Assessing Forest Vulnerability to Climate Change Combining Remote Sensing and Tree-Ring Data: Issues, Needs and Avenues
Abstract
1. Introduction
| Reference | Species | Variables | Correlation |
|---|---|---|---|
| Moreno-Fernández et al., 2022 [24] | Pinus pinea L., 1753 | BAI, NDVI, NDWI | different responses to drought between indices and low BAI –VI correlation |
| Ogaya et al., 2015 [44] | Quercus ilex L., 1753 | Defoliation, BAI, NDVI, EVI | positive correlation between defoliation and vegetation indices |
| Coluzzi et al., 2020 [45] | Mixed forest of oaks and ash trees | Defoliation, NDVI | positive correlation |
| Brehaut et al., 2018 [47] | Picea glauca (Moench) Voss, 1907 Picea mariana (Mill.) Britton, Sterns & Poggenb., 1888 Salix glauca L., 1753 Alnus crispa (Aiton) Pursh, 1814 Populus tremuloides Michx., 1803, at different sites | TRW, NDVI | low correlation |
| Vicente-Serrano et al., 2020 [50] | 15 species in different biomes | TRW, GPP, NDVI | site-dependent relationships |
| Wang et al., 2021 [51] | Pinus densiflora Siebold & Zucc., 1842 | RWI, NDVI | positive correlation |
| Castellaneta et al., 2022 [52] | Pinus sylvestris L., 1753 Quercus pubescens Willd., 1805 Quercus frainetto Ten., 1813 Juniperus phoenicea L., 1753 | BAI, NDVI | positive correlations |
| Gazol et al., 2018 [53] | 11 tree species between gymnosperms and angiosperms | TRWi, NDVI | positive correlation |
| D’Andrea et al., 2022 [54] | Picea abies (L.) H.Karst., 1881 | RWI, NDVI | inconsistent trend |
| Lapenis et al., 2013 [55] | Picea abies (L.) H.Karst., 1881 | TRW, NDVI | inconsistent trend |
| Vicente-Serrano et al., 2016 [56] | 100 tree species in different biomes | TRW, NDVI | different relationships between growth and vegetation indices; stronger correlation in dry sites |
| Beck et al., 2013 [57] | Treeline vegetation mix in different forests | TRW, MXD, NDVI | positive NDVI-MXD correlation |
| D’Arrigo et al., 2000 [58] | Picea glauca (Moench) Voss, 1907 Larix gmelinii (Rupr.) Kuzen, 1854 | MXD, NDVI | good correlation |
2. Resilience Indexes to Assess the Vulnerability of Forests
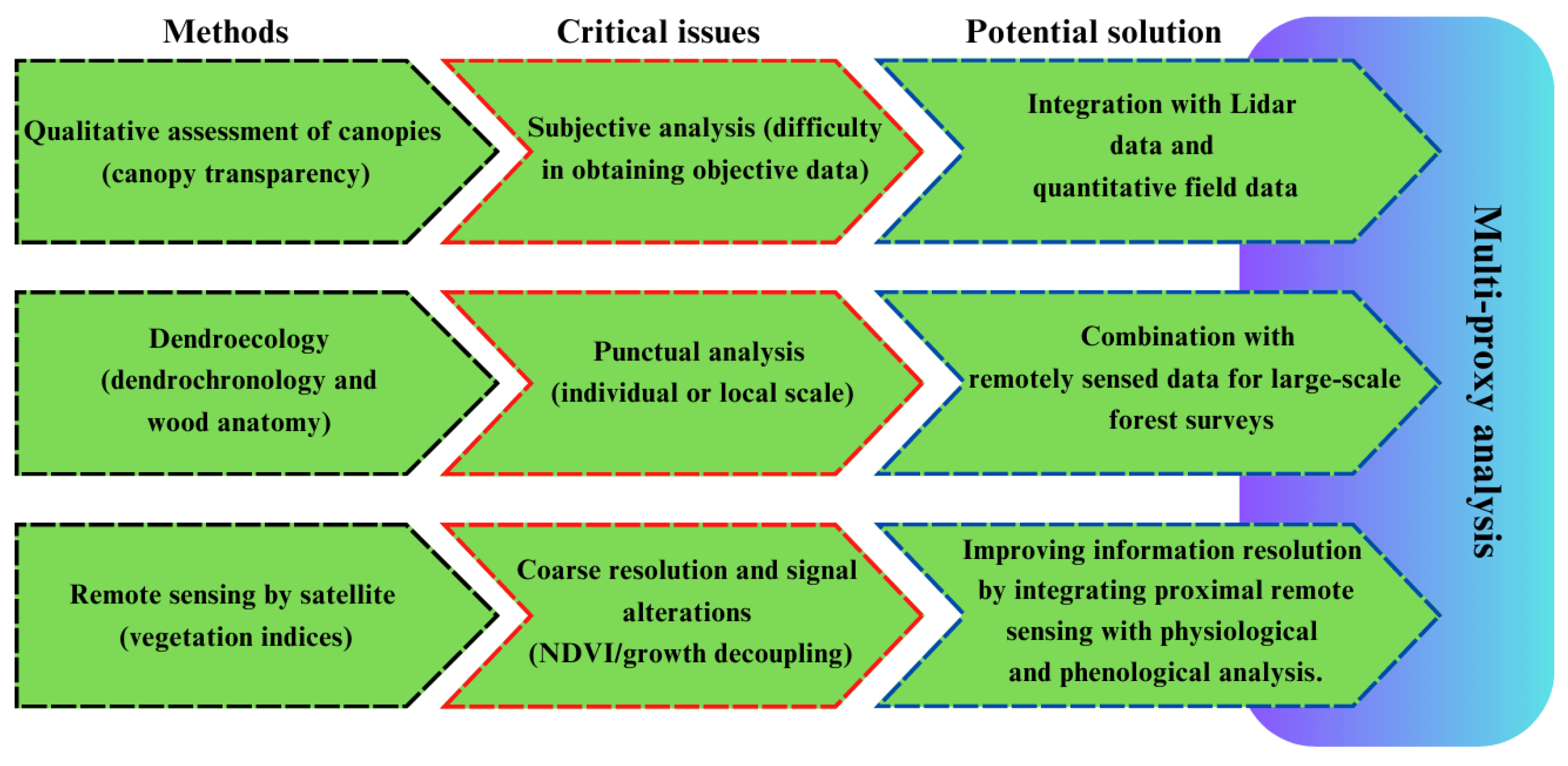
3. Methods for Monitoring and Studying Forest Vulnerability
3.1. Tree Crown Evaluations
3.2. Dendroecology
3.3. Remote Sensing
3.3.1. Decoupling of NDVI–Growth Relationship
3.3.2. Low Spatial Resolution and Remote Sensing Signal Anomalies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cramer, W.; Guiot, J.; Fader, M.; Garrabou, J.; Gattuso, J.P.; Iglesias, A.; Lange, M.A.; Lionello, P.; Llasat, M.C.; Paz, S. Climate change and interconnected risks to sustainable development in the Mediterranean. Nat. Clim. Change. 2018, 8, 972–980. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers. In Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Shukla, P.R., Skea, J., Calvo Buendia, E., Masson-Delmotte, V., Pörtner, H.O., Roberts, D.C., Zhai, P., Slade, R., Connors, S., Van Diemen, R., et al., Eds.; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2019; ISBN 978-92-9169-154-8. [Google Scholar]
