Habitat Distribution Pattern of Rare and Endangered Plant Magnolia wufengensis in China under Climate Change
Abstract
:1. Introduction
2. Materials and Methods
2.1. Species Distribution Points
2.2. Environmental Factors
2.3. Model Simulation
2.4. Model Evaluation
2.5. Suitable Habitat Partitions under Current and Future Conditions
3. Results
3.1. Prediction Results of Four Models
3.2. Model Accuracy Evaluation
3.3. Evaluation of Environmental Factors
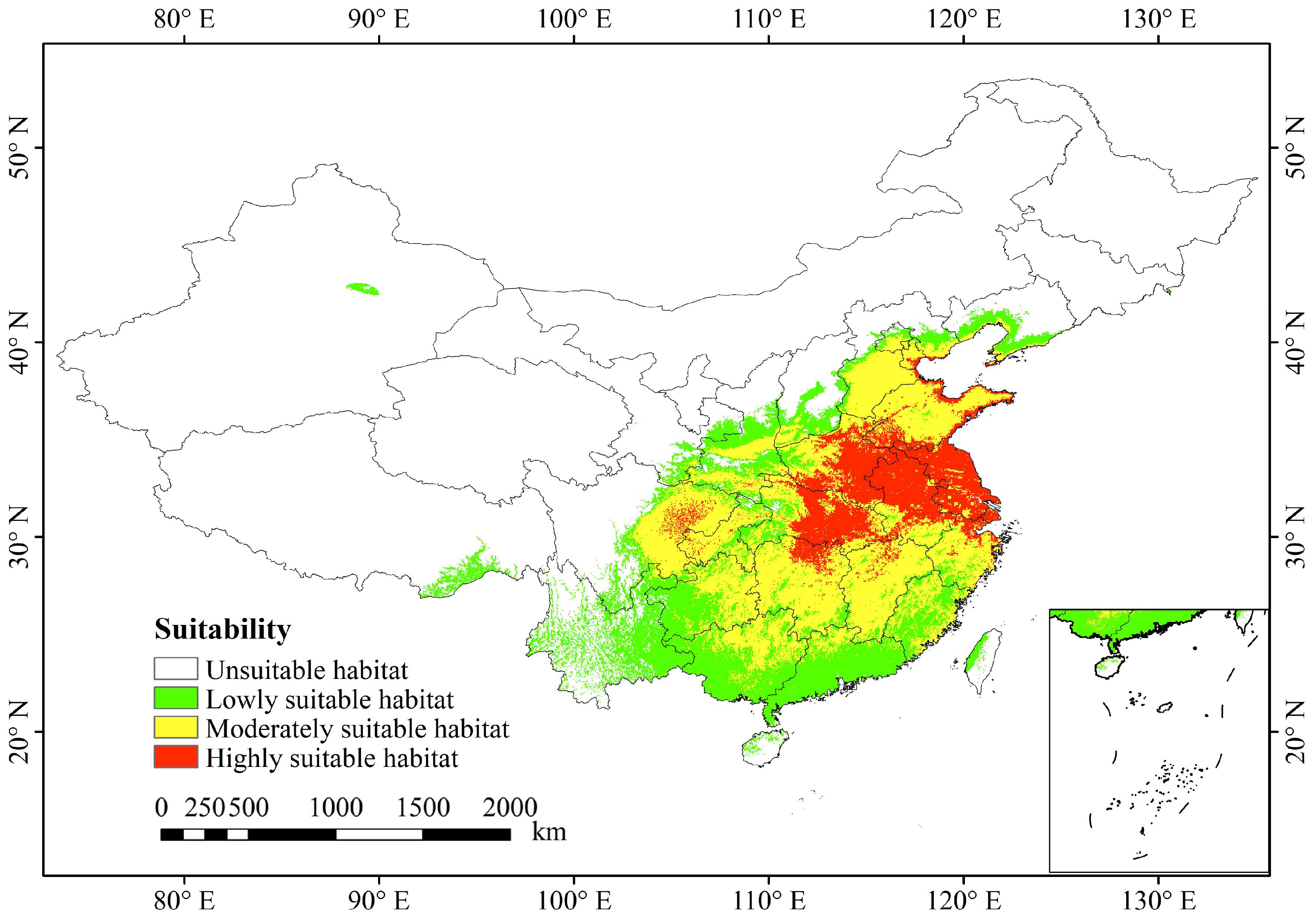
3.4. Current Potential Suitable Habitats in China
3.5. Potential Suitable Habitat and Dynamic Changes in the Future
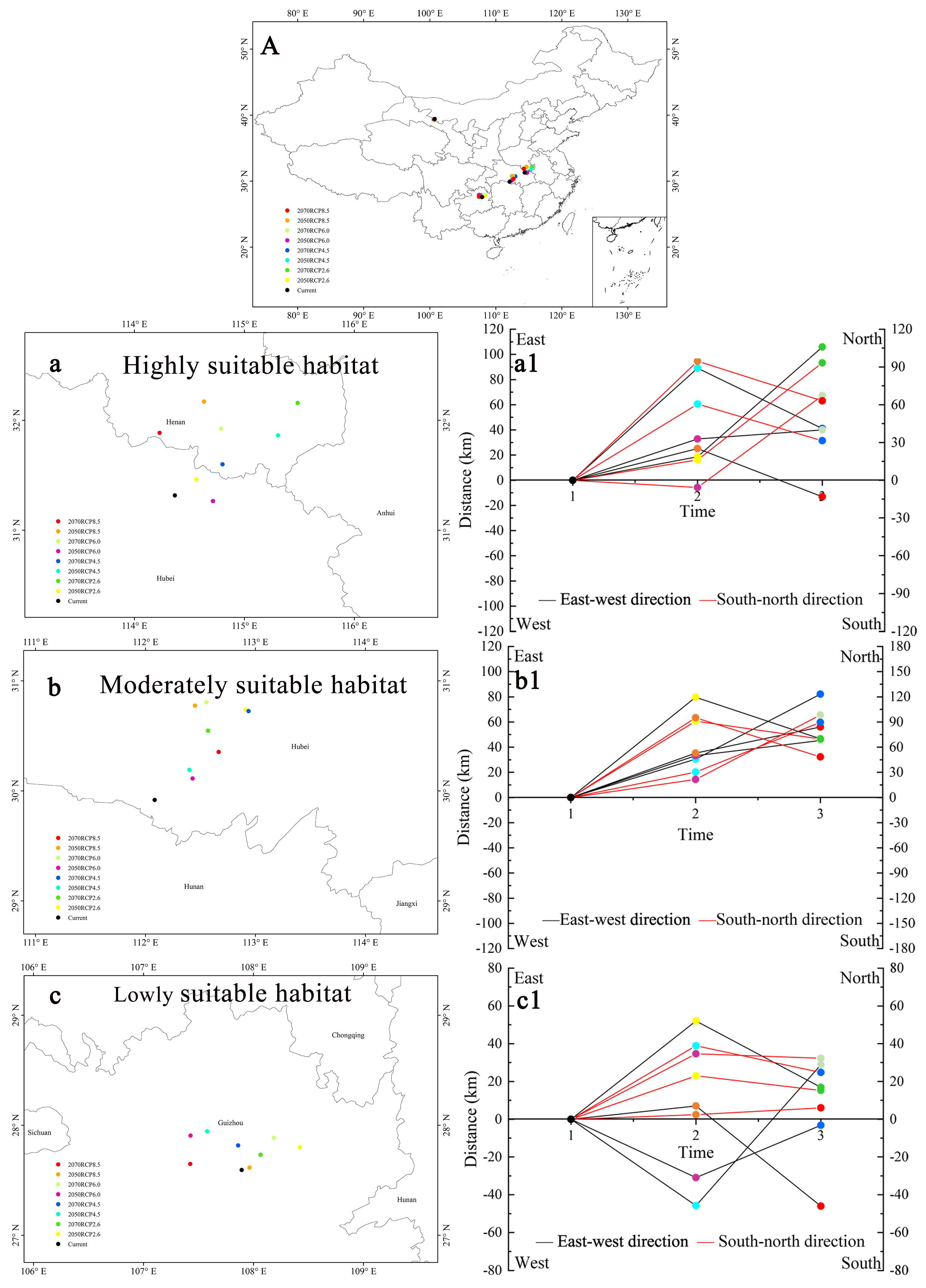
3.6. Centroid Shifts in Different Suitable Habitats
4. Discussion
4.1. Model Performance
4.2. Dominant Environmental Factors
4.3. Current Suitable Habitats
4.4. Changes to the Suitable Habitats in the Future
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change In Press; IPCC: Geneva, Switzerland, 2021. [Google Scholar]
- Yang, X.; Liu, B.; Bussmann, R.W.; Guan, X.; Xu, W.; Xue, T.; Yu, S. Integrated plant diversity hotspots and long-term stable conservation strategies in the unique karst area of southern China under global climate change. Forest Ecol. Manag. 2021, 498, 119540. [Google Scholar] [CrossRef]
- Shi, M.; Wu, H.; Jiang, P.; Zheng, K.; Liu, Z.; Dong, T.; He, P.; Fan, X. Food-water-land-ecosystem nexus in typical Chinese dryland under different future scenarios. Sci. Total Environ. 2023, 880, 163183. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Zou, M.; Qin, Y.; Tan, G.; Huang, S.; Quan, H.; Zhou, J.; Liao, H. Modeling of the Potential Geographical Distribution of Three Fritillaria Species Under Climate Change. Front. Plant Sci. 2022, 12, 749838. [Google Scholar] [CrossRef]
- Wang, W.; Li, Z.J.; Zhang, Y.L.; Xu, X.Q. Current Situation, Global Potential Distribution and Evolution of Six Almond Species in China. Front. Plant Sci. 2021, 12, 619883. [Google Scholar] [CrossRef]
- Wang, G.Z.; Gen, Q.F.; Xiao, M.Y.; Zhang, M.Y.; Zhang, Y.Y.; Wang, Z.S. Predicting Pseudolarix amabilis potential habitat based on four Niche models. Acta. Ecol. Sin. 2020, 40, 6096–6104. [Google Scholar]
- Dee, L.E.; Cowles, J.; Isbell, F.; Pau, S.; Gaines, S.D.; Reich, P.B. When do ecosystem services depend on rare species? Trends Ecol. Evol. 2019, 34, 746–758. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Song, X.; Ye, Y.; Gu, J.; Wang, R.; Zhao, D.; Shao, X. Climate Change May Pose Additional Threats to the Endangered Endemic Species Encalypta buxbaumioidea in China. Diversity 2023, 15, 269. [Google Scholar] [CrossRef]
