Soil Bacteria and Soil Fungi Respond Differently to the Changes in Aboveground Plants along Slope Aspect in a Subalpine Coniferous Forest
Abstract
1. Introduction
2. Materials and Methods
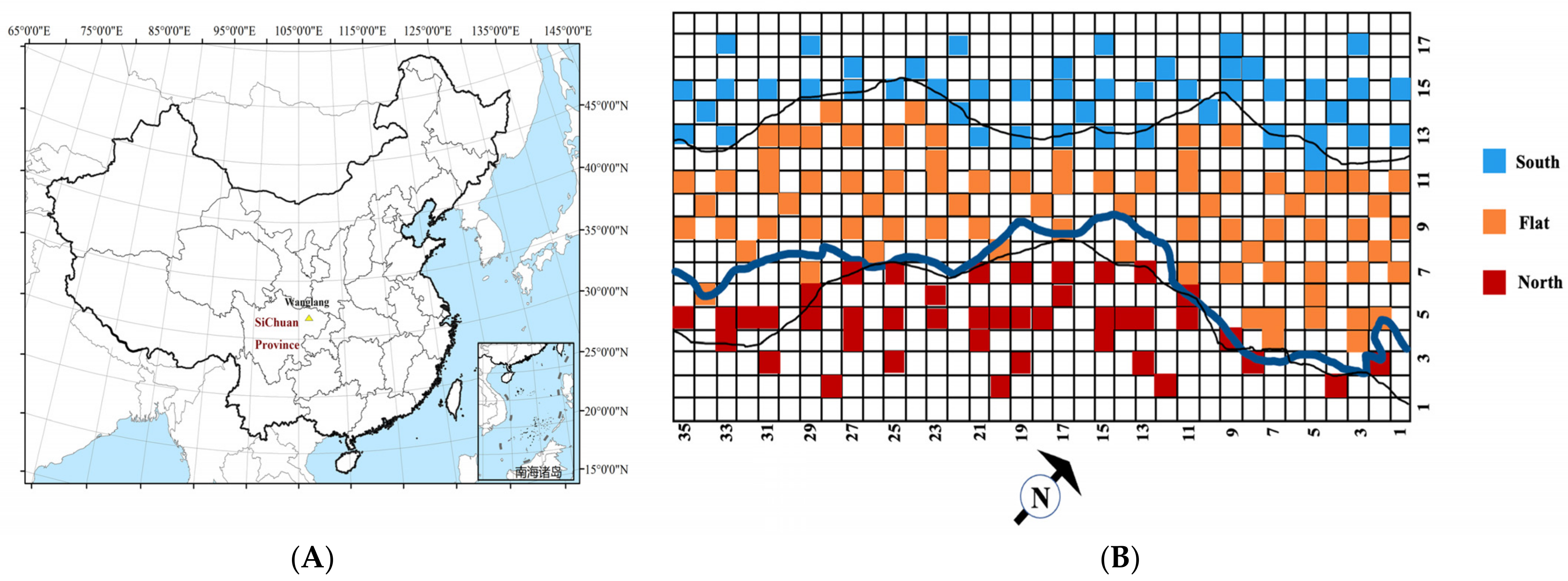
2.1. Study Area and Site Description
2.2. Vegetation and Soil Sampling
2.3. Determination of Soil Properties
2.4. DNA Extraction, Amplification, High-Throughput Sequencing, and Bioinformatics Analysis
2.5. Data Analysis
3. Results
3.1. Soil Properties across the Sampled Sites
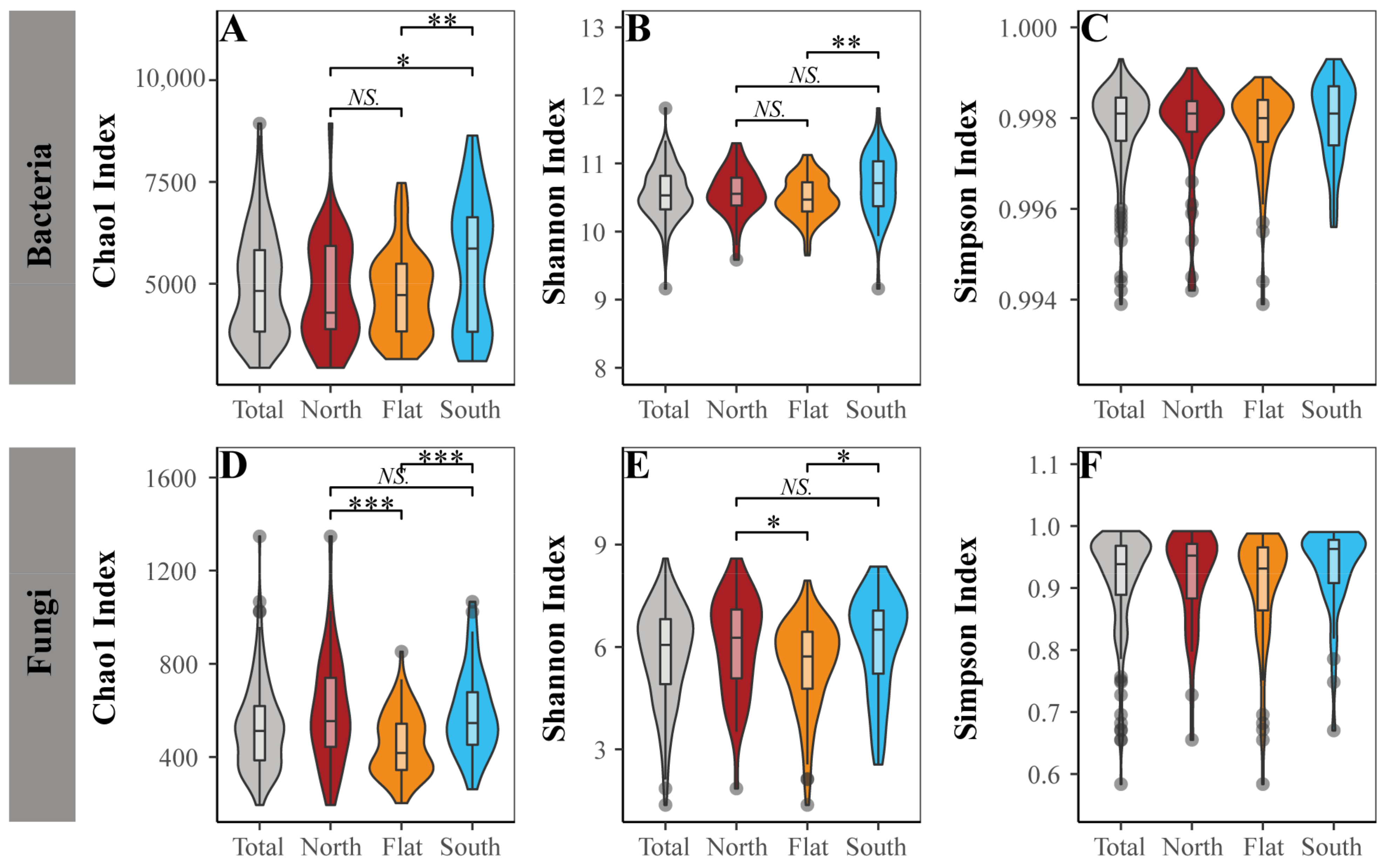
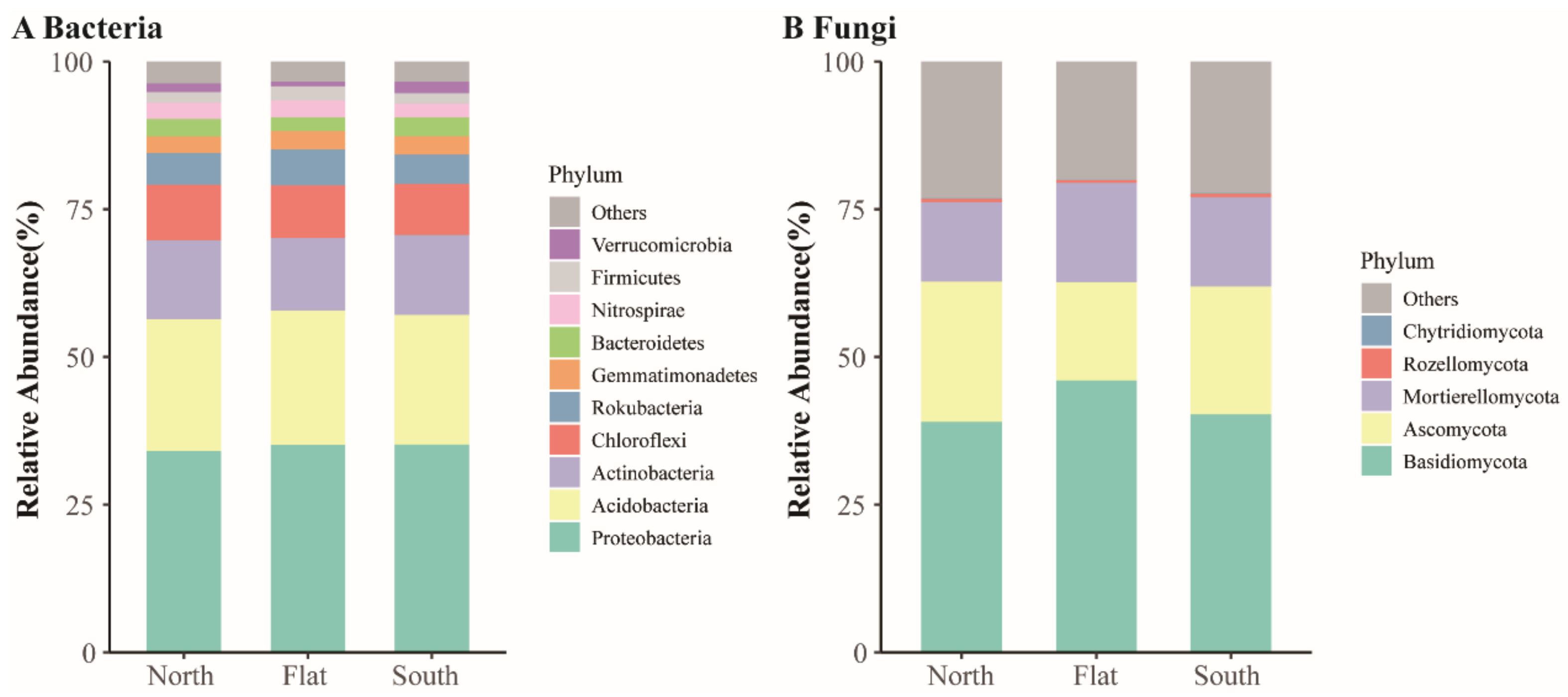
3.2. Community Characteristics of Vegetation and Soil Bacteria and Fungi across the Sample Sites
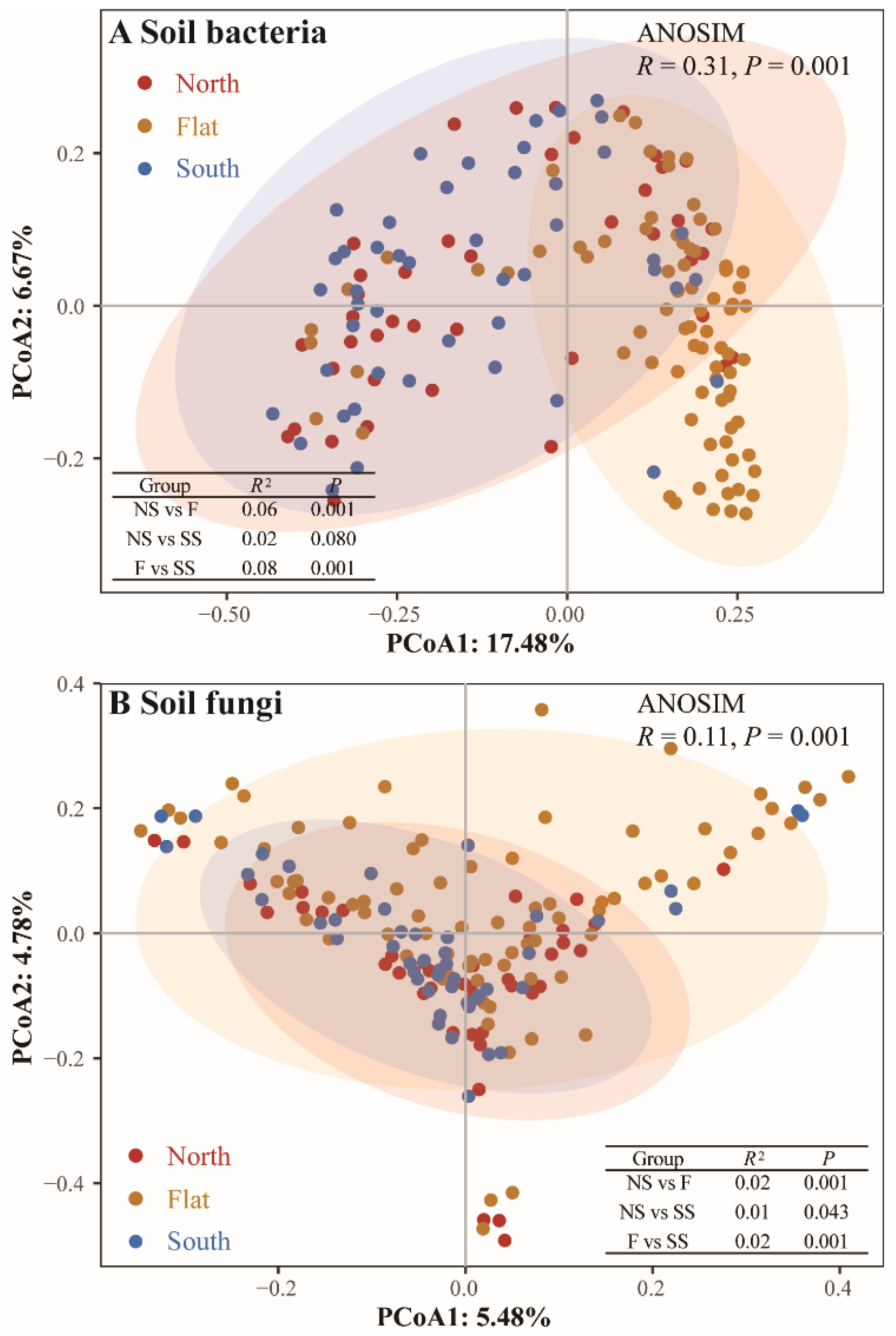
3.3. Factors Influencing the Community Composition of Plants and Soil Bacteria and Fungi
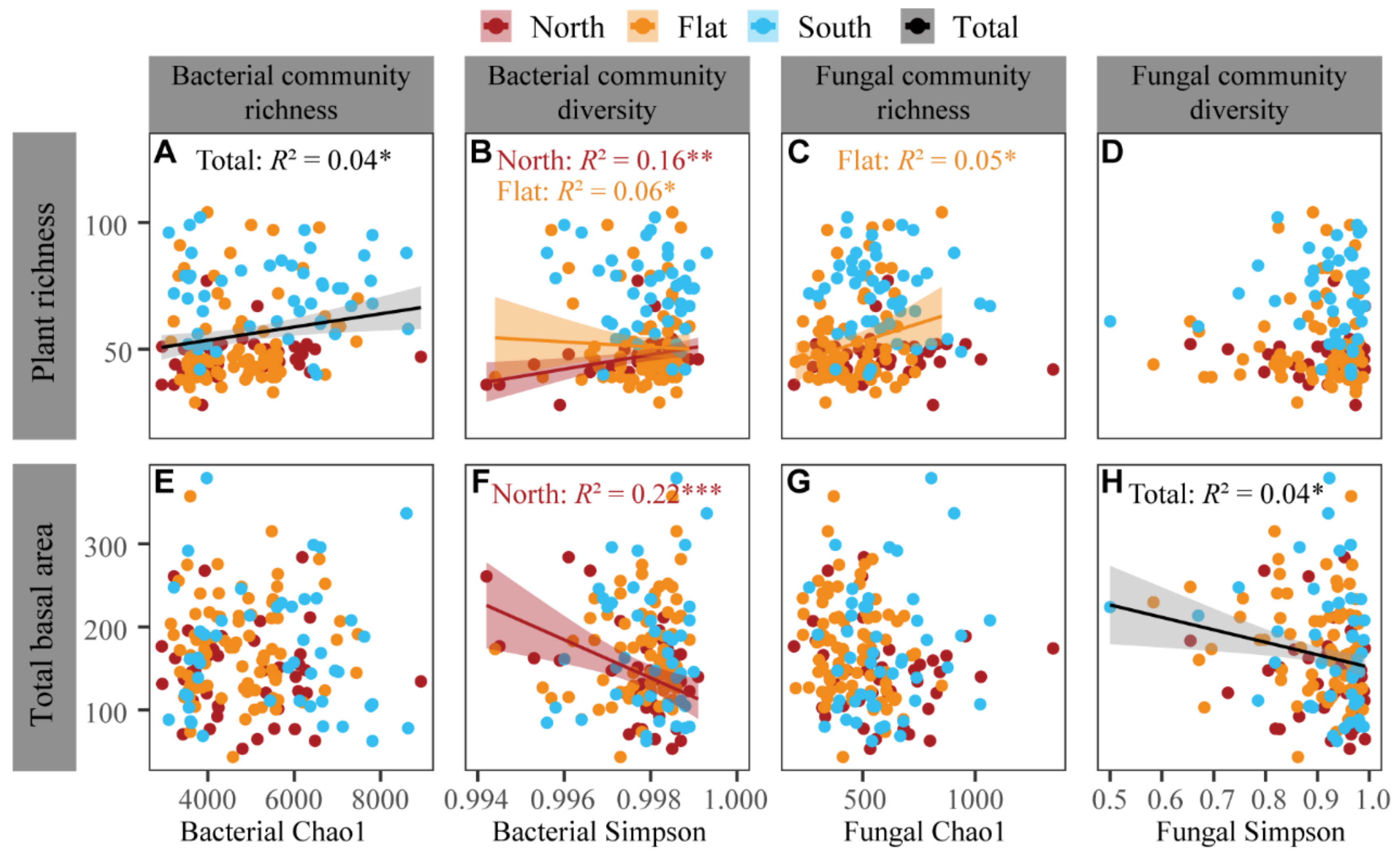
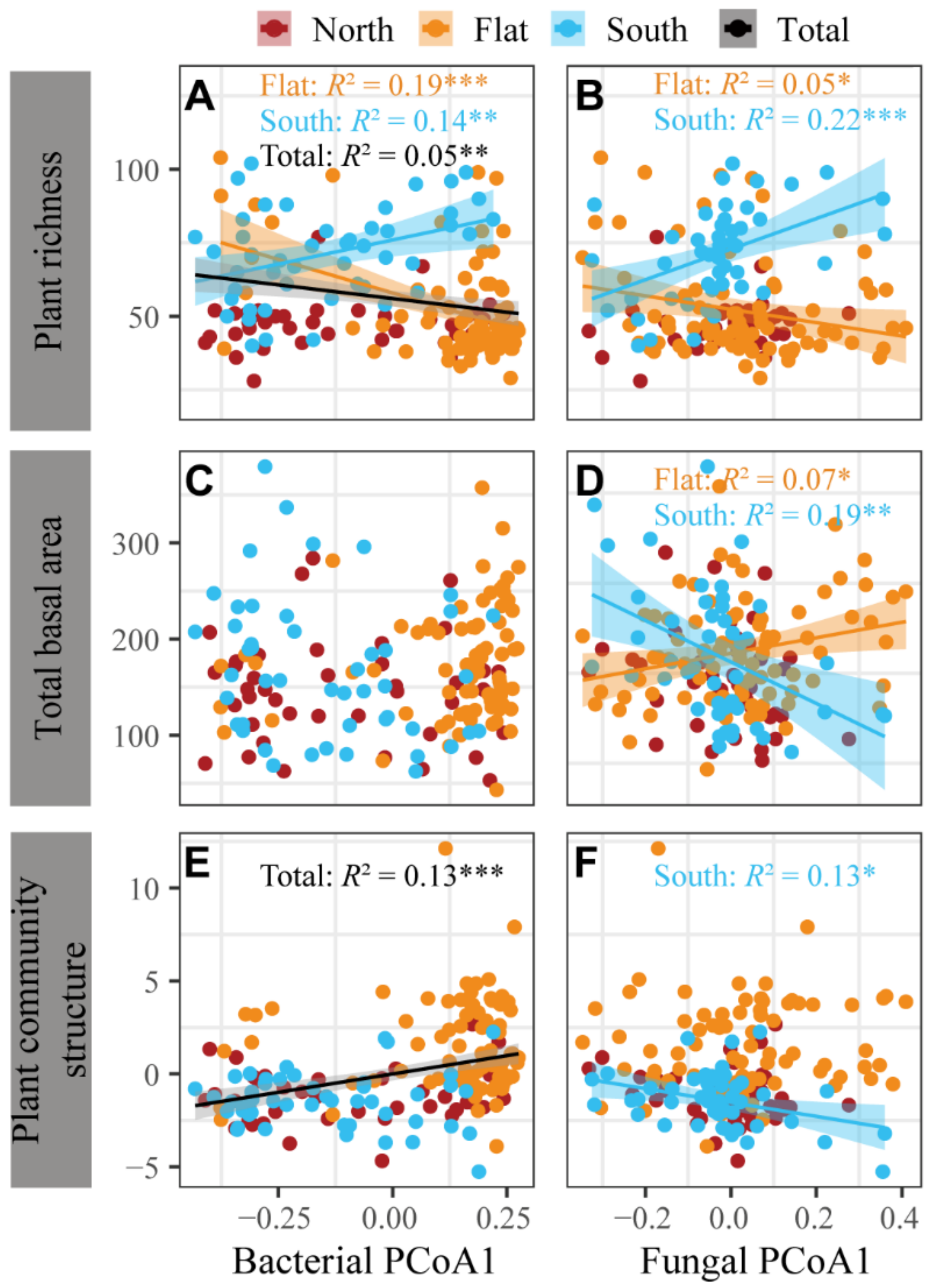
3.4. Correlations between Plant Community and Soil Bacteria and Fungi across the Sample Sites
4. Discussion
4.1. Differentiation of Aboveground Plants and Belowground Soil Bacteria and Soil Fungi Caused by Slope Aspects
4.2. Effect of Slope Aspect on the Relationships between Aboveground Plants and Belowground Soil Bacteria and Soil Fungi
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bakker, M.G.; Bradeen, J.M.; Kinkel, L.L. Effects of Plant Host Species and Plant Community Richness on Streptomycete Community Structure. FEMS Microbiol. Ecol. 2013, 83, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.; Wolf, A.B.; Jennings, S.J.; Kowalchuk, G.A. Micro-Scale Determinants of Bacterial Diversity in Soil. FEMS Microbiol. Rev. 2013, 37, 936–954. [Google Scholar] [CrossRef]
- van der Putten, W.H.; Bradford, M.A.; Pernilla Brinkman, E.; van de Voorde, T.F.J.; Veen, G.F. Where, When and How Plant-Soil Feedback Matters in a Changing World. Funct. Ecol. 2016, 30, 1109–1121. [Google Scholar] [CrossRef]
- Krashevska, V.; Klarner, B.; Widyastuti, R.; Maraun, M.; Scheu, S. Impact of Tropical Lowland Rainforest Conversion into Rubber and Oil Palm Plantations on Soil Microbial Communities. Biol. Fertil. Soils 2015, 51, 697–705. [Google Scholar] [CrossRef]
