1. Introduction
A major component of current climate change is the increase in climate variability leading to a higher occurrence of climate extremes [
1]. Under global warming, enhanced variability in temperature results in more frequent, intense and lasting heat waves, but also cold spells, despite trends towards fewer cold days. These climate extremes will impact forest growth, productivity and composition in different ways than normal climatic variability does [
2]. Note that a climate extreme defined according to statistical or climatological criteria (e.g., any event rarer than the 10th or 90th percentiles) may not necessarily incur tree responses [
3]. Therefore, adequate assessments of the impacts of climate extremes on forests are needed to determine if acclimate thresholds are surpassed and they are linked to meteorological anomalies defined by the tails of a distribution for a climate parameter. Long-term perspectives, as those provided by tree rings, are required to determine if the climate extreme pushes forests outside the range of variability [
4]. These long-term approaches are particularly necessary in seasonally dry areas with a high climate variability such as the Mediterranean basin, where droughts and heat waves are frequent but cold spells could also occur [
5].
Under climate warming, more frequent freeze–thaw cycles in winter may lead to frost damage and tree death, even in Mediterranean regions [
4]. Cold-induced forest dieback after very cold and/or dry winter conditions has been frequently described in boreal and subalpine conifer forests [
6], but there are also study cases at mid-elevation and mid-latitude sites subjected to seasonal drought [
7]. However, few studies exist in Mediterranean regions [
4] despite the fact that these rare climate extremes may also impact forests by impairing spring flush and constraining their capacity to recover after summer drought. Many Mediterranean tree species are of subtropical origin, and they are not well adapted to withstand rare cold spells [
4].
The main drivers of plant responses to winter conditions are the variability of air and soil temperatures, phenological timing (e.g., tissue hardening) and physiological sensitivity of tissues to rapid drops in air temperatures [
8]. These drivers must be considered when assessing the impacts of winter cold spells on forests. Here, we assessed the impacts of a rapid winter temperature drop on Mediterranean pine and oak forests following a retrospective, tree-ring-based approach. This allowed us to quantify the growth recovery after such a winter climate extreme.
From 8 to 10 January 2021, Storm Filomena crossed the Iberian Peninsula causing the biggest snowfalls in decades in central and eastern Spain and leading to record low temperatures during that period and afterwards [
9]. The large amount of snowfall and the low temperatures caused huge economic losses in the Madrid metropolitan area but also affected agricultural (e.g., olive production) and ecological systems [
9]. In eastern Spain, many of the affected forested areas are dominated by Mediterranean tree species such as Aleppo pine (
Pinus halepensis Mill.), which are vulnerable to snow and wind damage, causing the breakage and death of big branches [
10].
We followed a multi-proxy approach to assess the impacts of the cold spell on forests. To assess changes in forest cover and productivity, the Normalized Difference Vegetation Index (NDVI) was calculated using remote sensing imagery [
11]. To evaluate changes in tree radial growth, we analyzed tree-ring width series of defoliated vs. non-defoliated planted
P. halepensis trees. For comparison, we also sampled coexisting
Pinus pinaster Ait., which showed lower damage (crown defoliation) after the cold spell as compared to
P. halepensis. Lastly, to evaluate changes in carbon reserves, we analyzed the concentrations of non-structural carbohydrates (NSC) in leaves and stem sapwood in defoliated and non-defoliated
P. halepensis and
Quercus ilex L. individuals, since these were the most abundant planted and native tree species, respectively. We analyzed sapwood and leaf NSC concentrations because they represent a major pool of carbon in trees, correspond to C sinks (sapwood) and sources (leaves), and have been shown to respond to stressors such as drought and insect defoliation [
12]. We hypothesize that tree species which are dominant in Mediterranean regions, where they avoid or tolerate drought and are considered of subtropical origin, such as
P. halepensis or
Q. ilex will show stronger damages attributed to the cold spell than
P. pinaster, which can be found in colder mountain or northern locations. We also expect that the defoliated
P. halepensis trees will show a stronger growth reduction in 2021 and lower NSC concentrations than non-defoliated
P. halepensis trees and also a lower ability to recover growth after the 2021 winter cold spell. We expect a similar difference in leaf and sapwood NSCs when comparing defoliated and non-defoliated
