Tetraploid Induction with Leaf Morphology and Sunburn Variation in Sorbus pohuashanensis (Hance) Hedl
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Colchicine Treatment
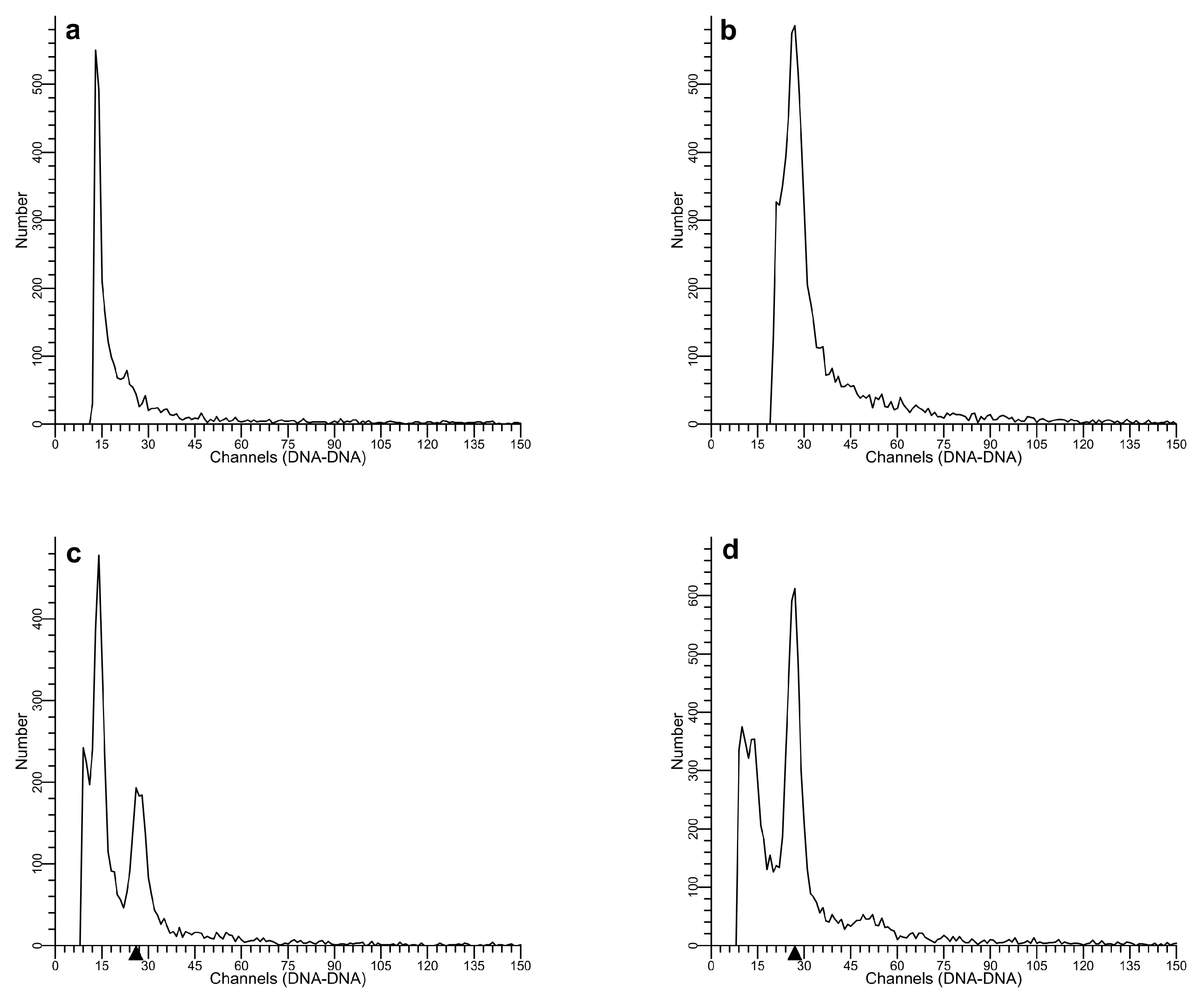
2.3. Ploidy Identification
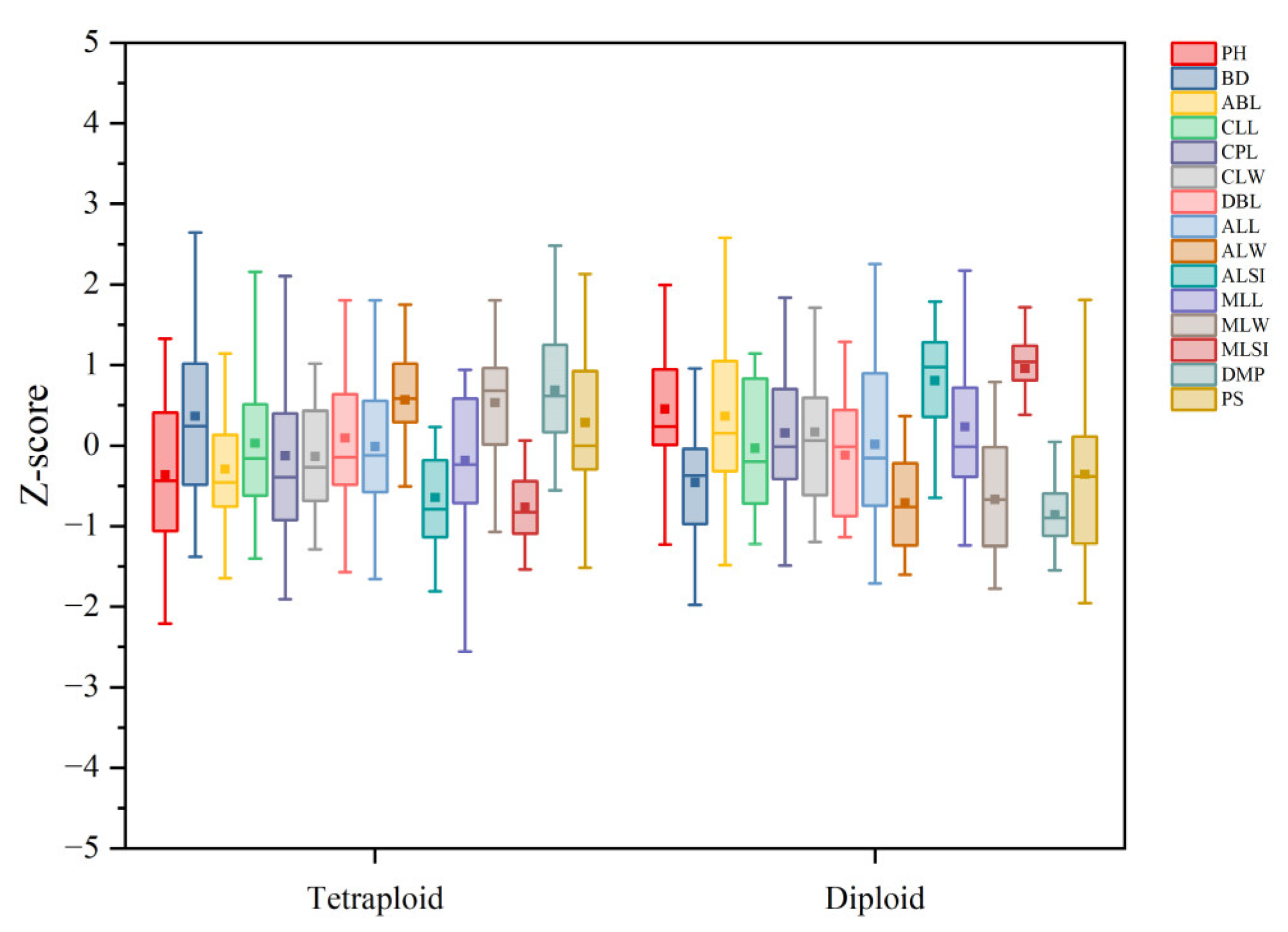
2.4. Plant Morphological Characteristics
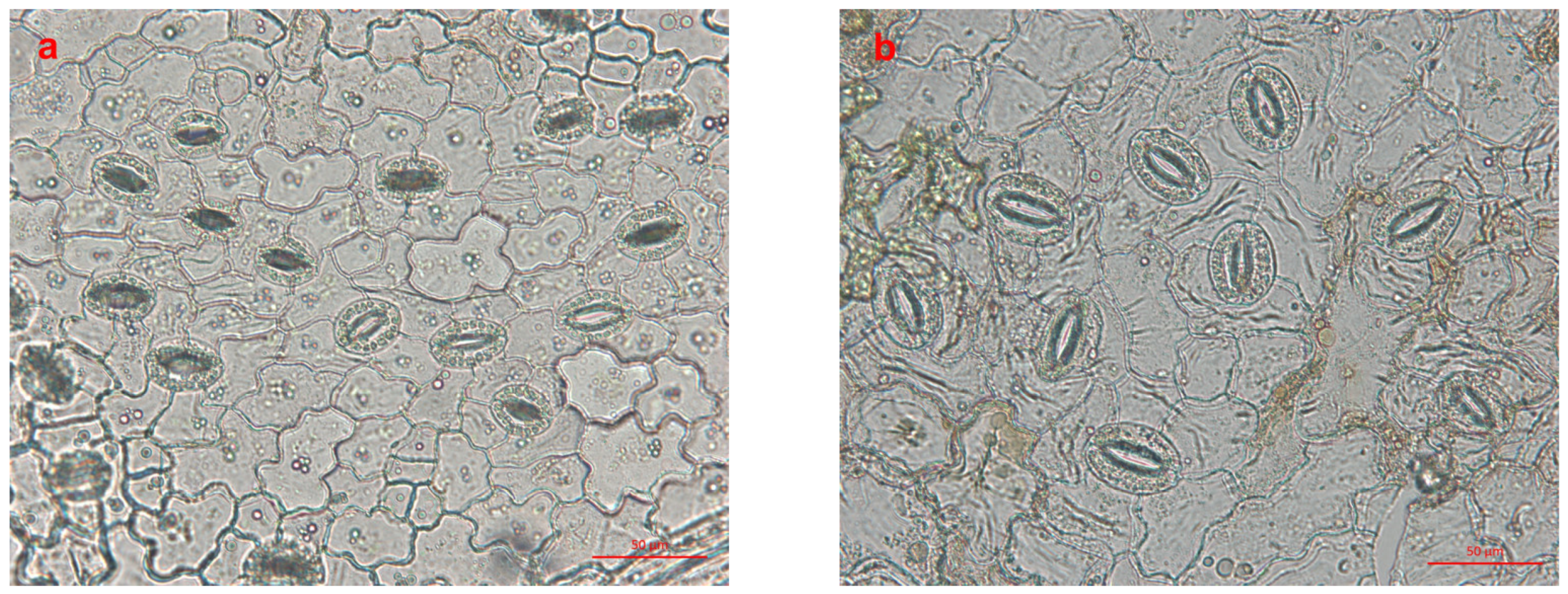
2.5. Stomata Observation
2.6. Statistical Analysis
3. Results
3.1. Effect of Colchicine on Seed Germination
3.2. Tetraploid Induction and Determination
3.3. Stomata Analysis
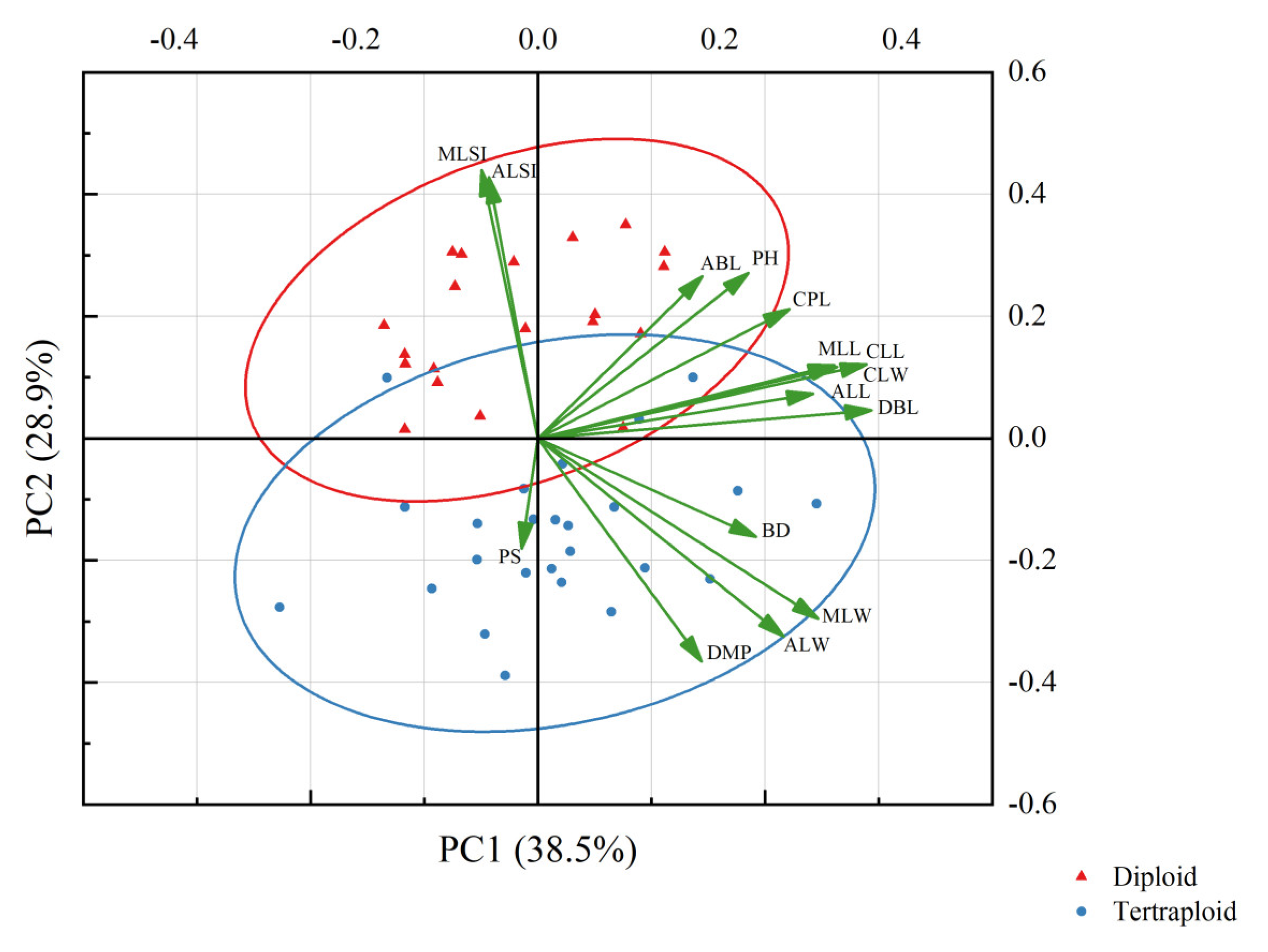
3.4. Plant Morphological Characteristics
4. Discussion
4.1. Colchicine Induced Tetraploids
4.2. Relationship between Phenotype and Polyploidy
4.3. Polyploidy and Plant Resistance
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

| SOV | df | MS | F | p-Value |
|---|---|---|---|---|
| Treatment time (h) | 2 | 1167.485 | 80.528 | 0.000 * |
| Colchicine concentration (%) | 4 | 336.601 | 23.217 | 0.000 * |
| Colchicine Conc. × Treatment time | 8 | 10.111 | 0.697 | 0.691 |
| Error | 30 | 14.498 |
| Year | SOV | Percentage | df | MS | F | p-Value |
|---|---|---|---|---|---|---|
| 2020 | Stratification time (d) | Tetraploid (%) | 2 | 266.819 | 5.449 | 0.100 |
| Mixoploid (%) | 2 | 43.126 | 0.833 | 0.515 | ||
| Induction percentage (%) | 2 | 168.241 | 1.878 | 0.296 | ||
| Concentration (%) | Tetraploid (%) | 1 | 115.120 | 0.814 | 0.418 | |
| Mixoploid (%) | 1 | 112.827 | 3.507 | 0.134 | ||
| Induction percentage (%) | 1 | 216.842 | 2.233 | 0.209 | ||
| 2021 | Stratification time (d) | Tetraploid (%) | 1 | 44.389 | 0.261 | 0.625 |
| Mixoploid (%) | 1 | 139.045 | 1.362 | 0.270 | ||
| Induction percentage (%) | 1 | 254.789 | 1.038 | 0.332 | ||
| Concentration (%) (w/v) | Tetraploid (%) | 1 | 1106.149 | 17.310 | 0.002 * | |
| Mixoploid (%) | 1 | 596.349 | 10.588 | 0.009 * | ||
| Induction percentage (%) | 1 | 1604.584 | 14.527 | 0.010 * |
| SOV | Percentage | df | MS | F | p-Value |
|---|---|---|---|---|---|
| Provenances | Tetraploid (%) | 1 | 50.439 | 0.628 | 0.446 |
| germination rate (%) | 1 | 637.491 | 32.404 | 0.000 * | |
| Concentration (%) (w/v) | Tetraploid (%) | 1 | 491.881 | 13.620 | 0.004 * |
| germination rate (%) | 1 | 41.355 | 0.522 | 0.487 | |
| Provenances × Concentration | Tetraploid (%) | 1 | 16.297 | 0.443 | 0.524 |
| germination rate (%) | 1 | 24.638 | 1.508 | 0.254 |
| Principal Component | |||
|---|---|---|---|
| 1 | 2 | 3 | |
| BDL | 0.882 | 0.095 | 0.108 |
| CLL | 0.869 | 0.252 | 0.164 |
| CLW | 0.791 | 0.245 | −0.400 |
| MLL | 0.784 | 0.250 | −0.448 |
| MLW | 0.741 | −0.613 | −0.125 |
| ALL | 0.728 | 0.152 | −0.127 |
| CPL | 0.664 | 0.440 | 0.391 |
| ALW | 0.651 | −0.675 | 0.050 |
| BD | 0.576 | −0.335 | −0.013 |
| PH | 0.556 | 0.565 | 0.169 |
| ABL | 0.435 | 0.554 | 0.551 |
| DMP | 0.433 | −0.759 | −0.063 |
| PS | −0.043 | −0.376 | 0.516 |
| ALSI | −0.130 | 0.891 | −0.122 |
| MLSI | −0.149 | 0.915 | −0.204 |
| Eigenvalue | 5.776 | 4.332 | 1.246 |
| Contribution rate (%) | 38.505 | 28.879 | 8.304 |
| Cumulative contribution rate (%) | 38.505 | 67.384 | 75.688 |
References
- Phipps, J.B.; Robertson, K.R.; Smith, P.G.; Rohrer, J.R. A checklist of the subfamily Maloideae (Rosaceae). Can. J. Bot. 1990, 68, 2209–2269. [Google Scholar] [CrossRef]