- Thomas, F.M.; Blank, R.; Hartman, G. Abiotic and biotic factors and their interactions as causes of oak decline in Central Europe. For. Pathol. 2002, 32, 277–307. [Google Scholar] [CrossRef]
- Jones, M.W.; Abatzoglou, J.T.; Veraverbeke, S.; Andela, N.; Lasslop, G.; Forkel, M.; Smith, A.J.P.; Burton, C.; Betts, R.A.; van der Werf, G.R.; et al. Global and regional trends and drivers of fire under climate change. Rev. Geophys. 2022, 60, e2020RG000726. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Yepez, E.A. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Sala, A.; Asensio, D.; Galiano, L.; Hoch, G.; Palacio, S.; Lloret, F. Dynamics of non-structural carbohydrates in terrestrial plants: A global synthesis. Ecol. Monogr. 2016, 86, 495–516. [Google Scholar] [CrossRef]
- Hartmann, H. Carbon starvation during drought-induced tree mortality–are we chasing a myth? J. Plant Hydraul. 2015, 2, e005. [Google Scholar] [CrossRef]
- González de Andrés, E.; Gazol, A.; Querejeta, J.I.; Igual, J.M.; Colangelo, M.; Sánchez-Salguero, R.; Linares, J.C.; Camarero, J.J. The role of nutritional impairment in carbon-water balance of silver fir drought-induced dieback. Glob. Change. Biol. 2022, 28, 4439–4458. [Google Scholar] [CrossRef]
- McDowell, N.G. Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiol. 2011, 155, 1051–1059. [Google Scholar] [CrossRef]
- Adams, H.D.; Zeppel, M.J.; Anderegg, W.R.; Hartmann, H.; Landhäusser, S.M.; Tissue, D.T.; McDowell, N.G. A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat. Ecol. Evol. 2017, 1, 1285–1291. [Google Scholar] [CrossRef]
- Levanič, T.; Čater, M.; McDowell, N.G. Associations between growth, wood anatomy, carbon isotope discrimination and mortality in a Quercus robur forest. Tree Physiol. 2011, 31, 298–308. [Google Scholar] [CrossRef]
- Colangelo, M.; Camarero, J.J.; Borghetti, M.; Gazol, A.; Gentilesca, T.; Ripullone, F. Size Matters a Lot: Drought-Affected Italian Oaks Are Smaller and Show Lower Growth Prior to Tree Death. Front. Plant Sci. 2017, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Patterson, D.T.; Westbrook, J.K.; Joyce, R.J.V.; Lingren, P.D.; Rogasik, J. Weeds, insects, and diseases. Clim. Change. 1999, 43, 711–727. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- DeSoto, L.; Cailleret, M.; Sterck, F.; Jansen, S.; Kramer, K.; Robert, E.M.R.; Aakala, T.; Amoroso, M.M.; Bigler, C.; Camarero, J.J.; et al. Low growth resilience to drought is related to future mortality risk in trees. Nat. Commun. 2020, 11, 545. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Cobb, N. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, 1–55. [Google Scholar] [CrossRef]