- Jeong, H.M.; Kim, H.R.; Hong, S.; You, Y.H. Effects of elevated CO2 concentration and increased temperature on leaf quality responses of rare and endangered plants. J. Ecol. Environ. 2018, 42, 1. [Google Scholar] [CrossRef]
- Markham, J. Rare species occupy uncommon niches. Sci. Rep. 2014, 4, 6012. [Google Scholar] [CrossRef]
- Yang, Z.; Bai, Y.; Alatalo, J.M.; Huang, Z.; Yang, F.; Pu, X.; Wang, R.; Yang, W.; Guo, X. Spatio-temporal variation in potential habitats for rare and endangered plants and habitat conservation based on the maximum entropy model. Sci. Total Environ. 2021, 784, 147080. [Google Scholar] [CrossRef]
- Rivers, M.; Newton, A.C.; Oldfield, S. Global Tree Assessment Contributors. Scientists’ warning to humanity on tree extinctions. Plants People Planet 2023, 5, 466–482. [Google Scholar] [CrossRef]
- Albert, J.S.; Destouni, G.; Duke-Sylvester, S.M.; Magurran, A.E.; Oberdorff, T.; Reis, R.E.; Winemiller, K.O.; Ripple, W.J. Scientists’ warning to humanity on the freshwater biodiversity crisis. Ambio 2021, 50, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.Y.; Wang, L.R.; He, S.C.; Liu, X.; Wang, X.Q. A new species of Magnolia (Magnoliaceae) from Hubei, China. Bull Bot. Res. 2006, 26, 4–7. [Google Scholar]
- Ma, L.Y.; Wang, L.R.; He, S.C.; Liu, X.; Wang, X.Q. A new variety of Magnolia (Magnoliaceae) from Hubei, China. Bull Bot. Res. 2006, 26, 516–519. [Google Scholar]
- Liang, D.W. Select on superior tree Magnolia wufengensis and cultivar classification. Beijing For. Univ. 2010, 3–8. [Google Scholar]
- Ma, L.Y. Breeding of Magnolia wufengensis and its application in Land Greening. Land Green 2019, 3, 54–56. [Google Scholar]
- Sang, Z.Y.; Ma, L.Y.; Chen, F.J.; Zhang, P.; Zhu, Y.C. Protection status and utilization countermeasure of germplasm resources of the Magnolia wufengensis in Wufeng County. Hubei Agric. Sci. 2011, 50, 1564–1567. [Google Scholar]
- Liu, H.; Ren, H.; Liu, Q.; Wen, X.; Maunder, M.; Gao, J. Translocation of threatened plants as a conservation measure in China. Conserv. Biol. 2015, 29, 1537–1551. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, M.; Wen, X.; Li, N.; Ren, H. Strengthen ex situ conservation of plants and promote protection and utilization of plant resources. Bul. Chin. Acad. Sci. (China Ver.) 2021, 36, 417–424. [Google Scholar]