- Li, J.B.; Zhu, K.L.; Wang, Q.; Chen, X. Genome size variation and karyotype diversity in eight taxa of Sorbus sensu stricto (Rosaceae) from China. Comp. Cytogenet. 2021, 15, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J. Evaluation, Conservation and Domestication of Genetic Resources of Sorbus pohuashanensis. Ph.D. Thesis, Chinese Academy of Forestry, Beijing, China, 2008. [Google Scholar]
- Yu, X.; Wang, Z.; Shu, Z.; Li, Z.; Ning, Y.; Yun, K.; Bai, H.; Liu, R.; Liu, W. Effect and mechanism of Sorbus pohuashanensis (Hante) Hedl. Flavonoids protect against arsenic trioxide-induced cardiotoxicity. Biomed. Pharmacother. 2017, 88, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Zhang, Y.; Li, H.; Zhao, Y.; Cai, E.; Zhu, H.; Li, P.; Liu, J. Triterpenoids from fruits of Sorbus pohuashanensis inhibit acetaminophen-induced acute liver injury in mice. Biomed. Pharmacother. 2019, 109, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.M.; Yu, X.D.; Zheng, Y.Q.; Zhang, T.; Xia, X.H.; Fu, Q.D.; Zhang, C.H. Study on nutritive substances and medicinal components of Sorbus pohuashanensis. For. Res. 2020, 33, 154–160. [Google Scholar] [CrossRef]
- Zhao, D.X.; Zhang, Y.; Lu, Y.Z.; Fan, L.Q.; Zhang, Z.B.; Zheng, J.; Chai, M. Genome sequence and transcriptome of Sorbus pohuashanensis provide insights into population evolution and leaf sunburn response. J. Genet. Genomics 2022, 49, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.X.; Qi, X.Y.; Zhang, Y.; Zhang, R.L.; Wang, C.; Sun, T.X.; Zheng, J.; Lu, Y.Z. Genome-wide analysis of the heat shock transcription factor gene family in Sorbus pohuashanensis (Hance) Hedl identifies potential candidates for resistance to abiotic stresses. Plant. Physiol. Biochem. 2022, 175, 68–80. [Google Scholar] [CrossRef]
- Racskó, J.; Szabó, T.; Nyéki, J.; Soltész, M.; Nagy, P.T. Characterization of sunburn damage to apple fruits and leaves. Int. J. Hortic. Sci. 2010, 16, 15–20. [Google Scholar] [CrossRef][Green Version]
- Gu, Y.P.; Zhang, Z.R.; Sun, T.; Han, Q.J.; Li, N.N.; Lu, Y.Z.; Dou, D.Q.; Zheng, J. Hybrid identification and genetic relationship analysis of F1 hybrid population of Sorbus pohuashanensis and Sorbus hupehensis var. paucijuga based on EST-SSR marker. J. Plant Resour. Environ. 2022, 31, 65–73. [Google Scholar]
- Comai, L. The advantages and disadvantages of being polyploid. Nat. Rev. Genet. 2005, 6, 836–846. [Google Scholar] [CrossRef]
- Leitch, A.R.; Leitch, I.J. Genomic plasticity and the diversity of polyploid plants. Science 2008, 320, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Wickett, N.J.; Ayyampalayam, S.; Chanderbali, A.S.; Landherr, L.; Ralph, P.E.; Tomsho, L.P.; Hu, Y.; Liang, H.; Soltis, P.S.; et al. Ancestral polyploidy in seed plants and angiosperms. Nature 2011, 473, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.R.; Feng, Z.Y.; Zhang, X.H.; Song, Z.J.; Cai, D.T. A new way of rice breeding: Polyploid rice breeding. Plants 2021, 10, 422. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.Q.; Jin, J.J.; Zhao, H.; Li, K.L. Drought stress tolerance analysis of Populus ussuriensis clones with different ploidies. J. Forestry Res. 2018, 30, 1267–1275. [Google Scholar] [CrossRef]