- Colangelo, M.; Camarero, J.J.; Ripullone, F.; Gazol, A.; Sánchez-Salguero, R.; Oliva, J.; Redondo, M.A. Drought Decreases Growth and Increases Mortality of Coexisting Native and Introduced Tree Species in a Temperate Floodplain Forest. Forests 2018, 9, 205. [Google Scholar] [CrossRef]
- Pericolo, O.; Camarero, J.J.; Colangelo, M.; Valeriano, C.; Sánchez-Salguero, R.; Borghetti, M.; Castellaneta, M.; Nola, P.; Ripullone, F. Species specific vulnerability to increased drought in temperate and Mediterranea floodplain forests. Agric. For. Meteorol. 2023, 328, 109238. [Google Scholar] [CrossRef]
- Camarero, J.J.; Colangelo, M.; Gazol, A.; Azorín-Molina, C. Drought and cold spells trigger dieback of temperate oak and beech forests in northern Spain. Dendrochronologia 2021, 66, 125812. [Google Scholar] [CrossRef]
- Gentilesca, T.; Camarero, J.J.; Colangelo, M.; Nolè, A.; Ripullone, F. Drought induced oak decline in the western Mediterranean region: An overview on current evidences, mechanisms and management options to improve forest resilience. iForest 2017, 10, 796–806. [Google Scholar] [CrossRef]
- Colangelo, M.; Camarero, J.J.; Battipaglia, G.; Borghetti, M.; De Micco, V.; Gentilesca, T.; Ripullone, F. A multi-proxy assessment of dieback causes in a Mediterranean oak species. Tree Physiol. 2017, 37, 617–631. [Google Scholar] [CrossRef]
- Navarro-Cerrillo, R.M.; Rodriguez-Vallejo, C.; Silveiro, E.; Hortal, A.; Palacios-Rodríguez, G.; Duque-Lazo, J.; Camarero, J.J. Cumulative Drought Stress Leads to a Loss of Growth Resilience and Explains Higher Mortality in Planted than in Naturally Regenerated Pinus pinaster Stands. Forests 2018, 9, 358. [Google Scholar] [CrossRef]
- Moreno-Fernández, D.; Camarero, J.J.; García, M.; Lines, E.R.; Sánchez-Dávila, J.; Tijerín, J.; Ruiz-Benito, P. The Interplay of the Tree and Stand-Level Processes Mediate Drought-Induced Forest Dieback: Evidence from Complementary Remote Sensing and Tree-Ring Approaches. Ecosystems 2022, 25, 1738–1753. [Google Scholar] [CrossRef]
- Camarero, J.J.; Gazol, A.; Sangüesa-Barreda, G.; Oliva, J.; Vicente-Serrano, S.M. To die or not to die: Early warnings of tree dieback in response to a severe drought. J. Ecol. 2015, 103, 44–57. [Google Scholar] [CrossRef]
- Camarero, J.J.; Bigler, C.; Linares, J.C.; Linares, J.C.; Gil-Pelegrín, E. Synergistic effects of past historical logging and drought on the decline of Pyrenean silver fir forests. For. Ecol. Manag. 2011, 262, 759–769. [Google Scholar] [CrossRef]
- Gazol, A.; Camarero, J.J.; Gutiérrez, E.; Popa, I.; Andreu-Hayles, L.; Motta, R.; Nola, P.; Ribas, M.; Sangüesa-Barreda, G.; Urbinati, C.; et al. Distinct effects of climate warming on populations of silver fir (Abies alba) across Europe. J. Biogeogr. 2015, 42, 1150–1162. [Google Scholar] [CrossRef]