- Deng, Q.; Cheng, X.; Hui, D.; Zhang, Q.; Li, M.; Zhang, Q. Soil Microbial Community and Its Interaction with Soil Carbon and Nitrogen Dynamics Following Afforestation in Central China. Sci. Total Environ. 2016, 541, 230–237. [Google Scholar] [CrossRef]
- Hagedorn, F.; Gavazov, K.; Alexander, J.M. Above- and Belowground Linkages Shape Responses of Mountain Vegetation to Climate Change. Science 2019, 365, 1119–1123. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Zobel, M. How Mycorrhizal Associations Drive Plant Population and Community Biology. Science 2020, 367, eaba1223. [Google Scholar] [CrossRef]
- Van der Putten, W.H.; Vet, L.E.M.; Harvey, J.A.; Wäckers, F.L. Linking Above- and Belowground Multitrophic Interactions of Plants, Herbivores, Pathogens, and Their Antagonists. Trends Ecol. Evol. 2001, 16, 547–554. [Google Scholar] [CrossRef]
- Bezemer, T.M.; De Deyn, G.B.; Bossinga, T.M.; Van Dam, N.M.; Harvey, J.A.; Van der Putten, W.H. Soil Community Composition Drives Aboveground Plant-Herbivore-Parasitoid Interactions. Ecol. Lett. 2005, 8, 652–661. [Google Scholar] [CrossRef]
- Latz, E.; Eisenhauer, N.; Rall, B.C.; Allan, E.; Roscher, C.; Scheu, S.; Jousset, A. Plant Diversity Improves Protection against Soil-Borne Pathogens by Fostering Antagonistic Bacterial Communities. J. Ecol. 2012, 100, 597–604. [Google Scholar] [CrossRef]
- Ushio, M.; Wagai, R.; Balser, T.C.; Kitayama, K. Variations in the Soil Microbial Community Composition of a Tropical Montane Forest Ecosystem: Does Tree Species Matter? Soil Biol. Biochem. 2008, 40, 2699–2702. [Google Scholar] [CrossRef]
- Bainard, L.D.; Hamel, C.; Gan, Y. Edaphic Properties Override the Influence of Crops on the Composition of the Soil Bacterial Community in a Semiarid Agroecosystem. Appl. Soil Ecol. 2016, 105, 160–168. [Google Scholar] [CrossRef]
- Schlatter, D.C.; Bakker, M.G.; Bradeen, J.M.; Kinkel, L.L. Plant Community Richness and Microbial Interactions Structure Bacterial Communities in Soil. Ecology 2015, 96, 134–142. [Google Scholar] [CrossRef]
- Lyons, C.L.; Lindo, Z. Above- and Belowground Community Linkages in Boreal Peatlands. Plant Ecol. 2020, 221, 615–632. [Google Scholar] [CrossRef]