- Wu, W.Q.; Liao, T.; Du, K.; Wei, H.R.; Kang, X.Y. Transcriptome comparison of different ploidy reveals the mechanism of photosynthetic efficiency superiority of triploid poplar. Genomics 2021, 113, 2211–2220. [Google Scholar] [CrossRef]
- Xu, T.T.; Zhang, S.W.; Du, K.; Yang, J.; Kang, X.Y. Insights into the molecular regulation of lignin content in triploid poplar leaves. Int. J. Mol. Sci 2022, 23, 4603. [Google Scholar] [CrossRef]
- Zhu, Z.D.; Lin, H.B.; Kang, X.Y. Studies on allotriploid breeding of Populus tomentosa B301 clones. Sci. Silv. Sin. 1995, 31, 499–505+579. [Google Scholar]
- Liu, Q.Z.; Yuan, K.J.; Zhang, L.S.; Zhao, H.J.; Liu, P.; Han, Z.H. Growth and fruit characteristics of autotetraploid plants of Royal Gala apple. J. Fruit Sci. 2006, 23, 102–104. [Google Scholar]
- Wang, X.L.; Yu, M.D.; Lu, C.; Wu, C.R.; Jing, C.J. Study on breeding and photosynthetic characteristics of new polyploidy variety for leaf and fruit-producing Mulberry (Morus L.). Sci. Agric. Sin. 2011, 44, 562–569. [Google Scholar]
- Chen, S.Y.; Zhang, Y.; Zhang, T.; Zhan, D.J.; Pang, Z.W.; Zhao, J.; Zhang, J.F. Comparative transcriptomic, anatomical and phytohormone analyses provide new insights into hormone-mediated tetraploid dwarfing in hybrid sweetgum (Liquidambar styraciflua × L. formosana). Front. Plant Sci. 2022, 13, 924044. [Google Scholar] [CrossRef]
- Ma, Y.; Xue, H.; Zhang, L.; Zhang, F.; Ou, C.Q.; Wang, F.; Zhang, Z.H. Involvement of auxin and brassinosteroid in dwarfism of autotetraploid apple (Malus × domestica). Sci. Rep. 2016, 6, 26719. [Google Scholar] [CrossRef]
- Austin, R.B.; Morgan, C.L.; Ford, M.A.; Bhagwat, S.G. Flag leaf photosynthesis of Triticum aestivum and related diploid and tetraploid species. Ann. Bot. 1982, 49, 177–189. [Google Scholar] [CrossRef]
- Xu, C.P.; Zhang, Y.; Han, Q.; Kang, X.Y. Molecular Mechanism of Slow Vegetative Growth in Populus Tetraploid. Genes 2020, 11, 1417. [Google Scholar] [CrossRef] [PubMed]
- Blakeslee, A.F.; Avery, A.G. Methods of inducing doubling of chromosomes in plants by treatment with colchicine. J. Hered. 1937, 28, 393–411. [Google Scholar] [CrossRef]
- Zhang, Y. Tetraploid Induction and Transcriptome Analysis of Variation with the Regenerated Root and Stem in Hybrid Sweetgum. Ph.D. Thesis, Beijing Forestry University, Beijing, China, 2019. [Google Scholar]
- Li, Y.L.; Yan, S.B.; Mao, X.H.; Wang, C.Y.; Wang, J.N.; Liu, C.L.; Zhang, Q.; Qiao, Y.H. Polyploidy induction by colchicine in forest trees: Research progress. J. Agric. 2022, 12, 55–61. [Google Scholar]
- Tao, D.H.; Liu, M.Y.; Xiao, J.Z.; Deng, J.P. Advances in the research on induction means of biopolyploid. Life Sci. Res. 2007, 11, 6–13. [Google Scholar] [CrossRef]
- Xing, S.H.; Guo, X.B.; Wang, Q.; Pan, Q.F.; Tian, Y.S.; Liu, P.; Zhao, J.Y.; Wang, G.F.; Sun, X.F.; Tang, K.X. Induction and flow cytometry identification of tetraploids from seed-derived explants through colchicine treatments in Catharanthus roseus (L.) G. Don. J. Biomed. Biotechnol. 2011, 2011, 793198. [Google Scholar] [CrossRef]
- Luo, Z.; Iaffaldano, B.J.; Cornish, K. Colchicine-induced polyploidy has the potential to improve rubber yield in Taraxacum kok-saghyz. Ind. Crops Prod. 2018, 112, 75–81. [Google Scholar] [CrossRef]
