How Are Pine Species Responding to Soil Drought and Climate Change in the Iberian Peninsula?
Abstract
1. Introduction
2. Dataset and Methodology
2.1. Study Area and Dendrochronological Dataset
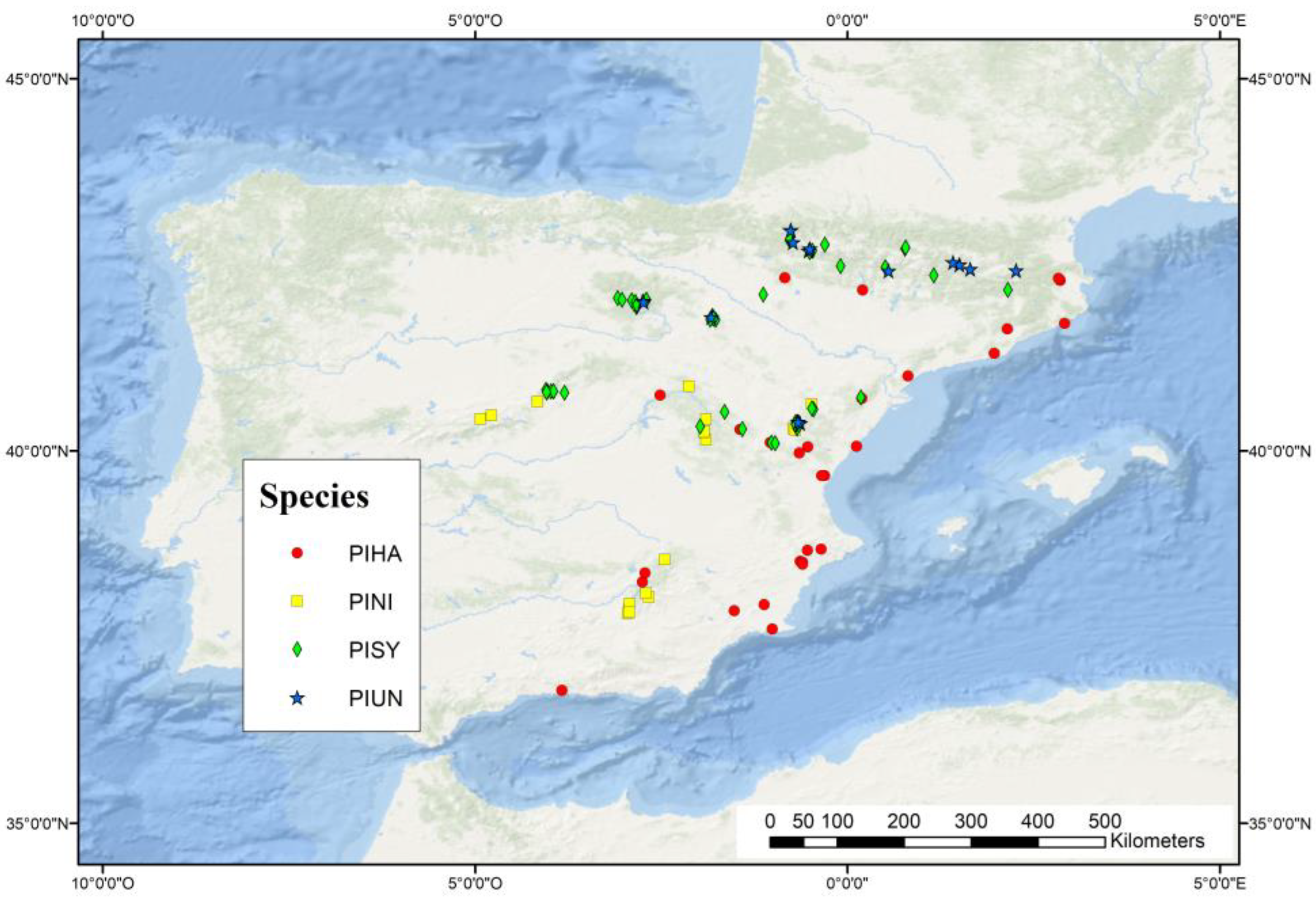
2.2. ERA5-Land
2.3. Analysis
3. Results and Discussion
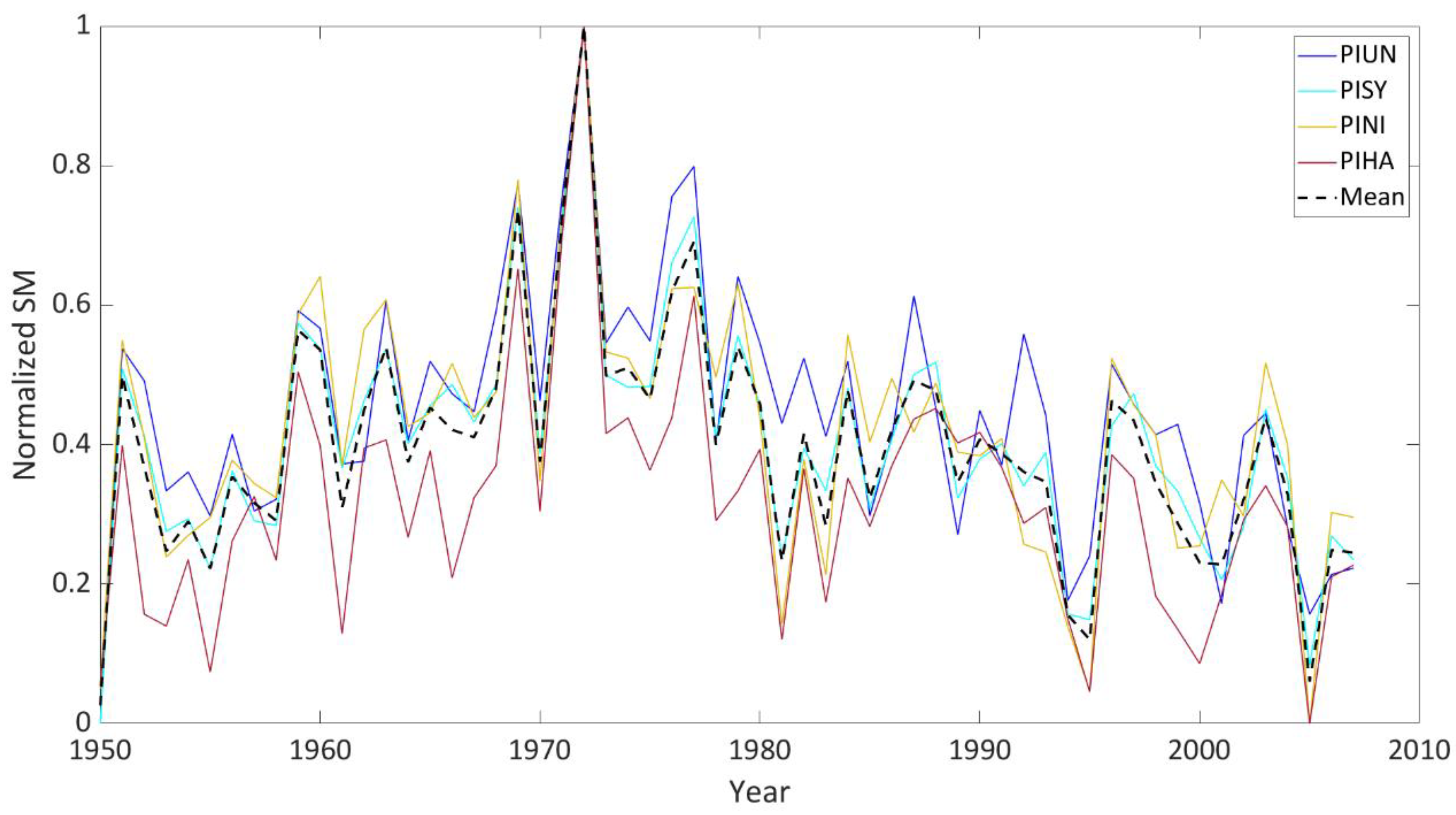
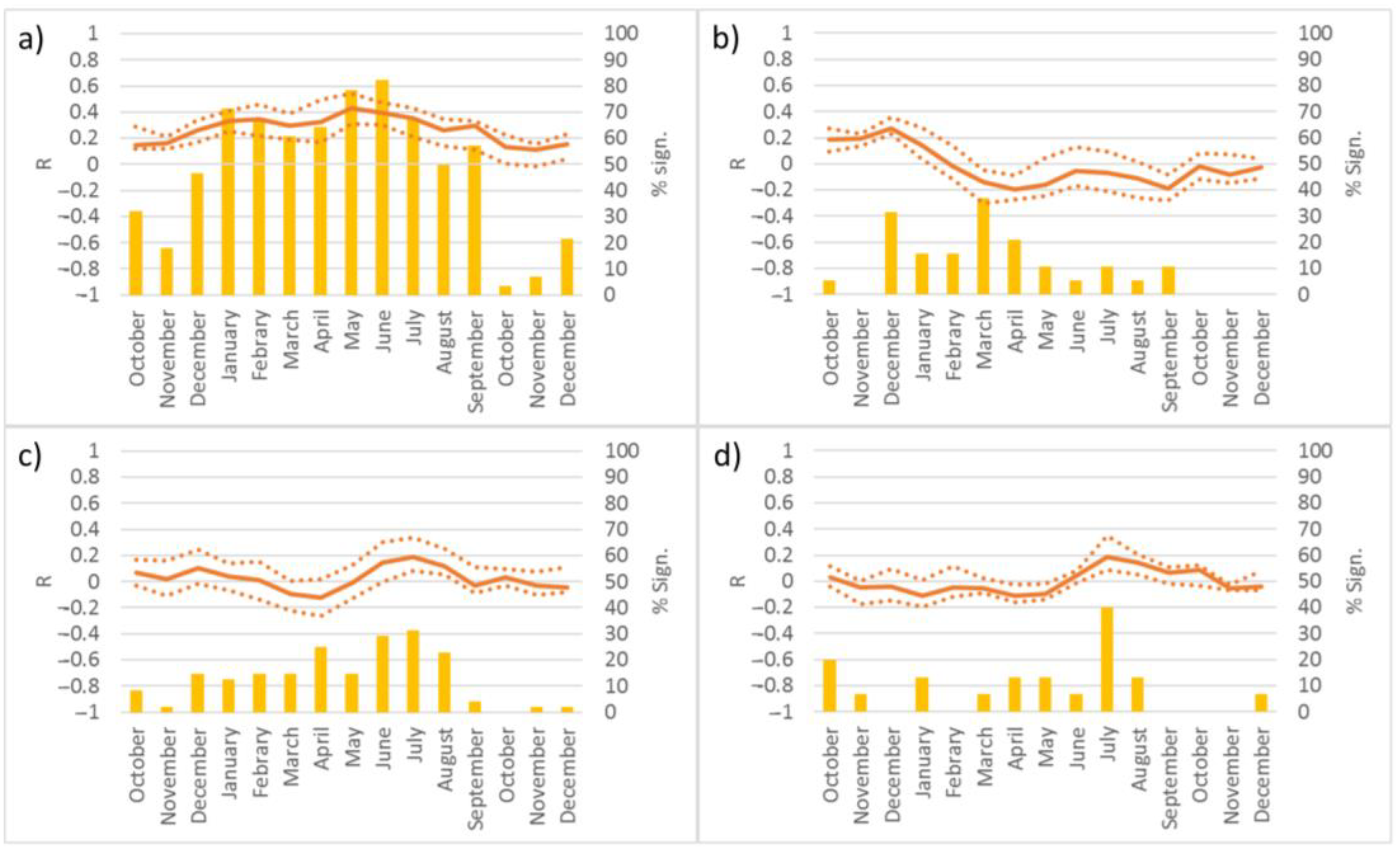
3.1. Relationship between Soil Moisture and Tree Growth
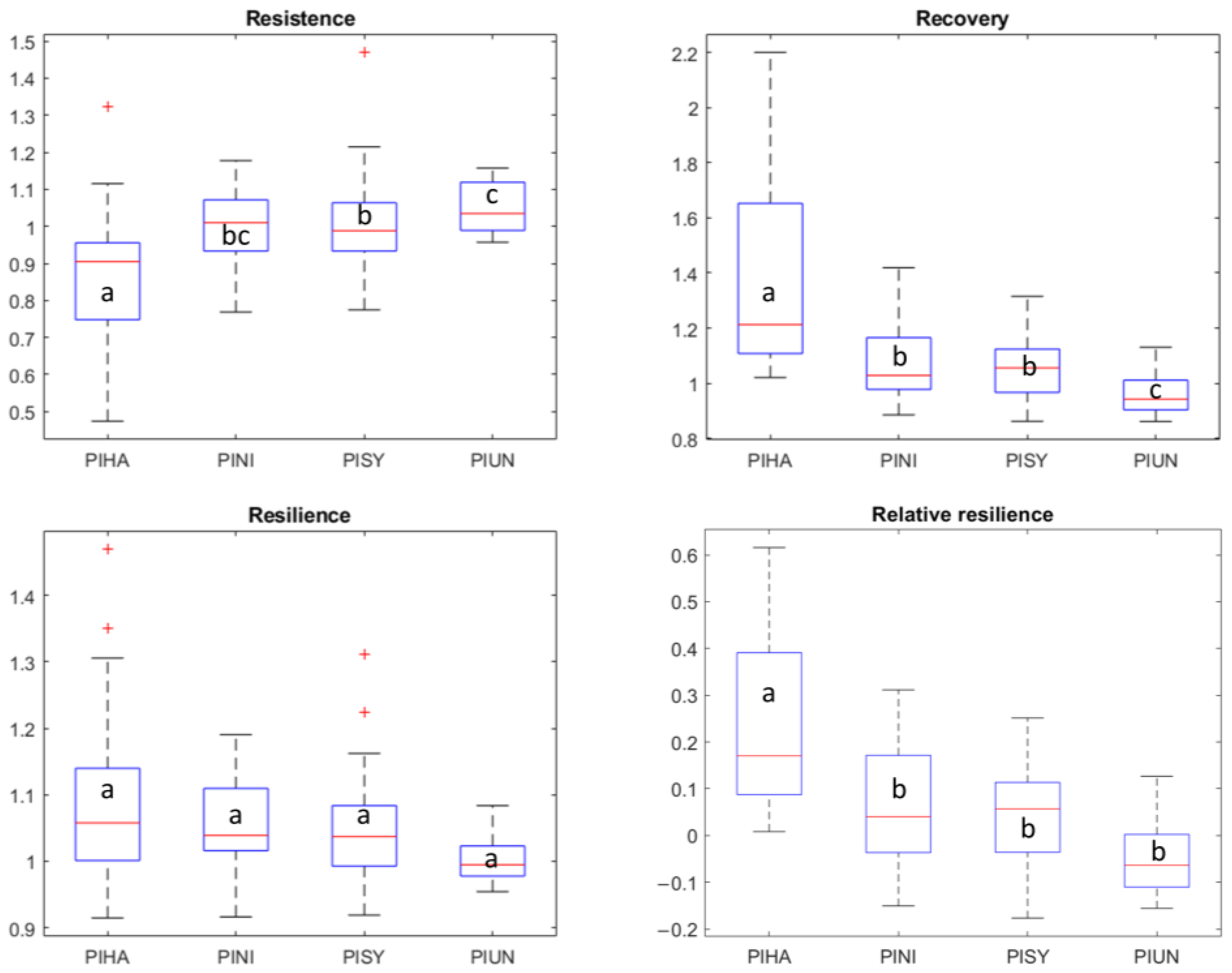
3.2. Tree Species’ Response to Droughts
3.3. Environmental Modulation of Pine Resilience
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Phillips, O.L.; Aragão, L.E.O.C.; Lewis, S.L.; Fisher, J.B.; Lloyd, J.; López-González, G.; Malhi, Y.; Monteagudo, A.; Peacock, J.; Quesada, C.A.; et al. Drought sensitivity of the Amazon rainforest. Science 2009, 323, 1344–1347. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, art129. [Google Scholar] [CrossRef]
- Adams, H.D.; Luce, C.H.; Breshears, D.D.; Allen, C.D.; Weiler, M.; Hale, V.C.; Smith, A.M.S.; Huxman, T.E. Ecohydrological consequences of drought- and infestation-triggered tree die-off: Insights and hypotheses. Ecohydrology 2012, 5, 145–159. [Google Scholar] [CrossRef]
- DeSoto, L.; Cailleret, M.; Sterck, F.; Jansen, S.; Kramer, K.; Robert, E.M.R.; Aakala, T.; Amoroso, M.M.; Bigler, C.; Camarero, J.J.; et al. Low growth resilience to drought is related to future mortality risk in trees. Nat. Commun. 2020, 11, 545. [Google Scholar] [CrossRef] [PubMed]
- Lasch, P.; Lindner, M.; Erhard, M.; Suckow, F.; Wenzel, A. Regional impact assessment on forest structure and functions under climate change—The Brandenburg case study. For. Ecol. Manag. 2002, 162, 73–86. [Google Scholar] [CrossRef]
- Brang, P.; Spathelf, P.; Larsen, J.B.; Bauhus, J.; Ina, A.B.; Chauvin, C.; Drössler, L.; García-Güemes, C.; Heiri, C.; Kerr, G.; et al. Suitability of close-to-nature silviculture for adapting temperate European forests to climate change. Forestry 2014, 87, 492–503. [Google Scholar] [CrossRef]
- Sohn, J.A.; Saha, S.; Bauhus, J. Potential of forest thinning to mitigate drought stress: A meta-analysis. For. Ecol. Manag. 2016, 380, 261–273. [Google Scholar] [CrossRef]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogée, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A.; et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef]
- Bréda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef]
- Italiano, S.S.; Camarero, J.J.; Colangelo, M.; Borghetti, M.; Castellaneta, M.; Pizarro, M.; Ripullone, F. Assessing Forest Vulnerability to Climate Change Combining Remote Sensing and Tree-Ring Data: Issues, Needs and Avenues. Forests 2023, 14, 1138. [Google Scholar] [CrossRef]
- Hoerling, M.; Eischeid, J.; Perlwitz, J.; Quan, X.; Zhang, T.; Pegion, P. On the increased frequency of Mediterranean drought. J. Clim. 2012, 25, 2146–2161. [Google Scholar] [CrossRef]