Q. ilex.
2. Materials and Methods
2.1. Study Sites, Field Sampling and Climate Data
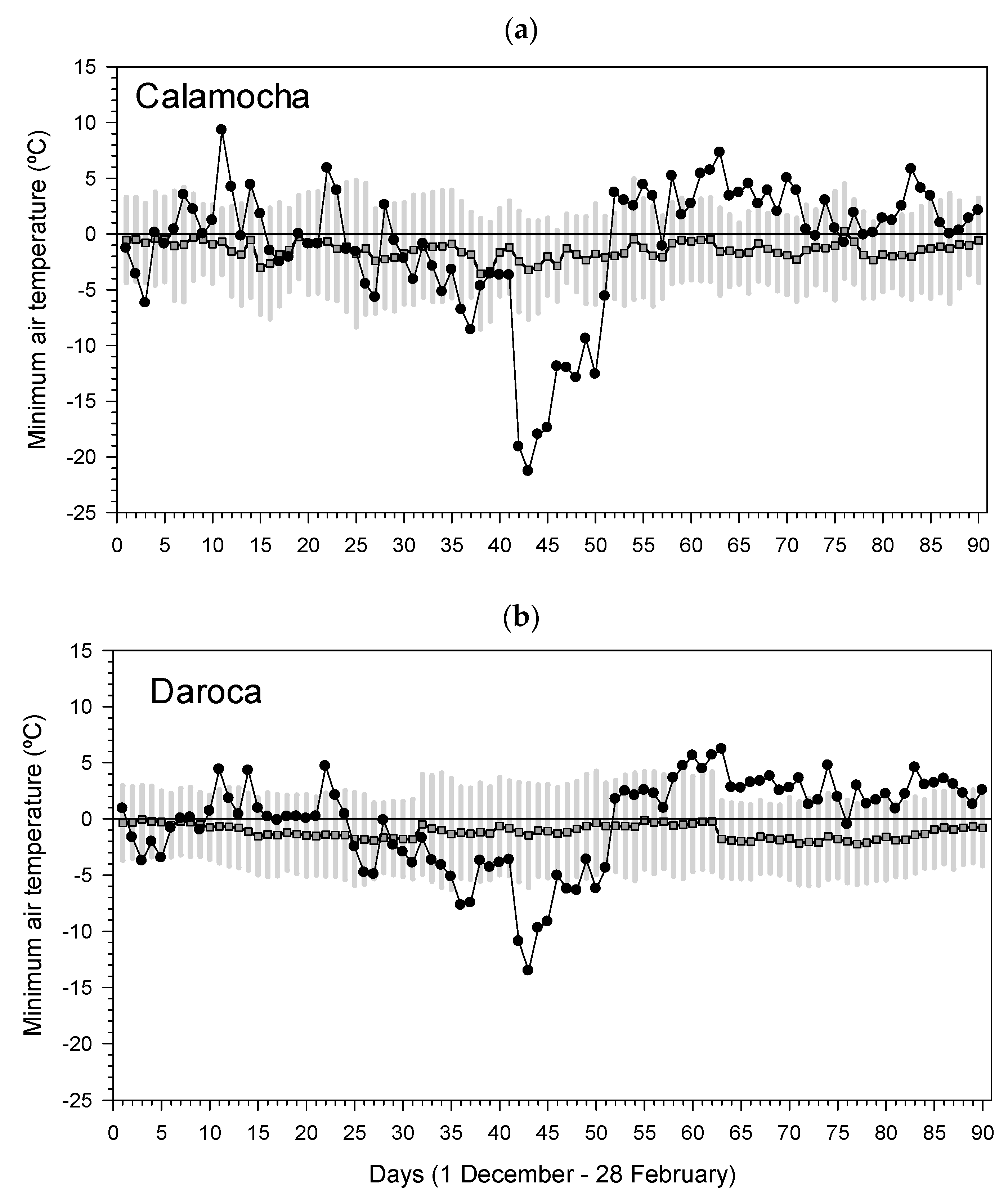
We sampled a study area situated in eastern Spain (Jiloca basin), near the towns of Calamocha and Daroca, subjected to continental Mediterranean conditions (cold winters, dry summers). In this area, very low minimum temperatures were recorded on 12 January 2021, about 12–18 °C lower than the long-term means (
Figure 1).
We selected three mature pine stands for tree-ring analyses. They corresponded to pine plantations located around the “Cabezo Gordo” peak, located near Luco de Jiloca village (Teruel province, Aragón, eastern Spain). First, a stand of defoliated P. halepensis trees was selected (40.996° N, 1.306° W, 925 m a.s.l.). Second, a nearby P. halepensis stand (40.995° N, 1.305° W, 974 m a.s.l.) showing low defoliation was sampled for comparison. Both stands showed W-SW aspect and intermediate to steep slopes (10–30°). In each P. halepensis stand, six trees were sampled. Third, a nearby Pinus pinaster Ait. stand with low defoliation (40.998° N, 1.298° W, 1001 m a.sl) was sampled (n = 12 trees). This last stand showed intermediate slopes (15–20°) and SE aspect.
The study area is covered by plantations of drought-tolerant conifers (P. halepensis, P. pinaster, Pinus pinea L., Cupressus arizonica Greene, Thuja spp.) carried out since the early 20th century to reduce soil erosion and avoid flood damage. The plantations were undertaken in formerly cultivated terraced lands with a mean distance between pines of 6.5 m. Scattered almond trees (Prunus dulcis (Mill.) D.A. Webb) of former croplands appear near pines. The natural vegetation of the area is dominated by Q. ilex with minor hardwood species (Crataegus monogyna Jacq.) and typical Mediterranean shrubs (Genista scorpius (L.) DC., Thymus spp.). Soils are acid and formed on shales.
We used daily and monthly climate data (maximum and minimum temperatures, total precipitation) from the nearby Daroca (41.11° N, 1.41° W, 779 m a.s.l.; period 1920–2021) and Calamocha (40.93° N, 1.29° W, 890 m a.s.l.; period 1993–2021) meteorological stations. Climate in the study area is Mediterranean and continental, i.e., subject to dry summers and cold winters (
Figure S1). The mean annual temperature varies from 11.0°C to 12.0 °C, with the warmest conditions in summer (mean temperatures of 20–22 °C) and the coldest ones in winter (mean temperatures of 1–3 °C). Mean minimum temperatures are negative in January and February (−0.2–−0.1 °C) when frosts are common. Mean annual precipitation is in the range of 415–436 mm with a climate water deficit of 230–259 mm. Such a dry period occurs from July to September, whereas soil moisture recharge mainly occurs in winter. In 2021, both the prior winter and summer were wet with values above the long-term averages. Thermal summer conditions were also normal. The 2021 cold spell was preceded by dry conditions in the 2005 and 2012 years [
4].
Crown defoliation estimates were undertaken by the first two authors by comparing every tree with a reference tree with the maximum amount of foliage at each site and considering fallen, brown and red (dead) needles. Tree defoliation was assessed in September 2021 when trees were tagged and their diameter at breast height was measured at 1.3 m.
Sampling of tree cores was conducted in November 2022 when two tree cores were taken at 1.3 m from each selected tree separated by 180° and perpendicular to the slope using a 5 mm Pressler increment borer. Dendrochronological analyses were not conducted on Q. ilex because of their small size and more difficult cross-dating of annual rings.
