Abstract
Osmanthus fragrans is an evergreen shrub or tree of the Oleaceae family with a long history of cultivation in Asian countries and is one of the ten traditionally famous flowers in China, with important cultural and economic value. The unique floral color and fragrance of O. fragrans are formed by a variety of endogenous metabolites that distinguish it from other flowers and exhibit extraordinary ornamental value. However, many studies on the flower color and fragrance of this plant have been mainly based on bioactive extracts and physiological characteristics, leading to a notable lack of molecular machinery and systematic research. In this review, recent advances in bioactive ingredients associated with the underlying regulatory mechanisms, as well as the prospect for industrial utilization, are comprehensively presented and critically evaluated. In particular, the isolated components and essential genes required for flower color and fragrance are also well summarized, which will provide a scientific basis for molecular breeding for ornamental applications and facilitate the discovery of novel natural products for the future industrial development of O. fragrans. In prospect, we plan to use genetic research and high-throughput omics to analyze the genes related to the flower color and fragrance of O. fragrans, and at the same time, we will hybridize and breed excellent O. fragrans varieties that are resistant to low temperature.
1. Introduction
1.1. Osmanthus fragrans Flower Cultivar Diversity
Osmanthus fragrans Lour. is a famous tree species with a long cultivation history, as well as flowers of high medicinal and edible value [1]. Bibliometric analysis of research on O. fragrans flower color and fragrance based on the Web of Science Core Collection database reveals that the number of O. fragrans studies has increased in the last decade. In Figure 1, years and keywords are juxtaposed in a heatmap, where flower color and fragrance are the main research highlights. Figure 1 is helpful for understanding the hot trends in O. fragrans research, updating research techniques, and accelerating research on the flower color and fragrance of O. fragrans. According to the “Flora of China”, O. fragrans is native to southwest China, and its distribution is concentrated in the vast central and subtropical areas north of the South Ridge to the south of the Qinling Mountains [2]. In other parts of the world, O. fragrans was first introduced from Guangzhou to England, and was planted at Ningjia Kew in England in 2007, followed by other European countries one after another; finally, some countries along the Mediterranean were successful in introducing it, and grow it in open fields [3]. After long-term breeding and hard cultivation by many gardeners, the O. fragrans flower has formed a rich variety of cultivars. Generally, it can be divided into four major cultivar groups, namely, the Albus group, Asiatiacus group, Luteus group, and Aurantiacus group. O. fragrans plants are often low and bushy shrub-like; their growing environment is warm and humid, with high temperatures. and sunshine. Shorter sunlight periods and lower humidity are conducive to the opening of O. fragrans. The four groups are divided by their flower color and flowering time (Figure 2). Except for the Asiatiacus group, which can bloom throughout the year and appears light yellow, the flowering period among the other three groups is mainly in autumn, and the flower color becomes the main difference instead of the flowering time. The Albus group flowers are a light silver white color. The Luteus group flowers are golden yellow [4]. The Aurantiacus group flowers are darker in color, an orange-red. In recent years, new colored groups have emerged with high ornamental value that are differentiated by leaf color phenotypes. The main technique for forming colored groups is cutting and grafting. Leaf color, leaf width, and leaf cross-sectional area are generally the main criteria for colored group classification. A new cultivar, ‘Qiannan Guifei’, changes leaf color from red to yellow-green and ultimately to green [5], enriching the landscape function of O. fragrans.
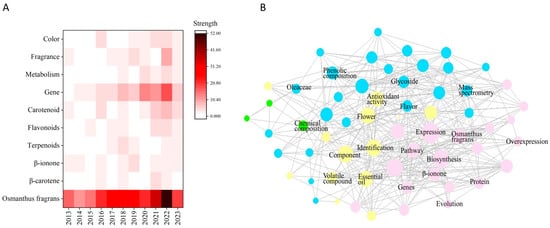
Figure 1.
Bibliometric analysis of research on O. fragrans flower color and fragrance based on the Web of Science Core Collection database. (A) Heatmap of bibliometric analysis, the number of documents with “Osmanthus fragrans”, “β-carotene”, “β-ionone”, “Terpenoids”, “Flavonoids”, “Carotenoid”, “Gene”, “Metabolism”, “Fragrance”, and “Color” as the keywords in the Web of Science Core Collection database. (B) Network diagram for bibliometric analysis of O. fragrans research based on the Web of Science Core Collection database.

Figure 2.
Picture of petals from four representative O. fragrans. Based on color and flowering time, O. fragrans is divided into four species groups, (A) the Albus group; (B) Asiatiacus group; (C) Luteus group; (D) Aurantiacus group. Scale bar = 5 mm.
1.2. Osmanthus Resource Distribution
Osmanthus are mainly distributed in eastern and southeastern Asia, with a few species extending southeast to the islands of New Caledonia in Oceania, and subtypes distributed intermittently in tropical Asia, Oceania, and central South America. There are approximately 35 species of Osmanthus, two of which are hybrids (O. × fortunei and O. × burkwoodii), and 33 natural species. According to the geographical distribution characteristics, four main distribution areas are distinguished within their worldwide distribution in East Asia and Southeast North America [6] and the Asian distribution type is the main distribution center of Osmanthus. The total number of plants in this region is 28, accounting for 84.9% of the total plants.
China is the country with the most abundant O. fragrans resource in the world. The species of O. fragrans is native to the southwestern region of China, and a small amount is distributed in Southeast Asian countries such as Vietnam, Cambodia, and India. O. fragrans is rarely planted in the northern regions due to its cold intolerant growth characteristics, and the southern regions are not suitable for the vernalization of O. fragrans due to the high temperature in winter, where potted O. fragrans is often used. After continuous cultivation, breeding, climate change, and other factors, the area suitable for O. fragrans has begun to gradually shift to the southwest [7]. Subsequently, through continuous on-site inspections, researchers discovered wild O. fragrans varieties mainly in Zhejiang, Anhui, Jiangxi, Hunan, Guangxi, Fujian, and other places. The discovery of new wild varieties can expand the understanding of O. fragrans and has a positive effect on breeding new excellent cultivated varieties to expand the group of O. fragrans varieties for further functional genome research [8].
1.3. The Value and Utilization of Osmanthus fragrans
O. fragrans is one of the ten famous traditional Chinese flowers. The cultivation history in China began in the Han Dynasty and lasted for 2500 years. O. fragrans flowers often appear in Chinese traditional myths and ancient Chinese poems, deriving many images that are deeply rooted in the heart of people, such as “Wu Gang cuts Gui”, “Chan Gong Zhe Gui”, and other myths that have been circulating in the folks. The image of O. fragrans harbors many noble spirits in modern times, including friendship, honor, and auspiciousness [9,10]. O. fragrans has great economic value with wide applications. Ancient Chinese medicine uses the organs of O. fragrans, including roots, branches, flowers, and fruits, as a prescription to relieve dampness and cold [11]. Food made from O. fragrans flower is very popular due to its unique flavor, gradually forming a rich and diverse food industry in modern times. The various secondary metabolites contained in O. fragrans have great potential for the cosmetics industry, and the acetone extract of O. fragrans used as a raw material has antioxidant and antiseptic functions, which can postpone the aging of skin. O. fragrans can also be used in garden landscaping. Different varieties often have different floral characteristics, including beautiful shapes, sweet aromas and auspicious symbols [12].
