The Environmental and Genetic Controls of Increment Suggest a Limited Adaptability of Native Populations of Norway Spruce to Weather Extremes
Abstract
:1. Introduction
2. Material and Methods
2.1. Trials and Sampling
2.2. Data Analysis
3. Results
4. Discussion
4.1. Representativity of the Datasets
4.2. Weather Controls of Increment
4.3. Genetic Controls over Sensitivity of Growth
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Buras, A.; Menzel, A. Projecting tree species composition changes of European forests for 2061–2090 under RCP 4.5 and RCP 8.5 Scenarios. Front. Plant Sci. 2019, 9, 1986. [Google Scholar] [CrossRef] [PubMed]
- Klisz, M.; Buras, A.; Sass-Klaassen, U.; Puchałka, R.; Koprowski, M.; Ukalska, J. Limitations at the limit? Diminishing of genetic effects in Norway spruce provenance trials. Front. Plant Sci. 2019, 10, 306. [Google Scholar] [CrossRef] [PubMed]
- Knoke, T.; Gosling, E.; Thom, D.; Chreptun, C.; Rammig, A.; Seidl, R. Economic losses from natural disturbances in Norway spruce forests—A quantification using Monte-Carlo simulations. Ecol. Econ. 2021, 185, 107046. [Google Scholar] [CrossRef]
- Garzón, M.B.; Robson, T.M.; Hampe, A. Δ Trait SDMs: Species distribution models that account for local adaptation and phenotypic plasticity. New Phytol. 2019, 222, 1757–1765. [Google Scholar] [CrossRef] [PubMed]
- Valladares, F.; Matesanz, S.; Guilhaumon, F.; Araújo, M.B.; Balaguer, L.; Benito-Garzón, M.; Cornwell, W.; Gianoli, E.; van Kleunen, M.; Naya, D.E.; et al. The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. Ecol. Lett. 2014, 17, 1351–1364. [Google Scholar] [CrossRef] [PubMed]
- Reyer, C.; Lasch-Born, P.; Suckow, F.; Gutsch, M.; Murawski, A.; Pilz, T. Projections of regional changes in forest net primary productivity for different tree species in Europe driven by climate change and carbon dioxide. Ann. For. Sci. 2014, 71, 211–225. [Google Scholar] [CrossRef]
- Liepe, K.J.; van der Maaten, E.; van der Maaten-Theunissen, M.; Liesebach, M. High phenotypic plasticity, but low signals of local adaptation to climate in a large-scale transplant experiment of Picea abies (L.) Karst. in Europe. Front. For. Glob. Chang. 2022, 5, 804857. [Google Scholar] [CrossRef]
- Moran, E.; Lauder, J.; Musser, C.; Stathos, A.; Shu, M. The genetics of drought tolerance in conifers. New Phytol. 2017, 216, 1034–1048. [Google Scholar] [CrossRef]
- Chevin, L.-M.; Hoffmann, A.A. Evolution of phenotypic plasticity in extreme environments. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160138. [Google Scholar] [CrossRef]
- Li, Y.; Suontama, M.; Burdon, R.D.; Dungey, H.S. Genotype by environment interactions in forest tree breeding: Review of methodology and perspectives on research and application. Tree Genet. Genomes 2017, 13, 60. [Google Scholar] [CrossRef]