- Liu, F.M.; Mu, H.Z.; Liu, Z.J.; Li, Z.X.; Jiang, J.; Liu, G.F. Inducing tetraploid of Betula platyphylla with different generations of seeds by colchicine. J. Beijing For. Univ. 2013, 35, 84–89. [Google Scholar] [CrossRef]
- Xi, L.L.; Li, J.B.; Zhu, K.L.; Qi, Q.; Chen, X. Variation in genome size and stomatal traits among three Sorbus species. Plant Sci. J. 2020, 38, 32–38. [Google Scholar] [CrossRef]
- Pellicer, J.; Clermont, S.; Houston, L.; Rich, T.C.; Fay, M.F. Cytotype diversity in the Sorbus complex (Rosaceae) in Britain: Sorting out the puzzle. Ann. Bot. 2012, 110, 1185–1193. [Google Scholar] [CrossRef]
- Tavan, M.; Mirjalili, M.H.; Karimzadeh, G. In vitro polyploidy induction: Changes in morphological, anatomical and phytochemical characteristics of Thymus persicus (Lamiaceae). Plant Cell Tissue Organ Cult. 2015, 122, 573–583. [Google Scholar] [CrossRef]
- Hu, Y.L.; Sun, D.Q.; Hu, H.G.; Zuo, X.D.; Xia, T.; Xie, J.H. A comparative study on morphological and fruit quality traits of diploid and polyploid carambola (Averrhoa carambola L.) genotypes. Sci. Hortic. 2021, 277, 109843. [Google Scholar] [CrossRef]
- Wu, J.; Sang, Y.R.; Zhou, Q.; Zhang, P.D. Colchicine in vitro tetraploid induction of Populus hopeiensis from leaf blades. Plant Cell Tissue Organ Cult. 2020, 141, 339–349. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, B.B.; Qi, S.Z.; Dong, M.L.; Wang, Z.W.; Li, Y.X.; Chen, S.Y.; Li, B.L.; Zhang, J.F. Ploidy and hybridity effects on leaf size, cell size and related genes expression in triploids, diploids and their parents in Populus. Planta 2019, 249, 635–646. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.Z.; Qiu, B.F.; Ma, Z.C.; Lu, T.; Kang, X.y.; Yang, J. Induction and characterization of tetraploid through zygotic chromosome doubling in Eucalyptus urophylla. Front. Plant Sci. 2022, 13, 870698. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Chen, K.; Wang, W.; Liu, G.F.; Yang, C.P.; Jiang, J. Differences in leaf morphology and related gene expression between diploid and tetraploid birch (Betula pendula). Int. J. Mol. Sci. 2022, 23, 12966. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.M.; Zhou, Y.; Sun, Y.J.; Li, X.; Zhang, J.J. Polyploidy induction of Lilium spp. by colchicine and ploidy identification by flow cytometry. Acta Agric. Shanghai 2018, 34, 81–87. [Google Scholar] [CrossRef]
- Shao, B.J.; Wan, S.Q.; Liu, J.M.; Xu, J.X.; Zhao, H.E. Polyploidy induction and identification of Lycium ruthenicum and Lycium barbarum. Mol. Plant Breed. 2018, 16, 2593–2599. [Google Scholar] [CrossRef]
- Fu, Y.; Li, N.; Shi, L.L.; Wang, Y.; Zhang, J.F. Preliminary study on induction of polyploidy in platycladus orientalis by colchicine treatment. Chin. Agric. Sci. Bull. 2007, 23, 194–197. [Google Scholar]
- Cowan, C.R.; Cande, W.Z. Meiotic telomere clustering is inhibited by colchicine but does not require cytoplasmic microtubules. J. Cell Sci. 2002, 115, 3747–3756. [Google Scholar] [CrossRef] [PubMed]
- Eigsti, O.J. A Cytological Study of Colchicine Effects in the Induction of Polyploidy in Plants. Proc. Natl. Acad. Sci. USA 1938, 24, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Sax, K.; Sax, H.J. Stomata size and distribution in diploid and polyploid plants. J. Arnold Arbor. 1937, 18, 164–172. [Google Scholar] [CrossRef]
