The Carbon Sequestration Potential of Silky Oak (Grevillea robusta A.Cunn. ex R.Br.), a High-Value Economic Wood in Thailand
Abstract
:1. Introduction
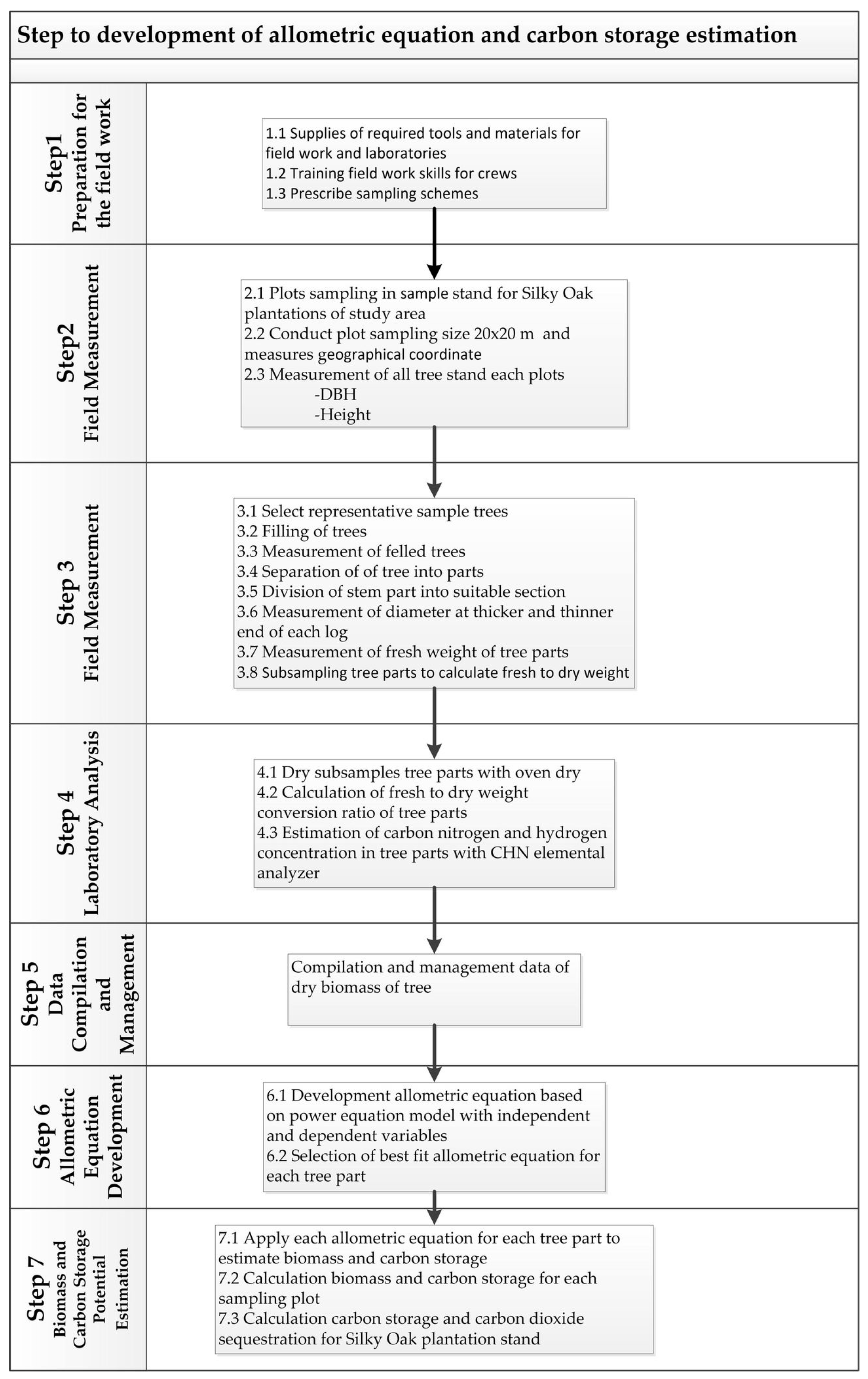
2. Materials and Methods
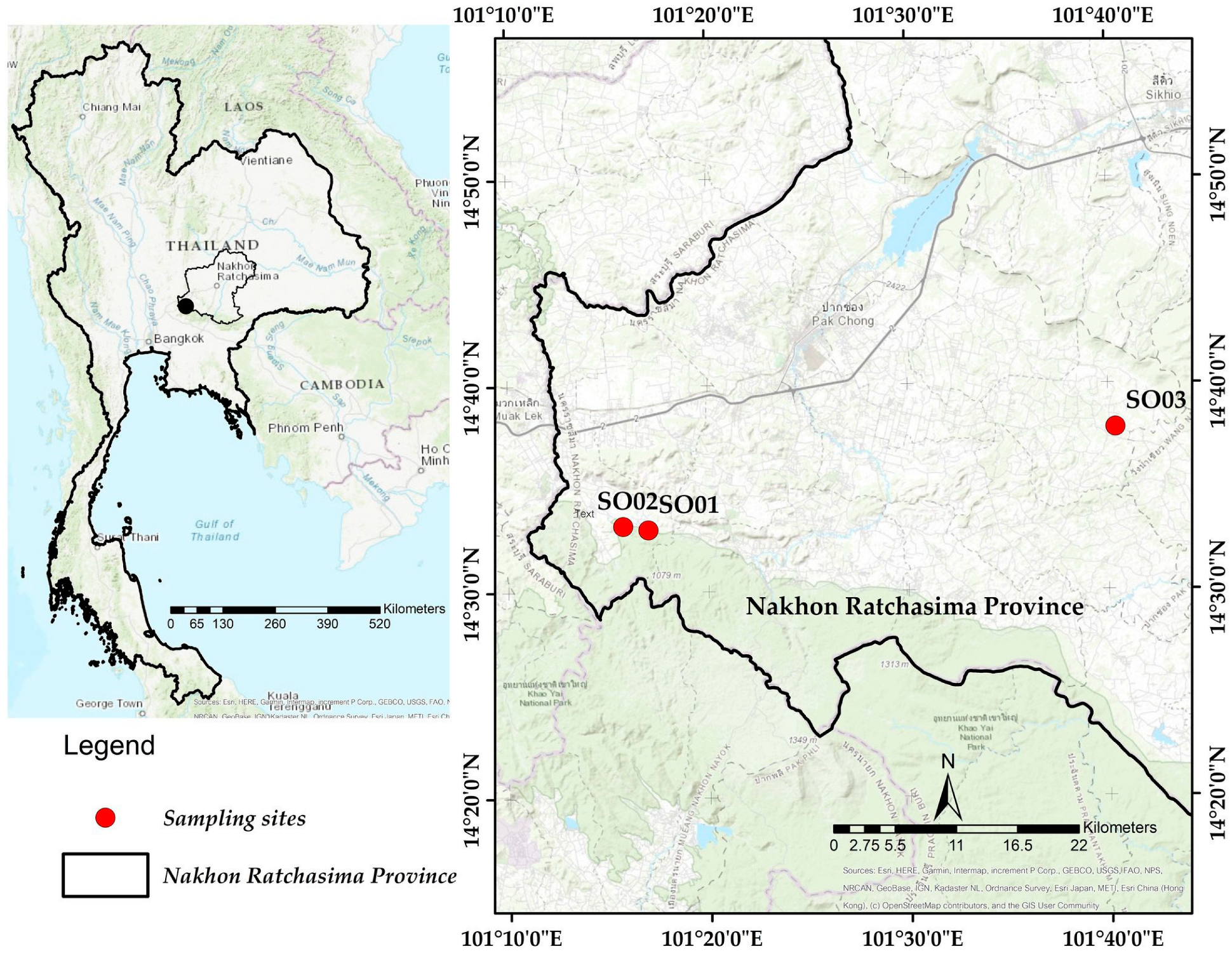
2.1. Study Area
2.2. Cutting of the Sample Trees
- We randomly selected 1–2 sample Silky Oak trees to represent all sites before measuring their DBH; trees with a small to large DBH were selected, based on information on the DBH distribution collected in a field survey.
- We used a chainsaw to cut the sample trees at a height of 0.3 m from ground level.
- We measured the height of the cut trees from bolt to top before cutting logs.
- We cut all branches from the stems after detaching all leaves from the branches and gathering them on a canvas or lining material. The branches and leaves were weighed, and the fresh weights in Kg were recorded in a Field Survey Record Form (FSRF).
- We cut each stem into equal sections, 1 m or 2 m in length, based on the height of stem, to facilitate their weighing, with the exception of one section that was cut to 1.3 m in length.
- 6.
- Tree cutting was performed at a marked position. After cutting, the diameter of the lower end of each log was measured.
- 7.
- We weighed each log and recorded its weight in kg.
- 8.
- We dug and collected all sample roots. These roots were cleaned by spraying water on them before aerating them. However, they were not dried in the sun to avoid losing the moisture in the wood. Subsequently, all roots were weighed.
2.3. Preparation of the Sample Portions for Dry Weighing
- Stem: the lower part of each log was cut to obtain sections with an approximate thickness of 2–4 cm, or an approximate weight of 100–1000 g. Subsequently, each section was marked with a code indicating the sample tree and the log. Each sample was packed separately and stored in a sampling bag before fresh weighing with a semi-micro balance. The fresh weight of each sample section was recorded in g.
