Coppicing Abilities of Decapitated Elite Tree Trunks of Selected Acacia Species Genotypes in Mixed-Species Plantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Plant Material
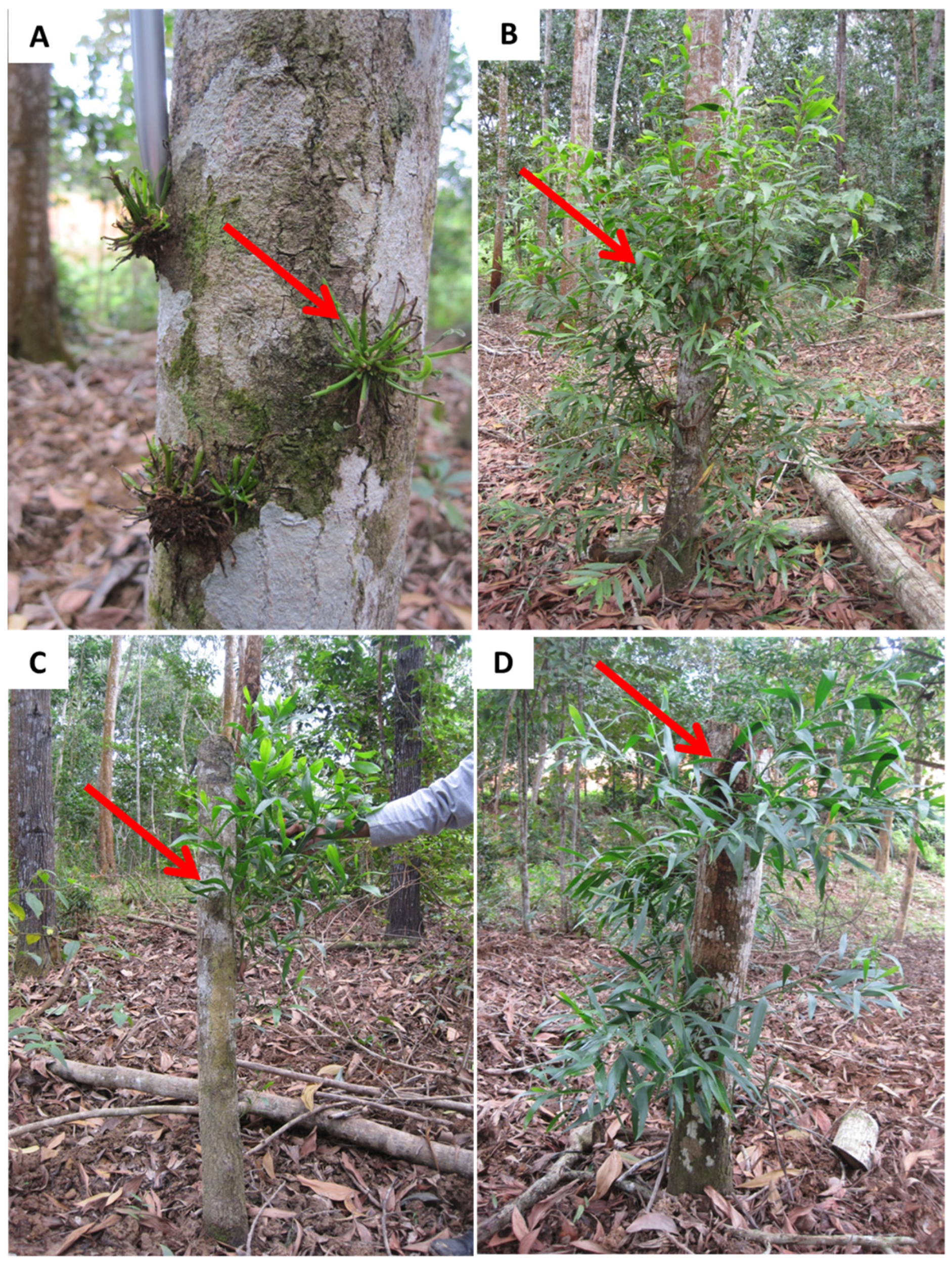
2.3. Shoot Sprout of Stumping/Epicormic/Coppicing
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greenwood, M.S. Rejuvenation of forest trees. Plant Growth Regul. 1987, 6, 1–12. [Google Scholar] [CrossRef]
- Poethig, R.S. Phase change and the regulation of shoot morphogenesis in plants. Science 1990, 250, 923–930. [Google Scholar] [CrossRef]
- Franclet, A.; Boulay, M.; Bekkaoui, F.; Fouret, Y.; Verschoore-Martouzet, B.; Walker, N. Rejuvenation. In Cell and Tissue Culture in Forestry; Bonga, J.M., Durzan, D.J., Eds.; Martinus Nijhoff Publishers: Dordrecht, The Netherlands; Boston, MA, USA; Lancaster, UK, 1987; Volume 1, pp. 232–248. [Google Scholar]
- Bonga, J.M. Vegetative propagation in relation to juvenility, maturity, and rejuvenation. In Tissue Culture in Forestry; Bonga, J.M., Durzan, D.J., Eds.; Martinus Nijhoff: The Hague, The Netherlands; Dr. W. Junk: The Hague, The Netherlands, 1982; pp. 387–412. [Google Scholar]
- Bonga, J.M.; von Aderkas, P. Rejuvenation of tissues from mature conifers and its implications for clonal propagation in vitro. In Clonal Forestry: Genetics, Biotechnology and Application; Ahuja, M.R., Libby, W.J., Eds.; Springer: New York, NY, USA, 1993; pp. 182–199. [Google Scholar]
- Read, P.E.; Bavougian, C.M. In vitro rejuvenation of woody species. In Protocols for Micropropagation of Selected Economically-Important Horticultural Plants; Springer: Berlin/Heidelberg, Germany, 2013; pp. 383–395. [Google Scholar] [CrossRef]
- Koguta, K.V.; Flores, P.C.; Alcantara, G.B.; Higa, A.R. Serial micropropagation and cuttings as rejuvenation methods for Cryptomeria japonica (L.f) D.Don. Rev. Espac. 2017, 38, 7. [Google Scholar]
- Zhang, Z.; Sun, Y.; Li, Y. Plant rejuvenation: From phenotypes to mechanisms. Plant Cell Rep. 2020, 39, 1249–1262. [Google Scholar] [CrossRef] [PubMed]
- Muniandi, S.K.; Muhammad, N.; Md Ariff, F.F.; Taheri, Y. Improved clonal propagation through rejuvenation of mature branch cutting of four important Acacia species. Forests 2022, 13, 1403. [Google Scholar] [CrossRef]