2. Osmanthus fragrans Flower Color
The color of O. fragrans flowers is one of the most important ornamental features during the flowering period. The floral components contained in different varieties of O. fragrans are highly associated with their colors. The main pigment substances that form the flower color of O. fragrans are carotenoids and flavonoids, the orange flowers are synthesized mainly by carotenoids, while the yellow and white flowers are mainly caused by flavonoid compounds, and carotenoids have a greater impact on the formation and variation of the floral color [13,14]. The main biosynthetic pathways involved in O. fragrans color formation are phenylpropanoid biosynthesis, flavonoid biosynthesis, terpenoid backbone biosynthesis, carotenoid biosynthesis, astaxanthin biosynthesis, and zeatin biosynthesis (Figure 3), which are described in detail below. The classic bioactive substance and the physicochemical effects related to O. fragrans flower color is shown in Table 1 [15,16].
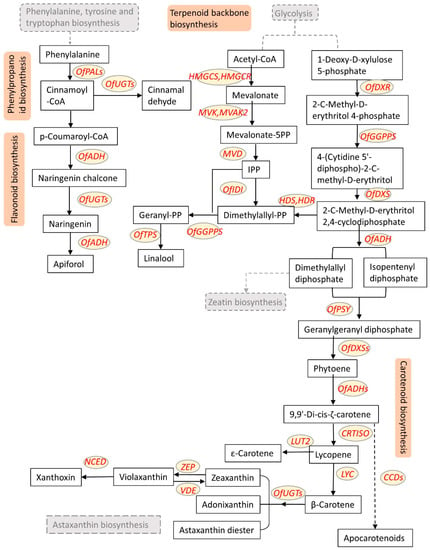
Figure 3.
The basic pathway map for the regulation of O. fragrans flower color and floral fragrance. The main biosynthetic pathways involved are phenylpropanoid biosynthesis, flavonoid biosynthesis, terpenoid backbone biosynthesis, carotenoid biosynthesis, astaxanthin biosynthesis, and zeatin biosynthesis. The genes involved are OfPSY (Phytoene synthase), OfZ-ISO (ζ-carotene isomerase), OfCRTISO (Carotenoid isomerase), OfPAL (Phenylalanine ammonia-lyase), OfCHI (Chalcone isomerase), OfTPS (Terpene synthase), OfUGTs (UDP-glucosyl transferase), OfADH (Alcohol dehydrogenase), OfMECS (2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase), OfHDR (4-hydroxy-3-methylbut-2-enyl diphosphate reductase), OfDXS (1-Deoxy-D-xylulose-5-phosphate synthase), OfGGPPS (Geranylgeranyl pyrophosphate synthase), OfNCED (9-cis Epoxycarotenoid dioxygenases), OfCCDs (Carotenoid cleavage dioxygenase), etc.

Table 1.
Bioactive substance and the physicochemical effects related to flower color and fragrance.
2.1. Major Compounds of Coloration in Osmanthus fragrans
2.1.1. Carotenoids
Carotenoids are compounds that include carotenoids and lutein, which are the main color-presenting substances for yellow and orange types of O. fragrans [14,17]. Carotenoids have medicinal value in antioxidant, immunomodulatory, antiaging, and anticancer activities [18]. Carotenoids are also an important class of photosynthetic pigments that play an indispensable regulatory role in photosynthesis and protection of plants from bright light [19]. Carotenoids in O. fragrans are mainly synthesized in the plastids of cells, and the carotenoid content in plants can be regulated through the hydroxylase pathway, the dioxygenase cleavage pathway and the oxidase pathway, resulting in color changes in different plant tissues [20]. Currently, the carotenoid biosynthesis pathway (CBP) has been well characterized in plants. Carotenoid compositions in O. fragrans consist of α-carotene, β-carotene, α-cryptoxanthin, β-cryptoxanthin, lutein, and zeaxanthin, but carotenoid accumulation patterns during the flowering process are different. The petals of the yellow-white cultivars exhibited high contents of β-carotene, lutein and α-carotene, whereas the petals of the orange-red cultivars mainly contained β-carotene and α-carotene [14]. O. fragrans cultivars vary in pigment composition and concentrations due to differential expression regulation of the downstream genes involved in the carotenoid synthesis and degradation pathway [21]. Therefore, carotenoids play an indispensable role in floral color formation, and a detailed metabolic pathway can be found in Figure 3.
2.1.2. Flavonoids
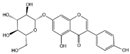
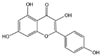
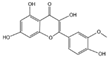
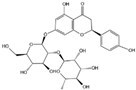
Flavonoids are a major class of secondary metabolites in plants, which have the basic structure of phenylchromenes and are mainly found in the vesicles of plant cells and to a lesser extent in various organs of plants, such as roots, stems, leaves, and flowers [22]. Some studies have proven that flavonoids have important medicinal effects in inhibiting free radical oxidation reactions and preventing tumors and cardiovascular diseases [23]. Flavonoids are also the most important pigment group in flower color, and flavonoids are involved in the widest range of colors [24]. All flower colors in the world, from pale yellow to blue-violet, are regulated by flavonoids, which are important pigment substances in the petals of O. fragrans flowers [25]. Among the flavonoids [26,27,28], there are mainly two categories of red and yellow compounds, among which red substances are mainly anthocyanins and other flavonoids basically belong to the yellow composition, such as dark yellow chalcone and aurora ketone, light yellow as well as nearly colorless flavonoids and flavonols. It has been shown that the content of flavonoids in O. fragrans varies with the flowering period [29], the total flavonoid content in unopened O. fragrans is significantly higher than that in opened O. fragrans and dried O. fragrans, and the amount of total flavonoids in dried O. fragrans decreases significantly, probably due to the decrease in the content of secondary metabolites produced in O. fragrans when it enters the aging stage. Six flavonoids and one phenylethanoid glycoside were isolated from the ethanol extract of O. fragrans flower residues, identified as quercetin (1), rutin (2), verbascoside (3), genistin (4), kaempferol (5), isorhamnetin (6) and naringin (7) [30]. Eleven flavonoids were identified or tentatively identified. ‘Xiaoye Sugui’, ‘Boye Jingui’, ‘Wuyi Dangui’, ‘Yingye Dangui’, ‘Danzhuang’, ‘Foding Zhu’, and ‘Tianxiang Taige’, which are enriched in rutin and total flavonoids, and ‘Sijigui’ contained the highest amounts of kaempferol glycosides and apigenin 7-O-glucoside and could be selected as potential pharmaceutical resources [13]. Liquid chromatography-mass spectrometry (LC-MS) was used to detect the main flavonoids contained in the floral extract of O. fragrans [31], including miconioside B, egonol gentiobioside, and camellianin A.
Anthocyanins are the main chromogenic substances among flavonoids [32], involving the red, blue and purple coloration of flowers. There are more than 100 species of anthocyanins known worldwide, and there are 6 species of anthocyanins commonly found in O. fragrans flowers, including geranophyllin, cornflowerin, delphiniumin, paeoniflorin, petuniain, and mallowin [33]. Among them, geranophyllin, cornflowerin, and delphinidin are the core pigments of anthocyanins, while paeoniflorin, petunidin, and mallowin are the three derived pigments. Among them, geranophyllin dominating the orange-red presentation.