- Branches: the branches were classified into 4 groups according to their size, which was related to their moisture content. The size was determined by measuring the diameter of each branch, and the branches were classified into the following groups: >10 cm, 5–10 cm, 2–5 cm, and <2 cm. Sampling was conducted by cutting the branches and placing them in sample bags marked with the code of each tree and a number identifying the part of the sample. Subsequently, each sample was weighed using a micro-balance to determine the fresh weight. The fresh weight of each group was recorded in g and was 500–1000 g, depending on the group.
- Leaves: the leaves were randomly selected from all samples and placed in a sample bag marked with the code of the tree before determining their fresh weight. The weight of the dried leaves had to be around 500–1000 g. If the number of the sampled leaves was small, the total number of leaves from the entire sample was used. The fresh weight of the samples was recorded in g.
- Roots: the roots were classified into 3 groups, including primary root (PR), secondary root (SR), and find root (FR). Samples were collected from the primary root based on their size and length. The roots were cut into 3–5 logs, each with a length of 2–5 cm. If the number of secondary roots was large, 3–5 roots were randomly selected as representatives of all large secondary roots. Each root was cut into 3–5 logs with a length of 2–5 cm, and they were marked with the code of the tree. The code of the root group indicated whether they were primary roots or secondary roots, and a code specifying the order of the root and that of the of log was also noted. Subsequently, each sample part was weighed to determine its fresh weight, which was recorded in g. The samples were stored in sampling bags, separately.
2.4. Sample Drying
2.5. Preparation of the Samples for the Analysis and Determination of Carbon in the Wood
2.6. Calculation of the Dry Weight of the Field Samples
2.7. Development of the Allometric Equation for Evaluating the Biomass of Silky Oak
2.8. Biomass and Carbon Storage Potential Estimation of the Planted Sampling Area
3. Results and Discussion
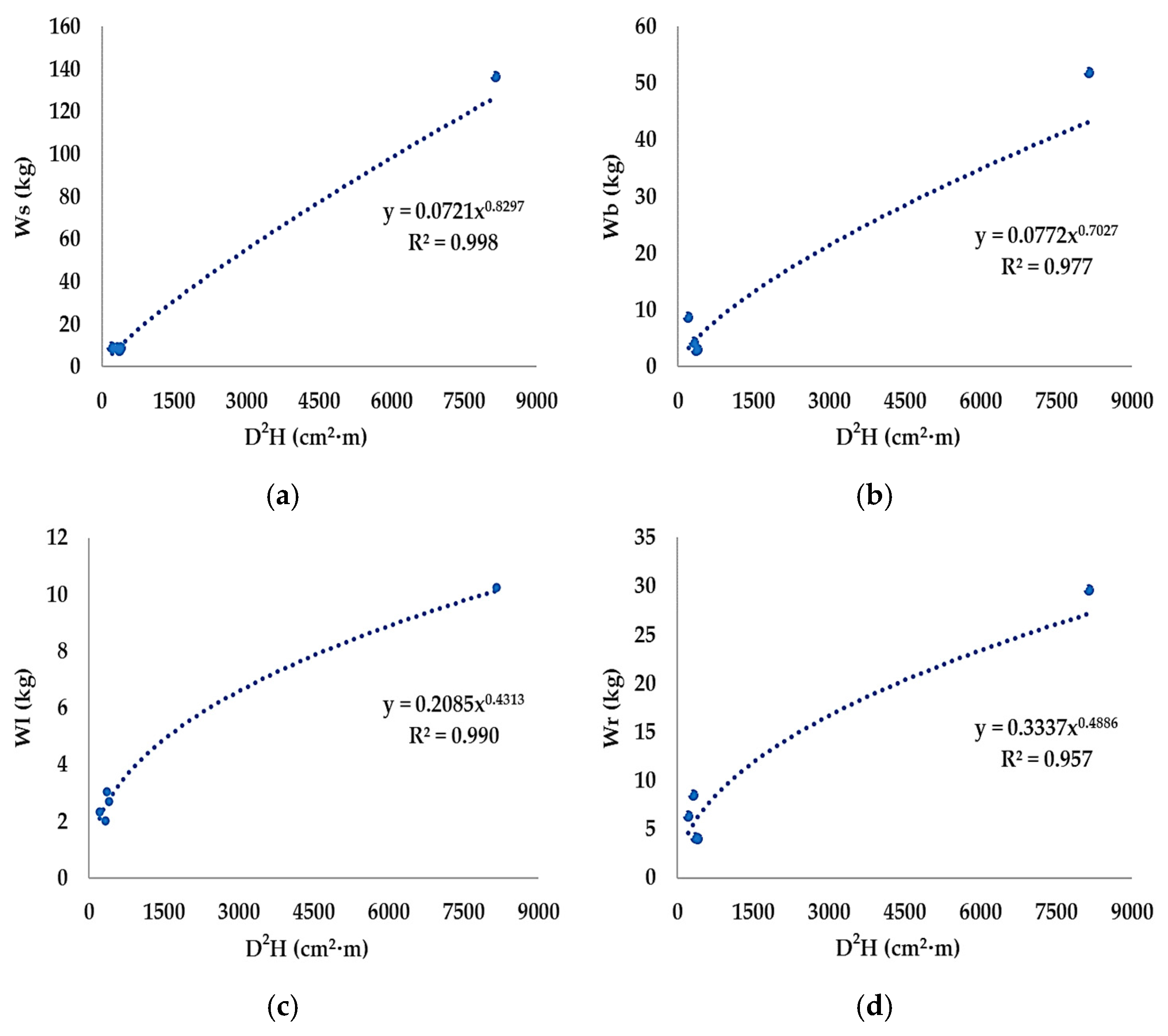
3.1. Development of the Allometric Equation
3.2. Carbon Storage in the Biomass
3.3. Evaluation of Carbon Storage in Silky Oak Sites Using the Developed Allometric Equation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Resources Institute: World GHG Emissions Flow Chart. Available online: http://pdf.wri.org/world_greenhouse_gas_emissions_flowchart.pdf (accessed on 10 May 2023).
- Tian, J.; Shan, Y.; Zheng, H.; Lin, X.; Liang, X.; Guan, D. Structural patterns of city-level CO2 emissions in Northwest China. J. Clean. Prod. 2019, 223, 553–593. [Google Scholar] [CrossRef]
- Sugsaisakon, S.; Kittipongvises, S. Citywide Energy-Related CO2 Emissions and Sustainability Assessment of the Development of Low-Carbon Policy in Chiang Mai, Thailand. Sustainability 2021, 13, 6789. [Google Scholar] [CrossRef]