2.2. Remote Sensing Information
The NDVI is a commonly used vegetation index, which measures changes in vegetation cover and photosynthetic active tissues (greenness), derived from the near-infrared and red channels of remotely sensed imagery [
12,
13]. We calculated the kernel version of the NDVI (kNDVI) at annual resolution and for the period 2000–2022 using the Google Earth Engine, a cloud-based platform for multitemporal analysis of satellite imagery. We analyzed 30 m resolution Landsat 7 ET+ images (USGS, NASA) in this study (images available at
https://www.usgs.gov/landsat-missions/, accessed on 15 March 2023).
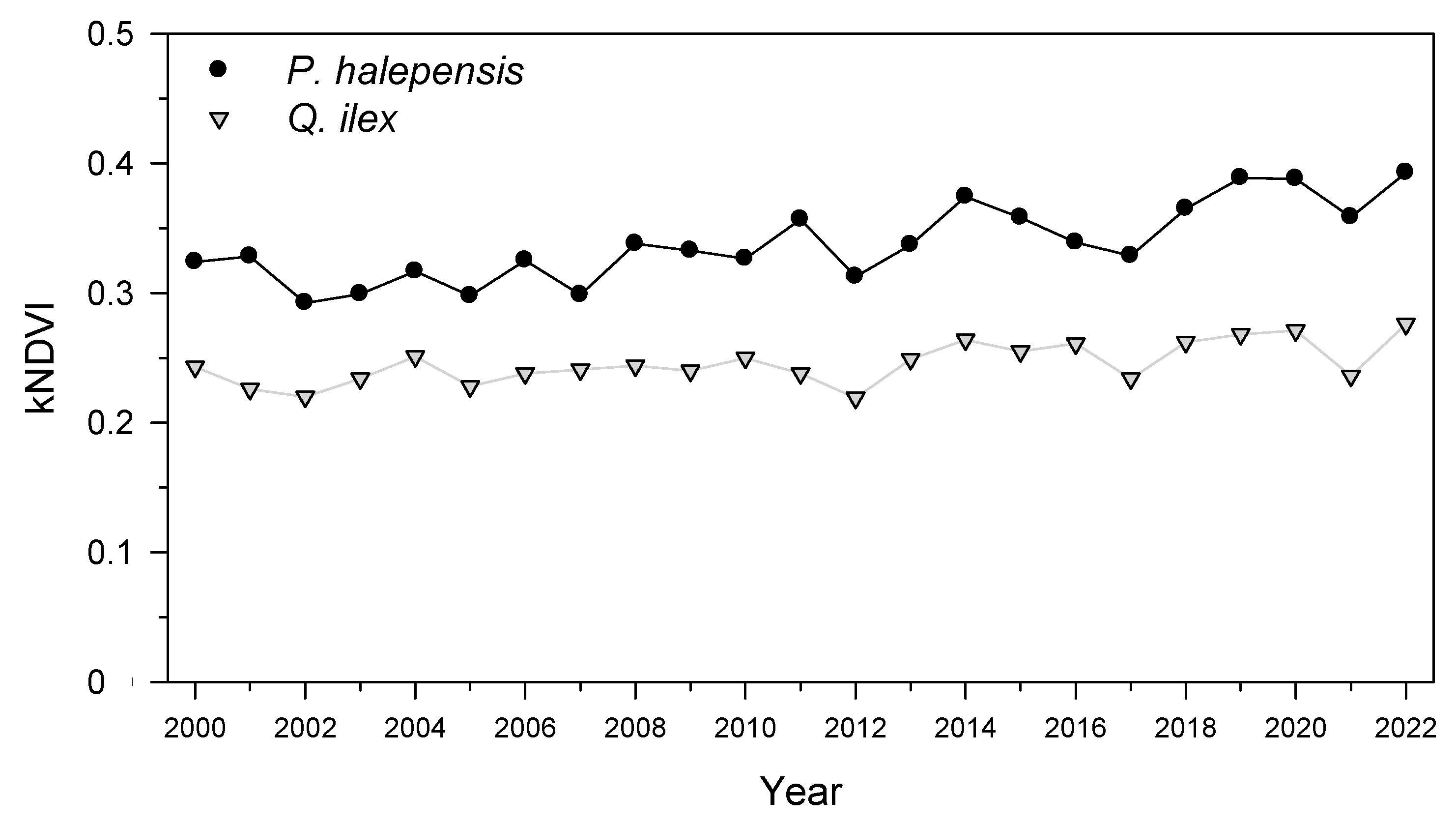
We obtained the kNDVI values for the two most affected areas dominated by
P. halepensis and
Q. ilex [
14,
15]. We eliminated cloudy images and harmonized different Landsat sensors (TM, ETM+, OLI) to obtain the annual kNDVI values, which were calculated as means of monthly values. The kNDVI values were obtained considering 50 m circular buffers centered in the two selected patches. The kNDVI is an improved version of the NDVI, which corrects part of saturation effects, phenological cycles and seasonal variations, and shows stronger correlations than the NDVI with gross primary production [
15].
2.3. Tree-Ring Width Data
We used dendrochronology to quantify changes in radial growth and growth responses to drought, including resilience [
16]. Cores were air-dried in the laboratory, glued into wooden supports, and their surface was sanded to enhance the visibility of tree-ring limits. Then, cores were scanned at 2400 dpi using a high-resolution scanner (Epson Expression 10.000 XL, Seiko Epson Corp., Suwa, Japan). Ring widths were measured with a 0.001 mm resolution using CDendro and CooRecorder software ver. 9.3.1 (Cybis Elektronik & Data AB, Stockholm, Sweden) [
17]. Cross-dating was checked using COFECHA software [
18], which allowed calculating moving correlations between the individual series and the mean series of each treatment.