- Ho, I.; Wan, Y.; Widholm, J.M.; Rayburn, A.L. The Use of Stomatal Chloroplast Number for Rapid Determination of Ploidy Level in Maize. Plant Breed. 1990, 105, 203–210. [Google Scholar] [CrossRef]
- Ewald, D.; Ulrich, K.; Naujoks, G.; Schröder, M.B. Induction of tetraploid poplar and black locust plants using colchicine: Chloroplast number as an early marker for selecting polyploids in vitro. Plant Cell Tissue Organ Cult. 2009, 99, 353–357. [Google Scholar] [CrossRef]
- Zlesak, D.C.; Thill, C.A.; Anderson, N.O. Trifluralin-mediated polyploidization of Rosa chinensis minima (Sims) Voss seedlings. Euphytica 2005, 141, 281–290. [Google Scholar] [CrossRef]
- Blasco, M.; Badenes, M.L.; Naval, M.M. Colchicine-induced polyploidy in loquat (Eriobotrya japonica (Thunb.) Lindl.). Plant Cell Tissue Organ Cult. 2014, 120, 453–461. [Google Scholar] [CrossRef]
- Feng, Y.; Ren, Y.H.; Li, X.Y.; Sun, Y.H.; Deng, Y.P.; Zhu, J.H.; Yang, H.; Li, Y. Artificial induction of polyploid Robinia pseudoacacia L. and identification of its ploidy. Mol. Plant Breed. 2018, 16, 7124–7131. [Google Scholar] [CrossRef]
- Xue, H.; Zhang, B.; Tian, J.R.; Chen, M.M.; Zhang, Y.Y.; Zhang, Z.H.; Ma, Y. Comparison of the morphology, growth and development of diploid and autotetraploid ‘Hanfu’ apple trees. Sci. Hortic. 2017, 225, 277–285. [Google Scholar] [CrossRef]
- Dang, J.B.; Liang, G.L.; Li, C.; Wu, D.; Guo, Q.G.; Liang, S.L.; Wang, P. Polyploid rootstock of fruit tree: Research status and prospects. Acta Hortic. Sin. 2019, 46, 1701–1710. [Google Scholar] [CrossRef]
- Liu, Q.Z.; Ma, H.Y.; Wei, H.R.; Ai, C.X.; Zhang, L.S.; Zhao, Y.; Robert, E.D.; Han, Z.H. Regeneration of hexaploid plants of cherry dwarf rootstock ‘Gisela 6’ from in vitro leaves treated with colchicine. Acta Hortic. Sin. 2008, 35, 285–288. [Google Scholar] [CrossRef]
- Dudits, D.; Torok, K.; Cseri, A.; Paul, K.; Nagy, A.V.; Nagy, B.; Sass, L.; Ferenc, G.; Vankova, R.; Dobrev, P.; et al. Response of organ structure and physiology to autotetraploidization in early development of energy willow Salix viminalis. Plant Physiol. 2016, 170, 1504–1523. [Google Scholar] [CrossRef] [PubMed]
- Li, L.G.; He, P.; Ou, C.Q.; Li, H.F.; Zhang, Z.H. In vitro induction of tetraploid from mature embryos of ‘Golden Delicious’ apple. Acta Hortic. Sin. 2007, 34, 1129–1134. [Google Scholar] [CrossRef]
- Zheng, J.S.; Sun, C.Z.; Yan, L.Y.; Feng, Z.H. Development of cucumber autotetraploids and their phenotypic characterization. Cytologia 2019, 84, 359–365. [Google Scholar] [CrossRef]
- Bhattarai, K.; Kareem, A.; Deng, Z. In vivo induction and characterization of polyploids in gerbera daisy. Sci. Hortic. 2021, 282, 110054. [Google Scholar] [CrossRef]
- Cho, S.H.; Kang, K.; Lee, S.H.; Lee, I.J.; Paek, N.C. OsWOX3A is involved in negative feedback regulation of the gibberellic acid biosynthetic pathway in rice (Oryza sativa). J. Exp. Bot. 2016, 67, 1677–1687. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Tanaka, S.Y.; Masumoto, Y.; Nobori, N.; Ishii, H.; Hibara, K.; Itoh, J.; Tanisaka, T.; Taketa, S. Barley NARROW LEAFED DWARF1 encoding a WUSCHEL-RELATED HOMEOBOX 3 (WOX3) regulates the marginal development of lateral organs. Breed. Sci. 2016, 66, 416–424. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, C.Q.; Zhao, B.L.; He, L.L.; Zhou, S.L.; Liu, Y.; Zhao, W.Y.; Guo, S.Q.; Wang, R.R.; Bai, Q.Z.; Li, Y.H.; et al. The WOX family transcriptional regulator SlLAM1 controls compound leaf and floral organ development in Solanum lycopersicum. J. Exp. Bot. 2021, 72, 1822–1835. [Google Scholar] [CrossRef]