- Samek, J.H.; Skole, D.L.; Butthep, C.; Navanugraha, C.; Uttaruk, P.; Laosuwan, T. Inpang carbon bank in northeast Thailand: A Community Effort in Carbon Trading from Agroforestry Projects. In Carbon Sequestration Potential of Agroforestry Systems; Springer: Berlin/Heidelberg, Germany, 2011; pp. 263–280. [Google Scholar]
- Uttaruk, Y.; Laosuwan, T. Development of Prototype Project for Carbon Storage and Greenhouse Gas Emission Reduction from Thailand’s Agricultural Sector. Sains Malays. 2019, 48, 2083–2092. [Google Scholar] [CrossRef]
- Manabe, S. Role of greenhouse gas in climate change. Tellus A Dyn. Meteorol. Oceanogr. 2019, 71, 1–13. [Google Scholar] [CrossRef]
- Community Forestry and Development. Manual of Carbon Sequestration Studies and Biodiversity in Community Forests. Available online: https://www.forest.go.th/community-development/wp-content/uploads/sites/105/2020/03/คู่มือคาร์บอน.pdf (accessed on 10 April 2023).
- Duncanson, L.; Rourke, O.; Dubayah, R. Small sample sizes yield biased allometric equations in temperate forests. Sci. Rep. 2015, 5, 17153. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Chen, Q.; Wang, G.; Liu, L.; Li, G.; Moran, E. A survey of remote sensing-based aboveground biomass estimation methods in forest ecosystems. Int. J. Digit. Earth 2016, 9, 63–105. [Google Scholar] [CrossRef]
- Pascarella, A.E.; Giacco, G.; Rigiroli, M.; Marrone, S.; Sansone, C. ReUse: REgressive Unet for Carbon Storage and Above-Ground Biomass Estimation. J. Imaging 2023, 9, 61. [Google Scholar] [CrossRef]
- Alongi, D.M. Impacts of Climate Change on Blue Carbon Stocks and Fluxes in Mangrove Forests. Forests 2022, 13, 149. [Google Scholar] [CrossRef]
- Byun, C.; Lee, S.H.; Kang, H. Estimation of carbon storage in coastal wetlands and comparison of different management schemes in South Korea. J. Ecol. Environ. 2019, 43, 8. [Google Scholar] [CrossRef]
- Repo, A.; Rajala, T.; Henttonen, H.M.; Lehtonen, A.; Peltoniemi, M.; Heikkinen, J. Age-Dependence of Stand Biomass in Managed Boreal Forests Based on the Finnish National Forest Inventory Data. For. Ecol. Manag. 2021, 498, 119507. [Google Scholar] [CrossRef]
- He, G.; Zhang, Z.; Zhu, Q.; Wang, W.; Peng, W.; Cai, Y. Estimating Carbon Sequestration Potential of Forest and Its Influencing Factors at Fine Spatial-Scales: A Case Study of Lushan City in Southern China. Int. J. Environ. Res. Public Health 2022, 19, 9184. [Google Scholar] [CrossRef] [PubMed]
- Xi, F.; Lin, G.; Zhao, Y.; Li, X.; Chen, Z.; Cao, C. Land Use Optimization and Carbon Storage Estimation in the Yellow River Basin, China. Sustainability 2023, 15, 11278. [Google Scholar] [CrossRef]
- Rotjanakusol, T.; Laosuwan, T. Model of Relationships between Land Surface Temperature and Urban Built-Up Areas in Mueang Buriram District, Thailand. Pol. J. Environ. Stud. 2020, 29, 3783–3790. [Google Scholar] [CrossRef]
- Encyclopedia. Deforestation and Global Warming. Available online: https://encyclopedia.pub/entry/37075 (accessed on 15 April 2023).