- Chrysopolitou, V.; Apostolakis, A.; Avtzis, D.; Avtzis, N.; Diamandis, S.; Kemitzoglou, D.; Dafis, S. Studies on forest health and vegetation changes in Greece under the effects of climate changes. Biodivers. Conserv. 2013, 22, 1133–1150. [Google Scholar] [CrossRef]
- Christopoulou, A.; Sazeides, C.I.; Fyllas, N.M. Size-mediated effects of climate on tree growth and mortality in Mediterranean Brutia pine forests. Sci. Total Environ. 2022, 812, 151463. [Google Scholar] [CrossRef]
- Kastridis, A.; Kamperidou, V.; Stathis, D. Dendroclimatological Analysis of Fir (A. borisii-regis) in Greece in the frame of Climate Change Investigation. Forests 2022, 13, 879. [Google Scholar] [CrossRef]
- Batllori, E.; Lloret, F.; Aakala, T.; Anderegg, W.R.; Aynekulu, E.; Bendixsen, D.P.; Zeeman, B. Forest and woodland replacement patterns following drought-related mortality. Proc. Natl. Acad. Sci. USA 2020, 117, 29720–29729. [Google Scholar] [CrossRef]
- Manion, P.D. Forest Decline Concepts; American Phytopathological Society: Saint Paul, MN, USA, 1992; ISBN 10 0890541434. [Google Scholar]
- Rita, A.; Camarero, J.J.; Nolè, A.; Borghetti, M.; Brunetti, M.; Pergola, N.; Serio, C.; Vicente-Serrano, S.M.; Tramutoli, V.; Ripullone, F. The impact of drought spells on forests depends on site conditions: The case of 2017 summer heat wave in southern Europe. Glob. Change. Biol. 2020, 26, 851–863. [Google Scholar] [CrossRef]
- Lloret, F.; Lobo, A.; Estevan, H.; Maisongrande, P.; Vayreda, J.; Terradas, J. Woody plant richness and NDVI response to drought events in Catalonian (Northeastern Spain) forests. Ecology 2007, 88, 2270–2279. [Google Scholar] [CrossRef] [PubMed]
- Pretzsch, H.; Schütze, G.; Uhl, E. Resistance of European tree species to drought stress in mixed versus pure forests: Evidence of stress release by inter-specific facilitation. Plant Biol. 2013, 15, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Trugman, A.T.; Detto, M.; Bartlett, M.K.; Medvigy, D.; Anderegg, W.R.L.; Schwalm, C.; Schaffer, B.; Pacala, S.W. Tree carbon allocation explains forest drought-kill and recovery patterns. Ecol. Lett. 2018, 21, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zhou, T.; Yi, C.; Fang, W.; Hendrey, G.; Zhao, X. Forest drought resistance distinguished by canopy height. Environ. Res. Lett. 2018, 13, 075003. [Google Scholar] [CrossRef]
- Ripullone, F.; Camarero, J.J.; Colangelo, M.; Voltas, J. Variation in the access to deep soil water pools explains tree-to-tree differences in drought-triggered dieback of Mediterranean oaks. Tree Physiol. 2020, 40, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Lloret, F.; Keeling, E.G.; Sala, A. Components of tree resilience: Effects of successive low-growth episodes in old ponderosa pine forests. Oikos 2011, 120, 1909–1920. [Google Scholar] [CrossRef]
- Colangelo, M.; Camarero, J.J.; Gazol, A.; Piovesan, G.; Borghetti, M.; Baliva, M.; Gentilesca, T.; Rita, A.; Schettino, A.; Ripullone, F. Mediterranean old-growth forests exhibit resistance to climate warming. Sci. Total Environ. 2021, 801, 149684. [Google Scholar] [CrossRef]