- Pauli, H.; Gottfried, M.; Dullinger, S.; Abdaladze, O.; Akhalkatsi, M.; Alonso, J.L.B.; Coldea, G.; Dick, J.; Erschbamer, B.; Calzado, R.F.; et al. Recent plant diversity changes on Europe’s mountain summits. Science 2012, 336, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Serrano, S.M.; Camarero, J.J.; Azorín-Molina, C. Diverse responses of forest growth to drought time-scales in the Northern Hemisphere. Glob. Ecol. Biogeogr. 2014, 23, 1019–1030. [Google Scholar] [CrossRef]
- Camarero, J.J.; Gazol, A.; Sangüesa-Barreda, G.; Oliva, J.; Vicente-Serrano, S.M. To die or not to die: Early-warning signals of dieback in response to a severe drought. J. Ecol. 2015, 103, 44–57. [Google Scholar] [CrossRef]
- Gazol, A.; Ribas, M.; Gutiérrez, E.; Camarero, J.J. Aleppo pine forests from across Spain show drought-induced growth decline and partial recovery. Agric. For. Meteorol. 2017, 232, 186–194. [Google Scholar] [CrossRef]
- Gea-Izquierdo, G.; Nicault, A.; Battipaglia, G.; Dorado-Liñán, I.; Gutiérrez, E.; Ribas, M.; Guiot, J. Risky future for Mediterranean forests unless they undergo extreme carbon fertilization. Glob. Chang. Biol. 2017, 23, 2915–2927. [Google Scholar] [CrossRef]
- Senf, C.; Buras, A.; Zang, C.S.; Rammig, A.; Seidl, R. Excess forest mortality is consistently linked to drought across Europe. Nat. Commun. 2020, 11, 6200. [Google Scholar] [CrossRef]
- Peltier, D.M.P.; Fell, M.; Ogle, K. Legacy effects of drought in the southwestern United States: A multi-species synthesis. Ecol. Monogr. 2016, 86, 312–326. [Google Scholar] [CrossRef]
- Greenwood, S.; Ruiz-Benito, P.; Martínez-Vilalta, J.; Lloret, F.; Kitzberger, T.; Allen, C.D.; Kraft, N.J. Tree mortality across biomes is promoted by drought intensity, lower wood density and higher specific leaf area. Ecol. Lett. 2017, 20, 539–553. [Google Scholar] [CrossRef]
- Yin, J.; Bauerle, T.L. A global analysis of plant recovery performance from water stress. Oikos 2017, 126, 1377–1388. [Google Scholar] [CrossRef]
- Andreu-Hayles, L.A.I.A.; Planells, O.; Gutierrez, E.; Muntan, E.; Helle, G.; Anchukaitis, K.J.; Schleser, G.H. Long tree-ring chronologies reveal 20th century increases in water-use efficiency but no enhancement of tree growth at five Iberian pine forests. Glob. Chang. Biol. 2011, 17, 2095–2112. [Google Scholar] [CrossRef]
- Veuillen, L.; Prévosto, B.; Alfaro-Sánchez, R.; Badeau, V.; Battipaglia, G.; Beguería, S.; Bravo, F.; Boivin, T.; Camarero, J.J.; Čufar, K.; et al. Pre-and post-drought conditions drive resilience of Pinus halepensis across its distribution range. Agric. For. Meteorol. 2023, 339, 109577. [Google Scholar] [CrossRef]
- Fatichi, S.; Leuzinger, S.; Korner, C. Moving beyond photosynthesis: From carbon source to sink-driven vegetation modeling. N. Phytol. 2014, 201, 1086–1095. [Google Scholar] [CrossRef]
- Klein, T. The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric and anisohydric behaviours. Funct. Ecol. 2014, 28, 1313–1320. [Google Scholar] [CrossRef]