- Ceres. Deforestation and Land Use Change. Available online: https://engagethechain.org/deforestation-and-land-use-change (accessed on 17 April 2023).
- Othman, M.; Zakaria, R.; Fernando, W. Strategic Planning on Carbon Capture from Coal Fired Plants in Malaysia and Indonesia: A Review. Energy Policy 2009, 37, 1718–1735. [Google Scholar] [CrossRef]
- Jorat, M.E.; Aziz, M.A.; Marto, A.; Zaini, N.; Jusoh, S.N.; Manning, D.A.C. Sequestering Atmospheric CO2 Inorganically: A Solution for Malaysia’s CO2 Emission. Geosciences 2018, 8, 483. [Google Scholar] [CrossRef]
- Küßner, R.; Mosandl, R. Comparison of direct and indirect estimation of leaf area index in mature Norway spruce stands of eastern Germany. Can. J. For. Res. 2011, 30, 440–447. [Google Scholar] [CrossRef]
- University of Idaho. Direct Measures of Biomass. Available online: https://www.webpages.uidaho.edu/veg_measure/Modules/Lessons/Module%207(Biomass&Utilization)/7_3_Direct%20Methods.htm (accessed on 25 April 2023).
- Barmpoutis, P.; Stathaki, T.; Kamperidou, V. Estimation of extent of trees and biomass infestation of the suburban forest of Thessaloniki (Seich Sou) using UAV imagery and combining R-CNNs and multichannel texture analysis. In Proceedings of the SPIE-The International Society for Optical Engineering This Link Is Disabled, Amsterdam, The Netherlands, 31 January 2020; Volume 11433, p. 114333C. [Google Scholar]
- Lambert, M.C.; Ung, C.H.; Raulier, F. Canadian national tree aboveground biomass equations. Can. J. For. Res. 2005, 35, 1996–2018. [Google Scholar] [CrossRef]
- Xiao, X.; White, E.P.; Hooten, M.B.; Durham, S.L. On the use of log-transformation vs. nonlinear regression for analyzing biological power laws. Ecology 2011, 92, 1887–1894. [Google Scholar] [CrossRef]
- Chave, J.; Rejou-Mechain, M.; Burquez, A.; Chidumayo, E.; Colgan, M.S.; Delitti, W.B.; Duque, A.; Eid, T.; Fearnside, P.M.; Goodman, R.C.; et al. Improved allometric models to estimate the aboveground biomass of tropical trees. Glob. Chang. Biol. 2014, 20, 3177–3190. [Google Scholar] [CrossRef]
- Li, Y.D.; Liu, Q.; Meng, S.; Zhou, G. Allometric biomass equations of Larix sibirica in the Altay Mountains, Northwest China. J. Arid. Land 2019, 11, 608–622. [Google Scholar] [CrossRef]
- Fradette, O.; Marty, C.; Tremblay, P.; Lord, D.; Boucher, J.-F. Allometric Equations for Estimating Biomass and Carbon Stocks in Afforested Open Woodlands with Black Spruce and Jack Pine, in the Eastern Canadian Boreal Forest. Forests 2021, 12, 59. [Google Scholar] [CrossRef]
- United States Department of Agriculture. Silk-Oak. Available online: https://www.srs.fs.usda.gov/pubs/misc/ag_654/volume_2/grevillea/robusta.htm (accessed on 30 April 2023).
- Post Today. Silk Oak. Available online: https://www.posttoday.com/post-next/be-greener/694264 (accessed on 2 May 2023).