- Beck, S.L.; Dunlop, R.; Van Staden, J. Rejuvenation and micropropagation of adult Acacia mearnsii using coppice material. Plant Growth Regul. 1998, 26, 149–153. [Google Scholar] [CrossRef]
- Haliza, I.; Ahmad Fauzi, M.S.; Siti Suhaila, A.R.; Nor Hasnida, H.; Nazirah, A.; Muhd Fuad, Y. In vitro propagation of Khaya ivorensis from coppiced shoots. J. Trop. For. Sci. 2014, 26, 298–301. [Google Scholar]
- Chang, S.H.; Donald, D.G.M.; Jacobs, G. Micropropagation of Eucalyptus radiata ssp. radiata using explants from mature and coppice material. South Afr. For. J. 1992, 162, 43–47. [Google Scholar] [CrossRef]
- Singh, S. Clonal Multiplication of Teak (Tectona grandis L.f.). In Clonal Forestry—Principles and Practices; Narendra Publishing House: New Delhi, India, 2023; pp. 101–120. ISBN 9789356512276. [Google Scholar]
- Del Tredici, P. Sprouting in temperate trees: A morphological and ecological review. Bot. Rev. 2001, 67, 121–140. [Google Scholar] [CrossRef]
- Sakai, A.; Sakai, S.; Ohsawa, T.; Ohsawa, M. Adaptive significance of sprouting of Eupteleapolyandra, a deciduous tree growing on steep slopes with shallow soil. J. Plant Res. 1995, 108, 377–386. [Google Scholar] [CrossRef]
- Sakai, A.; Sakai, S.; Akiyama, F. Do sprouting tree species on erosion-prone sites carry large reserves of resources? Ann. Bot. 1997, 79, 625–630. [Google Scholar] [CrossRef]
- Sakai, A.; Sakai, S. A test for the resource remobilization hypothesis: Tree sprouting using carbohydrates from above-ground parts. Ann. Bot. 1998, 82, 213–216. [Google Scholar] [CrossRef]
- Pate, J.S.; Froend, R.H.; Bowen, B.J.; Hansen, A.; Kuo, J. Seedling growth and storage characteristics of seeder and resprouter species of mediterranean-type ecosystems of S.W. Australia. Ann. Bot. 1990, 65, 585–601. [Google Scholar] [CrossRef]
- Canadell, J.; Zedler, P.H. Underground structures of woody plants in Mediterranean ecosystems of Australia, California, and Chile. In Ecology and Biogeography of Mediterranean Ecosystems in Chile, California, and Australia; Kalin Arroya, M.T., Zedler P., H., Fox, M.D., Eds.; Springer: New York, NY, USA, 1994; pp. 177–210. [Google Scholar]
