Enhanced Natural Regeneration Potential of Sessile Oak in Northern Hungary: Role of Artificially Increased Density of Insectivorous Birds
Abstract
1. Introduction
- Is there a direct effect of increased bird population on Lepidoptera populations?
- Is there an indirect effect of increased bird population on acorn crop and oak regeneration potential?
2. Materials and Methods
2.1. Study Site
2.2. Data Collection
2.3. Data Analysis
2.3.1. Sticky Belt Trap
2.3.2. Funnel Trap Baited with FLO
2.3.3. Funnel Trap Baited with NAA
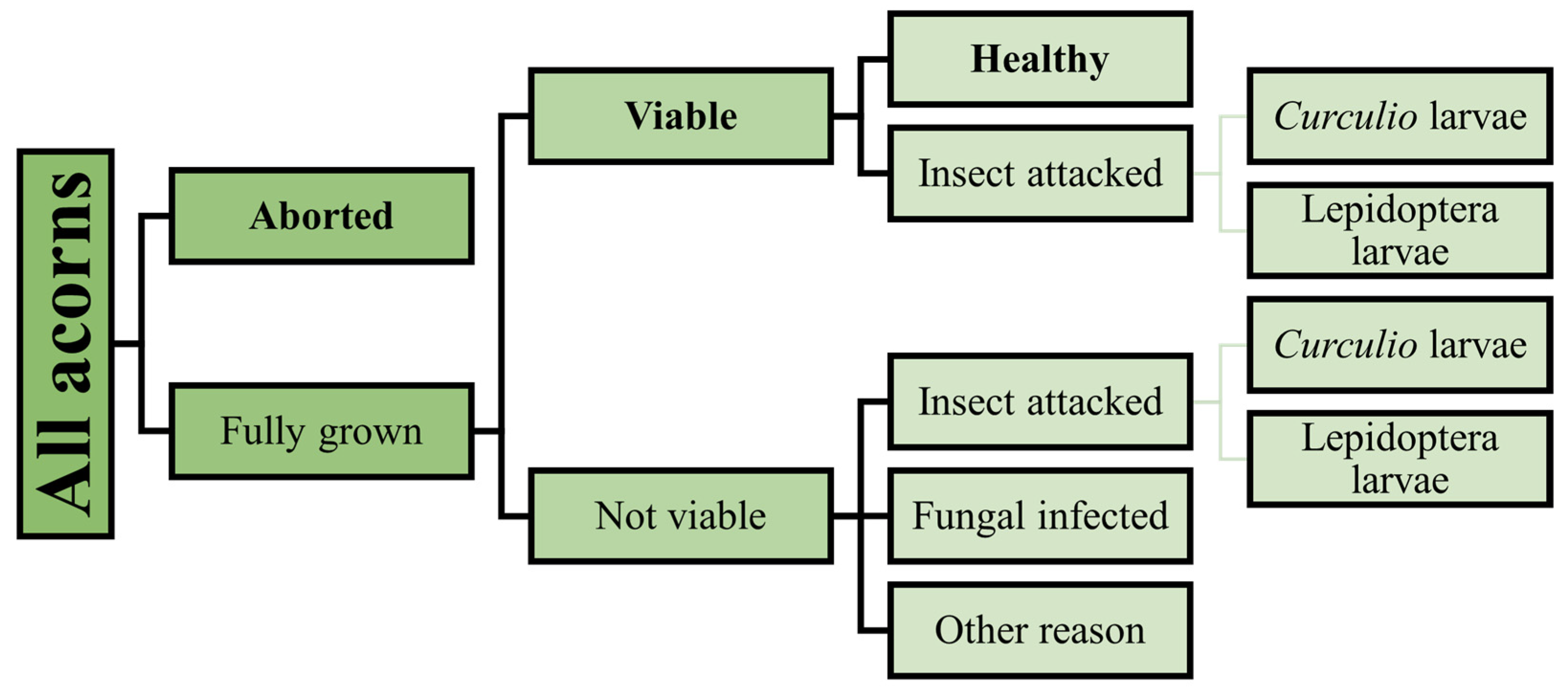
2.3.4. Acorn Crop
2.3.5. Seedling Counting
3. Results
3.1. Bird Abundance Data and Estimated Caterpillar Consumptionnesting Data
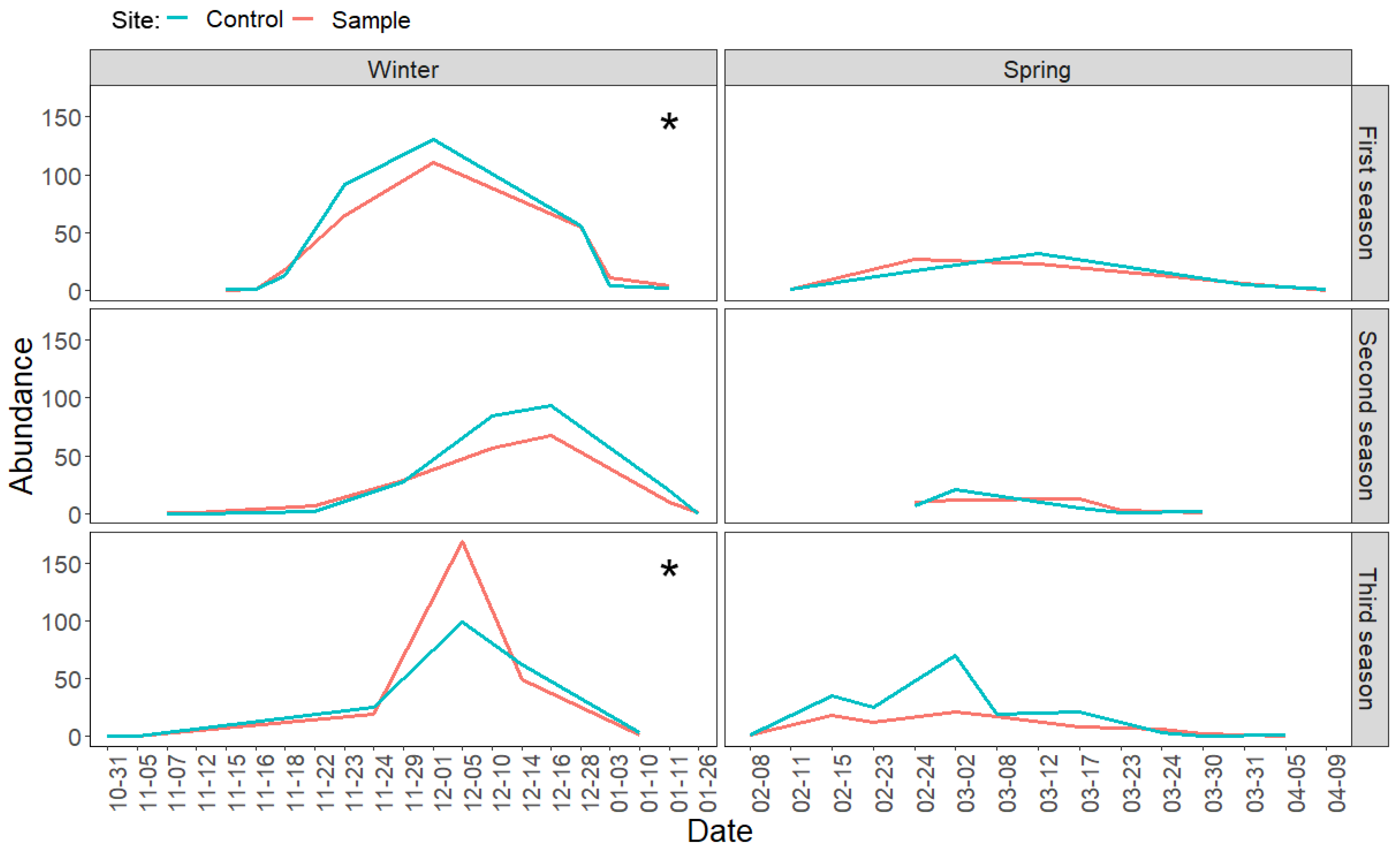
3.2. Direct Effects of Increased Bird Abundancies on Lepidoptera Populations
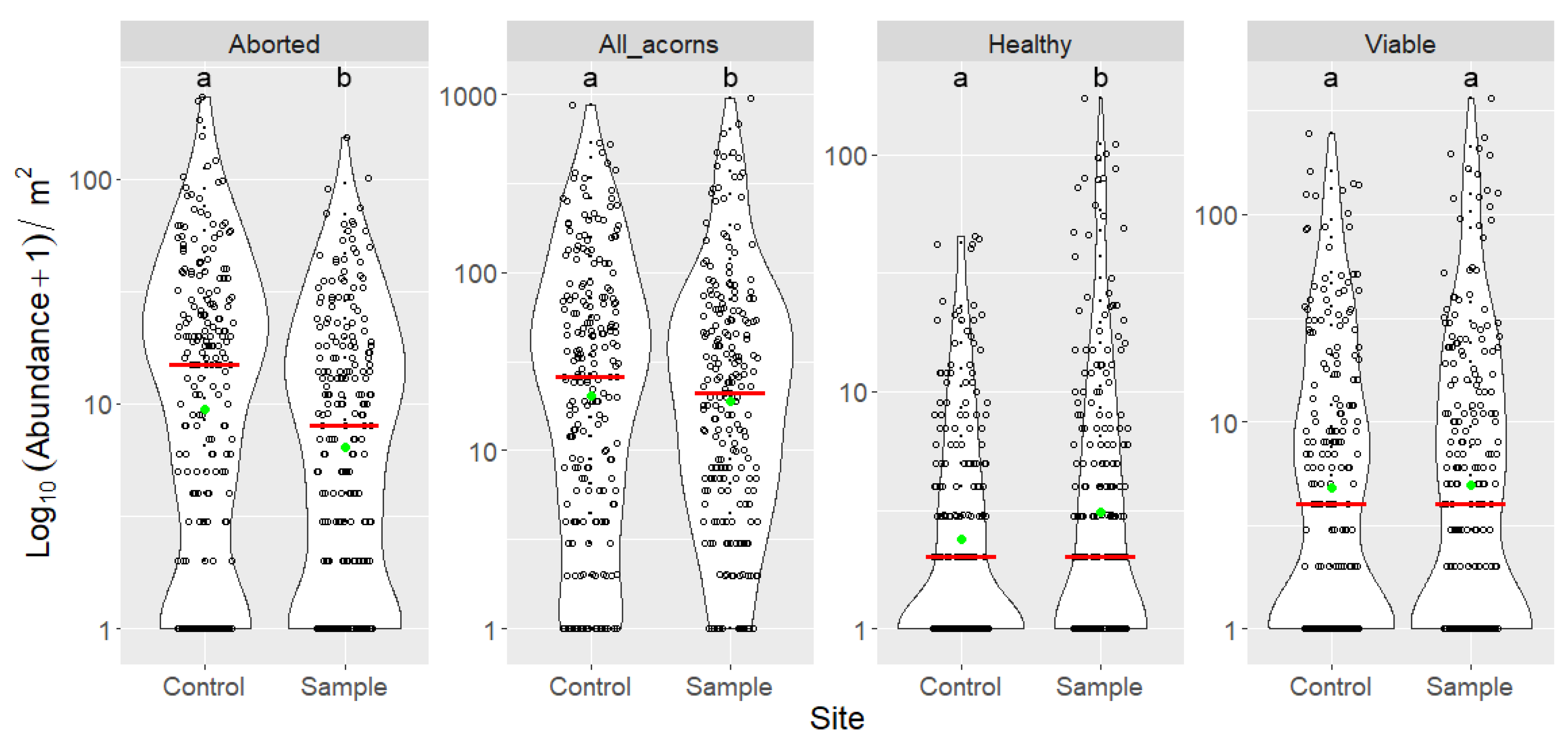
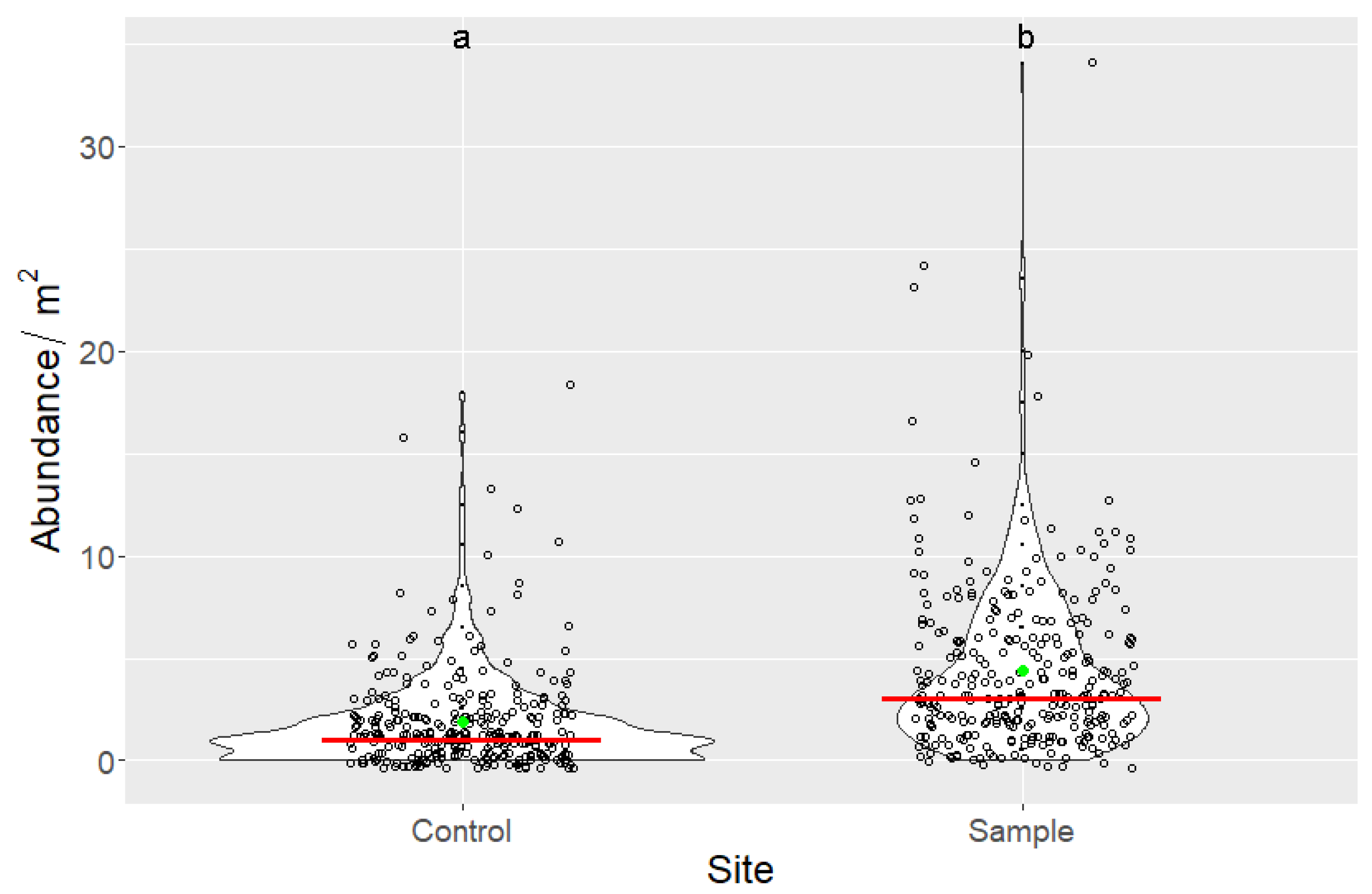
3.3. Indirect Effects of Increased Bird Abundancies on Acorn Crop and Regeneration Potential
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Csóka, G.; Koltay, A.; Hirka, A.; Janik, G. The effect of drought on the health state of our sessile oak and beech stands. Klíma-21 Füzetek 2009, 57, 64–73. (In Hungarian) [Google Scholar]
- Mátyás, C.; Berki, I.; Bidló, A.; Csóka, G.; Czimber, K.; Führer, E.; Gálos, B.; Gribovszki, Z.; Illés, G.; Hirka, A.; et al. Sustainability of Forest Cover under Climate Change on the Temperate-Continental Xeric Limits. Forests 2018, 9, 489. [Google Scholar] [CrossRef]
- Berki, I.; Móricz, N.; Rasztovits, E.; Gulyás, K.; Garamszegi, B.; Horváth, A.; Balázs, P. Mortality and accelerating growth in sessile oak sites. Erdészettudományi Közlemények 2018, 8, 119–130. (In Hungarian) [Google Scholar] [CrossRef]
- Móricz, N.; Illés, G.; Mészáros, I.; Garamszegi, B.; Berki, I.; Bakacsi, Z.; Kámpel, J.; Szabó, O.; Rasztovits, E.; Cseke, K.; et al. Different Drought Sensitivity Traits of Young Sessile Oak (Quercus petraea (Matt.) Liebl.) and Turkey Oak (Quercus cerris L.) Stands along a Precipitation Gradient in Hungary. For. Ecol. Manag. 2021, 492, 119165. [Google Scholar] [CrossRef]
- Németh, T.M.; Szabó, O.; Móricz, N. Comparative drought sensitivity analysis of young sessile oak and turkey oak trees in Somogy county (Hungary). Erdészettudományi Közlemények 2021, 11, 27–40. (In Hungarian) [Google Scholar] [CrossRef]
- Klapwijk, M.J.; Csóka, G.; Hirka, A.; Björkman, C. Forest Insects and Climate Change: Long-Term Trends in Herbivore Damage. Ecol. Evol. 2013, 3, 4183–4196. [Google Scholar] [CrossRef]
- Csóka, G.; Hirka, A.; Mutun, S.; Glavendekić, M.; Mikó, Á.; Szőcs, L.; Paulin, M.; Eötvös, C.B.; Gáspár, C.; Csepelényi, M.; et al. Spread and Potential Host Range of the Invasive Oak Lace Bug [Corythucha Arcuata (Say, 1832)—Heteroptera: Tingidae] in Eurasia. Agric. For. Entomol. 2020, 22, 61–74. [Google Scholar] [CrossRef]
- Csóka, G.; Hirka, A.; Csepelényi, M.; Szőcs, L.; Molnár, M.; Tuba, K.; Hillebrand, R.; Lakatos, F. Response of forest insects to the climate change (case studies). Erdészettudományi Közlemények 2018, 8, 149–162. (In Hungarian) [Google Scholar] [CrossRef]
- Demeter, L.; Molnár, Á.P.; Öllerer, K.; Csóka, G.; Kiš, A.; Vadász, C.; Horváth, F.; Molnár, Z. Rethinking the natural regeneration failure of pedunculate oak: The pathogen mildew hypothesis. Biol. Conserv. 2021, 253, 108928. [Google Scholar] [CrossRef]