- Shi, X.D.; Ma, L.Y.; Duan, J.; Sang, Z.Y.; Zhu, Z.L.; Yao, N.; Zhou, M.M.; Jia, Z.K. Potential climatically adaptive regions for the introduction of Magnolia wufengensis. J. Zhejiang A&F Univ. 2018, 35, 705–715. [Google Scholar]
- Vincent, H.; Bornand, C.N.; Kempel, A.; Fischer, M. Rare species perform worse than widespread species under changed climate. Biol. Conserv. 2020, 246, 108586. [Google Scholar] [CrossRef]
- Ye, X.Z.; Zhao, G.H.; Zhang, M.Z.; Cui, X.Y.; Fan, H.H.; Liu, B. Distribution pattern of endangered plant Semiliquidambar cathayensis (Hamamelidaceae) in response to climate change after the last interglacial period. Forests 2020, 11, 434. [Google Scholar] [CrossRef]
- Booth, T.H. Estimating potential range and hence climatic adaptability in selected tree species. Forest Ecol. Manag. 2016, 366, 175–183. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Y.; Zhang, D.; Yu, W.; Chen, G.; Xie, T.; Liu, Z.; Ma, Z.; Du, J.; Chao, B.; et al. Mapping the potential of mangrove forest restoration based on species distribution models: A case study in China. Sci. Total Environ. 2020, 748, 142321. [Google Scholar] [CrossRef]
- Chang, Q.X.; Zhong, Y.F.; Zhang, Z.; Zhao, Y.; Song, X.Q. Visual Analysis of Research Progress on Species Distribution Prediction Based on Citespace. Forest Inv. Plan. 2022, 47, 20–33. [Google Scholar]
- Susset, E.C.; Magro, A.; Hemptinnej, L. Using species distribution models to locate animal aggregations: A case study with Hippodamia undecimnotata (Schneider) overwintering aggregation sites. Ecol. Entomol. 2017, 42, 345–354. [Google Scholar] [CrossRef]
- Anibaba, Q.A.; Dyderski, M.K.; Jagodziński, A.M. Predicted range shifts of invasive giant hogweed (Heracleum mantegazzianum) in Europe. Sci. Total Environ. 2022, 825, 154053. [Google Scholar] [CrossRef]
- Li, G.; Huang, J.; Guo, H.; Du, S. Projecting species loss and turnover under climate change for 111 Chinese tree species. Forest Ecol. Manag. 2020, 477, 118488. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Ma, J.F.; Li, X.Q.; Wang, Y.F.; Cao, S.; Xie, A.T.; Ye, S.F.; Dong, B.X.; Zhao, W.X.; Qin, Y.X.; et al. The distribution of Athetis lepigone and prediction of its potential distribution based on GARP and MaxEnt. J. Appl. Entomol. 2017, 141, 431–440. [Google Scholar] [CrossRef]