- Babst, F.; Bouriaud, O.; Poulter, B.; Trouet, V.; Girardin, M.P.; Frank, D.C. Twentieth century redistribution in climatic drivers of global tree growth. Sci. Adv. 2019, 5, eaat4313. [Google Scholar] [CrossRef]
- Babst, F.; Bodesheim, P.; Charney, N.; Friend, A.D.; Girardin, M.P.; Klesse, S.; Moore, D.J.P.; Seftigen, K.; Björklund, J.; Bouriaud, O.; et al. When tree rings go global: Challenges and opportunities for retro-and prospective insight. Quat. Sci. Rev. 2018, 197, 1–20. [Google Scholar] [CrossRef]
- Gazol, A.; Camarero, J.J.; Vicente-Serrano, S.M.; Sánchez-Salguero, R.; Gutiérrez, E.; de Luis, M.; Sangüesa-Barreda, G.; Novak, K.; Rozas, V.; Tíscar, P.A.; et al. Forest resilience to drought varies across biomes. Glob. Chang. Biol. 2018, 24, 2143–2158. [Google Scholar] [CrossRef] [PubMed]
- Nicault, A.; Rathgeber, C.; Tessier, L.; Thomas, A. Observations sur la mise en place du cerne chez le pin d’Alep (Pinus halepensis Mill.): Confrontation entre les mesures de croissance radiale, de densité et les facteurs climatiques. Ann. For. Sci. 2001, 58, 769–784. [Google Scholar] [CrossRef]
- de Luis, M.; Novak, K.; Raventós, J.; Gričar, J.; Prislan, P.; Čufar, K. Climate factors promoting intra-annual density fluctuations in Aleppo pine (Pinus halepensis) from semiarid sites. Dendrochronologia 2011, 29, 163–169. [Google Scholar] [CrossRef]
- de Luis, M.; Čufar, K.; di Filippo, A.; Novak, K.; Papadopoulos, A.; Piovesan, G.; Rathgeber, C.B.K.; Raventós, J.; Saz, M.A.; Smith, K.T. Plasticity in Dendroclimatic Response across the Distribution Range of Aleppo Pine (Pinus halepensis). PLoS ONE 2013, 8, e83550. [Google Scholar] [CrossRef] [PubMed]
- Novak, K.; de Luis, M.; Raventós, J.; Čufar, K. Climatic signals in tree-ring widths and wood structure of Pinus halepensis in contrasted environmental conditions. Trees 2013, 27, 927–936. [Google Scholar] [CrossRef]
- Attolini, M.R.; Calvani, F.; Galli, M.; Nanni, T.; Ruggiero, L.; Schaer, E.; Zuanni, F. The relationship between climatic variables and wood structure in Pinus halepensis Mill. Theor. Appl. Climatol. 1990, 41, 121–127. [Google Scholar] [CrossRef]
- Requena-Mullor, J.M.; Steiner, A.; Keppel-Aleks, G.; Ibáñez, I. Tradeoffs in forest resilience to satellite-based estimates of water and productivity losses. Remote Sens. Environ. 2023, 285, 113414. [Google Scholar] [CrossRef]
- Nikinmaa, L.; Lindner, M.; Cantarello, E.; Jump, A.S.; Seidl, R.; Winkel, G.; Muys, B. Reviewing the use of resilience concepts in forest sciences. Curr. For. Rep. 2020, 6, 61–80. [Google Scholar] [CrossRef]
- Manrique-Alba, A.; Ruiz-Yanetti, S.; Moutahir, H.; Novak, K.; de Luis, M.; Bellot, J. Soil moisture and its role in growth-climate relationships across an aridity gradient in semiarid Pinus halepensis forests. Sci. Total Environ. 2017, 574, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Fernández, J.; Almendra-Martín, L.; de Luis, M.; González-Zamora, A.; Herrero-Jiménez, C. Tracking tree growth through satellite soil moisture monitoring: A case study of Pinus halepensis in Spain. Remote Sens. Environ. 2019, 235, 111422. [Google Scholar] [CrossRef]
