Genetic Characterisation and Core Collection Construction of European Larch (Larix decidua Mill.) from Seed Orchards in Romania
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Orchard Characteristics and Location
2.2. SSR Analysis
2.3. Data Analysis
3. Results
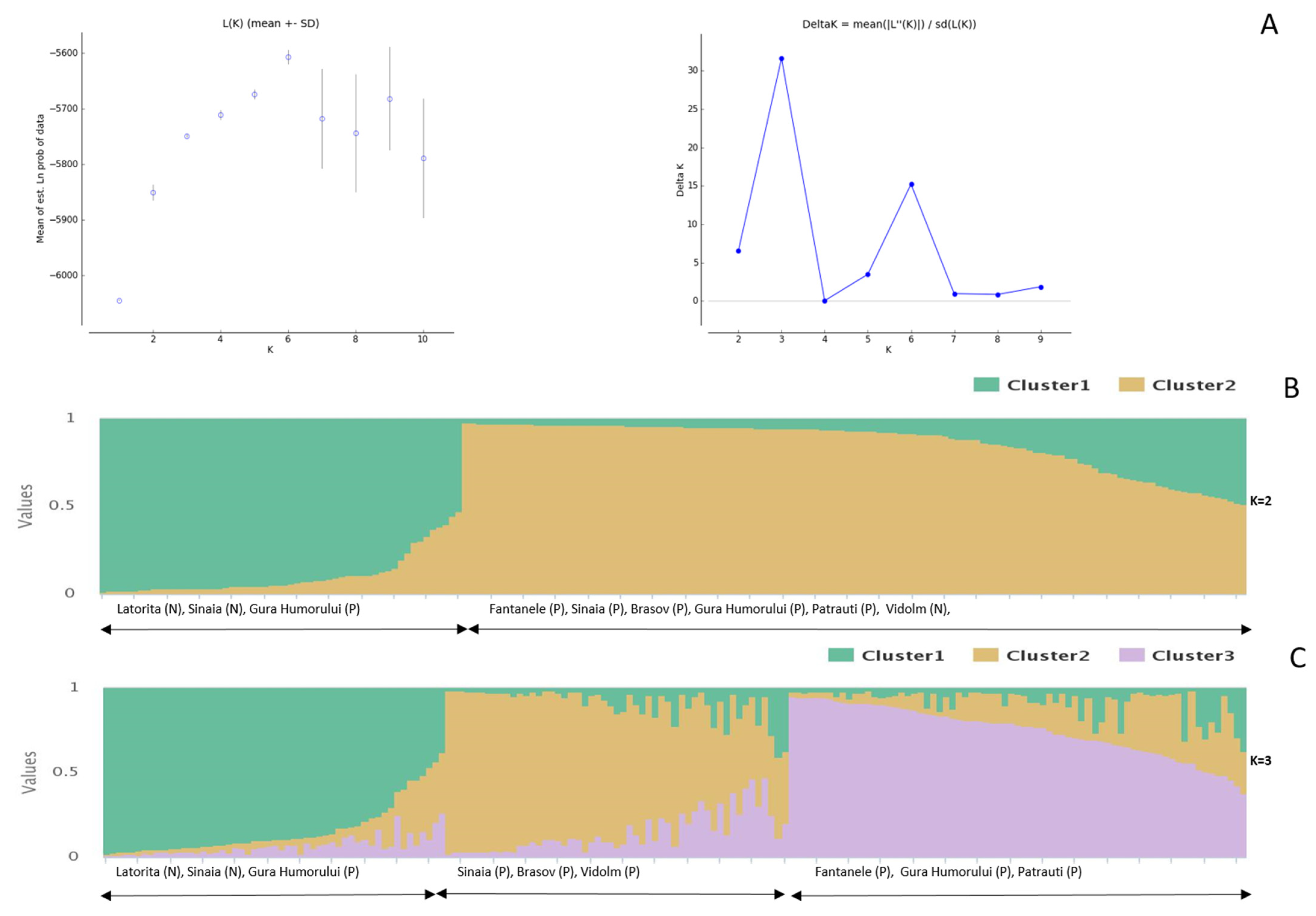
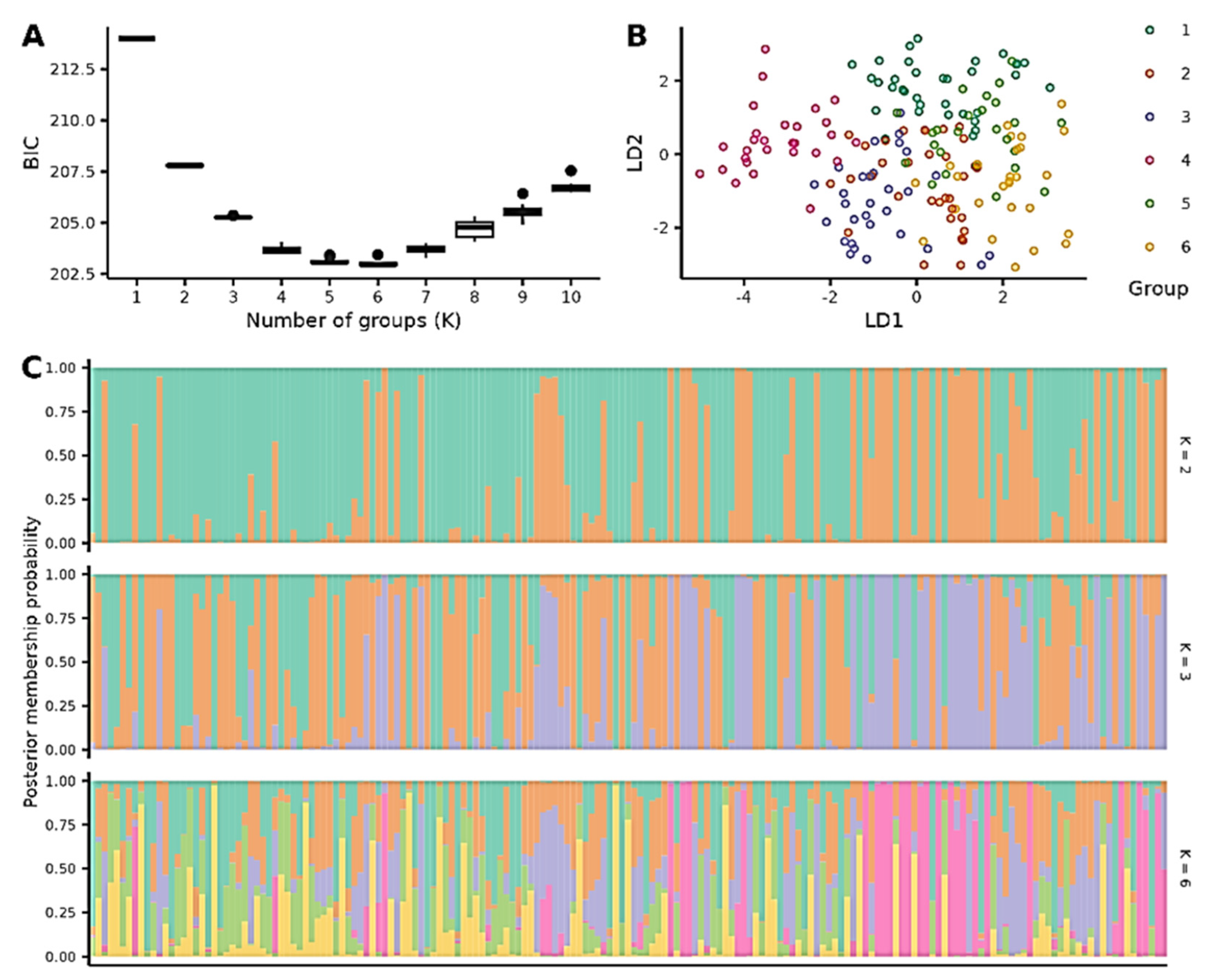
3.1. Genetic Structure of the Seed Orchards
3.2. Construction of the European Larch Core Collection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fady, B.; Cottrell, J.; Ackzell, L.; Alía, R.; Muys, B.; Prada, A.; González-Martínez, S.C. Forests and Global Change: What Can Genetics Contribute to the Major Forest Management and Policy Challenges of the Twenty-First Century? Reg. Environ. Chang. 2016, 16, 927–939. [Google Scholar] [CrossRef]
- Leites, L.; Garzón, M.B. Forest tree species adaptation to climate across biomes: Building on the legacy of ecological genetics to anticipate responses to climate change. Glob. Chang. Biol. 2023. [Google Scholar] [CrossRef]
- Potter, K.M.; Jetton, R.M.; Bower, A.; Jacobs, D.F.; Man, G.; Hipkins, V.D.; Westwood, M. Banking on the Future: Progress, Challenges and Opportunities for the Genetic Conservation of Forest Trees. N. For. 2017, 48, 153–180. [Google Scholar] [CrossRef]
- van Hintum, T.J.L.; Brown, A.H.D.; Spillane, C. Core Collections of Plant Genetic Resources; Bioversity International: Rome, Italy, 2000; ISBN 978-92-9043-454-2. [Google Scholar]
- Guo, Q.; Liu, J.; Li, J.; Cao, S.; Zhang, Z.; Zhang, J.; Zhang, Y.; Deng, Y.; Niu, D.; Su, L.; et al. Genetic Diversity and Core Collection Extraction of Robinia Pseudoacacia L. Germplasm Resources Based on Phenotype, Physiology, and Genotyping Markers. Ind. Crops Prod. 2022, 178, 114627. [Google Scholar] [CrossRef]
- Wu, H.; Duan, A.; Wang, X.; Chen, Z.; Zhang, X.; He, G.; Zhang, J. Construction of a Core Collection of Germplasms from Chinese Fir Seed Orchards. Forests 2023, 14, 305. [Google Scholar] [CrossRef]
- Li, N.; Yang, Y.; Xu, F.; Chen, X.; Wei, R.; Li, Z.; Pan, W.; Zhang, W. Genetic Diversity and Population Structure Analysis of Castanopsis Hystrix and Construction of a Core Collection Using Phenotypic Traits and Molecular Markers. Genes 2022, 13, 2383. [Google Scholar] [CrossRef]