- Bellingham, P.J.; Sparrow, A.D. Resprouting as a life history strategy in woody plant communities. Oikos 2000, 89, 409–416. [Google Scholar] [CrossRef]
- Diller, O.D.; Marshall, E.D. The relation of stump height to the sprouting of Ostrya virginiana in northern Indiana. J. For. 1937, 35, 1116–1119. [Google Scholar]
- Borchert, R. The concept of juvenility in woody plants. Acta Hort. 1976, 56, 21–36. [Google Scholar] [CrossRef]
- Fontanler, E.J.; Jonkers, H. Juvenility and maturity of plants as influenced by their ontogenetical and physiological aging. Acta Hort. 1976, 56, 37–44. [Google Scholar]
- Roth, E.R.; Hepting, G.H. Prediction of butt rot in newly regenerated sprout oak stands. J. For. 1969, 67, 756–760. [Google Scholar]
- Johnson, P.S. Growth and structural development of red oak sprout clumps. For. Sci. 1975, 21, 413–418. [Google Scholar]
- Burns, R.M.; Honkala, B.H. (Eds.) Silvics of North America; U.S. Forest Service Handbooks; US Department of Agriculture, Forest Service: Washington, DC, USA, 1990; 2 Volumes; p. 654.
- Cook, J.E.; Sharik, T.L. Oak regeneration in the southern Appalachians: Potential, problems, and possible solutions. Southern J. Appl. For. 1998, 22, 11–18. [Google Scholar] [CrossRef]
- Ahirwar, J.R. The study on coppicing capacity of Cordia myxa (Lasora). Int. Res. J. Biol. Sci. 2014, 3, 48–50. [Google Scholar]
- Solomon, D.S.; Blum, B.M. Stump Sprouting of Four Northern Hardwoods; U.S. Forest Service Research Paper NE-59; Northeastern Forest Experiment Station, Forest Service, US Department of Agriculture: Washington, DC, USA, 1967.
- Sander, I.L. Height growth of new oak sprouts depends on size of advance reproduction. J. For. 1971, 69, 809–811. [Google Scholar]
- Wendel, G.W. Stump Sprout Growth and Quality of Several Appalachian Hardwood Species after Clear Cutting; U.S. Forest Service Research Paper NE-329; Northeastern Forest Experiment Station, Forest Service, US Department of Agriculture: Washington, DC, USA, 1975.
- Johnson, P.S. Predicting Oak Stump Sprouting and Sprout Development in the Missouri Ozarks; U.S. Forest Service Research Paper NC-149; Northeastern Forest Experiment Station, Forest Service, US Department of Agriculture: Washington, DC, USA, 1978.