- González-Zamora, Á.; Almendra-Martín, L.; de Luis, M.; Martínez-Fernández, J. Influence of soil moisture vs. climatic factors in Pinus halepensis growth variability in Spain: A study with remote sensing and modeled data. Remote Sens. 2021, 13, 757. [Google Scholar] [CrossRef]
- Gumuzzio, A.; Brocca, L.; Sánchez, N.; González-Zamora, Á.; Martínez-Fernández, J. Comparison of SMOS, modelled and in situ long-term soil moisture series in the NW of Spain. Hydrol. Sci. J. 2016, 61, 2610–2625. [Google Scholar] [CrossRef]
- Almendra-Martín, L.; Martínez-Fernández, J.; González-Zamora, Á.; Benito-Verdugo, P.; Herrero-Jiménez, C.M. Agricultural drought trends on the Iberian Peninsula: An analysis using modeled and reanalysis soil moisture products. Atmosphere 2021, 12, 236. [Google Scholar] [CrossRef]
- Almendra-Martín, L.; Martínez-Fernández, J.; Piles, M.; González-Zamora, A. Comparison of gap-filling techniques applied to the CCI soil moisture database in Southern Europe. Remote Sens. Environ. 2021, 258, 112377. [Google Scholar] [CrossRef]
- Ministerio para la Transición Ecológica y el Reto Demográfico (MTERD). Cuarto Inventario Forestal Nacional; Ministerio para la Transición Ecológica y el Reto Demográfico, Ed.; Ministerio para la Transición Ecológica y el Reto Demográfico: Madrid, Spain, 2022. [Google Scholar]
- do Amaral Franco, J. Pinus L. in Flora Ibéria; Castroviejo, S., Lain, M., López González, G., Monserrat, P., Muñoz-Garmendia, F., Paiva, J., Villar, L., Eds.; Real Jardín Botánico, CSIC: Madrid, Spain, 1986; Volume I. [Google Scholar]
- Cano, E.; Cano-Ortiz, A.; Fuentes, J.C.P.; Quinto-Canas, R.; Igbareyeh, J.; del Río, S.; Gomes, C.J.P. Ecological and Syntaxonomic Analysis of Pinus halepensis Mill. in the Iberian Peninsula and Balearic Islands. Land 2022, 11, 369. [Google Scholar] [CrossRef]
- Ovenden, T.S.; Perks, M.P.; Clarke, T.K.; Mencuccini, M.; Jump, A.S. Life after recovery: Increased resolution of forest resilience assessment sheds new light on post-drought compensatory growth and recovery dynamics. J. Ecol. 2021, 109, 3157–3170. [Google Scholar] [CrossRef]
- Schwarz, J.; Skiadaresis, G.; Kohler, M.; Kunz, J.; Schnabel, F.; Vitali, V.; Bauhus, J. Quantifying growth responses of trees to drought—A critique of commonly used resilience indices and recommendations for future studies. Curr. For. Rep. 2020, 6, 185–200. [Google Scholar] [CrossRef]
- Serra-Maluquer, X.; Mencuccini, M.M.; Martínez-Vilalta, J. Changes in tree resistance, recovery and resilience across three successive extreme droughts in the Northeast Iberian Peninsula. Oecologia 2018, 187, 343–354. [Google Scholar] [CrossRef]
- Manrique-Alba, A.; Beguería, S.; Camarero, J.J. Long-term effects of forest management on post-drought growth resilience: An analytical framework. Sci. Total Environ. 2022, 810, 152374. [Google Scholar] [CrossRef]
