Effects of Ficus carica L. Water Extract on Taxus cuspidata Sieb. et Zucc. Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Reagents
2.2. Effects of Extracts from Different Parts of F. carica on the Growth of T. cuspidata
2.2.1. Sample Collection and Processing
2.2.2. Preparation of Water Extracts from Different Parts of F. carica
2.2.3. Pot Experiment of T. cuspidata Irrigated with Water Extracts from Different Parts of F. carica
2.3. Effects of F. carica Root Water Extract on the Growth of T. cuspidata
2.3.1. Preparation of F. carica Root Water Extract
2.3.2. Irrigation of T. cuspidata with Water Extract of F. carica Root
2.3.3. Determination of Plant Height and Base Diameter of T. cuspidata
2.3.4. Determination of Gas Exchange Parameters
2.3.5. Determination of Chlorophyll Contents
- C: Chlorophyll concentration (mg·L−1);
- V: Total extract volume (mL);
- M: Fresh leaf weight (g).
2.3.6. Determination of Protective Enzyme Activity and MDA Contents
2.4. Effects of F. carica Root Extract on Soil Properties of T. cuspidata
2.4.1. Collection of Soil Samples
2.4.2. Measurement of Soil Physicochemical Property
2.4.3. Determination of Soil Enzyme Activity
2.4.4. Determination of Soil Microbial Diversity
2.5. Identification of Potential Chemicals in Water Extract of T. cuspidata
2.6. Statistical Analysis
3. Results
3.1. Effects of Extracts from Different Parts of F. carica on the Growth of T. cuspidata
3.2. Effect of F. carica Water Extract on the Growth of T. cuspidata
3.2.1. Effect of F. carica Water Extract on the Plant Height and Base Diameter of T. cuspidata
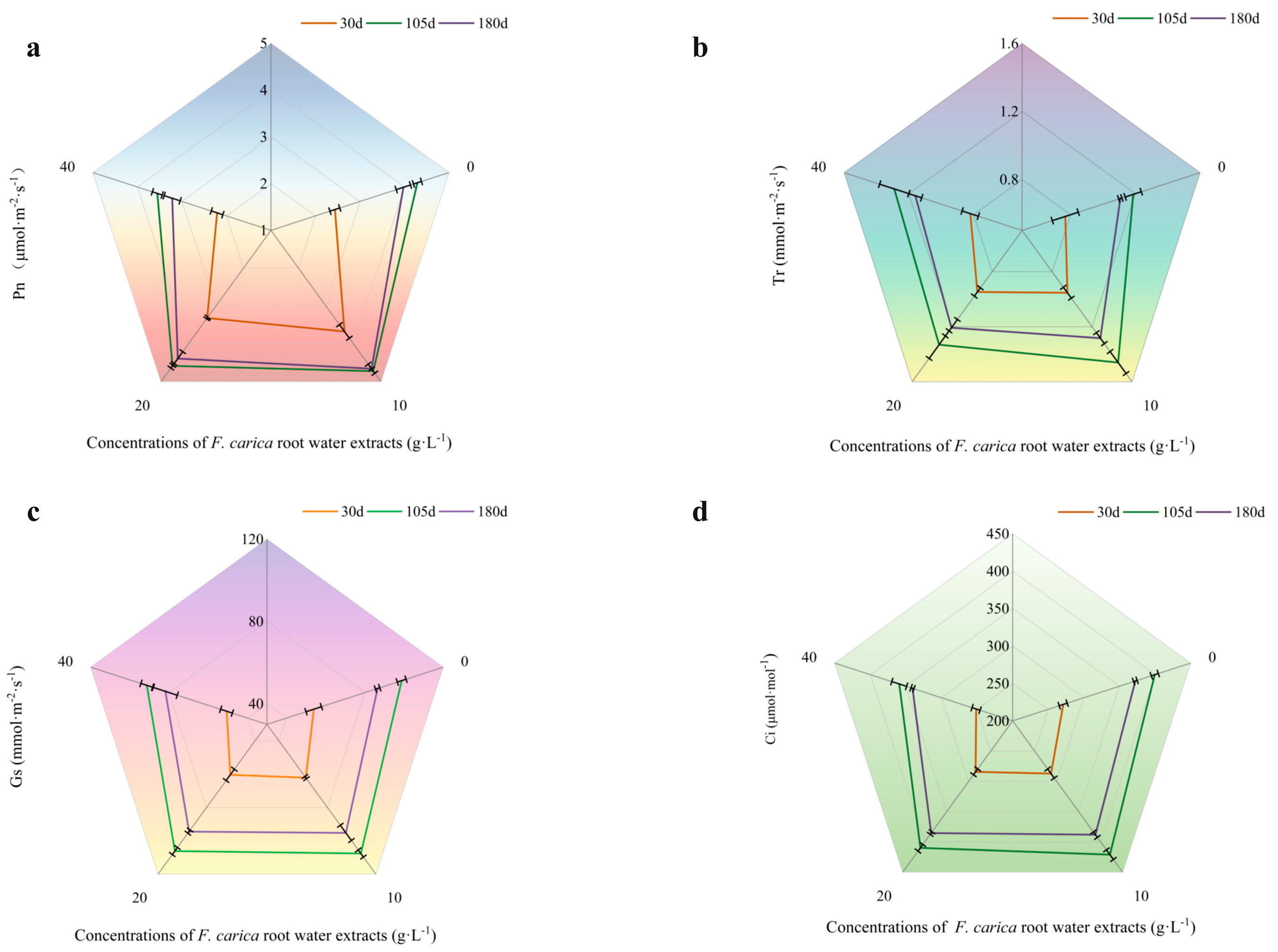
3.2.2. Effects of F. carica Water Extract on Gas Exchange Parameters of T. cuspidata
3.2.3. Effect of F. carica Water Extract on Chlorophyll Contents of T. cuspidata
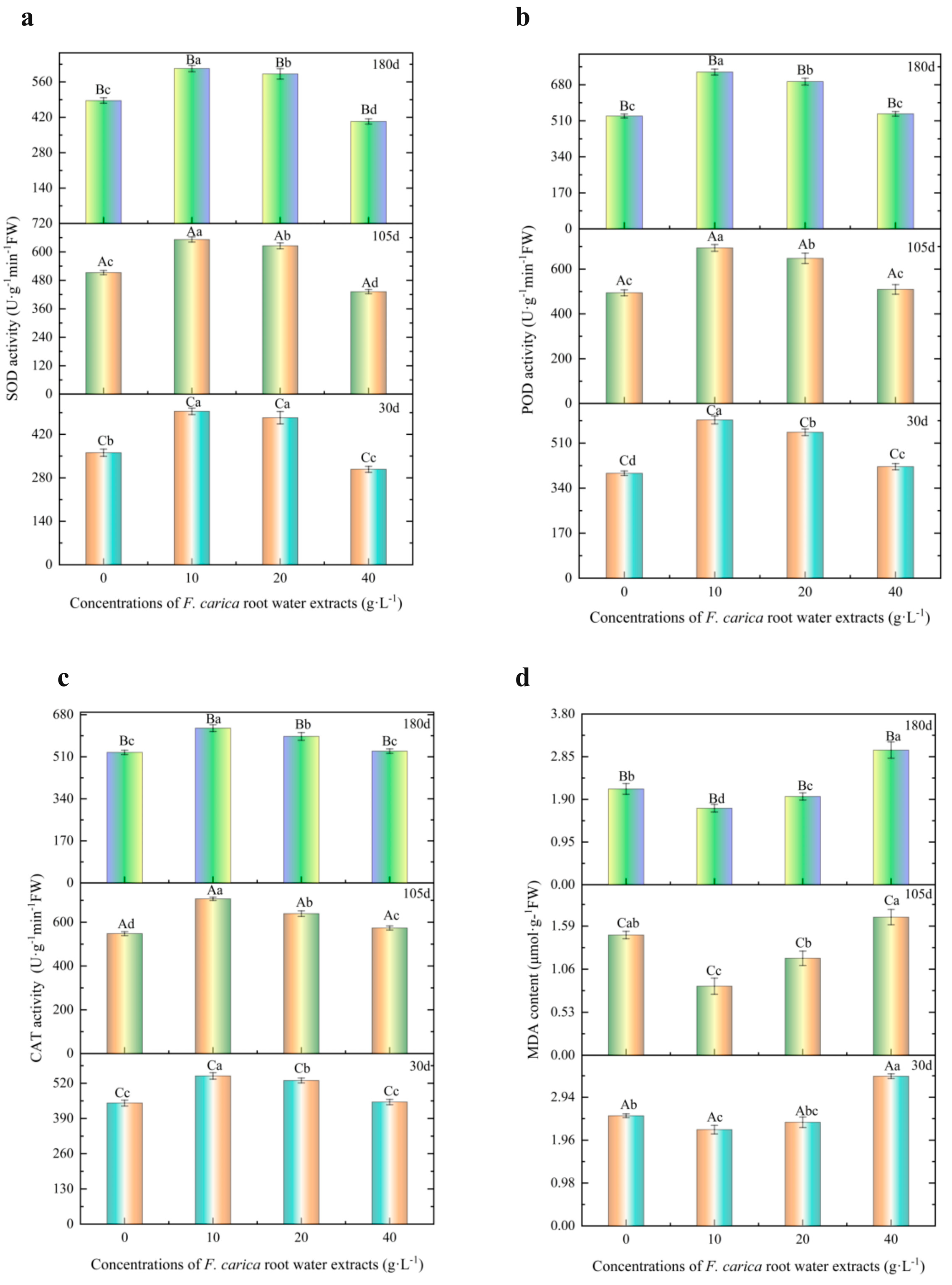
3.2.4. Effects of F. carica Water Extract on Enzymatic Activity and Malondialdehyde Contents of T. cuspidata
3.3. Effects of F. carica Water Extract on Soil Properties of T. cuspidata
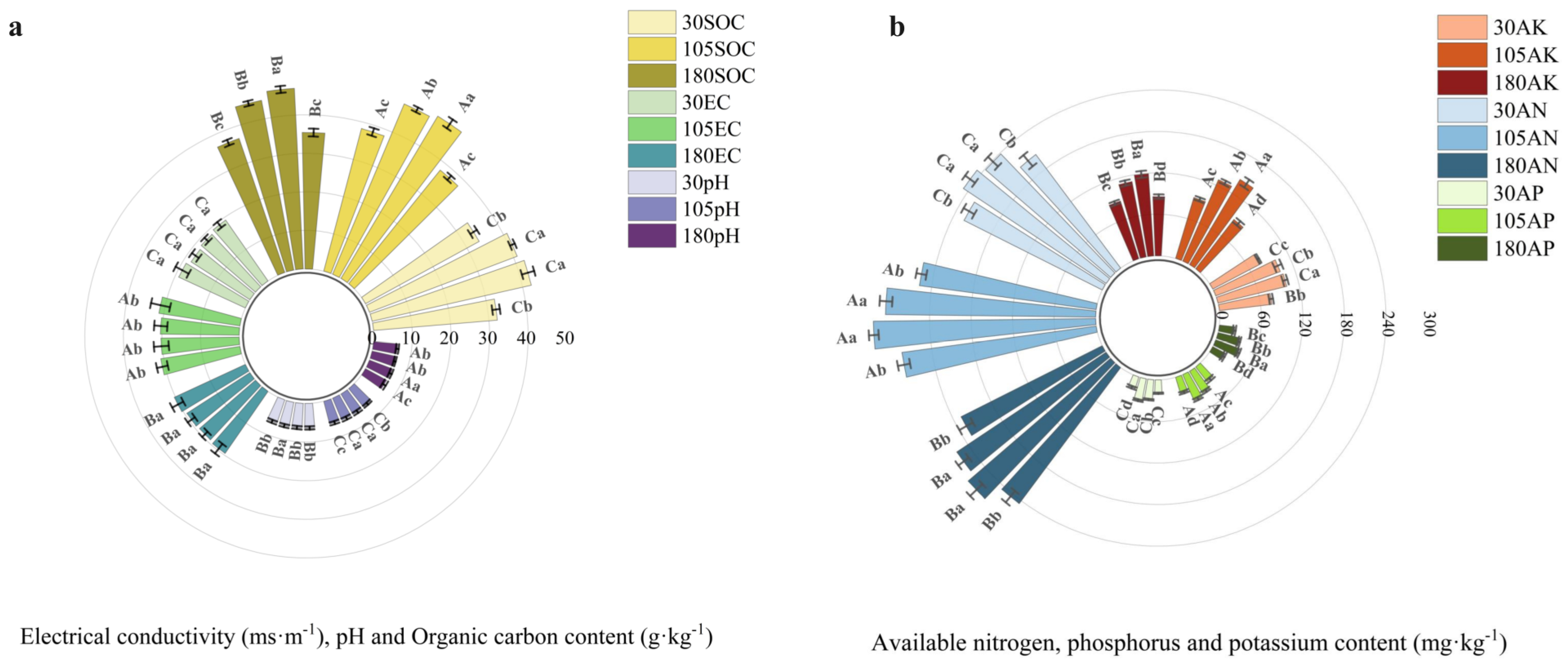
3.3.1. Changes in Soil Physicochemical Properties
3.3.2. Changes in Soil Enzymatic Activities
3.3.3. Effect of Water Extract on Soil Microbial Community
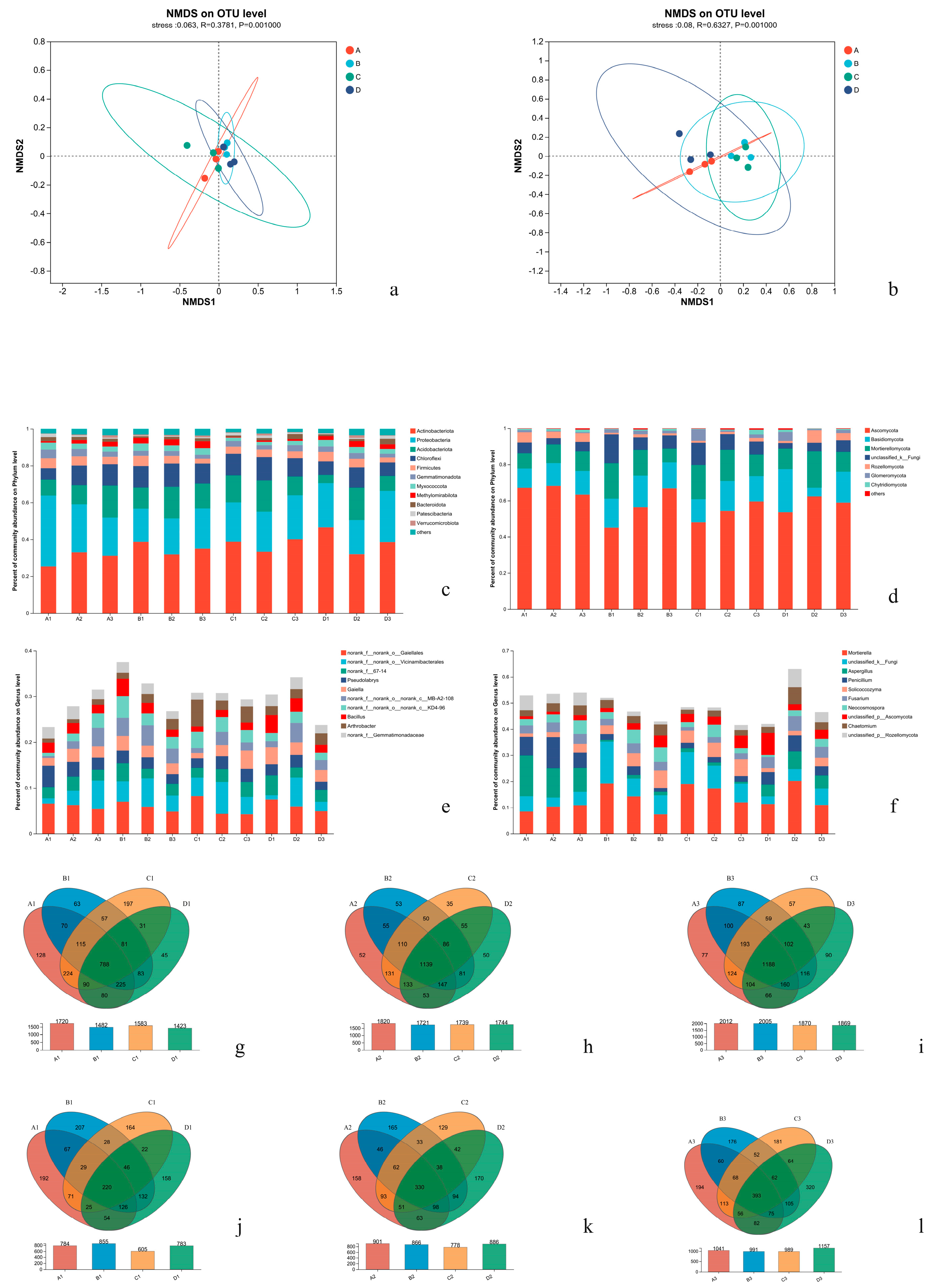
Effects of Different Treatments on α Diversity of Soil Microorganisms
Effects of Different Treatments on β Diversity of Soil Microorganisms
Effects of Different Treatments on Soil Microbial Community Composition and Abundance
3.4. Determination of Potential Growth-Promoting Chemicals in Extracts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hazrati, R.; Zare, N.; Asghari, R.; Sheikhzadeh, P.; Johari-Ahar, M. Biologically synthesized CuO nanoparticles induce physiological, metabolic, and molecular changes in the hazel cell cultures. Appl. Microbiol. Biotechnol. 2022, 106, 6017–6031. [Google Scholar] [CrossRef]
- White, H.B.; Batcheller, G.R.; Boggess, E.K.; Brown, C.L.; Butfiloski, J.W.; Decker, T.A.; Erb, J.D.; Fall, M.W.; Hamilton, D.A.; Hiller, T.L.; et al. Best Management Practices for Trapping Furbearers in the United States. Wildl. Monogr. 2021, 207, 3–59. [Google Scholar] [CrossRef]
- Morovati, M.R.; Ghanbari-Movahed, M.; Barton, E.M.; Farzaei, M.H.; Bishayee, A. A systematic review on potential anticancer activities of Ficus carica L. with focus on cellular and molecular mechanisms. Phytomedicine 2022, 105, 154333. [Google Scholar] [CrossRef]