- Broughton, L.C.; Gross, K.L. Patterns of Diversity in Plant and Soil Microbial Communities along a Productivity Gradient in a Michigan Old-Field. Oecologia 2000, 125, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Van der Heijden, M.G.A.; Bardgett, R.D.; Van Straalen, N.M. The Unseen Majority: Soil Microbes as Drivers of Plant Diversity and Productivity in Terrestrial Ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, N.; Beßler, H.; Engels, C.; Gleixner, G.; Habekost, M.; Milcu, A.; Partsch, S.; Sabais, A.C.W.; Scherber, C.; Steinbeiss, S.; et al. Plant Diversity Effects on Soil Microorganisms Support the Singular Hypothesis. Ecology 2010, 91, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Sauheitl, L.; Glaser, B.; Dippold, M.; Leiber, K.; Weigelt, A. Amino Acid Fingerprint of a Grassland Soil Reflects Changes in Plant Species Richness. Plant Soil 2010, 334, 353–363. [Google Scholar] [CrossRef]
- Santonja, M.; Rancon, A.; Fromin, N.; Baldy, V.; Hättenschwiler, S.; Fernandez, C.; Montès, N.; Mirleau, P. Plant Litter Diversity Increases Microbial Abundance, Fungal Diversity, and Carbon and Nitrogen Cycling in a Mediterranean Shrubland. Soil Biol. Biochem. 2017, 111, 124–134. [Google Scholar] [CrossRef]
- Otsing, E.; Barantal, S.; Anslan, S.; Koricheva, J.; Tedersoo, L. Litter Species Richness and Composition Effects on Fungal Richness and Community Structure in Decomposing Foliar and Root Litter. Soil Biol. Biochem. 2018, 125, 328–339. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, K.; Wurzburger, N.; Zhang, J. Relationships between Plant Diversity and Soil Microbial Diversity Vary across Taxonomic Groups and Spatial Scales. Ecosphere 2020, 11, e02999. [Google Scholar] [CrossRef]
- Zak, D.R.; Holmes, W.E.; White, D.C.; Peacock, A.D.; Tilman, D. Plant Diversity, Soil Microbial Communities, and Ecosystem Function: Are There Any Links? Ecology 2003, 84, 2042–2050. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Liang, C.; Hao, Z.; Zhou, L.; Ma, S.; Li, X.; Yang, S.; Yao, F.; Jiang, Y. Aboveground-Belowground Biodiversity Linkages Differ in Early and Late Successional Temperate Forests. Sci. Rep. 2015, 5, 12234. [Google Scholar] [CrossRef]