- Abedi-Tizaki, M.; Zafari, D. Geographic distribution of phylogenetic species of the Fusarium graminearum species complex and their 8-ketotrichothecene chemotypes on wheat spikes in Iran. Mycotoxin Res. 2017, 33, 245–259. [Google Scholar] [CrossRef]
- Deb, J.C.; Phinn, S.; Butt, N.; Mcalpine, C.A. Climatic-induced shifts in the distribution of Teak (Tectona grandis) in tropical Asia: Implications for forest management and planning. Environ. Manag. 2017, 60, 422–435. [Google Scholar] [CrossRef]
- Bai, J.J.; Hou, P.; Zhao, Y.H.; Xu, H.T.; Zhang, B. Research progress of species habitat suitability models and their verification. Chin. J. Ecol. 2022, 41, 1423–1432. [Google Scholar]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Shi, X.D.; Yin, Q.; Sang, Z.Y.; Zhu, Z.L.; Ma, L.Y.; Jia, Z.K. Prediction of potentially suitable areas for the introduction of magnolia wufengensis under climate change. Ecol. Indic. 2021, 127, 107762. [Google Scholar] [CrossRef]
- Song, C.; Liu, H. Habitat differentiation and conservation gap of Magnolia biondii, M. denudata, and M. sprengeri in China. Peer J. 2019, 6, e6126. [Google Scholar] [CrossRef]
- Chen, Y.H.; Lu, Y.W.; Yin, X.J. Predicting habitat suitability due to climate change of southwest coniferous forest tree species. J. Nanjing Univ. 2019, 43, 1–10. [Google Scholar]
- Zhang, X.W.; Li, Y.; Xie, Y.P.; Bao, X.M.; Fang, Y.M. Effect of climate change on potential geographical distribution of Sorbus amabilis. J. Plant Resour. Environ. 2018, 27, 31–41. [Google Scholar]
- IPCC. Climate Change and Land 2019: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; IPCC: Geneva, Switzerland, 2019; pp. 99–100. [Google Scholar]
- Aguirre-Gutiérrez, J.; Carvalheiro, L.G.; Polce, C.; van Loon, E.E.; Raes, N.; Reemer, M.; Biesmeijer, J.C. Fit-for-purpose: Species distribution model performance depends on evaluation criteria-Dutch hoverflies as a case study. PLoS ONE 2013, 8, e63708. [Google Scholar] [CrossRef]
- Gao, M.; Ni, S.P.; Shen, L. Analysis of global potential ecological suitable producing area for Salvia miltiorrhiza based on MaxEnt model. China Pharm. 2018, 29, 2243–2247. [Google Scholar]
- Dai, G.H.; Yan, J.; Huang, C.H.; Sun, C.W.; Jia, L.M.; Ma, L.Y. The effects of climate change on the development of tree plantations for biodiesel production in China. Forests 2017, 8, 207. [Google Scholar] [CrossRef]
- Anderson, R.P.; Lew, D.; Peterson, A.T. Evaluating predictive models of species’ distributions: Criteria for selecting optimal models. Ecol. Model. 2003, 162, 211–232. [Google Scholar] [CrossRef]