- Chmura, D.J.; Barzdajn, W.; Kowalkowski, W.; Guzicka, M.; Rożkowski, R. Analysis of genotype-by-environment interaction in a multisite progeny test with Scots pine for supporting selection decisions. Eur. J. For. Res. 2021, 140, 1457–1467. [Google Scholar] [CrossRef]
- Leites, L.P.; Rehfeldt, G.E.; Robinson, A.P.; Crookston, N.L.; Jaquish, B. Possibilities and limitations of using historic provenance tests to infer forest species growth responses to climate change. Nat. Resour. Model. 2012, 25, 409–433. [Google Scholar] [CrossRef]
- Meier, H.E.M.; Kniebusch, M.; Dieterich, C.; Gröger, M.; Zorita, E.; Elmgren, R.; Myrberg, K.; Ahola, M.P.; Bartosova, A.; Bonsdorff, E.; et al. Climate change in the Baltic Sea region: A summary. Earth Syst. Dyn. 2022, 13, 457–593. [Google Scholar] [CrossRef]
- Reyer, C.P.O.; Bathgate, S.; Blennow, K.; Borges, J.G.; Bugmann, H.; Delzon, S.; Faias, S.P.; Garcia-Gonzalo, J.; Gardiner, B.; Gonzalez-Olabarria, J.R.; et al. Are forest disturbances amplifying or canceling out climate change-induced productivity changes in European forests? Environ. Res. Lett. 2017, 12, 034027. [Google Scholar] [CrossRef] [PubMed]
- Lloret, F.; Keeling, E.G.; Sala, A. Components of tree resilience: Effects of successive low-growth episodes in old ponderosa pine forests. Oikos 2011, 120, 1909–1920. [Google Scholar] [CrossRef]
- Schwarz, J.; Skiadaresis, G.; Kohler, M.; Kunz, J.; Schnabel, F.; Vitali, V.; Bauhus, J. Quantifying growth responses of trees to drought—A critique of commonly used resilience indices and recommendations for future studies. Curr. For. Rep. 2020, 6, 185–200. [Google Scholar] [CrossRef]
- Nabuurs, G.J.; Verkerk, P.J.; Schelhaas, M.J.; González Olabarria, J.R.; Trasobares, A.; Cienciala, E. Climate-Smart Forestry: Mitigation Impacts in Three European Regions. 2018. Available online: https://www.efi.int/sites/default/files/files/publication-bank/2018/efi_fstp_6_2018.pdf (accessed on 14 July 2023).
- Ansarifar, J.; Akhavizadegan, F.; Wang, L. Performance prediction of crosses in plant breeding through genotype by environment interactions. Sci. Rep. 2020, 10, 11533. [Google Scholar] [CrossRef]
- Baliuckas, V.; Pliūra, A.; Eriksson, G. Forest tree breeding strategies in Nordic and Baltic countries and the possible implications on Lithuanian tree breeding strategy. Balt. For. 2004, 10, 95–103. [Google Scholar]
- Jansons, Ā.; Donis, J.; Danusevičius, D.; Baumanis, I. Differential analysis for next breeding cycle for Norway spruce in Latvia. Balt. For. 2015, 21, 285–297. [Google Scholar]
