Characteristics of Microbial Abundance in Rhizosphere and Non-Rhizosphere Soils of Permafrost Peatland, Northeast China
Abstract
:1. Introduction
2. Materials and Methods
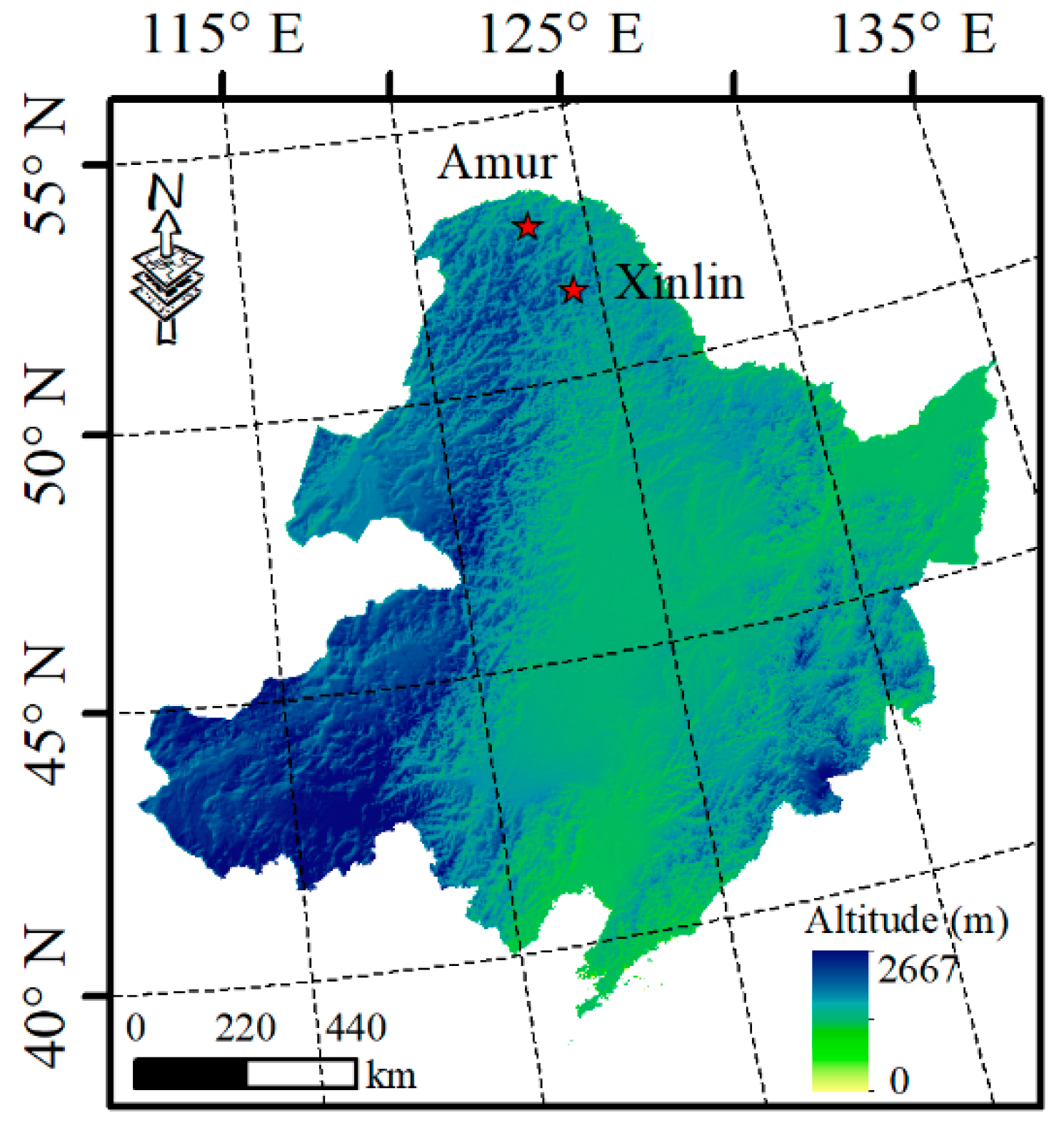
2.1. Site Description and Sampling
2.2. Soil Carbon, Nitrogen, Phosphorus, and pH Determination
2.3. DNA Extraction and Functional Gene Abundance Assay
2.4. Statistical Analyses
3. Results
3.1. SOC, TN, TP, and pH Values
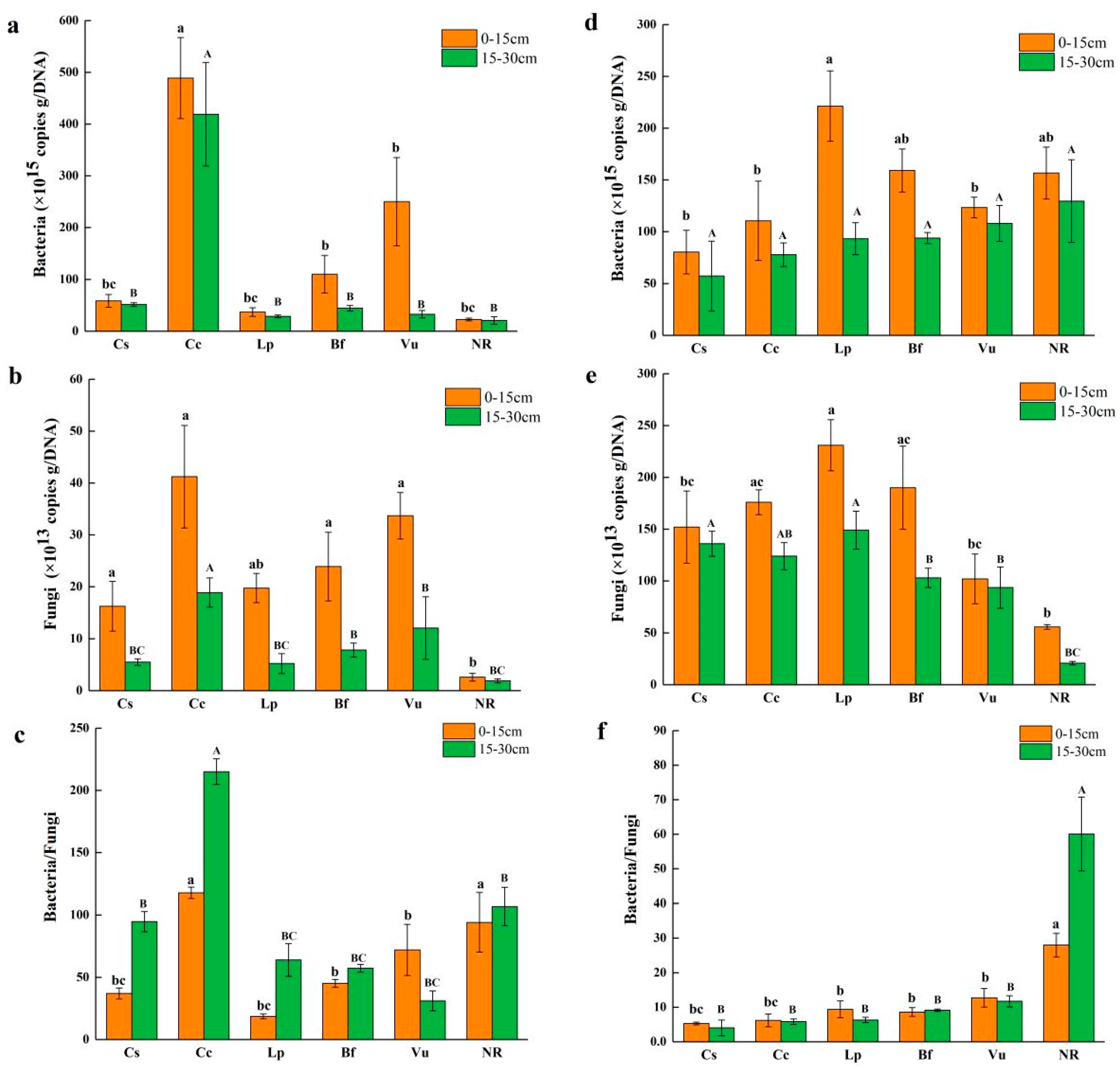
3.2. Abundances of Bacteria and Fungi
3.3. Abundance of Denitrifying Bacteria
3.4. Abundance of N-Fixing Bacteria
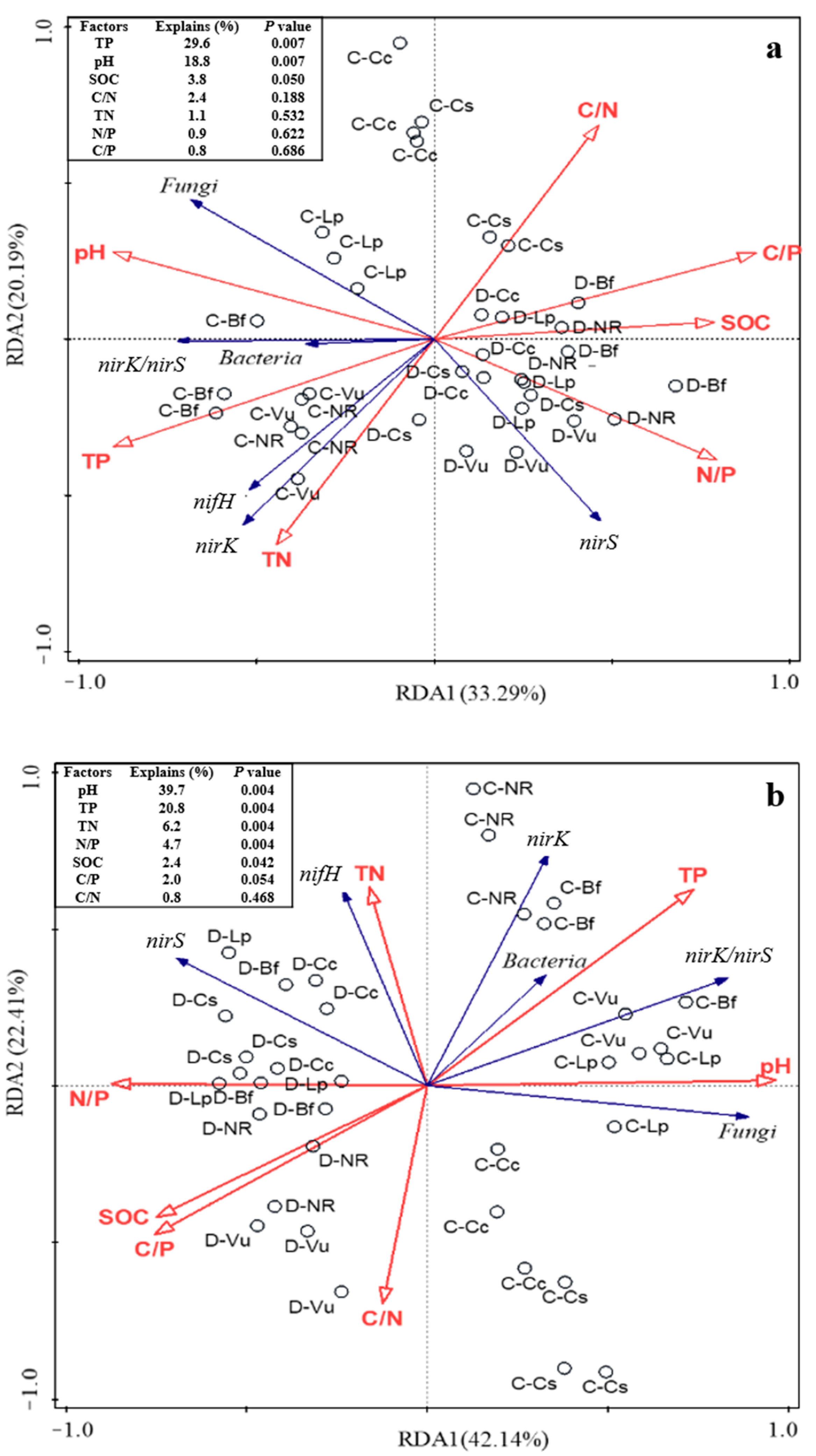
3.5. Effects of Vegetation Type, Soil Depth, and Permafrost Type on Soil Bacteria, Fungi, and Functional Gene Abundances
4. Discussion
4.1. Differences in Soil Microbial Abundance between Rhizosphere and Non-Rhizosphere Soil
4.2. Differences of Soil Microbial Abundance in Topsoil and Subsoil
4.3. Differences in Soil Microbial Abundances in Different Types of Permafrost
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Templer, P.H.; Groffman, P.M.; Flecker, A.S.; Power, A.G. Land use change and soil nutrient transformations in the Los Haitises region of Dominican Republic. Soil Biol. Biochem. 2005, 37, 215–225. [Google Scholar] [CrossRef]
- Kalbitz, K.; Solinger, S.; Park, J.H.; Michalzik, B.; Matzner, E. Controls on the dynamics of dissolved organic matter in soils: A review. Soil Sci. 2000, 165, 277–304. [Google Scholar] [CrossRef]
- Dazzo, F.B.; Garoutte, A.; Hartmann, A. Rhizosphere. In Encyclopedia of Microbiology, 4th ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 147–163. [Google Scholar]
- Bashir, O.; Khan, K.; Hakeem, K.R.; Mir, N.A.; Rather, G.H.; Mohiuddin, R. Soil Microbe Diversity and Root Exudates as Important Aspects of Rhizosphere Ecosystem. In Plant, Soil and Microbes; Springer International Publishing: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Hütsch, B.W.; Augustin, J.; Merbach, W. Plant rhizodeposition–An important source for carbon turnover in soils. J. Plant Nutr. Soil Sci. 2002, 165, 397–407. [Google Scholar] [CrossRef]