- Booth, T.H.; Nix, H.A.; Busby, J.R.; Hutchinson, M.F. Bioclim: The first species distribution modelling package, its early applications and relevance to most current MaxEnt studies. Diver. Distrib. 2013, 20, 1–9. [Google Scholar] [CrossRef]
- Carpenter, G.; Gillison, A.N.; Winter, J. DOMAIN: A flexible modelling procedure for mapping potential distributions of plants and animals. Biodivers Conserv. 1993, 2, 667–680. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, D.F.; Wu, M.L.; Guo, J.; Sun, C.Z.; Xie, C.X. Predicting the global areas for potential distribution of Gastrodia elata based on ecological niche models. Chin. J. Plant Ecol. 2017, 41, 770–778. [Google Scholar]
- Jayasinghe, S.L.; Kumar, L. Modeling the climate suitability of tea [Camellia sinensis (L.) o. Kuntze] in Sri Lanka in response to current and future climate change scenarios. Agric. Meteorol. 2019, 272–273, 102–117. [Google Scholar] [CrossRef]
- Adhikari, P.; Shin, M.S.; Jeon, J.Y.; Kim, H.W.; Hong, S.; Seo, C. Potential impact of climate change on the species richness of subalpine plant species in the mountain national parks of South Korea. J. Ecol. Environ. 2018, 42, 36. [Google Scholar] [CrossRef]
- Wang, S.Q. Genetic diversity and population structure of the endangered species Paeonia decomposita endemic to China and implications for its conservation. BMC Plant Biol. 2020, 20, 510. [Google Scholar] [CrossRef]
- Wang, Y.S.; Xie, B.Y.; Wang, F.H.; Xiao, Q.M.; Dai, L.Y. Application of ROC curve analysis in evaluating the performance of alien species’ potential distribution models. Biodiversity Sci. 2007, 15, 365–372. [Google Scholar]
- Wang, J. The Application of Kappa in Assessing Agreement. Master’s Thesis, Sichuan University, Chengdu, China, 2006. [Google Scholar]
- Yang, F.R.; Zhang, Q.; Sun, C.Z.; Xie, C.X.; Song, J.Y. Comparative evaluation of multiple models for predicting the potential distribution areas of Astragalus membranaceus var. mongholicu. Plant Sci. J. 2019, 37, 136–143. [Google Scholar]
- Zhu, G.P.; Qiao, H.J. Effect of the Maxent model’s complexity on the prediction of species potential distributions. Biodiversity Sci. 2016, 24, 1189–1196. [Google Scholar] [CrossRef]
- Jia, X.; Wang, C.; Jin, H.; Zhao, Y.; Liu, L.J.; Chen, Q.H.; Li, B.Y.; Xiao, Y.; Yin, H. Suitable distribution areas assessments of Pinus koraiensis based on an optimized MaxEnt model. Chin. J Ecol. 2019, 38, 2570–2576. [Google Scholar]