- MacDonald, J.E.; Powell, G.R. Relationship between stump sprouting and parent-tree diameter in sugar maple in the first year following clear-cutting. Canad. J. For. Res. 1983, 13, 390–394. [Google Scholar] [CrossRef]
- Ahmad, D.H.; Thompson, S.; Pirrie, A. Vegetative Propagation of Acacia mangium by Stem Cutting: The Effect of Seedling Age and Phyllode Number on Rooting. J. Trop For. Sci. 1990, 2, 274–279. [Google Scholar]
- Radhi, I.M.; Hussein, K.A. The Role of Ascorbic Acid and IBA in Improvement of Rooting Response of Difficult to Root Cuttings of Acacia spp. In Proceedings of the 8th International Conference on Applied Science and Technology (ICAST 2020), Karbala, Iraq, 15–16 April 2020; AIP Conference Proceedings. AIP Publishing: College Park, MD, USA, 2020; pp. 020003-1–020003-5. [Google Scholar]
- Hu, T.W.; Shen, T.A. Vegetative propagation of Acacia auriculiformis by leafy cuttings under mist spray. Nitrogen Fixing Tree Res. Rep. 1986, 4, 44–45. [Google Scholar]
- Kumar, M.; Parameswari, N.; Chin, C.F.; Baharum, Z.; Olalekan, K.; Nor Aini, A. Selection and screening of superior genotypes for quality planting stock based on vegetative growth performance of some selected 12-year-old Acacia species. Open J. For. 2016, 6, 217–229. [Google Scholar] [CrossRef][Green Version]
- IBM Corp. IBM SPSS Statistics for Windows, version 26; IBM Corp.: Armonk, NY, USA, 2019. [Google Scholar]
- Harwood, C. Strengthening the tropical Acacia plantation value chain: The role of research. J. Trop. For. Sci. 2011, 23, 1–3. [Google Scholar]
- Griffin, A.; Kumar, S.M.; Shukor, N.A.A. In Vitro Regeneration of Acacia crassicarpa A. Cunn Ex Benth through Organogenesis from Juvenile Sources. J. Food Agric. Environ. 2014, 12, 375–382. [Google Scholar]
- Ismail, H.; Muniandi, S.K.; Mohd Yusoff, A.; Hasnida, N.; Ab Shukor, N. In Vitro micropropagation of Acacia auriculiformis from selected juvenile sources. Dendrobiology 2016, 75, 157–165. [Google Scholar] [CrossRef]
- Ooyama, N. Studies on Promotion of Rooting Ability of the Cutting from Tree Species Difficult to Root; Bulletin Government Forest Experimental Station: Meguro, Tokyo, 1962; Volume 145, pp. 1–141. [Google Scholar]
- Hess, C.E. Internal and external factors regulating root initiation. In Root Growth; Whittington, J.W., Ed.; William Clove & Sons, Ltd.: London, UK, 1968; pp. 42–45. [Google Scholar]
- Ramasamy, Y.; Ramasamy, S.; Gurumurthi, K. Micropropagation for quality propagule production in plantation forestry. Indian J. Biotechnol. 2004, 3, 159–170. [Google Scholar]
- Eldoma, A.; Muniandi, S.K.; Ab Shukor, N. Stimulation of Multiple Leader Formation in Some Genotypes of Acacia mangium and Acacia auriculiformis with 6-Benzylaminopurine (BAP). Open J. For. 2015, 5, 637–650. [Google Scholar]
- Savill, P.S. Silviculture: Silvicultural Systems. In Encyclopedia of Forest Sciences; Burley, J., Ed.; Elsevier: Oxford, UK, 2004; pp. 1003–1011. [Google Scholar] [CrossRef]
- Ryan, P.A.; Bell, R.E. Growth, coppicing and flowering of Australian tree species in trials in southeast Queensland, Australia. In Trees for the Tropics; Boland, D.J., Ed.; ACIAR: Canberra, Australia, 1989; pp. 49–68. [Google Scholar]
- Rinaudo, T. Utilizing the underground forest. Farmer managed natural regeneration of trees. In Combating Desertification with Plants; Pasternak, D., Schlissel, A., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2001; pp. 325–336. [Google Scholar]
- Larwanou, M.; Abdoulaye, M.; Reij, C. Etude de la Regeneration Naturelle Assistee Dans la Region de Zinder (Niger); United States Agency for International Development (USAID/EGAT), International Resources Group: Washington, DC, USA, 2006.