- Chen, H.; Geekiyanage, N.; Wen, B.; Cao, K.-F.; Goodale, U.M. Regeneration responses to water and temperature stress drive recruitment success in hemiepiphytic fig species. Tree Physiol. 2021, 41, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; Li, C.; Li, Q.; Qiao, B.; Shi, S.; Zhao, C. Enhancement of Interplanting of Ficus carica L. with Taxus cuspidata Sieb. et Zucc. on Growth of Two Plants. Agriculture 2021, 11, 1276. [Google Scholar] [CrossRef]
- Molisch, H. Der Einfluss Einer Pflanze auf die Andere Allelopathie; Gustav Fischer Verlag: Jena, Germany, 1937. [Google Scholar]
- Hierro, J.L.; Callaway, R.M. The Ecological Importance of Allelopathy. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 25–45. [Google Scholar] [CrossRef]
- Wu, J.W.; Li, F.L.; Yao, S.K.; Zhao, Z.Y.; Feng, X.; Chen, R.Z.; Xu, Y.Q. Iva xanthiifolia leaf extract reduced the diversity of indigenous plant rhizosphere bacteria. BMC Plant Biol. 2023, 23, 297. [Google Scholar] [CrossRef]
- Li, L.L.; Zhao, H.H.; Kong, C.H. (-)-Loliolide, the most ubiquitous lactone, is involved in barnyardgrass-induced rice allelopathy. J. Exp. Bot. 2020, 71, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Bolouri, P.; Salami, R.; Kouhi, S.; Kordi, M.; Lajayer, B.A.; Hadian, J.; Astatkie, T. Applications of Essential Oils and Plant Extracts in Different Industries. Molecules 2022, 27, 8999. [Google Scholar] [CrossRef]
- Wang, C.Y.; Li, L.L.; Meiners, S.J.; Kong, C.H. Root placement patterns in allelopathic plant–plant interactions. New Phytol. 2023, 237, 563–575. [Google Scholar] [CrossRef]
- Pouresmaeil, M.; Nojadeh, M.S.; Movafeghi, A.; Maggi, F. Exploring the bio-control efficacy of Artemisia fragrans essential oil on the perennial weed Convolvulus arvensis: Inhibitory effects on the photosynthetic machinery and induction of oxidative stress. Ind. Crops Prod. 2020, 155, 112785. [Google Scholar] [CrossRef]
- Guo, J.; Bao, G.; Yang, Y.; Xi, J.; Zhang, X.; Pan, X.; Zhao, H.; Li, G.; Fan, C. Impact of repeated freeze-thaw cycles environment on the allelopathic effect to Secale cereale L. seedlings. Chemosphere 2022, 308, 136476. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, Y.; Song, X.; Jiang, M.; Zhao, X.; Cao, X. The inhibitory effects of simulated light sources on the activity of algae cannot be ignored in photocatalytic inhibition. Chemosphere 2022, 309, 136611. [Google Scholar] [CrossRef] [PubMed]
- Randé, H.; Michalet, R.; Nemer, D.; Sappin-Didier, V.; Delerue, F. Contrasting soil-and canopy-nurse effects in metalliferous systems may be explained by dominant plant functional strategies. J. Appl. Ecol. 2022, 60, 278–290. [Google Scholar] [CrossRef]
- Guan, B.; Xie, B.; Yang, S.; Hou, A.; Chen, M.; Han, G. Effects of five years’ nitrogen deposition on soil properties and plant growth in a salinized reed wetland of the Yellow River Delta. Ecol. Eng. 2019, 136, 160–166. [Google Scholar] [CrossRef]
- Ablimit, R.; Li, W.; Zhang, J.; Gao, H.; Zhao, Y.; Cheng, M.; Meng, X.; An, L.; Chen, Y. Altering microbial community for improving soil properties and agricultural sustainability during a 10-year maize-green manure intercropping in Northwest China. J. Environ. Manag. 2022, 321, 115859. [Google Scholar] [CrossRef]
- Williams, A.; Langridge, H.; Straathof, A.L.; Fox, G.; Muhammadali, H.; Hollywood, K.A.; Xu, Y.; Goodacre, R.; de Vries, F.T. Comparing root exudate collection techniques: An improved hybrid method. Soil Biol. Biochem. 2021, 161, 108391. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, L.L. Using an IR camera to improve leaf area and temperature measurements: A new method for increasing the accuracy of photosynthesis-related parameters. Agric. For. Meteorol. 2022, 322, 109005. [Google Scholar] [CrossRef]