- Fritts, H.C. Tree Rings and Climate; Academic Press: New York, NY, USA, 1976. [Google Scholar]
- Anderegg, W.R.L.; Schwalm, C.; Biondi, F.; Camarero, J.J.; Koch, G.; Litvak, M.; Ogle, K.; Shaw, J.D.; Shevliakova, E.; Williams, A.P.; et al. Pervasive drought legacies in forest ecosystems and their implications for carbon cycle models. Science 2015, 349, 528–532. [Google Scholar] [CrossRef]
- Navarro-Cerrillo, R.M.; Sanchez-Salguero, R.; Rodriguez, C.; Lazo, J.D.; Moreno-Rojas, J.M.; Palacios-Rodriguez, G.; Camarero, J.J. Is thinning an alternative when trees could die in response to drought? The case of planted Pinus nigra and P. Sylvestris stands in southern Spain. For. Ecol. Manag. 2019, 433, 313–324. [Google Scholar] [CrossRef]
- Lloret, F.; Keeling, E.G.; Sala, A. Components of tree resilience: Effects of successive low-growth episodes in old ponderosa pine forests. Oikos 2011, 120, 1909–1920. [Google Scholar] [CrossRef]
- Li, X.; Piao, S.; Wang, K.; Wang, X.; Wang, T.; Ciais, P.; Chen, A.; Lian, X.; Peng, S.; Peñuelas, J. Temporal trade-off between gymnosperm resistance and resilience increases forest sensitivity to extreme drought. Nat. Ecol. Evol. 2020, 4, 1075–1083. [Google Scholar] [CrossRef]
- Zheng, T.; Martinez-Vilalta, J.; Garcia-Valdes, R.; Gazol, A.; Camarero, J.J.; Mencuccini, M. Disentangling biology from mathematical necessity in twentieth-century gymnosperm resilience trends. Nat. Ecol. Evol. 2021, 5, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Borja, M.E.; Andivia, E.; Candel-Pérez, D.; Linares, J.C.; Camarero, J.J. Long term forest management drives drought resilience in Mediterranean black pine forest. Trees 2021, 35, 1651–1662. [Google Scholar] [CrossRef]
- Royo-Navascues, M.; del Castillo, E.M.; Tejedor, E.; Serrano-Notivoli, R.; Longares, L.A.; Saz, M.A.; Novak, K.; de Luis, M. The Imprint of Droughts on Mediterranean Pine Forests. Forests 2022, 13, 1396. [Google Scholar] [CrossRef]
- Tejedor, E.; Saz, M.A.; Cuadrat, J.M.; Esper, J.; de Luis, M. Temperature variability in the Iberian Range since 1602 inferred from tree-ring records. Clim. Past. 2017, 13, 93–105. [Google Scholar] [CrossRef]
- Royo-Navascues, M.; del Castillo, E.M.; Serrano-Notivoli, R.; Tejedor, E.; Novak, K.; Longares, L.A.; Saz, M.A.; de Luis, M. When density matters: The spatial balance between early and latewood. Forests 2021, 12, 818. [Google Scholar] [CrossRef]
- del Castillo, E.M.; Tejedor, E.; Serrano-Notivoli, R.; Novak, K.; Saz, M.Á.; Longares, L.A.; de Luis, M. Contrasting patterns of tree growth of Mediterranean pine species in the Iberian Peninsula. Forests 2018, 9, 416. [Google Scholar] [CrossRef]
- Holmes, R. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Bunn, A.; Korpela, M.; Biondi, F.; Campelo, F.; Mérian, P.; Qeadan, F. dplR: Dendrochronology Program Library in R [online], R package version 1.6.9; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://CRAN.R-project.org/package=dplR (accessed on 1 July 2023).