To calculate climate–growth relationships, we removed age-, disturbance- or size-related influences on tree-ring width through detrending [
16]. Individual tree-ring width series were detrended using a cubic smoothing spline with a 50% frequency response cutoff at 2/3 length of series. Then, autoregressive models were applied to each detrended series to remove the first-order autocorrelation and to obtain residual or pre-whitened ring-width chronologies. These indexed series were averaged by using a bi-weight robust mean to develop site-level mean series or chronologies for the three study sites.
We calculated several statistics to characterize growth variability, namely: the first-order autocorrelation (AR1) of ring-width series to measure the persistence in growth and the mean sensitivity (MSx), which is the relative change in width between consecutive rings of indexed, non-prewhitened data, i.e., standard chronologies [
16,
19]. We also obtained the mean inter-series correlation (Rbar), which measures the growth coherence among trees [
19]. These variables were calculated over the common and best-replicated period of 1980–2021. Comparisons of growth and NSCs between stands or species were carried out using Mann–Whitney tests.
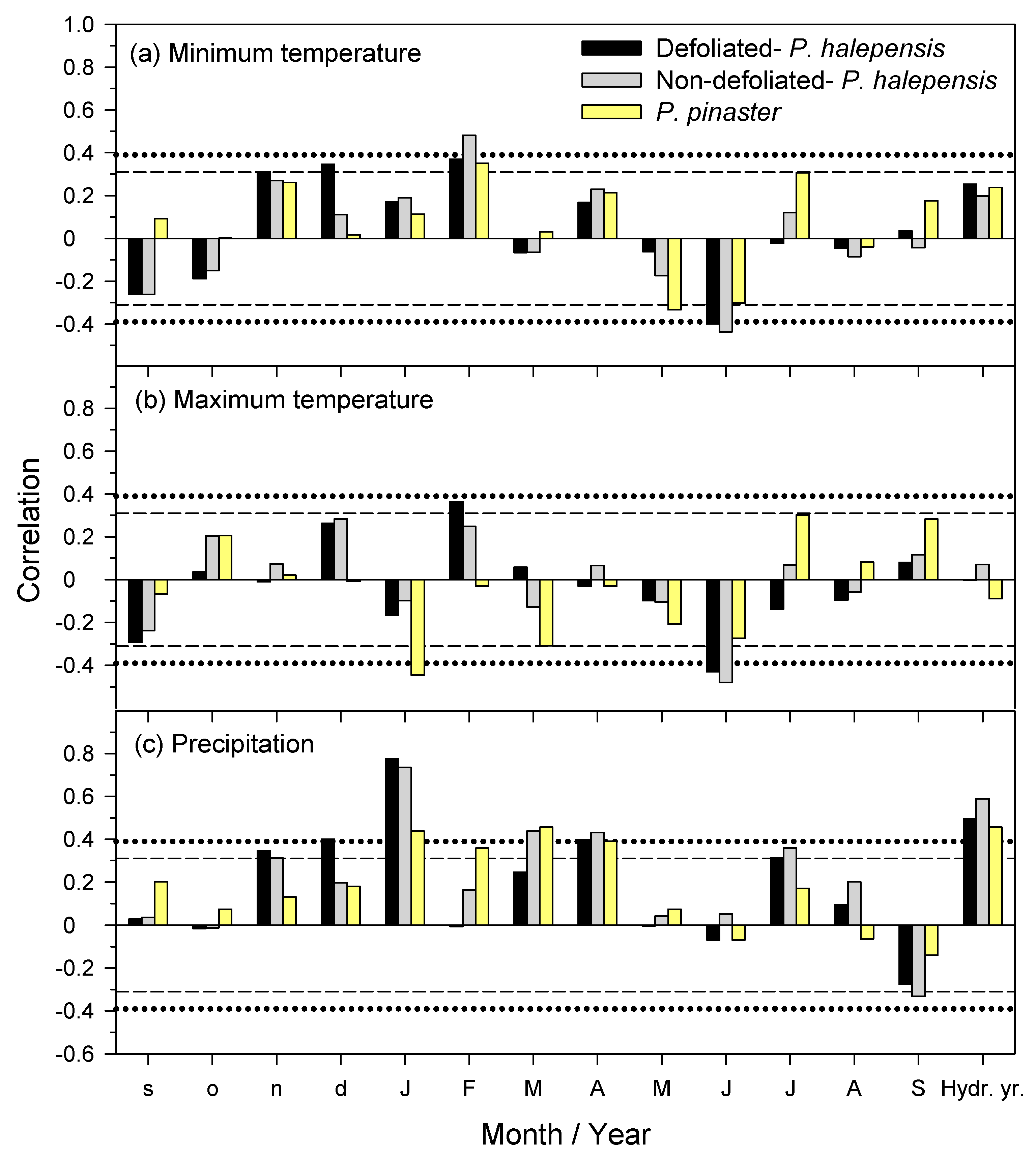
Climate–growth relationships were assessed by calculating Pearson correlations between monthly or annual (hydrological year) climate variables (mean temperature, total precipitation) from the Daroca station, which has a longer and more homogeneous record than Calamocha (
Figure S2), and series of residual ring-width indices. Correlations were calculated as follows: (i) considering the best-replicated period 1980–2020, and (ii) from the prior to current September, since climate conditions in the previous year influence growth in the following year [
16]. All analyses were carried out using the R statistical package ver. 4.1.3 [
20]. Detrending, chronology building and the calculation of dendrochronological statistics were performed using the dplR package [
21]. The resilience indices were calculated using the pointRes package [
22]. Climate–growth correlations were calculated using the treeclim package [
23].