- Sun, Y.; Dong, S.; Liu, Q.; Chen, J.; Pan, J.; Zhang, J. Selection of a Core Collection of Prunus sibirica L. Germplasm by a Stepwise Clustering Method Using Simple Sequence Repeat Markers. PLoS ONE 2021, 16, e0260097. [Google Scholar] [CrossRef]
- Gu, R.; Fan, S.; Wei, S.; Li, J.; Zheng, S.; Liu, G. Developments on Core Collections of Plant Genetic Resources: Do We Know Enough? Forests 2023, 14, 926. [Google Scholar] [CrossRef]
- Wang, X.; Cao, Z.; Gao, C.; Li, K. Strategy for the Construction of a Core Collection for Pinus Yunnanensis Franch. To Optimize Timber Based on Combined Phenotype and Molecular Marker Data. Genet. Resour. Crop Evol. 2021, 68, 3219–3240. [Google Scholar] [CrossRef]
- Kelblerová, R.; Dvořák, J.; Korecký, J. Genetic Diversity Maximization as a Strategy for Resilient Forest Ecosystems: A Case Study on Norway Spruce. Forests 2022, 13, 489. [Google Scholar] [CrossRef]
- Kalinowski, S.T. Do Polymorphic Loci Require Large Sample Sizes to Estimate Genetic Distances? Heredity 2005, 94, 33–36. [Google Scholar] [CrossRef]
- Schoen, D.J.; Brown, A.H. Conservation of Allelic Richness in Wild Crop Relatives Is Aided by Assessment of Genetic Markers. Proc. Natl. Acad. Sci. USA 1993, 90, 10623–10627. [Google Scholar] [CrossRef]
- Thachuk, C.; Crossa, J.; Franco, J.; Dreisigacker, S.; Warburton, M.; Davenport, G.F. Core Hunter: An Algorithm for Sampling Genetic Resources Based on Multiple Genetic Measures. BMC Bioinform. 2009, 10, 243. [Google Scholar] [CrossRef]
- Kempf, M.; Hebda, A.; Bodziarczyk, J. A Nature Reserve as a Repository of Genetic Richness–The Case of European Larch from the Gorce Mountains. J. Nat. Conserv. 2023, 74, 126440. [Google Scholar] [CrossRef]
- Raffl, H.; Konrad, H.; Curtu, L.A.; Geburek, T. Genetic Evidence of Human Mediated, Historical Seed Transfer from the Tyrolean Alps to the Romanian Carpathians in Larix decidua (Mill.) Forests. Ann. For. Sci. 2018, 75, 98. [Google Scholar] [CrossRef]
- Lewandowski, A.; Burczyk, J. Mating System and Genetic Diversity in Natural Populations of European Larch (Larix decidua) and Stone Pine (Pinus cembra) Located at Higher Elevations. Silvae Genet. 2000, 49, 158–160. [Google Scholar]
- Mosca, E.; Eckert, A.J.; Di Pierro, E.A.; Rocchini, D.; La Porta, N.; Belletti, P.; Neale, D.B. The Geographical and Environmental Determinants of Genetic Diversity for Four Alpine Conifers of the European Alps. Mol. Ecol. 2012, 21, 5530–5545. [Google Scholar] [CrossRef]