- Anderson, R.P.; Gonzalez, I. Species-specific tuning increases robustness to sampling bias in models of species distributions: An implementation with maxent. Ecol. Model. 2011, 222, 2796–2811. [Google Scholar] [CrossRef]
- Alberto, J.A.; Decae, A.E.; Arnedo, M.A. Environmental suitability of new reported localities of the funnelweb spider Macrothele calpeiana: An assessment using potential distribution modelling with presence-only techniques. J. Biogeog. 2011, 38, 1213–1223. [Google Scholar]
- Stockwell, D.; Peters, D.P. The GARP modelling system: Problems and solutions to automated spatial prediction. Int. J. Geog. Inf. Sci. 1999, 13, 143–158. [Google Scholar] [CrossRef]
- Srivastava, V.; Griess, V.C.; Padalia, H. Mapping invasion potential using ensemble modelling. A case study on Yushania maling in the Darjeeling Himalayas. Ecol. Model. 2018, 385, 35–44. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, H.X.; Wang, H. Potential geographical distribution of Populus euphratica in China under future climate change scenarios based on Maxent model. Acta. Ecol. Sin. 2020, 40, 6552–6563. [Google Scholar]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Ray, D.; Behera, M.D.; Jacob, J. Evaluating Ecological Niche Models: A Comparison Between Maxent and GARP for Predicting Distribution of Hevea brasiliensis in India. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2018, 88, 1337–1343. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, L.; Tian, C.M.; Li, T.; Wang, R.; Yang, Q.Q. Predicting the distribution of dwarf mistletoe (Arceuthobium sichuanense) with GARP and MaxEnt models. J. Beijing For. Univ. 2016, 38, 23–32. [Google Scholar]
- Duan, Y.Z.; Wang, H.T.; Wang, C.; Du, Z.Y. Potential distribution of endangered plant Helianthemum songaricum in China under climate change. J. Plant Resour. Environ. 2020, 29, 55–68. [Google Scholar]
- Yang, Y. Studies on the ecological adaptability and overwintering technique of Magnolia wufengensis seedlings in Beijing. Beijing For. Univ. 2015, 28–97. [Google Scholar]
- Zhu, Z.L. Study on the limiting factors and winter protection methods of Magnolia wufengensis after introduction to Beijing. Beijing For. Univ. 2012, 41–48. [Google Scholar]
- Yan, Y.H.; Cen, Y.F.; Zhang, P.Y.; Zhang, Y.; Liu, X.; Li, C.Y.; Xu, S. Predicting distribution pattern and future change of Pinus massoniana in China based on MaxEnt model. Chin. J. Ecol. 2019, 38, 2896–2901. [Google Scholar]