- Lloret, F.; Escudero, A.; Iriondo, O.M.; Martìnez-Vilalta, J.; Valladares, F. Extreme climatic events and vegetation: The role of stabilizing processes. Glob. Change. Biol. 2012, 18, 797–805. [Google Scholar] [CrossRef]
- Bussotti, F.; Pollastrini, M.; Holland, V.; Brüggemann, W. Functional traits and adaptive capacity of European forests to climate change. Environ. Exp. Bot. 2015, 111, 91–113. [Google Scholar] [CrossRef]
- Camarero, J.J.; Sangüesa-Barreda, G.; Vergarechea, M. Prior height, growth, and wood anatomy differently predispose to drought-induced dieback in two Mediterranean oak species. Ann. For. Sci. 2016, 73, 341–351. [Google Scholar] [CrossRef]
- Ogaya, R.; Barbeta, A.; Basnou, C.; Peñuelas, J. Satellite data as indicators of tree biomass growth and forest dieback in a Mediterranean holm oak forest. Ann. For. Sci. 2015, 72, 135–144. [Google Scholar] [CrossRef]
- Coluzzi, R.; Fascetti, S.; Imbrenda, V.; Italiano, S.S.P.; Ripullone, F.; Lanfredi, M. Exploring the Use of Sentinel-2 Data to Monitor Heterogeneous Effects of Contextual Drought and Heatwaves on Mediterranean Forests. Land 2020, 9, 325. [Google Scholar] [CrossRef]
- Michelot, A.; Simard, S.; Rathgeber, C.; Dufrêne, E.; Damesin, C. Comparing the intra-annual wood formation of three European species (Fagus sylvatica, Quercus petraea and Pinus sylvestris) as related to leaf phenology and non-structural carbohydrate dynamics. Tree Physiol. 2012, 32, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Brehaut, L.; Danbya, R.K. Inconsistent relationships between annual tree ring-widths and satellite measured NDVI in a mountainous subarctic environment. Ecol. Indic. 2018, 91, 698–711. [Google Scholar] [CrossRef]
- Chen, X.; Wang, D.; Chen, J.; Wang, C.; Shen, M. The mixed pixel effect in land surface phenology: A simulation study. Remote Sens. Environ. 2018, 211, 338–344. [Google Scholar] [CrossRef]
- Wang, H.; Muller, J.D.; Tatarinov, F.; Yakir, D.; Rotenberg, E. Disentangling Soil, Shade, and Tree Canopy Contributions to Mixed Satellite Vegetation Indices in a Sparse Dry Forest. Remote Sens. 2022, 14, 3681. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Martin-Hernandez, N.; Camarero, J.J.; Gazol, A.; Sanchez-Salguero, R.; Pena-Gallardo, M.; El Kenawy, A.; Domínguez-Castro, F.; Tomas-Burguera, M.; Gutiérrez, E.; et al. Linking tree-ring growth and satellite-derived gross primary growth in multiple forest biomes. Temporal-scale matters. Ecol. Indic. 2020, 108, 105753. [Google Scholar] [CrossRef]
- Wang, Z.; Lyu, L.; Liu, L.; Liang, H.; Huang, J.; Zhang, Q.B. Topographic patterns of forest decline as detected from tree rings and NDVI. Catena 2021, 198, 105011. [Google Scholar] [CrossRef]