- Maslin, B.R.; McDonald, M.W. A Key to Useful Australian Acacias for the Seasonally Dry Tropics; CSIRO Forestry and Forest Products: Canberra, Australia, 1996; p. 80. [Google Scholar]
- McDonald, M.W. Revision of Acacia tumida (Leguminoseae: Mimosoideae) and close allies, including the description of three rare taxa. Aust. Syst. Bot. 2003, 16, 139–164. [Google Scholar] [CrossRef]
- Rockwood, D.L.; Geary, T.F. Genetic variation in biomass productivity and coppicing of intensively grown Eucalyptus grandis in southern Florida. In Proceedings of the 7th North American Forest Biology Workshop, Lexington, KY, USA, 26–28 July 1982; pp. 400–405. [Google Scholar]
- Reddy, K.V.; Rockwood, D.L. Breeding strategies for coppice production in a Eucalyptus grandis base population with four generation of selection. Silvae Genet. 1989, 38, 3–4. [Google Scholar]
- Ducrey, M.; Turrel, M. Influence of cutting methods and dates on stump sprouting in Holm oak (Quercus ilex L) coppice. Ann. Sci. For. 1992, 49, 449–464. [Google Scholar] [CrossRef]
- Johansson, T. Sprouting of 10- to 50-year-old Betula pubescens in relation to felling time. For. Ecol. Manag. 1992, 53, 283–296. [Google Scholar] [CrossRef]
- Gardiner, E.S.; Helmig, L.M. Development of water oak stump sprouts under a partial overstory. New For. 1997, 14, 55–62. [Google Scholar] [CrossRef]
- Randall, C.K.; Duryea, M.L.; Vince, S.W.; English, R.J. Factors influencing stump sprouting by pondcypress (Taxodium distichum var. nutans (Ait.) Sweet). New For. 2005, 29, 245–260. [Google Scholar] [CrossRef]
- Luoga, J.E.; Witkowski, E.T.F.; Balkwill, K. Regeneration by coppicing (resprouting) of miombo (African savanna) trees in relation to land use. For. Ecol. Manag. 2004, 189, 23–35. [Google Scholar] [CrossRef]
- Wu, L.; Shinzato, T.; Chen, C.; Aramoto, M. Sprouting characteristics of a subtropical evergreen broad-leaved forest following clear-cutting in Okinawa, Japan. New For. 2008, 36, 239–246. [Google Scholar] [CrossRef]
- Atwood, C.J.; Fox, T.R.; Loftis, D.L. Effects of alternative silviculture on stump sprouting in the southern Appalachians. For. Ecol. Manag. 2009, 257, 1305–1313. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, W.; Zhou, J.; Ma, C.; Ma, L. Effects of stump diameter, stump height, and cutting season on Quercus variabilis stump sprouting. Scan. J. For. Res. 2013, 28, 223–231. [Google Scholar] [CrossRef]
- Shackleton, C.M. Managing regrowth of an indigenous savanna tree species (Terminalia sericea) for fuelwood: The influence of stump dimensions and post-harvest coppice pruning. Biomass Bioenergy 2001, 20, 261–270. [Google Scholar] [CrossRef]
- Kaschula, S.A.; Twine, W.C.; Scholes, M.C. The effect of catena position and stump characteristics on the coppice response of three savannah fuelwood species. Environ. Conserv. 2005, 32, 76–84. [Google Scholar] [CrossRef]