- Wagner, S.; Liepelt, S.; Gerber, S.; Petit, R.J. Within-Range Translocations and Their Consequences in European Larch. PLoS ONE 2015, 10, e0127516. [Google Scholar] [CrossRef]
- Dostálek, J.; Frantík, T.; Pospíšková, M.; Křížová, M. Population Genetic Structure and Delineation of Conservation Units in European Larch (Larix decidua Mill.) Across Its Native Range. Flora 2018, 246–247, 26–32. [Google Scholar] [CrossRef]
- Matsushita, M.; Nishikawa, H.; Tamura, A.; Takahashi, M. Effects of light intensity and girdling treatments on the production of female cones in Japanese larch (Larix kaempferi (Lamb.) Carr.): Implications for the management of seed orchards. Forests 2020, 11, 1110. [Google Scholar] [CrossRef]
- Inventarul Forestier Național. Available online: https://roifn.ro/site/ (accessed on 15 April 2023).
- Haralamb, A.M. Cultura Speciilor Forestiere; Editura Agrosilvică, Bucureşti: Bucharest, Romania, 1967; 755p. [Google Scholar]
- Rubtov, S. Arealul si ecologia Laricelui–Centrele de raspindire naturala a laricelui in Romania. The distribution area and ecology of larch–the natural distribution centers of larch in Romania. In Laricele–Ecologia si Cultura; Rubţov, Ș., Ed.; Editura Agro–Silvica: Bucharest, Romania, 1965; pp. 65–74. [Google Scholar]
- Jansen, S.; Geburek, T. Historic Translocations of European Larch (Larix decidua Mill.) Genetic Resources Across Europe—A Review from the 17th Until the Mid-20th Century. For. Ecol. Manag. 2016, 379, 114–123. [Google Scholar] [CrossRef]
- Mihai, G.; Alexandru, A.; Mirancea, I. Genetic Variation and Early Selection in Larix decidua Mill. From Progeny Test in Romania. Ann. For. Sci. 2019, 76, 81. [Google Scholar] [CrossRef]
- Gramazio, P.; Plesa, I.M.; Truta, A.M.; Sestras, A.F.; Vilanova, S.; Plazas, M.; Vicente, O.; Boscaiu, M.; Prohens, J.; Sestras, R.E. Highly Informative SSR Genotyping Reveals Large Genetic Diversity and Limited Differentiation in European Larch (Larix decidua) Populations from Romania. Turk. J. Agric. For. 2018, 42, 165–175. [Google Scholar] [CrossRef]
- Pârnuță, G.; Budeanu, M.; Stuparu, E.; Scărlătescu, V.; Chesnoiu, E.N.; Tudoroiu, M.; Filat, M.; Nica, M.S.; Teodosiu, M.; Lorent, A.; et al. Catalogul Național al Materialelor de bază Pentru Producerea Materialelor Forestiere de Reproducere; Eitura Silvică: București, Romania, 2012; 304p. [Google Scholar]