- Castellaneta, M.; Rita, A.; Camarero, J.J.; Colangelo, M.; Ripullone, F. Declines in canopy greenness and tree growth are caused by combined climate extremes during drought-induced dieback. Sci. Total Environ. 2022, 813, 152666. [Google Scholar] [CrossRef]
- Gazol, A.; Camarero, J.J.; Vicente-Serrano, S.M.; Sánchez-Salguero, R.; Gutiérrez, E.; de Luis, M.; Galván, J.D. Forest resilience to drought varies across biomes. Glob. Change. Biol. 2018, 24, 2143–2158. [Google Scholar] [CrossRef]
- D’Andrea, G.; Šimůnek, V.; Castellaneta, M.; Vacek, Z.; Vacek, S.; Pericolo, O.; Ripullone, F. Mismatch between annual tree-ring width growth and NDVI index in Norway spruce stands of Central Europe. Forests 2022, 13, 1417. [Google Scholar] [CrossRef]
- Lapenis, A.G.; Lawrence, G.B.; Heim, A.; Zheng, C.; Shortle, W. Climate warming shifts carbon allocation from stemwood to roots in calcium-depleted spruce forests. Glob. Biogeochem. Cycles 2013, 27, 101–107. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Camarero, J.J.; Olanob, J.M.; Martín-Hernández, N.; Peña-Gallardo, M.; Tomás-Burguera, M.; Gazol, A.; Azorin-Molina, C.; Bhuyan, U.; El Kenawy, A. Diverse relationships between forest growth and the Normalized Difference Vegetation Index at a global scale. Remote Sens. Environ. 2016, 187, 14–29. [Google Scholar] [CrossRef]
- Beck, P.S.A.; Andreu-Hayles, L.; D’Arrigo, R.; Anchukaitis, K.J.; Tucker, C.J.; Pinzón, J.E.; Goetz, S.J. A large-scale coherent signal of canopy status in maximum latewood density of tree rings at arctic treeline in North America. Glob. Planet. Change. 2013, 100, 109–118. [Google Scholar] [CrossRef]
- D’Arrigo, R.D.; Malmstrom, C.M.; Jacoby, G.C.; Los, S.O.; Bunker, D.E. Correlation between maximum latewood density of annual tree rings and NDVI based estimates of forest productivity. Int. J. Remote Sens. 2000, 21, 2329–2336. [Google Scholar] [CrossRef]
- Schwarz, J.; Skiadaresis, G.; Kohler, M.; Kunz, J.; Schnabel, F.; Vitali, V.; Bauhus, J. Quantifying Growth Responses of Trees to Drought-a Critique of Commonly Used Resilience Indices and Recommendations for Future Studies. Curr. For. Rep. 2020, 6, 185–200. [Google Scholar] [CrossRef]
- Schweingruber, F.H. Tree Rings: Basics and Applications of Dendrochronology; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1988. [Google Scholar] [CrossRef]
- Mannerucci, F.; Sicoli, G.; Luisi, N. Oak decline in Apulia, southern Italy: An ecological indicator of landscape evolution? In Patterns and Processes in Forest Landscapes. Consequences of Human Management; Lafortezza, R., Sanesi, G., Eds.; Locorotondo: Bari, Italy, 2006. [Google Scholar]
- Dobbertin, M. Tree growth as indicator of tree vitality and of tree reaction to environmental stress: A review. Eur. J. Forest Res. 2005, 124, 319–333. [Google Scholar] [CrossRef]