2.4. Analyses of NSCs in Leaves and Sapwood
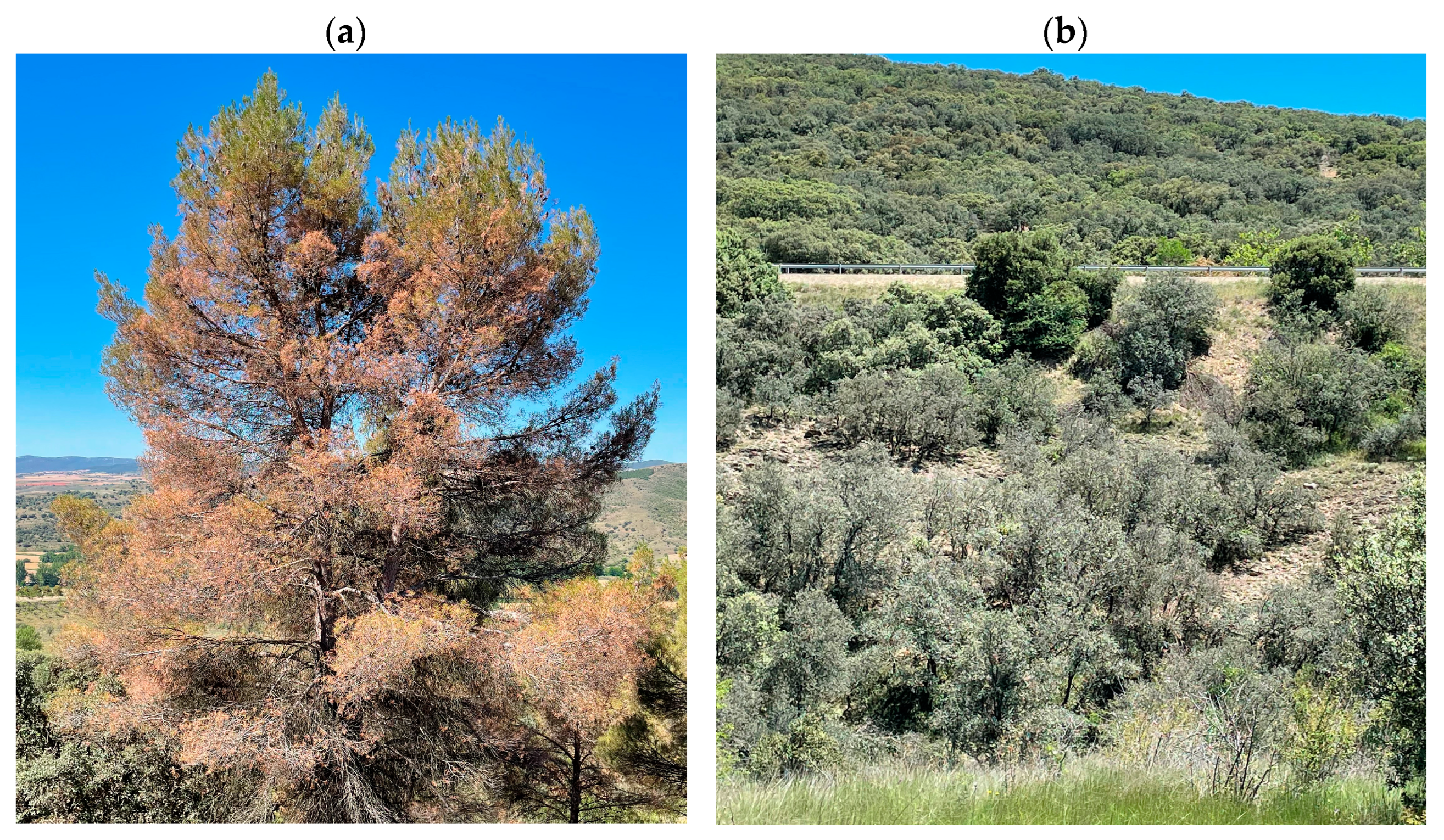
To analyze NSCs, sampling was conducted in the most defoliated
P. halepensis stand and in a nearby
Q. ilex population (40.960° N, 1.305° W, 873 m a.s.l., aspect W, slope 5°) which also showed defoliation after the 2021 cold spell (