- Muñoz-Sabater, J.; Dutra, E.; Agustí-Panareda, A.; Albergel, C.; Arduini, G.; Balsamo, G.; Boussetta, S.; Choulga, M.; Harrigan, S.; Hersbach, H.; et al. ERA5-Land: A state-of-the-art global reanalysis dataset for land applications. Earth Syst. Sci. Data 2021, 13, 4349–4383. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration-Guidelines for Computing Crop Water Requirements-FAO Irrigation and Drainage Paper 56; FAO: Rome, Italy, 1998; Volume 300, p. D05109. [Google Scholar]
- Wang, Y.; Shao, M.A.; Liu, Z.; Warrington, D.N. Regional spatial pattern of deep soil water content and its influencing factors. Hydrol. Sci. J. 2012, 57, 265–281. [Google Scholar] [CrossRef]
- Williams, N.D.; Walling, D.E.; Leeks, G.J.L. An analysis of the factors contributing to the settling potential of fine fluvial sediment. Hydrol. Process. 2008, 22, 4153–4162. [Google Scholar] [CrossRef]
- Yuan, L.; Li, L.; Zhang, T.; Chen, L.; Liu, W.; Hu, S.; Yang, L. Modeling Soil Moisture from Multisource Data by Stepwise Multilinear Regression: An Application to the Chinese Loess Plateau. ISPRS Int. J. Geoinf. 2021, 10, 233. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Automat. Contr. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Akaike, H. Information theory and an extension of the maximum likelihood principle. In Selected Papers of Hirotugu Akaike; Springer: New York, NY, USA, 1998; pp. 199–213. [Google Scholar]
- Anderson, D.R.; Burnham, K.P. Avoiding pitfalls when using information-theoretic methods. J. Wildl. Manag. 2002, 66, 912–918. [Google Scholar] [CrossRef]
- Groemping, U. Relative Importance for Linear Regression in R: The Package relaimpo. J. Stat. Softw. 2006, 17, 1–27. [Google Scholar]
- Sabaté, S.; Gracia, C.A.; Sánchez, A. Likely effects of climate change on growth of Quercus ilex, Pinus halepensis, Pinus pinaster, Pinus sylvestris and Fagus sylvatica forests in the Mediterranean region. For. Ecol. Manag. 2002, 162, 23–37. [Google Scholar] [CrossRef]
- Swidrak, I.; Gruber, A.; Kofler, W.; Oberhuber, W. Effects of environmental conditions on onset of xylem growth in Pinus sylvestris under drought. Tree Physiol. 2011, 31, 483–493. [Google Scholar] [CrossRef]
- Oberhuber, W.; Gruber, A. Climatic influences on intra-annual stem radial increment of Pinus sylvestris (L.) exposed to drought. Trees 2010, 24, 887–898. [Google Scholar] [CrossRef]
- Camarero, J.J.; Guerrero-Campo, J.; Gutiérrez, E. Tree-ring growth and structure of Pinus uncinata and Pinus sylvestris in the Central Spanish Pyrenees. Arct. Antarct. Alp. Res. 1998, 30, 1–10. [Google Scholar] [CrossRef]
- Sanmiguel-Vallelado, A.; Camarero, J.J.; Gazol, A.; Morán-Tejeda, E.; Sangüesa-Barreda, G.; Alonso-González, E.; Gutiérrez, E.; Alla, A.Q.; Galván, J.D.; López-Moreno, J.I. Detecting snow-related signals in radial growth of Pinus uncinata mountain forests. Dendrochronologia 2019, 57, 125622. [Google Scholar] [CrossRef]
- Gazol, A.; Camarero, J.J.; Anderegg, W.R.L.; Vicente-Serrano, S.M. Impacts of droughts on the growth resilience of Northern Hemisphere forests. Glob. Ecol. Biogeogr. 2017, 26, 166–176. [Google Scholar] [CrossRef]
- Prislan, P.; Gričar, J.; de Luis, M.; Novak, K.; del Castillo, E.M.; Schmitt, U.; Koch, G.; Štrus, J.; Mrak, P.; Žnidarič, M.T.; et al. Annual Cambial Rhythm in Pinus halepensis and Pinus sylvestris as Indicator for Climate Adaptation. Front. Plant Sci. 2016, 7, 1923. [Google Scholar] [CrossRef] [PubMed]
- Novak, K.; de Luis, M.; Gričar, J.; Prislan, P.; Merela, M.; Smith, K.T.; Čufar, K. Missing and dark rings associated with drought in Pinus halepensis. IAWA J. 2016, 37, 260–274. [Google Scholar] [CrossRef]
- Sperlich, D.; Chang, C.T.; Peñuelas, J.; Gracia, C.; Sabaté, S. Seasonal variability of foliar photosynthetic and morphological traits and drought impacts in a Mediterranean mixed forest. Tree Physiol. 2015, 35, 501–520. [Google Scholar] [CrossRef] [PubMed]
- García-Forner, N.; Adams, H.D.; Sevanto, S.; Collins, A.D.; Dickman, L.T.; Hudson, P.J.; Zeppel, M.J.B.; Jenkins, M.W.; Powers, H.; Martínez-Vilalta, J.; et al. Responses of two semiarid conifer tree species to reduced precipitation and warming reveal new perspectives for stomatal regulation. Plant Cell Environ. 2016, 39, 38–49. [Google Scholar] [CrossRef]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Poyatos, R.; Aguadé, D.; Retana, J.; Mencuccini, M. A new look at water transport regulation in plants. N. Phytol. 2014, 204, 105–115. [Google Scholar] [CrossRef]