- Solberg, S.; Næsset, E.; Hanssen, K.H.; Christiansen, E. Mapping defoliation during a severe insect attack on Scots pine using airborne laser scanning. Remote Sens. Environ. 2006, 102, 364–376. [Google Scholar] [CrossRef]
- Meng, P.; Wang, H.; Qin, S.; Li, X.; Song, Z.; Wang, Y.; Yang, Y.; Gao, J. Health assessment of plantations based on LiDAR canopy spatial structure parameters. Int. J. Digit. Earth 2022, 15, 712–729. [Google Scholar] [CrossRef]
- Cailleret, M.; Jansen, S.; Robert, E.M.; Desoto, L.; Aakala, T.; Antos, J.A.; Beikircher, B.; Bigler, C.; Bugmann, H.; Caccianiga, M.; et al. A synthesis of radial growth patterns preceding tree mortality. Glob. Change. Biol. 2017, 23, 1675–1690. [Google Scholar] [CrossRef]
- Fonti, P.; Arx, G.; García-González, I.; Eilmann, B.; Sass-Klaassen, U.; Gärtner, H.; Eckstein, D. Studying global change through investigation of the plastic responses of xylem anatomy in tree rings. New Phytol. 2010, 185, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Serrano, S.M.; Gouveiab, C.; Camarero, J.J.; Begueríae, S.; Trigo, R.; López-Morenoa, J.I.; Azorín-Molina, C.; Pashoa, E.; Lorenzo-Lacruza, J.; Revueltoa, J.; et al. Response of vegetation to drought time-scales across global land biomes. Proc. Natl. Acad. Sci. USA 2013, 110, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Rouse, J.; Haas, R.; Schell, J.; Deering, D. Monitoring Vegetation Systems in the Great Plains with ERTS. Third ERTS Symp. NASA 1973, 1, 309–317. [Google Scholar]
- Bochenek, Z.; Ziolkowski, D.; Bartold, M.; Orlowska, K.; Ochtyra, A. Monitoring forest biodiversity and the impact of climate on forest environment using high resolution satellite images. Eur. J. Remote Sens. 2017, 51, 166–181. [Google Scholar] [CrossRef]
- Glenn, E.P.; Huete, A.R.; Nagler, P.L.; Nelson, S.G. Relationship Between Remotely-sensed Vegetation Indices, Canopy Attributes and Plant Physiological Processes: What Vegetation Indices Can and Cannot Tell Us About the Landscape. Sensors 2008, 8, 2136–2160. [Google Scholar] [CrossRef]
- Tang, L.; Shao, G. Drone remote sensing for forestry research and practices. J. For. Res. 2015, 26, 791–797. [Google Scholar] [CrossRef]
- Vivar-Vivar, E.D.; Pompa-García, M.; Martínez-Rivas, J.A.; Mora-Tembre, L.A. UAV-Based Characterization of Tree-Attributes and Multispectral Indices in an Uneven-Aged Mixed Conifer-Broadleaf Forest. Remote Sens. 2022, 14, 2775. [Google Scholar] [CrossRef]
- Guerschman, J.P.; Hill, M.J.; Renzullo, L.J.; Barrett, D.J.; Marks, A.S.; Botha, E.J. Estimating fractional cover of photosynthetic vegetation, non-photosynthetic vegetation and bare soil in the Australian tropical savanna region upscaling the EO-1 Hyperion and MODIS sensors. Remote Sens. Environ. 2009, 113, 928–945. [Google Scholar] [CrossRef]
- Nagler, P.L.; Inoueb, Y.; Glenna, E.P.; Russc, A.L.; Daughtry, C.S.T. Cellulose absorption index (CAI) to quantify mixed soil–plant litter scenes. Remote Sens. Environ. 2003, 87, 310–325. [Google Scholar] [CrossRef]
