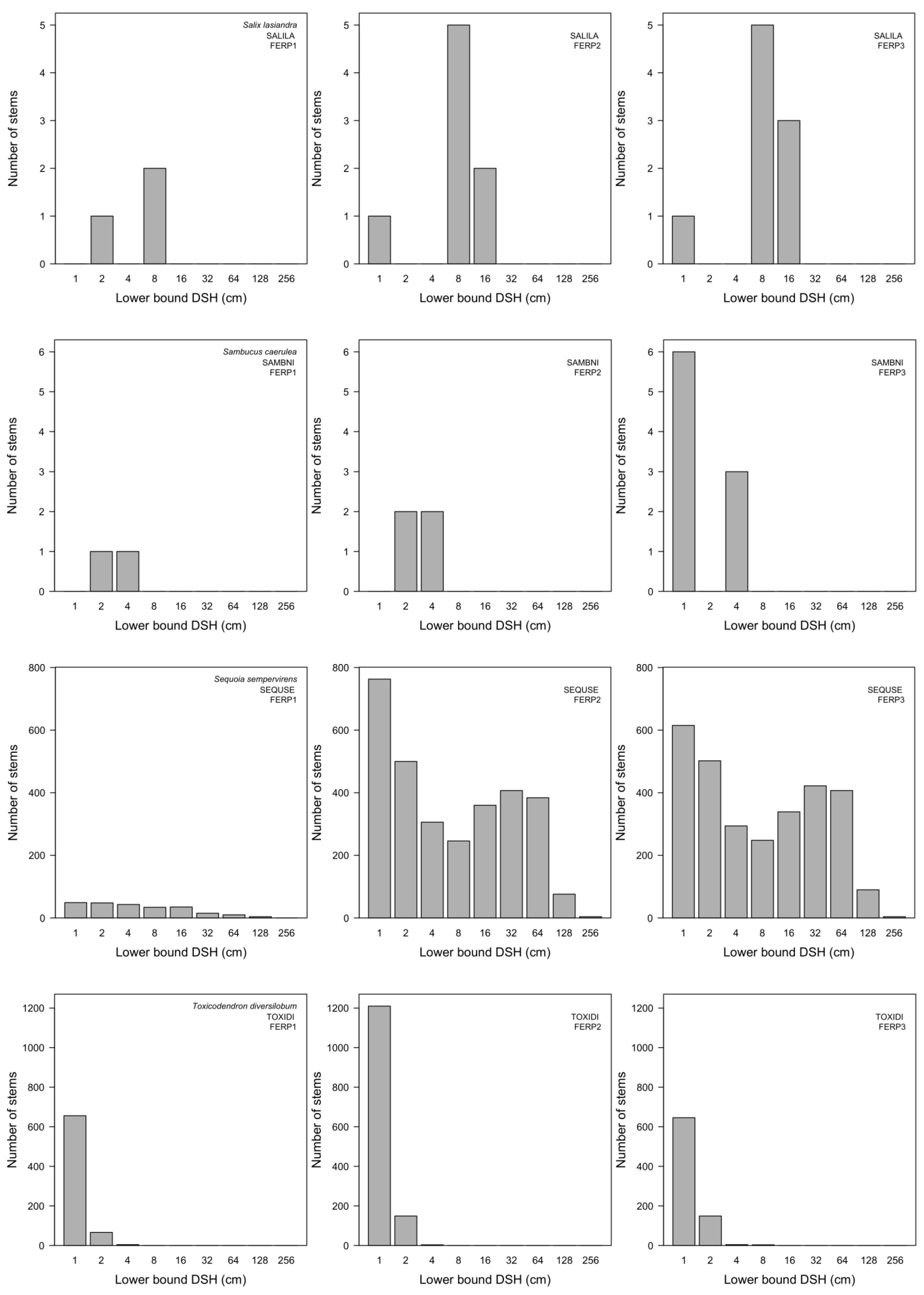
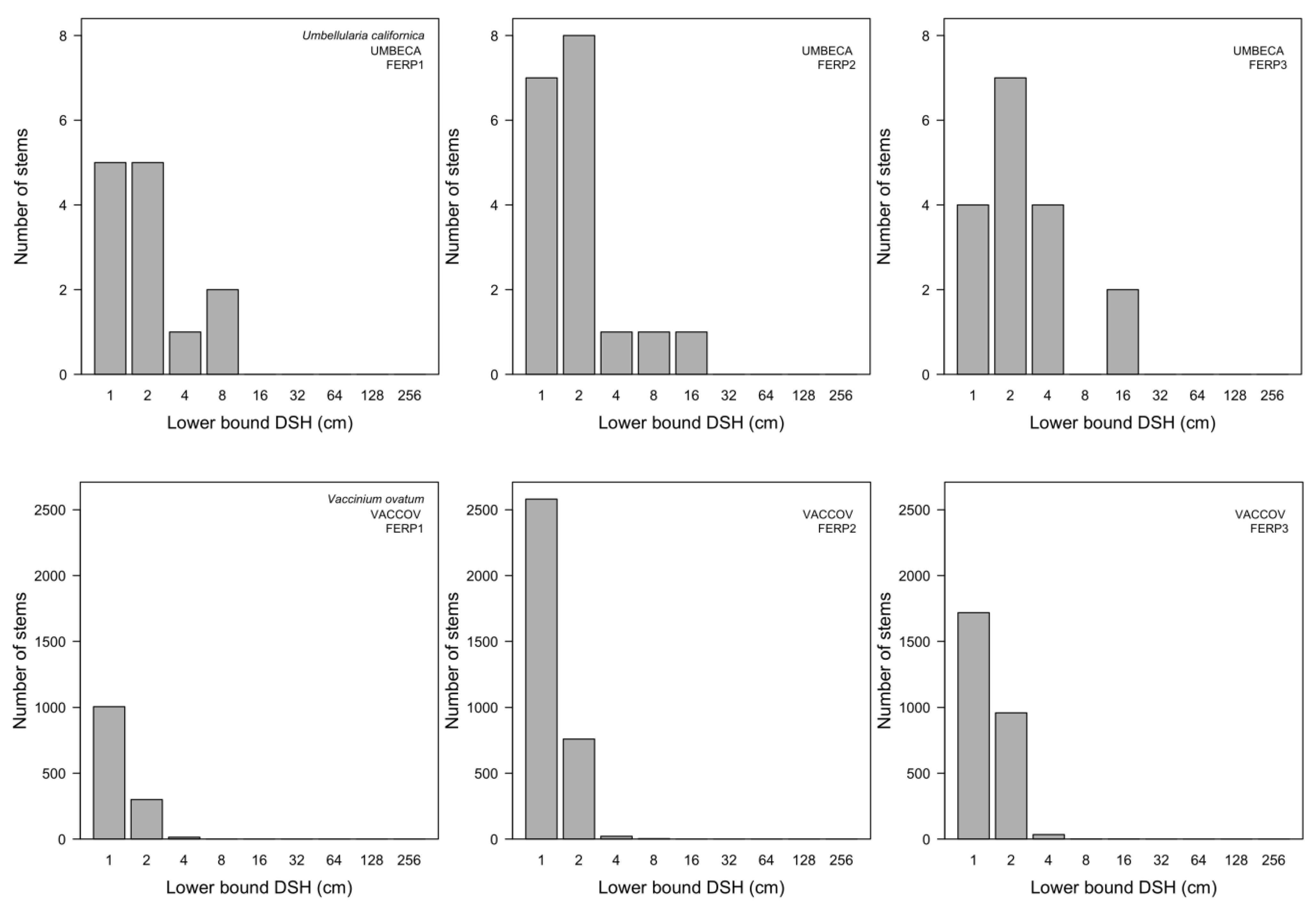
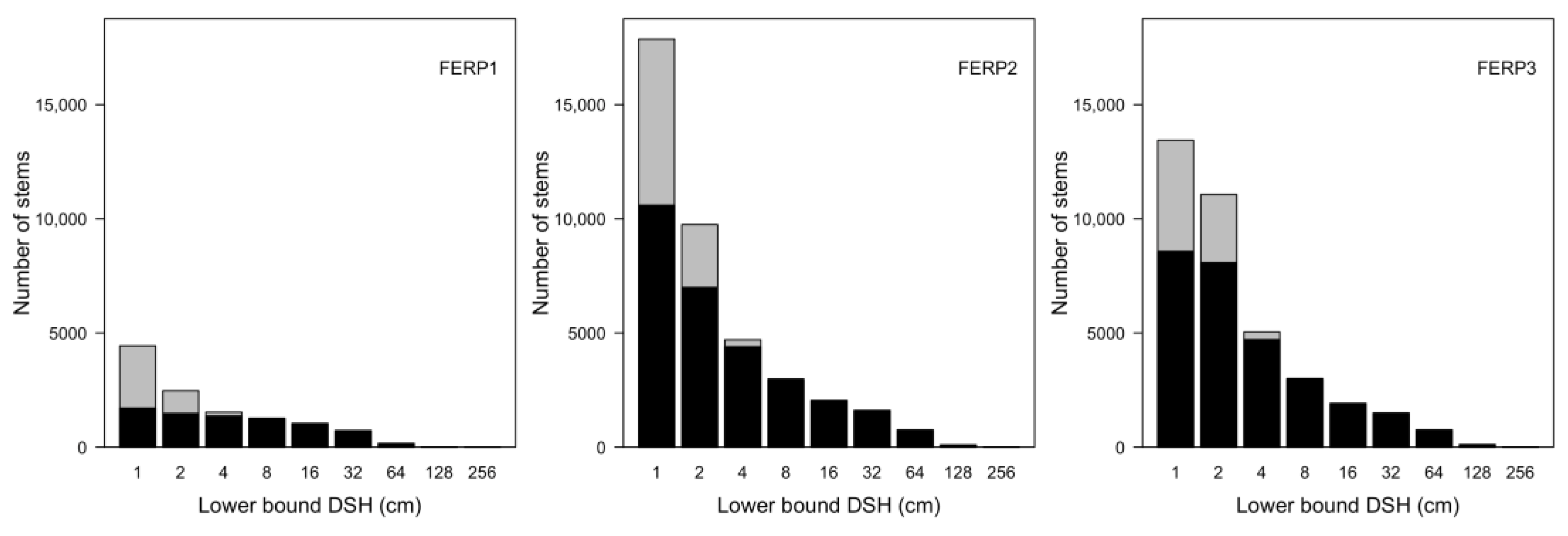
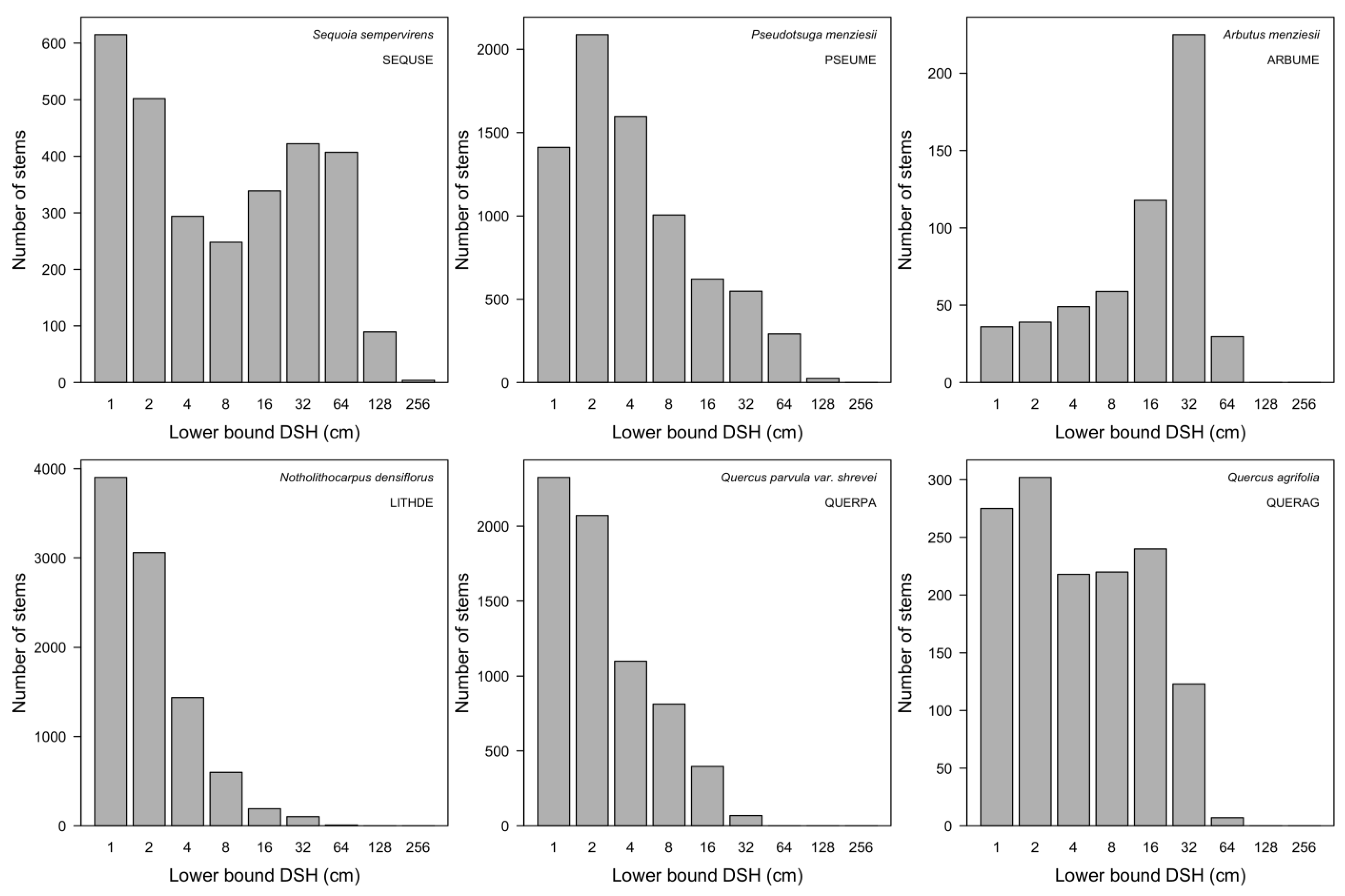
3.7. Patterns of Size Distribution
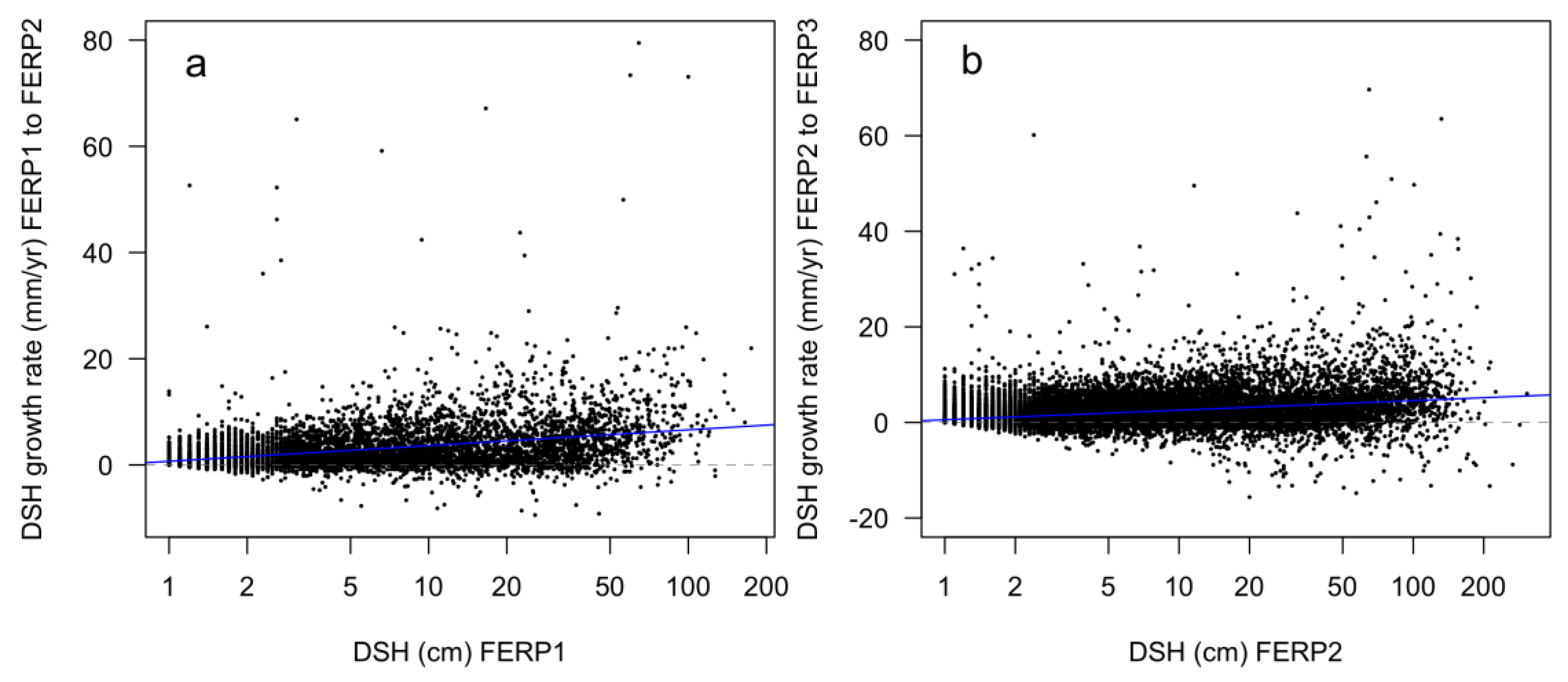
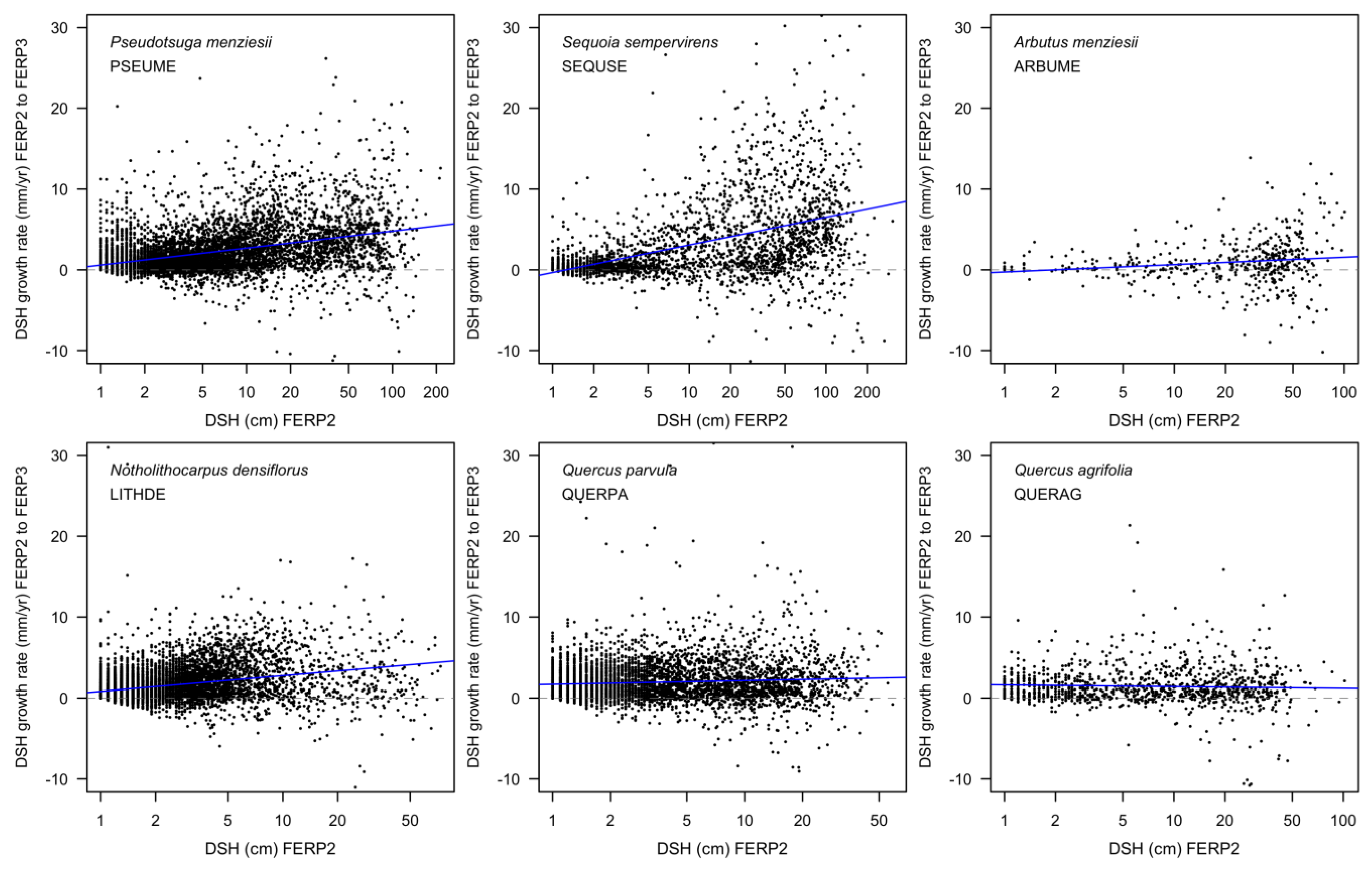
3.8. Patterns of Growth
4. Discussion
4.1. Species Composition
4.2. Abundance and Basal Area
4.3. Structure and Species Relative Abundance
4.4. Spatial Patterns
4.5. Patterns of Mortality
4.6. Patterns of Recruitment
4.7. Patterns of Size Distribution
4.8. Patterns of Growth
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B
- quadrat: Southwest corner of 20 m × 20 m quadrat, designated in meters E and N of the southwest corner of the FERP. For example, E080_N160 is 80 m east and 160 m north of the southwest corner.
- tag: Unique number for each individual tree, shrub, or liana with diameters larger than 1 cm. Tags are pre-stamped aluminum tags with sequential numbers from 1 through 37985. Tags for smaller individuals are tied to the base of the tree using horticultural tape; for larger trees, tags are nailed into the trunk at 2 m height on the north side of the tree.
- stemtag: Number that designates separate stems within a multistem individual. The stem with the tag is considered stemtag 1 but does not receive an additional stemtag. Each additional stem larger than 1 cm in diameter receives a write-on aluminum tag sequentially numbered, beginning with 2 within each individual, tied near the base of the stem. Stemtags were given to stems beginning in the FERP2 census.
- stemtag1: For FERP1, each stem of multistem individuals was measured but was not given a physical stemtag. These numbers are sequential, post hoc numbers, beginning at 102 within each individual, to designate each of the stems within a multistem individual. It is not possible to confidently make a direct correspondence between a particular stem of a multistem individual in FERP1 and a stem with a stemtag in FERP2.
- code6: Six-letter code designating the plant species. In general, the code is the first four letters of the genus and the first two letters of the species epithet. For species with taxonomic name changes after the FERP1 census, the original code6 was retained for consistency. Species names are as follows: ACERMA, Acer macrophyllum Pursh; ADENFA, Adenostoma fasciculatum Hook. & Arn.; ARBUME, Arbutus menziesii Pursh; ARCTAN, Arctostaphylos andersonii A. Gray; ARCTCR, Arctostaphylos crustacea subsp. crinita (J.E.Adams) V.T.Parker, M.C.Vasey & J.E.Keeley; BACCPI, Baccharis pilularis DC.; CEANTH, Ceanothus thyrsiflorus Eschsch.; CORYCO, Corylus cornuta subsp. californica Marshall (A.DC.) A.E.Murray; COTOFR, Cotoneaster franchetii Bois; COTOPA, Cotoneaster pannosus Franch.; CRATMO, Crataegus monogyna Jacq.; ERIOJA, Eriobotrya japonica (Thunb.) Lindl.; EUCAGL, Eucalyptus globulus Labill.; RHAMCA, Frangula californica (Eschsch.) A.Gray; HEDEHE, Hedera helix L.; HETEAR, Heteromeles arbutifolia (Lindl.) M.Roem; ILEXAQ, Ilex aquifolium L.; LONIHI, Lonicera hispidula (Lindl.) Douglas ex Torr. & A.Gray; MORECA, Morella californica (Cham.) Wilbur; LITHDE, Notholithocarpus densiflorus (Hook. & Arn.) Manos, C.H. Cannon & S.H.Oh; PINUAT, Pinus attenuata Lemmon; PINUPO, Pinus ponderosa var. pacifica J.R.Haller & Vivrette; PSEUME, Pseudotsuga menziesii (Mirb.) Franco; PYRAAN, Pyracantha angustifolia C.K.Schneid; QUERAG, Quercus agrifolia Née; QUERPA, Quercus parvula var. shrevei (C.H.Mull.) Nixon; RHODOC, Rhododendron occidentale (Torr. & A.Gray) A.Gray; RIBEDI, Ribes divaricatum Douglas; SALILA, Salix lasiandra Benth.; SAMBNI, Sambucus caerulea Raf.; SEQUSE, Sequoia sempervirens (D.Don) Endl.; TOXIDI, Toxicodendron diversilobum Greene; UMBECA, Umbellularia californica (Hook. & Arn.) Nutt.; VACCOV, Vaccinium ovatum Pursh.