- Li, H.; Xu, Z.; Yan, Q.; Yang, S.; Van Nostrand, J.D.; Wang, Z.; He, Z.; Zhou, J.; Jiang, Y.; Deng, Y. Soil Microbial Beta-Diversity Is Linked with Compositional Variation in Aboveground Plant Biomass in a Semi-Arid Grassland. Plant Soil 2018, 423, 465–480. [Google Scholar] [CrossRef]
- Uroz, S.; Buée, M.; Deveau, A.; Mieszkin, S.; Martin, F. Ecology of the Forest Microbiome: Highlights of Temperate and Boreal Ecosystems. Soil Biol. Biochem. 2016, 103, 471–488. [Google Scholar] [CrossRef]
- Chen, L.; Swenson, N.G.; Ji, N.; Mi, X.; Ren, H.; Guo, L.; Ma, K. Differential Soil Fungus Accumulation and Density Dependence of Trees in a Subtropical Forest. Science 2019, 366, 124–128. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, M.G.A.; Klironomos, J.N.; Ursic, M.; Moutoglis, P.; Streitwolf-Engel, R.; Boller, T.; Wiemken, A.; Sanders, I.R. Mycorrhizal Fungal Diversity Determines Plant Biodiversity, Ecosystem Variability and Productivity. Nature 1998, 396, 69–72. [Google Scholar] [CrossRef]
- Chen, L.; Xiang, W.; Wu, H.; Ouyang, S.; Zhou, B.; Zeng, Y.; Chen, Y.; Kuzyakov, Y. Tree Species Identity Surpasses Richness in Affecting Soil Microbial Richness and Community Composition in Subtropical Forests. Soil Biol. Biochem. 2019, 130, 113–121. [Google Scholar] [CrossRef]
- Qiu, H.; Ge, T.; Liu, J.; Chen, X.; Hu, Y.; Wu, J.; Su, Y.; Kuzyakov, Y. Effects of Biotic and Abiotic Factors on Soil Organic Matter Mineralization: Experiments and Structural Modeling Analysis. Eur. J. Soil Biol. 2018, 84, 27–34. [Google Scholar] [CrossRef]
- Qiang, W.; He, L.; Zhang, Y.; Liu, B.; Liu, Y.; Liu, Q.; Pang, X. Aboveground Vegetation and Soil Physicochemical Properties Jointly Drive the Shift of Soil Microbial Community during Subalpine Secondary Succession in Southwest China. Catena 2021, 202, 105251. [Google Scholar] [CrossRef]
- Cantón, Y.; Del Barrio, G.; Solé-Benet, A.; Lázaro, R. Topographic Controls on the Spatial Distribution of Ground Cover in the Tabernas Badlands of SE Spain. Catena 2004, 55, 341–365. [Google Scholar] [CrossRef]
- Lexer, M.J.; Bugmann, H. Mountain Forest Management in a Changing World. Eur. J. For. Res. 2017, 136, 981–982. [Google Scholar] [CrossRef]