- Li, Y.; Zhang, X.W.; Fang, Y.M. Responses of the distribution pattern of Quercus chenii to climate change following the last glacial maximum. Chin. J. Plant. Ecol. 2016, 40, 1164–1178. [Google Scholar]
- Hao, Y.; Peng, Z.D.; Ma, L.Y. Compare Studies on the Seedlings of Magnolia wufengensis and Magnolia denudate. Nor. Horticul. 2010, 4, 101–104. [Google Scholar]
- Liu, Y.H. A preliminary study on the taxonomy of the family Magnoliaceae. J. Syst. Evol. 1984, 22, 89–109. [Google Scholar]
- Liu, Y.H.; Xia, N.H.; Yang, H.Q. The origin, evolution and phytogeography of Magnoliaceae. J. Trop. Subtrop. Bot. 1995, 3, 1–12. [Google Scholar]
- Urban, M.C. Accelerating extinction risk from climate change. Science 2015, 348, 571–573. [Google Scholar] [CrossRef]
- Gao, X.; Liu, J.; Huang, Z. The impact of climate change on the distribution of rare and endangered tree Firmiana kwangsiensis using the Maxent modeling. Ecol. Evol. 2022, 12, e9165. [Google Scholar] [CrossRef]
- Li, Y.F.; Zhang, C.G.; Zhu, H.G.; Li, X.H.; Duan, Y.F. Analyses on suitable distribution areas and main climatic variables of Osmanthus yunnanensis and O. delavayi. J. Plant Res. Environ. 2019, 28, 71–78. [Google Scholar]
- Wu, Y.; Wang, H.F.; Mu, L.Q. Research progress and prospect of species distribution models. J. Sci. Teachers. Coll. Univ. 2022, 42, 66–70. [Google Scholar]

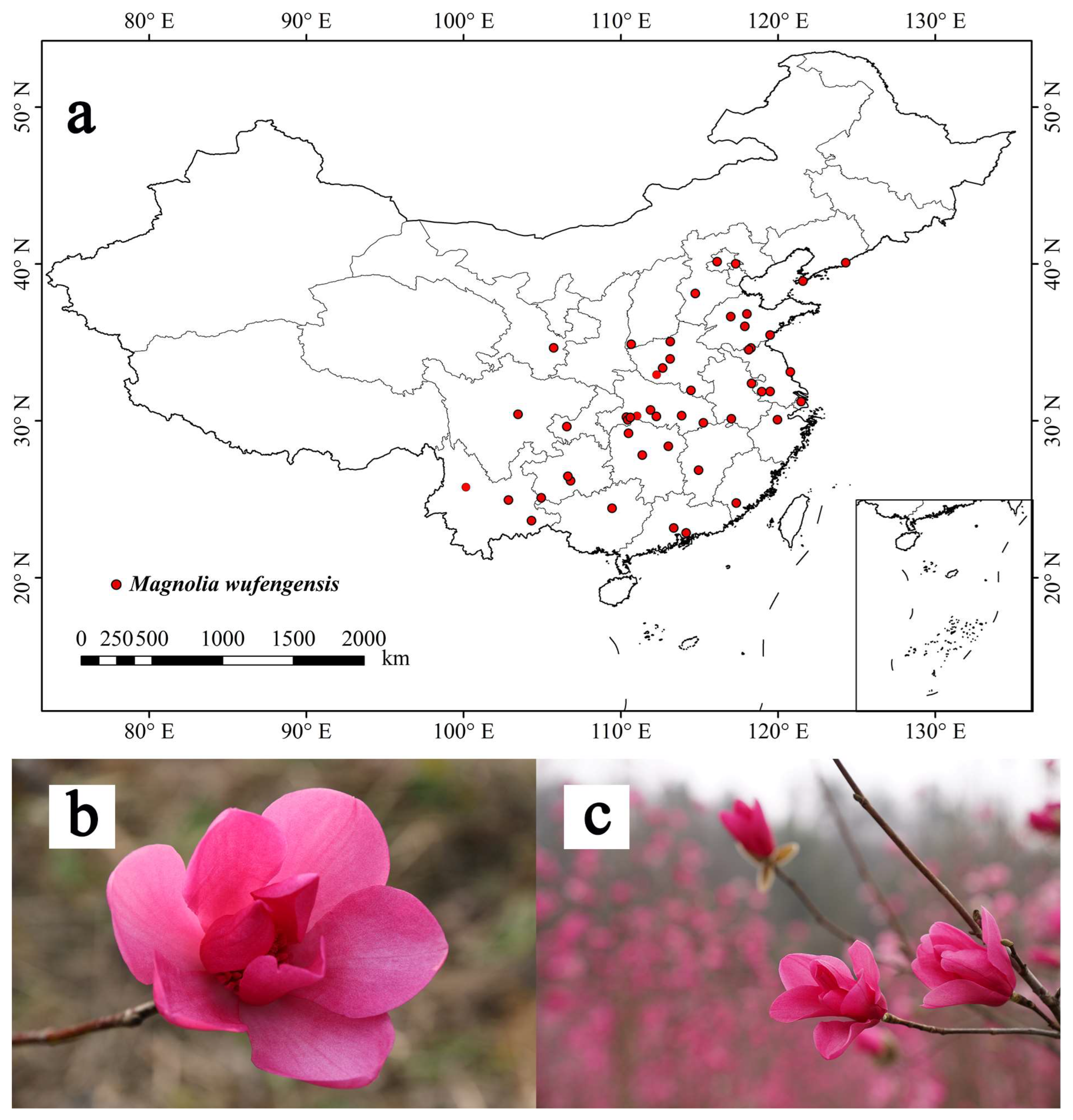
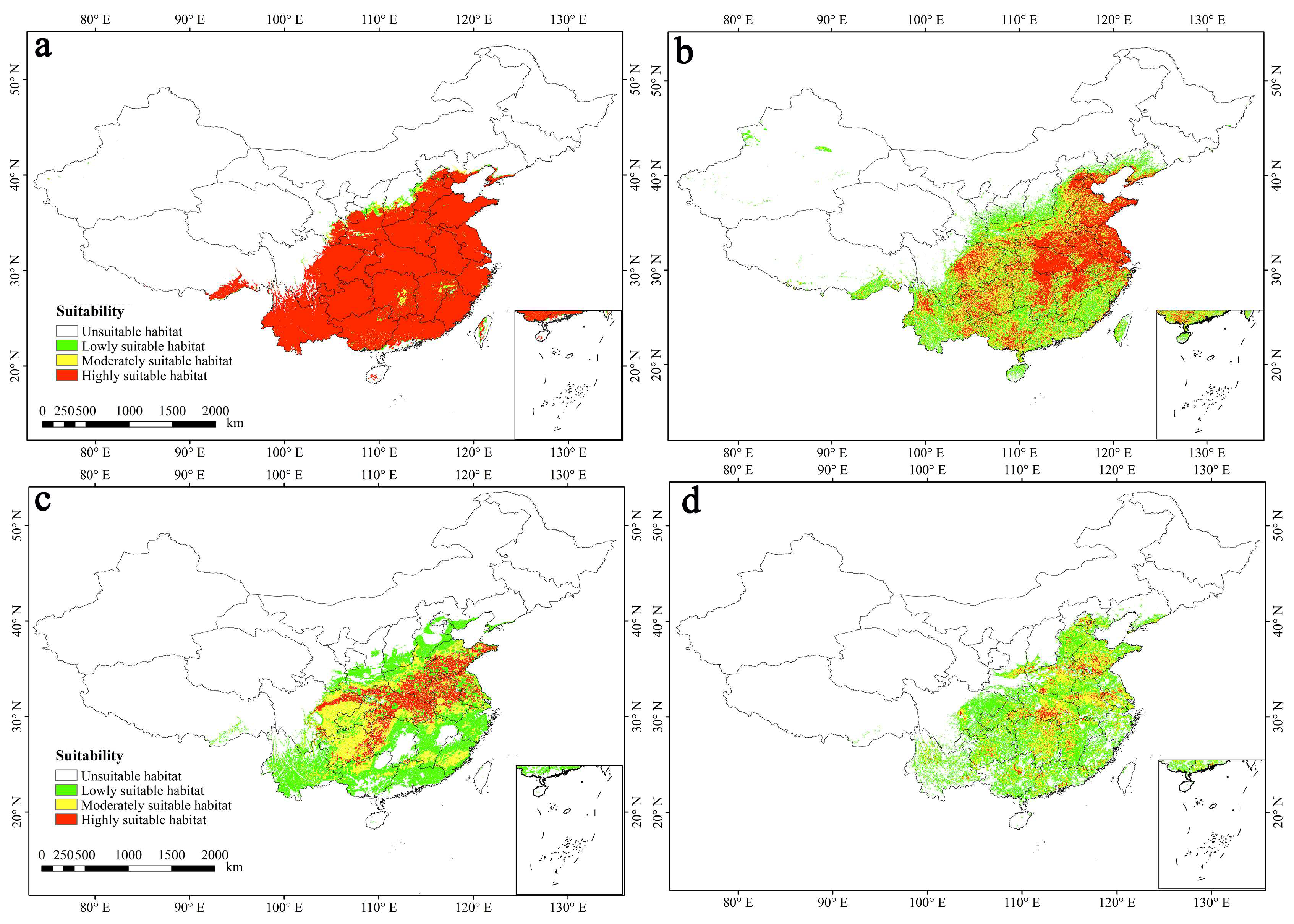
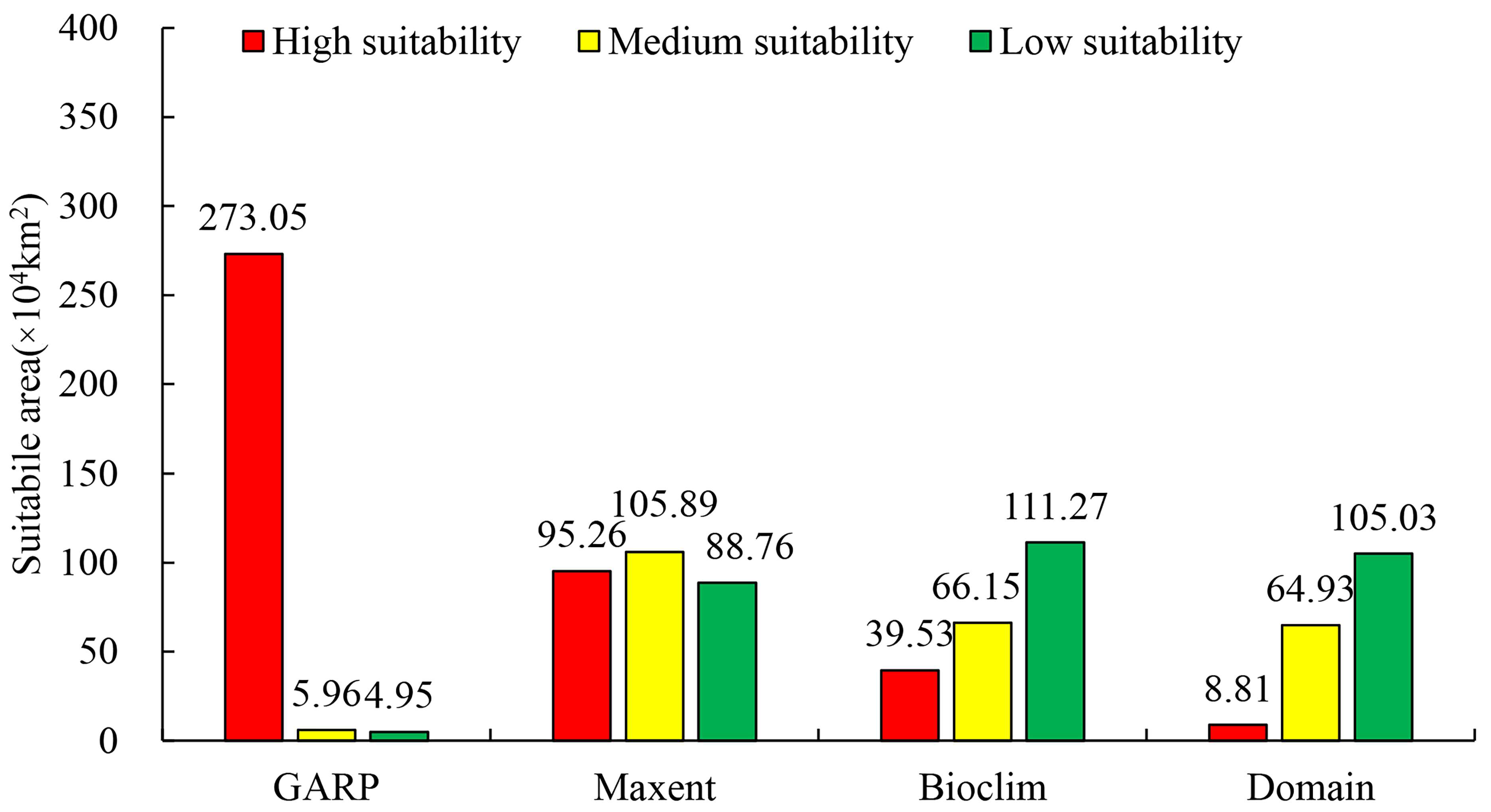
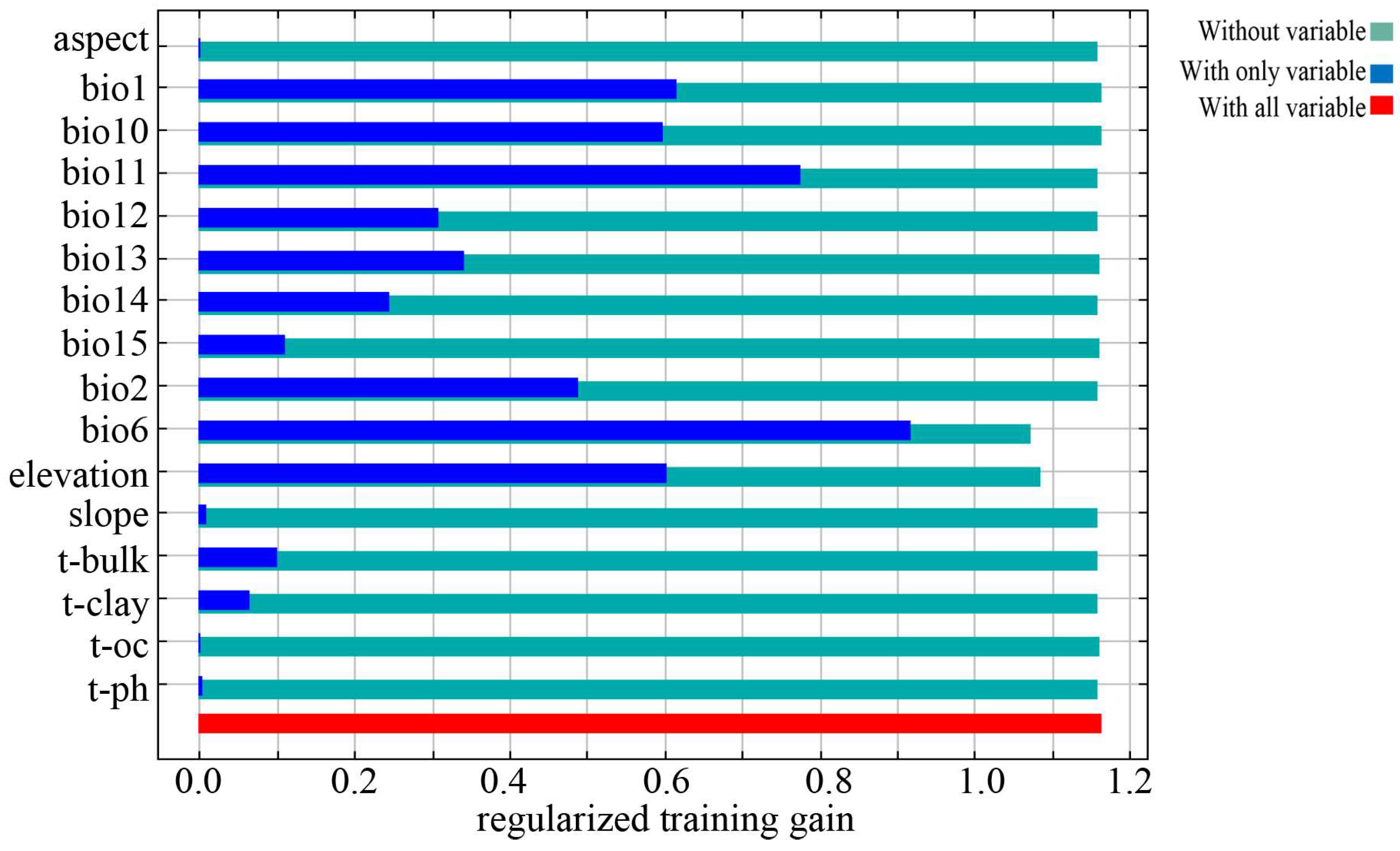



| Category | Variable | Description | Unit |
|---|---|---|---|
| Climate | bio1 | Annual mean temperature | °C |
| bio2 | Mean diurnal range (mean of monthly (max temp–min temp)) | °C | |
| bio6 | Min temperature of the coldest month | °C | |
| bio10 | Mean temperature of the warmest quarter | °C | |
| bio11 | Mean temperature of the coldest quarter | °C | |
| bio12 | Annual precipitation | mm | |
| bio13 | Precipitation of the wettest month | mm | |
| bio14 | Precipitation of the driest month | mm | |
| bio15 | Precipitation seasonality (coefficient of variation) | / | |
| Topographic | Elevation | m | |
| Slope | ° | ||
| Aspect | rad | ||
| Soil | t-bulk | Topsoil bulk density | kg/dm3 |
| t-ph | Topsoil pH (H2O) | / | |
| t-clay | Topsoil clay fraction | % | |
| t-oc | Topsoil organic carbon | % |
| Suitability | GARP | Maxent | Bioclim | Domain |
|---|---|---|---|---|
| Unsuitable | [0, 2] | [0, 0.2] | 0 | [0, 90] |