- Csóka, G.; Hirka, A.; Szőcs, L.; Móricz, N.; Rasztovits, E.; Podor, Z. Weather-dependent fluctuations in the abundance of the oak processionary moth, Thaumetopoea processionea (Lepidoptera: Notodontidae). Eur. J. Entomol. 2018, 115, 249–255. [Google Scholar] [CrossRef]
- Csóka, G. Increased insect damage in hungarian forests under drought impact. Biologia 1997, 52, 159–162. [Google Scholar]
- Jactel, H.; Petit, J.; Desprez-Loustau, M.-L.; Delzon, S.; Piou, D.; Battisti, A.; Koricheva, J. Drought effects on damage by forest insects and pathogens: A meta-analysis. Glob. Chang. Biol. 2012, 18, 267–276. [Google Scholar] [CrossRef]
- Wainhouse, D.; Inward, D.J.G. The Influence of Climate Change on Forest Insect Pests in Britain; FCRN021; Forestry Commission: Sydney, Australia, 2016; pp. 1–10. [Google Scholar]
- Kaliszewski, A. Cost analysis of artificial and natural oak regeneration in selected forest districts. For. Res. Pap. 2017, 78, 315–321. [Google Scholar] [CrossRef]
- Chudy, R.; Cubbage, F.; Siry, J.; Chudy, J. The profitability of artificial and natural regeneration: A forest investment comparison of Poland and the U.S. South. J. For. Bus. Res. 2022, 1, 1–20. [Google Scholar]
- Stimm, K.; Uhl, E.; Pretzsch, H. Chances and limitations of mixed oak regeneration under continuous canopy cover—Evidence from long-term observations. Forests 2022, 13, 2052. [Google Scholar] [CrossRef]
- Götmark, F.; Berglund, Å.; Wiklander, K. Browsing damage on broadleaved trees in semi-natural temperate forest in Sweden, with a focus on oak regeneration. Scand. J. For. Res. 2005, 20, 223–234. [Google Scholar] [CrossRef]
- Sork, V.L.; Bramble, J.; Sexton, O. Ecology of mast-fruiting in three species of North American deciduous oaks. Ecology 1993, 74, 528–541. [Google Scholar] [CrossRef]
- Cecich, R.A.; Sullivan, N.H. Influence of weather at time of pollination on acorn production of Quercus alba and Quercus velutina. Can. J. For. Res. 1999, 29, 1817–1823. [Google Scholar] [CrossRef]
- Askeyev, O.V.; Tischin, D.; Sparks, T.H.; Askeyev, I.V. The Effect of climate on the phenology, acorn crop and radial increment of pedunculate oak (Quercus robur) in the Middle Volga region, Tatarstan, Russia. Int. J. Biometeorol. 2005, 49, 262–266. [Google Scholar] [CrossRef]
- Abrahamson, W.G.; Layne, J.N. Long-term patterns of acorn production for five oak species in xeric Florida Uplands. Ecology 2003, 84, 2476–2492. [Google Scholar] [CrossRef]
- Martiník, A.; Dobrovolný, L.; Palátová, E. Tree growing space and acorn production of Quercus robur. Dendrobiology 2013, 71, 101–108. [Google Scholar] [CrossRef][Green Version]
- Crawley, M.J. Reduction of oak fecundity by low-density herbivore populations. Nature 1985, 314, 163–164. [Google Scholar] [CrossRef]
- Shaw, M.W. Factors affecting the natural regeneration of sessile oak (Quercus petraea) in North Wales: II. Acorn losses and germination under field conditions. J. Ecol. 1968, 56, 647. [Google Scholar] [CrossRef]
- Hirka, A. Investigations on Carpophagous Insects of Oaks in Hungary. Ph.D. Thesis, University of West Hungary, Sopron, Hungary, 2003. [Google Scholar]