- Walker, T.S.; Bais, H.P.; Grotewold, E.; Vivanco, J.M. Root exudation and rhizosphere biology. Plant. Physiol. 2003, 132, 44–51. [Google Scholar] [CrossRef]
- Turner, T.R.; Ramakrishnan, K.; Walshaw, J.; Heavens, D.; Alston, M.; Swarbreck, D.; Osbourn, A.; Grant, A.; Poole, P.S. Comparative metatranscriptomics reveals kingdom level changes in the rhizosphere microbiome of plants. ISME J. 2013, 7, 2248–2258. [Google Scholar] [CrossRef]
- Lynch, J.P. Root architecture and plant productivity. Plant Physiol. 1995, 109, 7–13. [Google Scholar] [CrossRef]
- He, Y.; Hu, W.G.; Ma, D.C.; Yang, Y.; Lan, H.Z.; Gao, Y. Diversity and abundance of ammonia-oxidizing microorganisms in relation to soil environment in rhizosphere soil of Halocnemum strobilaceum in Ebinur Lake wetland. Acta Sci. Circumstantiae 2017, 37, 1967–1975. [Google Scholar]
- Wang, C.H.; Wu, F.; Hu, W.G.; Mo, C.; Zhang, X.H. Community diversity of ammonia-oxidizing bacteria of three plants rhizosphere in Ebinur Lake wetland. Acta Microbiol. Sin. 2015, 55, 1190–1200. [Google Scholar]
- Liu, Y.; Shen, X.; Chen, Y.M.; Wang, L.F.; Chen, Q.M.; Zhang, J.; Xu, Z.F.; Tan, B.; Zhang, L.; Xiao, J.J.; et al. Litter chemical quality strongly affects forest floor microbial groups and ecoenzymatic stoichiometry in the subalpine forest. Ann. Forest Sci. 2019, 76, 106. [Google Scholar] [CrossRef]
- Moreno-Espíndola, I.P.; Ferrara-Guerrero, M.J.; Luna-Guido, M.L.; Ramírez-Villanueva, D.A.; León-Lorenzana, A.S.D.; Gómez-Acata, S.; González-Terreros, E.; Ramírez-Barajas, B.; Navarro-Noya, Y.E.; Sánchez-Rodríguez, L.M.; et al. The bacterial community structure and microbial activity in a traditional organic milpa farming system under different soil moisture conditions. Front. Microbiol. 2018, 9, 2737. [Google Scholar] [CrossRef]