- Bangkokbiznews. Silk Oak. Available online: https://www.bangkokbiznews.com/business/economic/1067157 (accessed on 2 May 2023).
- AVAFARM 888. Silk Oak. Available online: https://avafarm888.com/ (accessed on 5 May 2023).
- Silveroak. Silk Oak. Available online: https://silveroak.in.th/ (accessed on 5 May 2023).
- Mery, G.; Katila, P.; Galloway, G.; Alfaro, R.I.; Kanninen, M.; Lobovikov, M.; Varjo, J. Forests and Society–Responding to Global Drivers of Change; IUFRO World Series; IUFRO: Vienna, Austria, 2010; Volume 25, 509p. [Google Scholar]
- Díaz, S.; Settele, J.; Brondízio, E.S.; Ngo, H.T.; Guèze, M.; Agard, J.; Arneth, A.; Balvanera, P.; Brauman, K.A.; Butchart, S.H.M.; et al. (Eds.) Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; IPBES Secretariat: Bonn, Germany, 2019; 56p. [Google Scholar]
- Churkina, G.; Organschi, A.; Reyer, C.P.; Ruff, A.; Vinke, K.; Liu, Z.; Reck, B.K.; Graedel, T.E.; Schellnhuber, H.J. Buildings as a global carbon sink. Nat Sustain. 2020, 3, 269–276. [Google Scholar] [CrossRef]
- Kiyingi, I.; Ocama, D.; Mujuni, D.; Nyombi, K. A bioeconomic analysis of the carbon sequestration potential of agroforestry systems: A case study of Grevillea robusta in South Western Uganda. Uganda J. Agric. Sci. 2016, 17, 219–229. [Google Scholar] [CrossRef]
- Owate, O.; Mware, M.; Kinyanjui, M. Allometric Equations for Estimating Silk Oak (Grevillea robusta) Biomass in Agricultural Landscapes of Maragua Subcounty, Kenya. Int. J. For. Res. 2018, 2018, 6495271. [Google Scholar] [CrossRef]
- Jangra, R.; Gupta, S.R.; Kumar, R.; Sing, G. Carbon Sequestration in the Grevillea robudta Plantation on a Reclaimmed Sodic Soil at Karnal in Northern India. Int. J. Ecol. Environ. Sci. 2010, 36, 75–86. [Google Scholar]
- Yirdaw, M. Carbon Stock Sequestered by Selected Tree Species Plantations in Wondo Genet College, Ethiopia. J. Earth Sci. Clim. Change 2018, 9, 1000472. [Google Scholar]
- Cole, T.G.; Ewel, J.J. Allometric equations for four valuable tropical tree species. For. Ecol. Manag. 2006, 229, 351–360. [Google Scholar] [CrossRef]
- CHN628 Series: Instruction Manual. Available online: https://eu.leco.com/images/Analytical-Application-Library/CHN628_SULFUR_HYDROCARBONS_203-821-466.pdf (accessed on 27 April 2023).
- Paustian, K.; Ravindranath, N.H.; van Amstel, A.R. 2006 IPCC Guidelines for National Greenhouse Gas Inventories. Available online: http://www.ipcc-nggip.iges.or.jp/public/2006gl/index.htm (accessed on 30 April 2023).
- Viriyabuncha, C.; Chittachumnonk, P.; Sutthisrisinn, C.; Samran, S.; Peawsa-ad, K. Adjusting Equation to Estimate the Above-ground Biomass of Teak Plantation in Thailand. In Proceedings of the 7th Silvicultural Seminar, Faculty of Forestry, Kasetsart University, Thailand, 12–14 December 2001; pp. 239–260. [Google Scholar]
- Pretzsch, H.; Biber, P.; Schütze, G.; Kemmerer, J.; Uhl, E. Wood density reduced while wood volume growth accelerated in Central European forests since 1870. For. Ecol. Manag. 2018, 429, 589–616. [Google Scholar] [CrossRef]
- Grote, R.; Pretzsch, H. A model for individual tree development based on physiological processes. Plant Biol. 2002, 4, 167–180. [Google Scholar] [CrossRef]