- Preisler, Y.; Tatarinov, F.; Grünzweig, J.M.; Bert, D.; Ogee, J.; Wingate, L.; Rotenberg, E.; Rohatyn, S.; Her, N.; Moshe, I.; et al. Mortality versus survival in drought-affected Aleppo pine forest depends on the extent of rock cover and soil stoniness. Funct. Ecol. 2019, 33, 901–912. [Google Scholar] [CrossRef]
- Klein, T.; Cohen, S.; Yakir, D. Hydraulic adjustments underlying drought resistance of Pinus halepensis. Tree Physiol. 2011, 31, 637–648. [Google Scholar] [CrossRef]
- Grivet, D.; Sebastiani, F.; González-Martínez, S.C.; Vendramin, G.G. Patterns of polymorphism resulting from long-range colonization in the Mediterranean conifer Aleppo pine. N. Phytol. 2009, 184, 1016–1028. [Google Scholar] [CrossRef]
- Santini, F.; Climent, J.M.; Voltas, J. Phenotypic integration and life history strategies among populations of Pinus halepensis: An insight through structural equation modelling. Ann. Bot. 2019, 124, 1161–1171. [Google Scholar] [CrossRef]
- Almendra-Martín, L.; Martínez-Fernández, J.; Piles, M.; González-Zamora, Á.; Benito-Verdugo, P.; Gaona, J. Analysis of soil moisture trends in Europe using rank-based and empirical decomposition approaches. Glob. Planet. Change 2022, 215, 103868. [Google Scholar] [CrossRef]
- Mazza, G.; Markou, L.; Sarris, D. Species-specific growth dynamics and vulnerability to drought at the single tree level in a Mediterranean reforestation. Trees 2021, 35, 1697–1710. [Google Scholar] [CrossRef]
- Rodriguez de Prado, D.; Riofrío, J.; Aldea, J.; Bravo, F.; de Aza, C.H. Competition and climate influence in the basal area increment models for Mediterranean mixed forests. For. Ecol. Manag. 2022, 506, 119955. [Google Scholar] [CrossRef]
- Brunet, M.; Jones, P.D.; Sigró, J.; Saladié, Ó.; Aguilar, E.; Moberg, A.; Della-Marta, P.M.; Lister, D.; Walther, A.; López, D. Temporal and spatial temperature variability and change over Spain during 1850–2005. J. Geophys. Res. Atmos. 2007, 112, D12. [Google Scholar] [CrossRef]
- Serrano-Notivoli, R.; Beguería, S.; de Luis, M. STEAD: A high-resolution daily gridded temperature dataset for Spain. Earth Syst. Sci. Data 2019, 11, 1171–1188. [Google Scholar] [CrossRef]
- Esper, J.; Hartl, C.; Tejedor, E.; de Luis, M.; Günther, B.; Büntgen, U. High-resolution temperature variability reconstructed from Black pine tree ring densities in southern Spain. Atmosphere 2020, 11, 748. [Google Scholar] [CrossRef]
- Camarero, J.J.; Linares, J.C.; García-Cervigón, A.I.; Batllori, E.; Martínez, I.; Gutiérrez, E. Back to the future: The responses of alpine treelines to climate warming are constrained by the current ecotone structure. Ecosystems 2017, 20, 683–700. [Google Scholar] [CrossRef]
- Feuillet, T.; Birre, D.; Milian, J.; Godard, V.; Clauzel, C.; Serrano-Notivoli, R. Spatial dynamics of alpine tree lines under global warming: What explains the mismatch between tree densification and elevational upward shifts at the tree line ecotone? J. Biogeogr. 2020, 47, 1056–1068. [Google Scholar] [CrossRef]
- Lenz, A.; Hoch, G.; Körner, C. Early season temperature controls cambial activity and total tree ring width at the alpine treeline. Plant Ecol. Divers. 2013, 6, 365–375. [Google Scholar] [CrossRef]
- Martínez, I.; González-Taboada, F.; Wiegand, T.; Camarero, J.J.; Gutiérrez, E. Dispersal limitation and spatial scale affect model-based projections of Pinus uncinata response to climate change in the Pyrenees. Glob. Chang. Biol. 2012, 18, 1714–1724. [Google Scholar] [CrossRef]
- Grossiord, C.; Buckley, T.N.; Cernusak, L.A.; Novick, K.A.; Poulter, B.; Siegwolf, R.T.; Sperry, J.S.; McDowell, N.G. Plant responses to rising vapor pressure deficit. N. Phytol. 2020, 226, 1550–1566. [Google Scholar] [CrossRef]
- Sanginés de Cárcer, P.; Vitasse, Y.; Peñuelas, J.; Jassey, V.E.; Buttler, A.; Signarbieux, C. Vapor-pressure deficit and extreme climatic variables limit tree growth. Glob. Chang. Biol. 2018, 24, 1108–1122. [Google Scholar] [CrossRef]
- Noguera, I.; Vicente-Serrano, S.M.; Peña-Angulo, D.; Domínguez-Castro, F.; Juez, C.; Tomás-Burguera, M.; Lorenzo-Lacruz, J.; Azorín-Molina, C.; Halifa-Marín, A.; Fernández-Duque, B.; et al. Assessment of vapor pressure deficit variability and trends in Spain and possible connections with soil moisture. Atmos. Res. 2023, 285, 106666. [Google Scholar] [CrossRef]
- Gaona, J.; Quintana-Seguí, P.; Escorihuela, M.J.; Boone, A.; Llasat, M.C. Interactions between precipitation, evapotranspiration and soil-moisture-based indices to characterize drought with high-resolution remote sensing and land-surface model data. Nat. Hazards Earth Syst. Sci. 2022, 22, 3461–3485. [Google Scholar] [CrossRef]