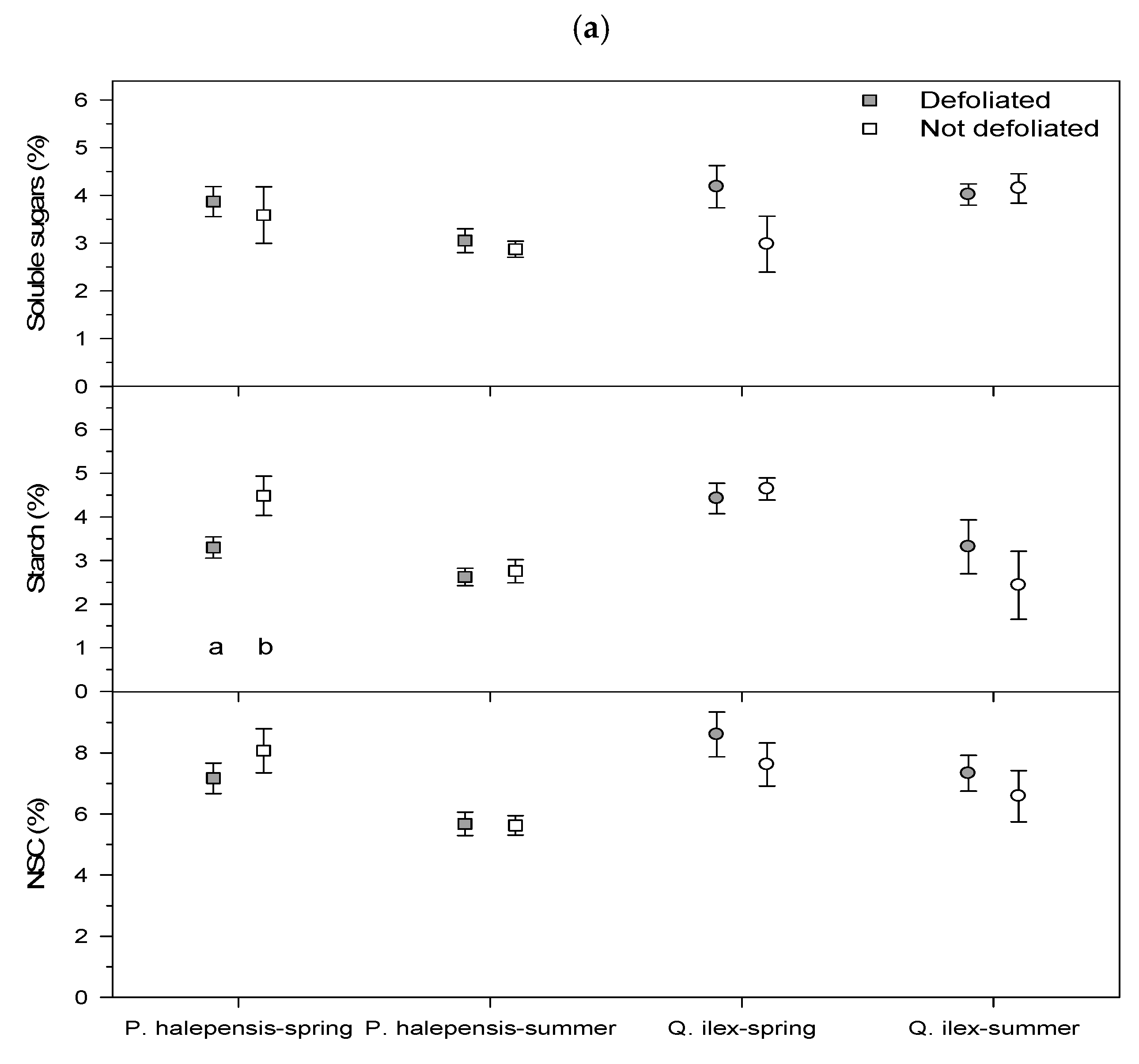
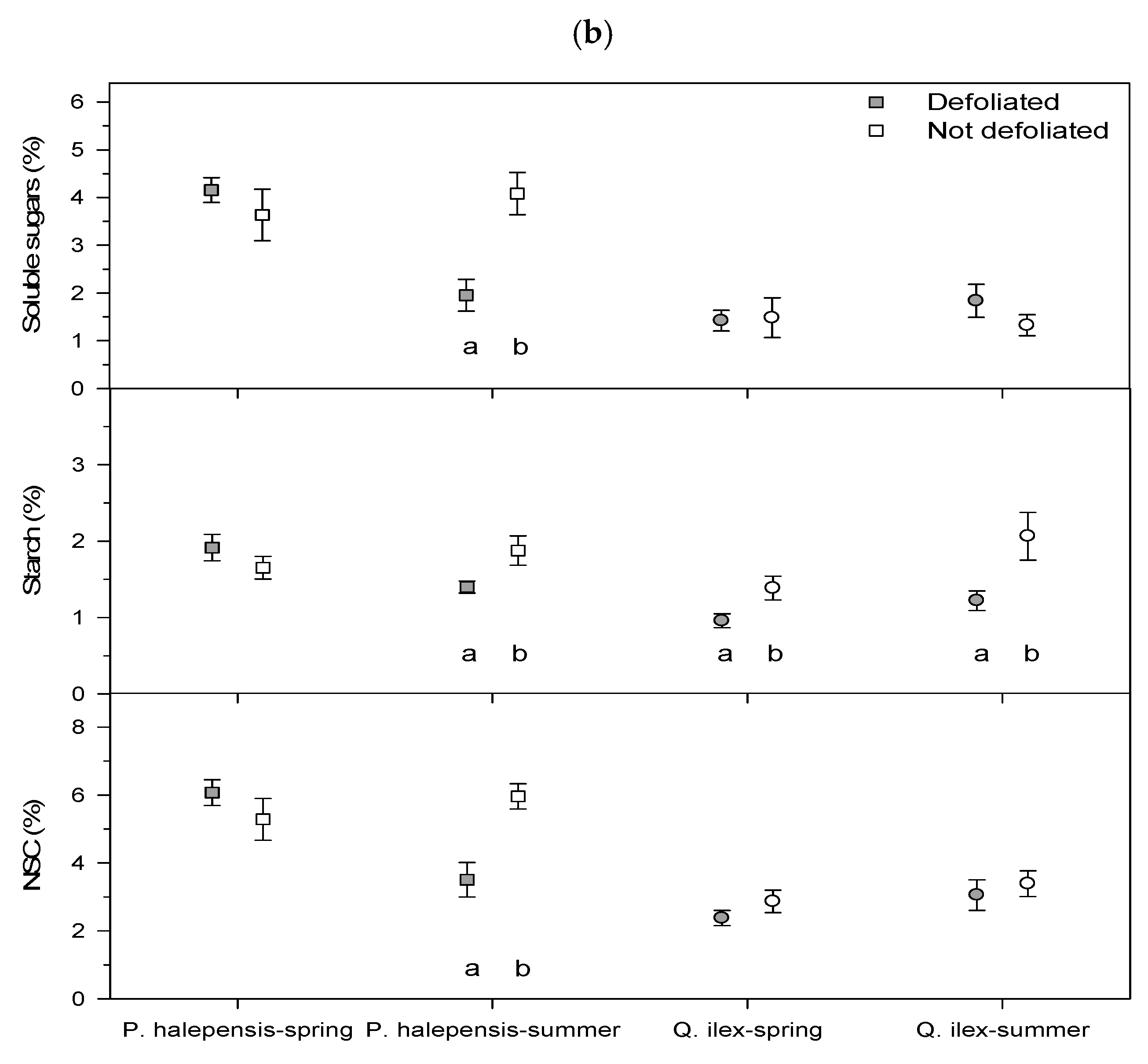
Figure 2). Sampling of leaves and sapwood for NSC analyses was carried out in early (29 June) and late summer (30 August) 2021, i.e., about 5.5 and 7.5 months after the January 2021 cold spell, respectively.