2.2. Regulation of Genes Involved in Osmanthus fragrans Flower Color Formation
A number of genes associated with the formation of flower color in O. fragrans have been isolated and identified. For example, β-ring hydroxylase (HYB) and zeaxanthin epoxidase (ZEP) are more strongly expressed in ‘Jingui’ petals than in ‘Chenghong Dangui’ petals, the flower color of ‘Jingui’ is golden yellow and ‘Chenghong Dangui’ is orange red, resulting in greater β-carotene contents. ε-ring cyclase (LCYE) is most strongly expressed, leading to lutein accumulation. The overexpression of carotenoid cleavage dioxygenase especially OfCCD1 and OfCCD4 in ‘Jingui’ is responsible for the near nonexistence of α-carotene and β-carotene in petals. The coloration of O. fragrans is positively correlated with carotenoid content. It shows The 15-cis-ζ-carotene isomerase gene (15-cis-ζ-carotene isomerase, Z-ISO) OfZ-ISO1 and OfZ-ISO2 are involved in carotenoid accumulation, and both genes have important practical significance to fully reveal the mechanism of coloration in O. fragrans [34] (in Chinese with English abstract).
CCDincludes four CCD subfamilies (CCD1, CCD4, CCD7, and CCD8) and five NCED subfamilies (NCED2, NCED3, NCED5, NCED6, NCED9) with a total of 9 members [35], especially CCD1 and CCD4, are key genes catalyzing many carotenoids to produce β-viologenone, α-viologenone, and unicinolide [36], determining pigmentation and the formation of aroma substances in O. fragrans. CCD1 is mainly an enzyme-activated protein that cleaves carotenoids to produce aroma substances [37]. CCD4 is involved in both the degradation pathway of carotenoids and the synthesis pathway of aroma components, including saffronin and β-viologenone [38]. The expression of CCD4 is strongly correlated with the difference between colored varieties [39]. The expression of OfCCD4a in O. fragrans is negatively correlated with carotenoid accumulation [40] (in Chinese with English abstract). The ERF2 transcription factor positively regulates CCD1 and CCD4, apparently changing flower color to form fragrance [41]. The overexpression of MYB43 promote the genes relating to the accumulation of β-carotenoids. The OfWRKY3 is a positive regulator of the OfCCD4 gene, and might partly account for the biosynthesis of beta-ionone in O. fragrans. the OfMYB1 may acted as a transcriptional activator to regulate expression of OfPAL gene [42,43,44] (Figure 4).
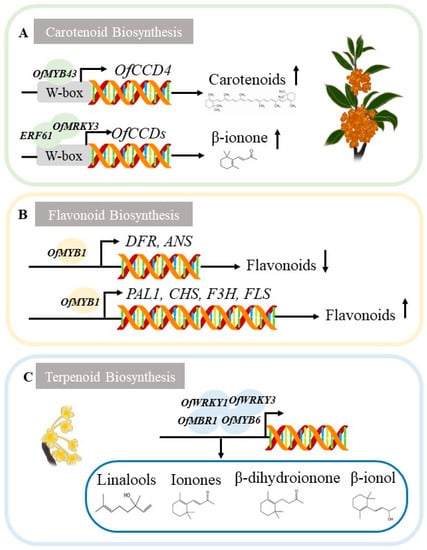
Figure 4.
Examples of gene regulation essential for O. fragrans flower color and floral fragrance. (A) Regulation of transcription factor OfMYB43 (MYB-related transcription factors), OfERF61 (Ethylene response factor), OfWRKY3 (WRKY transcription factors) associated with OfCCDs for carotenoid metabolism. (B) Regulation of transcription factor OfMYB1 involved in flavonoid biosynthesis. (C) Regulation of transcription factors and their roles in terpenoid biosynthesis.
Genes related to anthocyanin synthesis mainly include CHS, CHI, F3H, F3′H, F3′5′H, DFR, ANS, and UGT [45,46,47]. The expression abundance of related genes directly affects anthocyanin accumulation in plants, further influencing the color phenotype [48]. Chalcone isomerase (CHI) is the rate-limiting enzyme in anthocyanin biosynthesis. Taking ‘Orange-red Dangui’, ‘Jingui’, and ‘Early Yingui’ as samples to analyze the expression of CHI [49] (in Chinese with English abstracts), of which ‘Orange-red Dangui’ belongs to Aurantiacus group, ‘Jingui’ belongs to Luteus group, ‘Early Yingui’ belongs to Albus group. CHI expression positively correlates with the anthocyanin content among O. fragrans varieties and tissue specificity [49] (in Chinese with English abstracts), and the anthocyanin content aggravates flower color formation, causing the darkest color in ‘Orange-red Dangui’. The related genes involved in the flower coloration of O. fragrans are shown in Table 2.

Table 2.
List of genes involved in the regulation of O. fragrans flower color and floral fragrance.
3. Osmanthus fragrans Floral Fragrance
The fragrance of O. fragrans is popular among the public, giving it high ornamental and economic value. There is a difference between varieties because the aroma substances contained are different [61]. The main aroma substances in O. fragrans include ocimene, linalool and its oxides, and ionone compounds. According to the structure, the aroma components can be divided into alcohols, ketones, esters, and terpenes. The main substances include linalools, ionones, small amounts of alcohols, fatty acids, and esters [62]. The relative contents of β-ionone, α-ionone, and cis-linalool oxide accounted for 44.55%, 13.88%, and 3.96%, respectively [63] (in Chinese with English abstract). The classic bioactive substance and the physicochemical effects related to O. fragrans floral fragrance are shown in Table 1.
3.1. Fragrance Component Analysis
The main components of most O. fragrans flowers include linalool, ionone, a small amount of alcohols, fatty acids and esters and other compounds, resulting in O. fragrans having a unique floral fragrance compared with other flowers, and the difference in the floral fragrance between O. fragrans varieties is mainly attributed to a small amout of terpenoids and fatty acids.
3.1.1. Terpenoids
Terpenoids are important aroma active substances in O. fragrans. Its synthesis pathway consists of two metabolic pathways. One is the mevalonic acid pathway (MVA), which occurs in the cytoplasm, endoplasmic reticulum, and peroxidation. Among the targets, sesquiterpene compounds are mainly synthesized. The other is the methylerythritol phosphate pathway (MEP), which occurs in plastids and is mainly involved in the synthesis of monoterpenoids [64]. The MVA and MEP pathways in upstream section were separated. Recent studies have shown that isopentenyl pyrophosphate (IPP), the common precursor of terpenes, can be used as an energy source for monoterpenes or sesquiterpenes. Therefore, the MVA and MEP pathways can be crossed together [65].
3.1.2. Fatty Acid Compounds
The biosynthesis of fatty acid compounds is mainly synthesized through α-oxidation, β-oxidation, and lipoxygenase metabolism pathways [64]. Lipoxygenase (Lipoxygenase, LOX) can catalyze the production of fatty acid derivatives from phenolic glycerides in plants [66]. The fatty acid derivatives undergo α-oxidation and β-oxidation to further form alcohols, aldehydes, and ester compounds. Among them, α-ionone and β-trans-ionone are the main aroma active substances in O. fragrans [67].