- Jansson, G.; Hansen, J.K.; Haapanen, M.; Kvaalen, H.; Steffenrem, A. The genetic and economic gains from forest tree breeding programmes in Scandinavia and Finland. Scand. J. For. Res. 2017, 32, 273–286. [Google Scholar] [CrossRef]
- Burdon, R.D.; Klápště, J. Alternative selection methods and explicit or implied economic-worth functions for different traits in tree breeding. Tree Genet. Genomes 2019, 15, 79. [Google Scholar] [CrossRef]
- Hong, Z.; Fries, A.; Wu, H.X. High negative genetic correlations between growth traits and wood properties suggest incorporating multiple traits selection including economic weights for the future Scots pine breeding programs. Ann. For. Sci. 2014, 71, 463–472. [Google Scholar] [CrossRef]
- Trujillo-Moya, C.; George, J.-P.; Fluch, S.; Geburek, T.; Grabner, M.; Karanitsch-Ackerl, S.; Konrad, H.; Mayer, K.; Sehr, E.M.; Wischnitzki, E.; et al. Drought sensitivity of Norway spruce at the species’ warmest fringe: Quantitative and molecular analysis reveals high genetic variation among and within provenances. Genes Genom. Genet. 2018, 8, 1225–1245. [Google Scholar] [CrossRef] [PubMed]
- Goude, M.; Nilsson, U.; Mason, E.; Vico, G. Using hybrid modelling to predict basal area and evaluate effects of climate change on growth of Norway spruce and Scots pine stands. Scand. J. For. Res. 2022, 37, 59–73. [Google Scholar] [CrossRef]
- MacLachlan, I.R.; Wang, T.; Hamann, A.; Smets, P.; Aitken, S.N. Selective breeding of lodgepole pine increases growth and maintains climatic adaptation. For. Ecol. Manag. 2017, 391, 404–416. [Google Scholar] [CrossRef]
- Breed, M.F.; Stead, M.G.; Ottewell, K.M.; Gardner, M.G.; Lowe, A.J. Priority actions to improve provenance decision-making. BioScience 2018, 68, 510–516. [Google Scholar] [CrossRef]
- O’neill, G.A.; Stoehr, M.; Jaquish, B. Quantifying safe seed transfer distance and impacts of tree breeding on adaptation. For. Ecol. Manag. 2014, 328, 122–130. [Google Scholar] [CrossRef]
- Xu, K.; Wang, X.; Liang, P.; An, H.; Sun, H.; Han, W.; Li, Q. Tree-ring widths are good proxies of annual variation in forest productivity in temperate forests. Sci. Rep. 2017, 7, 1945. [Google Scholar] [CrossRef]
- Zhang, Z.; Babst, F.; Bellassen, V.; Frank, D.; Launois, T.; Tan, K.; Ciais, P.; Poulter, B. Converging climate sensitivities of European forests between observed radial tree growth and vegetation models. Ecosystems 2018, 21, 410–425. [Google Scholar] [CrossRef]
- Housset, J.M.; Nadeau, S.; Isabel, N.; Depardieu, C.; Duchesne, I.; Lenz, P.; Girardin, M.P. Tree rings provide a new class of phenotypes for genetic associations that foster insights into adaptation of conifers to climate change. New Phytol. 2018, 218, 630–645. [Google Scholar] [CrossRef]