- Abbasvand, E.; Hassannejad, S. Reduce the Adverse Effects of Dodder on Sweet Basil by Seed Priming with Salicylic Acid (SA) and Sown in Residues of Syrian Bean-Caper. J. Plant Growth Regul. 2022, 42, 2763–2775. [Google Scholar] [CrossRef]
- Zulfa, A.W.; Norizah, K.; Hamdan, O.; Zulkifly, S.; Faridah-Hanum, I.; Rhyma, P.P. Discriminating trees species from the relationship between spectral reflectance and chlorophyll contents of mangrove forest in Malaysia. Ecol. Indic. 2020, 111, 106024. [Google Scholar] [CrossRef]
- Abdelkader, M.; Geioushy, R.A.; Fouad, O.A.; Khaled, A.G.A.; Liudmila, P.V. Investigation the activities of photosynthetic pigments, antioxidant enzymes and inducing genotoxicity of cucumber seedling exposed to copper oxides nanoparticles stress. Sci. Hortic. 2022, 305, 111364. [Google Scholar] [CrossRef]
- Huang, T.; Yang, N.; Lu, C.; Qin, X.; Siddique, K.H.M. Soil organic carbon, total nitrogen, available nutrients, and yield under different straw returning methods. Soil Tillage Res. 2021, 214, 105171. [Google Scholar] [CrossRef]
- Raya-Moreno, I.; Canizares, R.; Domene, X.; Carabassa, V.; Alcaniz, J.M. Comparing current chemical methods to assess biochar organic carbon in a Mediterranean agricultural soil amended with two different biochars. Sci. Total. Environ. 2017, 598, 604–618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ai, N.; Liu, G.; Liu, C.; Qiang, F. Soil quality evaluation of various microtopography types at different restoration modes in the loess area of Northern Shaanxi. Catena 2021, 207, 105633. [Google Scholar] [CrossRef]
- Shen, L.; Wang, C.; Chen, J. Photometric determination of the activity of cellulase and xylanase via measurement of formation of gold nanoparticles. Microchim. Acta 2017, 184, 163–168. [Google Scholar] [CrossRef]
- Shi, S.; Gao, Y.; Li, C.; Zhang, J.; Guan, J.; Zhao, C.; Fu, Y. Allelopathy of Taxus chinensis var. mairei on Camptotheca acuminata seedling growth and identification of the active principles. J. Plant Interact. 2021, 17, 33–42. [Google Scholar] [CrossRef]
- Xin, J.; Zhao, C.; Li, Y.; Ma, S.; Tian, R. Transcriptional, secondary metabolic, and antioxidative investigations elucidate the rapid response mechanism of Pontederia cordata to cadmium. Ecotoxicol. Environ. Saf. 2022, 232, 113236. [Google Scholar] [CrossRef] [PubMed]
- Schlüter, U.; Bräutigam, A.; Gowik, U.; Melzer, M.; Christin, P.-A.; Kurz, S.; Mettler-Altmann, T.; Weber, A.P.M. Photosynthesis in C3–C4 intermediate Moricandia species. J. Exp. Bot. 2017, 68, 191–206. [Google Scholar] [CrossRef]
- Xu, M.; Liu, R.; Chen, J.M.; Shang, R.; Liu, Y.; Qi, L.; Croft, H.; Ju, W.; Zhang, Y.; He, Y.; et al. Retrieving global leaf chlorophyll content from MERIS data using a neural network method. ISPRS J. Photogramm. Remote Sens. 2022, 192, 66–82. [Google Scholar] [CrossRef]
- Lyu, M.; Liu, J.; Xu, X.; Liu, C.; Qin, H.; Zhang, X.; Tian, G.; Jiang, H.; Jiang, Y.; Zhu, Z.; et al. Magnesium alleviates aluminum-induced growth inhibition by enhancing antioxidant enzyme activity and carbon-nitrogen metabolism in apple seedlings. Ecotoxicol. Environ. Saf. 2022, 249, 114421. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Bao, Q.; Chu, Y.; Sun, H.; Huang, Y. Jasmonic acid alleviates cadmium toxicity through regulating the antioxidant response and enhancing the chelation of cadmium in rice (Oryza sativa L.). Environ. Pollut. 2022, 304, 119178. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Cheng, J.; Ahmad, N.; Zhao, W.; Tian, M.; Yuan, Z.; Li, C.; Zhao, C. Effects of potential allelochemicals in a water extract of Abutilon theophrasti Medik. on germination and growth of Glycine max L., Triticum aestivum L., and Zea mays L. J. Sci. Food Agric. 2022, 103, 2155–2165. [Google Scholar] [CrossRef]