3.2. Regulation of Genes Related to Fragrance Formation
Studies on the MEP pathway related to monoterpene biosynthesis have shown that most of the gene expression levels in the MEP pathway, such as the MCT2 gene and CMK2 gene, are consistent with the level of monoterpene release. The expression of the key gene TPS in the last step of monoterpene synthesis is significant. The expression level of the TPS gene in different flowering stages and different tissues is significantly different, in the two cultivars ‘Gecheng’ and ‘Liuye’ there were 33 volatile compounds at the beginning of flowering (S2), increasing from tight bud stage (S1) to full flowering stage (S3) and decreasing at late full flowering stage (S4) [52], and the expression of some TPSs also has circadian rhythm differences [68]. Studies have shown that CCDs are one of the most important floral regulation genes. The transcription level of CCD4 is closely related to the accumulation of carotenoids. In O. fragrans, CCD4 is the key gene that causes carotenoid degradation, which in turn produces β-ionone, which is the key substance of floral fragrance [55]. The synthesis of floral scents involves the participation of multiple transcription factors. Studies have shown that the overexpression of the OfWRKY3 transcription factor promotes the expression of the OfCCD4 gene [43]. The expression trend of OfRAP2-12 is consistent with the release law of the floral substance ocimene and its derivatives [69] (in Chinese with English abstract). The ERF2 transcription factor combines with the related elements of the CCD1 and CCD4 gene promoters to promote the expression of the CCDs [70] (in Chinese with English abstract). OfMYB19 and OfMYB20 have a positive effect on the accumulation of the aroma substance cis-linalool oxides, while OfMYB51, OfMYB65, and OfMYB88 show a negative effect on one or more linalool oxides [71,72]. The related genes regulating the fragrance of O. fragrans are shown in Table 2. Several major pathways for fragrance component synthesis were summarized as shown in Figure 5.
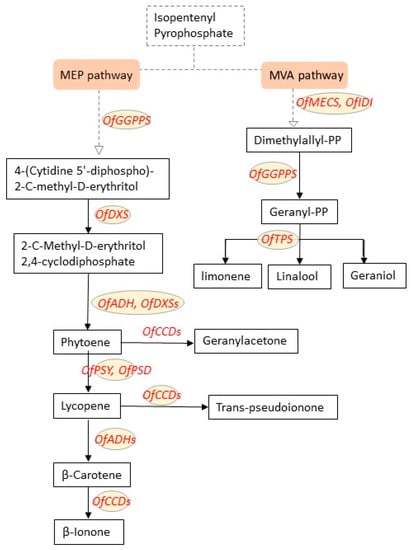
Figure 5.
Two main metabolic pathways involved in the formation of floral fragrance, where the compounds produced are mainly classified as terpenoids. The genes involved in the pathways are OfTPS, OfADH, OfMECS, OfDXS, OfGGPPS, OfCCDs, OfIDI (Isopentenyl diphosphate isomerase), OfPSY, OfPSD, etc.
4. The Relationship between Color and Floral Fragrance
Flower color and floral fragrance are important phenotypic traits of O. fragrans essential for its ornamental quality and economic value. Current studies have shown that there is an emerging connection between the metabolic pathways that regulate flower color and floral fragrance. The first is that the synthesis of anthocyanin and the benzene ring type/phenylpropanoid compound share the same predecessor on the shikimic acid pathway [73]. The second is that the MEP biosynthesis pathway in plants can simultaneously produce carotenoids and volatile terpenoids. Among them, the carotenoid cleavage dioxygenase gene CCDs are crucial regulatory genes that play a fine-tuning role in mediating flower color and floral fragrance [39,55]. CCD4 is a key enzyme in the degradation pathway of the petal pigment substance β-carotenoid, which catalyzes the formation of the floral substance β-ionone simultaneously. β-Ionone is the main aroma substance in O. fragrans, and the transcriptome sequencing and qRT-PCR results indicated that the high β-ionone content in the O. fragrans petals was accompanied by the upregulation of OfCCD1 and OfCCD4. Transcription factor ERF61 [39] can be combined with the OfCCD4 gene promoter to regulate the expression of OfCCD4 to coordinate the transformation from flower color to fragrance. The enzymatic activity assay confirmed that OfCCD4 functioned in catalyzing β-carotenoid into β-ionone [56].
5. High Value-Added Products of Osmanthus fragrans
With the trend of consumption upgrades becoming increasingly obvious, the demand for high-end consumer goods has shown explosive growth. As an O. fragrans product industry, the traditional advantages include ecological, catering and sustainable tourism development, and new development spaces should be expanded, such as essential oils and health care products, taking advantage of their medicinal and edible value [74]. High value-added products of O. fragrans are derived from at least four directions, including seedlings, primary processed products, tourism, and high value-added processed products (Figure 6). In some applications in the seedling industry, gene editing has been used to advance or delay the flowering of O. fragrans, while at the same time giving different flower colors on a single plant during different growth periods. Osmanthus extract is a brownish-yellow paste-like spice with a strong aroma that is extracted with petroleum ether, becoming a raw material for making perfumes. After mass production, the domestic price of osmanthus extract is more than 10,000 RMB per kilogram, and the price abroad is 1600 dollars per kilogram. Using different preservation methods will affect the extraction rate and the ratio of aroma components of O. fragrans essential oil. Compared with the traditional single enzyme extraction of O. fragrans essential oil, compound enzyme extraction is better. It increases the content of the main aroma substances, such as β-ionone, linalool, β-ionol, β-dihydroionone, geraniol, γ-decalactone, nerol, and perillyl alcohol, while reducing the content of harmful substances as phthalates, thereby improving the quality of O. fragrans essential oil to provide a basis for improving the extraction process of O. fragrans essential oil [16,75,76]. O. fragrans essential oil is an important raw material for perfumes, and high-grade cosmetics still have many unexplored effects. Potential neuroprotective and antitumor effects of the essential oil were also found. essential oil, which would benefit the development and utilization of O. fragrans [77]. How to adapt to the current market environment and seize market opportunities is a strategic issue that must be of great importance to the development of O. fragrans essential oil production enterprises in the future [74].
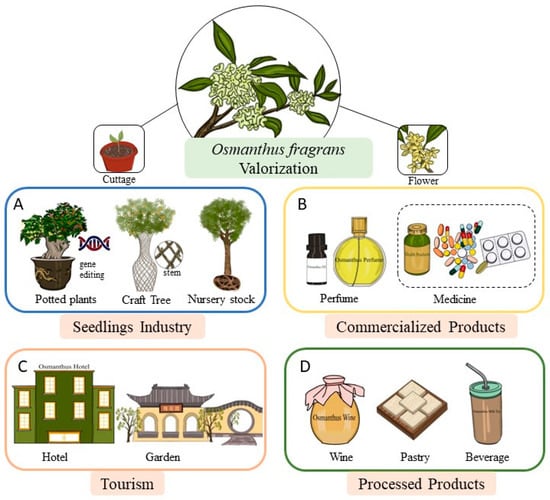
Figure 6.
High value-added products of O. fragrans. Products are developed from four directions, including (A) osmanthus seedlings, (B) primary commercialized products, (C) osmanthus tourism, and (D) high value-added processed products.
Research has implied that flowers are important natural sources of bioactive components with higher antioxidant capacities for use in the food and pharmaceutical industries [78]. O. fragrans, as a food flavor additive, is used extensively in the food industry, frequently appearing on the market, which includes wine, pastry, and beverages, and is widely praised by the public because of its seasonal characteristics and special floral flavor. The flower is often used as a food ingredient to add some color and fragrance and has nutritional value to food because of the bioactive substance it contains [79]. O. fragrans tea looks similar to other teas, but the unique scent of O. fragrans distinguishes it. Pharmacological studies have demonstrated that O. fragrans has a wide range of biological activities, such as antioxidant, antitumor, anti-inflammatory, anti-hyperglycemic, anti-thrombotic, anti-melanogenesis, neuroprotective, and hepatoprotective activities [1]. The medicinal value promotes the development of high value-added health care medicines of O. fragrans, which have the therapeutic effects of relieving cough, phlegm, dispelling cold, and pain. As an ornamental garden plant, O. fragrans has great potential in gardening and viewing. The stems of O. fragrans seedlings are twisted and wound, growing into a peculiar craftsmanship tree, which is used to provide creativity for garden design. With a great abundance of flower resources and massive potential in the tourism industry, China has seen the rise of flower-themed tourism markets. References can be made to form O. fragrans theme tourism, for instance, the China International Jasmine Cultural Festival in Hengxian and the Dounan Flower Market in Kunming all achieve great success around the world, leading to a substantial improvement of the local socioeconomic benefits [80]. The O. fragrans cultural heritage is used to create a themed industrial chain famous for its ornamental, medicinal, and edible characteristics.