- Damashek, J.; Francis, C.A. Microbial nitrogen cycling in estuaries: From genes to ecosystem processes. Estuaries Coasts 2018, 41, 626–660. [Google Scholar] [CrossRef]
- Huang, L.; Riggins, C.W.; Rodríguez-Zas, S.; Zabaloy, M.C.; Villamil, M.B. Long-term N fertilization imbalances potential N acquisition and transformations by soil microbes. Sci. Total Environ. 2019, 691, 562–571. [Google Scholar] [CrossRef]
- Linda, H.; Cécile, G.R.; Nicol, G.W.; Prosser, J.I. The consequences of niche and physiological differentiation of archaeal and bacterial ammon Aggravated phosphorus limitation on biomass production under increasing nitrogen loading: A meta-analysisia oxidisers for nitrous oxide emissions. ISME J. 2018, 12, 934–943. [Google Scholar]
- Berg, G.; Smalla, K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol. Ecol. 2009, 68, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Innes, L.; Hobbs, P.; Bardgett, R. The impacts of individual plant species on rhizosphere microbial communities in soils of different fertility. Biol. Fertil. Soils 2004, 40, 7–13. [Google Scholar] [CrossRef]
- Weinert, N.; Meincke, R.; Gottwald, C.; Heuer, H.; Gomes, N.C.M.; Schloter, M.; Berg, G.; Smalla, K. Rhizosphere communities of genetically modified Zeaxanthin-accumulating potato plants and their parent cultivar differ less than those of different potato cultivars. Appl. Environ. Microbiol. 2009, 75, 3859–3865. [Google Scholar] [CrossRef]
- Smalla, K.; Wieland, G.; Buchner, A.; Zock, A.; Parzy, J.; Kaiser, S.; Roskot, N.; Heuer, H.; Berg, G. Bulk and rhizosphere soil bacterial communities studied by denaturing gradient gel electrophoresis: Plant-dependent enrichment and seasonal shifts revealed. Appl. Environ. Microbiol. 2001, 67, 4742–4751. [Google Scholar] [CrossRef]
- Wickings, K.; Grandy, A.S.; Reed, S.C.; Cleveland, C.C. The origin of litter chemical complexity during decomposition. Ecol. Lett. 2012, 15, 1180–1188. [Google Scholar] [CrossRef]
- Xue, L.; Kuang, L.G.; Chen, H.Y.; Tan, S.M. Soil nutrients, microorganisms and enzyme activities of different stands. Acta Pedol. Sin. 2003, 40, 280–285. [Google Scholar]