- Matisons, R.; Schneck, V.; Jansone, D.; Bāders, E.; Dubra, S.; Zeltiņš, P.; Jansons, Ā. South-eastern Baltic provenances of Scots pine show heritable weather-growth relationships. Forests 2021, 12, 1101. [Google Scholar] [CrossRef]
- McCullough, I.M.; Davis, F.W.; Williams, A.P. A range of possibilities: Assessing geographic variation in climate sensitivity of ponderosa pine using tree rings. For. Ecol. Manag. 2017, 402, 223–233. [Google Scholar] [CrossRef]
- Jetschke, G.; van der Maaten, E.; van der Maaten-Theunissen, M. Towards the extremes: A critical analysis of pointer year detection methods. Dendrochronologia 2019, 53, 55–62. [Google Scholar] [CrossRef]
- Bräuning, A.; Bolte, A.; Nabais, C.; Rossi, S.; Sass-Klaassen, U. Studying tree responses to extreme events. Front. Plant Sci. 2017, 8, N506. [Google Scholar] [CrossRef]
- Jönsson, A.M.; Lagergren, F. Effects of climate and soil conditions on the productivity and defence capacity of Picea abies in Sweden—An ecosystem model assessment. Ecol. Model. 2018, 384, 154–167. [Google Scholar] [CrossRef]
- Bosela, M.; Tumajer, J.; Cienciala, E.; Dobor, L.; Kulla, L.; Marčiš, P.; Popa, I.; Sedmák, R.; Sedmáková, D.; Sitko, R.; et al. Climate warming induced synchronous growth decline in Norway spruce populations across biogeographical gradients since 2000. Sci. Total Environ. 2021, 752, 141794. [Google Scholar] [CrossRef]
- Jyske, T.; Mäkinen, H.; Kalliokoski, T.; Nöjd, P. Intra-annual tracheid production of Norway spruce and Scots pine across a latitudinal gradient in Finland. Agric. For. Meteorol. 2014, 194, 241–254. [Google Scholar] [CrossRef]
- Matisons, R.; Elferts, D.; Krišāns, O.; Schneck, V.; Gärtner, H.; Wojda, T.; Kowalczyk, J.; Jansons, Ā. Nonlinear weather-growth relationships suggest disproportional growth changes of Norway spruce in the eastern Baltic region. Forests 2021, 12, 661. [Google Scholar] [CrossRef]
- Tierney, G.L.; Fahey, T.J.; Groffman, P.M.; Hardy, J.P.; Fitzhugh, R.D.; Driscoll, C.T. Soil freezing alters fine root dynamics in a northern hardwood forest. Biogeochemistry 2001, 56, 175–190. [Google Scholar] [CrossRef]
- Seidl, R.; Rammer, W. Climate change amplifies the interactions between wind and bark beetle disturbances in forest landscapes. Landsc. Ecol. 2017, 32, 1485–1498. [Google Scholar] [CrossRef]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, 1–55. [Google Scholar] [CrossRef]
- Gailis, A. Norway spruce provenances in Latvia. In Proceedings of the Norway Spruce Provenances and Breeding: Proceedings of IUFRO (S2.2–11) Symposium, Riga, Latvia, 14–18 September 1993; pp. 44–49. [Google Scholar]