6. Conclusions and Prospect
6.1. Conclusions
This article reviews O. fragrans flower color and floral fragrance from 3 aspects: material component analysis, gene regulation, and characteristic valorization. Flower color substances in O. fragrans are mainly flavonoids and carotenoids, and the genes that regulate color formation mainly include CCDs, OfCRTISO, CHI, etc. The aroma substances leading to the unique fragrance of O. fragrans are mainly terpenes, benzene ring/phenylpropanoids, and fatty acids, and the genes that regulate the formation of floral aromas mainly include TPS, CCD, OfWRKY3, OfRAP2-12, ERF2, etc. Some transcription factors regulate genes to affect flower color and fragrance. Members of essential bioactive substances in O. fragrans have good pharmacological activities potential for clinical use to human health. Therefore, exploring the molecular regulatory mechanism responsible for these valuable metabolites would facilitate the effectiveness utilization from this important edible and medicinal plants and help interested researchers discover food and medicinal natural products from O. fragrans for further industrial development.
6.2. Prospect
With the development of next-generation sequencing and nanopore sequencing, sequencing data are accumulated rapidly and more and more analytical techniques are used for data mining. The flower color and fragrance of O. fragrans can be mined and analyzed by sequence characterization and chain analysis using bioinformatics [81,82], The integration of genomics, in combination with various histological tools such as transcriptomics, proteomics and metabolomics, may be the most powerful approach to reveal the molecular and biochemical basis for the biosynthesis of flower color and fragrance metabolites in O. fragrans. This line of research requires the identification of genes encoding enzymes involved in the synthesis of flower color and fragrance substances and the determination of how the expression of these genes is regulated, which may require the use of transgenic model plants [83]. Meanwhile, existing research shows that calli and others have carried out tissue culture of O. fragrans [84,85], but due to the low efficiency of callus tissue proliferation and the difficulty of differentiation of indefinite buds, the tissue culture and regeneration system of O. fragrans has not been successfully established [86]. The lack of complete sequencing of the O. fragrans genome may limit the cloning of O. fragrans genes and the development of gene transformation requires a stable O. fragrans tissue culture technology. Shen [87] used SWATH-MS to discover the post-transcriptional regulation of poplar response to lead. The use of genetic studies and high-throughput proteomics approaches to analyze the genes involved in flower color and fragrance in O. fragrans will further our understanding of the regulation of which in the plant. To date, the biosynthetic pathways associated with the flower color and fragrance of O. fragrans have been intensively analyzed. However, the extremely short flowering period and poor cold resistance of O. fragrans make it difficult to grow in areas north of the Yangtze River, which limits its ornamental and fragrant functions. It can be domesticated by planting some excellent O. fragrans species in the south and north area and selecting excellent low temperature tolerant species through hybridization to extend the flowering period to realize the southern species planted in the north.
Author Contributions
Conceptualization and methodology, Y.Q.; Acquisition of data, Y.Q. and L.S.; Writing—Original Draft, Y.Q. and L.S.; Investigation, Y.Q., R.Z., J.T. and C.Z.; Writing—Review & Editing, Y.Q., L.S., M.C., Y.D. and F.Z.; Visualization, Y.Q., L.S. and R.Z.; Validation, Y.Q., R.Z. and J.T.; Supervision, M.C.; Funding acquisition, Y.D.; Project administration, F.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Jiangsu Agricultural Science and Technology Innovation Fund (CX (21)2023), Natural Science Foundation of Jiangsu Province (SBK2020042924, BK20221334), the Science Technology and Innovation Committee of Shenzhen (JCYJ20210324115408023), Major project of natural science research in colleges of Jiangsu Province (20KJA220001).
Data Availability Statement
Data available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, B.J.; Luan, F.; Bao, Y.W.; Peng, X.; Rao, Z.L.; Tang, Q.; Zeng, N. Traditional uses, phytochemical constituents and pharmacological properties of Osmanthus fragrans: A review. J. Ethnopharmacol. 2022, 293, 25. [Google Scholar] [CrossRef]
- Zhao, H.B.; Hao, R.M.; Hu, S.Q. Geographic distribution and population characteristics of Osmanthus fragrans. Acta Hortic. Sin. 2015, 42, 1760–1770. [Google Scholar]
- Zang, D.K.; Xiang, Q.B.; Liu, Y.L.; Hao, R.M. The studying history and the application to International Cultivar Registration Authority of sweet osmanthus (Osmanthus fragrans Lour.). J. Plant Resour. Environ. 2003, 12, 49–53. [Google Scholar]
- Liu, Y.L. Cultivar classification of Osmanthus fragrans Lour. and the development of germplasm resources of Osmanthus Lour. J. Plant Resour. Environ. 1993, 2, 44–48. [Google Scholar]
- Cui, Q.; Huang, J.H.; Wu, F.; Li, D.Z.; Zheng, L.Q.; Hu, G.; Hu, S.Q.; Zhang, L. Biochemical and transcriptomic analyses reveal that critical genes involved in pigment biosynthesis influence leaf color changes in a new sweet osmanthus cultivar ‘Qiannan Guifei’. PeerJ 2021, 9, 28. [Google Scholar] [CrossRef]
- Xiang, Q.; Liu, L.; Wu, X. Studies on the Cultivar Classification of Osmanthus. In Proceedings of the 5th International Symposium on the Taxonomy of Cultivated Plants, Wageningen, The Netherlands, 15–19 October 2007. [Google Scholar]
- Kong, F.; Tang, L.; He, H.; Yang, F.X.; Tao, J.; Wang, W.C. Assessing the impact of climate change on the distribution of Osmanthus fragrans using Maxent. Environ. Sci. Pollut. Res. 2021, 28, 34655–34663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.R.; Fan, D.M.; Guo, S.Q.; Li, D.Z.; Zhang, Z.Y. Development of 29 microsatellite markers for Osmanthus fragrans (oleaceae), a traditional fragrant flowering tree of china. Am. J. Bot. 2011, 98, E356–E359. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.Y. History and cultural significance of sweet Osmanthus in Chinese gardenin. J. Beijing For. Univ. (Soc. Sci.) 2005, 25–29. [Google Scholar]