- Kuzyakov, Y.; Blagodatskaya, E. Microbial hotspots and hot moments in soil: Concept & review. Soil Biol. Biochem. 2015, 83, 184–199. [Google Scholar]
- Song, Y.Y.; Jiang, L.; Song, C.C.; Wang, X.W.; Ma, X.Y.; Zhang, H.; Tan, W.W.; Gao, J.L.; Hou, A.X. Microbial abundance and enzymatic activity from tussock and shrub soil in permafrost peatland after 6-year warming. Ecol. Indic. 2021, 126, 107589. [Google Scholar]
- Sun, L.; Li, X.Z.; Wang, X.W.; Lv, J.J.; Li, Z.M.; Hu, Y.M. Latitudinal pattern in species diversity and its response to global warming in permafrost wetlands in the Great Hing’an Mountains, China. Russ. J. Ecol. 2011, 42, 123–132. [Google Scholar]
- Sun, G.Y.; Yu, S.P.; Wang, H.X. Causes, south borderline and subareas of permafrost in Da Hinggan Mountains and Xiao Hinggan Mountains. Sci. Geogr. Sin. 2007, 27, 68–74. [Google Scholar]
- Bao, S.D. Soil Agricultural Chemistry Analysis, 3rd ed.; China Agricultural Press: Beijing, China, 2008. [Google Scholar]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef]
- Wang, H.; Yang, J.P.; Yang, S.H. Effect of a 10 degrees C-elevated temperature under different water contents on the microbial community in a tea orchard soil. Eur. J. Soil Biol. 2014, 62, 113–120. [Google Scholar]
- Gardes, M.; Bruns, T.D. Its primers with enhanced specificity for basidiomycetes-application of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [PubMed]
- Hallin, S.; Lindgren, P.E. PCR detection of genes encoding nitrile reductase in denitrifying bacteria. Appl. Environ. Microbiol. 1999, 65, 1652–1657. [Google Scholar] [CrossRef]
- Petersen, D.G.; Blazewicz, S.J.; Firestone, M.; Herman, D.J.; Turetsky, M.; Waldrop, M. Abundance of microbial genes associated with nitrogen cycling as indices of biogeochemical process rates across a vegetation gradient in Alaska. Environ. Microbiol. 2012, 14, 993–1008. [Google Scholar] [PubMed]