- Dumolin, S.; Demesure, B.; Petit, R.J. Inheritance of Chloroplast and Mitochondrial Genomes in Pedunculate Oak Investigated with an Efficient PCR Method. Theor. Appl. Genet. 1995, 91, 1253–1256. [Google Scholar] [CrossRef]
- Isoda, K.; Watanabe, A. Isolation and Characterization of Microsatellite Loci from Larix Kaempferi. Mol. Ecol. Notes 2006, 6, 664–666. [Google Scholar] [CrossRef]
- Wagner, S.; Gerber, S.; Petit, R.J. Two Highly Informative Dinucleotide SSR Multiplexes for the Conifer Larix decidua (European larch). Mol. Ecol. Resour. 2012, 12, 717–725. [Google Scholar] [CrossRef]
- Khasa, P.D.; Newton, C.H.; Rahman, M.H.; Jaquish, B.; Dancik, B.P. Isolation, Characterizaton, and Inheritance of Microsatellite Loci in Alpine Larch and Western Larch. Genome 2000, 43, 439–448. [Google Scholar] [CrossRef]
- Van Oosterhout, C.; Hutchinson, W.F.; Wills, D.P.M.; Shipley, P. MICRO-CHECKER: Software for Identifying and Correcting Genotyping Errors in Microsatellite Data. Mol. Ecol. Notes 2004, 4, 535–538. [Google Scholar] [CrossRef]
- Marshall, T.C.; Slate, J.; Kruuk, L.E.B.; Pemberton, J.M. Statistical Confidence for Likelihood-Based Paternity Inference in Natural Populations. Mol. Ecol. 1998, 7, 639–655. [Google Scholar] [CrossRef]
- Peakall, R.O.D.; Smouse, P.E. GENALEX 6: Genetic Analysis in Excel. Population Genetic Software for Teaching and Research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of Population Structure Using Multilocus Genotype Data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Earl, D.A.; VonHoldt, B.M. STRUCTURE HARVESTER: A Website and Program for Visualizing STRUCTURE Output and Implementing the Evanno Method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Jombart, T. Adegenet: A R Package for the Multivariate Analysis of Genetic Markers. Bioinformatics 2008, 24, 1403–1405. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- De Beukelaer, H.; Davenport, G.F.; Fack, V. Core Hunter 3: Flexible Core Subset Selection. BMC Bioinform. 2018, 19, 203. [Google Scholar] [CrossRef]
- Beukelaer, H.D.; Smýkal, P.; Davenport, G.F.; Fack, V. Core Hunter II: Fast Core Subset Selection Based on Multiple Genetic Diversity Measures Using Mixed Replica Search. BMC Bioinform. 2012, 13, 312. [Google Scholar] [CrossRef]