- Shang, F.D.; Yi, Y.J.; Xiang, Q.B. Osmanthus culture in China. J. Henan Univ. 2003, 136–139. [Google Scholar] [CrossRef]
- Fu, C.C.; Xu, F.Y.; Qian, Y.C.; Koo, H.L.; Duan, Y.F.; Weng, G.M.; Fan, T.P.; Chen, M.X.; Zhu, F.Y. Secondary metabolites of Osmanthus fragrans: Metabolism and medicinal value. Front. Pharmacol. 2022, 13, 922204. [Google Scholar] [CrossRef]
- Wang, C.R.; Liu, C.; Yuan, B.; Zeng, X.L.; Wang, C.Y. Cultivation history, traditional landscape arrangement and auspicious culture of Osmanthus fragrans in Chinese ancient garden. In Proceedings of the 29th International Horticultural Congress on Horticulture—Sustaining Lives, Livelihoods and Landscapes (IHC)/International Symposium on Impact of Asia-Pacific Horticulture—Resources, Technology and Social Welfare, Brisbane, Australia, 17–22 August 2014. [Google Scholar]
- Wang, Y.G.; Fu, J.X.; Zhang, C.; Zhao, H.B. HPLC-DAD-ESI-MS analysis of flavonoids from leaves of different cultivars of sweet osmanthus. Molecules 2016, 21, 1224. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.G.; Zhang, C.; Dong, B.; Fu, J.X.; Hu, S.Q.; Zhao, H.B. Carotenoid accumulation and its contribution to flower coloration of Osmanthus fragrans. Front. Plant. Sci. 2018, 9, 17. [Google Scholar] [CrossRef]
- Liu, J.; Nakamura, S.; Xu, B.; Matsumoto, T.; Ohta, T.; Fujimoto, K.; Ogawa, K.; Fukaya, M.; Miyake, S.; Yoshikawa, M.; et al. Chemical structures of constituents from the flowers of Osmanthus fragrans var. aurantiacus. J. Nat. Med. 2015, 69, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Wang, B.; Wang, M.; Wang, M. Ultrasound-assisted extraction of Osmanthus fragrans fruit oil and evaluation of its fatty acid composition, physicochemical properties and antioxidant activity. J. Appl. Res. Med. Aroma. 2021, 25, 100331. [Google Scholar] [CrossRef]
- Hermanns, A.S.; Zhou, X.; Xu, Q.; Tadmor, Y.; Li, L. Carotenoid pigment accumulation in horticultural plants. Hortic. Plant J. 2020, 6, 343–360. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- Hashimoto, H.; Uragami, C.; Yukihira, N.; Horiuchi, K.; Cogdell, R.J. Ultrafast laser spectroscopic studies on carotenoids in solution and on those bound to photosynthetic pigment-protein complexes. Methods Enzymol. 2022, 674, 1–51. [Google Scholar] [CrossRef]
- Guo, S.; Sun, H.; Zhang, H.; Liu, J.; Ren, Y.; Gong, G.; Jiao, C.; Zheng, Y.; Yang, W.; Fei, Z.; et al. Comparative transcriptome analysis of cultivated and wild watermelon during fruit development. PLoS ONE 2015, 10, e0130267. [Google Scholar] [CrossRef]
- Han, Y.J.; Wang, X.H.; Chen, W.C.; Dong, M.F.; Yuan, W.J.; Liu, X.; Shang, F.D. Differential expression of carotenoid-related genes determines diversified carotenoid coloration in flower petal of Osmanthus fragrans. Tree Genet. Genomes 2014, 10, 329–338. [Google Scholar] [CrossRef]
- Koes, R.; Verweij, W.; Quattrocchio, F. Flavonoids: A colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci. 2005, 10, 236–242. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Lu, W.; Shi, Y.; Wang, R.; Su, D.; Tang, M.; Liu, Y.; Li, Z. Antioxidant activity and healthy benefits of natural pigments in fruits: A review. Int. J. Mol. Sci. 2021, 22, 4945. [Google Scholar] [CrossRef]
- Yu, J.; Lou, Q.; Zheng, X.; Cui, Z.; Fu, J. Sequential combination of microwave- and ultrasound-assisted extraction of total flavonoids from Osmanthus fragrans Lour. flowers. Molecules 2017, 22, 2216. [Google Scholar] [CrossRef]
- Zhao, D.Q.; Tao, J. Recent advances on the development and regulation of flower color in ornamental plants. Front. Plant Sci. 2015, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Iwashina, T. Contribution to flower colors of flavonoids including anthocyanins: A review. Nat. Prod. Commun. 2015, 10, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, M.; Nakatsuka, T. Genetic engineering of flavonoid pigments to modify flower color in floricultural plants. Biotechnol. Lett. 2011, 33, 433–441. [Google Scholar] [CrossRef]
- Hao, J.F.; Li, Y.Y.; Jia, Y.S.; Wang, Z.J.; Rong, R.; Bao, J.; Zhao, M.Q.; Fu, Z.H.; Ge, G.T. Comparative analysis of major flavonoids among parts of Lactuca indica during different growth periods. Molecules 2021, 26, 7445. [Google Scholar] [CrossRef]
- Zhou, J.L.; Fang, X.Y.; Wang, J.Q.; Zhao, L.G.; Li, Y.; Tang, F.; Yue, Y.D. Structures and bioactivities of seven flavonoids from Osmanthus fragrans ‘Jinqiu’ essential oil extraction residues. Nat. Prod. Res. 2018, 32, 588–591. [Google Scholar] [CrossRef]
- Wu, L.C.; Chang, L.H.; Chen, S.H.; Fan, N.C.; Ho, J.A.A. Antioxidant activity and melanogenesis inhibitory effect of the acetonic extract of Osmanthus fragrans: A potential natural and functional food flavor additive. LWT-Food Sci. Technol. 2009, 42, 1513–1519. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, M.; Jin, J.; Zhao, L.; Xu, Z. Anthocyanins and their biosynthetic genes in three novel-colored Rosa rugosa cultivars and their parents. Plant Physiol. Bioch. 2018, 129, 421–428. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, T.; Dai, Z.; Dai, X.; Li, W.; Cao, M.; Li, C.; Tsai, W.; Wu, X.; Zhai, J. Comparative transcriptomics provides insight into floral color polymorphism in a Pleione limprichtii orchid population. Int. J. Mol. Sci. 2019, 21, 247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, Y.C.; Wang, Y.G.; Fu, J.X.; Zhao, H.B. Cloning and expression analysis of carotenoid isomerase gene in Osmanthus fragrans. Biotechnol. Bull. 2017, 33, 89. [Google Scholar]
- Auldridge, M.E.; McCarty, D.R.; Klee, H.J. Plant carotenoid cleavage oxygenases and their apocarotenoid products. Curr. Opin. Plant Biol. 2006, 9, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Auldridge, M.E.; Block, A.; Vogel, J.T.; Dabney-Smith, C.; Mila, I.; Bouzayen, M.; Magallanes-Lundback, M.; DellaPenna, D.; McCarty, D.R.; Klee, H.J. Characterization of three members of the Arabidopsis carotenoid cleavage dioxygenase family demonstrates the divergent roles of this multifunctional enzyme family. Plant J. 2006, 45, 982–993. [Google Scholar] [CrossRef]
- Floss, D.S.; Walter, M.H. Role of carotenoid cleavage dioxygenase 1 (CCD1) in apocarotenoid biogenesis revisited. Plant Signal. Behav. 2009, 4, 172–175. [Google Scholar] [CrossRef]