- Navarro-Cerrillo, R.M.; Cachinero-Vivar, A.M.; Ruiz-Gómez, F.J.; Camarero, J.J.; González-Pérez, J.A.; Pérez-Priego, Ó. Planted or Natural Pine Forests, Which One Will Better Recover after Drought? Insights from Tree Growth and Stable C and H Isotopes. Forests 2023, 14, 573. [Google Scholar] [CrossRef]
- MartÍn-Benito, D.; Beeckman, H.; Canellas, I. Influence of drought on tree rings and tracheid features of Pinus nigra and Pinus sylvestris in a mesic Mediterranean forest. Eur. J. For. Res. 2013, 132, 33–45. [Google Scholar] [CrossRef]
- Soto, A.; Robledo-Arnuncio, J.J.; González-Martínez, S.C.; Smouse, P.E.; Alía, R. Climatic niche and neutral genetic diversity of the six Iberian pine species: A retrospective and prospective view. Mol. Ecol. 2010, 19, 1396–1409. [Google Scholar] [CrossRef]
- de Luis, M.; Gonzalez-Hidalgo, J.C.; Brunetti, M.; Longares, L.A. Precipitation concentration changes in Spain 1946–2005. Nat. Hazards Earth Syst. Sci. 2011, 11, 1259–1265. [Google Scholar] [CrossRef]
- Ruiz-Labourdette, D.; Nogués-Bravo, D.; Ollero, H.S.; Schmitz, M.F.; Pineda, F.D. Forest composition in Mediterranean mountains is projected to shift along the entire elevational gradient under climate change. J. Biogeogr. 2012, 39, 162–176. [Google Scholar] [CrossRef]
- Klisz, M.; Jevšenak, J.; Prokopuk, Y.; Gil, W.; Mohytych, V.; Puchałka, R. Coping with Central European climate–xylem adjustment in seven non-native conifer tree species. Dendrobiology 2022, 88, 105–123. [Google Scholar] [CrossRef]
- Song, Y.; Sterck, F.; Sass-Klaassen, U.; Li, C.; Poorter, L. Growth resilience of conifer species decreases with early, long-lasting and intense droughts but cannot be explained by hydraulic traits. J. Ecol. 2022, 110, 2088–2104. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Domínguez-Castro, F.; Murphy, C.; Peña-Angulo, D.; Tomas-Burguera, M.; Noguera, I.; López-Moreno, J.I.; Juez, C.; Grainger, S.; Eklundh, L.; et al. Increased vegetation in mountainous headwaters amplifies water stress during dry periods. Geophys. Res. Lett. 2021, 48, e2021GL094672. [Google Scholar] [CrossRef]
- Goeking, S.A.; Tarboton, D.G. Variable Streamflow Response to Forest Disturbance in the Western US: A Large-Sample Hydrology Approach. Water Resour. Res. 2022, 58, e2021WR031575. [Google Scholar] [CrossRef]
- Krich, C.; Runge, J.; Miralles, D.G.; Migliavacca, M.; Perez-Priego, O.; El-Madany, T.; Carrara, A.; Mahecha, M.D. Estimating causal networks in biosphere-atmosphere interaction with the PCMCI approach. Biogeosciences 2020, 17, 1033–1061. [Google Scholar] [CrossRef]
- Orlowsky, B.; Seneviratne, S.I. Global changes in extreme events: Regional and seasonal dimension. Clim. Change 2012, 110, 669–696. [Google Scholar] [CrossRef]
- Russo, A.; Gouveia, C.M.; Dutra, E.; Soares, P.M.M.; Trigo, R.M. The synergy between drought and extremely hot summers in the Mediterranean. Environ. Res. Lett. 2019, 14, 014011. [Google Scholar] [CrossRef]

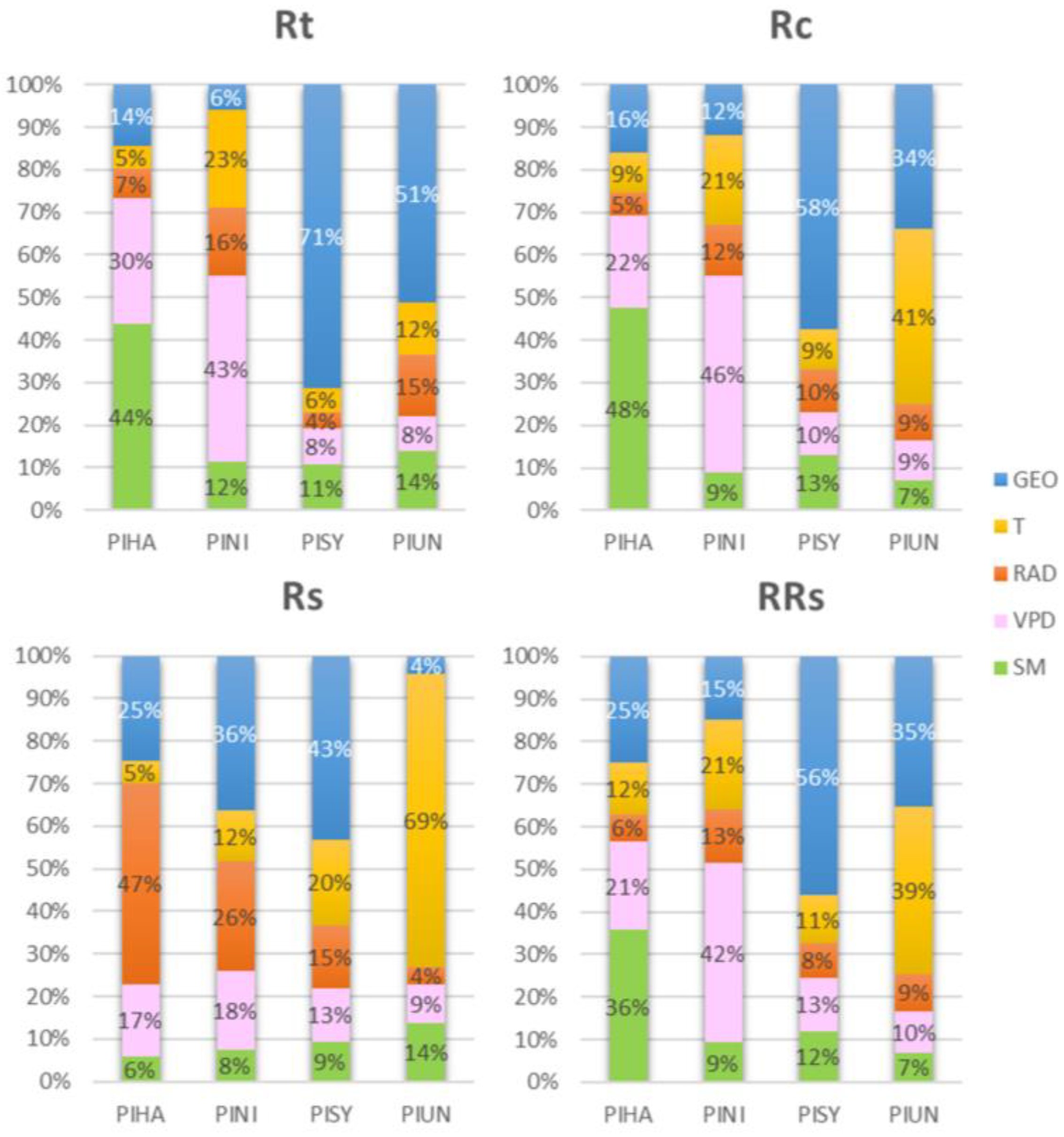
| R2 | Rt | Rc | Rs | RRs |
|---|---|---|---|---|
| PIHA | 0.26 | 0.79 | 0.35 | 0.09 |
| Geo. | Latitude | Latitude | Longitude | Longitude |
| PINI | 0.14 | 0.57 | 0.37 | 0.26 |
| Geo. | Coast | Latitude | Altitude | Latitude |
| PISY | 0.19 | 0.28 | 0.11 | 0.44 |
| Geo. | Longitude | Longitude | Coast | Longitude |
| PIUN | 0.11 | 0.58 | 0.39 | 0.28 |
| Geo. | Altitude | Altitude | Coast | Altitude |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Zamora, Á.; Almendra-Martín, L.; de Luis, M.; Gaona, J.; Martínez-Fernández, J. How Are Pine Species Responding to Soil Drought and Climate Change in the Iberian Peninsula? Forests 2023, 14, 1530. https://doi.org/10.3390/f14081530
González-Zamora Á, Almendra-Martín L, de Luis M, Gaona J, Martínez-Fernández J. How Are Pine Species Responding to Soil Drought and Climate Change in the Iberian Peninsula? Forests. 2023; 14(8):1530. https://doi.org/10.3390/f14081530
Chicago/Turabian StyleGonzález-Zamora, Ángel, Laura Almendra-Martín, Martín de Luis, Jaime Gaona, and José Martínez-Fernández. 2023. "How Are Pine Species Responding to Soil Drought and Climate Change in the Iberian Peninsula?" Forests 14, no. 8: 1530. https://doi.org/10.3390/f14081530
APA StyleGonzález-Zamora, Á., Almendra-Martín, L., de Luis, M., Gaona, J., & Martínez-Fernández, J. (2023). How Are Pine Species Responding to Soil Drought and Climate Change in the Iberian Peninsula? Forests, 14(8), 1530. https://doi.org/10.3390/f14081530