- east_m: Number of meters east (magnetic) of the western border of the FERP.
- north_m: Number of meters north (magnetic) of the southern border of the FERP.
- east_UTM: Easting UTM coordinates (zone 10, WGS84).
- north_UTM: Northing UTM coordinates (zone 10, WGS84).
- dsh1_mm: Diameter (in mm) at standard height (1.3 m) of stem in FERP1 census.
- dsh2_mm: Diameter (in mm) at standard height (1.3 m) of stem in FERP2 census
- dsh3_mm: Diameter (in mm) at standard height (1.3 m) of stem in FERP3 census.
- dsh1m_mm: Diameter (in mm) at standard height (1.3 m) of untagged multistem individuals in FERP1 census. Used for stems designated with stemtag1.
- date1: Date of observation in FERP1 census.
- date2: Date of observation in FERP2 census.
- date3: Date of observation in FERP3 census.
- status1: Indicated as “living” if stem was mapped and measured in the FERP1 census.
- condition1: Observation of status of stem during FERP1 census:
- “broken1.3” indicates stem is alive but broken below the standard height of 1.3 m;
- “leaning” indicates living trunk is leaning more than 30° from vertical;
- “prostrate” indicates stem is lying on the ground.
- status2: Survival status of stems in FERP2 census:
- “living” indicates stem was observed alive in the FERP2 census;
- “dead” indicates stem that was alive in FERP1 census but found dead or missing in FERP2;
- “nodata” indicates a missing observation in FERP2 for stems alive in FERP1.
- condition2: Observation of status of stem during FERP2 census:
- “broken1.3” indicates stem is alive but broken below the standard height of 1.3 m;
- “leaning” indicates living trunk is leaning more than 30° from vertical;
- “prostrate” indicates living stem is lying on the ground;
- “fallen” indicates the stem was found dead and found lying on the ground;
- “standing” indicates the stem was found dead and erect;
- “resprout” indicates the original stem was dead, with a living resprout not large enough to measure;
- “missing” indicates stems tagged in FERP1 could not be located after diligent search;
- “Tagonly” indicates aluminum tag was found not attached to a stem, and stem could not be found.
- status3: Survival status of stems in FERP3 census:
- “living” indicates stem was observed alive in the FERP3 census;
- “dead” indicates stem that was alive in FERP2 census but found dead or missing in FERP3;
- “nodata” indicates a missing observation in FERP3 for stems alive in FERP2.
- condition3: Observation of status of stem during FERP3 census:
- “broken1.3” indicates stem is alive but broken below the standard height of 1.3 m;
- “leaning” indicates living trunk is leaning more than 30° from vertical;
- “prostrate” indicates living stem is lying on the ground;
- “fallen” indicates the stem was found dead and found lying on the ground;
- “standing” indicates the stem was found dead and erect;
- “missing” indicates stems tagged in FERP1 could not be located after diligent search.
- first_census: The first census (1, 2, or 3) at which the stem was measured.
- irreg_dsh: Designates “irreg_dsh” if diameter measurement was made at a height other than the standard 1.3 m.
- hom_m: Height in m at which diameter was measured. The standard height is 1.3 m from the highest location where the ground meets the stem.
- multi1: “multi1” indicates that the individual had multiple stems larger than 1 cm in diameter in FERP1.
- stems1: Total number of stems in a multistem individual that was measured in FERP1.
- multi2: “multi2” indicates that the individual had multiple stems larger than 1 cm in diameter in FERP2.