- Prochazkova, Z.; Sikorova, A.; Peskova, V. Preliminary observations on the occurrence of Ciboria batschiana (Zopf) Buchwald in the Czech Republic. Work. Pap. Finnish For. Res. Inst. 2005, 11, 13–18. [Google Scholar]
- Markić, A.G.; Bogdan, S.; Gradečki Poštenjak, M.; Lanšćak, M.; Vujnović, Z.; Bogunović, S.; Ivanković, M. Acorn Yields and seed viability of pedunculate oak in a 10-year period in forest seed objects across Croatia. South-East Eur. For. 2022, 13, 2201. [Google Scholar] [CrossRef]
- Kohler, M.; Pyttel, P.; Kuehne, C.; Modrow, T.; Bauhus, J. On the knowns and unknowns of natural regeneration of silviculturally managed sessile oak (Quercus petraea (Matt.) Liebl.) forests—A literature review. Ann. For. Sci. 2020, 77, 101. [Google Scholar] [CrossRef]
- Csóka, G. Oak Defoliating insects in Hungary. In Proceedings of the Population Dynamics, Impacts, and Integrated Management of Forest Defoliating Insects, Banska Stiavnica, Slovakia, 18–23 August 1996; McManus, M.L., Liebhol, A.M., Eds.; USDA Forest Service, Northeastern Research Station: Madison, WI, USA, 1998; pp. 334–335. [Google Scholar]
- Seress, G.; Sándor, K.; Evans, K.L.; Liker, A. Food availability limits avian reproduction in the city: An experimental study on great tits Parus major. J. Anim. Ecol. 2020, 89, 1570–1580. [Google Scholar] [CrossRef]
- Nabity, P.D.; Zavala, J.A.; DeLucia, E.H. Indirect suppression of photosynthesis on individual leaves by arthropod herbivory. Ann. Bot. 2009, 103, 655–663. [Google Scholar] [CrossRef]
- Davidson, C.B.; Gottschalk, K.W.; Johnson, J.E. Tree mortality following defoliation by the European Gypsy moth (Lymantria dispar L.) in the United States: A review. For. Sci. 1999, 45, 74–84. [Google Scholar]
- Samson, D.A.; Werk, K.S. Size-dependent effects in the analysis of reproductive effort in plants. Am. Nat. 1986, 127, 667–680. [Google Scholar] [CrossRef]
- Canelo, T.; GaytÁn, Á.; GonzÁlez-Bornay, G.; Bonal, R. Seed loss before seed predation: Experimental evidence of the negative effects of leaf feeding insects on acorn production. Integr. Zool. 2018, 13, 238–250. [Google Scholar] [CrossRef]
- Paine, R.T. Food web complexity and species diversity. Am. Nat. 1966, 100, 65–75. [Google Scholar] [CrossRef]
- Şekercioğlu, Ç.H. Ecological significance of bird populations. In Handbook of the Birds of the World—Volume 11; del Hoyo, J., Elliott, A., Christie, D.A., Eds.; BirdLife International: Cambridge, UK; Lynx Edicions: Barcelona, Spain, 2006; pp. 15–51. [Google Scholar]
- Morse, D.H. The insectivorous bird as an adaptive strategy. Annu. Rev. Ecol. Syst. 2017, 2, 177–200. [Google Scholar] [CrossRef]
- Nyffeler, M.; Şekercioğlu, Ç.H.; Whelan, C.J. Insectivorous birds consume an estimated 400–500 million tons of prey annually. Sci. Nat. 2018, 105, 47. [Google Scholar] [CrossRef]
- Holmes, R.T.; Schultz, J.C.; Nothnagle, P. Bird predation on forest insects: An exclosure experiment. Science 1979, 206, 462–463. [Google Scholar] [CrossRef]
- Gilroy, J.J.; Anderson, G.Q.A.; Grice, P.V.; Vickery, J.A.; Watts, P.N.; Sutherland, W.J. Foraging habitat selection, diet and nestling condition in yellow wagtails Motacilla flava breeding on arable farmland. Bird Study 2009, 56, 221–232. [Google Scholar] [CrossRef]