- Wu, J.; Anderson, B.J.; Buckley, H.L.; Lewis, G.; Lear, G. Aspect Has a Greater Impact on Alpine Soil Bacterial Community Structure than Elevation. FEMS Microbiol. Ecol. 2017, 93, fiw253. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Jiang, S.; Guo, W.; Qin, M.; Pan, J.; Bahadur, A.; Shi, G.; Luo, J.; Jin, Z.; Liu, Y.; et al. The Effect of Slope Aspect on the Phylogenetic Structure of Arbuscular Mycorrhizal Fungal Communities in an Alpine Ecosystem. Soil Biol. Biochem. 2018, 126, 103–113. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Lu, J.; Chen, W.; Wei, G.; Lin, Y. Topography Affects the Soil Conditions and Bacterial Communities along a Restoration Gradient on Loess-Plateau. Appl. Soil Ecol. 2020, 150, 103471. [Google Scholar] [CrossRef]
- Chu, H.; Xiang, X.; Yang, J.; Adams, J.M.; Zhang, K.; Li, Y.; Shi, Y. Effects of Slope Aspects on Soil Bacterial and Arbuscular Fungal Communities in a Boreal Forest in China. Pedosphere 2016, 26, 226–234. [Google Scholar] [CrossRef]
- Måren, I.E.; Karki, S.; Prajapati, C.; Yadav, R.K.; Shrestha, B.B. Facing North or South: Does Slope Aspect Impact Forest Stand Characteristics and Soil Properties in a Semiarid Trans-Himalayan Valley? J. Arid Environ. 2015, 121, 112–123. [Google Scholar] [CrossRef]
- Mitchell, R.J.; Hester, A.J.; Campbell, C.D.; Chapman, S.J.; Cameron, C.M.; Hewison, R.L.; Potts, J.M. Explaining the Variation in the Soil Microbial Community: Do Vegetation Composition and Soil Chemistry Explain the Same or Different Parts of the Microbial Variation? Plant Soil 2012, 351, 355–362. [Google Scholar] [CrossRef]
- Gilliam, F.S.; Hédl, R.; Chudomelová, M.; McCulley, R.L.; Nelson, J.A. Variation in Vegetation and Microbial Linkages with Slope Aspect in a Montane Temperate Hardwood Forest. Ecosphere 2014, 5, 1–17. [Google Scholar] [CrossRef]
- Bennie, J.; Hill, M.O.; Baxter, R.; Huntley, B. Influence of Slope and Aspect on Long-Term Vegetation Change in British Chalk Grasslands. J. Ecol. 2006, 94, 355–368. [Google Scholar] [CrossRef]