- Zeltiņš, P.; Gailis, A.; Zariņa, I. Long-term performance of Norway spruce in two provenance trials in Latvia. Balt. For. 2021, 27, 195. [Google Scholar] [CrossRef]
- Cavin, L.; Jump, A.S. Highest drought sensitivity and lowest resistance to growth suppression are found in the range core of the tree Fagus sylvatica L. not the equatorial range edge. Glob. Chang. Biol. 2017, 23, 362–379. [Google Scholar] [CrossRef]
- van der Maaten-Theunissen, M.; Trouillier, M.; Schwarz, J.; Skiadaresis, G.; Thurm, E.A.; van der Maaten, E. pointRes 2.0: New functions to describe tree resilience. Dendrochronologia 2021, 70, 125899. [Google Scholar] [CrossRef]
- Harris, I.; Osborn, T.J.; Jones, P.; Lister, D. Version 4 of the CRU TS monthly high-resolution gridded multivariate climate dataset. Sci. Data 2020, 7, 1–18. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A multiscalar drought index sensitive to global warming: The standardized precipitation evapotranspiration index—SPEI. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Falconer, D.S.; Mackay, T.F.C. Introduction to Quantitative Genetics, 4th ed.; Longmans Green: Harlow, UK, 1996; 635p. [Google Scholar]
- Loha, A.; Tigabu, M.; Teketay, D.; Lundkvist, K.; Fries, A. Provenance variation in seed morphometric traits, germination, and seedling growth of Cordia africana Lam. New For. 2006, 32, 71–86. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 17 December 2023).
- Bunn, A.G. A dendrochronology program library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- Speer, J.H. Fundamentals of Tree-Ring Research; The University of Arizona Press: Tucson, AZ, USA, 2010; 333p. [Google Scholar]
- Cuny, H.E.; Fonti, P.; Rathgeber, C.B.K.; Von Arx, G.; Peters, R.L.; Frank, D.C. Couplings in cell differentiation kinetics mitigate air temperature influence on conifer wood anatomy. Plant Cell Environ. 2019, 42, 1222–1232. [Google Scholar] [CrossRef]
- Socha, J.; Solberg, S.; Tymińska-Czabańska, L.; Tompalski, P.; Vallet, P. Height growth rate of Scots pine in Central Europe increased by 29% between 1900 and 2000 due to changes in site productivity. For. Ecol. Manag. 2021, 490, 119102. [Google Scholar] [CrossRef]
- Milesi, P.; Berlin, M.; Chen, J.; Orsucci, M.; Li, L.; Jansson, G.; Karlsson, B.; Lascoux, M. Assessing the potential for assisted gene flow using past introduction of Norway spruce in southern Sweden: Local adaptation and genetic basis of quantitative traits in trees. Evol. Appl. 2019, 12, 1946–1959. [Google Scholar] [CrossRef]
- Strand, M.; Löfvenius, M.O.; Bergsten, U.; Lundmark, T.; Rosvall, O. Height growth of planted conifer seedlings in relation to solar radiation and position in Scots pine shelterwood. For. Ecol. Manag. 2006, 224, 258–265. [Google Scholar] [CrossRef]