- Orwa, C.; Mutua, A.; Kindt, R.; Jamnadass, R.; Simons, A. Agroforestree Database: A Tree Reference and Selection Guide, version 4.0; World Agroforestry Centre ICRAF: Nairobi, Kenya, 2009. [Google Scholar]
- Selby, C.; Watson, S.; Harvey, B.M.R. Morphogenesis in Sitka spruce (Picea sitchensis (Bong.) Carr.) bud cultures—Tree maturation and explants from epicormic shoots. Plant Cell Tissue Organ Cult. 2005, 83, 279–285. [Google Scholar] [CrossRef]
- Banik, R.L.; Islam, S.A.M.N. In vitro clonal propagation of hybrid Acacia (A. auriculiformis × A. mangium). Bangladesh J. For. Sci. 1997, 25, 1–7. [Google Scholar]
- Trindade, H.; Pais, M.S. In Vitro Studies on Eucalyptus globulus Rooting Ability. In Vitro Cell. Dev. Biol.-Plant 1997, 33, 1–5. [Google Scholar] [CrossRef]
| Species | CSIRO Section No | Provenances | Origin | Lat (0° S) | Long (0° E) | Alt (m) |
|---|---|---|---|---|---|---|
| A. mangium | 18,249 | Captain Billy Road (CBR) | QLD | 11° 41 | 142° 42 | 100 |
| 18,767 | Russell & Gap CK (R&GCK) | QLD | 15° 52 | 145° 19 | 60 | |
| 17,550 | Bansbech (B) | PNG | 85° 03 | 141° 17 | 25 | |
| 18,194 | SW of Boset WP (SWBWP) | PNG | 71° 07 | 141° 05 | 100 | |
| A. auriculiformis | 17,966 | Buggy Creek (BC) | QLD | 15° 52 | 144° 53 | 240 |
| 18,247 | Wenlock River (WR) | QLD | 13° 05 | 142° 51 | 120 | |
| 18,924 | Mibini (M) | PNG | 85° 00 | 141° 38 | 18 | |
| 18,932 | Bansbach (B) | PNG | 85° 03 | 141° 17 | 25 | |
| A. crassicarpa | 17,944 | Claudie River (CR) | QLD | 12° 48 | 143° 18 | 20 |
| 17,948 | Chilli Beach (CR) | QLD | 12° 38 | 143° 23 | 3 | |
| 18,940 | Bimadebum WP (BMWP) | PNG | 83° 08 | 142° 03 | 40 | |
| 18,947 | Bensbach WP (BWP) | PNG | 85° 03 | 141° 17 | 25 | |
| A. aulococarpa | 17,739 | 3K S Mt Larcom (3KSML) | QLD | 23° 05 | 151° 00 | 70 |
| 17,891 | Samford (S) | QLD | 27° 17 | 152° 51 | 50 | |
| 16,112 | W Morehead (WM) | PNG | 84° 02 | 141° 34 | 30 | |
| 16,995 | Arufi E Morehead WP (AEMWP) | PNG | 84° 03 | 141° 55 | 25 |
| No | Selection Criteria | Description |
|---|---|---|
| 1 | Diameter at breast height | Diameter of the tree stem at breast height (1.3 m above the ground) |
| 2 | Height | Total height of the tree from above the ground to the top of the tree |
| 3 | Clear bole height/merchantable height | Height of the tree from the ground to the first branch of the crown |
| 4 | Crown size | Diameter of the crown (length from outermost branch of the crown from one end to the other on ground level) |
| 5 | Number of stem | Number of stems produced from the base of the tree trunk |
| 6 | The bole form | score categories (six-point score)—six (circular/round in cross section) to one (severe flute and excessive taper) |
| 7 | The stem straightness | Score categories (six-point score)—six (very straight) to one (crooked with severe bends and kinks) |
| 8 | The forking ability | Score categories (six-point score)—six (single stem), five (fork > 6 m), four (fork at 4–6 m), three (fork at 2–4 m), two (fork < 2 m), and one (multiple leader) |