- Rubio, A.; Rambla, J.L.; Santaella, M.; Gómez, M.; Orzaez, D.; Granell, A.; Gómez-Gómez, L. Cytosolic and plastoglobule-targeted carotenoid dioxygenases from Crocus sativus are both involved in beta-ionone release. J. Biol. Chem. 2008, 283, 24816–24825. [Google Scholar] [CrossRef]
- Han, Y.; Wang, H.; Wang, X.; Li, K.; Dong, M.; Li, Y.; Zhu, Q.; Shang, F. Mechanism of floral scent production in Osmanthus fragrans and the production and regulation of its key floral constituents, beta-ionone and linalool. Hortic. Res. 2019, 6, 106. [Google Scholar] [CrossRef]
- Zeng, X.L. Research of TPS and CCD function analysis and their influence on petal color and scent in Osmanthus fragrans Lour. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2016. [Google Scholar]
- Han, Y.J.; Lu, M.M.; Yue, S.M.; Li, K.; Dong, M.F.; Liu, L.X.; Wang, H.Y.; Shang, F.D. Comparative methylomics and chromatin accessibility analysis in Osmanthus fragrans uncovers regulation of genic transcription and mechanisms of key floral scent production. Hortic. Res.-Engl. 2022, 9, 19. [Google Scholar] [CrossRef]
- Xi, W.; He, Y.H.; Zhu, L.L.; Hu, S.Y.; Xiong, S.Y.; Zhang, Y.; Zou, J.J.; Chen, H.G.; Wang, C.Y.; Zheng, R.R. CPTA treatment reveals potential transcription factors associated with carotenoid metabolism in flowers of Osmanthus fragrans. Hortic. Plant J. 2021, 7, 479–487. [Google Scholar] [CrossRef]
- Han, Y.J.; Wu, M.; Cao, L.Y.; Yuan, W.J.; Dong, M.F.; Wang, X.H.; Chen, W.C.; Shang, F.D. Characterization of OfWRKY3, a transcription factor that positively regulates the carotenoid cleavage dioxygenase gene OfCCD4 in Osmanthus fragrans. Plant Mol. Biol. 2016, 91, 485–496. [Google Scholar] [CrossRef]
- Han, Y.J.; Li, L.X.; Dong, M.F.; Yuan, W.J.; Shang, F.D. cDNA cloning of the phytoene synthase (PSY) and expression analysis of PSY and carotenoid cleavage dioxygenase genes in Osmanthus fragrans. Biologia 2013, 68, 258–263. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, P.; Lin, W.; Zheng, X.; Cai, M.; Peng, C. Sequencing of anthocyanin synthesis-related enzyme genes and screening of reference genes in leaves of four dominant subtropical forest tree species. Gene 2019, 716, 144024. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, B.; Liu, J.; Guo, D.; Hou, J.; Chen, S.; Song, B.; Xie, C. Analysis of structural genes and key transcription factors related to anthocyanin biosynthesis in potato tubers. Sci. Hortic. 2017, 225, 310–316. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Li, J.; Ding, Y.; Tian, J.L.; Wang, Z.; Xiong, B.; Xu, T.; Kou, G.; Zheng, Y.; et al. Analysis of anthocyanin accumulation and related gene expression during fig fruit development. Plant Mol. Biol. Rep. 2023. [Google Scholar] [CrossRef]
- Zhou, C.; Mei, X.; Rothenberg, D.O.N.; Yang, Z.; Zhang, W.; Wan, S.; Yang, H.; Zhang, L. Metabolome and transcriptome analysis reveals putative genes involved in anthocyanin accumulation and coloration in white and pink tea (Camellia sinensis) flower. Molecules 2020, 25, 190. [Google Scholar] [CrossRef]
- Zhang, S.M.; Tu, J.L.; Li, C.W.; Hu, Q.; Chen, L.; Dong, L.L. Cloning and expression analysis of chalcone isomerase OfCHI from Osmanthus fragrans. Acta Bot. Bor-Occid. Sin. 2016, 36, 1728–1734. [Google Scholar]
- Liu, Y.C.; Dong, B.; Zhang, C.; Yang, L.Y.; Wang, Y.G.; Zhao, H.B. Effects of exogenous abscisic acid (ABA) on carotenoids and petal color in Osmanthus fragrans ‘Yanhonggui’. Plants 2020, 9, 454. [Google Scholar] [CrossRef]
- Han, Y.; Chen, W.; Yang, F.; Wang, X.; Dong, M.; Zhou, P.; Shang, F. cDNA-AFLP analysis on 2 Osmanthus fragrans cultivars with different flower color and molecular characteristics of OfMYB1 gene. Trees-Struct. Funct. 2015, 29, 931–940. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, C.; Zheng, R.; Cai, X.; Luo, J.; Zou, J.; Wang, C. Emission and accumulation of monoterpene and the key terpene synthase (TPS) associated with monoterpene biosynthesis in Osmanthus fragrans Lour. Front. Plant Sci. 2016, 6, 1232. [Google Scholar] [CrossRef] [PubMed]
- Baldermann, S.; Kato, M.; Fleischmann, P.; Watanabe, N. Biosynthesis of alpha- and beta-ionone, prominent scent compounds, in flowers of Osmanthus fragrans. Acta Biochim. Pol. 2012, 59, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Baldermann, S.; Kato, M.; Kurosawa, M.; Kurobayashi, Y.; Fujita, A.; Fleischmann, P.; Watanabe, N. Functional characterization of a carotenoid cleavage dioxygenase 1 and its relation to the carotenoid accumulation and volatile emission during the floral development of Osmanthus fragrans Lour. J. Exp. Bot. 2010, 61, 2967–2977. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.C.; Molnar, P.; Schwab, W. Cloning and functional characterization of carotenoid cleavage dioxygenase 4 genes. J. Exp. Bot. 2009, 60, 3011–3022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Pei, J.; Zhao, L.; Tang, F.; Fang, X.; Xie, J. Overexpression and characterization of CCD4 from Osmanthus fragrans and beta-ionone biosynthesis from beta-carotene in vitro. J. Mol. Catal. B-Enzym. 2016, 134, 105–114. [Google Scholar] [CrossRef]
- Zheng, R.; Zhu, Z.; Wang, Y.; Hu, S.; Xi, W.; Xiao, W.; Qu, X.; Zhong, L.; Fu, Q.; Wang, C. UGT85A84 Catalyzes the glycosylation of aromatic monoterpenes in Osmanthus fragrans Lour. Flowers. Front. Plant Sci. 2019, 10, 1376. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, Z.; Chen, R.; He, H.; Ma, J.; Zhang, D. Gene cloning and gene expression characteristics of alcohol dehydrogenase in Osmanthus fragrans var. Semperflorens. Emir. J. Food Agr. 2018, 30, 820–827. [Google Scholar] [CrossRef]
- Chen, X.; Yang, X.; Xie, J.; Ding, W.; Li, Y.; Yue, Y.; Wang, L. Biochemical and comparative transcriptome analyses reveal key genes involved in major metabolic regulation related to colored leaf formation in Osmanthus fragrans ‘Yinbi Shuanghui’ during development. Biomolecules 2020, 10, 549. [Google Scholar] [CrossRef]
- Li, T.; Deng, Y.J.; Liu, J.X.; Duan, A.Q.; Liu, H.; Xiong, A.S. DcCCD4 catalyzes the degradation of alpha-carotene and beta-carotene to affect carotenoid accumulation and taproot color in carrot. Plant J. 2021, 108, 1116–1130. [Google Scholar] [CrossRef]
- Cai, X.; Mai, R.Z.; Zou, J.J.; Zhang, H.Y.; Zeng, X.L.; Zheng, R.R.; Wang, C.Y. Analysis of aroma-active compounds in three sweet osmanthus (Osmanthus fragrans) cultivars by GC-olfactometry and GC-MS. J. Zhejiang Univ.-SCI. B 2014, 15, 638–648. [Google Scholar] [CrossRef]