- Wilmoth, J.C.; Wang, S.; Tiwari, S.B.; Joshi, A.D.; Hagen, G.; Guilfoyle, T.J.; Alonso, J.M.; Ecker, J.R.; Reed, J.W. NPH4/ARF7 and ARF19 promote leaf expansion and auxin-induced lateral root formation. Plant J. 2005, 43, 118–130. [Google Scholar] [CrossRef]
- Liu, X. Effects of miRNA160 on Leaf Water Loss and Fruit Development in Tomato by SIARF10. Ph.D. Thesis, Shenyang Agricultural University, Shenyang, China, 2016. [Google Scholar]
- Li, L.C.; Kang, D.M.; Chen, Z.L.; Qu, L.J. Hormonal regulation of leaf morphogenesis in Arabidopsis. J. Integr. Plant Biol. 2007, 49, 75–80. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Khan, R.; Wu, X.Y.; Zhou, L.; Xu, N.; Du, S.S.; Ma, X.H. Exogenous application of brassinosteroids regulates tobacco leaf size and expansion via modulation of endogenous hormones content and gene expression. Physiol. Mol. Biol. Plants 2021, 27, 847–860. [Google Scholar] [CrossRef] [PubMed]
- Van de Peer, Y.; Mizrachi, E.; Marchal, K. The evolutionary significance of polyploidy. Nat. Rev. Genet. 2017, 18, 411–424. [Google Scholar] [CrossRef]
- Islam, M.M.; Deepo, D.M.; Nasif, S.O.; Siddique, A.B.; Hassan, O.; Siddique, A.B.; Paul, N.C. Cytogenetics and consequences of polyploidization on different biotic-abiotic stress tolerance and the potential mechanisms involved. Plants 2022, 11, 2684. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Lu, Z.W.; Wang, J.; Chen, T.; Gao, J.M.; Zheng, J.L.; Zhang, S.Q.; Xi, J.E.; Huang, X.; Guo, A.P.; et al. Induction of new tetraploid genotypes and heat tolerance assessment in Asparagus officinalis L. Sci. Hortic. 2020, 264, 109168. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Hu, C.G.; Yao, J.L. Tetraploidization of diploid Dioscorea results in activation of the antioxidant defense system and increased heat tolerance. J. Plant Physiol. 2010, 167, 88–94. [Google Scholar] [CrossRef]
- Liu, S.Y.; Chen, S.M.; Chen, Y.; Guan, Z.Y.; Yin, D.M.; Chen, F.D. In vitro induced tetraploid of Dendranthema nankingense (Nakai) Tzvel. shows an improved level of abiotic stress tolerance. Sci. Hortic. 2011, 127, 411–419. [Google Scholar] [CrossRef]
- Parisod, C.; Holderegger, R.; Brochmann, C. Evolutionary consequences of autopolyploidy. New Phytol. 2010, 186, 5–17. [Google Scholar] [CrossRef]
- Bickford, C.P. Ecophysiology of leaf trichomes. Funct. Plant Biol. 2016, 43, 807–814. [Google Scholar] [CrossRef]
- Majdi, M.; Karimzadeh, G.; Malboobi, M.A.; Omidbaigi, R.; Mirzaghaderi, G. Induction of tetraploidy to feverfew (Tanacetum parthenium Schulz-Bip.): Morphological, physiological, cytological, and phytochemical changes. HortScience 2010, 45, 16–21. [Google Scholar] [CrossRef]
- Li, Y.H.; Li, Z.; Xin, Z.M.; Liu, M.H.; Li, Y.L.; Hao, Y.G. Effects of leaf shape plasticity on leaf surface temperature. Chin. J. Plant Ecol. 2018, 42, 202–208. [Google Scholar] [CrossRef]
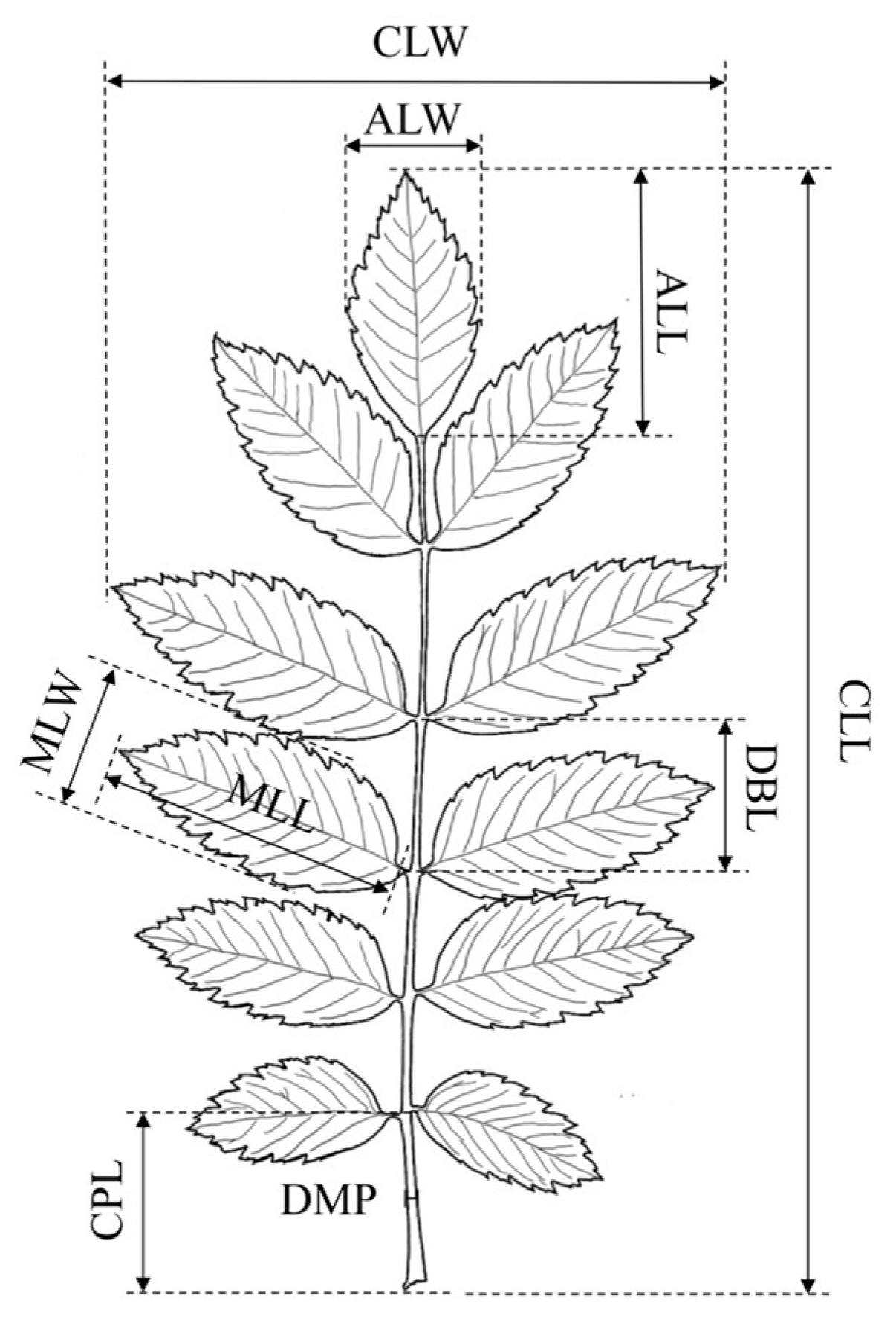


| Characteristics | Abbreviation |
|---|---|
| Compound leaf length | CLL |
| Compound leaf width | CLW |
| Common petiole length | CPL |