- Fan, L. Response of Diazotrophic Microbial Community to Nitrogen Input and Glyphosate Application in Soils Cropped to Soybean. Ph.D. Thesis, Auburn University, Auburn, AL, USA, 2013. [Google Scholar]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar]
- Hartmann, A.; Schmid, M.; van Tuinen, D.; Berg, G. Plant-driven selection of microbes. Plant Soil 2009, 321, 235–257. [Google Scholar]
- Patersen, E.; Gebbing, T.; Abel, C.; Sim, A.; Telfer, G. Rhizodeposition shapes rhizosphere microbial community structure in organic soil. New Phytologist. 2007, 173, 600–610. [Google Scholar]
- Jiao, R.Z.; Yang, C.D. The changes of the soil microorganism in rhizosphere and outside in different developing stages of the Chinese fir plantation. Sci. Silvae Sin. 1999, 35, 53–59. [Google Scholar]
- Elliott, D.R.; Caporn, S.J.M.; Nwaishi, F.; Nilsson, R.H.; Sen, R. Bacterial and Fungal Communities in a Degraded Ombrotrophic Peatland Undergoing Natural and Managed Re-Vegetation. PLoS ONE 2015, 10, e0124726. [Google Scholar] [CrossRef]
- Kemp, J.; Lotter, D.; Meyer, A.; Kleinert, A.; Pérez-Fernández, M.; Valentine, A. Variation in rhizosphere nutrient cycling affects the source of nitrogen acquisition in wild and cultivated Aspalathus linearis (N.L.Burm.) R.Dahlgren plants. Appl. Soil Ecol. 2018, 130, 26–33. [Google Scholar]
- Wang, Y.; Liu, S.; Guo, J.L.; Liu, B.B. Effects of different vegetation types on soil nutrients, enzyme activities and microorganisms on the Loess Plateau. Bull. Soil Water Conserv. 2018, 38, 62–68. (In Chinese) [Google Scholar]
- Broeckling, C.D.; Broz, A.K.; Bergelson, J. Root exudates regulate soil fungal community composition and diversity. Appl. Environ. Microbiol. 2008, 74, 738–744. [Google Scholar]
- Nie, S.A.; Xu, H.J.; Li, S.; Li, H.; Su, J.Q. Relationships between abundance of microbial functional genes and the status and fluxes of carbon and nitrogen in rice rhizosphere and bulk soils. Pedosphere 2014, 24, 645–651. [Google Scholar]