- Hyun, J.; Kim, Y.J.; Kim, A.; Plante, A.F.; Yoo, G. Ecosystem services-based soil quality index tailored to the metropolitan environment for soil assessment and management. Sci. Total. Environ. 2022, 820, 153301. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Jiang, C.; Chen, X.; Li, Y.; Li, C.; Zheng, L. Combining hydrochemistry and hydrogen and oxygen stable isotopes to reveal the influence of human activities on surface water quality in Chaohu Lake Basin. J. Environ. Manag. 2022, 312, 114933. [Google Scholar] [CrossRef]
- Yasmeen, T.; Arif, M.S.; Shahzad, S.M.; Riaz, M.; Tufail, M.A.; Mubarik, M.S.; Ahmad, A.; Ali, S.; Albasher, G.; Shakoor, A. Abandoned agriculture soil can be recultivated by promoting biological phosphorus fertility when amended with nano-rock phosphate and suitable bacterial inoculant. Ecotoxicol. Environ. Saf. 2022, 234, 113385. [Google Scholar] [CrossRef] [PubMed]
- Díaz-López, M.; Siles, J.A.; Ros, C.; Bastida, F.; Nicolás, E. The effects of ozone treatments on the agro-physiological parameters of tomato plants and the soil microbial community. Sci. Total Environ. 2022, 812, 151429. [Google Scholar] [CrossRef]
- Liu, S.; Wang, J.; Pu, S.; Blagodatskaya, E.; Kuzyakov, Y.; Razavi, B.S. Impact of manure on soil biochemical properties: A global synthesis. Sci. Total Environ. 2020, 745, 141003. [Google Scholar] [CrossRef]
- Li, H.; Yang, H.; Calderón-Urrea, A.; Li, Y.; Zhang, L.; Yu, Y.; Ma, J.; Shi, G. Microbial Fertilization Improves Soil Health When Compared to Chemical Fumigation in Sweet Lily. J. Fungi 2022, 8, 847. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, W.; Chen, J.; Zhou, Y. Allelopathic inhibitory effect on the growth of microcystis aeruginosa by improvedultrasonic-cellulase extract of Vallisneria. Chemosphere 2022, 298, 134245. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, Y.; Yuan, L.; Huang, J. Allelopathy of uncomposted and composted invasive aster (Ageratina adenophora) on ryegrass. J. Hazard. Mater. 2021, 402, 123727. [Google Scholar] [CrossRef]
- Liu, J.; Xu, G.; Yin, L.; Xu, X.; Armitage, D.W.; Dong, T. Invasive plants exert disproportionately negative allelopathic effects on the growth and physiology of the earthworm Eisenia fetida. Sci. Total Environ. 2020, 747, 141534. [Google Scholar] [CrossRef] [PubMed]
- Baresch, A.; Crifo, C.; Boyce, C.K. Competition for epidermal space in the evolution of leaves with high physiological rates. New Phytol. 2019, 221, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-R.; Ai, Y.; Du, J.-B.; Yu, L.; Wang, X.-C.; Yang, W.-Y.; Sun, X. Photosynthetic compensation of maize in heterogeneous light is impaired by restricted photosynthate export. Plant Physiol. Biochem. 2022, 192, 50–56. [Google Scholar] [CrossRef]