| Diameter of middle petiole | DMP |
| Distance between leaflets | DBL |
| Apical leaflet length | ALL |
| Apical leaflet width | ALW |
| Apical leaflet shape index | ALSI |
| Middle leaflet length | MLL |
| Middle leaflet width | MLW |
| Middle leaflet shape index | MLSI |
| Plant height | PH |
| Basal diameter | BD |
| Annual branch length | ABL |
| Percentage of sunburn | PS |
| Colchicine Concentration (%) (w/v) | Treatment Time (h) | |||
|---|---|---|---|---|
| 12 h | 24 h | 48 h | Mean | |
| 0 | 49.10 ± 1.41 abA | 41.92 ± 1.92 aB | 30.47 ± 1.22 abC | 40.50 ± 2.87 ab |
| 0.01 | 52.79 ± 1.86 aA | 40.65 ± 0.28 aB | 37.40 ± 0.47 aB | 43.61 ± 2.44 a |
| 0.05 | 49.48 ± 0.69 abA | 42.07 ± 0.42 aB | 32.50 ± 0.61 abC | 41.35 ± 2.48 ab |
| 0.1 | 43.85 ± 2.19 bcA | 31.29 ± 1.76 bB | 25.38 ± 3.50 bcB | 33.51 ± 3.15 bc |
| 0.2 | 39.21 ± 1.57 cA | 26.83 ± 1.21 bB | 20.91 ± 3.50 cB | 28.98 ± 3.05 c |
| Mean | 46.89 ± 1.48 A | 36.55 ± 1.79 B | 29.33 ± 1.86 C | |
| Treatment | Surviving Rate (%) | Tetraploid (%) | Mixoploid (%) | Induction Rate (%) | Tetraploid Induction Efficiency (%) | |
|---|---|---|---|---|---|---|
| Stratification time (d) | 14 | 38.92 ± 2.15 a | 8.82 ± 3.00 a | 9.65 ± 3.41 a | 14.18 ± 4.30 a | 5.04 ± 1.68 a |
| 21 | 38.15 ± 2.82 a | 11.38 ± 5.25 a | 14.19 ± 4.58 a | 20.32 ± 7.34 a | 5.40 ± 2.39 a | |
| Concentration (%) (w/v) | 0 | 43.82 ± 2.91 a | 0 ± 0 b | 0 ± 0 c | 0 ± 0 c | 0 ± 0 b |
| 0.1 | 40.48 ± 0.83 a | 5.55 ± 2.67 b | 10.83 ± 2.39 b | 14.31 ± 1.33 b | 3.58 ± 1.72 b | |
| 0.2 | 31.32 ± 2.07 b | 24.75 ± 3.76 a | 24.93 ± 3.61 a | 37.44 ± 5.92 a | 12.09 ± 1.43 a | |
| Provenances | Concentration (%) (w/v) | Tetraploid (%) | Mixoploid (%) | Induction Rate (%) | Germination Rate (%) |
|---|---|---|---|---|---|
| TS2018 | 0.1 | 5.55 ± 2.67 b | 10.83 ± 2.39 c | 14.31 ± 1.33 c | 40.87 ± 0.78 a |
| 0.2 | 24.75 ± 3.76 a | 24.93 ± 3.61 b | 37.44 ± 5.92 ab | 33.06 ± 1.04 b | |
| ZY2021 | 0.1 | 9.76 ± 4.88 b | 22.91 ± 4.05 b | 26.41 ± 3.18 bc | 22.95 ± 3.38 c |
| 0.2 | 24.9 ± 1.65 a | 40.08 ± 1.69 a | 50.42 ± 2.53 a | 22.10 ± 2.80 c |
| Characteristic | Diploid | Tetraploid |
|---|---|---|
| Stomatal length (μm) | 28.53 ± 0.41 | 37.10 ± 0.46 ** |
| Stomatal width (μm) | 19.56 ± 0.27 | 24.44 ± 0.31 ** |
| Stomatal density (number per mm2) | 156.37 ± 4.22 ** | 105.27 ± 2.38 |
| Chloroplast number in stomatal guard cells | 18.44 ± 0.25 | 29.73 ± 0.45 ** |
| Characteristic | First Year (2021) | Second Year (2022) | ||
|---|---|---|---|---|
| Diploid | Tetraploid | Diploid | Tetraploid | |
| Plant height (cm) | 44.76 ± 2.18 ** | 38.02 ± 3.35 | 71.96 ± 3.82 ** | 56.83 ± 3.39 |
| Basal diameter (mm) | 11.57 ± 0.34 | 12.28 ± 0.39 | 13.53 ± 0.36 | 15.3 ± 0.45 ** |
| Compound leaf length (cm) | 19.82 ± 0.52 | 18.43 ± 0.41 | 16.29 ± 0.51 | 16.47 ± 0.65 |
| Compound leaf width (cm) | 10.63 ± 0.37 | 10.08 ± 0.3 | 9.16 ± 0.32 | 8.72 ± 0.29 |
| Common petiole length (mm) | 40.31 ± 1.35 | 41.6 ± 4.08 | 36.85 ± 1.71 | 34.37 ± 1.93 |
| Distance between leaflets (mm) | 23.86 ± 0.77 | 24.33 ± 0.49 | 21.93 ± 0.66 | 22.74 ± 0.89 |
| Aapical leaflet length (mm) | 52.65 ± 1.71 | 47.59 ± 1.94 | 41.48 ± 1.42 | 41.33 ± 1.12 |
| Aapical leaflet width (mm) | 20.23 ± 0.71 | 24.18 ± 0.87 ** | 20.08 ± 0.06 | 25.93 ± 0.08 ** |