- Zheng, Y.L.; Hou, L.J.; Liu, M.; Yin, G.Y.; Gao, J.; Jiang, X.F.; Lin, X.B.; Li, X.F.; Yu, C.D.; Wang, R. Community composition and activity of anaerobic ammonium oxidation bacteria in the rhizosphere of salt-marsh grass Spartina alterniflora. Appl. Microbiol. Biot. 2016, 100, 8203–8212. [Google Scholar]
- Zhang, Z.X.; Zhang, W.Z.; Yang, H.C.; Sheng, R.; Wei, W.X.; Qin, H.L. Elevated N2O emission by the rice roots: Based on the abundances of narG and bacterial amoA genes. Environ. Sci. Pollut. Res. 2017, 24, 2116–2125. [Google Scholar]
- Bremer, C.; Braker, G.; Matthies, D.; Reuter, A.; Engels, C.; Conrad, R. Impact of plant functional group, plant species, and sampling time on the composition of nirK-type denitrififier communities in soil. Appl. Environ. Microbiol. 2007, 73, 6876–6884. [Google Scholar] [PubMed]
- Yang, Y. Characteristics of Denitrifying Microbial Community Structure and Niche Differentiation in Rhizosphere Soil of Dominant Plants in Ebinur Lake Wetland. Master’s thesis, Shihezi University, Shihezi, China, 2018. [Google Scholar]
- Uren, N.C. Types, amounts and possible functions of compounds released into the rhizosphere by soil grown plants. In The Rhizosphere: Biochemistry, and Organic Substances at the Soil Interface; Pinton, R., Varani, Z., Nanniperi, P., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2000; pp. 19–40. [Google Scholar]
- Rovira, A.D. Plant root excretions in relation to the rhizosphere effect. Plant Soil 2005, 7, 178–194. [Google Scholar]
- Vogel, T.M.; Simonet, P.; Jansson, J.K.; Hirsch, P.R.; Tiedje, J.M.; Elsas, J.D.V.; Bailey, M.J.; Nalin, R.; Philippot, L. Terra Genome: A consortium for the sequencing of a soil metagenome. Nat. Rev. Microbiol. 2009, 7, 252–253. [Google Scholar]
- Rasche, F.; Velvis, H.; Zachow, C.; Berg, G.; Elsas, J.D.V.; Sessitsch, A. Impact of transgenic potatoes expressing antibacterial agents on bacterial endophytes is comparable to effects of soil, wild type potatoes, vegetation stage and pathogen exposure. Can. J. Microbiol. 2006, 43, 555–566. [Google Scholar]