- Odong, T.L.; Jansen, J.; van Eeuwijk, F.A.; van Hintum, T.J.L. Quality of Core Collections for Effective Utilisation of Genetic Resources Review, Discussion and Interpretation. Theor. Appl. Genet. 2013, 126, 289–305. [Google Scholar] [CrossRef]
- Lv, J.; Li, C.; Zhou, C.; Chen, J.; Li, F.; Weng, Q.; Li, M.; Wang, Y.; Chen, S.; Chen, J.; et al. Genetic Diversity Analysis of a Breeding Population of Eucalyptus Cloeziana F. Muell. (Myrtaceae) and Extraction of a Core Germplasm Collection Using Microsatellite Markers. Ind. Crops Prod. 2020, 145, 112157. [Google Scholar] [CrossRef]
- Chapuis, M.-P.; Estoup, A. Microsatellite null alleles and estimation of population differentiation. Mol. Biol. Evol. 2007, 24, 621–631. [Google Scholar] [CrossRef]
- Pluess, A.R. Pursuing Glacier Retreat: Genetic Structure of a Rapidly Expanding Larix decidua Population. Mol. Ecol. 2011, 20, 473–485. [Google Scholar] [CrossRef]
- Nardin, M.; Musch, B.; Rousselle, Y.; Guérin, V.; Sanchez, L.; Rossi, J.-P.; Gerber, S.; Marin, S.; Pâques, L.E.; Rozenberg, P. Genetic Differentiation of European Larch Along an Altitudinal Gradient in the French Alps. Ann. For. Sci. 2015, 72, 517–527. [Google Scholar] [CrossRef]
- Litkowiec, M.; Lewandowski, A.; Burczyk, J. Genetic Status of Polish Larch (Larix decidua subsp. Polonica (Racib. Domin)) from Chełmowa Mountain: Implications for Gene Conservation. Dendrobiology 2018, 80, 101–111. [Google Scholar] [CrossRef]
- Nishimura, M.; Setoguchi, H. Homogeneous Genetic Structure and Variation in Tree Architecture of Larix Kaempferi Along Altitudinal Gradients on Mt. Fuji. J. Plant Res. 2011, 124, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Oreshkova, N.V.; Belokon, M.M.; Jamiyansuren, S. Genetic Diversity, Population Structure, and Differentiation of Siberian Larch, Gmelin Larch, and Cajander Larch on SSR-marker Data. Russ. J. Genet. 2013, 49, 178–186. [Google Scholar] [CrossRef]
- Wagner, S.; Litt, T.; Sánchez-Goñi, M.-F.; Petit, R.J. History of Larix decidua Mill. (European Larch) Since 130 Ka. Quat. Sci. Rev. 2015, 124, 224–247. [Google Scholar] [CrossRef]
- Vilcan, A.; Mihalte, L.; Sestras, A.F.; Holonec, L.; Sestras, R.E. Genetic Variation and Potential Genetic Resources of Several Romanian Larch Populations. Turk. J. Agric. For. 2017, 41, 82–91. [Google Scholar] [CrossRef]