- multi3: “multi3” indicates that the individual had multiple stems larger than 1 cm in diameter in FERP3.
- basalarea1_m2: For FERP1 only, the total basal area of all measured stems of an individual, in units of m2.
- code6fix: Notes that indicate that a stem identification in one census was corrected in a subsequent census.
- locfix: Indication that original mapping location of stem was in error and was fixed in a subsequent census. “loc_fixed” indicates that the stem was physically re-mapped to the correct location. “loc_rand_inQ” was used for 70 individuals (90 stems) for which location coordinates are missing but for which the 20 m × 20 m quadrat is known. For those stems, random easting and northing coordinates within the quadrat were assigned.
- notes2: Field notes indicating irregularities found in FERP2 census.
- notes3: Field notes indicating irregularities found in FERP3 census.
Appendix C
Appendix D
Appendix E

Appendix F

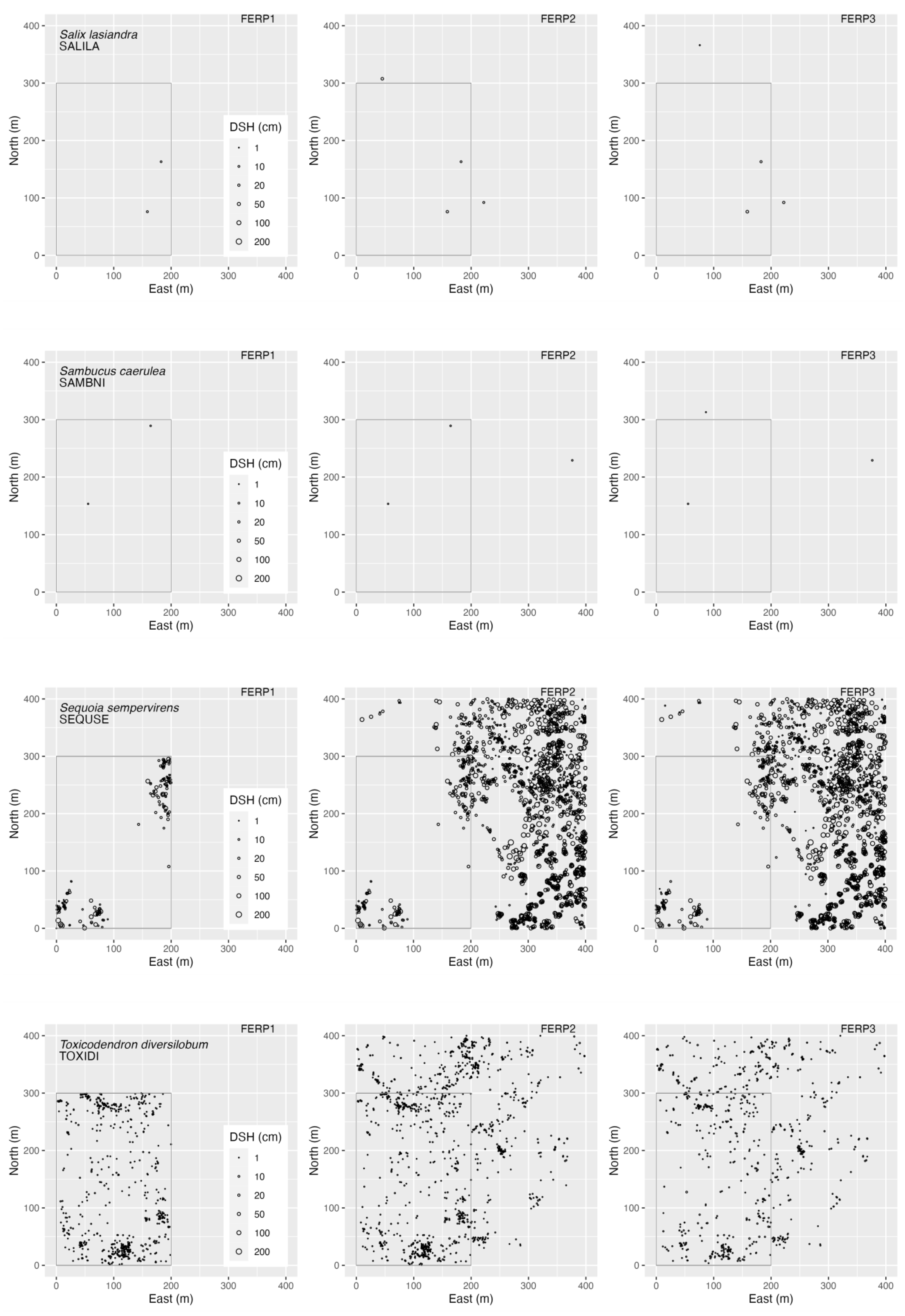
Appendix G
Appendix H

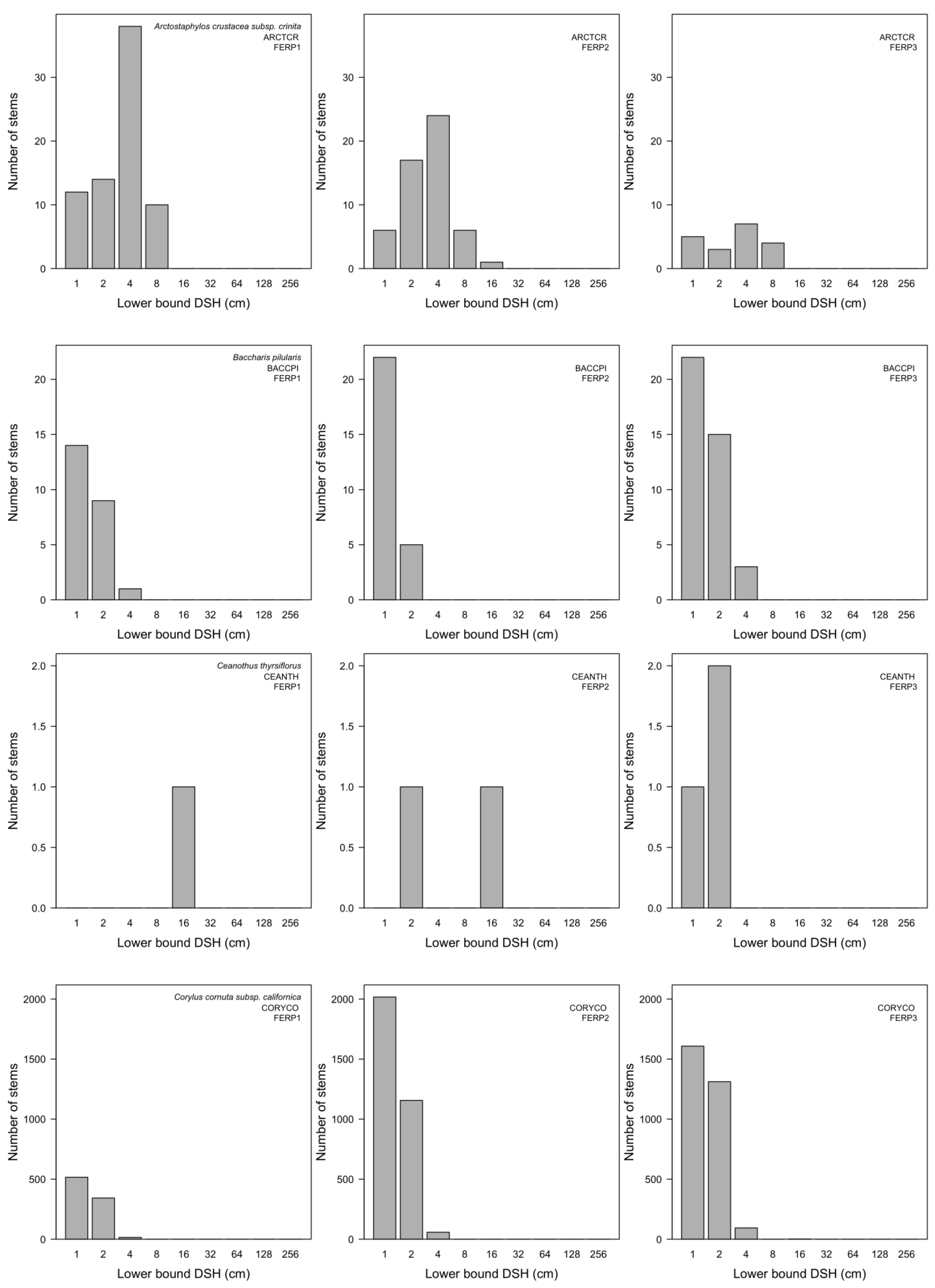
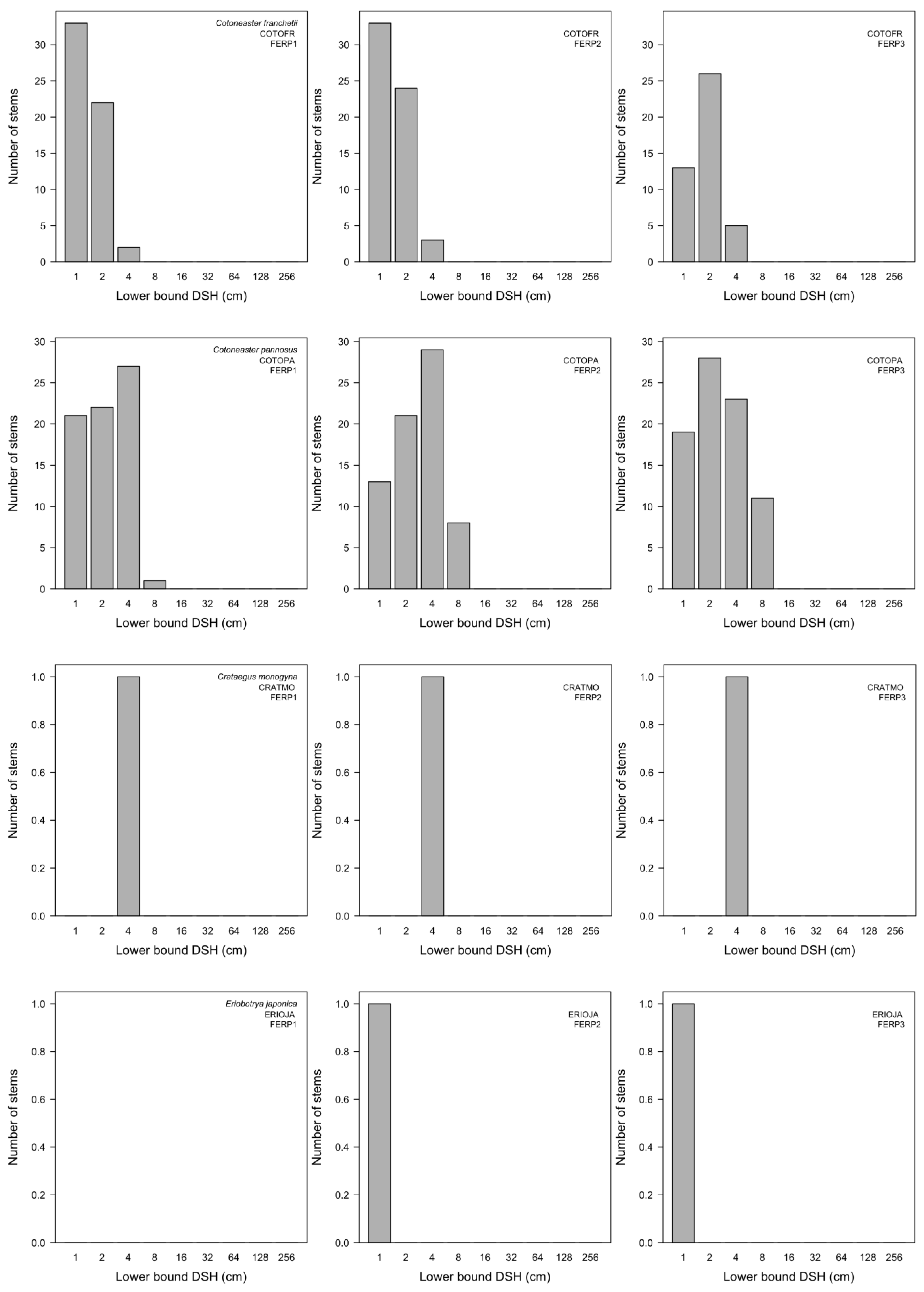
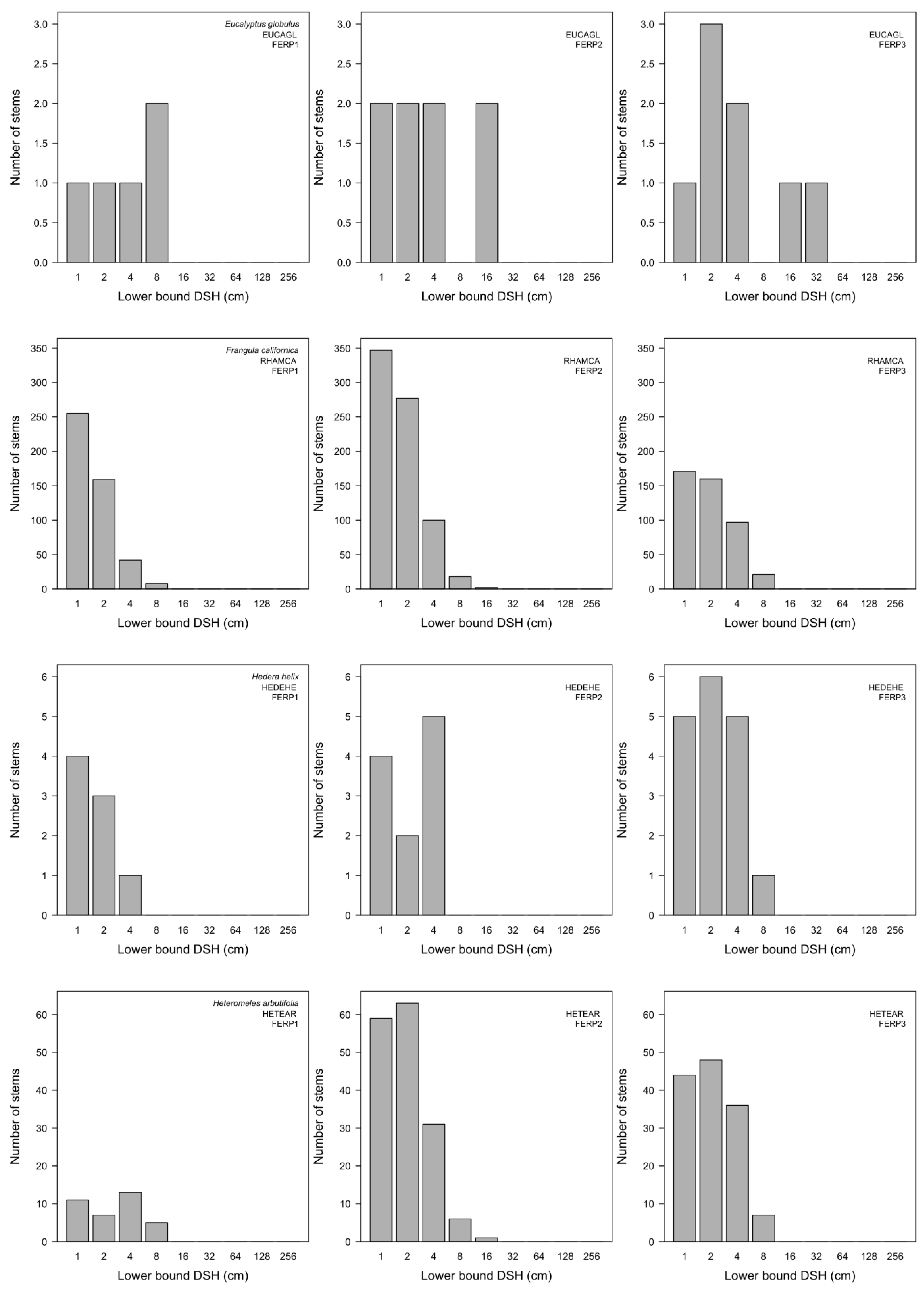
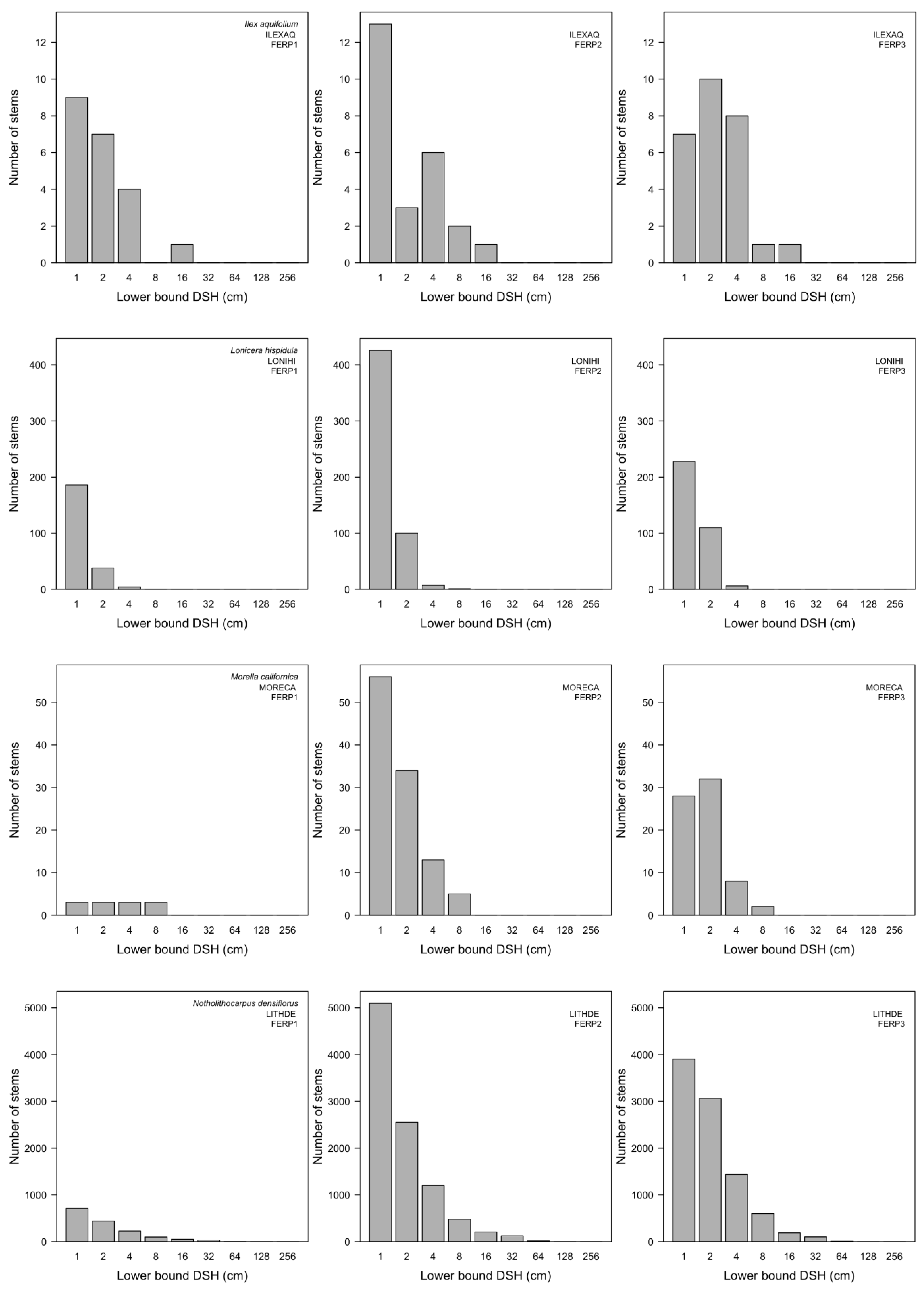
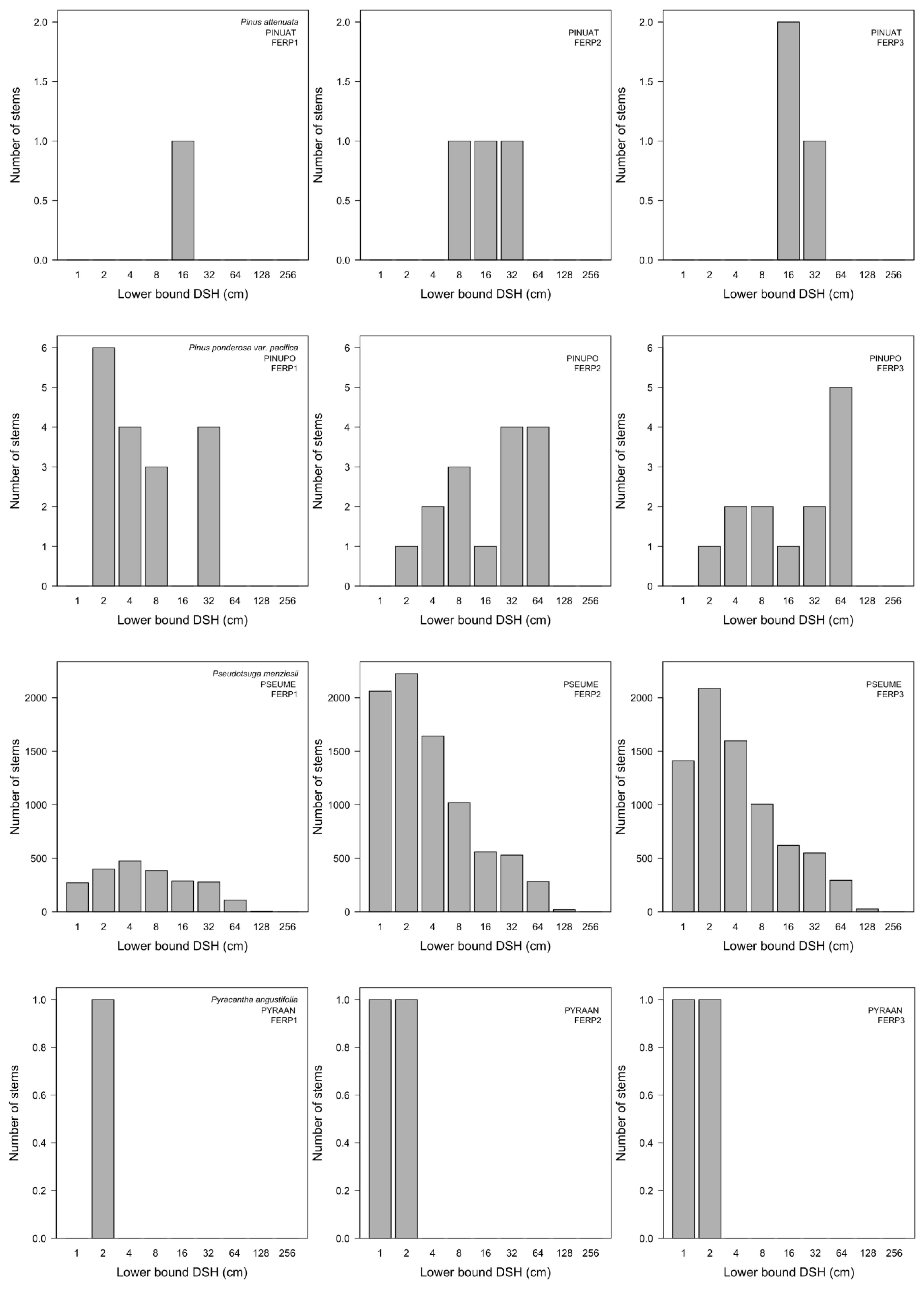
Appendix I
| Interval and Area | Code6 | Intercept | Slope | F | dfresid | R2adj | p |
|---|---|---|---|---|---|---|---|
| FERP1 to 2 6 ha | ARBUME | 0.0510 | 0.8264 | 2.266 | 284 | 0.004 | 0.13339 |
| FERP1 to 2 6 ha | CORYCO | 0.5430 | −0.4565 | 1.818 | 108 | 0.007 | 0.18042 |
| FERP1 to 2 6 ha | COTOFR | −0.1549 | 1.3384 | 1.628 | 11 | 0.050 | 0.22828 |
| FERP1 to 2 6 ha | COTOPA | 0.6771 | 0.8726 | 0.365 | 23 | −0.027 | 0.55159 |
| FERP1 to 2 6 ha | LITHDE | 0.7194 | 3.4134 | 145.686 | 834 | 0.148 | <0.00001 |
| FERP1 to 2 6 ha | LONIHI | 0.5059 | 0.0256 | 0.002 | 91 | −0.011 | 0.96401 |
| FERP1 to 2 6 ha | PSEUME | 0.0367 | 2.6748 | 241.779 | 1571 | 0.133 | <0.00001 |
| FERP1 to 2 6 ha | QUERAG | 1.6715 | −0.4876 | 4.648 | 597 | 0.006 | 0.03148 |
| FERP1 to 2 6 ha | QUERPA | 1.7115 | 0.3153 | 1.820 | 796 | 0.001 | 0.17771 |
| FERP1 to 2 6 ha | RHAMCA | 0.6775 | 2.7735 | 10.480 | 78 | 0.107 | 0.00177 |
| FERP1 to 2 6 ha | SEQUSE | −2.5797 | 8.1291 | 164.955 | 176 | 0.481 | <0.00001 |
| FERP1 to 2 6 ha | TOXIDI | 0.4083 | 0.0621 | 0.013 | 210 | −0.005 | 0.91000 |
| FERP1 to 2 6 ha | VACCOV | 0.7504 | 0.0311 | 0.003 | 166 | −0.006 | 0.95896 |
| FERP2 to 3 6 ha | ARBUME | 0.0668 | 0.7163 | 2.944 | 356 | 0.005 | 0.08707 |
| FERP2 to 3 6 ha | ARCTCR | 2.9091 | −3.8892 | 2.516 | 11 | 0.112 | 0.14102 |
| FERP2 to 3 6 ha | CORYCO | 0.6603 | −0.8901 | 31.354 | 685 | 0.042 | <0.00001 |
| FERP2 to 3 6 ha | COTOFR | 1.0233 | −1.1838 | 3.119 | 30 | 0.064 | 0.08754 |
| FERP2 to 3 6 ha | COTOPA | 1.7131 | −0.2399 | 0.088 | 48 | −0.019 | 0.76757 |
| FERP2 to 3 6 ha | HETEAR | 2.6800 | −2.0952 | 0.791 | 13 | −0.015 | 0.39005 |
| FERP2 to 3 6 ha | ILEXAQ | 0.3698 | 2.4993 | 6.654 | 10 | 0.340 | 0.02744 |
| FERP2 to 3 6 ha | LITHDE | 0.8468 | 3.1792 | 352.477 | 1528 | 0.187 | <0.00001 |
| FERP2 to 3 6 ha | LONIHI | 0.6395 | −0.2672 | 0.249 | 112 | −0.007 | 0.61906 |
| FERP2 to 3 6 ha | PSEUME | 0.5714 | 2.2955 | 225.324 | 1709 | 0.116 | <0.00001 |
| FERP2 to 3 6 ha | QUERAG | 1.6917 | −0.5010 | 8.601 | 738 | 0.010 | 0.00346 |
| FERP2 to 3 6 ha | QUERPA | 1.7007 | 0.2501 | 2.355 | 1264 | 0.001 | 0.12510 |
| FERP2 to 3 6 ha | RHAMCA | 1.6583 | 1.4043 | 5.420 | 171 | 0.025 | 0.02108 |
| FERP2 to 3 6 ha | SEQUSE | −1.6736 | 7.1713 | 231.511 | 234 | 0.495 | <0.00001 |
| FERP2 to 3 6 ha | TOXIDI | 0.5572 | −0.2852 | 0.293 | 307 | −0.002 | 0.58889 |
| FERP2 to 3 6 ha | VACCOV | 0.7292 | −0.7583 | 12.114 | 775 | 0.014 | 0.00053 |
| FERP2 to 3 16 ha | ARBUME | −0.2682 | 0.9131 | 7.357 | 486 | 0.013 | 0.00692 |
| FERP2 to 3 16 ha | ARCTCR | 3.0971 | −4.0288 | 2.944 | 12 | 0.130 | 0.11186 |
| FERP2 to 3 16 ha | CORYCO | 0.6802 | −1.0401 | 45.541 | 2450 | 0.018 | <0.00001 |
| FERP2 to 3 16 ha | COTOFR | 1.2554 | −1.6360 | 3.984 | 31 | 0.085 | 0.05478 |
| FERP2 to 3 16 ha | COTOPA | 1.5556 | −0.0346 | 0.002 | 51 | −0.020 | 0.96319 |
| FERP2 to 3 16 ha | HETEAR | 1.3254 | −0.3766 | 0.204 | 92 | −0.009 | 0.65222 |
| FERP2 to 3 16 ha | ILEXAQ | 0.3865 | 2.2146 | 4.476 | 14 | 0.188 | 0.05278 |
| FERP2 to 3 16 ha | LITHDE | 0.8149 | 1.9323 | 721.803 | 7337 | 0.089 | <0.00001 |
| FERP2 to 3 16 ha | LONIHI | 0.5321 | −0.5910 | 3.507 | 297 | 0.008 | 0.06208 |
| FERP2 to 3 16 ha | MORECA | 0.2690 | 1.7740 | 8.310 | 62 | 0.104 | 0.00541 |
| FERP2 to 3 16 ha | PINUPO | −5.6189 | 8.2716 | 43.530 | 10 | 0.795 | 0.00006 |
| FERP2 to 3 16 ha | PSEUME | 0.5880 | 2.1073 | 1016.673 | 6670 | 0.132 | <0.00001 |
| FERP2 to 3 16 ha | QUERAG | 1.6028 | −0.2101 | 1.495 | 1139 | 0.000 | 0.22174 |
| FERP2 to 3 16 ha | QUERPA | 1.6734 | 0.4623 | 27.311 | 4604 | 0.006 | 0.00001 |
| FERP2 to 3 16 ha | RHAMCA | 1.5007 | 1.1883 | 6.606 | 325 | 0.017 | 0.01061 |
| FERP2 to 3 16 ha | RHODOC | 0.2906 | −0.6306 | 6.276 | 435 | 0.012 | 0.01260 |
| FERP2 to 3 16 ha | SEQUSE | −0.3350 | 3.4143 | 578.488 | 2737 | 0.174 | <0.00001 |
| FERP2 to 3 16 ha | TOXIDI | 0.5124 | −0.7535 | 6.951 | 663 | 0.009 | 0.00857 |
| FERP2 to 3 16 ha | UMBECA | 2.7814 | −3.7592 | 2.553 | 13 | 0.100 | 0.13410 |
| FERP2 to 3 16 ha | VACCOV | 0.5880 | −0.8690 | 57.947 | 2417 | 0.023 | <0.00001 |
| Interval and Area | Code6 | Intercept | Slope | F | dfresid | R2adj | p |
|---|---|---|---|---|---|---|---|