- Kromdijk, J.; Głowacka, K.; Long, S.P. Photosynthetic efficiency and mesophyll conductance are unaffected in Arabidopsis thaliana aquaporin knock-out lines. J. Exp. Bot. 2020, 71, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Yuan, S.; Xie, B.; Li, Q.; Wang, Q.; Shao, M. IAA Plays an Important Role in Alkaline Stress Tolerance by Modulating Root Development and ROS Detoxifying Systems in Rice Plants. Int. J. Mol. Sci. 2022, 23, 14817. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Y.; Guo, Y.; Chen, Y.; Yang, M.; Fu, R.; Sun, Y. Physiological and iTRAQ-based proteomic analyses for yellowing of postharvest broccoli heads under elevated O2 controlled atmosphere. Sci. Hortic. 2022, 294, 110769. [Google Scholar] [CrossRef]
- Altınkaynak, Y.; Kural, B.; Akcan, B.A.; Bodur, A.; Özer, S.; Yuluğ, E.; Munğan, S.; Kaya, C.; Örem, A. Protective effects of L-theanine against doxorubicin-induced nephrotoxicity in rats. Biomed. Pharmacother. 2018, 108, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Tian, L.-L.; Xi, J.; Girimpuhwe, D.; Huang, C.; Ma, R.; Yao, X.; Shi, D.; Bai, Z.; Wu, Q.-X.; et al. Alterperylenol as a Novel Thioredoxin Reductase Inhibitor Induces Liver Cancer Cell Apoptosis and Ferroptosis. J. Agric. Food Chem. 2022, 70, 15763–15775. [Google Scholar] [CrossRef]
- Honeker, L.K.; Hildebrand, G.A.; Fudyma, J.D.; Daber, L.E.; Hoyt, D.; Flowers, S.E.; Gil-Loaiza, J.; Kübert, A.; Bamberger, I.; Anderton, C.R.; et al. Elucidating Drought-Tolerance Mechanisms in Plant Roots through 1H NMR Metabolomics in Parallel with MALDI-MS, and NanoSIMS Imaging Techniques. Environ. Sci. Technol. 2022, 56, 2021–2032. [Google Scholar] [CrossRef]
- Huérfano, X.; Estavillo, J.M.; Torralbo, F.; Vega-Mas, I.; González-Murua, C.; Fuertes-Mendizábal, T. Dimethylpyrazole-based nitrification inhibitors have a dual role in N2O emissions mitigation in forage systems under Atlantic climate conditions. Sci. Total Environ. 2022, 807, 150670. [Google Scholar] [CrossRef]
- Li, W.; Shi, Y.F.; Zhu, D.D.; Wang, W.Q.; Liu, H.W.; Li, J.Y.; Shi, N.N.; Ma, L.; Fu, S.L. Fine root biomass and morphology in a temperate forest are influenced more by the nitrogen treatment approach than the rate. Ecol. Indic. 2021, 130, 108031. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, X.; Wang, X.; Ge, J.; Cai, B. Elaeagnus angustifolia can improve salt-alkali soil and the health level of soil: Emphasizing the driving role of core microbial communities. J. Environ. Manag. 2022, 305, 114401. [Google Scholar] [CrossRef] [PubMed]
- Ivashchenko, K.; Sushko, S.; Selezneva, A.; Ananyeva, N.; Zhuravleva, A.; Kudeyarov, V.; Makarov, M.; Blagodatsky, S. Soil microbial activity along an altitudinal gradient: Vegetation as a main driver beyond topographic and edaphic factors. Appl. Soil Ecol. 2021, 168, 104197. [Google Scholar] [CrossRef]
- Zhao, S.; Schmidt, S.; Gao, H.; Li, T.; Chen, X.; Hou, Y.; Chadwick, D.; Tian, J.; Dou, Z.; Zhang, W.; et al. A precision compost strategy aligning composts and application methods with target crops and growth environments can increase global food production. Nat. Food 2022, 3, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Yansheng, C.; Fengliang, Z.; Zhongyi, Z.; Tongbin, Z.; Huayun, X. Biotic and abiotic nitrogen immobilization in soil incorporated with crop residue. Soil Tillage Res. 2020, 202, 104664. [Google Scholar] [CrossRef]
- Huang, X.; Wang, L.; Mei, Y.; Jia, Z.; Li, T.; Yu, G.; Ran, W. Towards a mechanistic understanding of microbial and nonmicrobial mediated topsoil organic carbon sequestration efficiency in a rice-wheat cropping system. Appl. Soil Ecol. 2022, 170, 104259. [Google Scholar] [CrossRef]
- Xu, W.; Tan, L.; Guo, X.; Wang, J. Isolation of anti-algal substances from Cylindrotheca closterium and their inhibition activity on bloom-forming Prorocentrum donghaiense. Ecotoxicol. Environ. Saf. 2020, 190, 110180. [Google Scholar] [CrossRef]