- Pagani-Núñez, E.; Renom, M.; Mateos-Gonzalez, F.; Cotín, J.; Senar, J.C. The diet of great tit nestlings: Comparing observation records and stable isotope analyses. Basic Appl. Ecol. 2017, 18, 57–66. [Google Scholar] [CrossRef]
- Tremblay, I.; Thomas, D.; Blondel, J.; Perret, P.; Lambrechts, M.M. The effect of habitat quality on foraging patterns, provisioning rate and nestling growth in Corsican blue tits Parus caeruleus. IBIS 2005, 147, 17–24. [Google Scholar] [CrossRef]
- Gibb, J.A.; Betts, M.M. Food and food supply of nestling tits (Paridae) in Breckland Pine. J. Anim. Ecol. 1963, 32, 489. [Google Scholar] [CrossRef]
- Török, J.; Tóth, L. Asymmetric competition between two tit species: A reciprocal removal experiment. J. Anim. Ecol. 1999, 68, 338–345. [Google Scholar] [CrossRef]
- Perrins, C.M. Tits and their caterpillar food supply. IBIS 2008, 133, 49–54. [Google Scholar] [CrossRef]
- Murdoch, W.W. Population regulation in theory and practice. Ecology 1994, 75, 271–287. [Google Scholar] [CrossRef]
- Sinclair, A.R.E. Population regulation in animals. In Ecological Concepts: The Contribution of Ecology to an Understanding of the Natural World, 29th Symposium of the British Ecological Society; Cherrett, J., Ed.; Blackwell Publishing Inc.: Oxford, UK, 1989; pp. 197–241. [Google Scholar]
- Begon, M.; Harper, J.L.; Townsend, C.R. Ecology; Blackwell Scientific: Boston, MA, USA, 1996. [Google Scholar]
- Hixon, M.A.; Pacala, S.W.; Sandin, S.A. Population regulation: Historical context and contemporary challenges of open vs. closed systems. Ecology 2002, 83, 1490–1508. [Google Scholar] [CrossRef]
- Runge, M.C.; Johnson, F.A. The importance of functional form in optimal control solutions of problems in population dynamics. Ecology 2002, 83, 1357. [Google Scholar] [CrossRef]
- Csörgő, T.; Haraszthy, L.; Kárpáti, L.; Molnár, L.; Schmidt, E. Identification Key for the Birds of Hungary; Haraszthy, L., Ed.; Mezőgazdasági Könyvkiadó Vállalat: Debrecen, Hungary, 1990. (In Hungarian) [Google Scholar]
- Szép, T.; Csörgő, T.; Halmos, G.; Lovászi, P.; Nagy, K.; Schmidt, A. Bird Atlas of Hungary, 2nd ed.; Agrárminisztérium, MME: Budapest, Hungary, 2022. (In Hungarian) [Google Scholar]
- Pigot, A.L.; Sheard, C.; Miller, E.T.; Bregman, T.P.; Freeman, B.G.; Roll, U.; Seddon, N.; Trisos, C.H.; Weeks, B.C.; Tobias, J.A. Macroevolutionary convergence connects morphological form to ecological function in birds. Nat. Ecol. Evol. 2020, 4, 230–239. [Google Scholar] [CrossRef]
- Ónodi, G.; Winkler, D. The role of dead wood in the formation of hollow breeder bird communities. In Silva Naturalis Vol. 5—A Holtfa; Csóka, G., Lakatos, F., Eds.; Nyugat-Magyarországi Egyetem: Sopron, Hungary, 2014; pp. 125–144. ISBN 978-963-334-207-7. (In Hungarian) [Google Scholar]
- Standovár, T.; Bán, M.; Kézdi, P. Forest health state evaluation in the middle height mountains of Hungary. In ROSALIA A Duna-Ipoly Nemzeti Park Igazgatóság Tanulmánykötetei 9; Standovár, T., Bán, M., Kézdi, P., Eds.; Duna-Ipoly Nemzeti Park Igazgatóság: Budapest, Hungary, 2017. (In Hungarian) [Google Scholar]
- Krebs, J.R. Territory and breeding density in the great tit, Parus major L. Ecology 1971, 52, 2–22. [Google Scholar] [CrossRef]
- Stauss, M.J.; Burkhardt, J.F.; Tomiuk, J. Foraging flight distances as a measure of parental effort in blue tits Parus caeruleus differ with environmental conditions. J. Avian Biol. 2005, 36, 47–56. [Google Scholar] [CrossRef]