| FERP1 to 2 6 ha | ARBUME | 0.0304 | −0.0104 | 10.5504 | 574 | 0.016 | 0.00123 |
| FERP1 to 2 6 ha | ARCTCR | −0.0140 | 0.0229 | 0.6400 | 20 | −0.017 | 0.43310 |
| FERP1 to 2 6 ha | CORYCO | 0.0031 | 0.0157 | 0.9613 | 128 | 0.000 | 0.32870 |
| FERP1 to 2 6 ha | COTOFR | −0.0035 | 0.0506 | 1.4018 | 15 | 0.024 | 0.25485 |
| FERP1 to 2 6 ha | COTOPA | 0.0334 | −0.0071 | 0.1076 | 26 | −0.034 | 0.74552 |
| FERP1 to 2 6 ha | LITHDE | 0.0876 | 0.0030 | 0.2948 | 1179 | −0.001 | 0.58725 |
| FERP1 to 2 6 ha | LONIHI | −0.0090 | 0.1456 | 43.2717 | 172 | 0.196 | <0.00001 |
| FERP1 to 2 6 ha | PSEUME | 0.0605 | −0.0214 | 118.0180 | 1979 | 0.056 | <0.00001 |
| FERP1 to 2 6 ha | QUERAG | 0.0458 | −0.0249 | 66.5674 | 777 | 0.078 | <0.00001 |
| FERP1 to 2 6 ha | QUERPA | 0.0600 | −0.0310 | 53.4449 | 1031 | 0.048 | <0.00001 |
| FERP1 to 2 6 ha | RHAMCA | 0.0154 | 0.1214 | 43.3036 | 215 | 0.164 | <0.00001 |
| FERP1 to 2 6 ha | SEQUSE | 0.0440 | 0.0028 | 0.2814 | 184 | −0.004 | 0.59642 |
| FERP1 to 2 6 ha | TOXIDI | −0.0015 | 0.1356 | 109.9723 | 494 | 0.180 | <0.00001 |
| FERP1 to 2 6 ha | UMBECA | 0.0012 | 0.0269 | 1.3054 | 8 | 0.033 | 0.28627 |
| FERP1 to 2 6 ha | VACCOV | −0.0050 | 0.0622 | 11.5952 | 222 | 0.045 | 0.00078 |
| FERP2 to 3 6 ha | ARBUME | 0.0207 | −0.0117 | 31.3512 | 356 | 0.078 | <0.00001 |
| FERP2 to 3 6 ha | ARCTCR | 0.0578 | −0.0629 | 9.6636 | 11 | 0.419 | 0.00995 |
| FERP2 to 3 6 ha | CORYCO | 0.0428 | −0.0813 | 106.1102 | 685 | 0.133 | <0.00001 |
| FERP2 to 3 6 ha | COTOFR | 0.0667 | −0.1142 | 13.9856 | 30 | 0.295 | 0.00078 |
| FERP2 to 3 6 ha | COTOPA | 0.0908 | −0.0890 | 22.9697 | 48 | 0.310 | 0.00002 |
| FERP2 to 3 6 ha | HETEAR | 0.0656 | −0.0617 | 1.3157 | 13 | 0.022 | 0.27204 |
| FERP2 to 3 6 ha | ILEXAQ | 0.0489 | −0.0230 | 0.5328 | 10 | −0.044 | 0.48217 |
| FERP2 to 3 6 ha | LITHDE | 0.0631 | −0.0242 | 46.5612 | 1528 | 0.029 | <0.00001 |
| FERP2 to 3 6 ha | LONIHI | 0.0461 | −0.0759 | 10.4039 | 112 | 0.077 | 0.00165 |
| FERP2 to 3 6 ha | PSEUME | 0.0593 | −0.0319 | 516.2390 | 1709 | 0.232 | <0.00001 |
| FERP2 to 3 6 ha | QUERAG | 0.0640 | −0.0445 | 366.4589 | 738 | 0.331 | <0.00001 |
| FERP2 to 3 6 ha | QUERPA | 0.0775 | −0.0565 | 435.9180 | 1264 | 0.256 | <0.00001 |
| FERP2 to 3 6 ha | RHAMCA | 0.0942 | −0.0750 | 17.9495 | 171 | 0.090 | 0.00004 |
| FERP2 to 3 6 ha | SEQUSE | 0.0283 | −0.0016 | 0.2948 | 234 | −0.003 | 0.58768 |
| FERP2 to 3 6 ha | TOXIDI | 0.0414 | −0.0895 | 20.1884 | 307 | 0.059 | 0.00001 |
| FERP2 to 3 6 ha | UMBECA | 0.0371 | 0.0010 | 0.0005 | 9 | −0.111 | 0.98227 |
| FERP2 to 3 6 ha | VACCOV | 0.0507 | −0.0998 | 114.2036 | 775 | 0.127 | <0.00001 |
| FERP2 to 3 16 ha | ARBUME | 0.0150 | −0.0082 | 20.9983 | 486 | 0.039 | 0.00001 |
| FERP2 to 3 16 ha | ARCTCR | 0.0614 | −0.0656 | 10.6471 | 12 | 0.426 | 0.00679 |
| FERP2 to 3 16 ha | CORYCO | 0.0409 | −0.0808 | 260.2206 | 2450 | 0.096 | <0.00001 |
| FERP2 to 3 16 ha | COTOFR | 0.0758 | −0.1320 | 13.7912 | 31 | 0.286 | 0.00080 |
| FERP2 to 3 16 ha | COTOPA | 0.0848 | −0.0811 | 21.6265 | 51 | 0.284 | 0.00002 |
| FERP2 to 3 16 ha | HEDEHE | 0.0842 | −0.0627 | 2.6679 | 8 | 0.156 | 0.14103 |
| FERP2 to 3 16 ha | HETEAR | 0.0594 | −0.0587 | 6.9670 | 92 | 0.060 | 0.00975 |
| FERP2 to 3 16 ha | ILEXAQ | 0.0524 | −0.0325 | 1.1025 | 14 | 0.007 | 0.31150 |
| FERP2 to 3 16 ha | LITHDE | 0.0562 | −0.0288 | 266.7038 | 7337 | 0.035 | <0.00001 |
| FERP2 to 3 16 ha | LONIHI | 0.0387 | −0.0808 | 26.9351 | 297 | 0.080 | <0.00001 |
| FERP2 to 3 16 ha | MORECA | 0.0304 | −0.0069 | 0.0687 | 62 | −0.015 | 0.79404 |
| FERP2 to 3 16 ha | PINUPO | 0.0222 | −0.0043 | 0.2605 | 10 | −0.072 | 0.62086 |
| FERP2 to 3 16 ha | PSEUME | 0.0556 | −0.0308 | 957.6681 | 6670 | 0.125 | <0.00001 |
| FERP2 to 3 16 ha | QUERAG | 0.0643 | −0.0445 | 328.8228 | 1139 | 0.223 | <0.00001 |
| FERP2 to 3 16 ha | QUERPA | 0.0881 | −0.0674 | 1114.4110 | 4604 | 0.195 | <0.00001 |
| FERP2 to 3 16 ha | RHAMCA | 0.0878 | −0.0725 | 26.4437 | 325 | 0.072 | <0.00001 |
| FERP2 to 3 16 ha | RHODOC | 0.0196 | −0.0568 | 24.3796 | 435 | 0.051 | <0.00001 |
| FERP2 to 3 16 ha | SEQUSE | 0.0266 | −0.0090 | 98.8974 | 2737 | 0.035 | <0.00001 |
| FERP2 to 3 16 ha | TOXIDI | 0.0384 | −0.1002 | 60.2448 | 663 | 0.082 | <0.00001 |
| FERP2 to 3 16 ha | UMBECA | 0.0775 | −0.0727 | 5.8115 | 13 | 0.256 | 0.03145 |
| FERP2 to 3 16 ha | VACCOV | 0.0418 | −0.0940 | 276.5557 | 2417 | 0.102 | <0.00001 |
References
- Bartels, S.F.; Chen, H.Y.; Wulder, M.A.; White, J.C. Trends in post-disturbance recovery rates of Canada’s forests following wildfire and harvest. For. Ecol. Manag. 2016, 361, 194–207. [Google Scholar] [CrossRef]
- Cole, L.E.; Bhagwat, S.A.; Willis, K.J. Recovery and resilience of tropical forests after disturbance. Nat. Commun. 2014, 5, 3906. [Google Scholar] [CrossRef]
- Hall, B.; Motzkin, G.; Foster, D.R.; Syfert, M.; Burk, J. Three hundred years of forest and land-use change in Massachusetts, USA. J. Biogeogr. 2002, 29, 1319–1335. [Google Scholar] [CrossRef]
- Almeida, D.R.; Stark, S.C.; Schietti, J.; Camargo, J.L.; Amazonas, N.T.; Gorgens, E.B.; Rosa, D.M.; Smith, M.N.; Valbuena, R.; Saleska, S. Persistent effects of fragmentation on tropical rainforest canopy structure after 20 yr of isolation. Ecol. Appl. 2019, 29, e01952. [Google Scholar] [CrossRef]