- Magalhães, A.P.; Jorge, P.; Pereira, M.O. Pseudomonas aeruginosa and Staphylococcus aureus communication in biofilm infections: Insights through network and database construction. Crit. Rev. Microbiol. 2019, 45, 712–728. [Google Scholar] [CrossRef]
- Benidire, L.; Madline, A.; Pereira, S.I.A.; Castro, P.M.L.; Boularbah, A. Synergistic effect of organo-mineral amendments and plant growth-promoting rhizobacteria (PGPR) on the establishment of vegetation cover and amelioration of mine tailings. Chemosphere 2021, 262, 127803. [Google Scholar] [CrossRef]
- Vaidya, B.P.; Hagmann, D.F.; Haramuniz, J.; Krumins, J.A.; Goodey, N.M. Artificial root exudates restore microbial functioning in a metal contaminated, barren, inactive soil. Environ. Pollut. 2022, 312, 120007. [Google Scholar] [CrossRef]
- Salehi, B.; Prakash-Mishra, A.; Nigam, M.; Karazhan, N.; Shukla, I.; Kiełtyka-Dadasiewicz, A.; Sawicka, B.; Głowacka, A.; Abu-Darwish, M.S.; Tarawneh, A.H.; et al. Ficus plants: State of the art from a phytochemical, pharmacological, and toxicological perspective. Phytotherapy Res. 2021, 35, 1187–1217. [Google Scholar] [CrossRef] [PubMed]



| Sample | Shannon | Simpson | Ace | Chao1 | Coverage |
|---|---|---|---|---|---|
| A: bacteria | |||||
| A1 | 6.34 ± 0.28 Aa | 0.0057 ± 0.0002 Ab | 3251.70 ± 137.24 Aa | 3237.81 ± 146.74 Aa | 0.9666 ± 0.0013 Aa |
| A2 | 6.54 ± 0.29 Aa | 0.0037 ± 0.0001 Cb | 3293.86 ± 148.76 Aa | 3230.11 ± 151.46 Aa | 0.9672 ± 0.008 Aa |
| A3 | 6.5 ± 0.32 Aa | 0.0041 ± 0.0001 Bc | 3185.11 ± 139.84 Aa | 3164.75 ± 144.11 Aa | 0.9681 ± 0.0012 Aa |
| B1 | 6.25 ± 0.40 Aa | 0.0055 ± 0.0002 Ab | 2953.54 ± 136.49 ABb | 3026.41 ± 153.06 Ab | 0.9696 ± 0.0009 Aa |
| B2 | 6.37 ± 0.39 Ab | 0.0046 ± 0.0001 Ba | 2851.46 ± 142.81 Bb | 2802.97 ± 142.12 Ab | 0.9723 ± 0.0016 Aa |
| B3 | 6.43 ± 0.45 Aa | 0.0043 ± 0.0001 Cbc | 3076.59 ± 140.93 Ab | 3027.90 ± 154.12 Aab | 0.9692 ± 0.0014 Aa |
| C1 | 6.03 ± 0.29 Bb | 0.0091 ± 0.0003 Aa | 2610.63 ± 118.32 Bc | 2526.66 ± 105.80 Bd | 0.9750 ± 0.0005 Aa |
| C2 | 6.47 ± 0.37 Aa | 0.0039 ± 0.0001 Cb | 3060.00 ± 147.33 Ab | 2933.94 ± 128.02 Ab | 0.9700 ± 0.0021 Aa |
| C3 | 6.27 ± 0.32 Ab | 0.0055 ± 0.0002 Ba | 2864.25 ± 133.27 Abc | 2880.64 ± 126.42 Ab | 0.9711 ± 0.0017 Aa |
| D1 | 6.21 ± 0.28 Aa | 0.0051 ± 0.0002 Ac | 2779.35 ± 130.21 Bbc | 2822.53 ± 114.68 Ac | 0.9717 ± 0.0010 Aa |
| D2 | 6.35 ± 0.26 Ab | 0.0048 ± 0.0001 ABa | 2997.85 ± 142.09 Ab | 2949.22 ± 125.48 Ab | 0.9701 ± 0.0005 Aa |
| D3 | 6.34 ± 0.35 Aa | 0.0045 ± 0.0001 Bb | 2660.82 ± 135.94 Bc | 2642.08 ± 137.26 Bc | 0.9749 ± 0.0014 Aa |
| B: fungi | |||||
| A1 | 4.84 ± 0.19 Aa | 0.0264 ± 0.0011 Aa | 951.53 ± 38.06 Bb | 953.92 ± 48.16 Bb | 0.9976 ± 0.0009 Aa |
| A2 | 4.67 ± 0.19 Ab | 0.0221 ± 0.0013 ABa | 1253.98 ± 52.16 Aa | 1240.38 ± 59.62 Aa | 0.9946 ± 0.0008 Aa |
| A3 | 4.94 ± 0.20 Aa | 0.0174 ± 0.0007 Ba | 1205.03 ± 48.20 Aa | 1207.17 ± 58.29 Aab | 0.9957 ± 0.0005 Aa |
| B1 | 4.79 ± 0.17 Bab | 0.0242 ± 0.0006 Aa | 1028.49 ± 45.14 Ba | 1026.60 ± 51.06 Ba | 0.9970 ± 0.008 Aa |
| B2 | 5.02 ± 0.18 Aa | 0.0169 ± 0.0012 Ab | 1015.63 ± 43.63 Bb | 1034.56 ± 41.04 Bb | 0.9975 ± 0.0002 Aa |
| B3 | 4.99 ± 0.21 Aa | 0.0174 ± 0.0015 Aa | 1133.12 ± 39.99 Ab | 1123.13 ± 43.69 Ab | 0.9963 ± 0.0007 Aa |
| C1 | 4.89 ± 0.19 Aa | 0.0191 ± 0.0018 Ab | 987.60 ± 37.63 Bab | 996.17 ± 39.89 Cb | 0.9968 ± 0.0009 Aa |