- Naef-Daenzer, B. Patch time allocation and patch sampling by foraging great and blue tits. Anim. Behav. 2000, 59, 989–999. [Google Scholar] [CrossRef]
- Kašová, M.; Naďo, L.; Kaňuch, P. Structure of tree vegetation may reduce costs of territory defence in Eurasian nuthatch Sitta europaea. Bird Study 2014, 61, 413–420. [Google Scholar] [CrossRef]
- Cowie, R.J.; Hinsley, S.A. Feeding ecology of great tits (Parus major) and blue tits (Parus caeruleus), breeding in suburban gardens. J. Anim. Ecol. 1988, 57, 611. [Google Scholar] [CrossRef]
- Krist, M. Short- and long-term effects of egg size and feeding frequency on offspring quality in the collared flycatcher (Ficedula albicollis). J. Anim. Ecol. 2009, 78, 907–918. [Google Scholar] [CrossRef]
- Cauchard, L.; Macqueen, E.I.; Lilley, R.; Bize, P.; Doligez, B. Inter-individual variation in provisioning rate, prey size and number, and links to total prey biomass delivered to nestlings in the collared flycatcher (Ficedula albicollis). Avian Res. 2021, 12, 15. [Google Scholar] [CrossRef]
- Krištín, A. Die Nestlingsnahrung des Kleibers (Sitta europaea L.) in Buchenwäldern. Acta Ornithoecol. 1992, 4, 341–349. [Google Scholar]
- Wesołowski, T.; Rowiński, P.; Neubauer, G. Food of nuthatch Sitta europaea young in a primeval forest: Effects of varying food supply and age of nestlings. Acta Ornithol. 2019, 54, 85. [Google Scholar] [CrossRef]
- Bereczki, K.; Ódor, P.; Csóka, G.; Mag, Z.; Báldi, A. Effects of forest heterogeneity on the efficiency of caterpillar control service provided by birds in temperate oak forests. For. Ecol. Manag. 2014, 327, 96–105. [Google Scholar] [CrossRef]
- Bates, D.M. Lme4: Mixed-Effects Modeling with R; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.H.; White, J.-S.S.; Henry, M.; Stevens, H.; White, J.-S.S. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; Sage Publications: Thousand Oaks, CA, USA, 2018; ISBN 9781544336473. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; ISBN 0387954570. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. R Core Team Nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-140. Available online: https://cran.r-project.org/package=nlme (accessed on 24 July 2023).
- Wolfinger, R. Laplace’s approximation for nonlinear mixed models. Biometrika 1993, 80, 791–795. [Google Scholar] [CrossRef]
- Breslow, N.E.; Clayton, D.G. Approximate inference in generalized linear mixed models. J. Am. Stat. Assoc. 1993, 88, 9. [Google Scholar] [CrossRef]
- Sanz, J.J. Experimentally increased insectivorous bird density results in a reduction of caterpillar density and leaf damage to Pyrenean oak. Ecol. Res. 2001, 16, 387–394. [Google Scholar] [CrossRef]
- Atlegrim, O. Exclusion of birds from bilberry stands: Impact on insect larval density and damage to the bilberry. Oecologia 1989, 79, 136–139. [Google Scholar] [CrossRef]
- Sam, K.; Tahadlova, M.; Freiberga, I.; Mrazova, A.; Sreekar, R. The impact of ants and vertebrate predators on arthropods and plants: A meta-analysis. bioRxiv 2022. [Google Scholar] [CrossRef]
- Olmos-Moya, N.; Díaz-Siefer, P.; Pozo, R.A.; Fontúrbel, F.E.; Lavandero, B.; Abades, S.; Celis-Diez, J.L. The use of cavity-nesting wild birds as agents of biological control in vineyards of Central Chile. Agric. Ecosyst. Environ. 2022, 334, 107975. [Google Scholar] [CrossRef]