- Liu, J.; Coomes, D.A.; Gibson, L.; Hu, G.; Liu, J.; Luo, Y.; Wu, C.; Yu, M. Forest fragmentation in China and its effect on biodiversity. Biol. Rev. 2019, 94, 1636–1657. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, J.; Wu, W.; Liu, J. Global forest fragmentation change from 2000 to 2020. Nat. Commun. 2023, 14, 3752. [Google Scholar] [CrossRef]
- Langmaier, M.; Lapin, K. A systematic review of the impact of invasive alien plants on forest regeneration in European temperate forests. Front. Plant Sci. 2020, 11, 524969. [Google Scholar] [CrossRef] [PubMed]
- Oswalt, C.M.; Fei, S.; Guo, Q.; Iannone, B.V., III; Oswalt, S.N.; Pijanowski, B.C.; Potter, K.M. A subcontinental view of forest plant invasions. NeoBiota 2015, 24, 49–54. [Google Scholar] [CrossRef]
- Profetto, G.; Howard, J.J. Plant community responses to kudzu (Pueraria montana) invasion in a southern upland forest. J. Torrey Bot. Soc. 2021, 149, 30–39. [Google Scholar] [CrossRef]
- Canelles, Q.; Aquilué, N.; James, P.M.; Lawler, J.; Brotons, L. Global review on interactions between insect pests and other forest disturbances. Landsc. Ecol. 2021, 36, 945–972. [Google Scholar] [CrossRef]
- Ghelardini, L.; Pepori, A.L.; Luchi, N.; Capretti, P.; Santini, A. Drivers of emerging fungal diseases of forest trees. For. Ecol. Manag. 2016, 381, 235–246. [Google Scholar] [CrossRef]
- Haavik, L.J.; Billings, S.A.; Guldin, J.M.; Stephen, F.M. Emergent insects, pathogens and drought shape changing patterns in oak decline in North America and Europe. For. Ecol. Manag. 2015, 354, 190–205. [Google Scholar] [CrossRef]
- McDowell, N.G.; Allen, C.D.; Anderson-Teixeira, K.; Aukema, B.H.; Bond-Lamberty, B.; Chini, L.; Clark, J.S.; Dietze, M.; Grossiord, C.; Hanbury-Brown, A. Pervasive shifts in forest dynamics in a changing world. Science 2020, 368, eaaz9463. [Google Scholar] [CrossRef]
- Johnstone, J.F.; Allen, C.D.; Franklin, J.F.; Frelich, L.E.; Harvey, B.J.; Higuera, P.E.; Mack, M.C.; Meentemeyer, R.K.; Metz, M.R.; Perry, G.L. Changing disturbance regimes, ecological memory, and forest resilience. Front. Ecol. Environ. 2016, 14, 369–378. [Google Scholar] [CrossRef]
- Hart, S.J.; Henkelman, J.; McLoughlin, P.D.; Nielsen, S.E.; Truchon-Savard, A.; Johnstone, J.F. Examining forest resilience to changing fire frequency in a fire-prone region of boreal forest. Glob. Change Biol. 2019, 25, 869–884. [Google Scholar] [CrossRef]
- Takahashi, M.; Feng, Z.; Mikhailova, T.A.; Kalugina, O.V.; Shergina, O.V.; Afanasieva, L.V.; Heng, R.K.J.; Abd Majid, N.M.; Sase, H. Air pollution monitoring and tree and forest decline in East Asia: A review. Sci. Total Environ. 2020, 742, 140288. [Google Scholar] [CrossRef]
- Emberson, L. Effects of ozone on agriculture, forests and grasslands. Philos. Trans. R. Soc. A 2020, 378, 20190327. [Google Scholar] [CrossRef]
- Etzold, S.; Ferretti, M.; Reinds, G.J.; Solberg, S.; Gessler, A.; Waldner, P.; Schaub, M.; Simpson, D.; Benham, S.; Hansen, K. Nitrogen deposition is the most important environmental driver of growth of pure, even-aged and managed European forests. For. Ecol. Manag. 2020, 458, 117762. [Google Scholar] [CrossRef]
- Brienen, R.J.; Caldwell, L.; Duchesne, L.; Voelker, S.; Barichivich, J.; Baliva, M.; Ceccantini, G.; Di Filippo, A.; Helama, S.; Locosselli, G.M. Forest carbon sink neutralized by pervasive growth-lifespan trade-offs. Nat. Commun. 2020, 11, 4241. [Google Scholar] [CrossRef]
- Schnitzer, S.A.; Bongers, F. Increasing liana abundance and biomass in tropical forests: Emerging patterns and putative mechanisms. Ecol. Lett. 2011, 14, 397–406. [Google Scholar] [CrossRef]
- Clark, J.S.; Iverson, L.; Woodall, C.W.; Allen, C.D.; Bell, D.M.; Bragg, D.C.; D’Amato, A.W.; Davis, F.W.; Hersh, M.H.; Ibanez, I. The impacts of increasing drought on forest dynamics, structure, and biodiversity in the United States. Glob. Change Biol. 2016, 22, 2329–2352. [Google Scholar] [CrossRef] [PubMed]
- Zellweger, F.; De Frenne, P.; Lenoir, J.; Vangansbeke, P.; Verheyen, K.; Bernhardt-Römermann, M.; Baeten, L.; Hédl, R.; Berki, I.; Brunet, J. Forest microclimate dynamics drive plant responses to warming. Science 2020, 368, 772–775. [Google Scholar] [CrossRef]
- Hiura, T.; Go, S.; Iijima, H. Long-term forest dynamics in response to climate change in northern mixed forests in Japan: A 38-year individual-based approach. For. Ecol. Manag. 2019, 449, 117469. [Google Scholar] [CrossRef]
- McRoberts, R.E.; Bechtold, W.A.; Patterson, P.L.; Scott, C.T.; Reams, G.A. The enhanced Forest Inventory and Analysis program of the USDA Forest Service: Historical perspective and announcement of statistical documentation. J. For. 2005, 103, 304–308. [Google Scholar]
- Blundo, C.; Carilla, J.; Grau, R.; Malizia, A.; Malizia, L.; Osinaga-Acosta, O.; Bird, M.; Bradford, M.; Catchpole, D.; Ford, A. Taking the pulse of Earth’s tropical forests using networks of highly distributed plots. Biol. Conserv. 2021, 260, 108849. [Google Scholar]
- Mauri, A.; Strona, G.; San-Miguel-Ayanz, J. EU-Forest, a high-resolution tree occurrence dataset for Europe. Sci. Data 2017, 4, 160123. [Google Scholar] [CrossRef]
- Davies, S.J.; Abiem, I.; Salim, K.A.; Aguilar, S.; Allen, D.; Alonso, A.; Anderson-Teixeira, K.; Andrade, A.; Arellano, G.; Ashton, P.S. ForestGEO: Understanding forest diversity and dynamics through a global observatory network. Biol. Conserv. 2021, 253, 108907. [Google Scholar] [CrossRef]
- ForestGEO. ForestGEO Global Eath Observatory Network. Available online: https://forestgeo.si.edu/ (accessed on 1 December 2023).