- Matisons, R.; Krišāns, O.; Jansons, Ā.; Kondratovičs, T.; Elferts, D.; Ievinsh, G. Norway spruce seedlings from an Eastern Baltic provenance show tolerance to simulated drought. Forests 2021, 12, 82. [Google Scholar] [CrossRef]
- Prendin, A.L.; Mayr, S.; Beikircher, B.; von Arx, G.; Petit, G. Xylem anatomical adjustments prioritize hydraulic efficiency over safety as Norway spruce trees grow taller. Tree Physiol. 2018, 38, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Ögren, E. Relationship between temperature, respiratory loss of sugar and premature dehardening in dormant Scots pine seedlings. Tree Physiol. 1997, 17, 47–51. [Google Scholar] [CrossRef]
- Matisons, R.; Jansone, D.; Elferts, D.; Adamovičs, A.; Schneck, V.; Jansons, Ā. Plasticity of response of tree-ring width of Scots pine provenances to weather extremes in Latvia. Dendrochronologia 2019, 54, 1–10. [Google Scholar] [CrossRef]
- Klesse, S.; DeRose, R.J.; Guiterman, C.H.; Lynch, A.M.; O’connor, C.D.; Shaw, J.D.; Evans, M.E.K. Sampling bias overestimates climate change impacts on forest growth in the southwestern United States. Nat. Commun. 2018, 9, 5336. [Google Scholar] [CrossRef] [PubMed]
- Neale, D.B.; Martínez-García, P.J.; De La Torre, A.R.; Montanari, S.; Wei, X.-X. Novel insights into tree biology and genome evolution as revealed through genomics. Annu. Rev. Plant Biol. 2017, 68, 457–483. [Google Scholar] [CrossRef] [PubMed]
- Schaberg, P.G.; DeHayes, D.H.; Hawley, G.J.; Nijensohn, S.E. Anthropogenic alterations of genetic diversity within tree populations: Implications for forest ecosystem resilience. For. Ecol. Manag. 2008, 256, 855–862. [Google Scholar] [CrossRef]
- de la Mata, R.; Zas, R.; Bustingorri, G.; Sampedro, L.; Rust, M.; Hernandez-Serrano, A.; Sala, A. Drivers of population differentiation in phenotypic plasticity in a temperate conifer: A 27-year study. Evol. Appl. 2022, 15, 1945–1962. [Google Scholar] [CrossRef] [PubMed]
- Isaac-Renton, M.; Montwé, D.; Hamann, A.; Spiecker, H.; Cherubini, P.; Treydte, K. Northern forest tree populations are physiologically maladapted to drought. Nat. Commun. 2018, 9, 5254. [Google Scholar] [CrossRef] [PubMed]
- Zeltiņš, P.; Katrevičs, J.; Gailis, A.; Maaten, T.; Desaine, I.; Jansons, Ā. Adaptation capacity of Norway spruce provenances in Western Latvia. Forests 2019, 10, 840. [Google Scholar] [CrossRef]
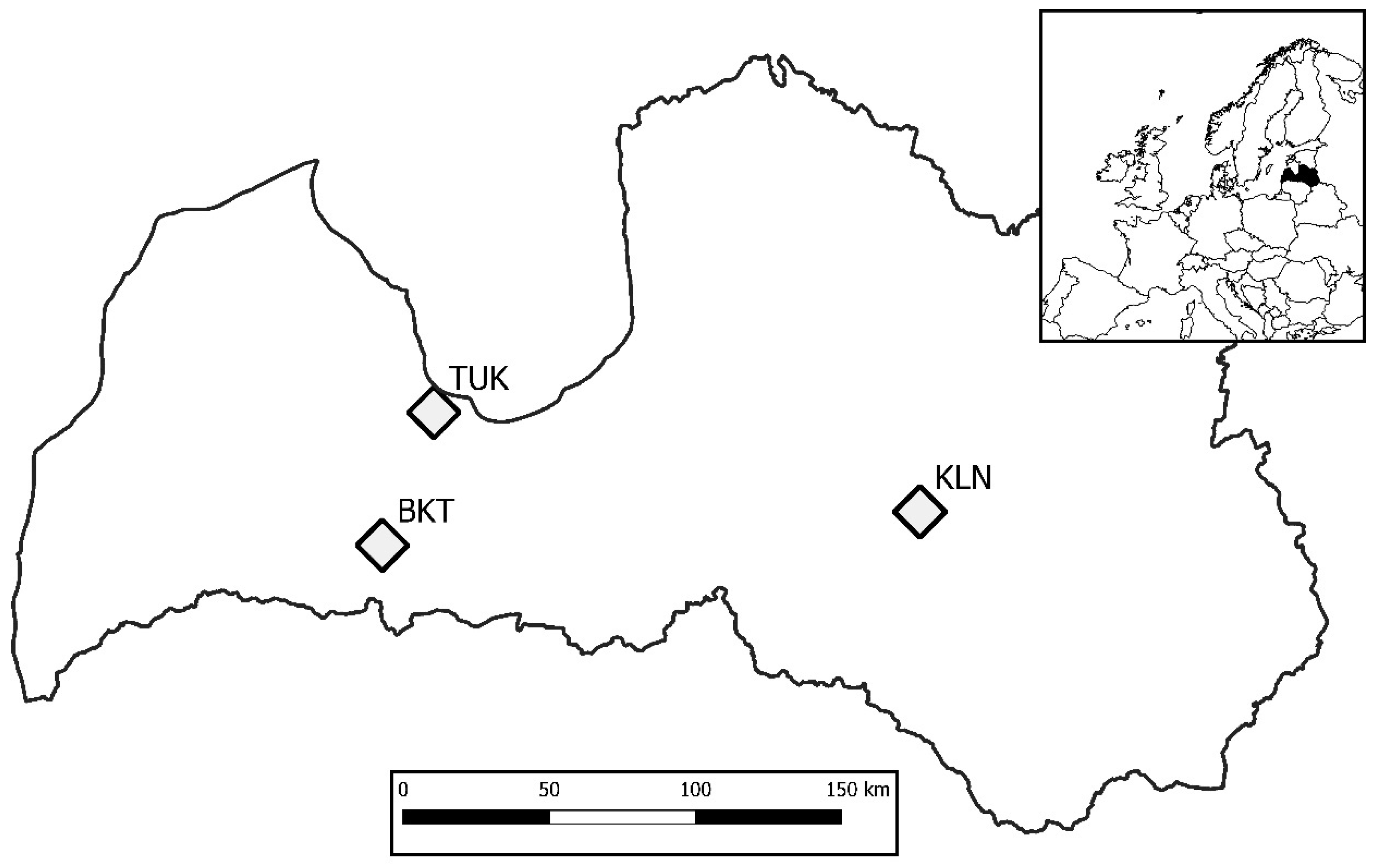


| Trial | Kalsnava (KLN) | Tukums (TUK) | Biksti (BKT) |
|---|---|---|---|
| Number of clones | 19 | 20 | 77 |
| Timespan of series | 1969–2015 | 1971–2015 | 1981–2018 |
| Total number of cross-dated trees | 211 | 335 | 629 |