- García, D.; Miñarro, M.; Martínez-Sastre, R. Enhancing ecosystem services in apple orchards: Nest boxes increase pest control by insectivorous birds. J. Appl. Ecol. 2021, 58, 465–475. [Google Scholar] [CrossRef]
- Oppel, S.; Burns, F.; Vickery, J.; George, K.; Ellick, G.; Leo, D.; Hillman, J.C. Habitat-specific effectiveness of feral cat control for the conservation of an endemic ground-nesting bird species. J. Appl. Ecol. 2014, 51, 1246–1254. [Google Scholar] [CrossRef]
- Karhu, K.J. Green Islands—Top-down and bottom-up effects of wood ants in forests under folivore attack. Ann. Univ. Turku 1969, 107, 82. [Google Scholar]
- Ehnström, B. Leaving dead wood for insects in boreal forests—Suggestions for the future. Scand. J. For. Res. 2001, 16, 91–98. [Google Scholar] [CrossRef]
- Jabin, M.; Mohr, D.; Kappes, H.; Topp, W. Influence of deadwood on density of soil macro-arthropods in a managed oak–beech forest. For. Ecol. Manag. 2004, 194, 61–69. [Google Scholar] [CrossRef]
- Poveda, J. Insect frass in the development of sustainable agriculture. A review. Agron. Sustain. Dev. 2021, 41, 5. [Google Scholar] [CrossRef]
- Frost, C.J.; Hunter, M.D. Insect herbivores and their frass affect Quercus rubra leaf quality and initial stages of subsequent litter decomposition. Oikos 2008, 117, 13–22. [Google Scholar] [CrossRef]
- Madritch, M.D.; Donaldson, J.R.; Lindroth, R.L. Canopy herbivory can mediate the influence of plant genotype on soil processes through frass deposition. Soil Biol. Biochem. 2007, 39, 1192–1201. [Google Scholar] [CrossRef]
- Grüning, M.M.; Simon, J.; Rennenberg, H.; lM-Arnold, A. Defoliating insect mass outbreak affects soil N fluxes and tree N nutrition in scots pine forests. Front. Plant Sci. 2017, 8, 954. [Google Scholar] [CrossRef]
- De Souza Vandenberghe, L.P.; Garcia, L.M.B.; Rodrigues, C.; Camara, M.C.; de Melo Pereira, G.V.; De Oliveira, J.; Soccol, C.R. Potential applications of plant probiotic microorganisms in agriculture and forestry. AIMS Microbiol. 2017, 3, 629–648. [Google Scholar] [CrossRef]
- Eötvös, C.B.; Hirka, A.; Gimesi, L.; Lövei, G.L.; Gáspár, C.; Csóka, G. No long-term decrease in caterpillar availability for invertivorous birds in deciduous forests in Hungary. Forests 2021, 12, 1070. [Google Scholar] [CrossRef]
- Zúbrik, M.; Hajek, A.; Pilarska, D.; Špilda, I.; Georgiev, G.; Hrašovec, B.; Hirka, A.; Goertz, D.; Hoch, G.; Barta, M.; et al. The potential for Entomophaga maimaiga to regulate Gypsy moth Lymantria dispar (L.) (Lepidoptera: Erebidae) in Europe. J. Appl. Entomol. 2016, 140, 565–579. [Google Scholar] [CrossRef]
- Georgiev, G.; Mirchev, P.; Rossnev, B.; Petkov, P.; Georgieva, M.; Pilarska, D.; Golemansky, V.; Pilarski, P.; Hubenov, Z. Potential of Entomophaga maimaiga Humber, Shimazu and Soper (Entomophthorales) for suppressing Lymantria dispar (Linnaeus) outbreaks in Bulgaria. Comptes Rendus L’Academie Bulg. Des. Sci. 2013, 66, 1025–1032. [Google Scholar] [CrossRef]
- Hajek, A.E.; Butler, L.; Wheeler, M.M. Laboratory bioassays testing the host range of the Gypsy moth fungal pathogen Entomophaga maimaiga. Biol. Control 1995, 5, 530–544. [Google Scholar] [CrossRef]