- Anderson-Teixeira, K.J.; Davies, S.J.; Bennett, A.C.; Gonzalez-Akre, E.B.; Muller-Landau, H.C.; Wright, S.J.; Abu Salim, K.; Zambrano, A.M.A.; Alonso, A.; Baltzer, J.L.; et al. CTFS-ForestGEO: A worldwide network monitoring forests in an era of global change. Glob. Change Biol. 2015, 21, 528–549. [Google Scholar] [CrossRef]
- Rundel, P.W.; Arroyo, M.T.; Cowling, R.M.; Keeley, J.E.; Lamont, B.B.; Vargas, P. Mediterranean biomes: Evolution of their vegetation, floras, and climate. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 383–407. [Google Scholar] [CrossRef]
- Gilbert, G.S.; Howard, E.; Ayala-Orozco, B.; Bonilla-Moheno, M.; Cummings, J.; Langridge, S.; Parker, I.M.; Pasari, J.; Schweizer, D.; Swope, S. Beyond the tropics: Forest structure in a temperate forest mapped plot. J. Veg. Sci. 2010, 21, 388–405. [Google Scholar] [CrossRef]
- Rizzo, M.A. We Are Not Animals. Indigenous Politics of Survival, Rebellion, and Reconstitution in Nineteenth-Century California; University of Nebraska Press: Lincoln, NE, USA, 2022; p. 536. [Google Scholar]
- Lewis, W.J. Rancho De la Cañada Del Rincón En el Río San Lorenzo De Sta. Cruz: Finally Confirmed to Don Pedro Sansevaine. The Upper Quarry Amphitheater Collection. 1855. Available online: https://quarryamphitheater.library.ucsc.edu/items/show/3 (accessed on 8 December 2023).
- Anonymous. Forest Fire Burns 10 Acres of UCSC Site. Santa Cruz Sentinel, 13 April 1964, p. 1.
- Haff, T.M.; Brown, M.T.; Tyler, W.B. The Natural History of the UC Santa Cruz Campus, 2nd ed.; Environmental Studies, UC Santa Cruz: Santa Cruz, CA, USA, 2008. [Google Scholar]
- UCSC. University of California Santa Cruz 2021 Long Range Development Plan. Available online: https://lrdp.ucsc.edu/2021/lrdp.html (accessed on 8 November 2023).
- Condit, R. Tropical Forest Census Plots; Springer: Berlin/Heidelberg, Germany, 1998; p. 211. [Google Scholar]
- Lewis, S.L.; Phillips, O.L.; Sheil, D.; Vinceti, B.; Baker, T.R.; Brown, S.; Graham, A.W.; Higuchi, N.; Hilbert, D.W.; Laurance, W.F. Tropical forest tree mortality, recruitment and turnover rates: Calculation, interpretation and comparison when census intervals vary. J. Ecol. 2004, 92, 929–944. [Google Scholar] [CrossRef]
- Lin, Z.; Fan, D.; Ma, S.; Miao, W.; Wang, X. Relative growth rate for trees at the growth stage is coordinated with leaf bulk modulus of elasticity and osmotic potential in a subtropical forest of China. Ecol. Indic. 2022, 141, 109135. [Google Scholar] [CrossRef]
- California Native Plants Society. A Manual of California Vegetation, Online Edition. Available online: http://www.cnps.org/cnps/vegetation/ (accessed on 10 November 2023).
- Dyderski, M.K.; Jagodziński, A.M. Impact of invasive tree species on natural regeneration species composition, diversity, and density. Forests 2020, 11, 456. [Google Scholar] [CrossRef]
- Jiménez-Hernández, H.A.; Bonilla-Valencia, L.; Martínez-Orea, Y.; Zamora-Almazan, M.J.; Espinosa-García, F.J.; Castillo-Argüero, S. Effects of Hedera helix L. removal on the understory early regeneration in an oak temperate forest in Mexico City. Ecol. Process. 2023, 12, 31. [Google Scholar] [CrossRef]
- Farmer, S.; Ward, J.R.; Horton, J.L.; Clarke, H.D. Southern Appalachian urban forest response to three invasive plant removal treatments. Manag. Biol. Invasions 2016, 7, 329–342. [Google Scholar] [CrossRef]
- Stokes, D.L.; Church, E.D.; Cronkright, D.M.; Lopez, S. Pictures of an invasion: English holly (Ilex aquifolium) in a semi-natural Pacific Northwest forest. Northwest Sci. 2014, 88, 75–93. [Google Scholar] [CrossRef]
- Lutz, J.A.; Furniss, T.J.; Johnson, D.J.; Davies, S.J.; Allen, D.; Alonso, A.; Anderson-Teixeira, K.J.; Andrade, A.; Baltzer, J.; Becker, K.M.L.; et al. Global importance of large-diameter trees. Glob. Ecol. Biogeogr. 2018, 27, 849–864. [Google Scholar] [CrossRef]
- Hubbell, S.P.; Foster, R.B.; O’Brien, S.T.; Harms, K.E.; Condit, R.; Wechsler, B.; Wright, S.J.; de Lao, S.L. Light-gap disturbances, recruitment limitation, and tree diversity in a neotropical forest. Science 1999, 283, 554–557. [Google Scholar] [CrossRef]
- Hunter, J.; Parker, V.; Barbour, M. Understory light and gap dynamics in an old-growth forested watershed in coastal California. Madroño 1999, 46, 1–6. [Google Scholar]
- Lutz, J.A.; Larson, A.J.; Freund, J.A.; Swanson, M.E.; Bible, K.J. The importance of large-diameter trees to forest structural heterogeneity. PLoS ONE 2013, 8, e82784. [Google Scholar] [CrossRef]
- Dodd, R.S.; Afzal-Rafii, Z. Selection and dispersal in a multispecies oak hybrid zone. Evolution 2004, 58, 261–269. [Google Scholar] [PubMed]
- Waring, R.; Major, J. Some vegetation of the California coastal redwood region in relation to gradients of moisture, nutrients, light, and temperature. Ecol. Monogr. 1964, 34, 167–215. [Google Scholar] [CrossRef]
- Davidson, J.M.; Wickland, A.C.; Patterson, H.A.; Falk, K.R.; Rizzo, D.M. Transmission of Phytophthora ramorum in mixed-evergreen forest in California. Phytopathology 2005, 95, 587–596. [Google Scholar] [CrossRef]
- Baguskas, S.A.; Peterson, S.H.; Bookhagen, B.; Still, C.J. Evaluating spatial patterns of drought-induced tree mortality in a coastal California pine forest. For. Ecol. Manag. 2014, 315, 43–53. [Google Scholar] [CrossRef]
- Madakumbura, G.D.; Goulden, M.L.; Hall, A.; Fu, R.; Moritz, M.A.; Koven, C.D.; Kueppers, L.M.; Norlen, C.A.; Randerson, J.T. Recent California tree mortality portends future increase in drought-driven forest die-off. Environ. Res. Lett. 2020, 15, 124040. [Google Scholar] [CrossRef]
- Choat, B.; Brodribb, T.J.; Brodersen, C.R.; Duursma, R.A.; López, R.; Medlyn, B.E. Triggers of tree mortality under drought. Nature 2018, 558, 531–539. [Google Scholar] [CrossRef]
- Sturrock, R.; Frankel, S.; Brown, A.; Hennon, P.; Kliejunas, J.; Lewis, K.; Worrall, J.; Woods, A. Climate change and forest diseases. Plant Pathol. 2011, 60, 133–149. [Google Scholar] [CrossRef]
- Cobb, R.C.; Haas, S.E.; Kruskamp, N.; Dillon, W.W.; Swiecki, T.J.; Rizzo, D.M.; Frankel, S.J.; Meentemeyer, R.K. The magnitude of regional-scale tree mortality caused by the invasive pathogen Phytophthora ramorum. Earth’s Future 2020, 8, e2020EF001500. [Google Scholar] [CrossRef]
- McPherson, B.A.; Mori, S.R.; Wood, D.L.; Storer, A.J.; Svihra, P.; Kelly, N.M.; Standiford, R.B. Sudden oak death in California: Disease progression in oaks and tanoaks. For. Ecol. Manag. 2005, 213, 71–89. [Google Scholar] [CrossRef]
- Elliott, M.; Edmonds, R.L.; Mayer, S. Role of fungal diseases in decline of Pacific madrone. Northwest Sci. 2002, 76, 293–303. [Google Scholar]
- Comita, L.S.; Aguilar, S.; Hubbell, S.P.; Pérez, R. Long-term seedling and small sapling census data from the Barro Colorado Island 50 ha Forest Dynamics Plot, Panama. Ecology 2023, 104, e4140. [Google Scholar] [CrossRef]
- Qiu, T.; Andrus, R.; Aravena, M.-C.; Ascoli, D.; Bergeron, Y.; Berretti, R.; Berveiller, D.; Bogdziewicz, M.; Boivin, T.; Bonal, R. Limits to reproduction and seed size-number trade-offs that shape forest dominance and future recovery. Nat. Commun. 2022, 13, 238. [Google Scholar] [CrossRef]
- de Lima, R.A.F.; Batista, J.L.F.; Prado, P.I. Modeling tree diameter distributions in natural forests: An evaluation of 10 statistical models. For. Sci. 2015, 61, 320–327. [Google Scholar] [CrossRef]
- Zenner, E.K. Development of tree size distributions in Douglas-fir forests under differing disturbance regimes. Ecol. Appl. 2005, 15, 701–714. [Google Scholar] [CrossRef]
- Coomes, D.A.; Allen, R.B. Mortality and tree-size distributions in natural mixed-age forests. J. Ecol. 2007, 95, 27–40. [Google Scholar] [CrossRef]
- Lorimer, C.G.; Krug, A.G. Diameter distributions in even-aged stands of shade-tolerant and midtolerant tree species. Am. Midl. Nat. 1983, 109, 331–345. [Google Scholar] [CrossRef]
- Wright, S.J.; Muller-Landau, H.C.; Condit, R.; Hubbell, S.P. Gap-dependent recruitment, realized vital rates, and size distributions of tropical trees. Ecology 2003, 84, 3174–3185. [Google Scholar] [CrossRef]
- Pommerening, A.; Muszta, A. Methods of modelling relative growth rate. For. Ecosyst. 2015, 2, 5. [Google Scholar] [CrossRef]
- Prado-Junior, J.A.; Schiavini, I.; Vale, V.S.; Raymundo, D.; Lopes, S.F.; Poorter, L. Functional traits shape size-dependent growth and mortality rates of dry forest tree species. J. Plant Ecol. 2017, 10, 895–906. [Google Scholar] [CrossRef]
- Yan, Y.-M.; Fan, Z.-X.; Fu, P.-L.; Chen, H.; Lin, L.-X. Size dependent associations between tree diameter growth rates and functional traits in an Asian tropical seasonal rainforest. Funct. Plant Biol. 2020, 48, 231–240. [Google Scholar] [CrossRef]