- Åström, M.; Dynesius, M.; Hylander, K.; Nilsson, C. Slope Aspect Modifies Community Responses to Clear-Cutting in Boreal Forests. Ecology 2007, 88, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Nadal-Romero, E.; Petrlic, K.; Verachtert, E.; Bochet, E.; Poesen, J. Effects of Slope Angle and Aspect on Plant Cover and Species Richness in a Humid Mediterranean Badland. Earth Surf. Process. Landf. 2014, 39, 1705–1716. [Google Scholar] [CrossRef]
- Liu, M.; Zheng, R.; Bai, S.; Bai, Y.; Wang, J. Slope Aspect Influences Arbuscular Mycorrhizal Fungus Communities in Arid Ecosystems of the Daqingshan Mountains, Inner Mongolia, North China. Mycorrhiza 2017, 27, 189–200. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Liu, Q. Ecological Research on Subalpine Coniferous Forests in China; Sichuan University Press: Chengdu, China, 2002. [Google Scholar]
- Xie, L.; Yin, C. Seasonal Variations of Soil Fungal Diversity and Communities in Subalpine Coniferous and Broadleaved Forests. Sci. Total Environ. 2022, 846, 157409. [Google Scholar] [CrossRef]
- Taylor, A.H.; Zisheng, Q.; Jie, L. Structure and Dynamics of Subalpine Forests in the Wang Lang Natural Reserve, Sichuan, China. Vegetatio 1996, 124, 25–38. [Google Scholar] [CrossRef]
- Xiong, X.; Zhu, J.; Li, S.; Fan, F.; Cai, Q.; Ma, S.; Su, H.; Ji, C.; Tang, Z.; Fang, J. Aboveground Biomass and Its Biotic and Abiotic Modulators of a Main Food Bamboo of the Giant Panda in a Subalpine Spruce-Fir Forest in Southwestern China. J. Plant Ecol. 2022, 15, 1–12. [Google Scholar] [CrossRef]
- Condit, R. Tropical Forest Census Plots: Methods and Results from Barro Colorado Island, Panama and a Comparison with Other Plots; Springer: Berlin, Germany, 1998. [Google Scholar]
- Lu, R.K. Soil and Agricultural Chemistry Analysis Method; China Agriculture Science and Technology Press: Beijing, China, 2002. [Google Scholar]
- Peng, X.; Wang, W. Stoichiometry of Soil Extracellular Enzyme Activity along a Climatic Transect in Temperate Grasslands of Northern China. Soil Biol. Biochem. 2016, 98, 74–84. [Google Scholar] [CrossRef]
- Hu, L.; Xiang, Z.; Wang, G.; Rafique, R.; Liu, W.; Wang, C. Changes in Soil Physicochemical and Microbial Properties along Elevation Gradients in Two Forest Soils. Scand. J. For. Res. 2016, 31, 242–253. [Google Scholar] [CrossRef]