| 9 | The branch size (BS) | Score categories (four-point score)—four (<1/4 of the main stem), three (1/4 to 1/2 of the main stem), two (between 1/2 and 3/4 of the main stem), and one (between 3/4 and 1 of the main stem) |
| 10 | The branch angle (BA) | Score categories (four-point score)—four (angle between 65° and 90° to the main stem), three (angle between 45° and 65° to the main stem), two (angle between 25° and 45° to the main stem), and one (angle < 25° to the main stem) |
| Species | Source of Variation | df | SP | SN | SL | SG | NL |
|---|---|---|---|---|---|---|---|
| Acacia mangium | Provenance (P) | 3 | 0.51 ns | 1.58 ns | 4.53 * | 2.63 * | 1.50 ns |
| Height (H) | 1 | 76.19 * | 12.10 * | 1.21 * | 7.15 * | 5.40 * | |
| (P) × (H) | 3 | 5.33 * | 0.43 ns | 1.39 ns | 0.84 ns | 0.11 ns | |
| Acacia auriculiformis | Provenance (P) | 3 | 5.16 * | 1.53 ns | 1.65 ns | 0.60 ns | 0.56 ns |
| Height (H) | 1 | 33.64 * | 28.30 * | 5.78 * | 35.93 * | 10.00 * | |
| (P) × (H) | 3 | 1.21 ns | 0.76 ns | 1.23 ns | 1.38 ns | 0.59 ns | |
| Acacia crassicarpa | Provenance (P) | 3 | 0.67 ns | 1.38 ns | 1.41 ns | 1.45 ns | 1.38 ns |
| Height (H) | 1 | 2.00 ns | 1.04 ns | 1.37 ns | 0.91 ns | 1.03 ns | |
| (P) × (H) | 3 | 0.67 ns | 1.03 ns | 1.26 ns | 1.88 ns | 1.04 ns | |
| Acacia aulococarpa | Provenance (P) | 3 | 2.98 * | 10.47 * | 3.50 * | 3.69 * | 4.26 * |
| Height (H) | 1 | 6.37 * | 9.56 * | 2.74 ns | 3.56 * | 12.78 * | |
| (P) × (H) | 3 | 0.3 ns | 2.63 ns | 1.96 ns | 2.34 ns | 1.30 ns |
| Species | SP (%) | SN | SL (cm) | SG (mm) | NL |
|---|---|---|---|---|---|
| Acacia mangium | 75.00 ± 4.17 b | 8.50 ± 0.43 b | 6.61 ± 0.33 a | 3.14 ± 0.14 a | 18.05 ± 0.76 b |
| Acacia auriculiformis | 83.75 ± 3.60 a | 13.50 ± 0.64 a | 4.75 ± 0.26 b | 2.62 ± 0.15 b | 26.43 ± 1.12 a |
| Acacia crassicarpa | 1.67 ± 1.15 d | 0.03 ± 0.02 d | 0.09 ± 0.05 c | 0.02 ± 0.01 d | 0.07 ± 0.03 d |
| Acacia aulococarpa | 40.00 ± 4.30 c | 4.86 ± 0.46 c | 5.59 ± 0.53 b | 1.32 ± 0.14 c | 9.96 ± 0.86 c |
| Species | Provenance | SP (%) | SN | SL (cm) | SG (mm) | NL |
|---|---|---|---|---|---|---|
| Acacia mangium | Bensbach WP | 78.33 ± 4.01 ab | 7.10 ± 0.78 c | 5.44 ± 0.60 b | 3.11 ± 0.31 a | 15.60 ± 1.48 bc |
| SW of Boset WP | 75.00 ± 7.19 ab | 8.93 ± 0.95 c | 7.78 ± 0.67 a | 2.97 ± 0.28 ab | 17.80 ± 1.64 bc | |
| Captain Billy Road | 71.67 ± 11.67 b | 9.53 ± 0.85 c | 7.74 ± 0.61 a | 3.76 ± 0.22 a | 19.00 ± 1.23 bc | |
| Russel & Gap CK | 75.00 ± 10.57 ab | 8.43 ± 0.84 c | 5.49 ± 0.63 b | 2.72 ± 0.31 abc | 19.80 ± 1.63 b | |
| Acacia auriculiformis | Mibini | 85.00 ± 7.19 ab | 13.87 ± 1.16 ab | 5.49 ± 0.53 b | 2.67 ± 0.30 abc | 24.77 ± 1.62 a |
| Bansbach | 90.00 ± 6.83 a | 15.27 ± 1.31 a | 3.96 ± 0.38 b | 2.72 ± 0.26 abc | 28.57 ± 2.00 a | |
| Boggy Creek | 70.00 ± 8.56 b | 11.90 ± 1.29 b | 4.95 ± 0.64 b | 2.76 ± 0.37 abc | 25.73 ± 2.50 a | |
| Wenlock River | 90.00 ± 3.65 a | 13.00 ± 1.33 ab | 4.59 ± 0.47 b | 2.31 ± 0.30 bc | 26.67 ± 2.70 a | |