- Qiu, T.; Aravena, M.-C.; Andrus, R.; Ascoli, D.; Bergeron, Y.; Berretti, R.; Bogdziewicz, M.; Boivin, T.; Bonal, R.; Caignard, T.; et al. Is there tree senescence? The fecundity evidence. Proc. Natl. Acad. Sci. USA 2021, 118, e2106130118. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Lutz, J.A.; Guo, Q.; Hao, Z.; Wang, X.; Gilbert, G.S.; Mao, Z.; Orwig, D.A.; Parker, G.G.; Sang, W. Mycorrhizal type influences plant density dependence and species richness across 15 temperate forests. Ecology 2021, 102, e03259. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.S.; Andrus, R.; Aubry-Kientz, M.; Bergeron, Y.; Bogdziewicz, M.; Bragg, D.C.; Brockway, D.; Cleavitt, N.L.; Cohen, S.; Courbaud, B.; et al. Continent-wide tree fecundity driven by indirect climate effects. Nat. Commun. 2021, 12, 1242. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Andrus, R.; Bergeron, Y.; Bogdziewicz, M.; Bragg, D.C.; Brockway, D.; Cleavitt, N.L.; Courbaud, B.; Das, A.J.; Dietze, M.; et al. North American tree migration paced by climate in the West, lagging in the East. Proc. Natl. Acad. Sci. USA 2022, 119, e2116691118. [Google Scholar] [CrossRef] [PubMed]
- Sousa, D.; Fisher, J.B.; Galvan, F.R.; Pavlick, R.P.; Cordell, S.; Giambelluca, T.W.; Giardina, C.P.; Gilbert, G.S.; Imran-Narahari, F.; Litton, C.M. Tree canopies reflect mycorrhizal composition. Geophys. Res. Lett. 2021, 48, e2021GL092764. [Google Scholar] [CrossRef]
- Zhong, Y.; Chu, C.; Myers, J.A.; Gilbert, G.S.; Lutz, J.A.; Stillhard, J.; Zhu, K.; Thompson, J.; Baltzer, J.L.; He, F. Arbuscular mycorrhizal trees influence the latitudinal beta-diversity gradient of tree communities in forests worldwide. Nat. Commun. 2021, 12, 3137. [Google Scholar] [CrossRef]
- Chen, C.; Brodie, J.F.; Kays, R.; Davies, T.J.; Liu, R.; Fisher, J.T.; Ahumada, J.; McShea, W.; Sheil, D.; Agwanda, B. Global camera trap synthesis highlights the importance of protected areas in maintaining mammal diversity. Conserv. Lett. 2022, 15, e12865. [Google Scholar] [CrossRef]
- Menge, D.N.; Chisholm, R.A.; Davies, S.J.; Abu Salim, K.; Allen, D.; Alvarez, M.; Bourg, N.; Brockelman, W.Y.; Bunyavejchewin, S.; Butt, N. Patterns of nitrogen-fixing tree abundance in forests across Asia and America. J. Ecol. 2019, 107, 2598–2610. [Google Scholar] [CrossRef]
- Xiao, X.; McGlinn, D.J.; White, E.P. A strong test of the maximum entropy theory of ecology. Am. Nat. 2015, 185, E70–E80. [Google Scholar] [CrossRef]
- Chacon, A.I.; Baer, A.; Wheeler, J.K.; Pittermann, J. Two coastal Pacific evergreens, Arbutus menziesii Pursh. and Quercus agrifolia Née show little water stress during California’s exceptional drought. PLoS ONE 2020, 15, e0230868. [Google Scholar] [CrossRef]
- Crandall, S.G.; Gilbert, G.S. Meteorological factors associated with abundance of airborne fungal spores over natural vegetation. Atmos. Environ. 2017, 162, 87–99. [Google Scholar] [CrossRef]
- Crandall, S.G.; Saarman, N.; Gilbert, G.S. Fungal spore diversity, community structure, and traits across a vegetation mosaic. Fungal Ecol. 2020, 45, 100920. [Google Scholar] [CrossRef]
- Reid, R.E.; Greenwald, E.N.; Wang, Y.; Wilmers, C.C. Dietary niche partitioning by sympatric Peromyscus boylii and P. californicus in a mixed evergreen forest. J. Mammal. 2013, 94, 1248–1257. [Google Scholar] [CrossRef]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef]



| Scientific Name | Family | Common Name | Origin | Habit | Code 1 |
|---|---|---|---|---|---|
| Acer macrophyllum Pursh | Sapindaceae | Bigleaf maple | Native | Tree | ACERMA |
| Adenostoma fasciculatum Hook. & Arn. | Rosaceae | Chamise | Native | Shrub | ADENFA |
| Arbutus menziesii Pursh | Ericaceae | Pacific madrone | Native | Tree | ARBUME |
| Arctostaphylos andersonii A. Gray | Ericaceae | Santa Cruz manzanita | Native | Shrub | ARCTAN |
| Arctostaphylos crustacea subsp. crinita (J.E.Adams) V.T.Parker, M.C.Vasey & J.E.Keeley | Ericaceae | Brittle leaf manzanita | Native | Shrub | ARCTCR |
| Baccharis pilularis DC. | Asteraceae | Coyote brush | Native | Shrub | BACCPI |
| Ceanothus thyrsiflorus Eschsch. | Rhamnaceae | Blueblossom | Native | Shrub | CEANTH |
| Corylus cornuta subsp. californica Marshall (A.DC.) A.E.Murray | Betulaceae | Beaked hazelnut | Native | Shrub | CORYCO |
| Cotoneaster franchetii Bois | Rosaceae | Franchet cotoneaster | Intro | Shrub | COTOFR |
| Cotoneaster pannosus Franch. | Rosaceae | Woolly cotoneaster | Intro | Shrub | COTOPA |
| Crataegus monogyna Jacq. | Rosaceae | One-seed hawthorn | Intro | Shrub | CRATMO |
| Eriobotrya japonica (Thunb.) Lindl. | Rosaceae | Loquat | Intro | Tree | ERIOJA |
| Eucalyptus globulus Labill. | Myrtaceae | Blue gum | Intro | Tree | EUCAGL |
| Frangula californica (Eschsch.) A.Gray | Rhamnaceae | California coffeeberry | Native | Shrub | RHAMCA |
| Hedera helix L. | Araliaceae | English ivy | Intro | Liana | HEDEHE |
| Heteromeles arbutifolia (Lindl.) M.Roem | Rosaceae | Toyon | Native | Shrub | HETEAR |
| Ilex aquifolium L. | Aquifoliaceae | English holly | Intro | Tree | ILEXAQ |
| Lonicera hispidula (Lindl.) Douglas ex Torr. & A.Gray | Caprifoliaceae | Pink honeysuckle | Native | Liana | LONIHI |
| Morella californica (Cham.) Wilbur | Myricaceae | California wax myrtle | Native | Shrub | MORECA |
| Notholithocarpus densiflorus (Hook. & Arn.) Manos, C.H. Cannon & S.H.Oh | Fagaceae | Tanoak | Native | Tree | LITHDE |
| Pinus attenuata Lemmon | Pinaceae | Knobcone pine | Native | Tree | PINUAT |
| Pinus ponderosa var. pacifica J.R.Haller & Vivrette | Pinaceae | Pacific ponderosa pine | Native | Tree | PINUPO |
| Pseudotsuga menziesii (Mirb.) Franco | Pinaceae | Douglas fir | Native | Tree | PSEUME |
| Pyracantha angustifolia C.K.Schneid | Rosaceae | Firethorn | Intro | Shrub | PYRAAN |
| Quercus agrifolia Née | Fagaceae | Coast live oak | Native | Tree | QUERAG |
| Quercus parvula var. shrevei (C.H.Mull.) Nixon | Fagaceae | Shreve’s oak | Native | Tree | QUERPA |
| Rhododendron occidentale (Torr. & A.Gray) A.Gray | Ericaceae | Western azalea | Native | Shrub | RHODOC |
| Ribes divaricatum Douglas | Grossulariaceae | Spreading gooseberry | Native | Shrub | RIBEDI |
| Salix lasiandra Benth. | Salicaceae | Pacific willow | Native | Shrub | SALILA |
| Sambucus caerulea Raf. | Vibernaceae | Blue elderberry | Native | Shrub | SAMBNI |
| Sequoia sempervirens (D.Don) Endl. | Cupressaceae | Coast redwood | Native | Tree | SEQUSE |
| Toxicodendron diversilobum Greene | Anacardiaceae | Poison oak | Native | Liana | TOXIDI |
| Umbellularia californica (Hook. & Arn.) Nutt. | Lauraceae | California bay | Native | Tree | UMBECA |
| Vaccinium ovatum Pursh | Ericaceae | Evergreen huckleberry | Native | Shrub | VACCOV |
| 6 ha | 16 ha | |||||
|---|---|---|---|---|---|---|
| Density Measure | FERP1 | FERP2 | FERP3 | FERP2 | FERP3 | |
| Individuals ha−1 | 1363.3 | 1378.7 | 1158.2 | 1630.2 | 1558.7 | |
| Stems ha−1 | 1941.0 | 1992.3 | 1724.0 | 2488.8 | 2303.1 | |
| Basal area m2 ha−1 | 47.2 | 49.1 | 48.6 | 66.9 | 68.3 | |
| Individuals ha−1 (adj) | - | - | - | 1636.4 | 1564.7 | |
| Stems ha−1 (adj) | - | - | - | 2498.3 | 2311.9 | |
| Basal area m2 ha−1 (adj) | - | - | - | 67.2 | 68.6 | |
| 6 ha | 16 ha | |||||
|---|---|---|---|---|---|---|
| Code | FERP1 | FERP2 | FERP3 | FERP2 | FERP3 | |
| ACERMA | 2 | 2 | 2 | 10 | 9 | |
| ADENFA | 0 | 0 | 0 | 1 | 0 | |
| ARBUME | 687 | 597 | 305 | 845 | 415 | |
| ARCTAN | 11 | 7 | 1 | 9 | 11 | |
| ARCTCR | 39 | 23 | 11 | 28 | 13 | |
| BACCPI | 11 | 1 | 1 | 6 | 14 | |
| CEANTH | 1 | 1 | 0 | 2 | 2 | |
| CORYCO | 146 | 146 | 155 | 478 | 477 | |
| COTOFR | 18 | 21 | 17 | 22 | 19 | |
| COTOPA | 36 | 32 | 33 | 35 | 38 | |
| CRATMO | 1 | 1 | 1 | 1 | 1 | |
| ERIOJA | 0 | 0 | 0 | 1 | 1 | |
| EUCAGL | 5 | 5 | 5 | 6 | 6 | |
| HEDEHE | 8 | 7 | 12 | 11 | 16 | |
| HETEAR | 11 | 8 | 11 | 52 | 50 | |
| ILEXAQ | 10 | 9 | 11 | 13 | 16 | |
| LITHDE | 1260 | 1630 | 1504 | 6164 | 6314 | |
| LONIHI | 217 | 197 | 126 | 490 | 318 | |
| MORECA | 8 | 8 | 3 | 52 | 39 | |
| PINUAT | 1 | 0 | 0 | 3 | 3 | |
| PINUPO | 17 | 10 | 7 | 15 | 13 | |
| PSEUME | 2158 | 2121 | 1864 | 8005 | 7308 | |
| PYRAAN | 1 | 1 | 1 | 1 | 1 | |
| QUERAG | 908 | 856 | 749 | 1242 | 1159 | |
| QUERPA | 1196 | 1202 | 1192 | 3860 | 4747 | |
| RHAMCA | 293 | 268 | 148 | 388 | 236 | |
| RHODOC | 0 | 0 | 0 | 215 | 150 | |
| RIBEDI | 1 | 1 | 0 | 3 | 3 | |
| SALILA | 2 | 2 | 2 | 4 | 4 | |
| SAMBNI | 2 | 2 | 1 | 3 | 3 | |
| SEQUSE | 190 | 194 | 196 | 2001 | 2015 | |
| TOXIDI | 675 | 638 | 341 | 1229 | 732 | |
| UMBECA | 11 | 11 | 10 | 16 | 14 | |
| VACCOV | 254 | 271 | 240 | 872 | 792 | |
| Total | 8180 | 8272 | 6949 | 26,083 | 24,939 | |
| 6 ha | 16 ha | |||||
|---|---|---|---|---|---|---|
| Code | FERP1 | FERP2 | FERP3 | FERP2 | FERP3 | |
| ACERMA | 3 | 2 | 2 | 12 | 9 | |
| ADENFA | 0 | 0 | 0 | 1 | 0 | |
| ARBUME | 907 | 742 | 409 | 1060 | 556 | |
| ARCTAN | 24 | 9 | 1 | 14 | 32 | |
| ARCTCR | 74 | 46 | 15 | 54 | 19 | |
| BACCPI | 24 | 1 | 1 | 27 | 40 | |
| CEANTH | 1 | 1 | 0 | 2 | 3 | |
| CORYCO | 874 | 920 | 1092 | 3231 | 3016 | |
| COTOFR | 57 | 59 | 42 | 60 | 44 | |
| COTOPA | 71 | 65 | 76 | 71 | 81 | |
| CRATMO | 1 | 1 | 1 | 1 | 1 | |
| ERIOJA | 0 | 0 | 0 | 1 | 1 | |
| EUCAGL | 5 | 6 | 6 | 8 | 8 | |
| HEDEHE | 8 | 7 | 13 | 11 | 17 | |
| HETEAR | 36 | 23 | 27 | 160 | 135 | |
| ILEXAQ | 21 | 14 | 20 | 25 | 27 | |
| LITHDE | 1566 | 2313 | 2099 | 9675 | 9297 | |