- Reyer, C.; Lasch-Born, P.; Suckow, F.; Gutsch, M.; Murawski, A.; Pilz, T. Projections of regional changes in forest net primary productivity for different tree species in Europe driven by climate change and carbon dioxide. Ann. For. Sci. 2014, 71, 211–225. [Google Scholar] [CrossRef]
- Keenan, R.J. Climate change impacts and adaptation in forest management: A review. Ann. For. Sci. 2015, 72, 145–167. [Google Scholar] [CrossRef]
- Chmielewski, F.M.; Rötzer, T. Response of tree phenology to climate change across Europe. Agric. For. Meteorol. 2001, 108, 101–112. [Google Scholar] [CrossRef]
- Churkina, G.; Zaehle, S.; Hughes, J.; Viovy, N.; Chen, Y.; Jung, M.; Heumann, B.W.; Ramankutty, N.; Heimann, M.; Jones, C. Interactions between nitrogen deposition, land cover conversion, and climate change determine the contemporary carbon balance of Europe. Biogeosciences 2010, 7, 2749–2764. [Google Scholar] [CrossRef]
- Davis, W.J. The Relationship between Atmospheric Carbon Dioxide Concentration and Global Temperature for the Last 425 Million Years. Climate 2017, 5, 76. [Google Scholar] [CrossRef]
- Nunes, L.J.R. The Rising Threat of Atmospheric CO2: A Review on the Causes, Impacts, and Mitigation Strategies. Environments 2023, 10, 66. [Google Scholar] [CrossRef]
| Tree ID | Age (Year) | Ht (m) | DBH (cm) | Fresh Weight (kg) | |||
|---|---|---|---|---|---|---|---|
| Stem | Branch | Leaf | Root | ||||
| SO0101 | 7 | 18.5 | 21.00 | 283.6 | 89.6 | 22.8 | 50.3 |
| SO0201 | 2 | 6 | 5.97 | 18.8 | 15 | 5.2 | 10.8 |
| SO0202 | 2 | 6.1 | 7.32 | 18 | 7.4 | 4.5 | 14.4 |
| SO0301 | 3–4 | 7.8 | 7.18 | 18 | 5.2 | 6 | 6.8 |
| SO0302 | 3–4 | 7.2 | 7.14 | 15.8 | 5 | 6.8 | 7 |
| Tree ID | Age (Year) | % Moisture | |||
|---|---|---|---|---|---|
| Stem | Branch | Leaf | Root | ||
| SO0101 | 7 | 108.26 | 72.99 | 122.47 | 69.84 |
| SO0201 | 2 | 93.92 | 91.27 | 77.47 | 88.53 |
| SO0202 | 2 | 91.43 | 85.63 | 80.51 | 87.90 |
| SO0301 | 3–4 | 140.15 | 66.28 | 110.97 | 64.91 |
| SO0302 | 3–4 | 117.94 | 62.86 | 97.63 | 71.23 |
| Tree ID | Ht (m) | DBH (cm) | DBH2Ht (cm2·m) | Dry Weight (kg) | ||||
|---|---|---|---|---|---|---|---|---|
| Ws | Wb | Wl | Wr | AGB | ||||
| SO0101 | 18.5 | 21.00 | 8158.50 | 136.17 | 51.80 | 10.25 | 29.62 | 227.83 |
| SO0201 | 6 | 5.97 | 213.85 | 9.03 | 8.67 | 2.34 | 6.36 | 26.39 |
| SO0202 | 6.1 | 7.32 | 326.85 | 8.64 | 4.28 | 2.02 | 8.48 | 23.42 |
| SO0301 | 7.8 | 7.18 | 402.11 | 8.64 | 3.01 | 2.70 | 4.00 | 18.35 |
| SO0302 | 7.2 | 7.14 | 367.05 | 7.59 | 2.89 | 3.06 | 4.12 | 17.65 |
| Tree Part | Allometric Equation | R2 |
|---|---|---|
| Stem | Ws = 0.0721(D2H)0.8297 | 0.998 |
| Branch | Wb = 0.0772(D2H)0.7027 | 0.977 |
| Leaf | Wl = 0.2085(D2H)0.4313 | 0.990 |
| Root | Wr = 0.3337(D2H)0.4886 | 0.957 |
| Part of Tree | Sample ID | Carbon % | Hydrogen % | Nitrogen % |
|---|---|---|---|---|
| Stem | SSO0101 | 45.390 | 7.850 | 0.098 |
| SSO0102 | 45.654 | 7.826 | 0.195 | |
| SSO0201 | 44.908 | 7.733 | 0.026 | |
| SSO0301 | 44.328 | 7.724 | 0.067 | |
| SSO0302 | 45.060 | 7.747 | 0.117 | |
| Average | 45.068 | 7.776 | 0.101 | |
| Branch | BSO0101 | 45.530 | 7.852 | 0.198 |
| BSO0201 | 44.185 | 7.831 | 0.135 | |
| BSO0202 | 44.929 | 7.847 | 0.151 | |
| BSO0301 | 45.329 | 7.823 | 0.181 | |