- Li, Y.C.; Li, Z.; Li, Z.W.; Jiang, Y.H.; Weng, B.Q.; Lin, W.X. Variations of Rhizosphere Bacterial Communities in Tea (Camellia sinensis L.) Continuous Cropping Soil by High-Throughput Pyrosequencing Approach. J. Appl. Microbiol. 2016, 121, 787–799. [Google Scholar] [CrossRef]
- Landlinger, C.; Bašková, L.; Preuner, S.; Willinger, B.; Buchta, V.; Lion, T. Identification of Fungal Species by Fragment Length Analysis of the Internally Transcribed Spacer 2 Region. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet. J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.I.; Miyata, T. MAFFT: A Novel Method for Rapid Multiple Sequence Alignment Based on Fast Fourier Transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. Fasttree: Computing Large Minimum Evolution Trees with Profiles Instead of a Distance Matrix. Mol. Biol. Evol. 2009, 26, 1641–1650. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing Taxonomic Classification of Marker-Gene Amplicon Sequences with QIIME 2’s Q2-Feature-Classifier Plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Kõljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.S.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M.; et al. Towards a Unified Paradigm for Sequence-Based Identification of Fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Xue, R.; Yang, Q.; Miao, F.; Wang, X.; Shen, Y. Slope Aspect Influences Plant Biomass, Soil Properties and Microbial Composition in Alpine Meadow on the Qinghai-Tibetan Plateau. J. Soil Sci. Plant Nutr. 2018, 18, 1–12. [Google Scholar] [CrossRef]
- He, X.; Zhou, J.; Wu, Y.; Bing, H.; Sun, H.; Wang, J. Leaching Disturbed the Altitudinal Distribution of Soil Organic Phosphorus in Subalpine Coniferous Forests on Mt. Gongga, SW China. Geoderma 2018, 326, 144–155. [Google Scholar] [CrossRef]
- Liu, B.; Biswas, S.R.; Yang, J.; Liu, Z.; He, H.S.; Liang, Y.; Lau, M.K.; Fang, Y.; Han, S. Strong Influences of Stand Age and Topography on Post-Fire Understory Recovery in a Chinese Boreal Forest. For. Ecol. Manag. 2020, 473, 118307. [Google Scholar] [CrossRef]