- Schnitzer, S.A.; Klironomos, J.N.; HilleRisLambers, J.; Kinkel, L.L.; Reich, P.B.; Xiao, K.; Rillig, M.C.; Sikes, B.A.; Callaway, R.M.; Mangan, S.A.; et al. Soil microbes drive the classic plant diversity-productivity pattern. Ecology 2011, 92, 296–303. [Google Scholar]
- Bulgarelli, D.; Rot, M.; Schlaeppi, K. Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 2012, 488, 91–95. [Google Scholar] [PubMed]
- Lundberg, D.S.; Lebeis, S.L.; Paredes, S.H.; Yourstone, S.; Gehring, J.; Malfatti, S.; Tremblay, J.; Engelbrektson, A.; Kunin, V.; Rio, T.G.D.; et al. Defining the core Arabidopsis thaliana root microbiome. Nature 2012, 488, 86–90. [Google Scholar]
- Su, Y.G.; Huang, G.; Lin, Y.J.; Zhang, Y.M. No synergistic effects of water and nitrogen addition on soil microbial communities and soil respiration in a temperate desert. Catena 2016, 142, 126–133. [Google Scholar]
- Keiblinger, K.M.; Hall, E.K.; Wanek, W.; Szukics, U.; Hämmerle, I.; Ellersdorfer, G.; Böck, S.; Strauss, J.; Sterflinger, K.; Richter, A.; et al. The effect of resource quantity and resource stoichiometry on microbial carbon-use-efficiency. FEMS Microbiol. Ecol. 2010, 73, 430–440. [Google Scholar]



| Group | Primer | Sequence (5′—3′) | Amplification Details | Reference |
|---|---|---|---|---|
| Bacteria | Bacteria-338F | CCTACGGGAGGCAGCAG | 95 °C, 2 min, 35 cycles; 95 °C, 30 s; 60 °C, 30 s; 72 °C, 30 s; 80 °C, 15 s | [28] |
| Bacteria-518R | ATTACCGCGGCTGCTGG | |||
| Fungi | ITSIF | TCCGTAGGTGAACCTGCGG | 94 °C, 15 min; 94 °C, 30 s; 59.4 °C, 30 s; 72 °C, 30 s; 80 °C, 30 s, 35 cycles | [29] |
| 5.8S | CGCTGCGTTCTTCATCG | |||
| nirK | F1aCu | ATCATGGTSCTGCCGCG | 95 °C, 10 min; 6 touchdown cycles: 95 °C, 15 s; 63 °C, 30 s (−1 °C); 72 °C, 30 s; and 95 °C, 15 s; 58 °C, 30 s; 72 °C, 30 s; 80 °C, 30 s, 35 cycles | [30] |
| R3Cu | GCCTCGATCAGRTTGTGGTT | |||
| nirS | cd3aF | GTSAACGTSAAGGARACSGG | 95 °C, 10 min; 94 °C, 1 min; 57 °C, 1 min; 72 °C, 1 min; 83 °C, 30 s, 40 cycles | [31] |