| BSO0302 | 44.886 | 7.853 | 0.051 | |
| Average | 44.972 | 7.841 | 0.143 | |
| Leaf | LSO0101 | 47.806 | 7.970 | 0.722 |
| LSO0201 | 46.778 | 7.935 | 0.699 | |
| LSO0202 | 48.172 | 7.404 | 1.526 | |
| LSO0301 | 47.194 | 7.373 | 1.409 | |
| LSO0302 | 47.837 | 7.319 | 1.490 | |
| Average | 47.557 | 7.600 | 1.169 | |
| Root | RSO0101 | 46.442 | 7.861 | 0.004 |
| RSO0201 | 45.845 | 7.784 | 0.132 | |
| RSO0202 | 45.937 | 7.794 | 0.144 | |
| RSO0301 | 45.142 | 7.774 | 0.143 | |
| RSO0302 | 44.757 | 7.447 | 0.041 | |
| Average | 45.624 | 7.732 | 0.093 |
| Site ID | Biomass (Tons) | |||||
|---|---|---|---|---|---|---|
| Ws | Wb | Wl | Wr | AGB | TTB | |
| SO01 | 17.92 (63.61%) | 5.75 (20.41%) | 1.20 (4.26%) | 3.30 (11.17%) | 24.87 (88.29%) | 28.17 (100%) |
| SO02 | 0.36 (34.29%) | 0.21 (20.00%) | 0.15 (14.29%) | 0.32 (30.48) | 0.73 (69.52%) | 1.05 (100%) |
| SO03 | 1.65 (37.41%) | 0.90 (20.40%) | 0.59 (13.38%) | 1.27 (28.80%) | 3.14 (71.20%) | 4.41 (100%) |
| Total | 19.93 | 6.86 | 1.94 | 4.89 | 28.74 | 33.63 |
| Site ID | SO01 | SO02 | SO03 | Total |
|---|---|---|---|---|
| Total aboveground biomass (tons) | 24.87 | 0.73 | 3.14 | 28.74 |
| Total carbon storage in the sampling plots (tons C) | 11.69 | 0.34 | 1.48 | 13.51 |
| Number of plots | 4 | 1 | 4 | 9 |
| Sampling plot area (ha) | 0.16 | 0.04 | 0.16 | 0.36 |
| Carbon density (tC) | 73.07 | 8.52 | 9.23 | - |
| Stand area (ha) | 1.55 | 0.12 | 1.80 | 3.46 |
| Carbon in stands (tC) | 113.05 | 1.01 | 16.58 | 130.63 |
| CO2 in stands (tCO2eq) | 414.51 | 3.70 | 60.78 | 478.99 |
| Site ID | SO01 | SO02 | SO03 | Total |
|---|---|---|---|---|
| Total tree biomass (tons) | 28.17 | 1.05 | 4.41 | 33.63 |
| Total carbon storage in the sampling plots (tons C) | 13.24 | 0.49 | 2.07 | 15.80 |
| Number of plots | 4 | 1 | 4 | 9 |
| Sampling plot area (ha) | 0.16 | 0.04 | 0.16 | 0.36 |
| Carbon density (tC) | 82.75 | 12.30 | 12.95 | - |
| Stand area (ha) | 1.55 | 0.12 | 1.80 | 3.46 |
| Carbon in the stands (tC) | 128.03 | 1.46 | 23.26 | 152.75 |
| CO2 in stands (tCO2eq) | 469.46 | 5.34 | 85.30 | 560.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laosuwan, T.; Uttaruk, Y.; Sangpradid, S.; Butthep, C.; Leammanee, S. The Carbon Sequestration Potential of Silky Oak (Grevillea robusta A.Cunn. ex R.Br.), a High-Value Economic Wood in Thailand. Forests 2023, 14, 1824. https://doi.org/10.3390/f14091824
Laosuwan T, Uttaruk Y, Sangpradid S, Butthep C, Leammanee S. The Carbon Sequestration Potential of Silky Oak (Grevillea robusta A.Cunn. ex R.Br.), a High-Value Economic Wood in Thailand. Forests. 2023; 14(9):1824. https://doi.org/10.3390/f14091824
Chicago/Turabian StyleLaosuwan, Teerawong, Yannawut Uttaruk, Satith Sangpradid, Chetphong Butthep, and Smith Leammanee. 2023. "The Carbon Sequestration Potential of Silky Oak (Grevillea robusta A.Cunn. ex R.Br.), a High-Value Economic Wood in Thailand" Forests 14, no. 9: 1824. https://doi.org/10.3390/f14091824
APA StyleLaosuwan, T., Uttaruk, Y., Sangpradid, S., Butthep, C., & Leammanee, S. (2023). The Carbon Sequestration Potential of Silky Oak (Grevillea robusta A.Cunn. ex R.Br.), a High-Value Economic Wood in Thailand. Forests, 14(9), 1824. https://doi.org/10.3390/f14091824