| C2 | 4.94 ± 0.18 Aa | 0.0195 ± 0.0013 Aab | 1050.64 ± 48.52 Bb | 1078.28 ± 42.35 Bb | 0.9970 ± 0.0006 Aa |
| C3 | 5.06 ± 0.20 Aa | 0.0172 ± 0.0005 Ba | 1285.57 ± 58.57 Aa | 1311.17 ± 57.27 Aa | 0.9954 ± 0.0009 Aa |
| D1 | 4.70 ± 0.19 ABb | 0.0233 ± 0.0012 Aab | 663.10 ± 40.89 Cc | 668.57 ± 36.74 Cc | 0.9990 ± 0.0001 Aa |
| D2 | 4.60 ± 0.17 Bb | 0.0254 ± 0.0007 Aa | 996.87 ± 39.91 Bb | 987.83 ± 49.51 Bc | 0.9962 ± 0.0003 Aa |
| D3 | 4.98 ± 0.18 Aa | 0.0163 ± 0.0008 Ba | 1154.25 ± 54.63 Ab | 1167.26 ± 56.69 Ab | 0.9961 ± 0.0007 Aa |
| Type of Compound | RT (min) | Formula | Proposed Compound | F. carica Root Water Extract | F. carica Root Exudates |
|---|---|---|---|---|---|
| Coumarins | 3.94 | C9H6O4 | Esculetin | + | + |
| 4.88 | C11H6O4 | Xanthotoxol | + | + | |
| 4.89 | C11H6O4 | Bergaptol | + | + | |
| 5.57 | C9H6O3 | Umbelliferone | + | + | |
| 6.77 | C14H12O3 | Angenomalin | + | + | |
| 6.83 | C14H12O3 | Trioxsalen | + | − | |
| 8.17 | C14H14O4 | Nodakenetin | + | + | |
| 8.26 | C14H14O4 | Columbianetin | + | + | |
| 9.63 | C11H6O3 | Psoralen | + | + | |
| 11.21 | C12H8O4 | Bergapten | + | + | |
| 25.70 | C21H24O5 | Rutamarin | + | + | |
| 35.59 | C11H10O3 | 7- Ethoxycoumarin | + | + | |
| Terpenoids | 0.73 | C13H16N4O6 | Furaltadone | + | + |
| 0.75 | C3H6N6O5 | 1,3-Dinitro-5-nitroso-1,3,5-triazinane | + | - | |
| 3.66 | C20H24O10 | Ginkgolide B | − | + | |
| 4.22 | C11H15N3O8 | Polyoxin C | − | + | |
| 5.61 | C31H32N4O9 | Cotarnine phthalate | + | − | |
| 7.23 | C25H22N2O5 | Zanamivir Amine Triacetate Methyl Ester | + | + | |
| 10.57 | C28H22 | 9,10-Bis(4-methylphenyl)anthracene | + | + | |
| 11.20 | C9H3N3O3 | 1,3,5-Triisocyanatobenzene | + | − | |
| 15.30 | C26H26O9 | Gilvocarcin M | + | + | |
| 19.36 | C20H22O2 | Longistylin | − | + | |
| 24.10 | C14H26O4 | Tetradecaneioic acid | − | + | |
| 26.91 | C38H49N3O5 | Bemotrizinol | + | + | |
| 27.27 | C24H38O4 | Dioctyl phthalate | + | + | |
| 27.76 | C25H35NO4 | Norbuprenorphine | + | + | |
| 29.56 | C30H46O3 | Wilforlide A | + | − | |
| 31.46 | C15H22O4 | Zinniol | + | + | |
| 31.94 | C12H16O | 4-Cyclohexylphenol | − | + | |
| 34.61 | C17H26O5 | Decyl Gallate | + | + | |
| Alkaloids | 0.69 | C11H6O6 | 4-Azido-3,5-dinitrobenzonitrile | + | − |
| 0.82 | C14H21NO3 | 2,6-ditert-butyl-4-nitrophenol | − | + | |
| 0.90 | C21H16O6 | Justicidin B | + | + | |
| 0.93 | C4H2N6O5 | Azoxyfuroxan | + | + | |
| 1.22 | C13H20N2O6 | 5-Butyluridine | − | + | |
| 4.28 | C22H26O12 | Catalposide | + | − | |
| 4.98 | C32H18N8 | Phthalocyanine | + | − | |
| 5.55 | C20H24O10 | 4-[4-(3-Phenyl-2-quinoxalinyl)phthalonitrile | + | + | |
| 5.93 | C30H22N4O4 | Adozelesin | + | − | |
| 6.07 | C15H18O4 | Helenalin | + | + | |
| 7.15 | C19H20N6O6 | Azido-Thalidomide | + | − | |
| 7.30 | C18H26N2O9 | Nicametate citrate | + | − | |
| 7.38 | C37H28O10 | Pyranoamentoflavone | + | + | |
| 8.11 | C18H22N2O10 | Nitrophenyl 2-acetamido-3,6-di-O-acety-2-deoxyl-beta-D-glucopyranoside | + | + | |
| 13.89 | C19H38N2O3 | N-Pentadecyl-L-asparagine | + | − | |
| 13.94 | C16H35NO2 | Lauryldimethylammonium Acetate | + | + | |
| 14.03 | C14H31NO | Lauryldimethylamine oxide | + | + | |
| 16.29 | C18H39NO2 | Safingol | + | + | |
| 17.32 | C15H18N6O2 | Pimefylline | − | + | |
| 17.51 | C21H37N | N,N-Dimethylandrostan-1-amine | + | + | |
| 19.60 | C7H17N | N,N-dimethylpentan-3-amine | + | + | |
| 20.39 | C22H47NO2 | Ammonium Behenate | + | + | |
| 20.65 | C22H47N | Docosylamine | + | − | |
| 23.36 | C18H35NO | 9-Octadecenamide | + | − | |
| 25.33 | C18H37NO | Amide C18 | + | + | |