- George, J.-P.; Grabner, M.; Karanitsch-Ackerl, S.; Mayer, K.; Weißenbacher, L.; Schueler, S.; Mäkelä, A. Genetic Variation, Phenotypic Stability, and Repeatability of Drought Response in European Larch Throughout 50 Years in a Common Garden Experiment. Tree Physiol. 2017, 37, 33–46. [Google Scholar] [CrossRef]
- Lstibůrek, M.; Schueler, S.; El-Kassaby, Y.A.; Hodge, G.R.; Stejskal, J.; Korecký, J.; Škorpík, P.; Konrad, H.; Geburek, T. In situ Genetic Evaluation of European Larch Across Climatic Regions Using Marker-Based Pedigree Reconstruction. Front. Genet. 2020, 11, 28. [Google Scholar] [CrossRef]

| Seed Orchard Name | Seed Orchard ID * | Latitude (°N) | Longitude (°E) | Year of Installation | Area (ha) | No. of Clones | Sample Size |
|---|---|---|---|---|---|---|---|
| Siminicea | PS-LA-SV83 | 47°41′ | 26°22′ | 1983 | 5.0 | 56 | 119 |
| Gârcina | PS-LA-NT82 | 47°03′ | 26°26′ | 1982 | 6.8 | 56 | 152 |
| Hemeiuşi | PS-LA-BC67 | 46°37′ | 26°51′ | 1967 | 5.6 | 38 | 104 |
| Beizadele | PS-LA-PH82 | 44°53′ | 25°53′ | 1982 | 4.0 | 42 | 99 |
| Valea lui Ştefan | PS-LA-AG68 | 45°05′ | 25°04′ | 1968 | 5.0 | 54 | 149 |
| Pooled | 246 | 623 |
| Seed Orchard | Clone ID | Region of Provenances * | Forest District | Type of Stand |
|---|---|---|---|---|
| Hemeiuşi | 1–36, NB, NS | G3 | Fântânele | plantation |
| Gârcina | 1–34 | G3 | Fântânele | plantation |
| 83, 86 | G1 | Pătrăuţi | plantation | |
| 113, 119, 584, 586, 718–726, 1P-4P, NB, NP | A2 | Gura Humorului | plantation | |
| Siminicea | 1–36 | G3 | Fântânele | plantation |
| 77, 78, 83–88 | G1 | Pătrăuţi | plantation | |
| 102, 103, 112–115, 126 | A2 | Gura Humorului | plantation | |
| Beizadele | 2.1–2.18, 2.26 | B1 | Brasov | plantation |
| 3.6, 3.12 | E3 | Vidolm | natural | |
| 5.1–5.11 | B2 | Sinaia | natural | |
| Valea lui Ştefan | 1L–30L | C2 | Latorita | natural |
| 1S–24S | B2 | Sinaia | natural, plantation |
| Locus | Observed Allele Size (bp) (Multiplex) | A | Ne | Ho | He | FIS | PIC | fnull |
|---|---|---|---|---|---|---|---|---|
| Ld30 | 106–138 (A) | 12 | 4.59 | 0.641 | 0.778 | 0.174 | 0.771 | 0.102 *** |
| Ld31 | 113–143 (A) | 13 | 4.02 | 0.709 | 0.751 | 0.056 | 0.760 | 0.024 |
| bclK189 | 146–172 (A) | 12 | 6.88 | 0.823 | 0.854 | 0.037 | 0.845 | 0.038 *** |
| bclK211 | 188–232 (A) | 21 | 4.54 | 0.703 | 0.777 | 0.098 | 0.789 | 0.011 |
| bclK228 | 176–212 (A) | 17 | 8.67 | 0.841 | 0.884 | 0.048 | 0.885 | 0.024 |
| bclK253 | 204–226 (A) | 11 | 7.06 | 0.691 | 0.853 | 0.196 | 0.845 | 0.115 |