- Hajek, A.E.; Butler, L.; Walsh, S.R.A.; Silver, J.C.; Hain, F.P.; Hastings, F.L.; Odell, T.M.; Smitley, D.R. Host range of the Gypsy moth (Lepidoptera: Lymantriidae) pathogen Entomophaga maimaiga (Zygomycetes: Entomophthorales) in the field versus laboratory. Environ. Entomol. 1996, 25, 709–721. [Google Scholar] [CrossRef]
- Zúbrik, M.; Pilarska, D.; Kulfan, J.; Barta, M.; Hajek, A.E.; Bittner, T.D.; Zach, P.; Takov, D.; Kunca, A.; Rell, S.; et al. Phytophagous larvae occurring in Central and Southeastern European oak forests as a potential host of Entomophaga maimaiga (Entomophthorales: Entomophthoraceae)—A field study. J. Invertebr. Pathol. 2018, 155, 52–54. [Google Scholar] [CrossRef]
- Greenwood, P.J.; Harvey, P.H.; Perrins, C.M. The role of dispersal in the great tit (Parus major): The causes, consequences and heritability of natal dispersal. J. Anim. Ecol. 1979, 48, 123. [Google Scholar] [CrossRef]
- Nicolaus, M.; Michler, S.P.M.; Jalvingh, K.M.; Ubels, R.; van der Velde, M.; Komdeur, J.; Both, C.; Tinbergen, J.M. Social environment affects juvenile dispersal in great tits (Parus major). J. Anim. Ecol. 2012, 81, 827–837. [Google Scholar] [CrossRef]
- Hirka, A.; Csóka, G. Indirect effects of carpophagous insects on germination success of stored acorns. In Proceedings of the Ochrana Lesa a Lesnicka Fytopatologia, Sielnica, Slovakia, 4–6 September 2000; pp. 51–56. [Google Scholar]
- Burczyk, J.; Adams, W.T.; Birkes, D.S.; Chybicki, I.J. Using genetic markers to directly estimate gene flow and reproductive success parameters in plants on the basis of naturally regenerated seedlings. Genetics 2006, 173, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Nörr, R.; Baumer, M. Planting—A Risk for the Stand Stability? Bayerische Landesanstalt für Wald und Forstwirtschaft: Freising, Germany, 2002. (In German) [Google Scholar]
- Von Lüpke, B. Silvicultural methods of oak regeneration with special respect to shade tolerant mixed species. For. Ecol. Manag. 1998, 106, 19–26. [Google Scholar] [CrossRef]
- Kanjevac, B.; Krstić, M.; Babić, V.; Govedar, Z. Regeneration dynamics and development of seedlings in sessile oak forests in relation to the light availability and competing vegetation. Forests 2021, 12, 384. [Google Scholar] [CrossRef]







| Species | Year | Nest With Eggs | Successful Nests | Egg Number | Hatched Chicks | Fledged Chicks | Estimated Caterpillar Consumption (Individuals) |
|---|---|---|---|---|---|---|---|
| Cyanistes caeruleus L., 1758 | 2018 | 6 | 5 | 61 | 58 | 46 | 41,795 |
| 2019 | 13 | 8 | 109 | 91 | 77 | 66,872 | |
| 2020 | 7 | 6 | 62 | 50 | 49 | 50,154 | |
| 2021 | 12 | 7 | 114 | 78 | 73 | 58,513 | |
| 2022 | 3 | 3 | 34 | 33 | 31 | 25,077 | |
| Ficedula albicollis (Temminck, 1815) | 2018 | 25 | 16 | 121 | 91 | 61 | 91,872 |
| 2019 | 30 | 24 | 165 | 129 | 123 | 137,808 | |
| 2020 | 41 | 26 | 237 | 197 | 130 | 149,292 | |
| 2021 | 33 | 26 | 188 | 153 | 130 | 149,292 | |
| 2022 | 38 | 28 | 228 | 189 | 154 | 160,776 | |
| Parus major L., 1758 | 2018 | 16 | 14 | 155 | 123 | 119 | 49,448 |
| 2019 | 22 | 17 | 245 | 235 | 198 | 60,044 | |
| 2020 | 19 | 14 | 177 | 133 | 117 | 49,448 | |
| 2021 | 20 | 12 | 191 | 136 | 106 | 42,384 | |
| 2022 | 11 | 11 | 96 | 89 | 87 | 38,852 | |