- Carletti, P.; Vandramin, E.; Pizzeghello, D.; Concheri, G.; Zenella, A.; Nardi, S.; Squartini, A. Soil Humic Compounds and Microbial Communities in Six Spruce Forests as Function of Parent Material, Slope Aspect and Stand Age. Plant Soil 2009, 315, 47–65. [Google Scholar] [CrossRef]
- Geisen, S. The Bacterial-Fungal Energy Channel Concept Challenged by Enormous Functional Versatility of Soil Protists. Soil Biol. Biochem. 2016, 102, 22–25. [Google Scholar] [CrossRef]



| Variable | North-Facing Slope | Flat Site | South-Facing Slope | Total |
|---|---|---|---|---|
| Soil pH | 6.67 ± 0.67 a | 7.36 ± 0.5 c | 7.00 ± 0.49 b | 6.99 ± 0.05 |
| Soil water content (%) | 62.51 ± 0.07 c | 54.63 ± 0.11 a | 57.45 ± 0.04 b | 56.81 ± 0.01 |
| Soil total P (g kg−1) | 1.07 ± 0.24 b | 0.69 ± 0.18 a | 1.00 ± 0.19 b | 0.87 ± 0.02 |
| Soil-available P (mg g−1) | 28.57 ± 10.12 b | 18.17 ± 10.5 a | 28.24 ± 9.19 b | 23.52 ± 0.86 |
| Soil total N (g kg−1) | 8.54 ± 2.37 b | 5.62 ± 2.48 a | 8.71 ± 2.04 b | 7.20 ± 0.21 |
| Soil-available N (g kg−1) | 1.04 ± 0.26 b | 0.79 ± 0.35 a | 1.34 ± 0.39 c | 1.00 ± 0.03 |
| Soil organic matter (g kg−1) | 222.14 ± 63.73 b | 155.16 ± 52.16 a | 238.09 ± 62.66 b | 194.64 ± 5.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, L.; Ma, S.; Zhu, B.; Ji, C. Soil Bacteria and Soil Fungi Respond Differently to the Changes in Aboveground Plants along Slope Aspect in a Subalpine Coniferous Forest. Forests 2023, 14, 1389. https://doi.org/10.3390/f14071389
He L, Ma S, Zhu B, Ji C. Soil Bacteria and Soil Fungi Respond Differently to the Changes in Aboveground Plants along Slope Aspect in a Subalpine Coniferous Forest. Forests. 2023; 14(7):1389. https://doi.org/10.3390/f14071389
Chicago/Turabian StyleHe, Luoshu, Suhui Ma, Biao Zhu, and Chengjun Ji. 2023. "Soil Bacteria and Soil Fungi Respond Differently to the Changes in Aboveground Plants along Slope Aspect in a Subalpine Coniferous Forest" Forests 14, no. 7: 1389. https://doi.org/10.3390/f14071389
APA StyleHe, L., Ma, S., Zhu, B., & Ji, C. (2023). Soil Bacteria and Soil Fungi Respond Differently to the Changes in Aboveground Plants along Slope Aspect in a Subalpine Coniferous Forest. Forests, 14(7), 1389. https://doi.org/10.3390/f14071389