| Ld50 | 168–196 (B) | 14 | 5.82 | 0.687 | 0.809 | 0.148 | 0.815 | 0.098 *** |
| Ld58 | 140–174 (B) | 17 | 8.43 | 0.716 | 0.881 | 0.187 | 0.870 | 0.003 |
| Ld45 | 203–219 (B) | 9 | 4.48 | 0.673 | 0.767 | 0.117 | 0.755 | 0.070 ** |
| Ld42 | 178–194 (B) | 8 | 3.75 | 0.452 | 0.726 | 0.379 | 0.743 | 0.267 *** |
| Ld56 | 228–248 (B) | 10 | 6.01 | 0.815 | 0.831 | 0.023 | 0.840 | 0.021 |
| bclK263 | 185–243 (C) | 22 | 10.65 | 0.908 | 0.906 | −0.003 | 0.918 | 0.023 |
| bclk229 | 93–125 (C) | 8 | 3.09 | 0.603 | 0.675 | 0.109 | 0.611 | 0.070 ** |
| Ld101 | 190–198 (C) | 5 | 1.31 | 0.154 | 0.239 | 0.327 | 0.202 | 0.103 |
| UAKLLy6 | 229–239 (C) | 6 | 3.93 | 0.484 | 0.741 | 0.340 | 0.702 | 0.193 *** |
| Mean | 12.33 | 5.54 | 0.660 | 0.764 | 0.149 | 0.756 | 0.077 |
| Seed Orchard | N | Na | Ne | I | Ho | He | FIS |
|---|---|---|---|---|---|---|---|
| Hemeiuşi (HEM) | 37 | 8.90 | 5.15 | 1.75 | 0.725 | 0.774 | 0.072 |
| Gârcina (GAR) | 55 | 10.27 | 5.51 | 1.81 | 0.725 | 0.783 | 0.082 |
| Siminicea (SIM) | 40 | 9.72 | 5.52 | 1.83 | 0.729 | 0.789 | 0.076 |
| Beizadele (BEI) | 37 | 10.00 | 5.94 | 1.88 | 0.637 | 0.797 | 0.206 |
| Valea lui Ştefan (VST) | 50 | 10.00 | 6.10 | 1.90 | 0.633 | 0.809 | 0.223 |
| Mean | 44 | 9.78 | 5.64 | 1.83 | 0.690 | 0.790 | 0.132 |
| Source | df | SS | MS | Est. Var. | % | p |
|---|---|---|---|---|---|---|
| Among seed orchards | 4 | 43.669 | 10.917 | 0.068 | 2% | 0.001 |
| Among accessions within seed orchards | 214 | 1070.636 | 5.003 | 0.595 | 13% | 0.001 |
| Within accession | 219 | 835.248 | 3.814 | 3.814 | 85% | 0.001 |
| Total | 437 | 1949.553 | 4.476 | 100% | ||
| F-statistics value FST 0.015 FIS 0.135 FIT 0.148 |
| Hemeiuşi | Gârcina | Siminicea | Beizadele | Valea lui Ştefan | |
|---|---|---|---|---|---|
| 0.000 | Hemeiuşi | ||||
| 0.004 | 0.000 | Gârcina | |||
| 0.005 | 0.003 | 0.000 | Siminicea | ||
| 0.011 ** | 0.010 ** | 0.007 | 0.000 | Beizadele | |
| 0.046 *** | 0.043 *** | 0.030 *** | 0.022 *** | 0.000 | Valea lui Ştefan |
| Method | Subset Size | Sampling Intensity | Na | Ne | I | Ho | He |
|---|---|---|---|---|---|---|---|
| Whole | 177 | 100 | 13.2 | 6.57 | 2.01 | 0.699 | 0.820 |
| A-NE | 10 | 5 | 6.8 | 4.69 | 1.57 | 0.660 | 0.720 |
| 20 | 11 | 9.4 | 6.07 | 1.88 | 0.687 | 0.794 | |
| 30 | 16 | 10.2 | 6.02 | 1.90 | 0.711 | 0.799 | |
| 40 | 22 | 11.1 | 6.33 | 1.96 | 0.706 | 0.810 | |
| 50 | 28 | 10.9 | 6.22 | 1.96 | 0.699 | 0.810 | |