| Number of ramets (trees) per clone | 11.1 (6–18) | 16.7 (7–24) | 8.3 (5–22) |
| Mean tree ring width, mm | 4.00 (3.58–4.91) | 4.62 (3.74–5.44) | 5.68 (4.22–7.02) |
| Standard deviation in tree ring width, mm | 1.61 (1.28–2.16) | 1.49 (1.21–1.76) | 2.00 (1.33–2.99) |
| Mean sensitivity of time series | 0.22 (0.19–0.28) | 0.25 (0.21–0.29) | 0.23 (0.17–0.29) |
| Gini coefficient of time series | 0.13 (0.10–0.16) | 0.14 (0.12–0.15) | 0.12 (0.09–0.17) |
| First-order autocorrelation of time series | 0.13 (0.10–0.16) | 0.14 (0.12–0.15) | 0.12 (0.09–0.17) |
| Mean interseries correlation (r-bar) | 0.40 (0.22–0.57) | 0.48 (0.31–0.62) | 0.50 (0.20–0.68) |
| Expressed Population Signal (EPS) | 0.87 (0.75–0.94) | 0.93 (0.81–0.97) | 0.85 (0.82–0.97) |
| Signal-to-noise ratio (SNR) | 8.02 (3.00–17.25) | 16.21 (4.07–32.45) | 9.02 (3.73–38.06) |
| Year | Pointer Year | Almanack | Gridded Data, Monthly Variables |
|---|---|---|---|
| 1974 | neg., KLN | Cold and heat records in winter and summer | Prec. spring (−2.34) |
| 1975 | neg., KLN | Warmth records in winter, contrasting spring temp., dry summer | MAT (2.49), SPEI Mar. (−2.41) |
| 1978 | pos., TUK | Low temp. records in spring and summer, moist summer | Prec. Aug. (2.14) |
| 1980 | pos., TUK, KLN | Low. temp. records in winter and spring, cool spring, moist summer | Prec. prev. Jul. (2.19), SPEI Aug. (2.46) |
| 1981 | pos., KLN | Cold records in spring, heat records in summer, warm and moist summer | Prec. Mar. (2.17), prec. Jun. (2.25) |
| 1990 | pos., BKT, TUK | Warmth records in winter, warm winter, spring | Temp. Feb. (2.14), temp. Mar. (2.10), temp. winter (2.68) |
| 1993 | pos., BKT, TUK | Low temp. records in winter and summer, cold year | Temp. prev. Oct. (−2.31), temp. May (2.34), SPEI Mar. (2.04) |
| 1995 | pos., BKT | Warmth records in spring and summer, contrasting summer temp. | Temp. prev. Jul. (2.32), prec. prev. Jul. (−2.11) |
| 1998 | neg., KLN | Warmth records in winter, moist summer | Temp. prev. Aug. (2.46), prec. Jun. (2.1), prec. veg. seas. (2.07) |
| 2000 | neg., KLN | Warmth records in winter, spring, and summer | Temp. prev. Jun. (2.29), temp. Apr. (2.37), SPEI. Jun. (2.06) |
| 2006 | neg. | Cold records in winter, heat records in July | - |
| 2007 | neg., BKT, TUK | Temp. contrasts in winter and spring | Temp. prev. Dec. (2.26), temp Mar. (2.43), prec. Jan. (2.33) |
| 2014 | neg. | Warmth record in spring, contrasting temp. May, heat records in summer, warm and dry summer | SPEI. prev. Sep. (2.06), SPEI Jan. (2.30), SPEI. veg. seas (−2.55) |
| 2017 | neg., BKT | Warmth records in winter and spring, low temp. records in summer, warm winter, cool summer. | Prec. Sep. (2.26) |