| 25.58 | C20H41NO | N,N-Dimethylstearamide | + | + | |
| 35.14 | C19H30O5 | Dodecyl gallate | + | + | |
| Flavones | 0.70 | C30H22O10 | Isochamaejasmin | + | − |
| 0.85 | C30H26O13 | Proanthocyanidins | + | + | |
| 0.92 | C15H12O7 | Taxifolin | + | + | |
| 2.49 | C15H14O6 | (-)-Epicatechin | + | + | |
| 3.16 | C30H26O12 | Procyanidin B2 | + | + | |
| 3.31 | C21H23N3O7 | Amicycline | + | + | |
| 3.91 | C30H26O11 | Gambiriin C | + | + | |
| 4.45 | C30H26O12 | Procyanidin B3 | + | + | |
| 4.47 | C26H28O15 | Neocarlinoside | + | − | |
| 4.92 | C24H22O13 | Malonylgenistin | + | + | |
| 7.25 | C28H34O15 | Hesperidin | + | + | |
| 8.86 | C15H11O6 | Cyanidin cation | + | + | |
| 23.99 | C27H30O16 | Rutin | − | + | |
| 35.14 | C19H30O5 | Dodecyl gallate | + | − | |
| 35.56 | C27H32O14 | Naringin | + | + | |
| 35.60 | C28H34O15 | Neohesperidin | + | + | |
| Organic acids | 0.71 | C12H21N3O6 | Boc-Ala-Gly-Gly | + | − |
| 0.94 | C11H20N2O3 | Pro-leu | + | − | |
| 0.95 | C10H13NO2 | Phenprobamate | + | + | |
| 1.22 | C5H11NO2 | Valine | + | − | |
| 4.51 | C11H18N2O2 | Cyclo(leucylprolyl) | + | + | |
| 5.21 | C10H19N7O5 | neosaxitoxin conjugate acid | − | + | |
| 5.34 | C24H39N7O8 | L-Threonyl-L-histidyl-L-threonyl-L-valyl-L-proline | + | + | |
| 6.43 | C9H14N2O7 | Asp-Glu | + | − | |
| 9.36 | C6H12N2O4 | Glycyl-D-threonine | + | + | |
| 11.10 | C10H5O3 | Oxo-4-phenyl-3-butynoate | + | + | |
| 11.32 | C24H20O10 | Gyrophoric acid | + | − | |
| 13.35 | C10H16N2O8 | Ethylenediaminetetraacetic acid | + | + | |
| 19.67 | C24H40O2 | 5β-cholanoic acid | + | + | |
| 21.11 | C14H26N2O6 | BOC-L-DAB(BOC) | − | + | |
| 27.78 | C19H38N2O6 | 2,3-dihydroxybutanedioic acid | + | + | |
| 30.62 | C15H22O3 | gemfibrozil | + | + | |
| 32.15 | C44H58N2O3 | 4-[28-Oxo-28-{[2-(2-pyridinyl)ethyl]amino}lupa-2,20(29)-dien-3-yl]benzoic acid | + | + | |
| 32.22 | C39H58N4O5 | 3-hexanoyl-NBD Cholesterol | + | + | |
| 32.34 | C26H52O3 | 26-Hydroxyhexacosanoic acid | + | − | |
| 33.68 | C6H8O7 | Citric Acid | + | − | |
| Steroids | 0.74 | C11H15N5O3 | Lobucavir | + | − |
| 4.63 | C22H14 | Pentaphene | + | − | |
| 5.77 | C14H7NO5 | 1-Hydroxy-3-nitro-9,10-anthraquinone | − | + | |
| 15.37 | C24H30O6 | Meprednisone Acetate | + | + | |
| 27.63 | C19H26O6 | Arctiopicri | − | + | |
| Quinones | 5.20 | C32H28O9 | Shikometabolin B | + | − |
| 6.66 | C27H30O13 | Lignan P | + | − | |
| 32.18 | C17H26O4 | Embelin | + | + | |
| 35.53 | C19H30O4 | Decylubiquinone | + | + | |
| 35.63 | C11H8O3 | Plumbagin | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Huang, J.; Yang, X.; Gul, Z.; Sun, W.; Qiao, B.; Cheng, J.; Li, C.; Zhao, C. Effects of Ficus carica L. Water Extract on Taxus cuspidata Sieb. et Zucc. Growth. Forests 2023, 14, 1213. https://doi.org/10.3390/f14061213
Li Q, Huang J, Yang X, Gul Z, Sun W, Qiao B, Cheng J, Li C, Zhao C. Effects of Ficus carica L. Water Extract on Taxus cuspidata Sieb. et Zucc. Growth. Forests. 2023; 14(6):1213. https://doi.org/10.3390/f14061213
Chicago/Turabian StyleLi, Qianqian, Jin Huang, Xue Yang, Zarmina Gul, Wenxue Sun, Bin Qiao, Jiabo Cheng, Chunying Li, and Chunjian Zhao. 2023. "Effects of Ficus carica L. Water Extract on Taxus cuspidata Sieb. et Zucc. Growth" Forests 14, no. 6: 1213. https://doi.org/10.3390/f14061213
APA StyleLi, Q., Huang, J., Yang, X., Gul, Z., Sun, W., Qiao, B., Cheng, J., Li, C., & Zhao, C. (2023). Effects of Ficus carica L. Water Extract on Taxus cuspidata Sieb. et Zucc. Growth. Forests, 14(6), 1213. https://doi.org/10.3390/f14061213