| E-NE | 10 | 5 | 8.9 | 7.08 | 2.02 | 0.730 | 0.849 |
| 20 | 11 | 9.9 | 7.01 | 2.04 | 0.700 | 0.842 | |
| 30 | 16 | 11.1 | 7.19 | 2.08 | 0.687 | 0.844 | |
| 40 | 22 | 10.7 | 6.92 | 2.05 | 0.693 | 0.841 | |
| 50 | 28 | 11.8 | 7.02 | 2.08 | 0.703 | 0.843 | |
| Cov | 10 | 5 | 9.5 | 7.23 | 2.06 | 0.740 | 0.85 |
| 20 | 11 | 11.4 | 7.23 | 2.09 | 0.670 | 0.842 | |
| 30 | 16 | 12.4 | 7.41 | 2.11 | 0.702 | 0.84 | |
| 40 | 22 | 13 | 7.16 | 2.10 | 0.706 | 0.835 | |
| 50 | 28 | 13.1 | 6.99 | 2.09 | 0.704 | 0.836 | |
| He | 10 | 5 | 9.5 | 7.63 | 2.10 | 0.670 | 0.86 |
| 20 | 11 | 10.9 | 7.74 | 2.12 | 0.645 | 0.858 | |
| 30 | 16 | 11.4 | 7.78 | 2.14 | 0.667 | 0.857 | |
| 40 | 22 | 12.0 | 7.99 | 2.16 | 0.685 | 0.858 | |
| 50 | 28 | 12.1 | 7.98 | 2.15 | 0.691 | 0.857 | |
| Shannon | 10 | 5 | 9.3 | 7.44 | 2.07 | 0.680 | 0.856 |
| 20 | 11 | 11.2 | 8.02 | 2.15 | 0.675 | 0.859 | |
| 30 | 16 | 11.8 | 8.08 | 2.16 | 0.669 | 0.857 | |
| 40 | 22 | 12.2 | 8.09 | 2.17 | 0.688 | 0.856 | |
| 50 | 28 | 12.4 | 7.97 | 2.16 | 0.688 | 0.853 | |
| E-NE+Co | 10 | 5 | 8.8 | 7.05 | 2.05 | 0.650 | 0.855 |
| 20 | 11 | 11.1 | 7.60 | 2.13 | 0.695 | 0.849 | |
| 30 | 16 | 11.9 | 7.70 | 2.16 | 0.693 | 0.856 | |
| 40 | 22 | 12.4 | 7.48 | 2.14 | 0.710 | 0.849 | |
| 50 | 28 | 12.9 | 7.36 | 2.13 | 0.705 | 0.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teodosiu, M.; Mihai, G.; Ciocîrlan, E.; Curtu, A.L. Genetic Characterisation and Core Collection Construction of European Larch (Larix decidua Mill.) from Seed Orchards in Romania. Forests 2023, 14, 1575. https://doi.org/10.3390/f14081575
Teodosiu M, Mihai G, Ciocîrlan E, Curtu AL. Genetic Characterisation and Core Collection Construction of European Larch (Larix decidua Mill.) from Seed Orchards in Romania. Forests. 2023; 14(8):1575. https://doi.org/10.3390/f14081575
Chicago/Turabian StyleTeodosiu, Maria, Georgeta Mihai, Elena Ciocîrlan, and Alexandru Lucian Curtu. 2023. "Genetic Characterisation and Core Collection Construction of European Larch (Larix decidua Mill.) from Seed Orchards in Romania" Forests 14, no. 8: 1575. https://doi.org/10.3390/f14081575
APA StyleTeodosiu, M., Mihai, G., Ciocîrlan, E., & Curtu, A. L. (2023). Genetic Characterisation and Core Collection Construction of European Larch (Larix decidua Mill.) from Seed Orchards in Romania. Forests, 14(8), 1575. https://doi.org/10.3390/f14081575