| Sitta europaea L., 1758 | 2018 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2019 | 1 | 1 | 9 | 9 | 8 | 3059 | |
| 2020 | 2 | 2 | 17 | 17 | 17 | 6118 | |
| 2021 | 6 | 3 | 37 | 24 | 18 | 9177 | |
| 2022 | 1 | 1 | 9 | 9 | 9 | 3059 | |
| All species | 2018 | 47 | 35 | 337 | 272 | 226 | 183,115 |
| 2019 | 66 | 50 | 528 | 464 | 406 | 267,783 | |
| 2020 | 69 | 48 | 493 | 397 | 313 | 255,012 | |
| 2021 | 71 | 48 | 530 | 391 | 327 | 259,366 | |
| 2022 | 53 | 43 | 367 | 320 | 281 | 227,764 |
| Site | Categories | n | Min | Max | Mean | Median | SD | Sum |
|---|---|---|---|---|---|---|---|---|
| Control | Aborted | 225 | 0 | 231 | 22.880 | 14 | 33.619 | 5148 |
| All acorns | 225 | 0 | 868 | 66.631 | 25 | 109.628 | 14,992 | |
| Curculio attacked | 225 | 0 | 367 | 23.227 | 6 | 45.869 | 5226 | |
| Fully grown | 225 | 0 | 637 | 43.751 | 12 | 80.933 | 9844 | |
| Fungal infection | 225 | 0 | 65 | 5.080 | 1 | 9.359 | 1143 | |
| Healthy | 225 | 0 | 44 | 3.698 | 1 | 7.502 | 832 | |
| Insect attacked | 225 | 0 | 532 | 34.640 | 9 | 66.616 | 7794 | |
| Lepidoptera attacked | 225 | 0 | 165 | 11.413 | 4 | 21.578 | 2568 | |
| Other reason | 225 | 0 | 9 | 0.333 | 0 | 1.039 | 75 | |
| Viable | 225 | 0 | 247 | 14.711 | 3 | 30.978 | 3310 | |
| Sample | Aborted | 225 | 0 | 151 | 13.502 | 7 | 19.531 | 3038 |
| All acorns | 225 | 0 | 945 | 60.284 | 19 | 119.636 | 13,564 | |
| Curculio attacked | 225 | 0 | 330 | 21.093 | 5 | 46.665 | 4746 | |
| Fully grown | 225 | 0 | 855 | 46.782 | 12 | 106.319 | 10,526 | |
| Fungal infection | 225 | 0 | 147 | 6.320 | 1 | 16.461 | 1422 | |
| Healthy | 225 | 0 | 171 | 8.329 | 1 | 21.043 | 1874 | |
| Insect attacked | 225 | 0 | 536 | 31.929 | 10 | 70.143 | 7184 | |
| Lepidoptera attacked | 225 | 0 | 206 | 10.836 | 3 | 24.201 | 2438 | |
| Other reason | 225 | 0 | 5 | 0.204 | 0 | 0.592 | 46 | |
| Viable | 225 | 0 | 362 | 18.151 | 3 | 43.720 | 4084 |
| Site | n | Min | Max | Mean | Median | SD | Sum |
|---|---|---|---|---|---|---|---|
| Control | 300 | 0 | 18 | 1.873 | 1 | 2.424 | 562 |
| Sample | 300 | 0 | 34 | 4.383 | 3 | 4.131 | 1315 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eötvös, C.B.; Fürjes-Mikó, Á.; Paulin, M.; Gáspár, C.; Kárpáti, M.; Hirka, A.; Csóka, G. Enhanced Natural Regeneration Potential of Sessile Oak in Northern Hungary: Role of Artificially Increased Density of Insectivorous Birds. Forests 2023, 14, 1548. https://doi.org/10.3390/f14081548
Eötvös CB, Fürjes-Mikó Á, Paulin M, Gáspár C, Kárpáti M, Hirka A, Csóka G. Enhanced Natural Regeneration Potential of Sessile Oak in Northern Hungary: Role of Artificially Increased Density of Insectivorous Birds. Forests. 2023; 14(8):1548. https://doi.org/10.3390/f14081548
Chicago/Turabian StyleEötvös, Csaba Béla, Ágnes Fürjes-Mikó, Márton Paulin, Csaba Gáspár, Marcell Kárpáti, Anikó Hirka, and György Csóka. 2023. "Enhanced Natural Regeneration Potential of Sessile Oak in Northern Hungary: Role of Artificially Increased Density of Insectivorous Birds" Forests 14, no. 8: 1548. https://doi.org/10.3390/f14081548
APA StyleEötvös, C. B., Fürjes-Mikó, Á., Paulin, M., Gáspár, C., Kárpáti, M., Hirka, A., & Csóka, G. (2023). Enhanced Natural Regeneration Potential of Sessile Oak in Northern Hungary: Role of Artificially Increased Density of Insectivorous Birds. Forests, 14(8), 1548. https://doi.org/10.3390/f14081548