| Acacia crassicarpa | Bensbach WP | 3.33 ± 3.33 e | 0.07 ± 0.05 e | 0.16 ± 0.11 c | 0.02 ± 0.02 f | 0.13 ± 0.09 e |
| Bimadebum WP | 3.33 ± 3.33 e | 0.07 ± 0.05 e | 0.20 ± 0.14 c | 0.04 ± 0.03 f | 0.13 ± 0.09 e | |
| Chilli Beach | 0.00 ± 0.00 e | 0.00 ± 0.00 e | 0.00 ± 0.00 c | 0.00 ± 0.00 f | 0.00 ± 0.00 e | |
| Claudie River | 0.00 ± 0.00 e | 0.00 ± 0.00 e | 0.00 ± 0.00 c | 0.00 ± 0.00 f | 0.00 ± 0.00 e | |
| Acacia aulococarpa | Arufi E Morehead WP | 50.00 ± 5.77 c | 8.43 ± 1.14 c | 8.19 ± 1.23 a | 1.95 ± 0.36 cd | 14.62 ± 1.91 c |
| W Morehead | 51.67 ± 7.03 c | 4.37 ± 0.87 d | 4.72 ± 1.01 b | 1.09 ± 0.25 e | 8.21 ± 1.68 d | |
| 3K S Mt Larcom | 30.00 ± 8.56 d | 3.67 ± 0.59 d | 5.09 ± 0.92 b | 1.41 ± 0.26 de | 9.83 ± 1.51 d | |
| Samford | 28.33 ± 9.46 d | 2.97 ± 0.60 d | 4.30 ± 0.96 b | 0.82 ± 0.21 e | 7.27 ± 1.50 d |
| Species | Stump Height (m) | SP (%) | SN | SL (cm) | SG (mm) | NL |
|---|---|---|---|---|---|---|
| Acacia mangium | 1.0 | 58.33 ± 4.05 | 7.07 ± 0.64 | 6.95 ± 0.53 | 3.51 ± 0.23 | 16.32 ± 1.18 |
| 1.5 | 91.67 ± 2.41 | 9.93 ± 0.52 | 6.27 ± 0.39 | 2.77 ± 0.16 | 19.78 ± 0.90 | |
| Acacia auriculiformis | 1.0 | 71.67 ± 4.74 | 10.45 ± 0.73 | 5.35 ± 0.40 | 3.43 ± 0.21 | 23.00 ± 1.39 |
| 1.5 | 95.83 ± 0.00 | 16.57 ± 0.90 | 4.15 ± 0.31 | 1.81 ± 0.17 | 29.87 ± 1.64 | |
| Acacia crassicarpa | 1.0 | 0.00 ± 3.33 | 0.05 ± 0.03 | 0.14 ± 0.08 | 0.03 ± 0.02 | 0.10 ± 0.06 |
| 1.5 | 0.00 ± 2.29 | 0.02 ± 0.02 | 0.04 ± 0.04 | 0.01 ± 0.01 | 0.03 ± 0.03 | |
| Acacia aulococarpa | 1.0 | 30.83 ± 6.21 | 3.72 ± 0.66 | 6.39 ± 0.94 | 1.57 ± 0.24 | 7.18 ± 1.10 |
| 1.5 | 49.17 ± 4.84 | 6.00 ± 0.60 | 4.76 ± 0.47 | 1.07 ± 0.13 | 12.83 ± 1.21 | |
| Total | 1.0 | 40.00 ± 4.54 | 5.32 ± 0.38 | 4.71 ± 0.34 | 2.13 ± 0.14 | 11.65 ± 0.77 |
| 1.5 | 60.00 ± 5.67 | 8.13 ± 0.49 | 3.80 ± 0.23 | 1.42 ± 0.09 | 15.65 ± 0.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muniandi, S.K.; Muhammad, N.; Md Ariff, F.F.; Taheri, Y. Coppicing Abilities of Decapitated Elite Tree Trunks of Selected Acacia Species Genotypes in Mixed-Species Plantation. Forests 2024, 15, 9. https://doi.org/10.3390/f15010009
Muniandi SK, Muhammad N, Md Ariff FF, Taheri Y. Coppicing Abilities of Decapitated Elite Tree Trunks of Selected Acacia Species Genotypes in Mixed-Species Plantation. Forests. 2024; 15(1):9. https://doi.org/10.3390/f15010009
Chicago/Turabian StyleMuniandi, Sures Kumar, Norwati Muhammad, Farah Fazwa Md Ariff, and Yaghoob Taheri. 2024. "Coppicing Abilities of Decapitated Elite Tree Trunks of Selected Acacia Species Genotypes in Mixed-Species Plantation" Forests 15, no. 1: 9. https://doi.org/10.3390/f15010009
APA StyleMuniandi, S. K., Muhammad, N., Md Ariff, F. F., & Taheri, Y. (2024). Coppicing Abilities of Decapitated Elite Tree Trunks of Selected Acacia Species Genotypes in Mixed-Species Plantation. Forests, 15(1), 9. https://doi.org/10.3390/f15010009