| Apical leaflet shape index | 2.66 ± 0.07 ** | 1.99 ± 0.06 | 2.08 ± 0.05 ** | 1.62 ± 0.05 |
| Middle leaflet length (mm) | 61.33 ± 3.22 | 53.02 ± 1.43 | 49.63 ± 1.54 | 46.55 ± 1.5 |
| Middle leaflet width (mm) | 20.8 ± 0.51 | 25.07 ± 0.63 ** | 21.93 ± 0.81 | 27.63 ± 0.8 ** |
| Middle leaflet shape index | 2.94 ± 0.14 ** | 2.14 ± 0.06 | 2.28 ± 0.04 ** | 1.7 ± 0.04 |
| Annual branch length (cm) | —— | —— | 32.93 ± 3.56 ** | 23.02 ± 2.64 |
| Diameter of middle petiole (mm) | —— | —— | 1.52 ± 0.03 | 2.03 ± 0.05 ** |
| Percentage of sunburn | —— | —— | 44.08 ± 3.25 | 53.24 ± 2.57 ** |
| Ploidy | PH | BD | ABL | CLL | CPL | CLW | DBL | ALL | ALW | ALSI | MLL | MLW | MLSI | DMP | PS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ploidy | ||||||||||||||||
| PH | −0.412 ** | |||||||||||||||
| BD | 0.413 ** | 0.178 | ||||||||||||||
| ABL | −0.329 * | 0.709 ** | 0.083 | |||||||||||||
| CLL | 0.032 | 0.549 ** | 0.337 * | 0.489 ** | ||||||||||||
| CPL | −0.141 | 0.549 ** | 0.173 | 0.639 ** | 0.843 ** | |||||||||||
| CLW | −0.153 | 0.593 ** | 0.301 * | 0.286 | 0.665 ** | 0.428 ** | ||||||||||
| DBL | 0.106 | 0.416 ** | 0.449 ** | 0.456 ** | 0.870 ** | 0.708 ** | 0.601 ** | |||||||||
| ALL | −0.013 | 0.339 * | 0.373 * | 0.303 * | 0.614 ** | 0.500 ** | 0.545 ** | 0.601 ** | ||||||||
| ALW | 0.643 ** | −0.004 | 0.587 ** | −0.018 | 0.349 * | 0.139 | 0.270 | 0.487 ** | 0.496 ** | |||||||
| ALSI | −0.729 ** | 0.303 * | −0.321 * | 0.312 * | 0.115 | 0.254 | 0.141 | −0.039 | 0.241 | −0.709 ** | ||||||
| MLL | −0.211 | 0.574 ** | 0.295 * | 0.255 | 0.643 ** | 0.392 ** | 0.944 ** | 0.643 ** | 0.555 ** | 0.280 | 0.118 | |||||
| MLW | 0.605 ** | 0.096 | 0.560 ** | −0.083 | 0.455 ** | 0.166 | 0.525 ** | 0.553 ** | 0.417 ** | 0.869 ** | −0.654 ** | 0.527 ** | ||||
| MLSI | −0.865 ** | 0.404 ** | −0.351 * | 0.358 * | 0.056 | 0.154 | 0.209 | −0.050 | 0.015 | −0.728 ** | 0.849 ** | 0.251 | −0.679 ** | |||
| DMP | 0.777 ** | −0.231 | 0.420 ** | −0.226 | 0.210 | −0.095 | 0.230 | 0.311 * | 0.163 | 0.735 ** | −0.677 ** | 0.181 | 0.757 ** | −0.687 ** | ||
| PS | 0.323 * | −0.079 | 0.011 | −0.015 | −0.059 | −0.155 | −0.144 | −0.103 | −0.146 | 0.162 | −0.284 | −0.183 | 0.176 | −0.337 * | 0.292 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Zhang, Y.; Di, Z.; Zhang, R.; Mu, Y.; Sun, T.; Tian, Z.; Lu, Y.; Zheng, J. Tetraploid Induction with Leaf Morphology and Sunburn Variation in Sorbus pohuashanensis (Hance) Hedl. Forests 2023, 14, 1589. https://doi.org/10.3390/f14081589
Zhang Z, Zhang Y, Di Z, Zhang R, Mu Y, Sun T, Tian Z, Lu Y, Zheng J. Tetraploid Induction with Leaf Morphology and Sunburn Variation in Sorbus pohuashanensis (Hance) Hedl. Forests. 2023; 14(8):1589. https://doi.org/10.3390/f14081589
Chicago/Turabian StyleZhang, Zeren, Yan Zhang, Zexin Di, Ruili Zhang, Yanjuan Mu, Tao Sun, Zhihui Tian, Yizeng Lu, and Jian Zheng. 2023. "Tetraploid Induction with Leaf Morphology and Sunburn Variation in Sorbus pohuashanensis (Hance) Hedl" Forests 14, no. 8: 1589. https://doi.org/10.3390/f14081589
APA StyleZhang, Z., Zhang, Y., Di, Z., Zhang, R., Mu, Y., Sun, T., Tian, Z., Lu, Y., & Zheng, J. (2023). Tetraploid Induction with Leaf Morphology and Sunburn Variation in Sorbus pohuashanensis (Hance) Hedl. Forests, 14(8), 1589. https://doi.org/10.3390/f14081589