| Lowly suitable | (2, 4] | (0.2, 0.4] | (0, 5%] | [91, 93] |
| Moderately suitable | (4, 6] | (0.4, 0.6] | (5%, 10%] | [94, 96] |
| Highly suitable | (6, 10] | (0.6, 1.0] | (10%, 27%] | [97, 100] |
| Model | Maxent | GARP | Bioclim | Domain |
|---|---|---|---|---|
| AUC | 0.9479 ± 0.0080 a | 0.8719 ± 0.0039 b | 0.8512 ± 0.0177 c | 0.9367 ± 0.0287 a |
| Kappa | 0.8113 ± 0.0228 a | 0.6969 ± 0.0200 c | 0.7166 ± 0.0372 c | 0.7629 ± 0.0531 b |
| Climate Scenario | High Suitability | Medium Suitability | Low Suitability | No Suitability | ||||
|---|---|---|---|---|---|---|---|---|
| Area (104 km2) | Change Rate (%) | Area (104 km2) | Change Rate (%) | Area (104 km2) | Change Rate (%) | Area (104 km2) | Change Rate (%) | |
| Current | 46.59 | / | 122.82 | / | 96.36 | / | 694.83 | / |
| RCP2.6 (2050) | 32.63 | −29.98 | 130.74 | 6.45 | 117.43 | 21.86 | 679.81 | −2.16 |
| RCP4.5 (2050) | 33.48 | −28.15 | 154.86 | 26.09 | 97.10 | 0.77 | 675.16 | −2.83 |
| RCP6.0 (2050) | 36.34 | −22.00 | 156.09 | 27.09 | 92.26 | −4.25 | 675.91 | −2.72 |
| RCP8.5 (2050) | 37.85 | −18.77 | 144.75 | 17.86 | 101.48 | 5.31 | 676.53 | −2.63 |
| RCP2.6 (2070) | 33.58 | −27.94 | 147.68 | 20.24 | 103.54 | 7.45 | 675.81 | −2.74 |
| RCP4.5 (2070) | 34.01 | −27.02 | 149.15 | 21.44 | 102.21 | 6.07 | 675.23 | −2.82 |
| RCP6.0 (2070) | 36.65 | −21.33 | 143.50 | 16.84 | 100.99 | 4.80 | 679.46 | −2.21 |
| RCP8.5 (2070) | 44.48 | −4.54 | 144.27 | 17.46 | 99.31 | 3.06 | 672.55 | −3.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Yin, Q.; Sang, Z.; Zhu, Z.; Jia, Z.; Ma, L. Habitat Distribution Pattern of Rare and Endangered Plant Magnolia wufengensis in China under Climate Change. Forests 2023, 14, 1767. https://doi.org/10.3390/f14091767
Shi X, Yin Q, Sang Z, Zhu Z, Jia Z, Ma L. Habitat Distribution Pattern of Rare and Endangered Plant Magnolia wufengensis in China under Climate Change. Forests. 2023; 14(9):1767. https://doi.org/10.3390/f14091767
Chicago/Turabian StyleShi, Xiaodeng, Qun Yin, Ziyang Sang, Zhonglong Zhu, Zhongkui Jia, and Luyi Ma. 2023. "Habitat Distribution Pattern of Rare and Endangered Plant Magnolia wufengensis in China under Climate Change" Forests 14, no. 9: 1767. https://doi.org/10.3390/f14091767
APA StyleShi, X., Yin, Q., Sang, Z., Zhu, Z., Jia, Z., & Ma, L. (2023). Habitat Distribution Pattern of Rare and Endangered Plant Magnolia wufengensis in China under Climate Change. Forests, 14(9), 1767. https://doi.org/10.3390/f14091767