We collected leaves and sapwood within 10:00 and 12:00 h to avoid diurnal variability in NSC concentrations. Current-year leaves were collected from three branches located in the upper, light-exposed side of crowns since young needles account for a high proportion of total NSCs in conifers [
24]. Stem sapwood samples were taken at 1.3 m using a Pressler increment borer.
After being collected in the field, needle and wood samples were taken to the laboratory in a portable cooler. Needles were oven-dried at 60 °C for 72 h. Wood samples were subsequently frozen and stored at −20 °C until freeze-dried after removing the bark and phloem. All dried samples were weighted and milled to a fine powder in a ball mill (Retsch Mixer MM301, Leeds, UK) prior to chemical analyses.
Soluble sugars were extracted with 80% (
v/v) ethanol and their concentration determined colorimetrically at 490 nm, using the phenol–sulphuric method [
25,
26]. Starch and complex sugars remaining in the undissolved pellet after ethanol extractions were enzymatically reduced to glucose and analyzed [
27,
28]. To gelatinize starch, samples were incubated in a 4 cm
3 sodium acetate buffer in a shaking water bath at 100 °C for 1 h. After cooling to room temperature, 1 cm
3 of amyloglucosidase solution (0.5% amyloglucosidase 73.8 U/mg, Fluka 10,115, in acetate buffer) was added and the samples were incubated overnight for 16 h at 50 °C. After centrifugation, the concentration of starch and complex sugars as glucose equivalents was determined colorimetrically as described above using a spectrophotometer (Bio-Tek Instruments, Colmar, France). Enzyme blanks were included in each set of samples, and their absorption was subtracted from each sample’s absorbance prior to calculation of sugar content. The coefficient of variation of the extraction and measurement procedure determined from three independent replicates of the same powder did not exceed 8%.
NSCs measured after ethanol extraction are referred to as soluble sugars, and carbohydrates measured after enzymatic digestion are referred to as starch. Both are expressed in glucose equivalents. The sum of soluble sugars and starch is referred to as total NSCs.
4. Discussion
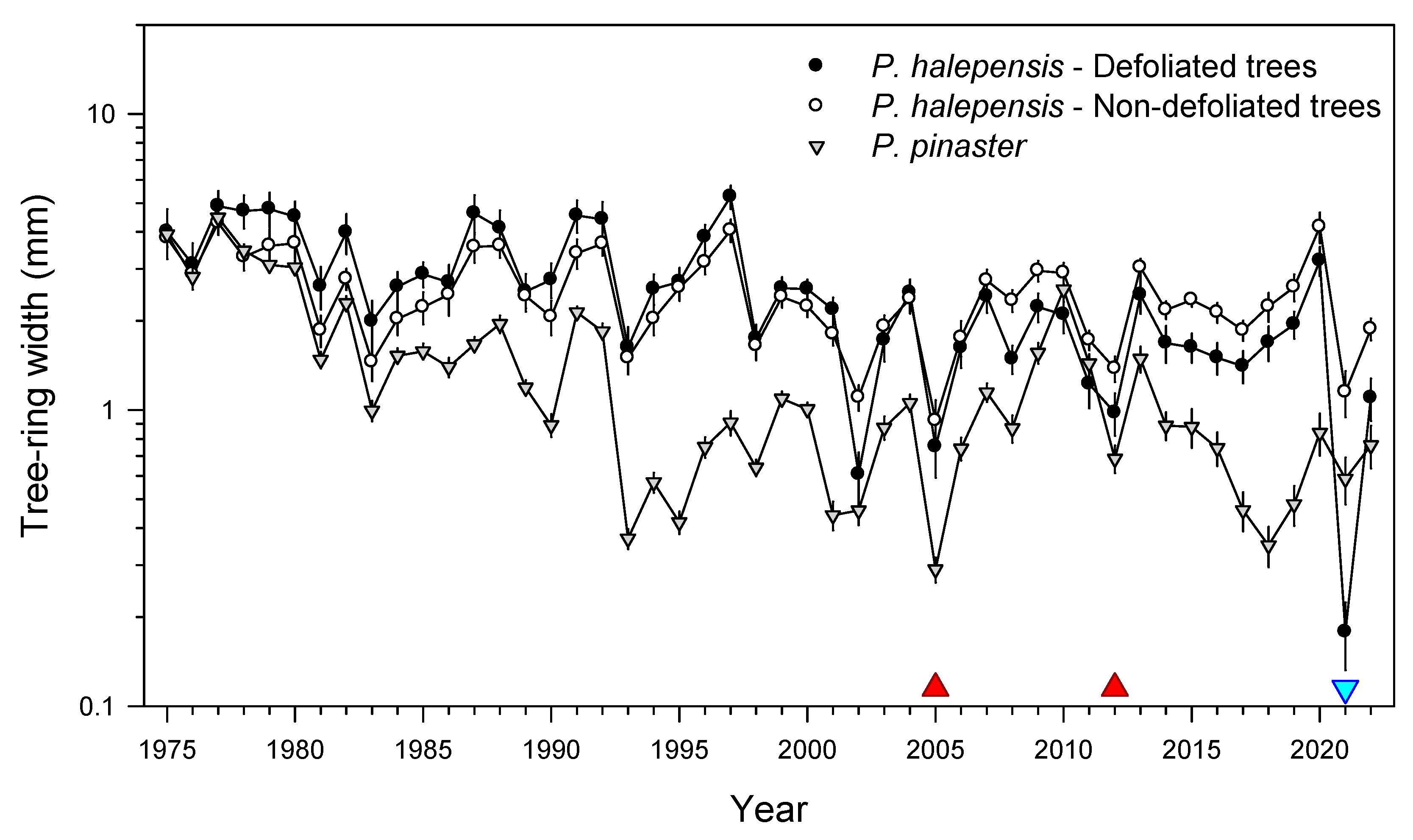
As hypothesized, tree species of subtropical origin dominant in Mediterranean forests, such as P. halepensis and Q. ilex, showed stronger losses in growth than P. pinaster, which can be found in colder sites. We found that the January 2021 cold spell led to a decrease in forest cover in the most defoliated pine (P. halepensis) and oak (Q. ilex) stands, reduced radial growth of defoliated P. halepensis and diminished sapwood NSC concentrations in P. halepensis and Q. ilex. As expected, the defoliated P. halepensis showed the strongest growth reduction in 2021 and lower NSC concentrations (leaf starch in spring, sapwood soluble sugars and starch in summer) than non-defoliated P. halepensis trees. However, they showed a high growth recovery capacity, comparable to that of non-defoliated conspecifics. In the case of Q. ilex, defoliated individuals presented lower starch sapwood concentrations than conspecifics in spring and summer. Some possible explanations for these findings are discussed below.