| LONIHI | 228 | 217 | 136 | 534 | 344 | |
| MORECA | 12 | 9 | 5 | 108 | 70 | |
| PINUAT | 1 | 0 | 0 | 3 | 3 | |
| PINUPO | 17 | 10 | 7 | 15 | 13 | |
| PSEUME | 2207 | 2176 | 1929 | 8339 | 7592 | |
| PYRAAN | 1 | 2 | 2 | 2 | 2 | |
| QUERAG | 1061 | 1007 | 874 | 1500 | 1385 | |
| QUERPA | 1678 | 1694 | 1680 | 5728 | 6776 | |
| RHAMCA | 464 | 470 | 258 | 744 | 449 | |
| RHODOC | 0 | 0 | 0 | 629 | 452 | |
| RIBEDI | 3 | 1 | 0 | 3 | 3 | |
| SALILA | 3 | 3 | 4 | 8 | 9 | |
| SAMBNI | 2 | 2 | 1 | 4 | 9 | |
| SEQUSE | 238 | 263 | 258 | 3046 | 2924 | |
| TOXIDI | 726 | 709 | 380 | 1362 | 802 | |
| UMBECA | 13 | 13 | 13 | 18 | 17 | |
| VACCOV | 1320 | 1169 | 993 | 3365 | 2713 | |
| Total | 11,646 | 11,954 | 10,344 | 39,821 | 36,849 | |
| 6 ha | 16 ha | |||||
|---|---|---|---|---|---|---|
| Code | FERP1 | FERP2 | FERP3 | FERP2 | FERP3 | |
| ACERMA | 0.38097 | 0.46521 | 0.53047 | 1.46086 | 1.5160 | |
| ADENFA | 0 | 0 | 0 | 0.00010 | 0 | |
| ARBUME | 67.04084 | 59.66385 | 39.75019 | 94.86421 | 57.87346 | |
| ARCTAN | 0.11528 | 0.04846 | 0.00018 | 0.05267 | 0.01887 | |
| ARCTCR | 0.20206 | 0.14434 | 0.05949 | 0.15199 | 0.06171 | |
| BACCPI | 0.00814 | 0.00023 | 0.00038 | 0.00549 | 0.01935 | |
| CEANTH | 0.02324 | 0.03048 | 0 | 0.03079 | 0.00106 | |
| CORYCO | 0.29372 | 0.30811 | 0.41493 | 1.04925 | 1.22132 | |
| COTOFR | 0.02203 | 0.02321 | 0.026 | 0.02336 | 0.02706 | |
| COTOPA | 0.09969 | 0.13451 | 0.16585 | 0.13604 | 0.16802 | |
| CRATMO | 0.00166 | 0.00173 | 0.00166 | 0.00173 | 0.00166 | |
| ERIOJA | 0 | 0 | 0 | 0.00013 | 0.00008 | |
| EUCAGL | 0.0244 | 0.08458 | 0.16591 | 0.08512 | 0.16724 | |
| HEDEHE | 0.00616 | 0.01061 | 0.02279 | 0.01144 | 0.02424 | |
| HETEAR | 0.09899 | 0.08072 | 0.08109 | 0.21873 | 0.20313 | |
| ILEXAQ | 0.03951 | 0.04201 | 0.06493 | 0.05557 | 0.06836 | |
| LITHDE | 9.39694 | 10.53444 | 10.39026 | 41.39863 | 36.90206 | |
| LONIHI | 0.05187 | 0.06133 | 0.05087 | 0.13584 | 0.10142 | |
| MORECA | 0.03905 | 0.02948 | 0.01946 | 0.10048 | 0.06096 | |
| PINUAT | 0.03079 | 0 | 0 | 0.29882 | 0.31127 | |
| PINUPO | 1.00556 | 1.49778 | 1.366 | 3.04374 | 3.15685 | |
| PSEUME | 132.73041 | 150.37244 | 166.0646 | 332.96542 | 357.46774 | |
| PYRAAN | 0.00057 | 0.00073 | 0.00076 | 0.00073 | 0.00076 | |
| QUERAG | 32.69696 | 30.07165 | 26.66856 | 36.19849 | 32.61653 | |
| QUERPA | 17.3345 | 14.04387 | 14.49981 | 34.71857 | 35.77295 | |
| RHAMCA | 0.29838 | 0.41742 | 0.33405 | 0.68199 | 0.52023 | |
| RHODOC | 0 | 0 | 0 | 0.14524 | 0.12705 | |
| RIBEDI | 0.00072 | 0.00008 | 0 | 0.00024 | 0.00032 | |
| SALILA | 0.01681 | 0.05385 | 0.10789 | 0.15586 | 0.18607 | |
| SAMBNI | 0.00244 | 0.00343 | 0.00126 | 0.00714 | 0.00652 | |
| SEQUSE | 20.55343 | 26.16681 | 30.32737 | 521.86279 | 563.20891 | |
| TOXIDI | 0.12645 | 0.13451 | 0.11196 | 0.23866 | 0.19235 | |
| UMBECA | 0.01869 | 0.01921 | 0.03316 | 0.08226 | 0.07376 | |
| VACCOV | 0.32824 | 0.29923 | 0.36747 | 0.84534 | 0.87761 | |
| Total | 282.9885 | 294.7443 | 291.6273 | 1071.0280 | 1092.9550 | |
| FERP1 to FERP2 | FERP2 to FERP3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Annual Mortality | Annual Mortality | |||||||||
| Code | Live | Surv | Lambda | Lower | Upper | Live | Surv | Lambda | Lower | Upper |
| ARBUME | 687 | 585 | 0.0337 | 0.0329 | 0.0348 | 597 | 294 | 0.1137 | 0.1083 | 0.1179 |
| ARCTCR | 39 | 23 | 0.1106 | 0.1081 | 0.1142 | 23 | 10 | 0.1337 | 0.1274 | 0.1386 |
| CORYCO | 146 | 137 | 0.0133 | 0.013 | 0.0138 | 146 | 137 | 0.0102 | 0.0097 | 0.0106 |
| COTOFR | - | - | - | - | - | 21 | 17 | 0.0339 | 0.0323 | 0.0352 |
| COTOPA | 36 | 32 | 0.0247 | 0.0241 | 0.0255 | 32 | 31 | 0.0051 | 0.0049 | 0.0053 |
| LITHDE | 1260 | 1188 | 0.0123 | 0.012 | 0.0127 | 1630 | 1185 | 0.0512 | 0.0488 | 0.0531 |
| LONIHI | 217 | 174 | 0.0462 | 0.0452 | 0.0478 | 197 | 104 | 0.1025 | 0.0977 | 0.1063 |
| PSEUME | 2158 | 1988 | 0.0172 | 0.0168 | 0.0177 | 2121 | 1683 | 0.0371 | 0.0354 | 0.0385 |
| QUERAG | 908 | 785 | 0.0305 | 0.0298 | 0.0315 | 856 | 671 | 0.0391 | 0.0372 | 0.0405 |
| QUERPA | 1196 | 1048 | 0.0277 | 0.027 | 0.0286 | 1202 | 944 | 0.0388 | 0.0369 | 0.0402 |
| RHAMCA | 293 | 223 | 0.0572 | 0.0559 | 0.0590 | 268 | 119 | 0.1303 | 0.1241 | 0.1351 |
| SEQUSE | 190 | 188 | 0.0022 | 0.0022 | 0.0023 | 194 | 186 | 0.0068 | 0.0064 | 0.007 |
| TOXIDI | 675 | 499 | 0.0633 | 0.0618 | 0.0653 | 638 | 289 | 0.1271 | 0.1211 | 0.1318 |
| VACCOV | 254 | 229 | 0.0217 | 0.0212 | 0.0224 | 271 | 215 | 0.0372 | 0.0354 | 0.0385 |
| Annual Mortality | |||||
|---|---|---|---|---|---|
| Code | Live | Surv | Lambda | Lower | Upper |
| ARBUME | 845 | 395 | 0.1317 | 0.1159 | 0.1773 |
| ARCTCR | 28 | 11 | 0.1618 | 0.1424 | 0.2178 |
| CORYCO | 478 | 427 | 0.0195 | 0.0172 | 0.0263 |
| COTOFR | 22 | 18 | 0.0347 | 0.0306 | 0.0468 |
| COTOPA | 35 | 34 | 0.0050 | 0.0044 | 0.0068 |
| HETEAR | 52 | 38 | 0.0543 | 0.0478 | 0.0731 |
| LITHDE | 6164 | 5012 | 0.0358 | 0.0315 | 0.0482 |
| LONIHI | 490 | 276 | 0.0994 | 0.0875 | 0.1338 |
| MORECA | 52 | 36 | 0.0637 | 0.0561 | 0.0857 |
| PSEUME | 8005 | 6513 | 0.0357 | 0.0314 | 0.0481 |
| QUERAG | 1242 | 1003 | 0.0370 | 0.0326 | 0.0498 |
| QUERPA | 3860 | 3287 | 0.0278 | 0.0245 | 0.0375 |
| RHAMCA | 388 | 181 | 0.1320 | 0.1162 | 0.1777 |
| RHODOC | 215 | 145 | 0.0682 | 0.0600 | 0.0918 |
| SEQUSE | 2001 | 1919 | 0.0072 | 0.0064 | 0.0098 |
| TOXIDI | 1229 | 621 | 0.1182 | 0.104 | 0.1591 |
| VACCOV | 872 | 735 | 0.0296 | 0.0261 | 0.0398 |
| FERP1 to FERP2 6 ha | FERP2 to FERP3 6 ha | FERP2 to FERP3 16 ha | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Code | Live | Died | Recruit | R-D | Live | Died | Recruit | R-D | Live | Died | Recruit | R-D |
| ACERMA | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 10 | 1 | 0 | −1 |
| ADENFA | - 1 | - | - | - | - | - | - | - | 1 | 1 | 0 | −1 |
| ARBUME | 687 | 102 | 12 | −90 | 597 | 303 | 7 | −296 | 845 | 450 | 16 | −434 |
| ARCTAN | 11 | 4 | 0 | −4 | 7 | 7 | 1 | −6 | 9 | 8 | 10 | 2 |
| ARCTCR | 39 | 16 | 0 | −16 | 23 | 13 | 0 | −13 | 28 | 17 | 1 | −16 |
| BACCPI | 11 | 10 | 0 | −10 | 1 | 1 | 1 | 0 | 6 | 5 | 13 | 8 |
| CEANTH | 1 | 0 | 0 | 0 | 1 | 1 | 0 | −1 | 2 | 1 | 1 | 0 |
| CORYCO | 146 | 9 | 9 | 0 | 146 | 9 | 17 | 8 | 478 | 51 | 49 | −2 |
| COTOFR | 18 | 0 | 3 | 3 | 21 | 4 | 0 | −4 | 22 | 4 | 1 | −3 |
| COTOPA | 36 | 4 | 0 | −4 | 32 | 1 | 2 | 1 | 35 | 1 | 4 | 3 |
| CRATMO | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| ERIOJA | - | - | - | - | - | - | - | - | 1 | 0 | 0 | 0 |
| EUCAGL | 5 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 6 | 0 | 0 | 0 |
| HEDEHE | 8 | 2 | 1 | −1 | 7 | 0 | 5 | 5 | 11 | 1 | 6 | 5 |
| HETEAR | 11 | 5 | 2 | −3 | 8 | 0 | 3 | 3 | 52 | 14 | 12 | −2 |
| ILEXAQ | 10 | 1 | 0 | −1 | 9 | 1 | 2 | 1 | 13 | 2 | 4 | 2 |
| LITHDE | 1260 | 72 | 442 | 370 | 1630 | 445 | 305 | −140 | 6164 | 1152 | 1288 | 136 |
| LONIHI | 217 | 43 | 23 | −20 | 197 | 93 | 19 | −74 | 490 | 214 | 39 | −175 |
| MORECA | 8 | 1 | 1 | 0 | 8 | 5 | 0 | −5 | 52 | 16 | 3 | −13 |
| PINUAT | 1 | 1 | 0 | −1 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| PINUPO | 17 | 7 | 0 | −7 | 10 | 3 | 0 | −3 | 15 | 3 | 1 | −2 |
| PSEUME | 2158 | 170 | 133 | −37 | 2121 | 438 | 164 | −274 | 8005 | 1492 | 778 | −714 |
| PYRAAN | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| QUERAG | 908 | 123 | 71 | −52 | 856 | 185 | 68 | −117 | 1242 | 239 | 146 | −93 |
| QUERPA | 1196 | 148 | 151 | 3 | 1202 | 258 | 236 | −22 | 3860 | 573 | 1448 | 875 |
| RHAMCA | 293 | 70 | 45 | −25 | 268 | 149 | 27 | −122 | 388 | 207 | 53 | −154 |
| RHODOC | - | - | - | - | - | - | - | - | 215 | 70 | 5 | −65 |
| RIBEDI | 1 | 0 | 0 | 0 | 1 | 1 | 0 | −1 | 3 | 1 | 1 | 0 |
| SALILA | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 4 | 1 | 1 | 0 |
| SAMBNI | 2 | 0 | 0 | 0 | 2 | 1 | 0 | −1 | 3 | 1 | 1 | 0 |
| SEQUSE | 190 | 2 | 6 | 4 | 194 | 8 | 9 | 1 | 2001 | 82 | 95 | 13 |
| TOXIDI | 675 | 176 | 139 | −37 | 638 | 349 | 42 | −307 | 1229 | 608 | 101 | −507 |
| UMBECA | 11 | 0 | 0 | 0 | 11 | 1 | 0 | −1 | 16 | 2 | 0 | −2 |
| VACCOV | 254 | 25 | 42 | 17 | 271 | 56 | 17 | −39 | 872 | 137 | 49 | −88 |
| Total | 8180 | 991 | 1080 | 89 | 8272 | 2332 | 925 | −1407 | 26,083 | 5354 | 4126 | −1228 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilbert, G.S.; Carvill, S.G.; Krohn, A.R.; Jones, A.S. Three Censuses of a Mapped Plot in Coastal California Mixed-Evergreen and Redwood Forest. Forests 2024, 15, 164. https://doi.org/10.3390/f15010164
Gilbert GS, Carvill SG, Krohn AR, Jones AS. Three Censuses of a Mapped Plot in Coastal California Mixed-Evergreen and Redwood Forest. Forests. 2024; 15(1):164. https://doi.org/10.3390/f15010164
Chicago/Turabian StyleGilbert, Gregory S., Sarah G. Carvill, Alexander R. Krohn, and Alexander S. Jones. 2024. "Three Censuses of a Mapped Plot in Coastal California Mixed-Evergreen and Redwood Forest" Forests 15, no. 1: 164. https://doi.org/10.3390/f15010164
APA StyleGilbert, G. S., Carvill, S. G., Krohn, A. R., & Jones, A. S. (2024). Three Censuses of a Mapped Plot in Coastal California Mixed-Evergreen and Redwood Forest. Forests, 15(1), 164. https://doi.org/10.3390/f15010164