- Sheng, X.J.; Lin, Y.N.; Cao, J.M.; Ning, Y.; Pang, X.L.; Wu, J.H.; Kong, F.Y. Comparative evaluation of key aroma-active compounds in sweet osmanthus (Osmanthus fragrans Lour.) with different enzymatic treatments. J. Agr. Food Chem. 2021, 69, 332–344. [Google Scholar] [CrossRef]
- Chen, P.Z. Optimization of extraction technology and analysis of components of Osmanthus fragrans essential oil. Cereals Oils 2016, 29, 4. [Google Scholar]
- Orlova, I.; Marshall-Colon, A.; Schnepp, J.; Wood, B.; Varbanova, M.; Fridman, E.; Blakeslee, J.J.; Peer, W.A.; Murphy, A.S.; Rhodes, D.; et al. Reduction of benzenoid synthesis in petunia flowers reveals multiple pathways to benzoic acid and enhancement in auxin transport. Plant Cell. 2006, 18, 3458–3475. [Google Scholar] [CrossRef] [PubMed]
- Henry, L.K.; Gutensohn, M.; Thomas, S.T.; Noel, J.P.; Dudareva, N. Orthologs of the archaeal isopentenyl phosphate kinase regulate terpenoid production in plants. Proc. Natl. Acad. Sci. USA 2015, 112, 10050–10055. [Google Scholar] [CrossRef] [PubMed]
- Hudak, K.A.; Thompson, J.E. Subcellular localization of secondary lipid metabolites including fragrance volatiles in carnation petals. Plant Physiol. 1997, 114, 705–713. [Google Scholar] [CrossRef]
- Xu, C.; Yang, X.L.; Huang, S.C.; Chen, H.; Zhang, Y.B. Cloning and expression analysis of OfCCD1 and OfCCD4 genes in sweet osmanthus. Pak. J. Bot. 2023, 55, 563–569. [Google Scholar] [CrossRef]
- Sun, P.; Chen, X.Y.; Chantarasuwan, B.; Zhu, X.Y.; Deng, X.X.; Bao, Y.; Yu, H. Composition diversity and expression specificity of the TPS gene family among 24 Ficus species. Diversity 2022, 14, 721. [Google Scholar] [CrossRef]
- Ouyang, Q.X.; Ding, W.J.; Wu, X.Y.; Yue, Y.Z.; Yang, X.L.; Wang, L.G. Cloning and expression characteristic analysis of RAP2-12 in Osmanthus fragrans. Acta Bot. Bor-Occid. Sin. 2020, 40, 10. [Google Scholar]
- Liu, Y.C.; Zhang, C.; Dong, B.; Zhao, H.B. Advances of CCD subfamily in higher plants. J. Agric. Biotech. 2019, 027, 720–734. [Google Scholar]
- Li, H.Y.; Yue, Y.Z.; Ding, W.J.; Chen, G.W.; Li, L.; Li, Y.L.; Shi, T.T.; Yang, X.L.; Wang, L.G. Genome-wide identification, classification, and expression profiling reveals R2R3-MYB transcription factors related to monoterpenoid biosynthesis in Osmanthus fragrans. Genes 2020, 11, 353. [Google Scholar] [CrossRef]
- Yan, X.; Ding, W.J.; Wu, X.Y.; Wang, L.G.; Yang, X.L.; Yue, Y.Z. Insights into the MYB-Related transcription factors involved in regulating floral aroma synthesis in sweet osmanthus. Front. Plant Sci. 2022, 13, 14. [Google Scholar] [CrossRef]
- Yeon, J.Y.; Kim, W.S. Biosynthetic linkage between the color and scent of flowers: A review. Hortic. Sci. Technol. 2021, 39, 697–713. [Google Scholar] [CrossRef]
- Wang, S.G.; Feng, X.; Li, G.C. Research on the development environment of the extraction enterprises of sweet osmanthus essential oil. Ekoloji 2018, 27, 163–171. [Google Scholar]
- Pan, C.; Zhao, L.; Zhao, D. Microwave-assisted green extraction of antioxidant components from Osmanthus fragrans (Lour) flower using natural deep eutectic solvents. J. Appl. Res. Med. Aroma 2021, 20, 100285. [Google Scholar] [CrossRef]
- Usuki, T.; Munakata, K. Extraction of essential oils from the flowers of Osmanthus fragrans var. aurantiacus using an ionic liquid. Bull. Chem. Soc. Jpn. 2017, 90, 1105–1110. [Google Scholar] [CrossRef]
- Wang, L.; Tan, N.N.; Hu, J.Y.; Wang, H.; Duan, D.Z.; Ma, L.; Xiao, J.; Wang, X.L. Analysis of the main active ingredients and bioactivities of essential oil from Osmanthus fragrans var. thunbergii using a complex network approach. BMC Syst. Biol. 2017, 11, 12. [Google Scholar] [CrossRef]
- Chen, G.L.; Chen, S.G.; Xiao, Y.; Fu, N.L. Antioxidant capacities and total phenolic contents of 30 flowers. Ind. Crop. Prod. 2018, 111, 430–445. [Google Scholar] [CrossRef]
- Wu, L.P.; Liu, J.Y.; Huang, W.S.; Wang, Y.X.; Chen, Q.; Lu, B.Y. Exploration of Osmanthus fragrans Lour.’s composition, nutraceutical functions and applications. Food Chem. 2022, 377, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.Z.; Xu, Y.Y.; Zhou, Z.C. Management Systems of Flower-Themed Tourism in China: A Value Chain Analysis. In Proceedings of the 18th International Symposium on Horticultural Economics and Management, Alnarp, Sweden, 31 May–3 June 2015. [Google Scholar]
- Chen, M.X.; Zhang, K.L.; Gao, B.; Yang, J.F.; Tian, Y.; Das, D.; Fan, T.; Dai, L.; Hao, G.F.; Yang, G.F.; et al. Phylogenetic comparison of 5′ splice site determination in central spliceosomal proteins of the U1-70K gene family, in response to developmental cues and stress conditions. Plant J. 2020, 103, 357–378. [Google Scholar] [CrossRef]
- Song, T.; Das, D.; Ye, N.H.; Wang, G.Q.; Zhu, F.Y.; Chen, M.X.; Yang, F.; Zhang, J.H. Comparative transcriptome analysis of coleorhiza development in japonica and Indica rice. BMC Plant Biol. 2021, 21, 514. [Google Scholar] [CrossRef]
- Zhao, Y.; Xin, D.; Lu, W.; Zong, X.; Niu, Y.; Guo, X.; Ma, Y.; Qiang, W.; Su, H.; Zhang, S.; et al. PeMPK7 is induced in an ROS-dependent manner and confers poplar para-hydroxybenzoic acid stress resistance through the removal of ROS. Ind. Crop. Prod. 2022, 182, 114861. [Google Scholar] [CrossRef]
- Zou, J.J.; Gao, W.; Cai, X.; Zeng, X.L.; Wang, C.Y. Somatic embryogenesis and plant regeneration in Osmanthus fragrans Lour. Propag. Ornam. Plants 2014, 14, 32–39. [Google Scholar]
- Zhong, S.; Dong, B.; Zhou, J.; Miao, Y.; Yang, L.; Wang, Y.; Xiao, Z.; Fang, Q.; Wan, Q.; Zhao, H. Highly efficient transient gene expression of three tissues in Osmanthus fragrans mediated by Agrobacterium tumefaciens. Sci. Hortic. 2023, 310, 111725. [Google Scholar] [CrossRef]
- Gu, H.; Ding, W.; Shi, T.; Ouyang, Q.; Yang, X.; Yue, Y.; Wang, L. Integrated transcriptome and endogenous hormone analysis provides new insights into callus proliferation in Osmanthus fragrans. Sci. Rep. 2022, 12, 7609. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.C.; Chen, M.X.; Xiao, T.; Zhang, C.; Shang, J.; Zhang, K.L.; Zhu, F.Y. Global proteome response to Pb(II) toxicity in poplar using SWATH-MS-based quantitative proteomics investigation. Ecotox. Environ. Safe. 2021, 220, 8. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).