The extreme low air temperatures, which lasted several days, probably caused cold-induced xylem embolism, contributing to a loss of hydraulic conductivity and growth as has been observed in the alpine treeline [
29]. The cold spell was not preceded by a very warm autumn, which could have contributed to tissue dehardening, or by dry conditions or anticyclonic conditions leading to high radiation levels and pronounced leaf-to-air temperature differences enhancing water loss by transpiration [
30]. In addition, snow cover was deep in many sites, probably insulating shallow soils and avoiding rapid drops of soil temperatures which can also damage forests by constraining soil water uptake [
6]. Overall, the studied damages, basically crown defoliation, seem to be directly caused by the January 2021 cold spell, with 4–9 days having minimum temperatures lower than the long-term means. In addition, these damages were more frequent in concave microsites or in sites with W-SW exposure. If damage was higher in S exposure sites, freeze–thaw cycles would be the most plausible cause of hydraulic dysfunction [
29].
The fact that planted
P. halepensis individuals were more affected than co-occurring
P. pinaster trees agrees with their geographical distribution and ecological requirements. Mediterranean pines of subtropical origin inhabiting mild or cool winter locations, such as
P. halepensis, are vulnerable to frost damage, as several studies on seedlings have illustrated [
31,
32]. For instance,
P. halepensis seedlings had the lowest frost survival rate and the highest vulnerability to needle damage among several Iberian pine species [
31]. According to these experiments, seedling survival would be nil below −20 °C. However, in our study, temperatures below that threshold were measured in the Calamocha station and adult pines still survived. This discrepancy could be explained by a higher tolerance to cold damage of adults as compared to seedlings or to warmer microclimate conditions in the forest compared with the meteorological stations located in towns. More precise in situ measurements of microclimate conditions are required to test this idea.
At the individual scale, it is noteworthy that the defoliated
P. halepensis individuals grew more and showed a higher responsiveness to December–January precipitation than non-defoliated
P. halepensis and
P. pinaster prior to the cold spell. In a nearby site where
Pinus sylvestris L. stands experienced winter-drought dieback, the most affected trees also showed higher growth rates and lower water-use efficiency prior to the dieback [
33]. These patterns suggest that the most affected
P. halepensis trees were predisposed to the cold-spell damages because of their high growth rates and probably more of a “water-spender” strategy as compared with non-defoliated conspecifics, i.e., fast-growing trees were less likely to endure harsh winter cold spells. To test this hypothesis, tree-ring wood C and O isotopes and wood anatomical variables (e.g., lumen area) could be measured in those two types of trees. Furthermore, defoliated
P. halepensis trees started to grow less than non-defoliated conspecifics from 2008 onwards, which we interpreted as a differential response to the 2005 drought, which could have impaired the ability of the defoliated individuals to access deep soil water sources or to fully recover after a severe water shortage.
Despite the outer stem sapwood and leaves representing a large biomass amount in conifers and accounting for a high proportion of total NSC pools [
12], few differences in leaf NSC concentrations were found when comparing defoliated and non-defoliated trees, excepting
P. halepensis starch in spring, whereas more differences were found considering sapwood NSCs (soluble sugars and starch of
P. halepensis in summer, starch of
Q. ilex in spring and summer). The cold spell reduced the sapwood NSCs of
P. halepensis in summer, diminishing the resilience capacity of trees to withstand summer drought since soluble sugars can be used as osmolytes and provide freezing tolerance [
34]. The decrease of sapwood starch concentrations could also negatively impact growth (or sprouting capacity of
Q. ilex) since starch is a storage compound and it is particularly abundant in sprouts and roots [
34]. Nevertheless, carbon starvation is rare in defoliated trees showing severe growth reduction [
28]. To reach a critical survival threshold due to strong NSC drawdown (carbon starvation), crown defoliation should be higher than 75% and root NSC concentrations lower than 1.5% [
35]. This was not so in our case, since all sampled trees survived the 2021 cold spell and did not show such high defoliation levels. Therefore, differences were found in sink organs such as sapwood, where NSCs are stored in parenchyma cells, but not in C sources such as leaves, suggesting a rapid NSC replenishment of the main photosynthetic tissues. There could also be additional links between growth responses to climate and shifts in NSC concentrations. For instance, the positive effect on radial growth of high minimum February temperatures but the negative effect of high maximum January temperatures, at least for
P. pinaster, could be due to enhanced carbohydrate consumption triggered by a wide thermal range in winter as has been observed in other evergreen conifers [
36]. Nevertheless, the patterns of accumulation and loss of NSCs, especially starch, may or may not be correlated with cambial activity and radial growth [
37].