| R3cd | GASTTCGGRTGSGTCTTGA | |||
| nifH | PolF | TGCCAYCCSAARGCBGACTC | 95 °C, 10 min, 40 cycles; 95 °C, 15 s; 60 °C, 30 s; 72 °C, 30 s; 80 °C, 15 s | [32] |
| PolR | ATSGCCATCATYTCRCCGGA |
| Factor | Bacteria | Fungi | nirK | nirS | nifH |
|---|---|---|---|---|---|
| Vegetation Type | 3.814 ** | 3.819 ** | 13.470 ** | 2.671 * | 0.611 |
| Soil Depth | 8.640 ** | 0.862 | 76.677 ** | 1.323 | 1.636 |
| Permafrost Type | 0.360 | 1.591 | 47.942 ** | 0.123 | 0.000 |
| Vegetation Type × Permafrost Type | 0.699 | 0.606 | 48.258 ** | 2.728 * | 8.707 ** |
| Permafrost Type × Soil Depth | 9.520 ** | 6.22 * | 29.046 ** | 0.001 | 0.376 |
| Vegetation Type × Soil Depth | 2.274 * | 3.475 * | 52.467 ** | 1.979 | 2.312 * |
| Vegetation Type × Permafrost Type × Soil Depth | 1.005 | 1.756 | 17.412 ** | 5.283 ** | 4.101 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, C.; Ma, X.; Song, Y.; Zhang, D.; Zhu, M.; Wang, X.; Gao, S.; Gao, J.; Song, C. Characteristics of Microbial Abundance in Rhizosphere and Non-Rhizosphere Soils of Permafrost Peatland, Northeast China. Forests 2023, 14, 1794. https://doi.org/10.3390/f14091794
Gong C, Ma X, Song Y, Zhang D, Zhu M, Wang X, Gao S, Gao J, Song C. Characteristics of Microbial Abundance in Rhizosphere and Non-Rhizosphere Soils of Permafrost Peatland, Northeast China. Forests. 2023; 14(9):1794. https://doi.org/10.3390/f14091794
Chicago/Turabian StyleGong, Chao, Xiuyan Ma, Yanyu Song, Dan Zhang, Mengyuan Zhu, Xianwei Wang, Siqi Gao, Jinli Gao, and Changchun Song. 2023. "Characteristics of Microbial Abundance in Rhizosphere and Non-Rhizosphere Soils of Permafrost Peatland, Northeast China" Forests 14, no. 9: 1794. https://doi.org/10.3390/f14091794
APA StyleGong, C., Ma, X., Song, Y., Zhang, D., Zhu, M., Wang, X., Gao, S., Gao, J., & Song, C. (2023). Characteristics of Microbial Abundance in Rhizosphere and Non-Rhizosphere Soils of Permafrost Peatland, Northeast China. Forests, 14(9), 1794. https://doi.org/10.3390/f14091794