| 2018 | neg., BKT | Heat records in spring and summer, dry and warm summer | Temp. May (2.51), temp. summer (2.34) |
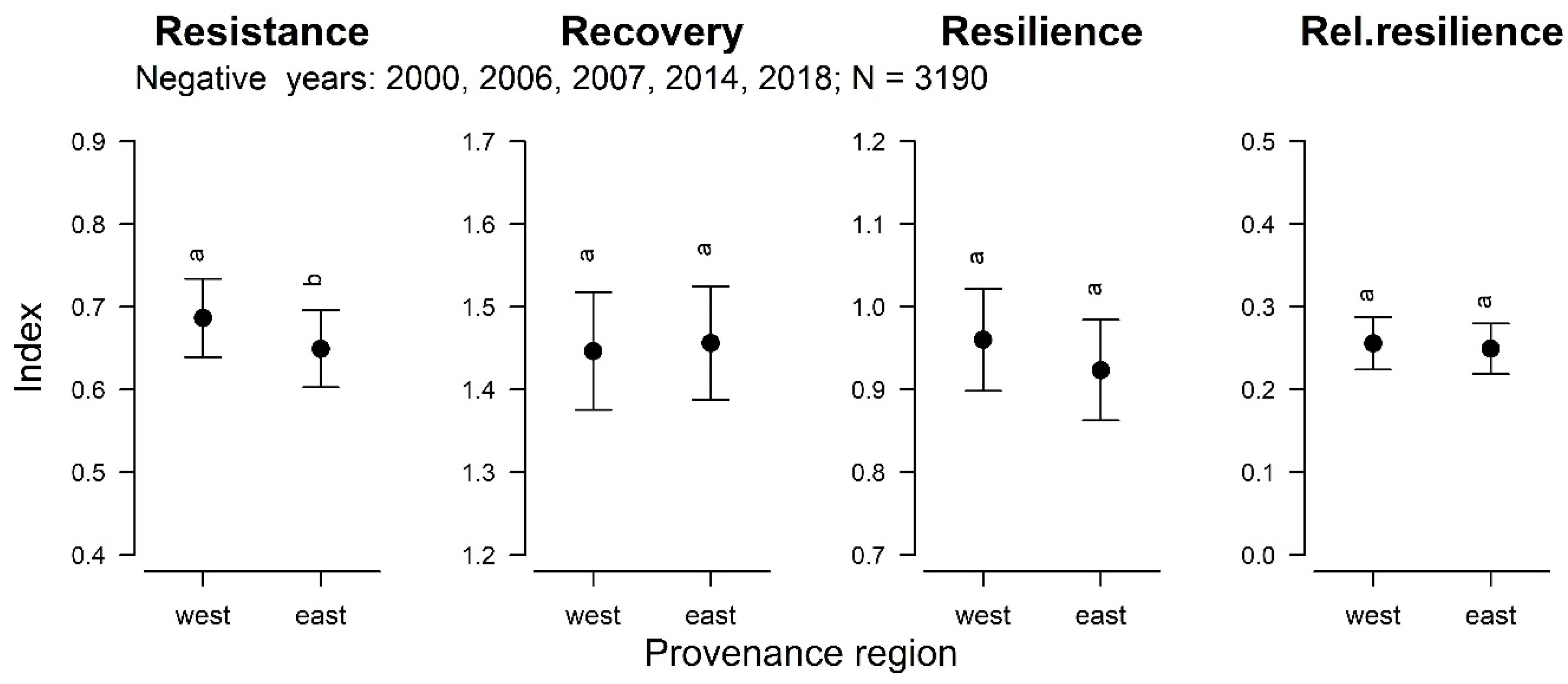
| Resistance | Recovery | Resilience | Relative Resilience | |||||
|---|---|---|---|---|---|---|---|---|
| Fixed effects | ||||||||
| χ2 | p-value | χ2 | p-value | χ2 | p-value | χ2 | p-value | |
| Local provenance | 4.7 | <0.05 | 0.1 | 0.82 | 2.8 | 0.09 | 0.1 | 0.74 |
| Stem diameter at breast height | 38.7 | <0.05 | 56.4 | <0.05 | 0.1 | 0.81 | 32.2 | <0.05 |
| Random effects, variance | ||||||||
| Tree | 0.0010 | 0.0001 | 0.0355 | 0.0076 | ||||
| Clone | 0.0022 | 0.0105 | 0.0013 | 0.0023 | ||||
| Provenance | 0.0001 | 0.0001 | 0.0001 | 0.0001 | ||||
| Year | 0.0029 | 0.0082 | 0.0023 | 0.0007 | ||||
| Trial | 0.0045 | 0.0035 | 0.0083 | 0.0014 | ||||
| Residual | 0.0353 | 0.2224 | 0.0156 | 0.0419 | ||||
| Model statistic, R2 | ||||||||
| Marginal | 0.018 | 0.025 | 0.005 | 0.017 | ||||
| Conditional | 0.245 | 0.114 | 0.753 | 0.238 | ||||
| Relative Changes | Resistance | Recovery | Resilience | Relative Resilience | |
|---|---|---|---|---|---|
| Variance components | |||||
| Clone-trial-year interaction | 0.0007 | 0.0178 | 0.0468 | 0.0001 | 0.0026 |
| Clone-year interaction | 0.0035 | 0.0164 | 0.0001 | 0.0221 | 0.0261 |
| Clone | 0.0002 | 0.0001 | 0.0001 | 0.0016 | 0.0001 |
| Residual | 0.0642 | 0.0680 | 0.1339 | 0.1260 | 0.0857 |
| Heritability estimates | |||||
| H2 | 0.0035 | 0.0001 | 0.0001 | 0.0104 | 0.0001 |
| CCV | 0.0160 | 0.0001 | 0.0001 | 0.0401 | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matisons, R.; Katrevičs, J.; Zeltiņš, P.; Jansone, D.; Jansons, Ā. The Environmental and Genetic Controls of Increment Suggest a Limited Adaptability of Native Populations of Norway Spruce to Weather Extremes. Forests 2024, 15, 15. https://doi.org/10.3390/f15010015
Matisons R, Katrevičs J, Zeltiņš P, Jansone D, Jansons Ā. The Environmental and Genetic Controls of Increment Suggest a Limited Adaptability of Native Populations of Norway Spruce to Weather Extremes. Forests. 2024; 15(1):15. https://doi.org/10.3390/f15010015
Chicago/Turabian StyleMatisons, Roberts, Juris Katrevičs, Pauls Zeltiņš, Diāna Jansone, and Āris Jansons. 2024. "The Environmental and Genetic Controls of Increment Suggest a Limited Adaptability of Native Populations of Norway Spruce to Weather Extremes" Forests 15, no. 1: 15. https://doi.org/10.3390/f15010015
APA StyleMatisons, R., Katrevičs, J., Zeltiņš, P., Jansone, D., & Jansons, Ā. (2024). The Environmental and Genetic Controls of Increment Suggest a Limited Adaptability of Native Populations of Norway Spruce to Weather Extremes. Forests, 15(1), 15. https://doi.org/10.3390/f15010015