- Huete, A.R. A soil-adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Qi, J.; Chehbouni, A.; Huete, A.R.; Kerr, Y.H.; Sorooshian, S. A modified soil adjusted vegetation index. Remote Sens. Environ. 1994, 48, 119–126. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Gilabert, M.A.; González-Piqueras, J.; Garcıa-Haro, F.J.; Meliá, J. A generalized soil-adjusted vegetation index. Remote Sens. Environ. 2002, 82, 303–310. [Google Scholar] [CrossRef]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Gitelson, A.A. Wide dynamic range vegetation index for remote quantification of biophysical characteristics of vegetation. J. Plant Physiol. 2004, 161, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Badgley, G.; Field, C.B.; Berry, J.A. Canopy near-infrared reflectance and terrestrial photosynthesis. Sci. Adv. 2017, 3, e1602244. [Google Scholar] [CrossRef]
- Liu, W.; Yamazaki, F. Object-Based Shadow Extraction and Correction of High-Resolution Optical Satellite Images. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2012, 5, 1296–1302. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers. In Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Shukla, P.R., Skea, J., Slade, R., Al Khourdajie, A., van Diemen, R., McCollum, D., Pathak, M., Some, S., Vyas, P., Fradera, R., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Hagedorn, F.; Joseph, J.; Peter, M.; Luster, J.; Pritsch, K.; Geppert, U.; Arend, M. Recovery of trees from drought depends on belowground sink control. Nat. Plants 2016, 2, 16111. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Italiano, S.S.P.; Camarero, J.J.; Colangelo, M.; Borghetti, M.; Castellaneta, M.; Pizarro, M.; Ripullone, F. Assessing Forest Vulnerability to Climate Change Combining Remote Sensing and Tree-Ring Data: Issues, Needs and Avenues. Forests 2023, 14, 1138. https://doi.org/10.3390/f14061138
Italiano SSP, Camarero JJ, Colangelo M, Borghetti M, Castellaneta M, Pizarro M, Ripullone F. Assessing Forest Vulnerability to Climate Change Combining Remote Sensing and Tree-Ring Data: Issues, Needs and Avenues. Forests. 2023; 14(6):1138. https://doi.org/10.3390/f14061138
Chicago/Turabian StyleItaliano, Santain S. P., Jesús Julio Camarero, Michele Colangelo, Marco Borghetti, Maria Castellaneta, Manuel Pizarro, and Francesco Ripullone. 2023. "Assessing Forest Vulnerability to Climate Change Combining Remote Sensing and Tree-Ring Data: Issues, Needs and Avenues" Forests 14, no. 6: 1138. https://doi.org/10.3390/f14061138
APA StyleItaliano, S. S. P., Camarero, J. J., Colangelo, M., Borghetti, M., Castellaneta, M., Pizarro, M., & Ripullone, F. (2023). Assessing Forest Vulnerability to Climate Change Combining Remote Sensing and Tree-Ring Data: Issues, Needs and Avenues. Forests, 14(6), 1138. https://doi.org/10.3390/f14061138









